Abstract
Important functions of the vascular endothelium, including permeability, production of antithrombotic factors, and control of vascular tone, are regulated by changes in intracellular Ca2+. The molecular identities and regulation of Ca2+ influx channels in the endothelium are incompletely understood, in part because of experimental difficulties associated with application of patch-clamp electrophysiology to native endothelial cells. However, advances in confocal and total internal reflection fluorescence microscopy and the development of fast, high-affinity Ca2+-binding fluorophores have recently allowed for direct visualization and characterization of single-channel transient receptor potential (TRP) channel Ca2+ influx events in endothelial cells. These events, called “TRP channel Ca2+ sparklets,” have been optically recorded from primary endothelial cells and the intact endothelium, and the biophysical properties and fundamental significance of these Ca2+ signals in vasomotor regulation have been characterized. This review will first briefly discuss the role of endothelial cell TRP channel Ca2+ influx in endothelium-dependent vasodilation, describe improved methods for recording unitary TRP channel activity using optical methods, and highlight discoveries regarding the regulation and physiological significance of TRPV4 Ca2+ sparklets in the vascular endothelium enabled by this new technology. Perspectives on the potential use of these techniques to evaluate changes in TRP channel Ca2+ influx activity associated with endothelial dysfunction are offered.
Keywords: endothelium, TRPV4, total internal reflection fluorescence microscopy, confocal microscopy, Ca2+ sparklet
the vascular endothelium, a thin monolayer of endothelial cells lining the lumen of all blood vessels, is critically important for angiogenesis, prevention of thrombogenesis, regulation of permeability, and control of arterial tone. Endothelial dysfunction is tightly associated with common cardiovascular diseases, such as atherosclerosis, stroke, and hypertension, demonstrating the critical importance of this tissue for maintaining vascular health. Because of their unique anatomic location, endothelial cells are able to directly detect blood-borne molecules and luminal shear stress. Endothelial cells convey these hemodynamic signals to underlying smooth muscle cells, thereby eliciting vasomotor responses. For example, increases in arterial shear stress stimulate the endothelium to produce nitric oxide (NO), which diffuses to underlying smooth muscle cells to evoke vasodilation (35, 69). Endothelial cell-mediated vasoregulatory pathways are highly dependent on changes in intracellular Ca2+ concentration ([Ca2+]), establishing an essential function for endothelial cell Ca2+ entry in the regulation of vascular tone. However, little is known about the molecular identities and regulation of the specific Ca2+ influx channels involved. Electrophysiological methods are frequently used to record Ca2+-permeable ion channel activity, but small, flat, native endothelial cells are difficult to study using this technique. Recently, alternative approaches utilizing fluorescence microscopy to record Ca2+ channel activity have been described (15). Cells containing sensitive, rapid Ca2+ indicators are imaged using total internal reflection fluorescence (TIRF) or high-speed confocal microscopy. With use of these methods, Ca2+ channel activity can be optically recorded as transient increases in fluorescence at the cell surface, and signal amplitude, spatial spread, and duration are determined.
Exciting studies have recently used these optical techniques to record transient receptor potential (TRP) channel activity in endothelial cells. Twenty-six of the 28 members of the TRP superfamily of ion channels are permeable to Ca2+, and many are present in the vascular endothelium (Table 1). Several TRP channels are known to be involved in endothelial cell Ca2+ influx and regulation of vascular tone (Table 1) (18, 19, 75, 105). Recently, optical recording methods were used to describe Ca2+ influx events through unitary transient receptor potential vanilloid (TRPV4) channels, termed “TRPV4 Ca2+ sparklets,” in vascular endothelial cells (78, 82). More interestingly, analysis of TRPV4 Ca2+ sparklets in the intact endothelium indicated that a small number of sparklets can evoke a near-maximal endothelium-dependent vasodilation in pressurized arteries (78). These pioneering studies indicate the essential role of unitary TRP channel Ca2+ microdomains in vascular function. This review discusses the role of TRP channels in endothelium-dependent dilation, methods for recording TRP channel Ca2+ sparklets in endothelial cells, and the significance of TRPV4 Ca2+ sparklets in the regulation of vascular tone.
Table 1.
TRP channels in vascular endothelial cells
| Channel | Selectivity PCa/PNa | Present in Endothelium? (98) | Involved in EDH |
|---|---|---|---|
| TRPA1 | 0.8–7.9 (79) | Yes | Yes (18) |
| TRPC1 | Nonselective (108) | Yes | |
| TRPC2 | 1–3 (47) | Yes | |
| TRPC3 | 1.6 (30, 33) | Yes | Yes (75) |
| TRPC4 | 1.1 (67) | Yes | |
| TRPC5 | 9 (67) | Yes | |
| TRPC6 | 5 (33) | Yes | |
| TRPC7 | 0.5–5.4 (63) | Yes | |
| TRPM1 | <1 (62) | Yes | |
| TRPM2 | 0.5–1.6 (66) | Yes | |
| TRPM3 | 0.1–10 (29) | Yes | |
| TRPM4 | Not Ca2+ permeable (<0.001) (43) | Yes | |
| TRPM5 | Not Ca2+ permeable (<0.05) (32) | No | |
| TRPM6 | PMg/PNa ∼6 (90) | Yes | |
| TRPM7 | 0.3–3 (70) | Yes | |
| TRPM8 | 1–3 (48) | Yes | |
| TRPML1 | Nonselective (42) | ND | |
| TRPML2 | ND | ND | |
| TRPML3 | ND | ND | |
| TRPP1 | 1–5 (41) | Yes | |
| TRPP2 | 4 (11) | ND | |
| TRPP3 | 1–5 (92) | Yes | |
| TRPV1 | 10 (7) | Yes | |
| TRPV2 | 1–3 (37) | Yes | |
| TRPV3 | 12 (101) | Yes (19, 50) | Yes (19) |
| TRPV4 | 6 (81) | Yes | Yes (105) |
| TRPV5 | 100 (61) | No | |
| TRPV6 | 100 (104) | No |
Relative Ca2+-to-Na+ permeability ratio (PCa/PNa) and presence in vascular endothelial cells were detected by RT-PCR, Western blotting, or immunohistochemistry [updated from Wong and Yao (98)] for each transient receptor potential (TRP) channel. EDH, endothelium-dependent hyperpolarization; ND, not determined. Reference numbers are in parentheses.
Changes in Intracellular [Ca2+] Regulate Vascular Tone
Intracellular [Ca2+] is a critical determinate of vascular tone. Ca2+ signals can be global (i.e., continuous throughout the cytosol) and long-lasting, or they can be spatially restricted to specific subcellular domains for brief periods of time. In arterial smooth muscle cells, membrane depolarization causes Ca2+ influx through voltage-dependent Ca2+ channels, resulting in elevated global cytosolic [Ca2+] and subsequent contraction. Endothelium-dependent relaxation of smooth muscle is also regulated by elevations in endothelial cell [Ca2+]. Three major pathways for endothelium-dependent vasodilation are widely recognized: 1) production of NO by endothelial NO synthase (NOS), 2) production of PGI2 by cyclooxygenase (COX), and 3) endothelium-derived hyperpolarizing factor (EDHF) (22) or endothelium-dependent hyperpolarization (EDH) (4, 27), which are defined as endothelium-dependent vasodilatory mechanisms that persist when NO and PGI2 synthesis is inhibited. The EDHF hypothesis follows the convention of the NO and PGI2 pathways and proposes that one or more additional endothelium-derived diffusible factor(s), such as epoxyeicosatrienoic acids (EETs) (6), K+ (21), C-type natriuretic peptide (9, 95), H2S (34, 106), and/or H2O2 (3), are responsible for smooth muscle cell hyperpolarization and subsequent vasodilation. In contrast, EDH, a “factorless” form of EDHF, is the result of endothelial cell plasma membrane hyperpolarization due to the efflux of K+ from small- and intermediate-conductance Ca2+-activated K+ (KCa2.3 and KCa3.1, respectively) channels (13, 89). Endothelial cell membrane hyperpolarization rapidly spreads to underlying smooth muscle cells via myoendothelial gap junctions, directly hyperpolarizing and relaxing arterial myocytes (12, 22). EDH responses may be augmented by inwardly rectifying K+ (KIR) channels, which conduct greater outward K+ currents at hyperpolarized membrane potential and amplify the original hyperpolarizing stimulus (18, 77). All these seemingly divergent pathways can be activated by increases in endothelial cell [Ca2+]: production of NO by endothelial NOS is elevated by binding to Ca2+-calmodulin (84), whereas liberation of arachidonic acid by Ca2+-sensitive PLA2 (5) provides substrate for the biosynthesis of PGI2 (5, 17) and EETs (6). Increases in endothelial cell [Ca2+] directly activate KCa channels to initiate EDH (4, 27).
Recent studies highlight the importance of myoendothelial junctions (MEJs) in EDH (18, 44, 68, 75, 78). MEJs are extensions of the endothelial cell plasma membrane that project through holes in the internal elastic lamina (IEL) and terminate proximal to the smooth muscle cell sarcolemma (51, 73). MEJs terminate in myoendothelial gap junctions, allowing for acute endothelial cell-smooth muscle cell communication (73). Not all membrane projections within IEL holes contain myoendothelial gap junctions (72), but the significance of these structures (called myoendothelial close contacts) is not known. Expression of components of the EDH pathway, including KCa channels, inositol 1,4,5-trisphophate receptors, gap junctions, and TRP channels, is enriched in authentic MEJs (18, 19, 39, 44, 73, 74). Furthermore, MEJs appear to be sites of dynamic localized Ca2+ signaling events that mediate EDH (44, 68).
Although the endothelium-dependent vasorelaxant pathways discussed above are functionally distinct, all can be initiated by an increase in endothelial cell intracellular [Ca2+]. Such increases can be accomplished by Ca2+ entry from the extracellular space, but our understanding of the Ca2+-permeable channels directly involved in endothelium-dependent vasodilation remains incomplete. Several classes of Ca2+ influx channels, including the cyclic nucleotide-gated (CNG) channel A1 (99, 103), the purinergic ligand-gated P2X4 channel (102), and TRP channels (8, 18, 19, 24, 91), have been reported in the vascular endothelium. Ca2+ and Na+ influx through CNG channels in endothelial cells occurs in response to store-operated Ca2+ entry (99, 103). P2X4 channels are activated by extracellular ATP and conduct Ca2+ into endothelial cells to cause vasodilation through increased NO synthesis (99, 102, 103). Although CNG and P2X4 channels may be involved in endothelium-dependent regulation under certain conditions, there is no apparent mechanism for these channels to respond to the multitude of stimuli presented to the intact endothelium. In contrast, Ca2+-permeable members of the TRP superfamily are sensitive to physical factors [mechanical force (85) and changes in osmolality (45)], temperature (7, 48, 64, 76, 79), hypoxia/hyperoxia (1, 86, 96), and chemical substances (7, 36, 100). A number of recent studies demonstrate the importance of particular TRP channels in endothelium-dependent vasodilatory responses.
TRP Channels in Endothelium-Dependent Vasodilation
All members of the TRP superfamily, with the exception of TRPM4 and TRPM5, are permeable to Ca2+ (Table 1). Expression of ≥20 Ca2+-permeable TRP channels, including TRPA1 (18), TRPC1 (8, 26), TRPC3 (8, 26), TRPC4 (24, 26), TRPC5 (26), TRPC6 (26), TRPV3 (19), and TRPV4 (97), has been detected in cultured and native endothelial cells (Table 1). Freichel et al. (24), who investigated the role of TRPC4 in store-operated Ca2+ entry in endothelial cells, reported the first functional evidence of the involvement of a TRP channel in endothelium-dependent vasodilation. Their study demonstrates that endothelial cells isolated from TRPC4 knockout (TRPC4−/−) mice did not display a store-operated Ca2+ entry current. More interestingly, endothelial cells isolated from TRPC4−/− mice exhibited reduced agonist-induced endothelial cell Ca2+ entry, and aortic ring sections displayed diminished endothelium-dependent vasorelaxation in response to acetylcholine, suggesting that TRPC4 channels are vitally important for endothelial cell function (24). Subsequent studies indicate that TRPV4, TRPC3, TRPV3, and TRPA1 channels are also critically involved in endothelium-dependent vasodilation (Table 1) (18, 19, 75, 105).
The contribution of TRPV4 channels to endothelium-dependent vasodilation is well described. In heterologous expression systems and endothelial cells, TRPV4 channels are activated by EETs (94), potent vasodilators produced by endothelial cells synthesized from arachidonic acid by cytochrome P-450 (CYP) epoxygenase enzymes (10, 38). EET-induced vasodilation of isolated mesenteric arteries was absent from TRPV4−/− mice, suggesting that the channel is involved in this response (20). The physiological significance of this pathway was reported by Mendoza and colleagues (49), who showed that TRPV4-mediated Ca2+ influx is critically important for flow-mediated dilation of human coronary arteries. Later studies demonstrate a similar role for TRPV4 in other vascular beds (31, 46). These studies imply that endothelial cell TRPV4 channels are activated by shear stress (45). However, although TRPV4 channels are activated by cell swelling, it has been reported that the channel is not inherently mechanosensitive (80). Further studies by Loot et al. (46) suggest that TRPV4 is indirectly activated by shear stress through flow-mediated production of arachidonic acid metabolites. Inhibition of CYP epoxygenase with N-(methylsulfonyl)-2-(2-propynyloxy)-benzenehexamide or block of TRPV4 with ruthenium red attenuated flow-induced dilation in mouse carotid arteries (46). However, TRPV4 blockade had no effect on flow-induced dilatory responses when CYP epoxygenase was downregulated (46). These results are supported by another study showing that flow-induced dilation was impaired by PLA2 inhibition, TRPV4 inhibition by ruthenium red, and TRPV4 knockout (31), suggesting that flow-induced dilation involves TRPV4 and CYP epoxygenase activity. Further work (20, 71, 105) indicates that Ca2+ influx and vasodilation following stimulation with cholinergic agonists in some, but not all vessels, is markedly reduced in TRPV4−/− mice, clearly indicating a role for the channel in this response. Oddly, although the studies cited above and others suggest a critical role for TRPV4 in vascular regulation, global TRPV4−/− mice have no obvious cardiovascular phenotype, other than slightly enhanced sensitivity to hypertensive stimuli (85). Further studies using tissue-specific and/or inducible knockout mice are warranted to resolve this apparent paradox.
Reports from several laboratories suggest involvement of TRPC3 in endothelium-mediated vascular responses. Gao et al. (25) provided evidence for expression of TRPC3 in the endothelial and smooth muscle layers of human internal mammary artery (IMA) and show that the TRPC3 blocker Pyr3 modestly diminished relaxation of precontracted IMA rings. Using knockout models, Kochukov et al. (40) demonstrated that TRPC1 and TRPC3 channels are involved in Ca2+ influx and vasorelaxation of aortic ring segments. The Sandow laboratory recently reported participation of TRPC3 channels in EDH of rat and mouse mesenteric arteries (75). This study demonstrates that TRPC3 channels are present in the mesenteric endothelium and that ∼70% of endothelial TRPC3 channels are localized within holes in the IEL. In the presence of the NOS inhibitor nitro-l-arginine methyl ester (l-NAME), the guanylyl cyclase inhibitor 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one, and the COX inhibitor indomethacin, Pyr3 blunted acetylcholine-induced vasorelaxation and endothelial cell hyperpolarization. Vasodilatory responses were further attenuated by block of KCa channels with TRAM34 and apamin. Together, these data suggest that TRPC3 channel activity can stimulate EDH in mesenteric arteries. In support of these findings, Kirby et al. (39) reported the presence of TRPC3, as well as KCa3.1 and KCa2.3, within IEL fenestrations of rat popliteal and first-order skeletal muscle arteries from the gastrocnemius muscle, suggesting that TRPC3 may contribute to EDH in this arterial bed as well, although this has not been directly demonstrated.
Our laboratory reported that TRPA1- and TRPV3-mediated Ca2+ influx provokes endothelium-dependent dilation of cerebral arteries (18, 19). TRPV3 channels are activated by the dietary molecule carvacrol, a substance derived from oregano (19). We found that carvacrol activates ruthenium red-sensitive cation currents and elevates intracellular Ca2+ levels in native cerebral artery endothelial cells (19). Carvacrol administration evoked endothelium-dependent vasodilation of cerebral arteries that was not altered by NOS and COX inhibition but was sensitive to block of KCa and KIR channels (19). TRPA1 channels are activated by a variety of electrophilic substances, including allicin, found in garlic, and allyl isothiocyanate (AITC), derived from mustard oil (36). Allicin can dilate mesenteric arteries by activating TRPA1 channels in perivascular nerves and causing the release of calcitonin gene-related peptide (2). We found that TRPA1 channels were also present in the cerebral artery endothelium (18). AITC caused robust endothelium-dependent dilation and smooth muscle cell membrane hyperpolarization that was blocked by the selective TRPA1 inhibitor HC-030031. These responses were insensitive to NOS and COX blockade but diminished by inhibition of KCa channels with TRAM34 and apamin, as well as block of KIR channels with BaCl2 (18). We also found that that TRPA1 channels are highly concentrated within IEL fenestrations in cerebral arteries and colocalize with KCa3.1 channels in this tissue (18). In contrast, TRPV3 channels are uniformly distributed (19), suggesting that TRPA1 and TRPV3 channels may elicit vasodilation by distinct molecular pathways. Insight into TRPA1-mediated endothelium-dependent vasodilation was provided by a recent study by Qian et al. (68), who demonstrated that activation of TRPA1 channels provokes dilation by recruiting large dynamic Ca2+ signals in the cerebral artery endothelium. Because food-borne molecules found in garlic and mustard can activate TRPV3 and TRPA1 channels, it is tempting to speculate that increased TRPV3 and/or TRPA1 activity in the endothelium could contribute to the putative cardioprotective benefits of certain dietary choices, but there is little direct evidence to support this idea. Further investigation into endogenous regulators of these channels is warranted.
Optical Methods for the Study of Ca2+ Influx Channels
Evidence cited above indicates that TRP channel activity can influence endothelium-dependent dilation, stimulating interest in the properties and regulation of TRP channels in the endothelium. Ion channels are traditionally studied using patch-clamp electrophysiology. However, small, flat cells, such as native endothelial cells, are difficult to patch successfully, and throughput using conventional methods can be painfully slow. Moreover, inherent limitations of the voltage-clamp technique can impede investigation of ion channel regulation in a physiological setting. For example, when the conventional whole cell patch-clamp configuration is employed, intracellular signaling pathways are disrupted when cells are dialyzed with the patch pipette solution. More serious disturbances in metabotropic regulatory mechanisms result from application of inside-out or outside-out patch-clamp methods. Although the intracellular environment is not disturbed when the on-cell patch-clamp configuration is employed, channel activity can only be recorded from the small area of membrane beneath the recording pipette, and voltage clamp cannot be maintained. These technical issues hamper electrophysiological investigation of Ca2+ influx pathways in native endothelial cells.
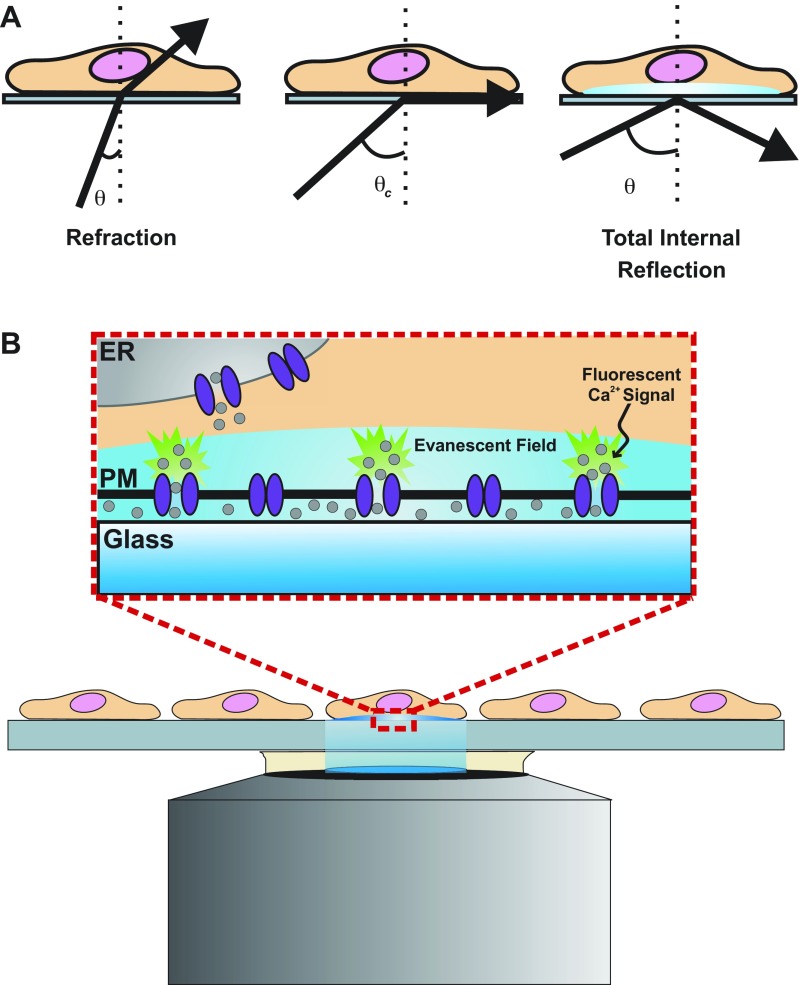
Fortunately, sophisticated imaging techniques have recently been developed to surmount the limitations of patch-clamp electrophysiology and global Ca2+-imaging techniques. This methodology, pioneered by Demuro and Parker (14, 16), uses high-speed, high-resolution confocal microscopy or TIRF microscopy to record single-channel Ca2+ signals in a variety of cell types. Confocal and TIRF microscopy use different approaches to achieve fluorescent excitation of a thin, isolated plane along the z-axis of a specimen (axial sectioning). High-resolution confocal microscopy employs an adjustable plane of illumination along the z-axis of a cell or tissue. This is achieved by scanning the specimen with a column of light so that only a small spot in the plane of focus is illuminated at a time. Because of light scattering, areas in out-of-focus planes are also illuminated with this setup alone. Therefore, the addition of a pinhole aperture proximal to the detector and conjugate to the in-focus plane prevents detection of light from out-of-focus planes, thus achieving a thin optical section of illumination. TIRF microscopy utilizes the total internal reflection of light that occurs when light travels through media of decreasing refractive indexes, e.g., when light travels through glass into an aqueous specimen, at an angle greater than a defined critical angle (relative to normal) (Fig. 1A). Total internal reflection of light creates a low-energy evanescent field of illumination along the surface of the aqueous specimen, generating a thin fluorescent plane of excitation that only penetrates ∼100 nm into the specimen (Fig. 1B). TIRF microscopy develops a thinner optical section than does high-resolution confocal microscopy (100 nm vs. 1 μm), which is advantageous for the study of unitary Ca2+ channel activity at the plasma membrane. However, this can also be a disadvantage for recording intracellular Ca2+ release events. Confocal microscopy is more useful for this purpose, as the plane of excitation can be focused within the cytosol. TIRF and confocal Ca2+ imaging rely on high-affinity fluorescent Ca2+ reporter molecules with rapid binding kinetics to resolve Ca2+ signals. The cell-permeable, nonratiometric Ca2+ indicators fluo 3-AM (52) and fluo 4-AM (28) are most commonly used to record transient Ca2+ signals. More recently, genetically encoded Ca2+ indicator proteins have been utilized in Ca2+-imaging experiments. Most of these molecules are green fluorescent protein-calmodulin (CaM) fusion proteins, such as Pericams (54), GCaMP2 (87, 88), and Cameleon (53). Targeted expression, allowing Ca2+ signals to be recorded from specific tissues without contamination from other cell types, constitutes a major advantage of genetically encoded vs. chemical Ca2+ indicators. In addition, genetic expression reduces loading variability associated with chemical indicators. Thus advances in Ca2+-imaging techniques and Ca2+ indicators have greatly improved the ability to optically resolve unitary Ca2+ channel activity and have expanded the type of information that can be obtained from such experiments.
Fig. 1.
Visualization of membrane Ca2+ channel activity using total internal reflection fluorescence (TIRF) microscopy. A: when light is directed toward a glass-cell interface at an angle (θ) greater than a critical angle (θc), light becomes totally, internally reflected away from the interface surface. The total internal reflection creates a low-energy plane of illumination (evanescent field) penetrating only ∼100 nm into a cell, which allows for visualization of plasma membrane (PM) Ca2+ channel activity without background cytosolic Ca2+ sources (B). Ca2+ (gray circles) that enters the cell will be bound by fluorescent Ca2+ indicators and produce a fluorescence signal at the site of entry. This technique allows for visualization of Ca2+ channel activity on the entire bottom surface of the cell. ER, endoplasmic reticulum.
Optical recording of Ca2+ channel activity presents distinct advantages over conventional electrophysiological methods. Spatial information regarding channel location on the plasma membrane is gained, and simultaneous channel activity can be recorded from the entire bottom surface of the plasma membrane. Moreover, optical methods are less invasive than patch-clamp techniques, allowing intracellular signaling pathways to remain intact. The main disadvantage of optical vs. patch-clamp recording is lack of membrane voltage clamp. To overcome this problem, the two techniques can be applied simultaneously. Ultimately, both methods will continue to be used to study Ca2+ influx pathways.
The most complete example of the power of these new optical approaches was reported in a sophisticated series of studies by the Santana laboratory that exploited TIRF microscopy to investigate unitary Ca2+ influx events through L-type [voltage-gated Ca2+ (CaV1.2)] channels in vascular smooth muscle cells (for review see Ref. 55). These events, called “L-type Ca2+ channel sparklets” (93), are recorded by imaging membrane Ca2+ signals in Ca2+ indicator-loaded cells with TIRF microscopy under voltage-clamp conditions while background cytosolic Ca2+ is buffered with the slow chelator EGTA. L-type Ca2+ channel sparklets are low-amplitude Ca2+ signals (Table 2); therefore, the thin fluorescent excitation plane generated by TIRF coupled with intracellular Ca2+ buffering is critical for resolution. Ca2+ sparklets are distinct from Ca2+ sparks, another well-described Ca2+ signal in smooth muscle cells (60). Ca2+ sparklets are recordings of Ca2+ influx through individual Ca2+-permeable ion channels on the plasma membrane and are abolished by removal of extracellular Ca2+ but unaffected by intracellular Ca2+ store depletion. Ca2+ sparks are Ca2+ release events from intracellular stores via ryanodine receptors located on the sarcoplasmic reticulum and are abolished by store depletion. Recordings of Ca2+ sparks and Ca2+ sparklets (and other localized Ca2+-signaling events) are easily distinguishable by their biophysical properties, such as amplitude and spatial spread (Table 2).
Table 2.
TRPV4 Ca2+ sparklets are distinct Ca2+ microdomains
| Ca2+ Microdomain | Amplitude | Duration, ms | Spatial Spread, μm2 | Frequency, Hz |
|---|---|---|---|---|
| Ca2+ sparks (60, 65) | 2 ± 0.1 F/F0 | 30 | 13.6 ± 1.2 | 0.5–1 |
| LTCC sparklets (56) | 18–280 nM | 30, 80 | 0.81 ± 0.01 | N/A |
| Ca2+ puffs (83) | 50–500 nM | 1,000 | 2–4 | N/A |
| Ca2+ pulsars (44) | 1.77 ± 0.10 F/F0 | 257 ± 12 | 15.9 ± 0.6 | 0.10 ± 0.02 |
| TRPV4 Ca2+ sparklets (fluo 4) (82) | 0.22 ± 0.01 F/F0 | 520 ± 40 | 4.8 ± 0.8 | 0.48 ± 0.05 |
| TRPV4 Ca2+ sparklets (GCaMP2) (78) | 0.19 F/F0 | 37.0 ± 0.7 | 11.2 ± 0.4 | 0.7 ± 0.2 |
Properties of smooth muscle cell Ca2+ sparks, L-type Ca2+ channel (LTCC) sparklets, Ca2+ puffs, Ca2+ pulsars, and TRPV4 Ca2+ sparklets were measured using fluo 4-AM, and properties of TRPV4 Ca2+ sparklets were measured using a GCaMP2 indicator. Frequency (Hz) represents number of Ca2+ events per second per cell. F/F0, relative fluorescence; N/A, not applicable. Reference numbers are in parentheses.
Application of the TIRF microscopy approach led to the discovery of several important aspects of L-type Ca2+ channel regulation in arterial myocytes. For example, although L-type Ca2+ channels are broadly distributed throughout the surface of smooth muscle cells, L-type Ca2+ channel sparklet activity is nonrandom; i.e., almost all channel activity occurs at a few persistently active sites on the sarcolemma (56). High-activity L-type Ca2+ channels were found to be modulated by PKA, PKCα, and calcineurin (55, 57, 59). PKA- and PKCα-dependent changes in L-type Ca2+ channel activity are partly due to their interaction with protein A kinase-anchoring proteins (AKAPs), which are required for PKA-dependent modification of L-type Ca2+ channels (58, 107) and for intracellular targeting of PKCα (55). Taken together, the actions of PKA, PKCα, AKAPs, and calcineurin account for the variation in L-type Ca2+ channel activity. This body of work highlights the potential of optical recoding methods to reveal fundamental aspects of Ca2+ influx mechanisms not detected by other means.
TRP Channel Ca2+ Sparklets
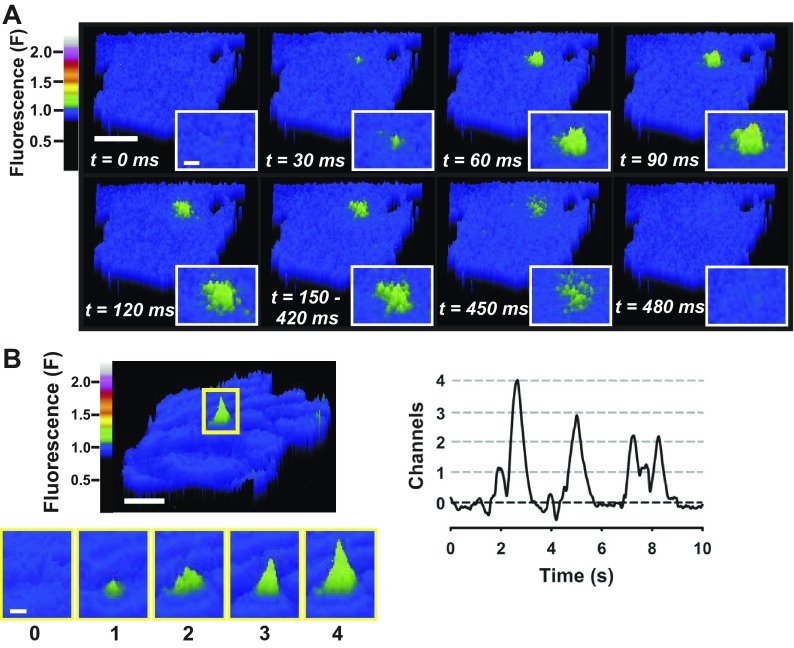
Optical techniques have recently been employed to record unitary Ca2+ influx events through TRP channels in vascular endothelial cells. Like L-type Ca2+ channel sparklets, unitary Ca2+ influx events through TRP channels are called “TRP channel Ca2+ sparklets,” and the molecular identity of the responsible TRP channel is specified (78, 82). For example, single-channel Ca2+ influx events through TRPV4 are called TRPV4 Ca2+ sparklets. TRPV4 Ca2+ sparklets were recently characterized in primary human microvascular endothelial cells (82). In this study, endothelial cells were loaded with the fast Ca2+ indicator dye fluo 4-AM, and Ca2+ sparklets at the plasma membrane, indicated by transient fluorescent signals, were recorded using TIRF microscopy (Fig. 2; see Supplemental Movie in Supplemental Material for this article available online at the Journal website). Ca2+ sparklet frequency was increased by the TRPV4 agonists 4α-phorbol 12,13-didecanoate (4α-PDD), GSK-1016790A, and 11,12-EET, inhibited by the TRPV4-selective antagonist HC-067047, and nearly abolished in the absence of extracellular Ca2+, demonstrating that these signals represent TRPV4-mediated Ca2+ influx (82). TRPV4 channel Ca2+ sparklets in endothelial cells are larger in amplitude than L-type Ca2+ channel sparklets in smooth muscle cells and can be recorded in the absence of exogenous cytosolic Ca2+ buffers and without voltage clamp. Custom software (LC_Pro) implemented as a plugin for ImageJ (23) was used to autodetect fluorescent signals and calculate the amplitude, duration, and spatial spread for each TRPV4 Ca2+ sparklet recorded. This method was used to characterize the biophysical properties of TRPV4 Ca2+ sparklets (Table 2). TRPV4 Ca2+ sparklets were rare under basal conditions but readily recruited upon stimulation of TRPV4 with an agonist (82). Additionally, even during maximal stimulation, only a few TRPV4 Ca2+ sparklet sites per cell (i.e., active TRPV4 channels) were detected, even though immunolabeling experiments revealed that TRPV4 protein was widely distributed (82). This is the first evidence demonstrating that most TRPV4 channels in primary endothelial cells are “silent,” suggesting a potential nonconducting role for TRPV4 protein.
Fig. 2.
Transient receptor potential vanilloid (TRPV4) Ca2+ sparklets in endothelial cells. A: pseudocolor time lapse of TIRF microscopy images of a primary human microvascular endothelial cell loaded with the Ca2+ indicator fluo 4-AM. Unitary TRPV4 Ca2+ influx events (i.e., TRPV4 Ca2+ sparklets) are observed as transient increases in fluorescence (green). Scale bar, 10 μm. Inset: magnifications of TRPV4 Ca2+ sparklet. Scale bar, 2 μm. B: pseudocolor image of a rat airway smooth muscle cell TRPV4 Ca2+ sparklet (top left; scale bar, 10 μm) and fluorescence signals produced from opening of 1, 2, 3, or 4 TRPV4 channels (bottom left; scale bar, 2 μm). Recording of fluorescence vs. time for region of interest (highlighted in top left) indicates opening of 1–4 TRPV4 channels (right).
The characteristics of endothelial cell TRPV4 Ca2+ sparklets are distinct from those of other Ca2+ signals recorded from vascular endothelial and smooth muscle cells (Table 2). TRPV4 Ca2+ sparklets recorded from endothelial cells of different vascular beds (dermal vs. mesenteric) and species (human vs. mouse) using different recording conditions (TIRF and fluo 4 vs. confocal and GCaMP2) and analysis methods displayed remarkable similarity in GSK-1016790A-stimulated frequency, amplitude, and spatial spread (Table 2). The duration of TRPV4 Ca2+ sparklets differed between the two studies. This discrepancy is due to differences in analysis method (manual vs. automated) or could be due to differences in the preparation (primary endothelial cells vs. intact endothelium). However, it is clear that TRPV4 Ca2+ sparklets are a novel Ca2+ microdomain in endothelial cells with definable biophysical properties that are distinct from Ca2+ microdomains that have been previously described.
TRPV4 Ca2+ Sparklets Cause Vasodilation
An elegant study by Sonkusare et al. (78) examined the functional significance of TRPV4 Ca2+ sparklets in the intact endothelium of mouse mesenteric arteries. A novel feature of this study was the use of Cx40BAC-GCaMP2 mice, a strain genetically modified to express a Ca2+-binding biosensor protein, GCaMP2, preferentially in the vascular endothelium. GCaMP2 mice allow for Ca2+ imaging of endothelial cell Ca2+ signals without background from other cell types, such as perivascular nerves or vascular smooth muscle cells (87, 88). Mesenteric arteries from Cx40BAC-GCaMP2 mice were isolated and pinned en face, and TRPV4 Ca2+ sparklets were recorded from the intact endothelial layer using high-speed, high-resolution confocal microscopy (78). Mesenteric artery endothelial TRPV4 Ca2+ sparklets were evoked by activators of TRPV4 (GSK-1016790A, 4α-PDD, and 11,12-EET), blocked by the TRPV4-specific inhibitor HC-067047, and absent from TRPV4−/− mice, providing the first evidence that unitary TRPV4 activity can be optically recorded from intact mesenteric arteries (78).
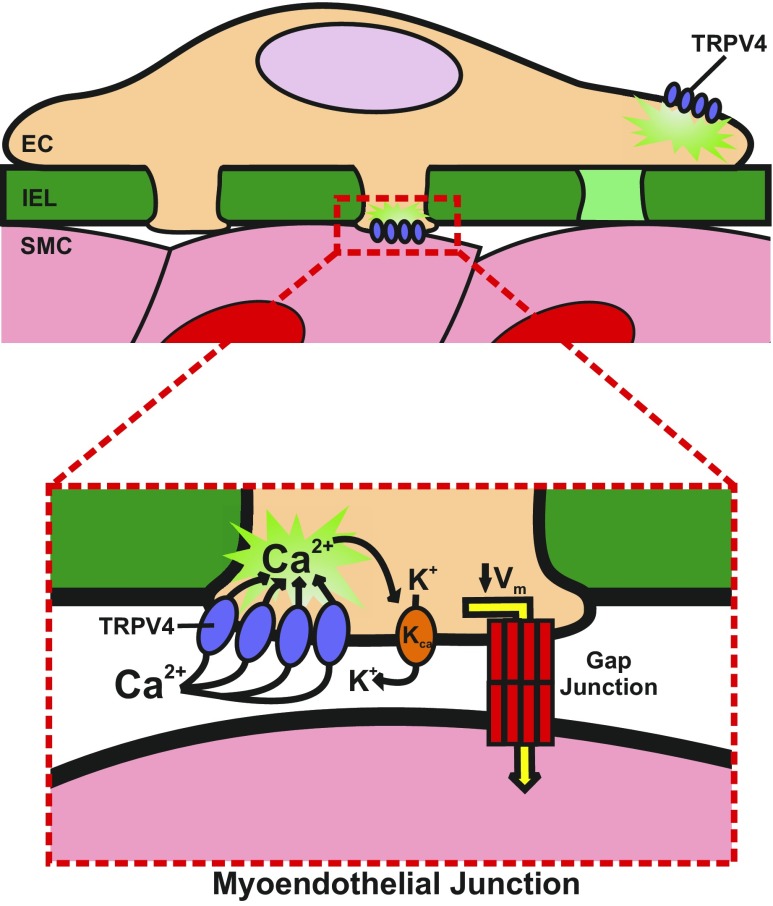
Interestingly, mesenteric artery TRPV4 Ca2+ sparklet recordings demonstrate cooperativity in terms of channel gating. The amplitudes of a majority of the recorded signals were consistent with four simultaneous channel openings, i.e., a “four-channel meta-structure” (78). In addition, similar to responses recorded from primary human endothelial cells (82), only four to eight TRPV4 channels per cell were active during near-maximal stimulation with the potent TRPV4-selective agonist GSK-1016790A (10 nM) (78). Surprisingly, the activity of these few TRPV4 channels is sufficient to hyperpolarize the endothelial cell plasma membrane and evoke maximal endothelium-dependent dilation of isolated, pressurized mesenteric arteries (78) by an EDH mechanism involving small- and intermediate-conductance Ca2+-sensitive K+ channels (Fig. 3) (78). High concentrations of GSK-1016790A (100 nM) resulted in excessive increases in global Ca2+ and severe vasomotion of the isolated arteries (78), suggesting that overstimulation of TRPV4 in the endothelium may have a pathophysiological role. Together, these findings indicate the significance of TRPV4 Ca2+ sparklets in EDH and vascular regulation in mouse mesenteric arteries.
Fig. 3.
TRPV4 Ca2+ sparklets in endothelial cells (ECs) initiate vasodilation. TRPV4 Ca2+ influx from the extracellular space through as few as 3–4 channels activates Ca2+-activated K+ (KCa) channels, resulting in K+ efflux from the cell. This hyperpolarizes the EC membrane, which directly hyperpolarizes smooth muscle cells (SMCs) via gap junctions located within myoendothelial junctions. IEL, internal elastic lamina; Vm, membrane potential.
Perspectives
Endothelial dysfunction is a hallmark of common cardiovascular diseases, such as atherosclerosis, stroke, and hypertension, establishing the critical nature of this fragile tissue for the maintenance of vascular health. Important functions of the endothelium are regulated by changes in intracellular Ca2+, emphasizing the significance of Ca2+ influx pathways. Recent improvements in confocal and TIRF microscopy used in conjunction with sensitive Ca2+ indicator molecules allow for the direct visualization of unitary TRP channel Ca2+ influx events, or TRP channel Ca2+ sparklets, in endothelial cells. Significant advantages of optical vs. traditional electrophysiological techniques of studying Ca2+ influx channels include higher throughput, preservation of intracellular Ca2+ signaling pathways, and acquisition of the number and subcellular distribution of active Ca2+ influx sites. Recent use of this new methodology has uncovered unexpected aspects of TRPV4 physiology in the endothelium, including evidence that gating is cooperative and that the activity of only a few channels per cell can yield maximal endothelium-dependent vasodilation. Application of this technology to the study of other Ca2+-permeable TRP channels involved in endothelium-dependent dilation, such as TRPC3, TRPA1, and TRPV3, is likely to yield further novel and surprising findings. Furthermore, optical recording may prove to be particularly useful for the assessment of changes in Ca2+ influx pathways associated with endothelial dysfunction and the evaluation of interventions designed to resolve this pathology.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant R01 HL-091905 (to S. Earley) and a Monfort Excellence Award from the Monfort Family Foundation (to S. Earley).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.N.S. and S.E. prepared the figures; M.N.S. and S.E. drafted the manuscript; M.N.S. and S.E. edited and revised the manuscript; M.N.S. and S.E. approved the final version of the manuscript.
Supplementary Material
REFERENCES
- 1.Aarts M, Iihara K, Wei WL, Xiong ZG, Arundine M, Cerwinski W, MacDonald JF, Tymianski M. A key role for TRPM7 channels in anoxic neuronal death. Cell 115: 863–877, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Bautista DM, Movahed P, Hinman A, Axelsson HE, Sterner O, Hogestatt ED, Julius D, Jordt SE, Zygmunt PM. Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci USA 102: 12248–12252, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beny JL, von der Weid PY. Hydrogen peroxide: an endogenous smooth muscle cell hyperpolarizing factor. Biochem Biophys Res Commun 176: 378–384, 1991. [DOI] [PubMed] [Google Scholar]
- 4.Bolton TB, Lang RJ, Takewaki T. Mechanisms of action of noradrenaline and carbachol on smooth muscle of guinea-pig anterior mesenteric artery. J Physiol 351: 549–572, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brotherton AF, Hoak JC. Role of Ca2+ and cyclic AMP in the regulation of the production of prostacyclin by the vascular endothelium. Proc Natl Acad Sci USA 79: 495–499, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res 78: 415–423, 1996. [DOI] [PubMed] [Google Scholar]
- 7.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389: 816–824, 1997. [DOI] [PubMed] [Google Scholar]
- 8.Chang AS, Chang SM, Garcia RL, Schilling WP. Concomitant and hormonally regulated expression of trp genes in bovine aortic endothelial cells. FEBS Lett 415: 335–340, 1997. [DOI] [PubMed] [Google Scholar]
- 9.Chauhan SD, Nilsson H, Ahluwalia A, Hobbs AJ. Release of C-type natriuretic peptide accounts for the biological activity of endothelium-derived hyperpolarizing factor. Proc Natl Acad Sci USA 100: 1426–1431, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen JK, Capdevila J, Harris RC. Cytochrome p450 epoxygenase metabolism of arachidonic acid inhibits apoptosis. Mol Cell Biol 21: 6322–6331, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen XZ, Vassilev PM, Basora N, Peng JB, Nomura H, Segal Y, Brown EM, Reeders ST, Hediger MA, Zhou J. Polycystin-L is a calcium-regulated cation channel permeable to calcium ions. Nature 401: 383–386, 1999. [DOI] [PubMed] [Google Scholar]
- 12.Coleman HA, Tare M, Parkington HC. Endothelial potassium channels, endothelium-dependent hyperpolarization and the regulation of vascular tone in health and disease. Clin Exp Pharmacol Physiol 31: 641–649, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Coleman HA, Tare M, Parkington HC. K+ currents underlying the action of endothelium-derived hyperpolarizing factor in guinea-pig, rat and human blood vessels. J Physiol 531: 359–373, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demuro A, Parker I. Imaging the activity and localization of single voltage-gated Ca2+ channels by total internal reflection fluorescence microscopy. Biophys J 86: 3250–3259, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demuro A, Parker I. “Optical patch-clamping”: single-channel recording by imaging Ca2+ flux through individual muscle acetylcholine receptor channels. J Gen Physiol 126: 179–192, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Demuro A, Parker I. Optical single-channel recording: imaging Ca2+ flux through individual N-type voltage-gated channels expressed in Xenopus oocytes. Cell Calcium 34: 499–509, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Dusting GJ, Moncada S, Vane JR. Prostacyclin (PGX) is the endogenous metabolite responsible for relaxation of coronary arteries induced by arachindonic acid. Prostaglandins 13: 3–15, 1977. [DOI] [PubMed] [Google Scholar]
- 18.Earley S, Gonzales AL, Crnich R. Endothelium-dependent cerebral artery dilation mediated by TRPA1 and Ca2+-activated K+ channels. Circ Res 104: 987–994, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Earley S, Gonzales AL, Garcia ZI. A dietary agonist of transient receptor potential cation channel V3 elicits endothelium-dependent vasodilation. Mol Pharmacol 77: 612–620, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Earley S, Pauyo T, Drapp R, Tavares MJ, Liedtke W, Brayden JE. TRPV4-dependent dilation of peripheral resistance arteries influences arterial pressure. Am J Physiol Heart Circ Physiol 297: H1096–H1102, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edwards G, Dora KA, Gardener MJ, Garland CJ, Weston AH. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature 396: 269–272, 1998. [DOI] [PubMed] [Google Scholar]
- 22.Edwards G, Feletou M, Weston AH. Endothelium-derived hyperpolarising factors and associated pathways: a synopsis. Pflügers Arch 459: 863–879, 2010. [DOI] [PubMed] [Google Scholar]
- 23.Francis M, Qian X, Charbel C, Ledoux J, Parker JC, Taylor MS. Automated region of interest analysis of dynamic Ca2+ signals in image sequences. Am J Physiol Cell Physiol 303: C236–C243, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freichel M, Suh SH, Pfeifer A, Schweig U, Trost C, Weissgerber P, Biel M, Philipp S, Freise D, Droogmans G, Hofmann F, Flockerzi V, Nilius B. Lack of an endothelial store-operated Ca2+ current impairs agonist-dependent vasorelaxation in TRP4−/− mice. Nat Cell Biol 3: 121–127, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Gao G, Bai XY, Xuan C, Liu XC, Jing WB, Novakovic A, Yang Q, He GW. Role of TRPC3 channel in human internal mammary artery. Arch Med Res 43: 431–437, 2012. [DOI] [PubMed] [Google Scholar]
- 26.Garcia RL, Schilling WP. Differential expression of mammalian TRP homologues across tissues and cell lines. Biochem Biophys Res Commun 239: 279–283, 1997. [DOI] [PubMed] [Google Scholar]
- 27.Garland CJ, Plane F, Kemp BK, Cocks TM. Endothelium-dependent hyperpolarization: a role in the control of vascular tone. Trends Pharmacol Sci 16: 23–30, 1995. [DOI] [PubMed] [Google Scholar]
- 28.Gee KR, Brown KA, Chen WN, Bishop-Stewart J, Gray D, Johnson I. Chemical and physiological characterization of fluo-4 Ca2+-indicator dyes. Cell Calcium 27: 97–106, 2000. [DOI] [PubMed] [Google Scholar]
- 29.Grimm C, Kraft R, Sauerbruch S, Schultz G, Harteneck C. Molecular and functional characterization of the melastatin-related cation channel TRPM3. J Biol Chem 278: 21493–21501, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Groschner K, Hingel S, Lintschinger B, Balzer M, Romanin C, Zhu X, Schreibmayer W. Trp proteins form store-operated cation channels in human vascular endothelial cells. FEBS Lett 437: 101–106, 1998. [DOI] [PubMed] [Google Scholar]
- 31.Hartmannsgruber V, Heyken WT, Kacik M, Kaistha A, Grgic I, Harteneck C, Liedtke W, Hoyer J, Kohler R. Arterial response to shear stress critically depends on endothelial TRPV4 expression. PLos One 2: e827, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hofmann T, Chubanov V, Gudermann T, Montell C. TRPM5 is a voltage-modulated and Ca2+-activated monovalent selective cation channel. Curr Biol 13: 1153–1158, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schultz G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature 397: 259–263, 1999. [DOI] [PubMed] [Google Scholar]
- 34.Hosoki R, Matsuki N, Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun 237: 527–531, 1997. [DOI] [PubMed] [Google Scholar]
- 35.Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci USA 84: 9265–9269, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 427: 260–265, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Kanzaki M, Zhang YQ, Mashima H, Li L, Shibata H, Kojima I. Translocation of a calcium-permeable cation channel induced by insulin-like growth factor-I. Nat Cell Biol 1: 165–170, 1999. [DOI] [PubMed] [Google Scholar]
- 38.Karara A, Dishman E, Falck JR, Capdevila JH. Endogenous epoxyeicosatrienoyl-phospholipids. A novel class of cellular glycerolipids containing epoxidized arachidonate moieties. J Biol Chem 266: 7561–7569, 1991. [PubMed] [Google Scholar]
- 39.Kirby BS, Bruhl A, Sullivan MN, Francis M, Dinenno FA, Earley S. Robust internal elastic lamina fenestration in skeletal muscle arteries. PLos One 8: e54849, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kochukov MY, Balasubramanian A, Noel RC, Marrelli SP. Role of TRPC1 and TRPC3 channels in contraction and relaxation of mouse thoracic aorta. J Vasc Res 50: 11–20, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koulen P, Cai Y, Geng L, Maeda Y, Nishimura S, Witzgall R, Ehrlich BE, Somlo S. Polycystin-2 is an intracellular calcium release channel. Nat Cell Biol 4: 191–197, 2002. [DOI] [PubMed] [Google Scholar]
- 42.LaPlante JM, Falardeau J, Sun M, Kanazirska M, Brown EM, Slaugenhaupt SA, Vassilev PM. Identification and characterization of the single channel function of human mucolipin-1 implicated in mucolipidosis type IV, a disorder affecting the lysosomal pathway. FEBS Lett 532: 183–187, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Launay P, Fleig A, Perraud AL, Scharenberg AM, Penner R, Kinet JP. TRPM4 is a Ca2+-activated nonselective cation channel mediating cell membrane depolarization. Cell 109: 397–407, 2002. [DOI] [PubMed] [Google Scholar]
- 44.Ledoux J, Taylor MS, Bonev AD, Hannah RM, Solodushko V, Shui B, Tallini Y, Kotlikoff MI, Nelson MT. Functional architecture of inositol 1,4,5-trisphosphate signaling in restricted spaces of myoendothelial projections. Proc Natl Acad Sci USA 105: 9627–9632, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liedtke W, Choe Y, Marti-Renom MA, Bell AM, Denis CS, Sali A, Hudspeth AJ, Friedman JM, Heller S. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 103: 525–535, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Loot AE, Popp R, Fisslthaler B, Vriens J, Nilius B, Fleming I. Role of cytochrome P450-dependent transient receptor potential V4 activation in flow-induced vasodilatation. Cardiovasc Res 80: 445–452, 2008. [DOI] [PubMed] [Google Scholar]
- 47.Lucas P, Ukhanov K, Leinders-Zufall T, Zufall F. A diacylglycerol-gated cation channel in vomeronasal neuron dendrites is impaired in TRPC2 mutant mice: mechanism of pheromone transduction. Neuron 40: 551–561, 2003. [DOI] [PubMed] [Google Scholar]
- 48.McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 416: 52–58, 2002. [DOI] [PubMed] [Google Scholar]
- 49.Mendoza SA, Fang J, Gutterman DD, Wilcox DA, Bubolz AH, Li R, Suzuki M, Zhang DX. TRPV4-mediated endothelial Ca2+ influx and vasodilation in response to shear stress. Am J Physiol Heart Circ Physiol 298: H466–H476, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mergler S, Valtink M, Coulson-Thomas VJ, Lindemann D, Reinach PS, Engelmann K, Pleyer U. TRPV channels mediate temperature-sensing in human corneal endothelial cells. Exp Eye Res 90: 758–770, 2010. [DOI] [PubMed] [Google Scholar]
- 51.Metz J, Weihe E. Intercellular junctions in the full term human placenta. II. Cytotrophoblast cells, intravillous stroma cells and blood vessels. Anat Embryol (Berl) 158: 167–178, 1980. [DOI] [PubMed] [Google Scholar]
- 52.Minta A, Kao JP, Tsien RY. Fluorescent indicators for cytosolic calcium based on rhodamine and fluorescein chromophores. J Biol Chem 264: 8171–8178, 1989. [PubMed] [Google Scholar]
- 53.Miyawaki A, Llopis J, Heim R, McCaffery JM, Adams JA, Ikura M, Tsien RY. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature 388: 882–887, 1997. [DOI] [PubMed] [Google Scholar]
- 54.Nagai T, Sawano A, Park ES, Miyawaki A. Circularly permuted green fluorescent proteins engineered to sense Ca2+. Proc Natl Acad Sci USA 98: 3197–3202, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Navedo MF, Amberg GC, Nieves M, Molkentin JD, Santana LF. Mechanisms underlying heterogeneous Ca2+ sparklet activity in arterial smooth muscle. J Gen Physiol 127: 611–622, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Navedo MF, Amberg GC, Votaw VS, Santana LF. Constitutively active L-type Ca2+ channels. Proc Natl Acad Sci USA 102: 11112–11117, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Navedo MF, Cheng EP, Yuan C, Votaw S, Molkentin JD, Scott JD, Santana LF. Increased coupled gating of L-type Ca2+ channels during hypertension and Timothy syndrome. Circ Res 106: 748–756, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Navedo MF, Nieves-Cintron M, Amberg GC, Yuan C, Votaw VS, Lederer WJ, McKnight GS, Santana LF. AKAP150 is required for stuttering persistent Ca2+ sparklets and angiotensin II-induced hypertension. Circ Res 102: e1–e11, 2008. [DOI] [PubMed] [Google Scholar]
- 59.Navedo MF, Takeda Y, Nieves-Cintron M, Molkentin JD, Santana LF. Elevated Ca2+ sparklet activity during acute hyperglycemia and diabetes in cerebral arterial smooth muscle cells. Am J Physiol Cell Physiol 298: C211–C220, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, Lederer WJ. Relaxation of arterial smooth muscle by calcium sparks. Science 270: 633–637, 1995. [DOI] [PubMed] [Google Scholar]
- 61.Nilius B, Vennekens R, Prenen J, Hoenderop JG, Bindels RJ, Droogmans G. Whole-cell and single channel monovalent cation currents through the novel rabbit epithelial Ca2+ channel ECaC. J Physiol 527: 239–248, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oancea E, Vriens J, Brauchi S, Jun J, Splawski I, Clapham DE. TRPM1 forms ion channels associated with melanin content in melanocytes. Sci Signal 2: ra21, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Okada T, Inoue R, Yamazaki K, Maeda A, Kurosaki T, Yamakuni T, Tanaka I, Shimizu S, Ikenaka K, Imoto K, Mori Y. Molecular and functional characterization of a novel mouse transient receptor potential protein homologue TRP7. Ca2+-permeable cation channel that is constitutively activated and enhanced by stimulation of G protein-coupled receptor. J Biol Chem 274: 27359–27370, 1999. [DOI] [PubMed] [Google Scholar]
- 64.Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A. A TRP channel that senses cold stimuli and menthol. Cell 108: 705–715, 2002. [DOI] [PubMed] [Google Scholar]
- 65.Perez GJ, Bonev AD, Patlak JB, Nelson MT. Functional coupling of ryanodine receptors to KCa channels in smooth muscle cells from rat cerebral arteries. J Gen Physiol 113: 229–238, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Perraud AL, Fleig A, Dunn CA, Bagley LA, Launay P, Schmitz C, Stokes AJ, Zhu Q, Bessman MJ, Penner R, Kinet JP, Scharenberg AM. ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature 411: 595–599, 2001. [DOI] [PubMed] [Google Scholar]
- 67.Philipp S, Cavalie A, Freichel M, Wissenbach U, Zimmer S, Trost C, Marquart A, Murakami M, Flockerzi V. A mammalian capacitative calcium entry channel homologous to Drosophila TRP and TRPL. EMBO J 15: 6166–6171, 1996. [PMC free article] [PubMed] [Google Scholar]
- 68.Qian X, Francis M, Solodushko V, Earley S, Taylor MS. Recruitment of dynamic endothelial Ca2+ signals by the TRPA1 channel activator AITC in rat cerebral arteries. Microcirculation 20: 138–148, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rubanyi GM, Romero JC, Vanhoutte PM. Flow-induced release of endothelium-derived relaxing factor. Am J Physiol Heart Circ Physiol 250: H1145–H1149, 1986. [DOI] [PubMed] [Google Scholar]
- 70.Runnels LW, Yue L, Clapham DE. TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science 291: 1043–1047, 2001. [DOI] [PubMed] [Google Scholar]
- 71.Saliez J, Bouzin C, Rath G, Ghisdal P, Desjardins F, Rezzani R, Rodella LF, Vriens J, Nilius B, Feron O, Balligand JL, Dessy C. Role of caveolar compartmentation in endothelium-derived hyperpolarizing factor-mediated relaxation: Ca2+ signals and gap junction function are regulated by caveolin in endothelial cells. Circulation 117: 1065–1074, 2008. [DOI] [PubMed] [Google Scholar]
- 72.Sandow SL, Goto K, Rummery NM, Hill CE. Developmental changes in myoendothelial gap junction mediated vasodilator activity in the rat saphenous artery. J Physiol 556: 875–886, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sandow SL, Hill CE. Incidence of myoendothelial gap junctions in the proximal and distal mesenteric arteries of the rat is suggestive of a role in endothelium-derived hyperpolarizing factor-mediated responses. Circ Res 86: 341–346, 2000. [DOI] [PubMed] [Google Scholar]
- 74.Sandow SL, Neylon CB, Chen MX, Garland CJ. Spatial separation of endothelial small- and intermediate-conductance calcium-activated potassium channels (KCa) and connexins: possible relationship to vasodilator function? J Anat 209: 689–698, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Senadheera S, Kim Y, Grayson TH, Toemoe S, Kochukov MY, Abramowitz J, Housley GD, Bertrand RL, Chadha PS, Bertrand PP, Murphy TV, Tare M, Birnbaumer L, Marrelli SP, Sandow SL. Transient receptor potential canonical type 3 channels facilitate endothelium-derived hyperpolarization-mediated resistance artery vasodilator activity. Cardiovasc Res 95: 439–447, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Smith GD, Gunthorpe MJ, Kelsell RE, Hayes PD, Reilly P, Facer P, Wright JE, Jerman JC, Walhin JP, Ooi L, Egerton J, Charles KJ, Smart D, Randall AD, Anand P, Davis JB. TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature 418: 186–190, 2002. [DOI] [PubMed] [Google Scholar]
- 77.Smith PD, Brett SE, Luykenaar KD, Sandow SL, Marrelli SP, Vigmond EJ, Welsh DG. KIR channels function as electrical amplifiers in rat vascular smooth muscle. J Physiol 586: 1147–1160, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sonkusare SK, Bonev AD, Ledoux J, Liedtke W, Kotlikoff MI, Heppner TJ, Hill-Eubanks DC, Nelson MT. Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science 336: 597–601, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 112: 819–829, 2003. [DOI] [PubMed] [Google Scholar]
- 80.Strotmann R, Harteneck C, Nunnenmacher K, Schultz G, Plant TD. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat Cell Biol 2: 695–702, 2000. [DOI] [PubMed] [Google Scholar]
- 81.Strotmann R, Schultz G, Plant TD. Ca2+-dependent potentiation of the nonselective cation channel TRPV4 is mediated by a C-terminal calmodulin binding site. J Biol Chem 278: 26541–26549, 2003. [DOI] [PubMed] [Google Scholar]
- 82.Sullivan MN, Francis M, Pitts NL, Taylor MS, Earley S. Optical recording reveals novel properties of GSK1016790A-induced vanilloid transient receptor potential channel TRPV4 activity in primary human endothelial cells. Mol Pharmacol 82: 464–472, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sun XP, Callamaras N, Marchant JS, Parker I. A continuum of InsP3-mediated elementary Ca2+ signalling events in Xenopus oocytes. J Physiol 509: 67–80, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Surks HK. cGMP-dependent protein kinase I and smooth muscle relaxation: a tale of two isoforms. Circ Res 101: 1078–1080, 2007. [DOI] [PubMed] [Google Scholar]
- 85.Suzuki M, Mizuno A, Kodaira K, Imai M. Impaired pressure sensation in mice lacking TRPV4. J Biol Chem 278: 22664–22668, 2003. [DOI] [PubMed] [Google Scholar]
- 86.Takahashi N, Kuwaki T, Kiyonaka S, Numata T, Kozai D, Mizuno Y, Yamamoto S, Naito S, Knevels E, Carmeliet P, Oga T, Kaneko S, Suga S, Nokami T, Yoshida J, Mori Y. TRPA1 underlies a sensing mechanism for O2. Nat Chem Biol 7: 701–711, 2011. [DOI] [PubMed] [Google Scholar]
- 87.Tallini YN, Brekke JF, Shui B, Doran R, Hwang SM, Nakai J, Salama G, Segal SS, Kotlikoff MI. Propagated endothelial Ca2+ waves and arteriolar dilation in vivo: measurements in Cx40BAC GCaMP2 transgenic mice. Circ Res 101: 1300–1309, 2007. [DOI] [PubMed] [Google Scholar]
- 88.Tallini YN, Ohkura M, Choi BR, Ji G, Imoto K, Doran R, Lee J, Plan P, Wilson J, Xin HB, Sanbe A, Gulick J, Mathai J, Robbins J, Salama G, Nakai J, Kotlikoff MI. Imaging cellular signals in the heart in vivo: cardiac expression of the high-signal Ca2+ indicator GCaMP2. Proc Natl Acad Sci USA 103: 4753–4758, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Taylor MS, Bonev AD, Gross TP, Eckman DM, Brayden JE, Bond CT, Adelman JP, Nelson MT. Altered expression of small-conductance Ca2+-activated K+ (SK3) channels modulates arterial tone and blood pressure. Circ Res 93: 124–131, 2003. [DOI] [PubMed] [Google Scholar]
- 90.Voets T, Nilius B, Hoefs S, van der Kemp AW, Droogmans G, Bindels RJ, Hoenderop JG. TRPM6 forms the Mg2+ influx channel involved in intestinal and renal Mg2+ absorption. J Biol Chem 279: 19–25, 2004. [DOI] [PubMed] [Google Scholar]
- 91.Voets T, Prenen J, Vriens J, Watanabe H, Janssens A, Wissenbach U, Bodding M, Droogmans G, Nilius B. Molecular determinants of permeation through the cation channel TRPV4. J Biol Chem 277: 33704–33710, 2002. [DOI] [PubMed] [Google Scholar]
- 92.Volk T, Schwoerer AP, Thiessen S, Schultz JH, Ehmke H. A polycystin-2-like large conductance cation channel in rat left ventricular myocytes. Cardiovasc Res 58: 76–88, 2003. [DOI] [PubMed] [Google Scholar]
- 93.Wang SQ, Song LS, Lakatta EG, Cheng H. Ca2+ signalling between single L-type Ca2+ channels and ryanodine receptors in heart cells. Nature 410: 592–596, 2001. [DOI] [PubMed] [Google Scholar]
- 94.Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature 424: 434–438, 2003. [DOI] [PubMed] [Google Scholar]
- 95.Wei CM, Hu S, Miller VM, Burnett JC., Jr Vascular actions of C-type natriuretic peptide in isolated porcine coronary arteries and coronary vascular smooth muscle cells. Biochem Biophys Res Commun 205: 765–771, 1994. [DOI] [PubMed] [Google Scholar]
- 96.Weissmann N, Dietrich A, Fuchs B, Kalwa H, Ay M, Dumitrascu R, Olschewski A, Storch U, Mederos y Schnitzler M., Ghofrani HA, Schermuly RT, Pinkenburg O, Seeger W, Grimminger F, Gudermann T. Classical transient receptor potential channel 6 (TRPC6) is essential for hypoxic pulmonary vasoconstriction and alveolar gas exchange. Proc Natl Acad Sci USA 103: 19093–19098, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wissenbach U, Bodding M, Freichel M, Flockerzi V. Trp12, a novel Trp related protein from kidney. FEBS Lett 485: 127–134, 2000. [DOI] [PubMed] [Google Scholar]
- 98.Wong CO, Yao X. TRP channels in vascular endothelial cells. Adv Exp Med Biol 704: 759–780, 2011. [DOI] [PubMed] [Google Scholar]
- 99.Wu S, Moore TM, Brough GH, Whitt SR, Chinkers M, Li M, Stevens T. Cyclic nucleotide-gated channels mediate membrane depolarization following activation of store-operated calcium entry in endothelial cells. J Biol Chem 275: 18887–18896, 2000. [DOI] [PubMed] [Google Scholar]
- 100.Xu H, Delling M, Jun JC, Clapham DE. Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat Neurosci 9: 628–635, 2006. [DOI] [PubMed] [Google Scholar]
- 101.Xu H, Ramsey IS, Kotecha SA, Moran MM, Chong JA, Lawson D, Ge P, Lilly J, Silos-Santiago I, Xie Y, DiStefano PS, Curtis R, Clapham DE. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature 418: 181–186, 2002. [DOI] [PubMed] [Google Scholar]
- 102.Yamamoto K, Korenaga R, Kamiya A, Qi Z, Sokabe M, Ando J. P2X4 receptors mediate ATP-induced calcium influx in human vascular endothelial cells. Am J Physiol Heart Circ Physiol 279: H285–H292, 2000. [DOI] [PubMed] [Google Scholar]
- 103.Yao X, Leung PS, Kwan HY, Wong TP, Fong MW. Rod-type cyclic nucleotide-gated cation channel is expressed in vascular endothelium and vascular smooth muscle cells. Cardiovasc Res 41: 282–290, 1999. [DOI] [PubMed] [Google Scholar]
- 104.Yue L, Peng JB, Hediger MA, Clapham DE. CaT1 manifests the pore properties of the calcium-release-activated calcium channel. Nature 410: 705–709, 2001. [DOI] [PubMed] [Google Scholar]
- 105.Zhang DX, Mendoza SA, Bubolz AH, Mizuno A, Ge ZD, Li R, Warltier DC, Suzuki M, Gutterman DD. Transient receptor potential vanilloid type 4-deficient mice exhibit impaired endothelium-dependent relaxation induced by acetylcholine in vitro and in vivo. Hypertension 53: 532–538, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J 20: 6008–6016, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhong J, Hume JR, Keef KD. Anchoring protein is required for cAMP-dependent stimulation of L-type Ca2+ channels in rabbit portal vein. Am J Physiol Cell Physiol 277: C840–C844, 1999. [DOI] [PubMed] [Google Scholar]
- 108.Zitt C, Zobel A, Obukhov AG, Harteneck C, Kalkbrenner F, Luckhoff A, Schultz G. Cloning and functional expression of a human Ca2+-permeable cation channel activated by calcium store depletion. Neuron 16: 1189–1196, 1996. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.