Abstract
Objective
To investigate the relationship among parity, length of the inter-pregnancy intervals and excessive pregnancy weight gain in the first pregnancy and the risk of obesity.
Methods
Using a prospective cohort study of 3422 non-obese, non-pregnant U.S. women aged 14–22 years at baseline, adjusted Cox models were used to estimate the association among parity, inter-pregnancy intervals, and excessive pregnancy weight gain in the first pregnancy and the relative hazard rate (HR) of obesity.
Results
Compared to nulliparous women, primiparous women with excessive pregnancy weight gain in the first pregnancy had a HR of obesity of 1.79 (95% CI: 1.40, 2.29); no significant difference was seen between primiparous without excessive pregnancy weight gain in the first pregnancy and nulliparous women. Among women with the same pregnancy weight gain in the first pregnancy and the same number of inter-pregnancy intervals (12 and 18 months or ≥ 18 months), the HR of obesity increased 2.43-fold (95% CI: (1.21, 4.89); p=0.01) for every additional inter-pregnancy interval of < 12 months; no significant association was seen for longer inter-pregnancy intervals. Among women with the same parity and inter-pregnancy interval pattern, women with excessive pregnancy weight gain in the first pregnancy had an HR of obesity 2.41 times higher (95% CI: (1.81, 3.21); p<0.001) than women without.
Conclusions
Primiparous and nulliparous women had similar obesity risk unless the primiparous women had excessive pregnancy weight gain in the first pregnancy, then their risk of obesity was greater. Multiparous women with the same excessive pregnancy weight gain in the first pregnancy and at least one additional short inter-pregnancy interval had a significant risk of obesity after childbirth. Perinatal interventions that prevent excessive pregnancy weight gain in the first pregnancy or lengthen the inter-pregnancy interval are necessary for reducing maternal obesity.
Keywords: pregnancy, inter-pregnancy interval, maternal obesity, parity, excessive pregnancy weight gain
INTRODUCTION
Length of the inter-pregnancy intervals, often defined as the interval between the end of one pregnancy and the start of the next pregnancy, is important to consider in the relationship between parity and maternal risk of obesity. The association of high parity and risk of obesity has not been consistently described in previous studies; some report that giving birth to one child compared to never having children appears to double the risk for major weight gain or obesity over a 5–10 year period, 1–4 while other studies show that multiparous women having at least two or more children compared to primiparous or nulliparous women have the greater increase in obesity. 2,5,6 A few studies have even reported a non-significant relationship between parity and obesity. 7,8 A possible explanation for the inconsistency in the findings in the relationship between parity and risk of obesity is the omission of the length of the interval between pregnancies from the analyses.
Research has shown that giving birth to two or more children in a short inter-pregnancy interval (defined as < 12 months) appears to increase the risk of adverse perinatal infant outcomes and increased maternal morbidity and mortality 9–14 However, the influence of the length of the inter-pregnancy interval on maternal weight outcomes, in particular obesity, is unknown. Conceivably, having short inter-pregnancy intervals and high parity may increase the risk of maternal obesity because weight may change significantly in the inter-pregnancy interval either because of weight retained from pregnancy or gained postpartum. Women who become obese during pregnancy remain significantly overweight or obese within five years after childbirth. 2 Additionally, a Swedish cohort found that an inter-pregnancy weight gain of 1–2 body mass index (BMI) units during an average of two years increases the risk of weight-related diseases, gestational hypertension and diabetes, by 20–40%. 15 Thus, understanding the contribution of parity, length of the inter-pregnancy interval, and excessive pregnancy weight gain is important for effectively reducing the risk of maternal obesity after childbirth. However, studying these relationships has been difficult due to the current lack of large diverse prospective cohorts (particularly in the U.S.) that have detailed perinatal data and sufficient follow-up for multiple births to have occurred. Prior studies have also been limited in the analytic approaches that were used because parity, length of the inter-pregnancy interval, and pregnancy weight gain were not treated as time-varying covariates.3,4,7,15,16 We addressed the limitations by analyzing data obtained from a national sample of U.S. women followed from adolescence into adulthood using an analytic approach that accounts for the time-varying nature of parity, length of the inter-pregnancy interval, and excessive pregnancy weight gain in the first pregnancy. Using a novel analytical approach, our study aim was to investigate the relationship between parity, length of inter-pregnancy intervals, and excessive pregnancy weight gain in the first pregnancy on the risk of obesity after childbirth. Our main hypothesis was that women with short inter-pregnancy intervals (< 12 months), a parity of greater than 2 children, and excessive pregnancy weight gain had higher risk of obesity, even after adjusting for demographic and socioeconomic factors.
MATERIALS AND METHODS
The National Longitudinal of Youth 1979 Survey (NLSY 79) is a national cohort study of 12,686 youth 14–21 years of age as of January 1, 1979. NLSY 79 was funded by the U.S. Department of Labor with the purpose of prospectively examining participants’ labor force experiences and investments in education and training. The National Institute for Child Health and Human Development provided additional funding to include perinatal and other health-related variables. Participants underwent yearly personal interviews from 1979–86 and 1988–94. Details of recruitment, sampling methods and study procedures have been published previously (http://www.bls.gov/nls).17 While several cohorts in other countries exist, 12,15,18 to our knowledge NLSY 79 is the largest nationally representative cohort in the U.S. with sufficient follow-up data collected on perinatal and clinical factors to investigate the relationship between perinatal factors and long-term weight change in women. Because the NLSY 79 dataset is de-identified and publically available, our study was exempted by the Institutional Review Board.
Sample
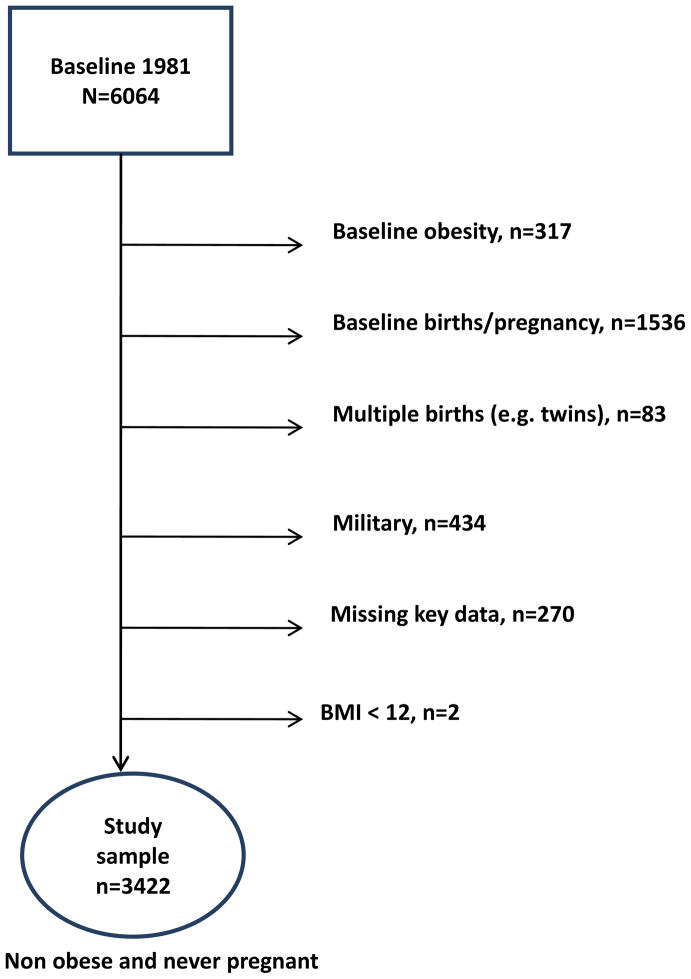
The NLSY 79 study was constructed from three independent multi-stage stratified area probability samples: a cross-sectional sample representative of the non-institutionalized U.S. civilian youth 14 to 22 years of age in 1979, a supplemental oversampling of Hispanics (n=977), African Americans (n=1472) and economically disadvantaged non-African American/non-Hispanics (n=901), and a military sample (n=456) of youths enlisted in the armed services in 1979. Of the 6064 women enrolled in NLSY 79, our study focused on a sample of 3422 (see Figure 1) nulliparous (no previous pregnancies or childbirths) and non-obese women followed from 1981 (when weight data were available) to 1990.
Figure 1.
Selection of study sample
Demographic variables
Age of each participant was calculated as the interview date in 1981 minus date of birth. Race/ethnicity was self-defined by each participant and was categorized as non-Hispanic white, non-Hispanic black, Hispanic or other (e.g. Asians, Native Americans and women who identified themselves as other). Marital status was dichotomized as married or not married (including single, divorced, separated or other). Place of residence was dichotomized as urban or rural. Because participants were adolescents or young adults at baseline, two proxy variables were used to define socioeconomic status: maternal education and poverty status. Maternal education was defined as the highest education level attained by the participant’s mother (no college degree or college degree or higher) and was selected because it has been shown to be strongly correlated with health risk behavior in adolescents.19 Family poverty status was defined as ‘in poverty’ if the family income for the family size was below the federal poverty income guidelines for the calendar year prior to the follow-up interview year. 17
Pregnancy related factors
Parity was defined as the number of live births. The date of the last menstrual period was not available and therefore, length of the inter-pregnancy interval was estimated as the length of time between the birth dates of each child minus 280 days (the average gestational age for a full-term infant) as done previous research.15,20 For each participant, inter-pregnancy intervals were then grouped by the number of inter-pregnancy intervals that were < 12 months (e.g. short inter-pregnancy interval), 12–18 months, and ≥ 18 months and were selected based on the literature.11 Excessive pregnancy weight gain was defined as weight gain in the first pregnancy that exceeded the upper range recommended by the Institute of Medicine’s guidelines for pre-pregnancy BMI, given by > 40 lbs for underweight, > 35 lbs for normal weight, > 25 lbs for overweight, and > 15 lbs for obese women. 21 For brevity, the term ‘excessive pregnancy weight gain’ will be used for the remainder of our discussion to refer to excessive pregnancy weight gain in the first pregnancy.
Main outcome
The main outcome was time to obesity, defined as the time from the baseline visit in 1981 to the visit when obesity was first detected. Obesity, calculated using self-reported heights and weights (collected in years 1981–82, 1985–1986, 1988–1990), was defined as a body mass index (BMI) ≥ 30 kg/m2 for adult women 17 years of age and older.22 Obesity for women younger than 17 years was defined as a BMI greater than the 95th percentile for age according to the age and gender specific BMI growth charts from the Centers from Disease Control and Prevention.23 The height in 1985 was used to calculate BMI for the later years because by then all women were assumed to have reached their adult height.
Statistical analyses
Baseline characteristics were summarized using means and standard deviations or percentages as appropriate. The cumulative incidence of obesity was estimated over the 9-year follow-up period using Kaplan-Meier curve estimates. To investigate the association between parity, length of the inter-pregnancy interval, and excessive pregnancy weight gain and the risk of obesity, we developed Cox proportional hazards regression models. Cox proportional hazards regression models estimate the effect of each covariate on the relative hazard rate of obesity (e.g., instantaneous risk of obesity for subjects relative to subjects with continuous covariates set to zero and categorical covariates set to their reference value) and are interpreted as the ratio of the relative hazard rates (or hazard ratio) per unit increase in a continuous covariate or the ratio of the relative hazard rate between levels of a categorical covariate. In our models, the number of IPIs in each of the 3 ranges, age, BMI and the number of years of mother’s education at baseline were treated as continuous covariates while excessive pregnancy weight gain, marital status, race, place of residence and poverty status were treated as categorical covariates. Additionally, our models also allowed covariates to be treated as fixed covariates that are measured at baseline only (and so do not change at subsequent visits) or time-varying covariates that are measured at the time of each visit. Fixed covariates included BMI, age, race, and maternal education at the baseline visit in 1981 and time-varying covariates included marital status and pregnancy-related factors (parity, length of the IPI and excessive pregnancy weight gain). Because we assumed that the place of residence and poverty status at both the baseline visit and at the time of each visit could potentially confound the association between IPI and the risk of obesity, each of these variables was included as both a fixed and time-varying covariate. A more detailed description of our model is provided in the supplemental digital content online.
Additional analyses also incorporated an interaction between race and each of the covariates used to describe parity and length of the inter-pregnancy interval to investigate if the association between parity and length of the inter-pregnancy interval and the risk of obesity varied by race. Similar analyses were conducted to investigate if each of the socioeconomic factors (such as marital status, place of residence, maternal education, and poverty status) modified the effect of the association between parity and length of the inter-pregnancy interval and the risk of obesity as well.
Cox proportional hazards models also assume that the hazard rate is proportional to the baseline hazard rate independently of time and so for each fixed covariate, the assumption was tested by estimating a separate hazard ratio of obesity for the periods from 1981–1983, 1984–1986, and 1987–1990. If no evidence of a significant difference among the hazard ratios was found, the assumption of proportional hazards was assumed to be valid.
All analyses assumed a two-sided significance level of 0.05 and were performed using SAS statistical software version 9.2.
Results
Study sample
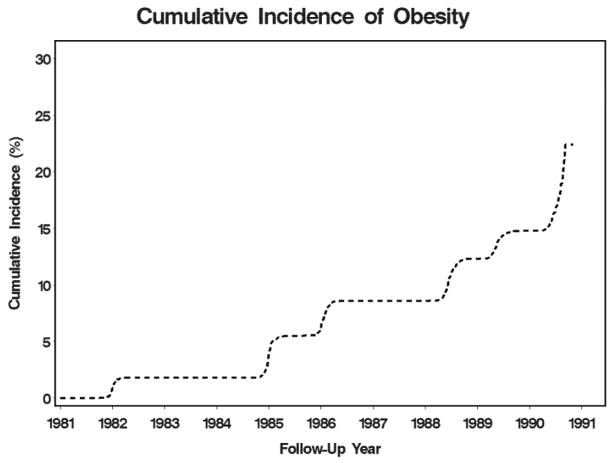
Table 1 displays the demographic and clinical characteristics for all 3422 participants at baseline and characteristics stratified by excessive pregnancy weight gain. Women were followed on average for 8.3 years (SD=2.5) and by the end of follow-up, 53% of women were nulliparous, 23% had one child and 24% had > 1 child. Among women who had at least one birth during the study, 41% had excessive weight gain and among women who had > 1 child, the average length of the first inter-pregnancy interval was 33.9 months (SD=17). Figure 2 provides the cumulative incidence of obesity over the 9-year follow-up period for all 3422 participants.
Table 1.
Demographic and clinical characteristics at baseline, expressed as mean (SD) or %
| Baseline demographics | Total n =3422 | Nulliparous2 n = 1803 | Parous and no excessive PWG2,3 n = 948 | Parous and excessive PWG2,3 n = 671 |
|---|---|---|---|---|
|
| ||||
| % BMI | ||||
| Underweight (BMI < 18.5) | 8 | 8 | 9 | 6 |
| Normal (BMI 18.5 – 24.9) | 79 | 76 | 84 | 81 |
| Overweight (BMI 25 – 29.9) | 13 | 16 | 6 | 14 |
| Age, mean (SD) | 19.7 (2.2) | 19.7(2.2) | 19.9 (2.2) | 19.7(2.2) |
| % Race | ||||
| Non-Hispanic white | 54 | 57 | 52 | 52 |
| Non-Hispanic black | 19 | 18 | 20 | 22 |
| Hispanic | 14 | 12 | 16 | 15 |
| Other1 | 12 | 13 | 12 | 11 |
| % Married | 13 | 9 | 18 | 16 |
| % Urban residence | 80 | 82 | 78 | 78 |
| % Live below poverty line | 18 | 16 | 22 | 19 |
| Number of years of maternal education, mean (SD) | 11.2 (3.1) | 11.5 (3.2) | 10.9 (3.2) | 10.9 (2.9) |
Other includes Native Americans, Asians, and women who identified self as other.
Parity and excessive pregnancy weight gain status at the end of follow-up
PWG=pregnancy weight gain during the first pregnancy
Figure 2.
Cumulative incidence of obesity for sample 3422
Association between pregnancy- related factors and relative hazard rate of obesity
Table 2 provides hazard ratios of obesity for parity, length of the inter-pregnancy interval, and excessive pregnancy weight gain, adjusting for demographic and socioeconomic variables. Race and socioeconomic factors were not modifiers of the association between parity and length of the inter-pregnancy interval and the relative hazard rate of obesity; therefore, no interactions between these factors were required in the adjusted model. For women with at least one birth and the same inter-pregnancy interval pattern, the adjusted relative hazard rate of obesity was 2.41 times higher (95% CI: (1.81, 3.21); p<0.001) in women who had excessive pregnancy weight gain compared to women who did not. For women that had the same excessive pregnancy weight gain and the same number of inter-pregnancy intervals (between 12 and 18 months or ≥ 18 months), the adjusted relative hazard rate of obesity increased 2.43-fold (95% CI: (1.21, 4.89); p=0.01) for every additional inter-pregnancy interval < 12 months. No significant difference was seen between the number of longer inter-pregnancy intervals (12–18 and ≥ 18 months) and the relative hazard rate of obesity.
Table 2.
Hazard ratio of obesity for demographic, socioeconomic status, and perinatal covariates of interest.
| Unadjusted Model1 | Adjusted Model2 | |||
|---|---|---|---|---|
| Participant characteristics | HR(95%CI) | p-value | HR(95%CI) | p-value |
| PERINATAL | ||||
| Number of IPI < 12 months (per every additional interval) | 2.55 (1.32, 4.91) | 0.005 | 2.43 (1.21, 4.89)3 | 0.01 |
| Number of IPI between 12 and 18 months (per every additional interval) | 1.42 (0.95, 2.13) | 0.09 | 1.25 (0.71, 2.19)3 | 0.43 |
| Number of IPI ≥ 18 months (per every additional interval) | 1.20 (0.96, 1.51) | 0.11 | 1.01 (0.60, 1.68)3 | 0.98 |
| Excessive PWG vs. no excessive PWG | 2.41 (1.81, 3.21)4 | < 0.001 | ||
| DEMOGRAPHICS | ||||
| Baseline BMI5 (per 1 unit increment) (1981 – 1983) | 1.99 (1.78, 2.22) | < 0.001 | 2.00 (1.79, 2.24) | < 0.001 |
| Baseline BMI5 (per 1 unit increment) (1984 – 1986) | 1.73 (1.64, 1.81) | < 0.001 | 1.72 (1.64, 1.81) | < 0.001 |
| Baseline BMI5 (per 1 unit increment) (1987 – 1989) | 1.47 (1.41, 1.54) | < 0.001 | 1.45 (1.38, 1.51) | < 0.001 |
| Baseline age (per 1 year increment) | 0.93 (0.90, 0.97) | 0.001 | ||
| Time-dependent married vs. not married | 1.13 (0.92, 1.38) | 0.25 | ||
| Non-Hispanic black vs. Non-Hispanic white | 1.53 (1.23, 1.90) | < 0.001 | ||
| Hispanic vs. Non-Hispanic white | 1.35 (1.02, 1.78) | 0.04 | ||
| Other vs. Non-Hispanic white | 1.20 (0.91, 1.59) | 0.20 | ||
| Baseline Urban vs. Rural5 (1981 – 1983) | 2.31 (1.01, 5.28) | 0.05 | ||
| Baseline Urban vs. Rural5 (1984 – 1986) | 1.35 (0.94, 1.96) | 0.11 | ||
| Baseline Urban vs. Rural5 (1987 – 1989) | 0.91 (0.66, 1.25) | 0.56 | ||
| Time-dependent urban vs. rural | 1.02 (0.80, 1.30) | 0.90 | ||
| SOCIOECONOMIC | ||||
| Number of years of mother’s education at baseline (per 1 year increment) | 0.99 (0.96, 1.02) | 0.55 | ||
| Baseline poverty vs. non-poverty | 1.08 (0.87, 1.36) | 0.47 | ||
| Time-dependent poverty vs. non-poverty | 0.96 (0.75, 1.23) | 0.76 | ||
Unadjusted model contains parity/IPI and baseline BMI variables.
Adjusted model contains variables in the unadjusted model and variables for demographic, socioeconomic and PWG characteristics.
Assuming all women have at least 2 births and the same PWG.
Assuming all women have at least 1 birth, the same parity, and the same IPI pattern.
BMI and residential status at baseline did not satisfy the proportional hazards assumption and so hazard ratios for each of the covariates were separately estimated for the periods from 1981–1983, 1984–1986, and 1987–1989.
Table 3 provides estimates of the adjusted hazard ratio of obesity comparing women with 1 or 2 births to nulliparous or primiparous women based on the adjusted model in Table 2. The adjusted relative hazard rate of obesity was 1.79 times higher (95% CI: (1.40, 2.29); p<0.001) in primiparous women with excessive pregnancy weight gain compared to nulliparous women whereas no significant difference was seen between primiparous women without excessive pregnancy weight gain and nulliparous women (HR=0.74; 95% CI: (0.55, 1.01); p=0.06). When comparing two groups of primiparous women, the women with excessive pregnancy weight gain had the greater risk of obesity (HR=2.41; 95% CI: (1.81, 3.21), p= <0.001). Regardless of IPI length, the adjusted relative hazard rate of obesity in multiparous women with 2 births with excessive pregnancy weight gain was approximately 2 to 4 times higher than in nulliparous and primiparous women without excessive pregnancy weight gain (p<0.001); women with short inter-pregnancy intervals had the highest risk obesity.
Table 3.
Adjusted hazard ratio1 (and 95% CI) of obesity comparing primiparous or multiparous women with 2 births (Group A) to nulliparous or primiparous women with and without excessive pregnancy weight gain (Group B).
| GROUP A | GROUP B (REFERENCE GROUP)
|
||
|---|---|---|---|
| Nulliparous3 | Primiparous No Excessive PWG2,3 | Primiparous Excessive PWG2,3 | |
|
| |||
| Primiparous ♀ with: | |||
| No excessive PWG2 | 0.74 (0.55,1.01) | 1 | -- |
| Excessive PWG2 | 1.79 (1.40,2.29)* | 2.41(1.81,3.21)* | 1 |
|
| |||
| Multiparous♀ had 2 births and no excessive PWG2 with one IPI: | |||
| Less than 12 m | 1.82 (0.77,4.29) | 2.45 (1.06,5.68)* | 1.02 (0.43,2.43) |
| 12 to18 m | 0.94 (0.55,1.59) | 1.26 (0.76,2.09) | 0.52 (0.30,0.92)* |
| 18 m or more | 0.75 (0.53,1.08) | 1.01 (0.75,1.38) | 0.42 (0.28,0.63)* |
|
| |||
| Multiparous♀ had 2 births with excessive PWG2 and one IPI: | |||
| Less than 12 m | 4.40 (1.88,10.3)* | 5.91 (2.40,14.6)* | 2.45 (1.06,5.68)* |
| 12 to18 m | 2.26 (1.35,3.79)* | 3.04 (1.67,5.53)* | 1.26 (0.76,2.09) |
| 18 m or more | 1.82 (1.32,2.52)* | 2.45 (1.59,3.76)* | 1.01 (0.75,1.38) |
Results are based on adjusted model defined in Table 2.
PWG= pregnancy weight gain. Statistical significance at 0.05 level is identified by a *.
Nulliparous women have no births during the study. Primiparous women have one birth during the study. Multiparous women have two births and one IPI during the study.
Table 4 provides estimates of the adjusted hazard ratio of obesity comparing women with 2 births with the same excessive pregnancy weight gain but with different inter-pregnancy interval patterns based on the adjusted model in Table 2. For instance, the relative hazard rate of obesity in women with an inter-pregnancy interval < 12 months was 2.42 times higher (95% CI: (1.08, 5.40); p=0.03) than in women with an inter-pregnancy interval ≥ 18 months. Alternatively, the relative hazard rate of obesity in women with an inter-pregnancy interval between 12 and 18 months did not differ from women with an inter-pregnancy < 12 months or from women with an inter-pregnancy interval ≥ 18 months.
Table 4.
Adjusted hazard ratio1 (and 95% CI) of obesity comparing two groups (Group A to Group B) of multiparous women with two births but different IPI patterns.
| GROUP A | GROUP B (REFERENCE GROUP) | ||
|---|---|---|---|
|
| |||
| Multiparous ♀ with two births and one IPI: | Multiparous♀ with two births and one IPI:
|
||
| Less than 12 m | 12 to 18 m | 18 m or more | |
|
| |||
| Less than 12 m | 1 | 1.94 (0.85,4.45) | 2.42 (1.08,5.40)* |
| 12 to18 m | -- | 1 | 1.24 (0.76,2.03) |
| 18 m or more | -- | -- | 1 |
Results are based on adjusted model defined in Table 2. Statistical significance at 0.05 level is identified by a *.
Table 5 shows the adjusted hazard ratios of obesity of multiparous women with the same excessive pregnancy weight gain but with differing parity (3 births vs 2 births) and inter-pregnancy intervals. For most cases, the relative hazard rate of obesity was significantly higher in the group that had at least one additional inter-pregnancy interval < 12 months than women with longer inter-pregnancy intervals.
Table 5.
Adjusted hazard ratio1 (and 95% CI) of obesity comparing multiparous women with three births (Group A) to women with two births (Group B).
| GROUP A | GROUP B (REFERENCE GROUP) | ||
|---|---|---|---|
|
| |||
| Multiparous♀ with three births and two IPIs: | Multiparous♀ with two births and one IPI:
|
||
| Less than 12 m | 12 to 18 m | 18 m or more | |
|
| |||
| Both are less than 12 m | 2.43 (1.21,4.89)* | 4.72 (1.13,19.7)* | 5.87 (1.42,24.2)* |
| Both are 12 to 18m | 0.64 (0.19,2.19) | 1.25 (0.71,2.19) | 1.55 (0.62,3.90) |
| Both are 18m or more | 0.42 (0.13,1.32) | 0.81 (0.35,1.87) | 1.01 (0.60,1.68) |
| 1 IPI <12 m and 1 IPI 12 –18 m | 1.25 (0.71,2.19) | 2.43 (1.21,4.89)* | 3.02 (1.28,7.14)* |
| 1 IPI < 12 m and 1 IPI ≥ 18 m | 1.01 (0.60,1.68) | 1.96 (0.83,4.59) | 2.43 (1.21,4.89)* |
| 1 IPI 12–18 m and 1 IPI ≥ 18 m | 0.52 (0.17,1.53) | 1.01 (0.60,1.68) | 1.25 (0.71,2.19) |
Results are based on adjusted model defined in Table 2. Statistical significance at 0.05 level is identified by a *.
Discussion
We found that multiparous women with short inter-pregnancy intervals, with or without excessive pregnancy weight gain, had a substantial risk of obesity after childbirth compared to multiparous women with longer inter-pregnancy intervals. Our findings are consistent with previous study by Zhu and colleagues suggesting an inter-pregnancy interval of 18–23 months may be optimal for preventing adverse maternal and infant health outcomes. 11 Short inter-pregnancy intervals of less than 12 months are concerning because they may not provide adequate time to appropriately lose weight gained during pregnancy, to replenish maternal nutritional deficiencies (e.g. folate and iron) that occur in pregnancy or to return to the “normal” pre-pregnancy metabolic state before the conception of the next pregnancy.24,25 The concomitant metabolic demands of breastfeeding and weight change during and after pregnancy also affect the nutritional status of the mother.26 This metabolic conundrum may limit the mothers’ ability to support the nutritional needs of both herself and a new growing fetus. Thus, giving birth to two or more children in short inter-pregnancy intervals contributes to anemia, a low birth weight or small for gestational age baby, premature birth, congenital malformations, and early fetal and maternal death;12–14 our findings suggest maternal obesity as well.
We also found that primiparous and nulliparous women had similar obesity risk unless the primiparous women had excessive pregnancy weight gain, then their risk of obesity was greater. Likewise, when comparing multiparous women to nulliparous and primiparous women, excessive pregnancy weight gain was associated with a higher risk for obesity in that group. Excessive pregnancy weight gain, particularly in the first pregnancy, has been reported to be a strong predictor of postpartum weight retention and maternal obesity. 2,27–29 Our findings build upon the previous studies by showing that the addition of short inter-pregnancy intervals and excessive weight gain in the first pregnancy further increased the risk for maternal obesity.
Maternal obesity remains concerning for not only the long-term risk of obesity and type 2 diabetes but its short-term risk of morbidity (such as gestational diabetes, operative complications, premature rupture of membranes, birth trauma, neonatal metabolic abnormalities) and death of a young mother and/or her baby during pregnancy, delivery and postpartum.6,30 Therefore, in addition to efforts to prevent excessive pregnancy weight gain (i.e. help women gain within the new 2009 IOM pregnancy weight gain guidelines), research and clinical practice should consider short inter-pregnancy intervals in addressing maternal obesity. Physicians and nurses can educate postpartum women on the perinatal hazards of short inter-pregnancy intervals for mother and baby. Once counseled, women could be offered long-term contraception such as IUDs and implants to help lengthen the inter-pregnancy interval.26 Additionally, clinical and population-based research using cohorts derived from electronic health records or birth certificate data are a feasible way to investigate the prevalence of and modifiable risk factors for short inter-pregnancy intervals.
As with all studies, several limitations deserve mention. First, factors of interest such as smoking did not have a consistent definition at each time point for incorporation as a time-varying covariate. Second, inherent in prospective designs, is the potential for residual confounding of other perinatal variables such as postpartum weight retention and gestational weight gain in subsequent pregnancies. We addressed the issue of confounding by adjusting for weight gain in the first pregnancy based on prior evidence that weight gain in the first pregnancy may have a greater effect on long-term obesity risk.2,31 Third, we were unable to account for pre-term births (prevalence of pre-term births in 1990 was about 10%) and based our findings on full term births (gestational age of 280 days). By doing so, we may have overestimated the length of the inter-pregnancy interval by approximately 21 days if 259 days was used as the estimate for gestational age for preterm births, which would attenuate the estimated hazard ratios. Fourth, treating time-to-obesity (measured annually rather than when obesity actually occurred) as a right censored, rather than as an interval censored, time-to-event outcome could potentially bias the hazard ratio because we assumed that obesity occurred at the annual visit time rather than some time in between the last annual visit when the subject may not have been obese. Additionally, no adjustment was made for the complex sampling design used to obtain the NLSY 1979 cohort which limits national generalizability. Finally, our results may underestimate the incidence of obesity due to the use of self-reported weight and height 32 and use of an older cohort in which the overall prevalence of obesity (16%–23%) was lower than current estimates (35.8% among adult women).33 However, due to limited number of national datasets in the U.S. with high retention rates (95–91%) 17, a diverse population of young woman, and all of the necessary perinatal and anthropometric variables, the NLSY 79 having all of these features best enabled us to illustrate our unique analytic approach.
Our study is the first to our knowledge to show and independent association between length of inter-pregnancy interval and maternal obesity. In the absence of randomized control trials, our results are consistent with a growing body of observational research indicating the need for clinical interventions targeting women at risk for short inter-pregnancy intervals and excessive pregnancy weight gain. Future investigations should focus on special subgroups such as adolescent mothers, mothers with substance use disorders, or with preterm births, all of whom may have significant risk for shorter IPI intervals. Physicians and clinical staff should discuss with their patients during preconception planning and prenatal care visits about the potential risks of poor perinatal outcomes due to close birth spacing.
Supplementary Material
Acknowledgments
This publication was made possible by the Case Western Reserve University/Cleveland Clinic CTSA Grant Number UL1 RR024989 from the National Center for Research Resources, a component of the National Institutes of Health and NIH roadmap for Medical Research. Dr. Esa Davis was supported by a Harold Amos Medical Faculty Award from the Robert Wood Johnson Foundation, grant #053501. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH or RWJ. This study has not received any funding support from a commercial organization.
References
- 1.Gunderson EP, Quesenberry CP, Jr, Lewis CE, et al. Development of overweight associated with childbearing depends on smoking habit: The Coronary Artery Risk Development in Young Adults (CARDIA) Study. Obes Res. 2004;12:2041–53. doi: 10.1038/oby.2004.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis EM, Zyzanski SJ, Olson CM, Stange KC, Horwitz RI. Racial, ethnic, and socioeconomic differences in the incidence of obesity related to childbirth. Am J Public Health. 2009;99:294–9. doi: 10.2105/AJPH.2007.132373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williamson DF, Madans J, Pamuk E, Flegal KM, Kendrick JS, Serdula MK. A prospective study of childbearing and 10-year weight gain in US white women 25 to 45 years of age. Int J Obes Relat Metab Disord. 1994;18:561–9. [PubMed] [Google Scholar]
- 4.Rosenberg L, Palmer JR, Wise LA, Horton NJ, Kumanyika SK, Adams-Campbell LL. A prospective study of the effect of childbearing on weight gain in African-American women. Obes Res. 2003;11:1526–35. doi: 10.1038/oby.2003.204. [DOI] [PubMed] [Google Scholar]
- 5.Coitinho DC, Sichieri R, D’Aquino Benicio MH. Obesity and weight change related to parity and breast-feeding among parous women in Brazil. Public Health Nutr. 2001;4:865–70. doi: 10.1079/phn2001125. [DOI] [PubMed] [Google Scholar]
- 6.Magann EF, Doherty DA, Chauhan SP, Klimpel JM, Huff SD, Morrison JC. Pregnancy, obesity, gestational weight gain, and parity as predictors of peripartum complications. Archives of gynecology and obstetrics. 2011;284:827–36. doi: 10.1007/s00404-010-1754-0. [DOI] [PubMed] [Google Scholar]
- 7.Brown JE, Kaye SA, Folsom AR. Parity-related weight change in women. Int J Obes Relat Metab Disord. 1992;16:627–31. [PubMed] [Google Scholar]
- 8.Heliovaara M, Aromaa A. Parity and obesity. J Epidemiol Community Health. 1981;35:197–9. doi: 10.1136/jech.35.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conde-Agudelo A, Belizan JM. Maternal morbidity and mortality associated with interpregnancy interval: cross sectional study. Bmj. 2000;321:1255–9. doi: 10.1136/bmj.321.7271.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conde-Agudelo A, Rosas-Bermudez A, Kafury-Goeta AC. Effects of birth spacing on maternal health: a systematic review. Am J Obstet Gynecol. 2007;196:297–308. doi: 10.1016/j.ajog.2006.05.055. [DOI] [PubMed] [Google Scholar]
- 11.Zhu BP, Haines KM, Le T, McGrath-Miller K, Boulton ML. Effect of the interval between pregnancies on perinatal outcomes among white and black women. Am J Obstet Gynecol. 2001;185:1403–10. doi: 10.1067/mob.2001.118307. [DOI] [PubMed] [Google Scholar]
- 12.Grisaru-Granovsky S, Gordon ES, Haklai Z, Samueloff A, Schimmel MM. Effect of interpregnancy interval on adverse perinatal outcomes--a national study. Contraception. 2009;80:512–8. doi: 10.1016/j.contraception.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Rodrigues T, Barros H. Short interpregnancy interval and risk of spontaneous preterm delivery. Eur J Obstet Gynecol Reprod Biol. 2008;136:184–8. doi: 10.1016/j.ejogrb.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 14.Zhu BP. Effect of interpregnancy interval on birth outcomes: findings from three recent US studies. Int J Gynaecol Obstet. 2005;89 (Suppl 1):S25–33. doi: 10.1016/j.ijgo.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Villamor E, Sparen P, Cnattingius S. Interpregnancy weight gain and the male-to-female sex ratio of the second pregnancy: a population-based cohort study. Fertil Steril. 2008;89:1240–4. doi: 10.1016/j.fertnstert.2007.07.1371. [DOI] [PubMed] [Google Scholar]
- 16.Coitinho DC, Sichieri R, D’Aquino Benicio MH. Obesity and weight change related to parity and breast-feeding among parous women in Brazil. Public Health Nutr. 2001;4:865–70. doi: 10.1079/phn2001125. [DOI] [PubMed] [Google Scholar]
- 17.US Department of Labor. NLSY79 User’s guide. Columbus, OH: Center for Human Resource Research; 2004. p. 393. [Google Scholar]
- 18.Kaharuza FM, Sabroe S, Basso O. Choice and chance: determinants of short interpregnancy intervals in Denmark. Acta obstetricia et gynecologica Scandinavica. 2001;80:532–8. [PubMed] [Google Scholar]
- 19.Miech R, Chilcoat H. Maternal education and adolescent drug use: a longitudinal analysis of causation and selection over a generation. Soc Sci Med. 2005;60:725–35. doi: 10.1016/j.socscimed.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 20.Kwon S, Lazo-Escalante M, Villaran MV, Li CI. Relationship between interpregnancy interval and birth defects in Washington State. Journal of perinatology: official journal of the California Perinatal Association. 2012;32:45–50. doi: 10.1038/jp.2011.49. [DOI] [PubMed] [Google Scholar]
- 21.Medicine Io. Nutrition During Pregnancy. Washington DC: National Academy Press; 1990. pp. 1–468. [Google Scholar]
- 22.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii. 1–253. [PubMed] [Google Scholar]
- 23.National Center for Health Statistics. 2000 Clinical Growth Charts for the United States. Center for Disease Control and Prevention; [Accessed June 8, 2010]. Available at www.cdc.gov/nchs/about/major/nhanes/growthcharts/clinical_charts.htm. [Google Scholar]
- 24.Dewey KG, Cohen RJ. Does birth spacing affect maternal or child nutritional status? A systematic literature review. Maternal & child nutrition. 2007;3:151–73. doi: 10.1111/j.1740-8709.2007.00092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.King JC. The risk of maternal nutritional depletion and poor outcomes increases in early or closely spaced pregnancies. The Journal of nutrition. 2003;133:1732S–6S. doi: 10.1093/jn/133.5.1732S. [DOI] [PubMed] [Google Scholar]
- 26.Council. IoMaNR. Weight Gain During Pregnancy: Reexamining the Guidelines. In: Rasmussen KM, Yaktine AL, editors. Weight Gain During Pregnancy: Reexamining the Guidelines. Washington (DC): The National Academies Press; 2009. [PubMed] [Google Scholar]
- 27.Olson CM, Strawderman MS, Hinton PS, Pearson TA. Gestational weight gain and postpartum behaviors associated with weight change from early pregnancy to 1 y postpartum. Int J Obes Relat Metab Disord. 2003;27:117–27. doi: 10.1038/sj.ijo.0802156. [DOI] [PubMed] [Google Scholar]
- 28.Viswanathan M, Siega-Riz AM, Moos MK, et al. Outcomes of maternal weight gain. Evidence report/technology assessment. 2008:1–223. [PMC free article] [PubMed] [Google Scholar]
- 29.Gunderson EP, Abrams B. Epidemiology of gestational weight gain and body weight changes after pregnancy. Epidemiologic reviews. 2000;22:261–74. doi: 10.1093/oxfordjournals.epirev.a018038. [DOI] [PubMed] [Google Scholar]
- 30.Chen A, Feresu SA, Fernandez C, Rogan WJ. Maternal obesity and the risk of infant death in the United States. Epidemiology. 2009;20:74–81. doi: 10.1097/EDE.0b013e3181878645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gunderson EP, Murtaugh MA, Lewis CE, Quesenberry CP, West DS, Sidney S. Excess gains in weight and waist circumference associated with childbearing: The Coronary Artery Risk Development in Young Adults Study (CARDIA) Int J Obes Relat Metab Disord. 2004;28:525–35. doi: 10.1038/sj.ijo.0802551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brunner Huber LR. Validity of self-reported height and weight in women of reproductive age. Maternal and child health journal. 2007;11:137–44. doi: 10.1007/s10995-006-0157-0. [DOI] [PubMed] [Google Scholar]
- 33.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. Jama. 2012;307:491–7. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.