SUMMARY
Adolescence is often described as a period of heightened reactivity to emotions paired with reduced regulatory capacities, a combination suggested to contribute to risk-taking and susceptibility to peer influence during puberty. However, no longitudinal research has definitively linked these behavioral changes to underlying neural development. Here, 38 neurotypical participants underwent two fMRI sessions across the transition from late childhood (10 years) to early adolescence (13 years). Responses to affective facial displays exhibited a combination of general and emotion-specific changes in ventral striatum (VS), ventromedial PFC, amygdala, and temporal pole. Furthermore, VS activity increases correlated with decreases in susceptibility to peer influence and risky behavior. VS and amygdala responses were also significantly more negatively coupled in early adolescence than in late childhood while processing sad and happy versus neutral faces. Together, these results suggest that VS responses to viewing emotions may play a regulatory role that is critical to adolescent interpersonal functioning.
INTRODUCTION
During the transition from childhood to adolescence, there is a dramatic increase in the amount of time spent with peers (Brown, 2004). This coincides with heightened reward sensitivity, sensation-seeking, preferences for risky behavior, a greater sense of the importance of conforming to peer group norms, and a growing divergence of peer and family values as peers begin to approve of more negative behaviors (Gardner and Stein-berg, 2005; Steinberg, 2008). Together, these changes create the sense that teenagers are less resistant to peer pressure than either children or adults, although susceptibility to peer influence per se gradually decreases over the course of adolescence (Steinberg and Monahan, 2007). Parental and societal concerns therefore abound regarding adolescent abilities to resist peer pressure, and whether a teenager’s lack thereof will precipitate his or her engagement in risky behaviors (such as early substance abuse, delinquency, or unsafe sexual activity). Although there are various social explanations for why peers are so influential during this period of development, researchers are increasingly focusing on biological factors that may underlie adolescents’ affective reactivity and emotion regulation ability during interactions with peers (Steinberg, 2008). These biological factors include not only hormonal changes that occur with the onset of puberty but also further brain development (Nelson et al., 2005). In particular, it is thought that subcortical neural systems typically associated with affective responding (e.g., amygdala; Adolphs, 2002; Fitzgerald et al., 2006) may mature earlier than the regions that adults use to regulate affective responses (e.g., prefrontal cortex, or PFC; Etkin et al., 2006; Somerville et al., 2010).
It is thus critical to understand how the adolescent brain responds to facial expressions of emotion, as peers’ emotional displays may exert a strong influence on subsequent behavior (e.g., Baird et al., 2010; Schlicht et al., 2010). Furthermore, it is important to determine whether changes in the neural response to emotional displays are indeed associated with changes in susceptibility to peer influence or engagement in risky behavior. Finally, it is vital to learn how these neural responses to emotional displays may be regulated, as this should enhance a teenager’s ability to resist peer pressure and diminish the possibility of engaging in risky behavior. Two recent cross-sectional studies reported that adolescents display more reactivity to affective facial displays, at a neural level, than either children or adults— specifically in the amygdala (Guyer et al., 2008; Hare et al., 2008). Further, adolescents also show less response to emotions in ventromedial PFC (VMPFC), a region whose functional connectivity with the amygdala is associated with habituation to emotional stimuli (Etkin et al., 2006; Hare et al., 2008). This suggests that teenagers may be more emotionally reactive, and also less capable of relying on PFC for affect regulation (see also Grosbras et al., 2007; Lévesque et al., 2004).
Although modulation of emotional responses via prefrontal circuitry may be less efficient during early adolescence, regulatory processes may be aided by subcortical involvement at this stage—particularly by the ventral striatum (VS). The VS is most frequently associated with reward-related processing (Delgado et al., 2000; Knutson et al., 2000; O’Doherty et al., 2003, 2004), but more recently has been implicated in responses to stimuli that are aversive (Becerra et al., 2001; Jensen et al., 2003; Levita et al., 2009; Rich et al., 2006), salient (Horvitz, 2000), or novel (Guitart-Masip et al., 2010). Critically with regard to the present investigation, evidence is also emerging that the VS may be specifically associated with emotion regulation. For example, increased activity in VS mediated successful positive reappraisal (Wager et al., 2008; for similar reports of striatal involvement in emotion regulation see also Hare et al., 2005; McRae et al., 2008). Complementing this finding, we previously observed that VS was more active in adolescents during social exclusion than in inclusion (Masten et al., 2009), and the VS response during exclusion was negatively correlated with the level of subjective distress adolescents reported following exclusion, and positively correlated with activity in prefrontal regions that are frequently associated with regulatory processes (just as in Wager et al., 2008). Finally, two recent studies examining reward anticipation and reward outcome in typically-developing adolescents and those with depression found that greater striatal activity during reward outcome was associated with greater subjective positive affect on a daily basis, and fewer depressive symptoms (Forbes et al., 2009,2010). Taken together, this growing body of work suggests that the VS may support affect regulation by compensating for and/or enhancing some of the roles typically carried out by prefrontal circuitry. Because PFC is known to undergo a prolonged period of maturation spanning adolescence (Shaw et al., 2008; Sowell et al., 2002,2004), VS involvement in affect regulation may be particularly critical during this period of development.
The current investigation was designed to document changes in affective reactivity at the neural level during the transition from late childhood to early adolescence, and how these changes may be related to changes in socioemotional functioning, specifically resistance to peer influence and engagement in risky or delinquent behaviors. Prior behavioral research suggests this is an especially important time window to examine, because susceptibility to peer influence is greatest during late elementary and early middle school, and risk preference, reward sensitivity, and sensation-seeking are reported to increase from 10 to approximately 13–16 years of age (Steinberg, 2008). This longitudinal fMRI study—to our knowledge, the first of its kind—thus affords a unique perspective on normative socioemotional development. Typically-developing participants completed two fMRI scans during which they observed exemplars of five different emotional expressions in a rapid event-related design, one session at age 10 (T1), and another session at age 13 (T2); they also completed self-report measures of resistance to peer influence (RPI; Steinberg and Monahan, 2007) and indicators of risk behavior and delinquency (IRBD; Gestsdóttir and Lerner, 2007) at both time points (see Experimental Procedures for fuller methodological details).
RESULTS
Overall patterns of brain activity elicited by affective facial displays at each time point were consistent with previously published reports, showing robust activity in regions including the fusiform gyrus, amygdala, hippocampus, and PFC (see Figure S1 available online). These results will not be discussed further here, as the primary aim of the current investigation was to examine longitudinal changes in neural responses to emotional expressions, with VS, VMPFC, and amygdala serving as our a priori regions of interest (ROIs) based on the previous research summarized in the Introduction.
Longitudinal Changes in Neural Response to Affective Displays
We first queried whether any brain regions evidenced longitudinal increases in BOLD signal during the observation of emotional expressions across the two time points representing late childhood and early adolescence. Several of our a priori ROIs demonstrated significantly different responses to affective facial displays as children transitioned into adolescence. Specifically, activity in VS and VMPFC increased from T1 to T2 (see Figure 1 and Table 1 for a complete list of significant increases), but there were no increases in amygdala activity at the whole-brain level of analysis. This analysis was conducted averaging across all facial expressions (including neutral) because recent research suggests that neutral facial expressions can actually elicit neural responses that do not significantly differ from those elicited by emotions like fear, happiness, and disgust (van der Gaag et al., 2007), although a recent meta-analysis suggests that emotion may consistently activate the amygdala relative to control states (Kober et al., 2008). In addition, studies have produced conflicting evidence about which expressions undergo the most change during human development, and/or which expression produces maximal amygdala activation in children and adolescents, such as fearful displays (Baird et al., 1999; Guyer et al., 2008; Hare et al., 2008; Monk et al., 2003) or neutral displays (Thomas et al., 2001). Given these prior mixed findings, all facial expressions were first compared to fixation and then examined individually (see below). These analyses confirmed that neutral facial expressions did elicit increased activity in several of our ROIs, thus precluding us from using neutral expressions as a meaningful baseline when exploring changes in responsivity to emotions over time (stronger T1 to T2 signal increases for emotional over neutral faces were only observed in the left temporal pole).
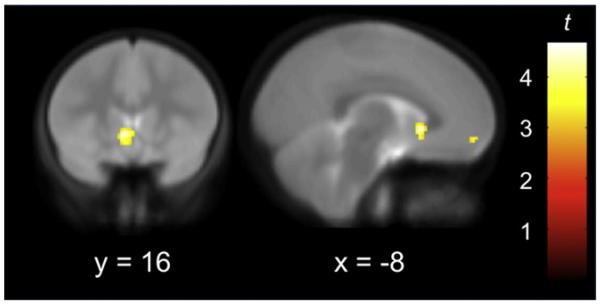
Figure 1. Longitudinal Increases from Late Childhood to Early Adolescence in BOLD Response to Affective Facial Displays.
Figure 1 depicts increased activity in ventral striatum (VS) and ventromedial prefrontal cortex (VMPFC) during the transition from late childhood to early adolescence. Imaging data are thresholded at p < 0.005 (t > 2.72), and displayed on the average coplanar high-resolution scan across both time points for all children. x and y refer to the Talairach coordinates corresponding to the left-right and anterior-posterior axes, respectively.
Table 1. Regions of Longitudinal Increase during Observation of Affective Facial Displays.
| Region | BA | x | y | z | t | |
|---|---|---|---|---|---|---|
| All expressions (t) | Ventral striatum | −6 | 16 | 0 | 4.67 | |
| Extrastriate visual cortex |
19 | 46 | −70 | 10 | 4.00 | |
| 37 | 38 | −58 | −6 | 3.69 | ||
| Superior parietal lobule |
7/40 | −32 | −56 | 52 | 3.43 | |
| VMPFC | 10 | −8 | 54 | −6 | 3.18 | |
| Midbrain (SN/VTA) | 10 | −18 | −14 | 3.25 | ||
| Cerebellum | 20 | −78 | −32 | 3.78 | ||
| 16 | −78 | −22 | 3.32 | |||
| Cingulate sulcus | 31 | 22 | −28 | 44 | 3.30 | |
| Medial temporal lobe |
36/37 | 40 | −24 | −10 | 3.49 | |
| Emotion > neutral (t) | Temporal pole | 38 | −52 | 2 | −6 | 4.71 |
Note: BA refers to putative Brodmann’s Area; x, y, and z refer to the left-right, anterior-posterior, and inferior-superior dimensions, respectively; t refers to the t statistic at those coordinates (local maxima or submax-ima); and SN and VTA refer to substantia nigra and ventral tegmental area, respectively.
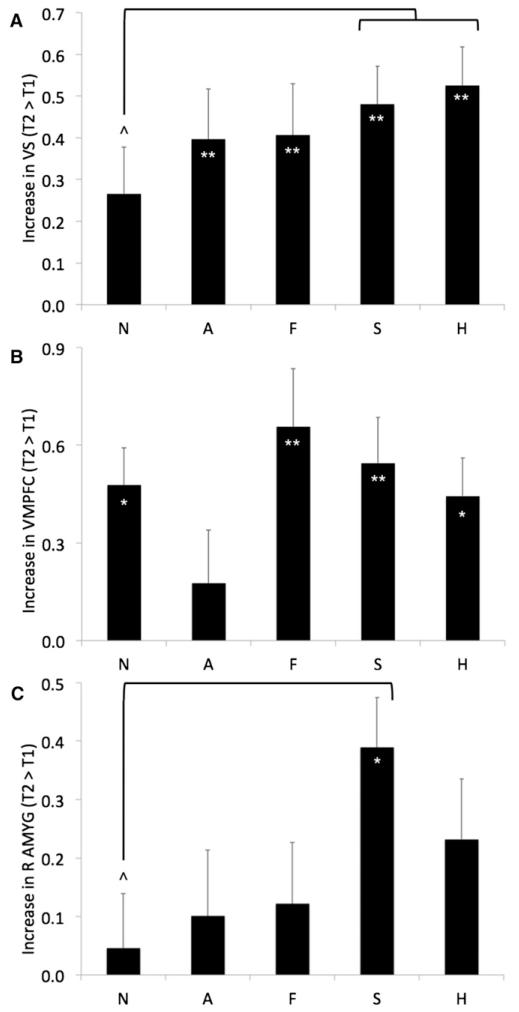
To further interrogate these longitudinal changes, mean parameter estimates were extracted for each type of facial expression at each time point from our a priori ROIs: the left and right amygdala (defined structurally), as well as the VS and VMPFC (using the clusters identified in the prior analysis as significantly increasing from T1 to T2). These parameter estimates were then included in full factorial repeated-measures ANOVAs (one for each ROI) with time and emotion as withinsubject factors. Significant modulation of signal increases by emotion type would indicate that the observed longitudinal effects cannot be merely ascribed to general developments in processing faces or complex visual stimuli (versus fixation). For the VS ROI, these analyses demonstrated that the increases from T1 to T2 were significant for all emotions and marginally significant for neutral expressions; however, VS responses increased over time significantly more for sad and happy expressions than for neutral ones (see Figure 2A). For the VMPFC ROI, these analyses showed that the increases over time were significant for all expressions except anger, with no significant differences between the other expressions (see Figure 2B). Finally, consistent with the whole-brain analysis, these analyses revealed that amygdala response did not significantly change when averaged across all emotions, although there was a trend toward increases in both left and right amygdala. However, a significant increase over time was detected in the right amygdala in response to sad faces; right amygdala responses also increased over time significantly more for sad expressions than for neutral ones (see Figure 2C).
Figure 2. Modulation by Facial Expression of Longitudinal Increases from Late Childhood to Early Adolescence in BOLD Response to Affective Displays.
(A), (B), and (C) depict longitudinal increases (means and standard errors) in activity for each expression in VS, VMPFC, and right amygdala (R AMYG), respectively, during the transition from late childhood to early adolescence. N = neutral, A = angry, F = fearful, S = sad, and H = happy expressions; (*) and (**) denote within-expression paired comparisons indicating increases over time that significantly differed at p < 0.05 and p < 0.005, respectively; (^) denotes paired comparisons indicating that change over time significantly differed between expressions at p < 0.05.
Longitudinal Relationships between Brain and Behavior
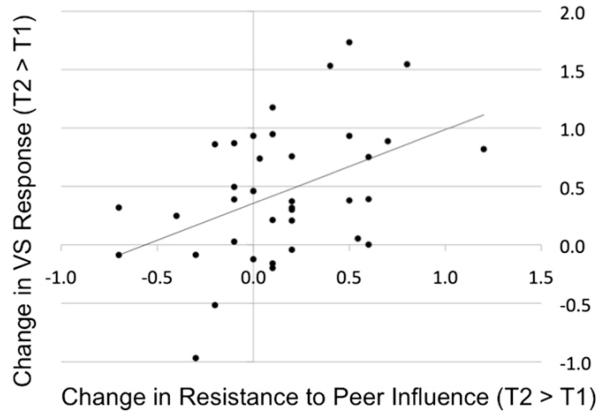
We next examined how these longitudinal changes in neural responses might be related to changes in socioemotional functioning by conducting both ROI and whole-brain regression analyses. Using the same parameter estimates of activity extracted from each a priori ROI (as described above), a difference score was first calculated to index change over time (T2 > T1) averaging across all expressions; these scores were then correlated with change over time in RPI and IRBD. The results of these analyses indicated that VS activity increases over time were positively correlated with RPI increases over time [r(36) = 0.44, p < .005; see Figure 3]. This finding was confirmed in an independent whole-brain regression analysis, wherein change over time in RPI scores was entered as a predictor of change over time in responses to all expressions, resulting in positive correlations in VS (at a nearly identical location), temporal pole, dorsal striatum, and the hippocampal gyrus, as well as a negative correlation in the periamgydala region (see Table 2). Interestingly, the relationship between VS activity and RPI was evident only in early adolescence and not late childhood, as the correlation between RPI at T1 and parameter estimates from this same VS cluster at T1 was not significant (r(36) = 0.01), whereas the correlation between RPI at T1 and VS at T2 was significant [r(36) = 0.31, p < .05]. Finally, although the base rate of self-reported risky behavior and delinquency was rather low, increases in IRBD from T1 to T2 correlated with decreases in VS response to all expressions [r(36) = 0.27, p = 0.05]. Such a relationship between more VS response and less engagement in risky behavior (as well as less susceptibility to peer influence) suggests that the VS response to affective facial displays may serve some protective function, at least during early adolescence. This might generally reflect normative neural responses to salient emotional information in the environment during this developmental stage, but given the prior research suggesting that the VS may support or index successful regulation, we suspected its role would reflect this capacity as well.
Figure 3. Longitudinal Increases in VS Response to Affective Facial Displays Correlate with Increases in Resistance to Peer Influence from Late Childhood to Early Adolescence.
Scatterplot depicts the positive correlation between increases in VS activity on the ordinate (mean parameter estimates, averaged across all voxels in the VS cluster shown to increase from T1 to T2; see Table 1 and Figure 1) and increases in resistance to peer influence on the abscissa.
Table 2. Correlation between Longitudinal Change in Brain Activity and Longitudinal Change in Resistance to Peer Influence.
| Region | BA | x | y | z | t | |
|---|---|---|---|---|---|---|
| Positive correlation |
Ventral striatum | −8 | 18 | 0 | 3.46 | |
| Dorsal striatum | −8 | 8 | 14 | 3.49 | ||
| Temporal pole | 38 | −46 | 4 | −14 | 4.03 | |
| Hippocampal gyrus | 36 | −18 | −28 | −24 | 3.52 | |
| 28/35 | −18 | −18 | −14 | 3.25 | ||
| Negative correlation |
Periamgydala | 20 | 0 | −8 | 3.68 | |
| Cerebellum | 38 | −62 | −24 | 3.68 |
Note: BA refers to putative Brodmann’s Area; x, y, and z refer to the left-right, anterior-posterior, and inferior-superior dimensions, respectively; and t refers to the t statistic at those coordinates (local maxima or sub-maxima). The ventral and dorsal striatum were observed as two distinct clusters, not submaxima within one larger cluster.
Psychophysiological Interaction Analysis
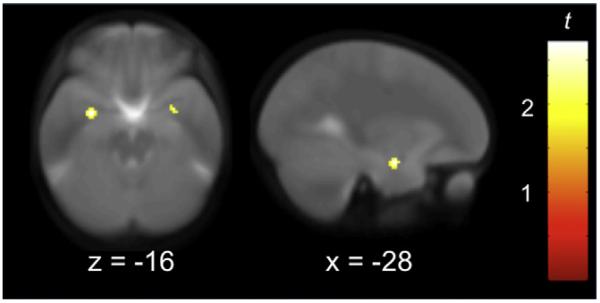
To provide further evidence in support of the notion that the increased VS response may reflect emotion regulation, as indicated by a dampening of the amygdala response to affective facial displays, a psychophysiological interaction (PPI) analysis was conducted. This technique identifies which areas of the brain are positively or negatively coupled with a specific brain region in a task-dependent fashion. We used the PPI analysis to ask the following specific question: is there enhanced negative functional connectivity between VS and amygdala in early adolescence as compared with late childhood? The results demonstrated that VS and amygdala activity were significantly more inversely related at T2 than T1 while processing both sad and happy expressions (relative to neutral ones), but not while processing angry or fearful expressions (see Figure 4).
Figure 4. Negative Coupling between VS and Amygdala Revealed by Psychophysiological Interaction Analysis.
Peaks in left ([−30 −4 −16], t = 2.70) and right ([30 0 −16], t = 2.28) amygdala represent significantly more negative coupling with VS while processing sad and happy expressions rather than neutral ones during early adolescence, compared with late childhood. Imaging data are thresholded at p < 0.05 (t > 1.69), and displayed on the average coplanar high-resolution scan across both time points for all children. x and z refer to the Talairach coordinates corresponding to the anterior-posterior and inferior-superior axes, respectively.
DISCUSSION
The results of this study provide not only evidence of longitudinal changes in neural responses to basic emotional stimuli, but also a demonstration of a relationship between these changes and important aspects of interpersonal functioning—resistance to peer influence and engagement in risky behavior—across a critical developmental transition. Responses to affective facial displays in VS and VMPFC increased from late childhood to early adolescence, with significantly greater VS reactions to sad and happy expressions (as compared with neutral ones). Notably, VS response increases to all expressions were correlated with increases in RPI and decreases in IRBD. Furthermore, VS and amygdala activity were significantly more negatively coupled at T2 than T1 while processing both sad and happy expressions relative to neutral ones. These longitudinal changes in responsivity to affective facial displays represent a combination of effects that generalize across a variety of expressions and emotion-specific trajectories. In particular, changes in response to sad and happy faces appear particularly prominent during this period of development.
Although activation in VS is typically associated with reward sensitivity and approach-related behavior (Delgado et al., 2000; Knutson et al., 2000; O’Doherty et al., 2003,2004), VS also responds to various aversive, salient, or novel stimuli (Gui-tart-Masip et al., 2010; Levita et al., 2009; Rich et al., 2006), and most importantly, this area has recently been implicated in successful emotion regulation and greater subjective positive affect in both adolescents and adults (Forbes et al., 2009,2010; Masten et al., 2009; Wager et al., 2008). Contrary to previous post hoc interpretations that increases in VS activity during adolescence represent a major risk factor, the current findings provide empirical evidence suggesting that increases in VS activity during adolescence are not necessarily a liability, but may instead be associated with relatively greater growth in abilities to resist peer pressure, as well as reductions in risky behavior and delinquency. The results of the PPI analysis provide support for the notion that VS facilitated or indexed greater regulation of amygdala responses to sad and happy expressions relative to neutral ones in early adolescence than in childhood. Better regulation of responses to emotional expressions may be associated with improved abilities to resist peer influence, because affective displays by peers can strongly impact behavior (Baird et al., 2010; Schlicht et al., 2010). The decomposition of amygdalostriatal interactions is an important direction for subsequent work exploring the development of emotion regulation. Future research should also attempt to better characterize the precise regulatory functions represented by VS responses during late childhood and early adolescence by, for example, contrasting patterns of brain activity during intentional emotion regulation tasks with those where any moderation of affective responses is incidental (as was the case in our design).
In contrast with two previous cross-sectional studies (Guyer et al., 2008; Hare et al., 2008), we did not find evidence of significant increases in amygdala activity across expressions during adolescence. Even when examining each expression independently, only sad faces elicited significantly greater amygdala activity over time. However, our results appear consistent with the prior research when one considers the age of our participants, who were just entering early adolescence, while the other studies examined amygdala responses throughout adolescence and into adulthood. In other words, upsurges in amygdala activity may be more extensive in middle adolescence, as suggested by inspecting the scatterplot from Hare et al. (2008) of amygdala reactivity to emotional expressions.
Several analyses suggested that two emotions evince the most change in subcortical activity during the transition from childhood to adolescence: sadness and happiness. The enhanced response to sadness may be related to its increased salience in adolescence, or the emergence of more advanced understandings of sadness. Rates of depression begin to increase during early adolescence, particularly for girls (Cyranowski et al., 2000; Chaplin et al., 2009). Next to surprise, the ability to recognize sadness appears to be relatively late in developing—a recent behavioral study demonstrated that 10-year-olds were least accurate at recognizing sad facial expressions from multiple vantage points (compared to the recognition of anger, disgust, and fear), and most accurate at recognizing happy expressions (Lau et al., 2009). Future research should continue to explore why sadness and happiness may evidence more change at the neural and/or behavioral levels than most basic emotions during this period.
Three other brain regions were identified in this study as demonstrating significant associations with increased resistance to peer pressure over time: temporal pole, dorsal striatum, and hippocampus. The temporal pole seems to play an important role in processing socioemotional information, including responding more to emotional than neutral facial expressions in adulthood (for a review, see Olson et al., 2007); our finding of longitudinal response increases in this region to emotional expressions versus neutral ones may pinpoint when this pattern first emerges. Although the dorsal striatum is typically thought of as a motor control region, it has been increasingly implicated in decision-making, executive functioning, and motivational learning (Balleine et al., 2007). Finally, the hippocampus is critical to the formation of episodic memories, and can influence amygdala responses to emotional stimuli (Phelps, 2004). Together, these regions may assist in the development and execution of strategies and behaviors that counteract peer pressure.
On a methodological note, our results emphasize the importance of exploring developmental changes in emotion reactivity and regulation using relatively tight age bands. Although relying on cross-sectional comparisons may be useful for initial descriptions of age-related trends, this may inadvertently miss important developmental processes, and does not help to differentiate the effects of age and puberty. Future investigations should explore longitudinal changes within this network during later developmental transitions, such as from early to middle or late adolescence, because these periods capture the windows of time when peer group norms transition to even greater behavioral misconduct, risk, and delinquency (Dishion and Tipsord, 2011; Steinberg and Monahan, 2007; Steinberg, 2008). In addition, similar studies should be conducted on more high-risk samples, to further test the relationships between longitudinal change in subcortical responses to various emotional expressions and pubertal development, peer pressure, and risky behavior.
In conclusion, the findings from this longitudinal fMRI study of normative socioemotional development provide us with a much more complete perspective on the role of subcortical systems in this crucial window of time, and perhaps beyond. On the one hand, the transition to adolescence does seem to be associated with greater subcortical reactivity to affective facial displays, particularly to sadness and happiness. On the other hand, not all adolescent increases in subcortical activity are indications of emotional chaos, limited willpower in the face of peer pressure, or propensity to engage in risky behavior. As conveyed via mass media, results of psychological and neuroscientific investigations may be perceived by laypersons as indicating that during adolescence, subcortical brain systems run amok and drive teenagers toward impulsive, emotional, and risky behavior. Our empirical findings provide a critical reminder that this is not always the case. Depending on the circumstances, subcortical activity may mark successful regulation of emotional responses to one’s environment. Perhaps if teenagers can better modulate their affective responses to a peer who is trying to persuade them to do something unwise (via nonverbal expressions of emotion, among other strategies), they will be less susceptible to that external influence. Importantly, the present findings also underline the need to explore whether basic training in emotion regulation techniques may support resistance to peer influence and prevent risky behavior or delinquency during the transition to adolescence and beyond, particularly for at-risk individuals with a history of behavioral misconduct and vulnerability to peer pressure.
EXPERIMENTAL PROCEDURES
Participants
Typically-developing children (n = 38, 24 girls) completed data collection at two visits (separated by 36 ± 10 months), and provided high-quality fMRI and behavioral data at both time points (average age = 10.0 ± 0.57 and 13.0 ± 0.67 years at T1 and T2, respectively). Participants and their parents provided written informed assent/consent according to guidelines specified by the Institutional Review Board at UCLA. Participants had no history of significant medical, psychiatric, or neurological disorders.
As a group, participants were ethnically and socioeconomically diverse. Fifty percent of participants were White, 25% Hispanic, 7.5% Asian, 5% Black, 5% Native American, and 2.5% Pacific Islander; 15% of this sample reported being multiethnic, including one participant (2.5%) who primarily identified as multiethnic. Parent reports of annual household income ranged from <$25,000 to >$400,000 (with an average income bracket of $100,000-$120,000). Full-scale IQ assessed by the Wechsler Intelligence Scale for Children (Wechsler, 1949) ranged from 86 to 144 (with an average IQ of 118).
The Pubertal Development Scale (PDS; Petersen et al., 1988) was completed at both time points; on this measure, participants self-report visible development of secondary sexual characteristics. There was a highly significant increase from T1 to T2 on the PDS [Ms = 1.58 and 2.56 at T1 and T2, respectively; t(1, 37) = 9.43, p ~0]. According to methods outlined by Shirtcliff et al. (2009), the PDS was transformed into values corresponding with Tanner stages on a gender-specific basis. These transformations suggested that the average level of development was similar between girls and boys in late childhood (T1Ms = 1.96 and 1.93 for girls and boys, respectively, indicating early pubertal status). By early adolescence, the girls were slightly more advanced (T2 Ms = 3.73 and 3.07 for girls and boys, indicating mid-to-late and midpubertal status, respectively).
Behavioral Measures
Participants also filled out the RPI Scale (Steinberg and Monahan, 2007), and indicators of risk behavior and delinquency as utilized in the Lerner 4-H Study of Positive Youth Development Survey (IRBD; Gestsdóttir and Lerner, 2007). The RPI is a self-report measure of resistance to peer influence that has been validated in nearly 4000 individuals ranging in age from 10–30 and varying in ethnicity and socioeconomic status. Important advantages of the RPI are that it targets primarily neutral, not antisocial or deviant influences, and minimizes socially desirable responding. On average, resistance to peer influence is lowest in late childhood and early adolescence, but then increases linearly beginning around 14 years of age. Our study thus presumably investigated the window of maximum susceptibility. The IRBD assesses self-reported substance use and delinquency, such as drinking, stealing, or getting into trouble with the police. In our sample, RPI evidenced a significant longitudinal increase (Ms = 2.7 and 2.9, SDs = 0.3 and 0.4, p < .05), but IRBD did not (Ms = 1.1 and 1.1, SDs = 0.1, not significant [n.s.]). These two constructs (RPI and IRBD) were significantly negatively correlated by age 13, but not at age 10 [r(36)s = 0.08 and −0.39, n.s., and p < .01, at T1 and T2, respectively]. Although it is common to assume adolescents are more susceptible to peer influence than children, the mean increase in RPI demonstrated in this sample was highly consistent with previously published reports; for example, in Steinberg and Monahan (2007), a nearly identical increase was found between age 10–11 (M = 2.8) and age 13 (M = 3.0).
Paradigm
During the fMRI scan, children passively observed full-color, whole-face emotional displays (angry, fearful, happy, sad, and neutral) from the NimStim set (Tottenham et al., 2009). Events lasted 2 s, with an interstimulus interval of variable (jittered) length ranging from 0.5–1.5 s (M = 1 s); events were presented in counterbalanced orders optimized for efficient detection of contrasts between emotions using a genetic algorithm (Wager and Nichols, 2003). A total of 96 whole-brain volumes were acquired on a Siemens Allegra 3.0 Tesla MRI scanner at each time point, including the 80 stimuli described above and an additional 16 null events (fixation crosses at eye-level).
fMRI Acquisition and Analysis
Data were acquired using a Siemens Allegra 3.0 Tesla MRI scanner. A 2D spinecho scout (TR = 4000 ms, TE = 40 ms, matrix size 256 by 256, 4 mm thick, 1 mm gap) was acquired in the sagittal plane to allow prescription of the slices to be obtained in the remaining scans. The scan lasted 4 min and 54 s (gradient-echo, TR = 3000 ms, TE = 25 ms, flip angle = 90°, matrix size 64 by 64, FOV = 20 cm, 36 slices, 3.125 mm in-plane resolution, 3 mm thick). For each participant, a high-resolution structural T2-weighted echo-planar imaging volume (spin-echo, TR = 5000 ms, TE = 33 ms, matrix size 128 by 128, FOV = 20 cm, 36 slices, 1.56 mm in-plane resolution, 3 mm thick) was also acquired coplanar with the functional scan. Stimuli were presented to participants through high-resolution magnet-compatible goggles (Resonance Technology, Inc.).
Using Automated Image Registration (Woods et al., 1998a, 1998b) implemented in the LONI Pipeline Processing Environment (http://pipeline.loni.ucla.edu/; Rex et al., 2003), all functional images were (1) realigned to correct for head motion and coregistered to their respective high-resolution structural images using a six-parameter rigid body transformation model, (2) spatially normalized into a Talairach-compatible MR atlas (Woods et al., 1999) using polynomial nonlinear warping, and (3) smoothed using a 6 mm FWHM isotropic Gaussian kernel. The quality of the data was extremely high: no participant averaged more than 1.5 mm of motion at either time point (Ms = 0.333 and 0.331 mm, SDs = 0.278 and 0.271, at T1 and T2, respectively), and no participant moved more than 2.0 mm between any image.
Statistical analyses were implemented in SPM8 (Wellcome Department of Cognitive Neurology, London, UK; (http://www.fil.ion.ucl.ac.uk/spm/) and MarsBaR (http://marsbar.sourceforge.net/; Brett et al., 2002). For each subject, condition effects were estimated according to the general linear model, using a canonical hemodynamic response function, high-pass filtering (128 s), AR(1), and no global scaling. Linear contrasts were employed to assess comparisons of interest within individual participants (all of the expressions versus null events, all of the emotional expressions versus neutral faces, and each of the five expressions versus null events) at the fixed-effects level. Random effects analyses were computed using the resulting contrast images generated for each subject. For all whole-brain analyses, results were reported that exceeded p < 0.005 for magnitude, uncorrected, and 20 contiguous voxels (a joint thresholding procedure that balances the risk of type I and type II errors; Lieberman and Cunningham, 2009).
Our a priori ROIs were driven by the prior research summarized in the Introduction and included the VS, VMPFC, and amygdala. For ROI analyses, mean parameter estimates of activity were extracted for each expression, at each time point, by averaging across every voxel in the ROI using MarsBaR. The exact same masks were used at T1 and T2 for all ROI analyses. The ROIs for VS and VMPFC were functionally defined as the clusters in VS and VMPFC that demonstrated significant increases over time (to all expressions) in the SPM analysis. Because the amygdala did not demonstrate a similar increase over time in this whole-brain analysis, the amygdala ROI was defined anatomically. When these mean parameter estimates of activity were subsequently correlated with behavioral measures, results were reported that exceeded p < 0.05.
The PPI analysis was conducted solely to determine if VS activity was more negatively coupled with amygdala activity in early adolescence than late childhood in an emotion-dependent manner. Volumes of interest (VOIs) were extracted at both T1 and T2 from the same VS mask used for the brain-behavior correlations, and then combined to create the PPI interaction term using the PPI function in SPM8. Rather than being performed on the whole brain, this analysis therefore utilized an explicit mask of the amygdala (the same mask used for the brain-behavior correlations), and activity was reported that exceeded p < 0.05 for magnitude, uncorrected.
Supplementary Material
ACKNOWLEDGMENTS
The authors wish to express their gratitude to Kristin McNealy, Larissa Borof-sky, Nicole Vazquez, Elliot Berkman, and the University of Oregon Developmental Social Neuroscience Lab, as well as three anonymous reviewers. For generous support the authors also wish to thank the Santa Fe Institute Consortium, Brain Mapping Medical Research Organization, Brain Mapping Support Foundation, Pierson-Lovelace Foundation, The Ahmanson Foundation, William M. and Linda R. Dietel Philanthropic Fund at the Northern Piedmont Community Foundation, Tamkin Foundation, Jennifer Jones-Simon Foundation, Capital Group Companies Charitable Foundation, Robson Family, and Northstar Fund. The project described was supported by grant numbers RR12169, RR13642, and RR00865 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH); its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCR or NIH.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes one figure and can be found with this article online at doi:10.1016/j.neuron.2011.02.019.
REFERENCES
- Adolphs R. Neural systems for recognizing emotion. Curr. Opin. Neurobiol. 2002;12:169–177. doi: 10.1016/s0959-4388(02)00301-x. [DOI] [PubMed] [Google Scholar]
- Baird AA, Gruber SA, Fein DA, Maas LC, Steingard RJ, Renshaw PF, Cohen BM, Yurgelun-Todd DA. Functional magnetic resonance imaging of facial affect recognition in children and adolescents. J. Am. Acad. Child Adolesc. Psychiatry. 1999;38:195–199. doi: 10.1097/00004583-199902000-00019. [DOI] [PubMed] [Google Scholar]
- Baird AA, Silver SH, Veague HB. Cognitive control reduces sensitivity to relational aggression among adolescent girls. Soc. Neurosci. 2010:1–14. doi: 10.1080/17470911003747386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. J. Neurosci. 2007;27:8161–8165. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra L, Breiter HC, Wise R, Gonzalez RG, Borsook D. Reward circuitry activation by noxious thermal stimuli. Neuron. 2001;32:927–946. doi: 10.1016/s0896-6273(01)00533-5. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox. NeuroImage. 2002;16:1140–1141. [Google Scholar]
- Brown B. Adolescents’ relationships with peers. In: Lerner R, Steinberg L, editors. Handbook of Adolescent Psychology. Wiley; New York: 2004. [Google Scholar]
- Chaplin TM, Gillham JE, Seligman ME. Gender, Anxiety, and Depressive Symptoms: A Longitudinal Study of Early Adolescents. J. Early Adolesc. 2009;29:307–327. doi: 10.1177/0272431608320125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyranowski JM, Frank E, Young E, Shear MK. Adolescent onset of the gender difference in lifetime rates of major depression: A theoretical model. Arch. Gen. Psychiatry. 2000;57:21–27. doi: 10.1001/archpsyc.57.1.21. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA. Tracking the hemodynamic responses to reward and punishment in the striatum. J. Neurophysiol. 2000;84:3072–3077. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- Dishion TJ, Tipsord JM. Peer contagion in child and adolescent social and emotional development. Annu. Rev. Psychol. 2011;62:189–214. doi: 10.1146/annurev.psych.093008.100412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving emotional conflict: A role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51:871–882. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Fitzgerald DA, Angstadt M, Jelsone LM, Nathan PJ, Phan KL. Beyond threat: Amygdala reactivity across multiple expressions of facial affect. Neuroimage. 2006;30:1441–1448. doi: 10.1016/j.neuroimage.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Hariri AR, Martin SL, Silk JS, Moyles DL, Fisher PM, Brown SM, Ryan ND, Birmaher B, Axelson DA, Dahl RE. Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. Am. J. Psychiatry. 2009;166:64–73. doi: 10.1176/appi.ajp.2008.07081336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Ryan ND, Phillips ML, Manuck SB, Worthman CM, Moyles DL, Tarr JA, Sciarrillo SR, Dahl RE. Healthy adolescents’ neural response to reward: Associations with puberty, positive affect, and depressive symptoms. J. Am. Acad. Child Adolesc. Psychiatry. 2010;49:162–172. e1–e5. doi: 10.1097/00004583-201002000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M, Steinberg L. Peer influence on risk taking, risk preference, and risky decision making in adolescence and adulthood: An experimental study. Dev. Psychol. 2005;41:625–635. doi: 10.1037/0012-1649.41.4.625. [DOI] [PubMed] [Google Scholar]
- Gestsdóttir S, Lerner RM. Intentional self-regulation and positive youth development in early adolescence: Findings from the 4-h study of positive youth development. Dev. Psychol. 2007;43:508–521. doi: 10.1037/0012-1649.43.2.508. [DOI] [PubMed] [Google Scholar]
- Grosbras MH, Jansen M, Leonard G, McIntosh A, Osswald K, Poulsen C, Steinberg L, Toro R, Paus T. Neural mechanisms of resistance to peer influence in early adolescence. J. Neurosci. 2007;27:8040–8045. doi: 10.1523/JNEUROSCI.1360-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guitart-Masip M, Bunzeck N, Stephan KE, Dolan RJ, Düzel E. Contextual novelty changes reward representations in the striatum. J. Neurosci. 2010;30:1721–1726. doi: 10.1523/JNEUROSCI.5331-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Monk CS, McClure-Tone EB, Nelson EE, Roberson-Nay R, Adler AD, Fromm SJ, Leibenluft E, Pine DS, Ernst M. A developmental examination of amygdala response to facial expressions. J. Cogn. Neurosci. 2008;20:1565–1582. doi: 10.1162/jocn.2008.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Davidson MC, Glover GH, Casey BJ. Contributions of amygdala and striatal activity in emotion regulation. Biol. Psychiatry. 2005;57:624–632. doi: 10.1016/j.biopsych.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol. Psychiatry. 2008;63:927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvitz JC. Mesolimbocortical and nigrostriatal dopamine responses to salient non-reward events. Neuroscience. 2000;96:651–656. doi: 10.1016/s0306-4522(00)00019-1. [DOI] [PubMed] [Google Scholar]
- Jensen J, McIntosh AR, Crawley AP, Mikulis DJ, Remington G, Kapur S. Direct activation of the ventral striatum in anticipation of aversive stimuli. Neuron. 2003;40:1251–1257. doi: 10.1016/s0896-6273(03)00724-4. [DOI] [PubMed] [Google Scholar]
- Knutson B, Westdorp A, Kaiser E, Hommer D. FMRI visualization of brain activity during a monetary incentive delay task. Neuroimage. 2000;12:20–27. doi: 10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]
- Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. Functional grouping and cortical-subcortical interactions in emotion: A meta-analysis of neuroimaging studies. Neuroimage. 2008;42:998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau JY, Burt M, Leibenluft E, Pine DS, Rijsdijk F, Shiffrin N, Eley TC. Individual differences in children’s facial expression recognition ability: The role of nature and nurture. Dev. Neuropsychol. 2009;34:37–51. doi: 10.1080/87565640802564424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévesque J, Joanette Y, Mensour B, Beaudoin G, Leroux JM, Bourgouin P, Beauregard M. Neural basis of emotional self-regulation in childhood. Neuroscience. 2004;129:361–369. doi: 10.1016/j.neuroscience.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Levita L, Hare TA, Voss HU, Glover G, Ballon DJ, Casey BJ. The bivalent side of the nucleus accumbens. Neuroimage. 2009;44:1178–1187. doi: 10.1016/j.neuroimage.2008.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: Re-balancing the scale. Soc. Cogn. Affect. Neurosci. 2009;4:423–428. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten CL, Eisenberger NI, Borofsky LA, Pfeifer JH, McNealy K, Mazziotta JC, Dapretto M. Neural correlates of social exclusion during adolescence: Understanding the distress of peer rejection. Soc. Cogn. Affect. Neurosci. 2009;4:143–157. doi: 10.1093/scan/nsp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae K, Reiman EM, Fort CL, Chen K, Lane RD. Association between trait emotional awareness and dorsal anterior cingulate activity during emotion is arousal-dependent. Neuroimage. 2008;41:648–655. doi: 10.1016/j.neuroimage.2008.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk CS, McClure EB, Nelson EE, Zarahn E, Bilder RM, Leibenluft E, Charney DS, Ernst M, Pine DS. Adolescent immaturity in attention-related brain engagement to emotional facial expressions. Neuroimage. 2003;20:420–428. doi: 10.1016/s1053-8119(03)00355-0. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Leibenluft E, McClure EB, Pine, D The social re-orientation of adolescence: A neuroscience perspective on the process and its relation to psychopathology. Psychol. Med. 2005;35:163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP, Dayan P, Friston K, Critchley H, Dolan RJ. Temporal difference models and reward-related learning in the human brain. Neuron. 2003;38:329–337. doi: 10.1016/s0896-6273(03)00169-7. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science. 2004;304:452–454. doi: 10.1126/science.1094285. [DOI] [PubMed] [Google Scholar]
- Olson IR, Plotzker A, Ezzyat Y. The Enigmatic temporal pole: A review of findings on social and emotional processing. Brain. 2007;130:1718–1731. doi: 10.1093/brain/awm052. [DOI] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. J. Youth Adolesc. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Phelps EA. Human emotion and memory: Interactions of the amygdala and hippocampal complex. Curr. Opin. Neurobiol. 2004;14:198–202. doi: 10.1016/j.conb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Rex DE, Ma JQ, Toga AW. The LONI Pipeline Processing Environment. Neuroimage. 2003;19:1033–1048. doi: 10.1016/s1053-8119(03)00185-x. [DOI] [PubMed] [Google Scholar]
- Rich BA, Vinton DT, Roberson-Nay R, Hommer RE, Berghorst LH, McClure EB, Fromm SJ, Pine DS, Leibenluft E. Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder. Proc. Natl. Acad. Sci. USA. 2006;103:8900–8905. doi: 10.1073/pnas.0603246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlicht EJ, Shimojo S, Camerer CF, Battaglia P, Nakayama K. Human wagering behavior depends on opponents’ faces. PLoS ONE. 2010;5:e11663. doi: 10.1371/journal.pone.0011663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, Greenstein D, Clasen L, Evans A, Rapoport JL, et al. Neurodevelopmental trajectories of the human cerebral cortex. J. Neurosci. 2008;28:3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Dahl RE, Pollak SD. Pubertal development: Correspondence between hormonal and physical development. Child Dev. 2009;80:327–337. doi: 10.1111/j.1467-8624.2009.01263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Jones RM, Casey BJ. A time of change: Behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain Cogn. 2010;72:124–133. doi: 10.1016/j.bandc.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Trauner DA, Gamst A, Jernigan TL. Development of cortical and subcortical brain structures in childhood and adolescence: A structural MRI study. Dev. Med. Child Neurol. 2002;44:4–16. doi: 10.1017/s0012162201001591. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Toga AW. Mapping changes in the human cortex throughout the span of life. Neuroscientist. 2004;10:372–392. doi: 10.1177/1073858404263960. [DOI] [PubMed] [Google Scholar]
- Steinberg L. A social neuroscience perspective on adolescent risk-taking. Dev. Rev. 2008;28:78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L, Monahan KC. Age differences in resistance to peer influence. Dev. Psychol. 2007;43:1531–1543. doi: 10.1037/0012-1649.43.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas KM, Drevets WC, Whalen PJ, Eccard CH, Dahl RE, Ryan ND, Casey BJ. Amygdala response to facial expressions in children and adults. Biol. Psychiatry. 2001;49:309–316. doi: 10.1016/s0006-3223(00)01066-0. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Marcus DJ, Westerlund A, Casey BJ, Nelson C. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Res. 2009;168:242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Gaag C, Minderaa RB, Keysers C. Facial expressions: What the mirror neuron system can and cannot tell us. Soc. Neurosci. 2007;2:179–222. doi: 10.1080/17470910701376878. [DOI] [PubMed] [Google Scholar]
- Wager TD, Nichols TE. Optimization of experimental design in fMRI: A general framework using a genetic algorithm. Neuroimage. 2003;18:293–309. doi: 10.1016/s1053-8119(02)00046-0. [DOI] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. The Wechsler Intelligence Scale for Children. Psychological Corp; New York: 1949. [Google Scholar]
- Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC. Automated image registration: I. General methods and intrasubject, intramodality validation. J. Comput. Assist. Tomogr. 1998a;22:139–152. doi: 10.1097/00004728-199801000-00027. [DOI] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Watson JD, Sicotte NL, Mazziotta JC. Automated image registration: II. Intersubject validation of linear and nonlinear models. J. Comput. Assist. Tomogr. 1998b;22:153–165. doi: 10.1097/00004728-199801000-00028. [DOI] [PubMed] [Google Scholar]
- Woods RP, Dapretto M, Sicotte NL, Toga AW, Mazziotta JC. Creation and use of a Talairach-compatible atlas for accurate, automated, nonlinear intersubject registration, and analysis of functional imaging data. Hum. Brain Mapp. 1999;8:73–79. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<73::AID-HBM1>3.0.CO;2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.