Abstract
Background/hypotheses
Doxorubicin is a standard adjuvant therapy for early-stage breast cancer and it significantly improves disease-free and overall survival. However, 3-20% of breast cancer patients develop chronic cardiomyopathic changes and congestive heart failure due to doxorubicin therapy. Doxorubicin-induced cardiotoxicity is thought to be due to increased generation of reactive oxygen species (ROS) within cardiac myocyte mitochondria. Coenzyme Q10 (CoQ10) is a lipid-soluble antioxidant that may protect against mitochondrial ROS, and thus prevent doxorubicin-induced cardiotoxicity. Despite the potential benefits of CoQ10 in preventing cardiotoxicity, it is unknown if CoQ10 diminishes the antineoplastic effects of doxorubicin therapy.
Study design
In vitro cell culture experiments.
Methods
Breast cancer cell lines (MDA-MB-468 and BT549) were tested for their ability to uptake exogenous CoQ10 using high performance liquid chromatography (HPLC). Breast cancer cell lines were then treated with doxorubicin and a range of CoQ10 concentrations to determine the effect of CoQ10 on doxorubicin’s cytotoxicity.
Results
This study demonstrated that intracellular and mitochondrial CoQ10 concentrations increased substantially as higher exogenous concentrations are administered to breast cancer cells. CoQ10 had no effect on the ability of doxorubicin to induce apoptosis or inhibit growth or colony formation in both cell lines tested when CoQ10 was applied over a wide dose range, which encompassed typical basal plasma levels as well as plasma levels above those typically achieved by supplemented patients.
Conclusion
The clinical testing of CoQ10 as supplement to prevent doxorubicin induced cardiotoxicity requires confidence that it does not decrease chemotherapy efficacy. These results support the hypothesis that CoQ10 does not alter the antineoplastic properties of doxorubicin. Further in vivo studies, as well as combination chemotherapy studies, would be reassuring before large scale clinical testing of CoQ10 as a cardioprotective drug.
Keywords: Doxorubicin, Adriamycin, Coenzyme Q10, cytotoxicity, breast cancer, in vitro
INTRODUCTION
Doxorubicin (adriamycin), an anthracycline antibiotic, plus cyclophosphamide (AC) is standard adjuvant therapy for early stage breast cancer. The combination of these therapies significantly improves disease-free and overall survival. Despite these benefits, 3-20% of doxorubicin treated breast cancer patients develop chronic cardiomyopathic changes and congestive heart failure due to doxorubicin therapy.1 Currently, no treatments are available to prevent this cardiotoxicity that have been proven to be safe and effective in breast cancer patients receiving the current standard dose of doxorubicin. A recent Cochrane review suggests that dexrazoxane may decrease the risk of cardiac toxicity from anthracyclines, but the majority of studies were not conducted in breast cancer patients.2 Effective agents need to be identified as being both safe and effective for the prevention of doxorubicin-induced cardiac toxicity.
The differential toxicity of doxorubicin to cardiac myocytes as compared with other tissues is hypothesized to be due an interaction between doxorubicin and a mitochondrial nicotinamide adenine dinucleotide dehydrogenase (NADH) enzyme specific to cardiac myocytes.3 In all cells the mitochondria are the site of ATP generation via the electron transport chain. Coenzyme Q10 (CoQ10, also known as ubiquinone) is located within the mitochondrial inner membrane and plays an integral role in generating intracellular ATP by serving as electron acceptor within the electron transport chain. CoQ10 also quenches intracellular oxidative stress by accepting electrons.
In most tissues, the mitochondrial inner membrane serves as a barrier to pro-oxidant doxorubicin metabolites. However, the mitochondrial inner membrane of cardiac myocytes uniquely contains a specific form of mitochondrial nicotinamide adenine dinucleotide (NADH) dehydrogenase which reduces doxorubicin to its semiquinone form and subsequently generates high concentrations of ROS within the mitochondrial cytosol.3 Doxorubicin semiquinones donate electrons to oxygen, which generates superoxide radicals, and are also autooxidized and cleaved to form aglycones. The aglycones displace CoQ10 from the mitochondrial inner membrane and serve as electron acceptors from complex I and II (which is a function of CoQ10). However, instead of transferring electrons to complex III as CoQ10 does, the aglycones transfer electrons to molecular oxygen, resulting in the formation of superoxide radicals. The cumulative high levels of ROS cause irreversible damage to mitochondrial DNA, which blocks the regenerative capability of the organelle and leads to cardiac myocyte apoptosis or necrosis. Animal and human studies support this proposed mechanism of doxorubicin-induced cardiotoxicity.3 During doxorubicin treatment, an acute rise in plasma CoQ104 is followed by a marked post-treatment decrease in the CoQ10 content of cardiac and skeletal muscle.5 Damage to the mitochondria of cardiac myocytes is one of the earliest and most prominent histological findings of doxorubicin-induced cardiomyopathy.
It has been hypothesized that dietary supplementation of CoQ10 prior to and during doxorubicin treatment can prevent doxorubicin-induced cardiotoxicity by preventing or slowing the displacement of CoQ10 by doxorubicin metabolites.3 CoQ10 is a lipid-soluble antioxidant that is synthesized in the mitochondria and in most intracellular membranes, and is ingested via the diet. Intake of CoQ10 via dietary supplements is generally considered to be safe at doses up to 1200 mg per day.6 A daily dose of 300 mg per day for 11 days has been shown to raise plasma CoQ10 concentrations by 300-400%.7
In vitro studies have shown that administering CoQ10 either before or during doxorubicin administration can prevent doxorubicin-induced changes including cardiac beat inhibition, increase in lipid peroxidation, and reduced cardiac contractile tension.3 Animal studies suggest that the impact of doxorubicin on mitochondria is cardioselective, that CoQ10 can prevent doxorubicin-induced mitochondrial damage, and that CoQ10 can prevent the progression of cardiomyopathy induced by doxorubicin.3 Clinical studies of CoQ10 administered before or during doxorubicin treatment have shown that CoQ10 prevented changes in left ventricular ejection fraction (LVEF), systolic time intervals (STI), fractional shortening, intraventricular septal wall thickening, cardiac output, and EKG changes.3 In addition, CoQ10 allowed for increased doses of doxorubicin to be administered.3 However, these studies were small and included patients of mixed tumor types. No studies have focused on aggressive early stage breast cancer in which doxorubicin containing chemotherapy regimens are a standard of care.
In addition to the doxorubicin cytotoxic mechanisms of topoisomerase II inhibition, DNA intercalation, and inhibition of DNA dependent RNA polymerase, doxorubicin treatment produces DNA damaging cytotoxic oxygen free radicals.8 Previous in vitro and animal studies in a lymphoma model suggested that high doses of vitamin C may diminish the cytotoxicity of doxorubicin, perhaps by preserving mitochondrial membrane potential.9 Another study found that blockade of CoQ10 synthesis increased the sensitivity of cancer cells to the chemotherapeutic camptothecin.10 Despite the potential benefits of CoQ10 in preventing doxorubicin-induced cardiotoxicity, there is concern that CoQ10 may decrease the desired prooxidant treatment effects of doxorubicin. One previous study assessed the effects of CoQ10 on the in vitro efficacy of doxorubicin. CoQ10 increased the cytotoxic activity of doxorubicin in L-1210 leukemia cells and had no effect in Ehrlich ascites carcinoma cells.3 Similar preclinical studies examining the effects of CoQ10 in breast cancer models are lacking. Here we report on a series of in vitro breast cancer cell culture experiments examining the effects of CoQ10 on doxorubicin cytotoxicity.
METHODS
Reagents
MDA-MB-468 and BT549 breast cancer cell lines were obtained from ATCC and cultured in DMEM and RPMI media, respectively, supplemented with 10% fetal bovine serum and penicillin and streptomycin. Doxorubicin hydrochloride was obtained from the medical center pharmacy and diluted in water. Powdered fermented CoQ10 was obtained via Thorne Research (Sandpoint, ID) (original source, Sinphar Group, Taiwan) and dissolved in 1-propanol.
Coenzyme Q10 quantification
Cells from both cell lines were grown to 80% confluency in 6-well dishes before treatment with CoQ10 and/or doxorubicin for 48 hours. All cells receiving both drugs were pretreated with CoQ10 for 24 hours before 48 hours of doxorubicin exposure. On the day of harvest for whole cell quantification, cells were washed three times with PBS, trypsinized and spun down. Cell pellets were stored at −20°C until CoQ10 level was quantified using a modified form of a previously established high performance liquid chromotography (HPLC) method.11,12 For mitochondrial fractions, Mitosciences Mitochondria Isolation Kit for Cultured Cells (MS852) was used to separate the mitochondrial portion of the cells before following the same HPLC protocol to quantify the CoQ10 levels. Immunoblotting was used to validate separation of mitochondria. For all samples, the Bio-Rad DC Protein Quantification Microplate Assay Protocol was used to quantify and normalize the amount of protein per sample.
Proliferation and IC50 assays
Cells (1 × 104) were plated on 48-well dishes. At 16 to 24 hours later, cells were treated with drug for 72 hours. For CoQ10 plus doxorubicin studies, cells were incubated with CoQ10 for 24 hours before doxorubicin was added. On the day of harvest, cells were stained with 0.05% crystal violet in 10% formalin, washed, and incubated with 10% acetic acid before a 590-nm absorbance was measured (BIO-TEK). The IC50 values were determined using the dose response curve fitting model y=A+((B-A)/(1+((C/x)^D))) designated as model 205 using XLfit4 with parameter A=0 [min. y value], B=100 [max. y value], C=IC50, and D=slope.
Colony formation assays
Cells (1-10 × 105) were plated on 6-well dishes and treated with drug for 48 hours. CoQ10 plus doxorubicin treated cells were pre-incubated with CoQ10 for 24 hours. Cells were collected and plated in 500-1000 dilutions on fresh 6-well dishes. After 10-12 days, media was removed and cells were fixed with cold methanol for 5 minutes before staining with 0.25% crystal violet in 10% formalin for 30 minutes. Cells were washed and colonies were counted using ImageJ software.
Immunoblotting
Whole-cell lysates and mitochondrial fractionations were used in immunoblots, as indicated. Cleaved Caspase-3 (Asp175) (#9661) antibody from Cell Signaling was diluted 1:1000 in 5% w/v nonfat dry milk, 1X TBS, 0.1% Tween-20 and incubated at 4°C overnight before incubating in anti-rabbit secondary diluted 1:5000 for one hour. COX IV (#4844) antibody from Cell Signaling was diluted 1:1000 in 5% w/v nonfat dry milk, 1X TBS, 0.1% Tween-20 and incubated at 4°C overnight before incubating in anti-rabbit secondary diluted 1:5000 for one hour.
Statistical analysis
One-way analysis of variance (ANOVA) was used to examine differences in CoQ10 concentration assessed using HPLC. T-tests were used to examine differences in the cell proliferation assays. Analyses were conducted using StatPlus:mac LE.2009 (AnalystSoft).
RESULTS
Exogenous CoQ10 increases cellular level of CoQ10
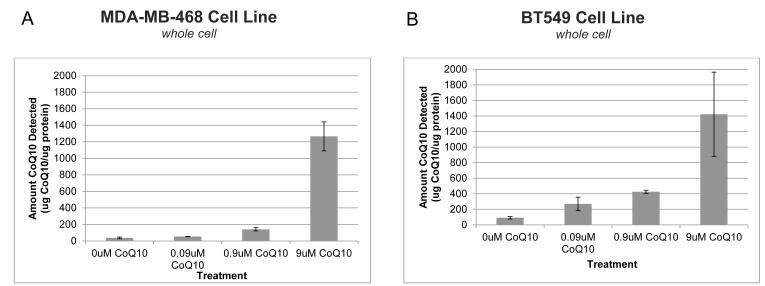
Triple-negative breast cancer cell lines MDA-MB-468 and BT549 were tested for their ability to uptake exogenous CoQ10 over a dose range of 0.09μM to 9μM CoQ10 (Fig. 1). In humans, the standard supplementation dose raises plasma CoQ10 levels from 0.5μM to 2μM; breast cancer patients have a typical plasma level of 0.9μM.13 Both cell lines demonstrated that in vitro cellular CoQ10 concentration increases as higher exogenous concentrations are administered to cells. In MDA-MB-468 cells, adding 0.09μM of CoQ10 increased the amount detected from 30.7μg CoQ10 per μg protein (when no CoQ10 was added) to 53.2μg CoQ10. Adding 0.9μM and 9μM CoQ10 increased detected levels even higher to 140.5μg and 1,265μg CoQ10 per μg protein, respectively. BT549 cells demonstrated a similar dose increase, with approximately 1,400μg CoQ10 detected per μg protein, over a 15 fold increase from the 91μg CoQ10 detected in the baseline cells.
Figure 1.
Exogenous CoQ10 increases cellular level of CoQ10 in breast cancer cells in vitro. MDA-MB-468 (A) and BT459 (B) triple negative breast cancer cells were exposed to CoQ10 for 48 hours at concentrations up to 9uM CoQ10. HPLC was used to quantify CoQ10 levels in whole-cell lysates. Three separate experiments were conducted in triplicate. Error bars represent one standard deviation.
Abbreviations: CoQ10, Coenzyme Q10; HPLC, high performance liquid chromatography
Exogenous CoQ10 increases CoQ10 levels in the mitochondria of breast cancer cells in vitro, even in the presence of doxorubicin
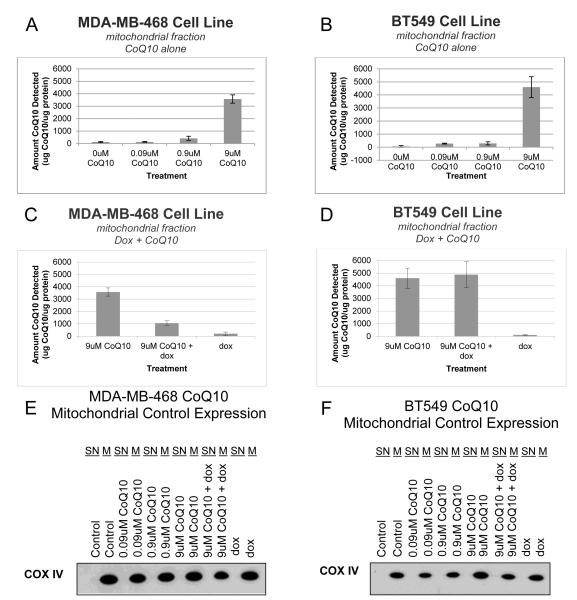
To determine if exogenous CoQ10 was able to concentrate in the mitochondria of the breast cancer cells, cellular fractionation was performed to separate out the mitochondria before testing the levels of CoQ10 after exogenous treatment (Fig. 2). In both cell lines, the highest treatment of CoQ10, 9μM, caused a substantial increase in CoQ10 detected over the baseline, up to a third of the amount measured in the whole cell lysates (Fig. 2A and 2B). The level of CoQ10 was also tested after exposure to doxorubicin alone and in combination with CoQ10 (Fig. 2C and 2D). In both cell lines, the level of CoQ10 detected after treatment with doxorubicin alone was similar to the baseline CoQ10 levels. However, the two lines differed in their mitochondrial CoQ10 levels after the combined treatment of CoQ10 and doxorubicin. MDA-MB-468 cells had a substantially decreased level of mitochondrial CoQ10 in the presence of doxorubicin, decreasing the amount of CoQ10 detected by approximately two-thirds (Fig. 2E). This decrease in mitochondrial CoQ10 was not observed in BT549 cells, where the amount of CoQ10 detected was the same with or without doxorubicin (Fig. 2F).
Figure 2.
Exogenous CoQ10 increases CoQ10 levels in the mitochondria of breast cancer cells in vitro, even in the presence of doxorubicin. MDA-MB-468 (A, C, E) and BT459 (B, D, F) triple negative breast cancer cells were exposed to drug before cell lysates were collected and the mitochondrial fraction was extracted. Cells were exposed to CoQ10 for 48 hours (A, B). Cells were pre-treated with CoQ10 for 24 hours before treatment with doxorubicin at the cell’s IC50 value (40nM for MDA-MB-468 and 200nM for BT549) (C, D). HPLC was used to quantify CoQ10 levels in whole-cell lysates. Three separate experiments were conducted in triplicate. Extracted cellular fractions were probed for COX IV expression to validate mitochondrial fractions were accurate (E, F). Error bars represent one standard deviation. For all experiments (A-D) P < 0.05.
Abbreviations: CoQ10, Coenzyme Q10; dox, doxorubicin; HPLC, high performance liquid chromatography; SN, supernatant; M, mitochondrial fraction
CoQ10 does not antagonize the in vitro growth inhibition effect of doxorubicin
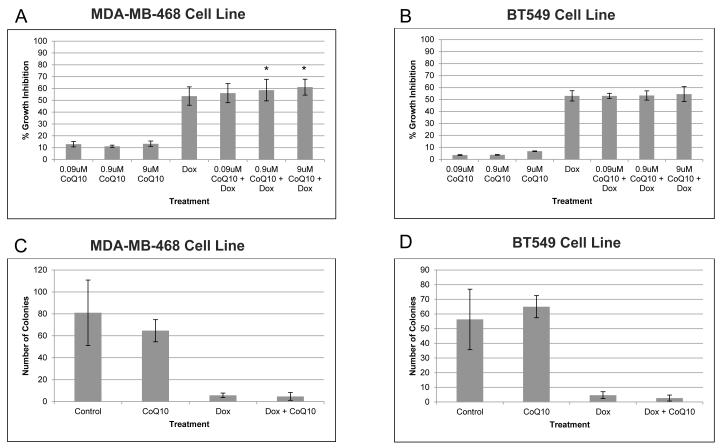
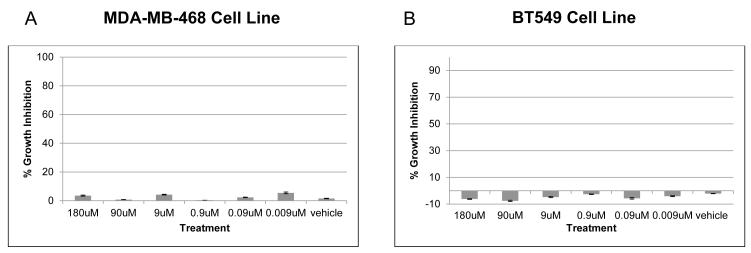
To determine if CoQ10 affects doxorubicin cytotoxicity, triple negative breast cancer cell lines MDAMB-468 and BT549 were treated with doxorubicin at each cell line’s IC50 concentration (40nM for MDA-MB-468 and 200nM for BT549 IC50 - each falling within the peak [5 μM] and steady-state [25-250 nM] plasma concentrations observed in patients after standard bolus infusions8) and a dose range of 0.09μM to 9μM of CoQ10 for 72 hours (Fig. 3A and 3B). In both cell lines CoQ10 did not alter the growth inhibition effect of doxorubicin, nor did CoQ10 alone significantly inhibit cell growth, even at high concentrations (Fig. 4). When treated with only doxorubicin, MDA-MB-468 growth inhibition was 53.6%. This inhibition did not change when any concentration of CoQ10 was added. The growth inhibition of doxorubicin alone for BT549 cell line of 53.1% was also not significantly altered by CoQ10 additions (Fig. 3A and 3B). When added alone, up to 180μM of CoQ10 did not inhibit cellular growth in either cell line (Fig. 4).
Figure 3.
CoQ10 does not antagonize in vitro growth inhibition effect of doxorubicin on breast cancer cell lines. MDA-MB-468 (A, C) and BT549 (B, D) cell lines were treated with CoQ10 for 24 hours before treatment with doxorubicin at IC50 value (40nM for MDA-MB-468 and 200nM for BT549) for 48 hours. Proliferation assay was used to determine growth inhibition effects of CoQ10 alone and in combination with doxorubicin (A, B). Additionally, colony formation assays were performed (C, D). Error bars represent one standard deviation. * Indicates P < 0.05.
Abbreviations: CoQ10, Coenzyme Q10; dox, doxorubicin
Figure 4.
CoQ10 alone does not affect growth of breast cancer cells. Proliferation assays were performed on MDA-MB-468 (A) and BT549 (B) breast cancer cells after treatment with increasing levels of CoQ10 for 72 hours. Error bars represent one standard deviation.
Abbreviation: CoQ10, Coenzyme Q10
To confirm our findings, colony formation assays were also performed on both cell lines after CoQ10 and doxorubicin treatments alone and in combination (Fig. 3C and 3D). Colony formation assays are a more stringent test of cell fitness as they measure the ability of every cell in the population to undergo unlimited growth and are therefore much more sensitive at identifying small differences between treatment groups.14 In both breast cancer cell lines doxorubicin severely reduced the number of colonies and the addition of CoQ10 did not alter doxorubicin’s inhibitory capacity. The addition of CoQ10 alone did not substantially affect the colony formation of the cells.
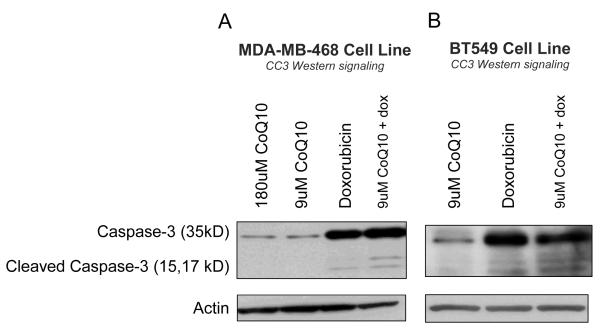
CoQ10 does not diminish apoptotic effect of doxorubicin
Given that doxorubicin kills cancer cells, at least in part, by inducing apoptosis,14 we directly measured whether or not CoQ10 altered the level of doxorubicin mediated apoptosis. Cells pretreated with CoQ10 were compared against cells pretreated with vehicle control for induction of cleaved caspase 3 after doxorubicin treatment for 24 hours (Fig. 5). Both cell lines showed substantial cleaved caspase-3 activity upon exposure to doxorubicin and this effect was not diminished with pretreatment of up to 9μM of CoQ10. MDA-MB-468 cells were exposed to excessive levels of CoQ10, 180μM, to determine if CoQ10 might induce apoptosis at high levels, but apoptosis was not detected.
Figure 5.
CoQ10 does not diminish apoptotic effect of doxorubicin. MDA-MB-468 (A) and BT549 (B) cells were treated with CoQ10 alone, doxorubicin alone or a combination of the two. Cleaved caspase-3 levels were evaluated using immunoblotting as a measure of apoptotic activity in the cells. MDA-MB-468 cells were treated with 800nM doxorubicin; BT549 cells were treated with 3.2uM doxorubicin.
Abbreviations: CoQ10, Coenzyme Q10; dox, doxorubicin
DISCUSSION
Doxorubicin-induced cardiotoxicity is a significant concern in the care of breast cancer patients, causing deaths, disability, and limiting the use of potentially more effective concurrent drug combinations (i.e. with trastuzumab). Effective treatments are needed to prevent this cardiotoxicity while not decreasing the anti-tumor effects of doxorubicin. We conducted a series of in vitro cell culture experiments to determine the effects of CoQ10 on doxorubicin cytotoxicity using two different triple negative breast cancer cell lines that are sensitive to doxorubicin treatment. We demonstrated that there was CoQ10 uptake by the breast cancer cells, that the CoQ10 entered the mitochondria, and that CoQ10 did not limit the cytotoxicity of doxorubicin.
The strengths of this work include our expertise and ability to measure and confirm the dose dependent delivery of CoQ10 to the mitochondria of breast cancer cells. In this setting we have demonstrated, using two separate assays (proliferation and colony formation), that cell death induced by clinically relevant doses of doxorubicin is not altered by CoQ10 administered over a wide dose range and up to doses higher than that achievable through oral supplementation. Our work is consistent with prior studies on non-breast cancer cell lines3. The observation that measured CoQ10 levels were decreased in the setting of doxorubicin treatment may be due to a doxorubicin induced increase in reactive oxygen species (ROS) production which has been observed in other cancer cell lines.10 Our work sets the stage for needed in vivo experimentation, requiring a large cohort of animals with a non-inferiority design and correlative studies showing the activity and effects of both the chemotherapy and CoQ10.
There is high use of antioxidant supplements by breast cancer patients after diagnosis, including during the time of treatment.15-17 Many patients use antioxidant supplements with the hope of decreasing treatment related side effects and preventing recurrence, but the long term effect of such use has not been rigorously studied for many supplement/disease/treatment combinations.15,18 Given the high cost of conducting long term clinical trials, combined with recent findings of harm from antioxidant supplements being tested for the purposes of primary cancer prevention,19,20 we need efficient methods to identify, screen and test dietary supplements that may hold benefit. Preclinical models, such as those presented here, can be used to efficiently screen agents for potential benefit or harm.
CONCLUSION
In a series of in vitro cell culture experiments, we found that CoQ10 did not inhibit doxorubicin cytotoxicity in breast cancer cell lines. These results are far from informing clinical practice. Rather, they can inform subsequent preclinical and clinical studies.
Acknowledgments
Funding: This work was supported by the National Cancer Institute (K23CA141052 to H.G.), the National Center for Research Resources (KL2RR024157 Career Development Awards to H.G. and to M.M.) and the Herbert Irving Comprehensive Cancer Center.
REFERENCES
- 1.Tan-Chiu E, Yothers G, Romond E, et al. Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2-overexpressing breast cancer: NSABP B-31. J Clin Oncol. 2005 Nov 1;23(31):7811–7819. doi: 10.1200/JCO.2005.02.4091. [DOI] [PubMed] [Google Scholar]
- 2.van Dalen EC, Caron HN, Dickinson HO, Kremer LC. Cardioprotective interventions for cancer patients receiving anthracyclines. Cochrane Database Syst Rev. 2011;(6):CD003917. doi: 10.1002/14651858.CD003917.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conklin KA. Coenzyme q10 for prevention of anthracycline-induced cardiotoxicity. Integr Cancer Ther. 2005 Jun;4(2):110–130. doi: 10.1177/1534735405276191. [DOI] [PubMed] [Google Scholar]
- 4.Eaton S, Skinner R, Hale JP, et al. Plasma coenzyme Q(10) in children and adolescents undergoing doxorubicin therapy. Clinica chimica acta; international journal of clinical chemistry. 2000 Dec;302(1-2):1–9. doi: 10.1016/s0009-8981(00)00316-8. [DOI] [PubMed] [Google Scholar]
- 5.Karlsson J, Folkers K, Astrom H. Effect of Adriamycin on heart and skeletal muscle coenzyme Q10 (CoQ10) in man. In: Folkers K, Yamamura Y, editors. Biomedical and Clinical Aspects of Coenzyme Q. Vol 5. Elsevier / North-Holland Biomedical Press; Amsterdam: 1986. pp. 241–245. [Google Scholar]
- 6.Hathcock JN, Shao A. Risk assessment for coenzyme Q10 (Ubiquinone) Regul Toxicol Pharmacol. 2006 Aug;45(3):282–288. doi: 10.1016/j.yrtph.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Mohr D, Bowry VW, Stocker R. Dietary supplementation with coenzyme Q10 results in increased levels of ubiquinol-10 within circulating lipoproteins and increased resistance of human low-density lipoprotein to the initiation of lipid peroxidation. Biochimica et biophysica acta. 1992 Jun 26;1126(3):247–254. doi: 10.1016/0005-2760(92)90237-p. [DOI] [PubMed] [Google Scholar]
- 8.Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev. 2004 Jun;56(2):185–229. doi: 10.1124/pr.56.2.6. [DOI] [PubMed] [Google Scholar]
- 9.Heaney ML, Gardner JR, Karasavvas N, et al. Vitamin C antagonizes the cytotoxic effects of antineoplastic drugs. Cancer Res. 2008 Oct 1;68(19):8031–8038. doi: 10.1158/0008-5472.CAN-08-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brea-Calvo G, Rodriguez-Hernandez A, Fernandez-Ayala DJ, Navas P, Sanchez-Alcazar JA. Chemotherapy induces an increase in coenzyme Q10 levels in cancer cell lines. Free radical biology & medicine. 2006 Apr 15;40(8):1293–1302. doi: 10.1016/j.freeradbiomed.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 11.Naini A, Lewis VJ, Hirano M, DiMauro S. Primary coenzyme Q10 deficiency and the brain. BioFactors (Oxford, England) 2003;18(1-4):145–152. doi: 10.1002/biof.5520180217. [DOI] [PubMed] [Google Scholar]
- 12.Quinzii C, Naini A, Salviati L, et al. A mutation in para-hydroxybenzoate-polyprenyl transferase (COQ2) causes primary coenzyme Q10 deficiency. Am J Hum Genet. 2006 Feb;78(2):345–349. doi: 10.1086/500092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Folkers K, Osterborg A, Nylander M, Morita M, Mellstedt H. Activities of vitamin Q10 in animal models and a serious deficiency in patients with cancer. Biochem Biophys Res Commun. 1997 May 19;234(2):296–299. doi: 10.1006/bbrc.1997.6522. [DOI] [PubMed] [Google Scholar]
- 14.Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C. Clonogenic assay of cells in vitro. Nat Protoc. 2006;1(5):2315–2319. doi: 10.1038/nprot.2006.339. [DOI] [PubMed] [Google Scholar]
- 15.Greenlee H, Hershman DL, Jacobson JS. Use of antioxidant supplements during breast cancer treatment: a comprehensive review. Breast Cancer Res Treat. 2009 Jun;115(3):437–452. doi: 10.1007/s10549-008-0193-0. [DOI] [PubMed] [Google Scholar]
- 16.Greenlee H, Gammon MD, Abrahamson PE, et al. Prevalence and predictors of antioxidant supplement use during breast cancer treatment: the Long Island Breast Cancer Study Project. Cancer. 2009 Jun 8;115(14):3271–3282. doi: 10.1002/cncr.24378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenlee H, Kwan ML, Kushi LH, et al. Antioxidant supplement use after breast cancer diagnosis and mortality in the Life After Cancer Epidemiology (LACE) cohort. Cancer. 2011 Sep 27; doi: 10.1002/cncr.26526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Velicer CM, Ulrich CM. Vitamin and mineral supplement use among US adults after cancer diagnosis: a systematic review. J Clin Oncol. 2008 Feb 1;26(4):665–673. doi: 10.1200/JCO.2007.13.5905. [DOI] [PubMed] [Google Scholar]
- 19.Mursu J, Robien K, Harnack LJ, Park K, Jacobs DR., Jr Dietary Supplements and Mortality Rate in Older Women: The Iowa Women’s Health Study. Arch Intern Med. 2011 Oct 10;171(18):1625–1633. doi: 10.1001/archinternmed.2011.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klein EA, Thompson IM, Jr., Tangen CM, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) Jama. 2011 Oct 12;306(14):1549–1556. doi: 10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]