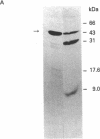
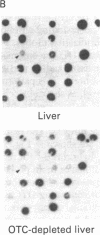
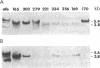
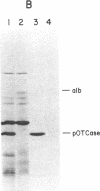
Abstract
Ornithine transcarbamoylase is a mitochondrial matrix enzyme composed of three identical subunits encoded on the X chromosome. The subunit is synthesized on cytoplasmic polysomes as a precursor that is cleaved during transport into mitochondria. We report here the isolation and characterization of cDNA clones containing sequences corresponding to the mRNA encoding the ornithine transcarbamoylase subunit. cDNA was synthesized using rat liver mRNA enriched by polysome immunoadsorption for the low-abundance messenger species encoding the enzyme subunit. After insertion of cDNA into plasmid pBR322 and cloning in Escherichia coli, identification of the desired plasmids was accomplished by (i) differential colony hybridization using cDNA probes synthesized from mRNA of various tissues; (ii) differential blot hybridization using cDNA probes synthesized from mRNA enriched for or depleted of the ornithine transcarbamoylase message; (iii) hybrid-selected translation assays; and (iv) most definitively, structural analysis, which matched 25 consecutive amino acid residues determined by sequential Edman analysis of the carboxyl-terminal portion of the purified enzyme subunit with coding sequence present in the insert of one of the plasmids.
Full text
PDF

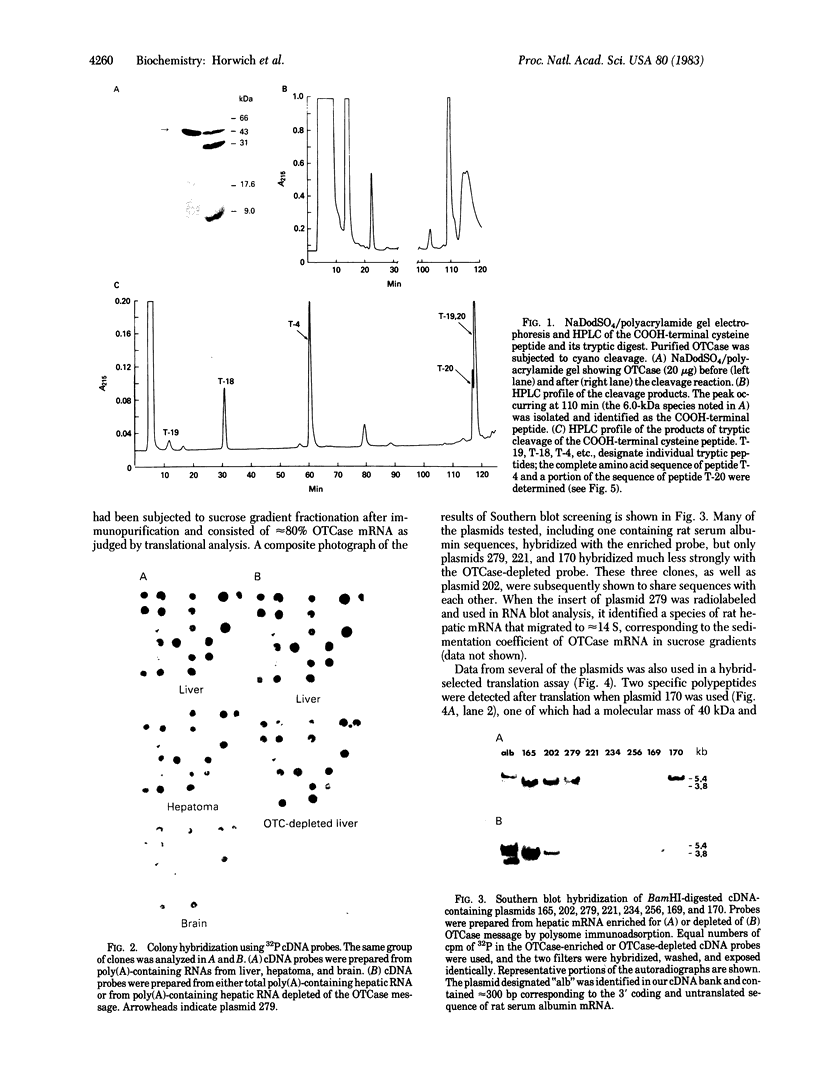
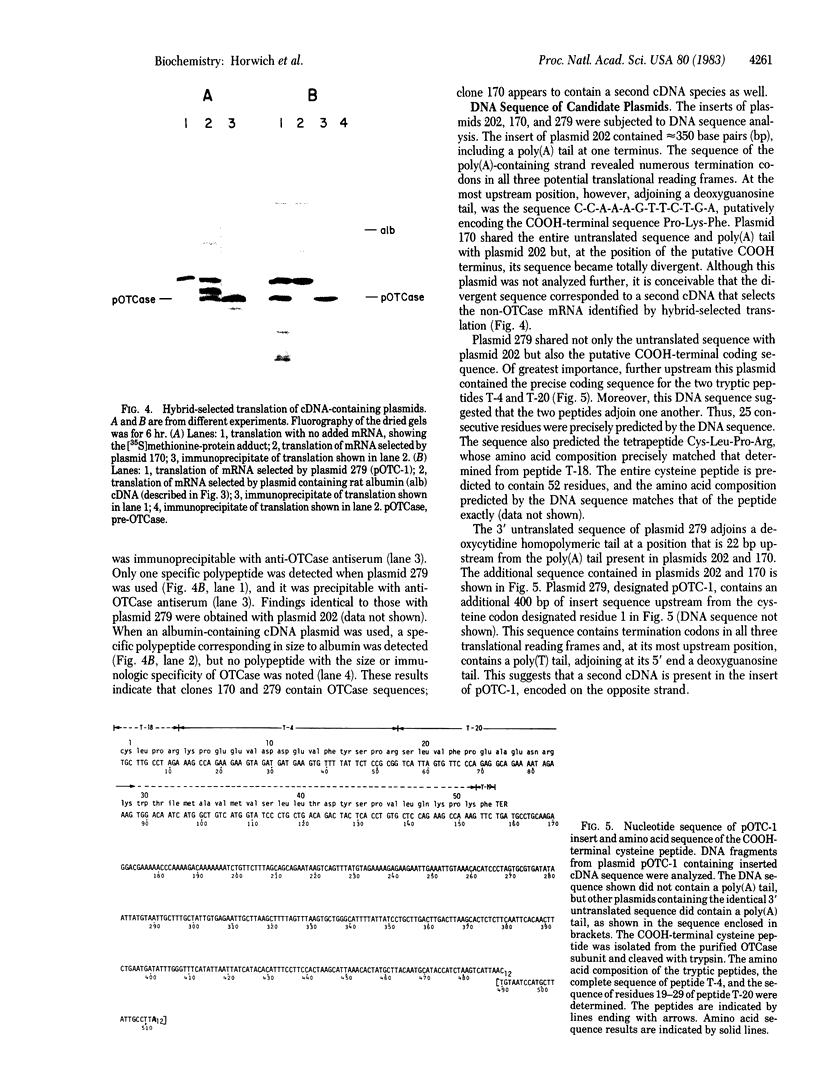

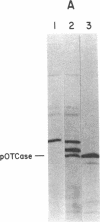
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolivar F., Backman K. Plasmids of Escherichia coli as cloning vectors. Methods Enzymol. 1979;68:245–267. doi: 10.1016/0076-6879(79)68018-7. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Effect of growth conditions on the formation of the relaxation complex of supercoiled ColE1 deoxyribonucleic acid and protein in Escherichia coli. J Bacteriol. 1972 Jun;110(3):1135–1146. doi: 10.1128/jb.110.3.1135-1146.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy J. G., Kalousek F., Rosenberg L. E. In vitro synthesis of a putative precursor of mitochondrial ornithine transcarbamoylase. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5724–5727. doi: 10.1073/pnas.76.11.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMars R., LeVan S. L., Trend B. L., Russell L. B. Abnormal ornithine carbamoyltransferase in mice having the sparse-fur mutation. Proc Natl Acad Sci U S A. 1976 May;73(5):1693–1697. doi: 10.1073/pnas.73.5.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogenraad N. J., Sutherland T. M., Howlett G. J. Purification of ornithine transcarbamylase from rat liver by affinity chromatography with immobilized transition-state analog. Anal Biochem. 1980 Jan 1;101(1):97–102. doi: 10.1016/0003-2697(80)90045-7. [DOI] [PubMed] [Google Scholar]
- Kolansky D. M., Conboy J. G., Fenton W. A., Rosenberg L. E. Energy-dependent translocation of the precursor of ornithine transcarbamylase by isolated rat liver mitochondria. J Biol Chem. 1982 Jul 25;257(14):8467–8471. [PubMed] [Google Scholar]
- Kraus J. P., Conboy J. G., Rosenberg L. E. Pre-ornithine transcarbamylase. Properties of the cytoplasmic precursor of a mitochondrial matrix enzyme. J Biol Chem. 1981 Nov 10;256(21):10739–10742. [PubMed] [Google Scholar]
- Kraus J. P., Rosenberg L. E. Purification of low-abundance messenger RNAs from rat liver by polysome immunoadsorption. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4015–4019. doi: 10.1073/pnas.79.13.4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusty C. J., Jilka R. L., Nietsch E. H. Ornithine transcarbamylase of rat liver. Kinetic, physical, and chemical properties. J Biol Chem. 1979 Oct 25;254(20):10030–10036. [PubMed] [Google Scholar]
- MacGillivray R. T., Degen S. J., Chandra T., Woo S. L., Davie E. W. Cloning and analysis of a cDNA coding for bovine prothrombin. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5153–5157. doi: 10.1073/pnas.77.9.5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall M., Cohen P. P. Ornithine transcarbamylases. Ordering of S-cyano peptides and location of characteristically reactive cysteinyl residues within the sequence. J Biol Chem. 1980 Aug 10;255(15):7287–7290. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M., Miura S., Morita T., Takiguchi M., Tatibana M. Ornithine transcarbamylase in liver mitochondria. Mol Cell Biochem. 1982 Nov 26;49(2):97–111. doi: 10.1007/BF00242488. [DOI] [PubMed] [Google Scholar]
- Mori M., Miura S., Tatibana M., Cohen P. P. Processing of a putative precursor of rat liver ornithine transcarbamylase, a mitochondrial matrix enzyme. J Biochem. 1980 Dec;88(6):1829–1836. doi: 10.1093/oxfordjournals.jbchem.a133158. [DOI] [PubMed] [Google Scholar]
- Parnes J. R., Velan B., Felsenfeld A., Ramanathan L., Ferrini U., Appella E., Seidman J. G. Mouse beta 2-microglobulin cDNA clones: a screening procedure for cDNA clones corresponding to rare mRNAs. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2253–2257. doi: 10.1073/pnas.78.4.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Ricciuti F. C., Gelehrter T. D., Rosenberg L. E. X-chromosome inactivation in human liver: confirmation of X-linkage of ornithine transcarbamylase. Am J Hum Genet. 1976 Jul;28(4):332–338. [PMC free article] [PubMed] [Google Scholar]
- Robson K. J., Chandra T., MacGillivray R. T., Woo S. L. Polysome immunoprecipitation of phenylalanine hydroxylase mRNA from rat liver and cloning of its cDNA. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4701–4705. doi: 10.1073/pnas.79.15.4701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K., Gallione C. J. Nucleotide sequences of the mRNA's encoding the vesicular stomatitis virus G and M proteins determined from cDNA clones containing the complete coding regions. J Virol. 1981 Aug;39(2):519–528. doi: 10.1128/jvi.39.2.519-528.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 1978 Mar 1;87(1):107–110. doi: 10.1016/0014-5793(78)80145-8. [DOI] [PubMed] [Google Scholar]
- Shields D., Blobel G. Cell-free synthesis of fish preproinsulin, and processing by heterologous mammalian microsomal membranes. Proc Natl Acad Sci U S A. 1977 May;74(5):2059–2063. doi: 10.1073/pnas.74.5.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short E. M., Conn H. O., Snodgrass P. J., Campbell A. G., Rosenberg L. E. Evidence for x-linked dominant inheritance of ornithine transcarbamylase deficiency. N Engl J Med. 1973 Jan 4;288(1):7–12. doi: 10.1056/NEJM197301042880102. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]