Abstract
Prolactin (PRL) is a hormone produced in the anterior pituitary but also synthesized extrapituitary where it can influence diverse cellular processes, including inflammatory responses. Females experience greater pain in certain inflammatory conditions, but the contribution of the PRL system to sex-dependent inflammatory pain is unknown. We found that PRL regulates transient receptor potential (TRP) channels in a sex-dependent manner in sensory neurons. At >20 ng/ml, PRL sensitizes TRPV1 in female, but not male, neurons. This effect is mediated by PRL receptor (PRL-R). Likewise, TRPA1 and TRPM8 were sensitized by 100 ng/ml PRL only in female neurons. We showed that complete Freund adjuvant (CFA) upregulated PRL levels in the inflamed paw of both male and female rats, but levels were higher in females. In contrast, CFA did not change mRNA levels of long and short PRL-R in the dorsal root ganglion or spinal cord. Analysis of PRL and PRL-R knockout (KO) mice demonstrated that basal responses to cold stimuli were only altered in females, and with no significant effects on heat and mechanical responses in both sexes. CFA-induced heat and cold hyperalgesia were not changed in PRL and PRL-R KO compared with wild-type (WT) males, whereas significant reduction of heat and cold post-CFA hyperalgesia was detected in PRL and PRL-R KO females. Attenuation of CFA-induced mechanical allodynia was observed in both PRL and PRL-R KO females and males. Thermal hyperalgesia in PRL KO females was restored by administration of PRL into hindpaws. Overall, we demonstrate a sex-dependent regulation of peripheral inflammatory hyperalgesia by the PRL system.
Keywords: prolactin receptor, transient receptor potential V1, transient receptor potential A1, transient receptor potential M8, female, inflammation, pain
many human chronic inflammatory conditions are associated with hyperprolactinemia, and this increase in prolactin (PRL) levels can lead to serious health issues related to cancer, infertility, inflammatory diseases, and body weight (11, 42, 48, 73). Human inflammatory conditions with increased PRL serum levels include the severe form of progressive systemic sclerosis (34, 69), the active phase of systemic lupus erythematosus (37, 74), rheumatoid arthritis (44), polymyalgia rheumatica (68), and autoimmune thyroid diseases (26). Unlike chronic inflammation, acute inflammation in animals triggers an accumulation of endogenous PRL at the site of inflammation, but not in blood serum (64). Altogether, these findings suggest that acute inflammation upregulates PRL via extrapituitary mechanisms, whereas chronic inflammation induces PRL via both pituitary and extrapituitary pathways (64, 75).
Elevated PRL can have modulatory effects on metabolic/endocrine and the immune systems. Thus, a positive feedback loop for PRL has been suggested, since PRL stimulates the immune system while products of immune cells can also stimulate PRL secretion (68). Accordingly, PRL modulates and activates T-lymphocytes and B-lymphocytes (9, 45). PRL also increases the secretion of cytokines, including IL-1β, IL-6, TNF-α, and interferon-γ from various cells (72). Some of the modulatory effects of the PRL system are sex-dependent since ablation of the PRL receptor (PRL-R) leads to moderate decreases in estradiol and progesterone in estrous females, whereas male animals lacking PRL-R do not show an alteration in serum levels of testosterone (20).
PRL is also capable of directly modulating central and peripheral neurons. The PRL-R has been detected in certain regions of the brain (58), where PRL can regulate tuberoinfundibular dopamine TIDA neurons and promote dopamine release (41). Peripherally, the transient receptor potential (TRP) V1 ion channel, a channel critically linked to pain and nociception and belonging to a family of other TRP channels important to pain mechanisms, including TRPA1 and TRPM8, was found to be sensitized by PRL in sensory neurons of ovariectomized rats with estradiol replacement (OVX-E), but not of ovariectomized (OVX) rats (27).
Because of the stimulatory effects of PRL on the immune system and certain neurons belonging to the pain pathway, direct and indirect pronociceptive roles of PRL have been suggested during inflammatory pain conditions (16, 40, 49, 64). The pronociceptive action of PRL could vary in males vs. females since PRL levels during inflammation are sex-dependent (31, 37, 44). Furthermore, females experience greater pain in some inflammatory pain conditions (4, 29, 53), and, although mechanisms contributing to sex-dependent differences in pain are not fully understood, these findings suggest the possible involvement of the PRL system.
Accordingly, the aims of this study were to examine 1) whether PRL regulates TRPV1, TRPA1, and TRPM8 channels in sensory neurons of female and male mice; 2) whether PRL and PRL-R are regulated locally in the periphery, in the dorsal root ganglion (DRG), and in the spinal cord after hindpaw CFA-induced inflammation, and 3) whether inflammatory hyperalgesia is altered in male and female mice with ablated PRL (PRL KO) or PRL-R (PRL-R KO) compared with wild-type (WT) mice. The first two aims of the study will evaluate the possible role of PRL in nociceptor sensitization and if PRL levels change in locations important to nociceptor sensitization after a peripheral inflammatory insult, whereas the third aim will evaluate the overall role of the PRL system in inflammatory hyperalgesia in males and females.
MATERIALS AND METHODS
Rats, TRPA1, PRL, and PRL-R null mutant mice.
All animal experiments confirmed to the American Physiological Society's Guiding Principles in the Care and Use of Vertebrate Animals in Research and Training and to protocols were approved by the University of Texas Health Science Center at San Antonio Animal Care and Use Committee. We also followed guidelines issued by the National Institutes of Health and the Society for Neuroscience to minimize the use of animals and their suffering.
Adult male and female Sprague-Dawley rats (200–250 g; Charles River Laboratories, Wilmington, MA) were housed three per cage under a 12:12-h light-dark cycle with food and water available ad libitum. Adult female and male TRPA1 null-mutant (TRPA1 KO), PRL null-mutant (PRL KO), PRL-R null-mutant (PRL-R KO), and corresponding littermate WT mice were obtained from The Jackson Laboratory (Bar Harbor, ME). PRL KO and PRL-R KO mice are viable, normal in size, and do not display any gross physical or behavioral abnormalities. The homozygous PRL KO females are infertile and have an irregular estrous cycle. Male and female homozygous PRL-R KO mice are completely sterile. The serum PRL levels are increased 60- to 100-fold in PRL-R KO males and females (56). The serum estradiol and progesterone levels are moderately decreased in estrous PRL-R KO females (estradiol: from 53 pg/ml for WT to 37 pg/ml for PRL-R KO; progesterone: from 17 ng/ml for WT to 7 ng/ml for PRL-R KO; see Ref. 20). The serum total testosterone levels are at similar levels in WT and PRL-R KO male mice (20).
PRL KO mice were generated via targeted disruption of coding exon 4, which results in a truncated 11-kDa PRL protein that lacks any detectable bioactivity (35). PRL-R KO mice were produced via creating an in-frame stop codon in exon 5 (56). The lack of functional PRL-R in homozygous mutant animals was confirmed using Northern, Western, and binding assays, and all demonstrated the lack of a functional receptor (56). PRL and PRL-R KO mice were produced in the C57BL/6J line.
Sensory neuron culture.
DRG were dissected from L3–L5 levels of adult WT male and female C57BL/6J mice. DRG neuronal culture was prepared as previously described (2) with some modifications. Thus, sensory neurons were maintained in DMEM supplemented with lower FBS (2%) and no nerve growth factor (NGF) addition. Sensory neurons were plated at moderate-to-low density on poly-d-lysine/laminin-coated cover slips (Clontech, Palo Alto, CA). Experiments were performed within 16–24 h postplating.
Ca2+ imaging.
The Ca2+-imaging experiments and ratiometric data conversion were basically performed as previously described (2). Fluorescence was detected by a Nikon TE 2000U microscope fitted with a ×20/0.9 NA Fluor objective. Data were collected and analyzed with MetaFluor Software (Universal Imaging, Downingtown, PA). The experiments were performed in standard extracellular solution (SES; see Electrophysiology). Ca2+-sensitive dye was fura 2-AM (2 μM; Molecular Probes, Carlsbad, CA). The net changes in Ca2+ influx were calculated by subtracting the basal intracellular Ca2+ concentration ([Ca2+]i, mean value collected for 60 s before agonist addition) from the peak [Ca2+]i value achieved after exposure to the agonists. Increases in [Ca2+]i above 100 nM were considered positive. This minimal threshold criterion was established by application of 0.1% DMSO as a vehicle. Ratiometric data were converted to [Ca2+]i (in nM) as previously described (36).
Electrophysiology.
Recordings were made in whole cell voltage-clamp (holding voltage (Vh) = −60 mV) mode at 22–24°C from the somata of small-to-medium DRG mouse neurons (20–35 pF) as described (2). Data were acquired and analyzed using an Axopatch 200B amplifier and pCLAMP9.0 software (Axon Instruments, Union City, CA). Recording data were filtered at 0.5 kHz and sampled at 2 kHz. Borosilicate pipettes (Sutter, Novato, CA) were polished to resistances of 4–7 MΩ in whole cell pipette solution. Access resistance was compensated (40–80%) when appropriate up to the value of 10–15 MΩ. Currents were considered positive when their amplitudes were fivefold bigger than displayed noise. SES contained (in mM): 140 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 d-glucose, and 10 HEPES, pH 7.4. The standard pipette solution (SIS) for the whole cell configurations contained (in mM): 140 KCl, 1 MgCl2, 1 CaCl2, 10 EGTA, 10 d-glucose, 10 HEPES, 2 GTP, and 5 ATP, pH 7.3. Drugs were applied using a fast, pressure-driven, and computer-controlled eight-channel system (ValveLink8; AutoMate Scientific, San Francisco, CA).
Induction of inflammation in hindpaw.
CFA-induced inflammation was induced by injection of 20 μl of 1:1 for saline-CFA (Sigma-Aldrich, St. Louis, MO) into hindpaw of mice with light isoflurane anesthesia (64). CFA-induced inflammation in rats was initiated in the same fashion, but 50 μl of 1:1 for saline-CFA were injected (64).
Measurement of endogenous PRL.
We selected male and female rats for measurement of endogenous PRL because ELISA PRL kit is optimized for rat tissues. Tissues and serum were collected 24 h after vehicle (saline) or CFA injection into hindpaws of male and female rats. Rat paw samples were collected with 6-mm biopsy punches (Healthlink; Fray, Buffalo, NY). Serum samples were collected by collecting trunk blood in blood collection tubes with sodium citrate (BD Biosciences, Franklin Lakes, NJ) followed by centrifugation at 1,100 g for 10 min. Protein extracts from tissues were generated by adding 200–500 μl T-PER solution (Thermo Scientific) to samples and disrupting them with TissueLyser LT (Millipore) at 50 oscillations for 5 min. Protein extract was stored at −20° C until assay with a commercially available rat PRL EIA kit (SPIbio, Montigny le Bretonneux, France, distributed by Cayman Chemical). Protein amount in extracts was measured by Bradford assay (64). Endogenous PRL levels in serum were presented as amount (ng) per milliliter of serum. PRL levels in tissues were presented as amount (ng) per milliliter of protein extract. These values were normalized by the total amount of proteins in extracts.
Real-time PCR.
DRG and spinal cord were collected in RNase Later (Qiagen, Valencia, CA) 24 h post-CFA. Tissues were homogenized in a TissueLyser LT (Qiagen) using 5-mm stainless steel beads for 5 min. Total RNA was extracted using the QIAzol lysis reagent and the RNAeasy Mini Kit (Qiagen) per the manufacturer's instructions. cDNA was prepared using the Superscript III First Strand Synthesis kit (Invitrogen). Quantitative real-time PCR (RT-PCR) was performed as described (62). Amplification of target sequences was detected by a sequence detector ABI 7500 Fast RTPCR system (Applied Biosystems, Foster City, CA) using TaqMan Fast Universal PCR Master Mix and PRL-R short form (assay no. Mm02017047_s1; Applied Biosystems) or PRL-R long form (assay no. Mm00619170_s1; Applied Biosystems) specific primers. The reactions were run in triplicates of 25 μl, including the endogenous control, mouse GAPDH (assay no. Mm03302249_g1), for each individual gene expression assay. For quantitative analysis, comparative delta-delta Ct was used to normalize the data based on the endogenous reference, and to express it as the relative fold change, after the exclusion criteria were verified by comparing primer efficiencies.
Behavior experiments.
All behavioral experiments were conducted by a blinded observer, and at least duplicated on a separate set of animals. Two main difficulties when performing behavior experiments on PRL KO and PRL KO mice are: 1) different breeding times can result in an age mismatch between animals, and 2) female PRLR KO mice have irregular cycles. To overcome these problems, we performed experiments (especially for evaluating mechanical allodynia) on small groups (n = 3–4) of mice in every trial. Age of animals was 4–7 mo. Thermal and mechanical nociception were measured in mice in estrous phase with a low-to-moderate level of estrogen. Furthermore, because PRL KO and PRL-R KO mice have irregular estrous cycles, we performed behavior experiments on females only during afternoon of the estrous phase, which was determined as described (17). However, 3 days post-CFA nociception in female mice was sometimes measured in diestrous 1 phase with low levels of estrogen. Mice were housed for at least 1 wk before handling and habituated to the testing environment for at least 1 h before testing.
Heat-induced nociception.
Heat nociception was assessed as previously described (33a). Briefly, mice were placed on a glass surface with temperature held constant at 20°C. Following habituation, thermal withdrawal latencies to a radiant heat beam were recorded at each time point (3× measurements at each time point, averaged to obtain the data value used in analyses). To prevent tissue damage, the stimulus was terminated after ≈20 s if the animal did not withdraw the hindpaw.
Cold-induced nociception.
Cold nociception in mice was measured using a modified IITC incremental hot/cold plate (IITC Life Sciences). The mice were placed on a metal plate maintained at room temperature. The mice were habituated to the apparatus for 45–60 min. The temperature was then decreased at a ramp of 10°C/min. To prevent any tissue damage resulting from extreme cold temperatures, the cutoff temperature was −5°C. The threshold temperatures manifested by escape reflex (jump) of mice were recorded as a response. One mouse at a time was used to measure cold nociception.
Mechanical stimulus-induced nociception.
To record withdrawal thresholds for mechanical stimulation, mice were habituated for 45–60 min, and then the baseline readings (3 readings/animal) were taken on the right hindpaw using the Dynamic Plantar Aesthesiometer (Ugo Basile). The instrument applies a constant ramp of increasing mechanical pressure to the animal right hindpaw (from 0 to 50 g in 10 s), and the reading provides withdrawal threshold in grams.
Anatomical analysis.
Male and female PRL-R KO and WT littermate mice (n = 1 of each) were anesthetized with an intraperitoneal injection of pentobarbital (2.5 mg) and then transcardially perfused with 20 ml/s of 0.9% saline in water followed by 30 ml/s of fixative consisting of 4% paraformaldehyde in 0.1 M phosphate buffer. The lumbar spinal cord was exposed, the L3–L5 region was removed and embedded in paraffin, and 15-μm transverse sections were stained with hematoxylin and eosin (H&E). Sections were evaluated, and images were obtained of the dorsal horn with a Nikon D-Eclipse light microscope. Images were processed for illustration purposes with Photoshop CS2.
Data analyses.
GraphPad Prism 5.0 (GraphPad, La Jolla, CA) was used for statistical analyses. The data in Figs. 1–7 were given as means ± SE, with the n value referring to the overall number of animals analyzed in all trials. All experiments were performed at least in duplicate. When only two sets of data were evaluated, the statistical differences were analyzed using unpaired t-test. Multiple sets of data were evaluated with one-way ANOVA with Bonferroni's multiple-comparison post hoc tests. Differences between groups were assessed by two-way analysis of variance (ANOVA) with Bonferroni's multiple-comparison post hoc tests. Select data analysis was performed with a three-way ANOVA with sex, genotype, and time as factors. A difference was accepted as statistically significant when P < 0.05. Significance levels were *P < 0.05, **P < 0.01, and ***P < 0.001.
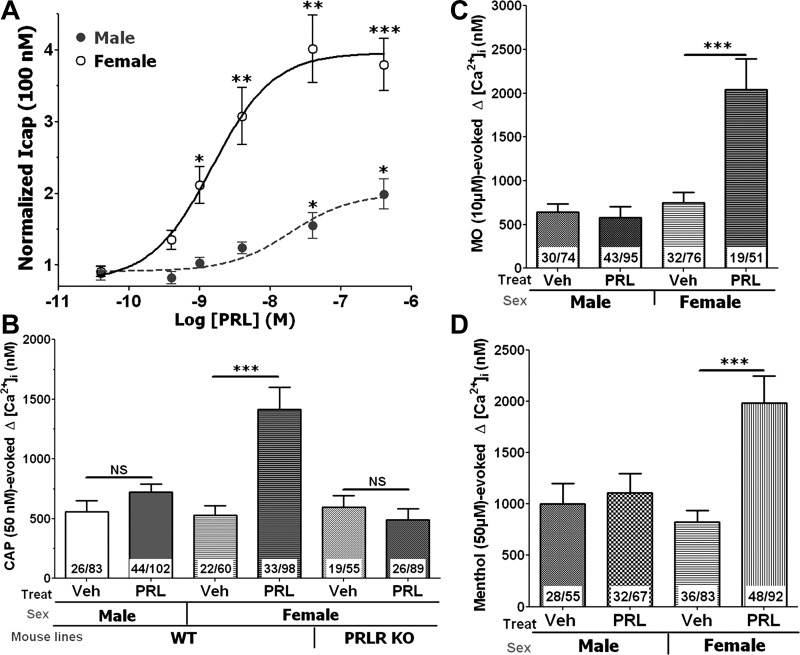
Fig. 1.
Regulation of transient receptor potential (TRP) V1, TRPA1, and TRPM8 by prolactin (PRL) in sensory neurons of female and male mice. Cells were pretreated by PRL for 20 min in all described experiments. A: concentration-response curve for PRL-induced sensitization of capsaicin (100 nM)-gated current (ICAP) in sensory neurons of wild-type (WT) female and male mice. Statistical significance was measured comparing ICAP from treatment groups with ICAP in vehicle (Veh)-treated sensory neurons (1-way ANOVA; *P < 0.05, **P < 0.01, and ***P < 0.001; n = 8–12 mice). B: regulation of capsaicin (50 nM)-evoked intracellular Ca2+ ([Ca2+]i) accumulation in sensory neurons of WT female and male mice as well as PRL receptor (PRL-R) knockout (KO) female mice (unpaired t-test; ***P < 0.001). NS, not significant. No. analyzed and capsaicin (CAP)-responsive cells are indicated within bars. C: regulation of mustard oil (MO; 10 μM)-evoked [Ca2+]i accumulation in sensory neurons of WT female and male mice (unpaired t-test; ***P < 0.001). No. analyzed and mustard oil-responsive cells are indicated within bars. PRLR, prolactin receptor. D: regulation of menthol (50 μM)-evoked [Ca2+]i accumulation in sensory neurons of TRPA1 KO female and male mice (unpaired t-test; ***P < 0.001). No. analyzed and menthol-responsive cells are indicated within bars. Mouse lines and sex are indicated below x-axis on B, C, and D.
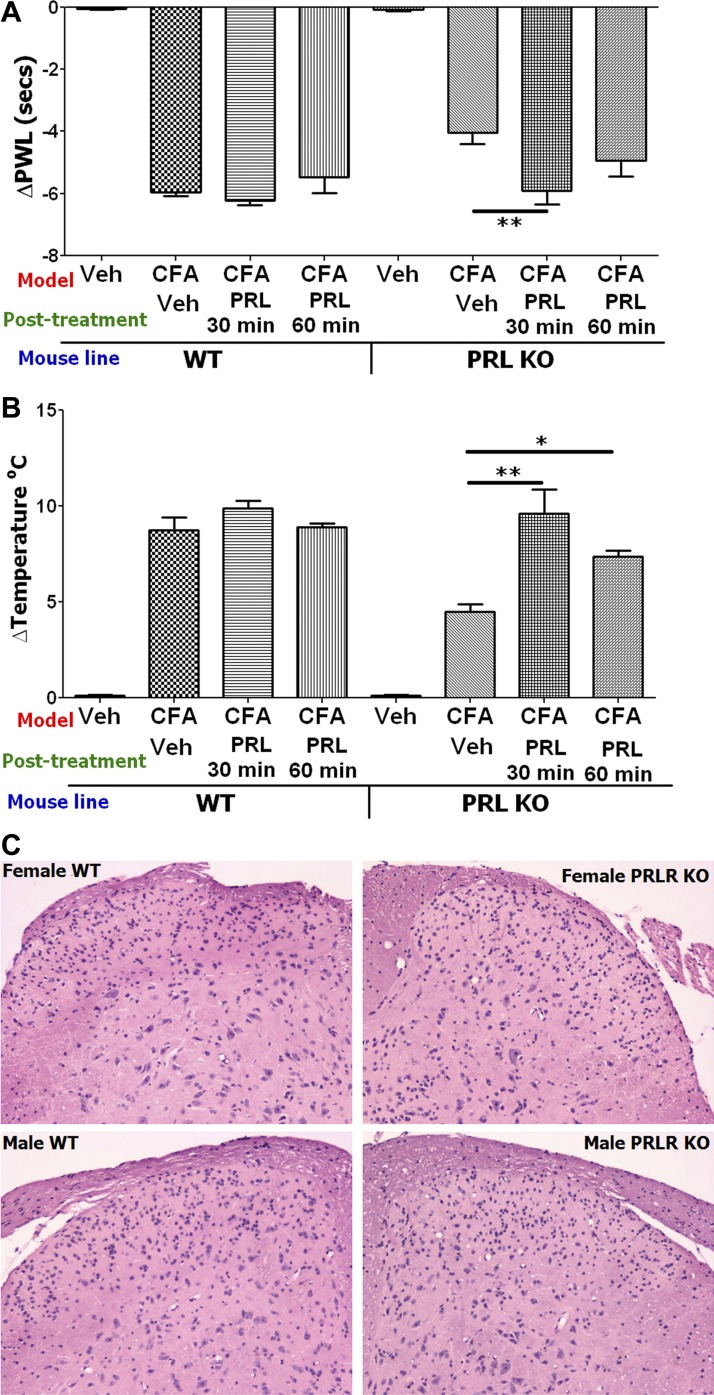
Fig. 7.
Restoration of thermal hyperalgesia in PRL KO female mice with hindpaw administration of mouse PR and lack of structural changes in PRL-R KO mice. A: CFA-induced heat hyperalgesia was partially restored in PRL KO but not WT female mice by administration of 10 μg mouse PRL in 6-h post-CFA time point into hindpaws (ΔPWL; **P < 0.01; 1-way ANOVA; n = 5). B: CFA-induced cold hyperalgesia was partially restored in PRL KO but not WT female mice by administration of 10 μg mouse PRL in CFA-injected hindpaws (ΔTemperature°C; *P < 0.05 and **P < 0.01; 1-way ANOVA; n = 5). Model, posttreatment with vehicle or PRL, and mouse lines are indicated below x-axis in A and B. Heat and cold nociception was measured 30 min after vehicle (saline) administration, and 30 or 60 min after PRL administration. C: no obvious structural changes are present in the dorsal horn of the lumbar spinal cord of PRL-R KO female and male mice compared with WT male or female mice. Transverse sections of the spinal cord sections were stained with hematoxylin and eosin.
RESULTS
Regulation of TRPV1, TRPA1, and TRPM8 by PRL in male and female mouse sensory neurons from DRG.
TRPV1, TRPA1, and TRPM8 play key roles in heat, cold, and mechanical inflammatory hyperalgesia (18, 22, 39). We have previously shown that 1 μg/ml PRL is able to sensitize TRPV1 in trigeminal neurons from OVX-E, but not in neurons from OVX rats (27). Here, we evaluated the concentrations of mouse PRL required to sensitize the activation of TRPV1 in DRG sensory neurons from male and estrous female WT mice. We also examined whether PRL-R is required to mediate PRL effects in sensory neurons. Finally, modulation of TRPA1 and TRPM8 by PRL in DRG neurons of male and female WT mice was also evaluated.
Figure 1A demonstrates that capsaicin-gated current (ICAP), which is mediated by the TRPV1 channel, is upregulated after mouse PRL (R&D Systems) pretreatment in WT male and female DRG neurons. ICAP in female sensory neurons is significantly regulated by >25 ng/ml (≈1 nM) of PRL, whereas at least >1 μg/ml (≈40 nM) of PRL is required to achieve substantial regulation of ICAP in male neurons (Fig. 1A). This effect of PRL on TRPV1 was confirmed with Ca2+ imaging as an alternative method. PRL at a concentration of 100 ng/ml significantly enhanced capsaicin-evoked Ca2+ influx in female but not male mouse DRG neurons (Fig. 1B). The effects of inflammatory mediators are usually mediated by their specialized receptors (25, 38, 54, 67). Similarly, the action of PRL is mediated by its short and/or long PRL-R isoform. Thus PRL (100 ng/ml) was not able to enhance capsaicin-evoked Ca2+ influx in DRG neurons from female PRL-R KO mice (Fig. 1B).
We next evaluated whether PRL modulates other TRP channels involved in inflammatory hyperalgesia. At 10 μM, mustard oil is considered as a selective agonist for the TRPA1 channel (2, 5). Figure 1C shows that 100 ng/ml PRL substantially enhanced mustard oil-evoked Ca2+ influx in female WT mouse DRG neurons (Fig. 1C). In contrast, this concentration of PRL does not alter TRPA1 activities in male WT mouse sensory neurons (Fig. 1C). Menthol at 50 μM concentration activates both TRPM8 and TRPA1 channels (3, 6, 46). Therefore, to examine regulation of TRPM8 by PRL, we performed experiments in TRPA1 KO DRG neurons, and 100 ng/ml PRL were also able to regulate TRPM8-mediated Ca2+ influx only in female DRG neurons (Fig. 1D).
Inflammation-induced regulation of endogenous PRL and PRL-R isoforms.
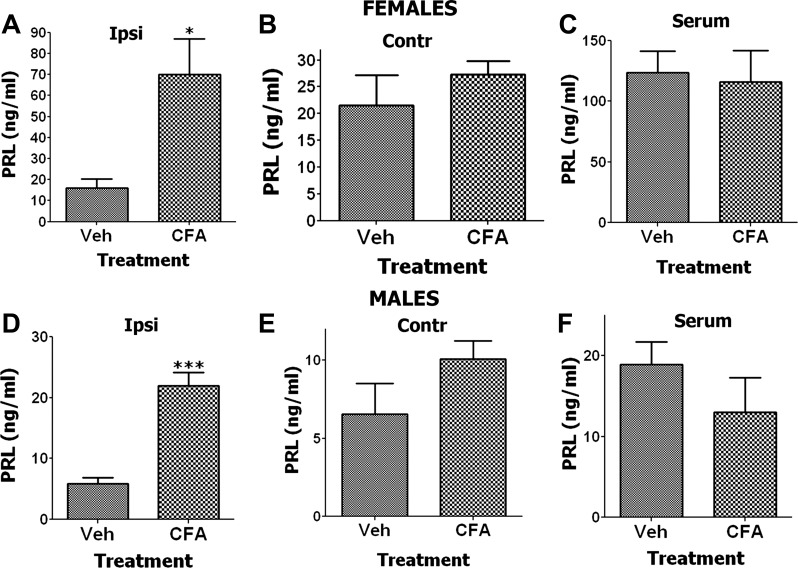
We have previously shown that inflammation can dramatically enhance local production of PRL in interstitial fluid from the inflamed hindpaw of OVX-E rats (64). Here we investigated whether CFA induces endogenous PRL production systemically (in serum) as well as in inflamed and noninflamed hindpaw tissues of estrous female and male rats. The data shown in Fig. 2 illustrate that CFA induces an increase of PRL levels in both female and male inflamed hindpaw tissues, but not in serum or in noninflamed hindpaws. The increase in PRL was sex-dependent and three times greater in females than males (70 ng/ml in female inflamed hindpaws vs. 23 ng/ml in males; Fig. 2, A and D). These PRL levels in both males and females are substantially lower than the levels observed in inflamed hindpaws of OVX-E rats (64). Importantly, the PRL level seen in the inflamed hindpaw of estrous females, but not males, is sufficient to enhance TRPV1, TRPA1, and TRPM8 channel activities (Fig. 1).
Fig. 2.
Endogenous PRL levels are regulated by complete Freund adjuvant (CFA) injection in hindpaws of female and male rats. Endogenous PRL levels are regulated 24 h post-CFA in ipsilateral (CFA-injected) hindpaw (n = 6; unpaired t-test, *P < 0.05) (A) but not contralateral hindpaw (B) and serum (C) of female rats (n = 6–8; unpaired t-test, P > 0.05). Endogenous PRL levels are also regulated 24 h post-CFA in ipsilateral hindpaw (n = 6–7; unpaired t-test, ***P < 0.001) (D) but not contralateral hindpaw (E) and serum (F) of male rats (n = 6–8; unpaired t-test, P > 0.05).
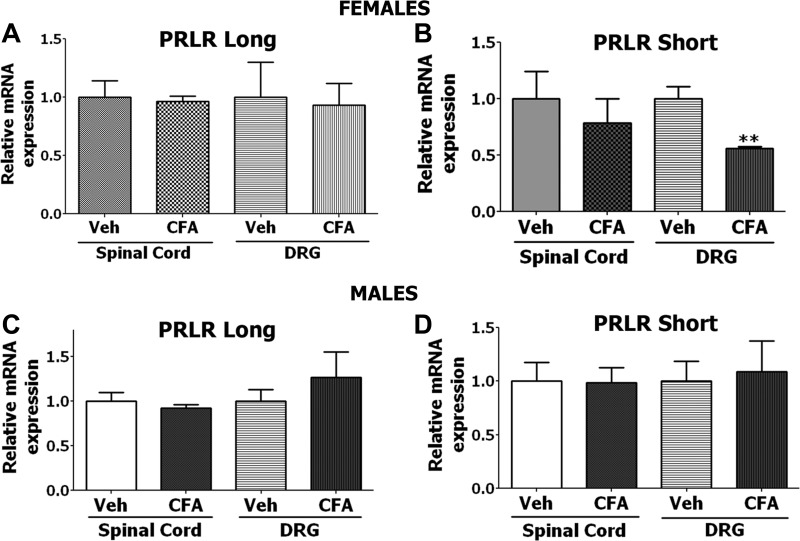
We next characterized CFA-induced transcriptional regulation of short and long PRL-R isoforms from DRG and spinal cord tissues of WT male and female mice. The data from Fig. 3 show a statistically significant change (reduction) observed only for the short PRL-R isoform mRNA in DRG of female mice (Fig. 3B). Interestingly, it was previously shown that the short isoform of the PRL-R can act as a dominant-negative molecule for the actions of the long PRL-R isoform in cellular differentiation (59, 71, 76).
Fig. 3.
Effect of CFA-induced inflammation on expression of PRL-R long and short forms in spinal cord and dorsal root ganglia (DRG) of female and male mice. Twenty microliters of 1:1 CFA to saline or saline (Veh) was injected in hindpaws of WT mice. Expression was measured with quantitative RT-PCR 24 h post-CFA injection. A: long PRL-R isoform expression in spinal cord and DRG of Veh and CFA-treated female mice (n = 3–4). B: short PRL-R isoform expression in spinal cord and DRG of Veh and CFA-treated female mice (**P < 0.01; unpaired t-test; n = 3–4). C: long PRL-R isoform expression in spinal cord and DRG of Veh and CFA-treated male mice (n = 3–4). D: short PRL-R isoform expression in spinal cord and DRG of Veh and CFA-treated male mice (n = 3–4). Each data set was normalized to the expression seen in uninflamed/control (Veh) animals.
Inflammatory heat hyperalgesia in male and female PRL KO and PRL-R KO mice.
PRL is a component of the inflammatory response that is upregulated during acute and chronic inflammatory conditions, and has a stimulatory effect on immune cells (Fig. 2). In addition, elevated PRL levels directly sensitize TRPV1 and TRPA1 channels in sensory neurons in a sex-dependent manner (Fig. 1; see Ref. 27). Such modulation by PRL implies that inhibition or ablation of the PRL system could influence heat inflammatory hyperalgesia. To investigate whether PRL and PRL-R regulate inflammation-induced heat hyperalgesia, we generated inflammation with hindpaw injection of CFA in WT, PRL KO, and PRL-R KO female and male mice and evaluated alterations in heat nociception at different post-CFA time points. Basal levels of heat nociception, which were measured before CFA injection, were not statistically different in PRL KO and PRL-R KO vs. WT male and female mice (Table 1). Data for heat hyperalgesia were presented as delta change in paw withdrawal latency (ΔPWL). Inflammatory heat hyperalgesia peaked at 3–6 h post-CFA in WT female and male mice (Fig. 4). WT male and estrous female littermate mice for PRL KO and PRL-R KO produced similar CFA-induced heat hyperalgesia across all post-CFA time points (3-way ANOVA with sex, genotype, and time as factors; n = 10–11). These results are in accordance with previously published data that showed that only female proestrous rats exhibited significant increase in hyperalgesia when compared with male, OVX, diestrous, and estrous female rats (15). Heat inflammatory hyperalgesia was not affected, at the tested post-CFA time points (1, 3, 6, 24 and 72 h), by the lack of PRL and PRL-R proteins in male mice (Fig. 4, B and D). However, heat inflammatory hyperalgesia characterized by changes in PWL was partially and significantly reversed at post-CFA 3-, 6-, and 24-h time points in PRL KO and PRL-R KO female mice (Fig. 4, A and C).
Table 1.
Baseline thermal and mechanical nociceptive thresholds before injection of CFA in WT, PRL KO, and PRL-R KO female and male mice
| WT vs. PRL KO |
WT vs. PRL-R KO |
|||||
|---|---|---|---|---|---|---|
| Heat, s | Cold, °C | Mechanical, g | Heat, s | Cold, °C | Mechanical, g | |
| Female | 8.85 ± 0.37 vs. 7.93 ± 0.41 (NS) | 3.92 ± 0.38 vs. 8.28 ± 0.57*** | 8.32 ± 0.43 vs. 7.62 ± 0.48 (NS) | 7.9 ± 0.27 vs. 7.22 ± 0.38 (NS) | 3.5 ± 0.31 vs. 6.27 ± 0.18*** | 7.84 ± 0.42 vs. 7.24 ± 0.54 (NS) |
| Male | 9.88 ± 0.46 vs. 9.25 ± 0.64 (NS) | 7.28 ± 0.59 vs. 8.46 ± 0.57 (NS) | 8.75 ± 0.43 vs. 8.13 ± 0.57 (NS) | 8.8 ± 0.45 vs. 8.33 ± 0.27 (NS) | 6.16 ± 0.53 vs. 7.25 ± 0.41 (NS) | 8.14 ± 0.3 vs. 7.62 ± 0.57 (NS) |
Values are means ± SE; n = 10–15 mice in each group. Heat nociception is measured as paw withdrawal latency in s. Cold nociception is measured in threshold temperature in °C. Mechanical nociception is measured in threshold force in g. CFA, complete Freund adjuvant; WT, wild type; PRL, prolactin; PRL-R, prolactin receptor; KO, knockout. Significant changes (unpaired t-test) are indicated: NS, nonsignificant and
P < 0.001.
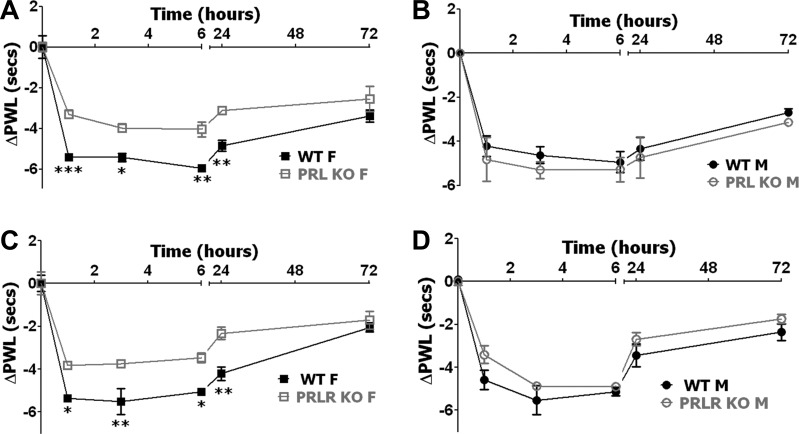
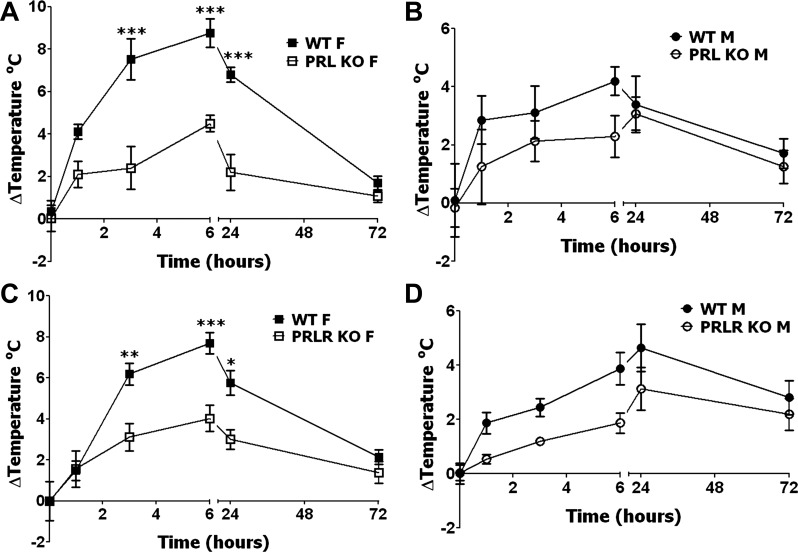
Fig. 4.
CFA-induced heat hyperalgesia in PRL KO and PRL-R KO female and male mice. A: CFA-induced heat hyperalgesia in WT and PRL KO female mice. Heat hyperalgesia is measured as changes (Δ) in paw withdrawal latency (ΔPWL; *P < 0.05, **P < 0.01, and ***P < 0.001; 2-way ANOVA; n = 5). B: CFA-induced heat hyperalgesia in WT and PRL KO male mice [ΔPWL; not significant (NS); 2-way ANOVA; n = 5]. C: CFA-induced heat hyperalgesia in WT and PRL-R KO female mice (ΔPWL; *P < 0.05 and **P < 0.01; 2-way ANOVA; n = 6). D: CFA-induced heat hyperalgesia in WT and PRL-R KO male mice (ΔPWL; NS; 2-way ANOVA; n = 6). Post-CFA time points are indicated above x-axis. Mouse lines and sex (i.e., F and M) are noted.
Inflammatory cold hyperalgesia in male and female PRL KO and PRL-R KO mice.
Next, we investigated the role of PRL and its receptor in inflammatory cold hyperalgesia, and, interestingly, inflammatory cold hyperalgesia in male and female mice has not been previously studied in detail. PRL KO and PRL-R KO female mice are dramatically more sensitive to cold stimuli than WT animals, whereas cold nociception was not changed in PRL KO and PRL-R KO male mice (Table 1). Interestingly, WT male mice responded to higher cold temperature than WT female mice (P < 0.01; Table 1). Although differences in the response to cold temperatures have not been previously characterized and reported in female and male animals, human male and female volunteers were shown to have similar cold nociception thresholds (52). To evaluate cold hyperlagesia, the data are presented as change from baseline (delta) in temperature thresholds of paw withdrawal. Inflammatory cold hyperalgesia peaked at 6 h post-CFA in WT female and male mice (Fig. 5). Unlike heat inflammatory hyperalgesia in female and male mice, WT female littermate mice for PRL KO and PRL-R KO produced significantly higher cold-induced post-CFA hyperalgesia than WT male mice at all post-CFA time points, except 1 h and 3 days post-CFA (P < 0.001 3 and 6 h post-CFA; P < 0.01 24 h post-CFA; 3-way ANOVA with sex, genotype, and time as factors; n = 10–11). Further analysis showed that cold inflammatory hyperalgesia was not statistically changed in male mice with ablated PRL and PRL-R genes (Fig. 5, B and D). In contrast, attenuation of cold hyperalgesia was pronounced in female mice with disrupted PRL and PRL-R genes at 3, 6, and 24 h post-CFA time points (Fig. 5, A and C). In summary, significant reduction in thermal (heat and cold) inflammatory hyperalgesia was only seen in female mice with PRL and PRL-R gene ablations.
Fig. 5.
CFA-induced cold hyperalgesia in PRL KO and PRL-R KO female and male mice. A: CFA-induced cold hyperalgesia in WT and PRL KO female (F) mice. Cold hyperalgesia reflects changes from baseline cold threshold responses measured before CFA application (ΔTemperature°C; ***P < 0.001; 2-way ANOVA; n = 5). B: CFA-induced cold hyperalgesia in WT and PRL KO male (M) mice (ΔTemperature°C; NS; 2-way ANOVA; n = 5). C: CFA-induced cold hyperalgesia in WT and PRL-R KO female mice (ΔTemperature°C; *P < 0.05, **P < 0.01, and ***P < 0.001; 2-way ANOVA; n = 6). D: CFA-induced cold hyperalgesia in WT and PRL-R KO male mice (ΔTemperature°C; NS; 2-way ANOVA; n = 6). Post-CFA time points are indicated below x-axis. Mouse lines and sex (i.e, F and M) are noted.
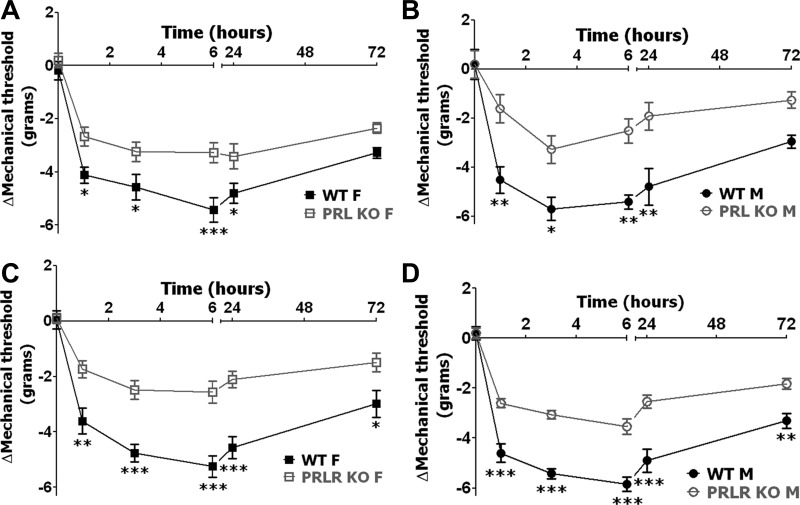
Inflammatory mechanical allodynia in male and female PRL KO and PRL-R KO mice.
To further characterize the role of PRL and PRL-R in inflammatory pain, we evaluated whether CFA-induced mechanical allodynia could be altered in male and female mice lacking PRL or PRL-R proteins. Basal behavioral responses to mechanical stimuli before CFA injection, as a measure for mechanical allodynia, were approximately equal in PRL KO and PRL-R KO vs. WT female as well as male mice (Table 1). Mechanical allodynia is shown as Δmechanical threshold (change from baseline) for paw withdrawal, so inflammatory hypersensitivity is consistently presented throughout this study. Mechanical allodynia in WT mice reached maximum values at 6 h post-CFA in both females and males (Fig. 6). Statistical differences between mechanical allodynia in WT male and female mice were not present at all post-CFA time points (Fig. 6; 3-way ANOVA with sex, genotype, and time as factors; n = 18–22). These results are in accordance with previously published data showing that CFA-induced mechanical allodynia is higher (i.e., lower mechanical threshold) only in proestrous but not estrous female rats compared with male rats (23). Interestingly, disruptions of PRL or PRL-R in both male and female mice significantly reduced inflammatory mechanical allodynia at 1, 3, 6, and 24 h post-CFA time points (Fig. 6, A–D). Altogether, attenuation of inflammation-triggered mechanical allodynia was equally affected (i.e., reduced) in both male and female PRL KO and PRL-KO mice.
Fig. 6.
CFA-induced mechanical allodynia in PRL KO and PRL-R KO female and male mice. A: CFA-induced mechanical allodynia in WT and PRL KO female mice. Mechanical allodynia reflects changes from baseline mechanical threshold responses measured before CFA application (ΔMechanical threshold; *P < 0.05 and ***P < 0.001; 2-way ANOVA; n = 11). B: CFA-induced mechanical allodynia in WT and PRL KO male mice (ΔMechanical threshold; *P < 0.05 and **P < 0.01; 2-way ANOVA; n = 8). C: CFA-induced mechanical allodynia in WT and PRL-R KO female mice (ΔMechanical threshold; *P < 0.05, **P < 0.01, and ***P < 0.001; 2-way ANOVA; n = 11). D: CFA-induced mechanical allodynia in WT and PRL-R KO male mice (ΔMechanical threshold; **P < 0.01 and ***P < 0.001; 2-way ANOVA; n = 9). Post-CFA time points are indicated above x-axis. Mouse lines and sex are noted.
Effects of application of exogenous PRL into PRL KO mouse hindpaws.
Because reductions in thermal and mechanical inflammatory hyperalgesia in PRL KO and PRL-R KO female mice could involve the direct modulation of peripheral nociceptors by PRL, we attempted to restore CFA-induced hyperalgesia at the 6-h post-CFA time point in female PRL KO mice with transient hindpaw administration of mouse PRL (10 μg). Figure 7, A and B, shows that CFA-induced heat and cold hyperalgesia is not significantly affected upon administration of PRL in the inflamed hindpaw of WT mice. In contrast, thermal (heat and cold) hyperalgesia was restored in PRL KO female mice 30 min after administration of exogenous mouse PRL (Fig. 7, A and B). Inflammation-induced mechanical allodynia was not restored in PRL KO female mice upon peripheral administration of PRL (data not shown).
This last observation implies that central mechanisms could play a vital role in modulation of inflammatory mechanical allodynia by the PRL system. One possibility is that the central processing of nociceptive information could be altered by structural changes in the dorsal horn of the spinal cord because of gene ablation in male or female PRL-R KO mice. Therefore, we analyzed for morphological/structural changes in dorsal horn of the lumbar spinal cord in PRL-R KO male and female mice and their corresponding WT littermates. Figure 7C shows that no obvious morphological or structural alterations were present in the dorsal horn of PRL-R KO male and female mice compared with WT mice in H&E-stained spinal cord sections.
DISCUSSION
The regulation of inflammatory pain by PRL and PRL-R could involve several different peripheral and central mechanisms. The PRL system could also indirectly affect the activation of pain pathways by regulating immune system responses within peripheral tissues and centrally in the spinal cord. This study identifies at least one direct peripheral mechanism involving the PRL sensitization of TRP channels in sensory neurons, which play key role in inflammatory pain. Furthermore, this sensitization in female sensory neurons from estrous mice was seen with a PRL concentration of >25 ng/ml, whereas >1 μg/ml was required to achieve a similar enhancement of TRP channel activities in male sensory neurons. This sex-dependent difference in PRL sensitization of TRP channels was also mirrored by a sex difference in PRL levels seen in the inflamed hindpaw (greater in females) that was at the >25 ng/ml level required to sensitize TRP channels in these same female mice. Indeed, it is well established that acute (<3 h in rodents; see Refs. 65 and 66) and chronic (>3–6 h in rodents; see Refs. 65 and 66) inflammation triggers an upregulation in PRL levels both locally in the vicinity of inflammation (64) and in the serum of animals and humans (7, 51, 68). Our data also find that PRL-R isoforms are not regulated by inflammation, thus again highlighting the importance of the sex-dependent PRL differences identified. Finally, local administration of exogenous PRL into the hindpaw of PRL KO female mice restored thermal hyperalgesia, but not mechanical allodynia, at levels usually observed in WT female mice. Taken together, these results identify a direct peripheral mechanism by which PRL can contribute to enhanced inflammatory thermal hyperalgesic responses in females.
Nevertheless, the observed reduction in thermal hyperalgesia in PRL KO and PRL-R KO female but not male mice could be explained by other mechanisms. For example, PRL has stimulatory effects on the immune system (45, 72), which could indirectly contribute to inflammatory hyperalgesia via peripheral and central mechanisms. Ablation of PRL-R could also trigger changes in the expression of certain genes in the spinal cord and brain since PRL-R has been implicated in epigenetic control mechanisms (13, 33, 70). To assess indirect effects of PRL, additional investigations involving utilization of tissue-specific KO mouse lines will be required. Furthermore, nonconditional ablation of the PRL system could lead to a change in metabolism and functioning of the endocrine system in PRL-R KO male and female mice. Thus, PRL levels are increased 60- to 100-fold in these animals (56). Similar to other inflammatory mediators such as bradykinin and cytokines that act via their specialized receptors, our data also show that PRL requires PRL-R to regulate channels in sensory neurons (25, 38, 54, 67). This implies that a 60- to 100-fold upregulation of PRL in PRL-R KO mice will have no effect on inflammatory hyperalgesia. Unlike testosterone, estradiol and progesterone are also mildly reduced in estrous PRL-R KO females (20). The difference in estradiol and progesterone levels between WT and PRL-R KO estrous females is much less than between WT estrous females and males. Our results and results of others demonstrate that inflammatory heat and mechanical hypersensitivity do not differ in estrous females compared with males (15, 23). Therefore, changes in sex hormones in PRL-R mice most likely do not account for the reduction in inflammatory heat and mechanical hyperalgesia seen in PRL KO and PRL-R KO mice, but rather because of PRL system gene ablations themselves.
Ablation of PRL and PRL-R in females resulted in a modest increased sensitivity to heat and mechanical stimuli, whereas cold sensitivity was substantially increased (Table 1). These findings imply that the physiological levels of PRL are mildly antinociceptive, and inflammation-induced PRL levels have hyperalgesic effects. Interestingly, systemic and prolonged treatments with supraphysiological concentrations of PRL, which are observed during lactation in females, could provide a protective effect for certain cell types (1, 50). This dual-effect phenomenon is reported for many trophic factors, and PRL could play such a role. For example, even though acute administration of glial cell line-derived neurotrophic factor and artemin produce hyperalgesia (19, 43), the long-term systemic administration of these trophic factors is associated with neuroprotection, antihyperalgesia, and antinociception (14, 30).
We found that mechanical allodynia was not restored by peripheral administration of PRL in PRL KO mice. Furthermore, mechanical allodynia was reduced in both PRL and PRL-R KO male and female mice. These observations could imply that effects of the PRL system on mechanical allodynia and thermal hyperalgesia may be mediated by central mechanisms during certain inflammatory conditions. There are several different possibilities that could be involved. First, inflammation could lead to an increase in the levels of PRL in the spinal cord and other regions of the central nervous system involved in the processing of nociceptive information. TRPV1 and TRPA1 play a role in inflammatory mechanical allodynia possibly via presynaptic mechanisms in the dorsal spinal cord (32, 57). A direct action of PRL on TRPs at the same site is possible, since activated microglia could release PRL during inflammation where this release could also contribute to the development of inflammatory hyperalgesia (50). Second, elevated levels of endogenous PRL during inflammation in the spinal cord could transiently sensitize channels at postsynaptic sites, resulting in enhanced neuronal activities. The function of PRL in the central nervous system could be evaluated by a pharmacological approach using the specific PRL-R antagonist ΔG129R-hPRL that could be applied locally or in spinal cord to define the specific site of action for the PRL system in inflammatory hyperalgesia. Third, inflammation can regulate PRL-R expression in a variety of cells (24, 61). Even though our results show that the short and long forms of PRL-R are not regulated at the transcriptional level in the DRG and spinal cord, membrane density of PRL-R in certain spinal cord cells could still be altered. Furthermore, detailed expression patterns of PRL-R, especially its short and long form, in nociceptive pathways are lacking. The use of RT-PCR may not be the optimal approach to evaluate changes in PRL-R expression, since PRL-R could be upregulated in sensory neurons and downregulated in nonneuronal cells, yet no change would be seen with RT-PCR. Fourth, PRL can have long-term trophic effects in various cell types via signaling pathways involving Jak/STAT5 and MAP kinases (8, 28). PRL also has transient effects (<5–15 min) on target cells (27), and it has been reported that PKC and phosphatidylinositol 3-kinase can also be activated by PRL in certain cell types (10, 21). These kinases are closely involved in the regulation of channel activity, including TRPV1, by certain inflammatory mediators (12). Collectively, PRL could potentially recruit two principally different mechanisms involved in the modulation of either transient or long-term cell activities and physiological processes. The data presented here mostly cover short-term actions (<5 days) of PRL and PRL-R and demonstrate that the disruption of PRL and PRL-R significantly decreases inflammatory hyperalgesia at 1–24 h post-CFA. Interestingly, short-term hyperprolactinemia increased inflammatory edema in male animals, whereas long-term 30-day hyperprolactinemia had anti-inflammatory effects (1, 55). Fifth, it has been shown that PRL produces antihyperalgesic effects in lactating females (60, 63). A recent study has shown that this paradoxical effect of the PRL system could be explained by the presence of unique PRL variants induced by suckling (47).
In conclusion, the mechanisms by which PRL and PRL-R regulate nociceptive processes in a plethora of painful conditions that are associated with changes in PRL remains an important topic. Further studies on the role of PRL-Rs in hyperalgesia should help in our understanding of the specific mechanisms that contribute to the origin of sex-dependent pain conditions by pathways other than those related to sex hormones such as testosterone, estrogen, and progesterone.
AKNOWLEDGMENTS
We thank Jackson Laboratory for providing TRPA1, PRL KO, and PRL-R KO female and male mice for our studies. We thank Jei Li for technical assistance.
GRANTS
This work was supported by the National Institute of Dental and Craniofacial Research (R01-DE-017696 to A. N. Akopian).
DISCLOSURES
There is no conflict of interest, financial or otherwise that could be perceived as prejudicing the impartiality of the research reported.
AUTHOR CONTRIBUTIONS
Author contributions: M.J.P., S.B.R., and M.A.H. performed experiments; M.J.P., S.B.R., M.A.H., and A.N.A. analyzed data; M.J.P., S.B.R., M.A.H., and A.N.A. interpreted results of experiments; M.J.P., S.B.R., M.A.H., and A.N.A. prepared figures; M.J.P. drafted manuscript; M.J.P., S.B.R., M.A.H., and A.N.A. edited and revised manuscript; M.J.P., S.B.R., M.A.H., and A.N.A. approved final version of manuscript; A.N.A. conception and design of research.
REFERENCES
- 1.Adan N, Guzman-Morales J, Ledesma-Colunga MG, Perales-Canales SI, Quintanar-Stephano A, Lopez-Barrera F, Mendez I, Moreno-Carranza B, Triebel J, Binart N, Martinez de la Escalera G, Thebault S, Clapp C. Prolactin promotes cartilage survival and attenuates inflammation in inflammatory arthritis. J Clin Invest 123: 3902–3913, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akopian AN, Ruparel NB, Jeske NA, Hargreaves KM. Transient receptor potential TRPA1 channel desensitization in sensory neurons is agonist dependent and regulated by TRPV1-directed internalization. J Physiol 583: 175–193, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 41: 849–857, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Barnabe C, Bessette L, Flanagan C, Leclercq S, Steiman A, Kalache F, Kung T, Pope JE, Haraoui B, Hochman J, Mosher D, Thorne C, Bykerk V. Sex differences in pain scores and localization in inflammatory arthritis: a systematic review and metaanalysis. J Rheumatol 39: 1221–1230, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 Mediates the Inflammatory Actions of Environmental Irritants and Proalgesic Agents. Cell 124: 1269–1282, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, Julius D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 448: 204–208, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Berczi I, Nagy E, Asa SL, Kovacs K. The influence of pituitary hormones on adjuvant arthritis. Arthritis Rheum 27: 682–688, 1984 [DOI] [PubMed] [Google Scholar]
- 8.Bernichtein S, Kayser C, Dillner K, Moulin S, Kopchick JJ, Martial JA, Norstedt G, Isaksson O, Kelly PA, Goffin V. Development of pure prolactin receptor antagonists. J Biol Chem 278: 35988–35999, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Bernton EW, Meltzer MS, Holaday JW. Suppression of macrophage activation and T-lymphocyte function in hypoprolactinemic mice. Science 239: 401–404, 1988 [DOI] [PubMed] [Google Scholar]
- 10.Bole-Feysot C, Goffin V, Edery M, Binart N, Kelly PA. Prolactin (PRL) and its receptor: actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr Rev 19: 225–268, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Bolyakov A, Paduch DA. Prolactin in men's health and disease. Curr Opin Urol 21: 527–534, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Bonnington JK, McNaughton PA. Signalling pathways involved in the sensitisation of mouse nociceptive neurones by nerve growth factor. J Physiol 551: 433–446, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Book McAlexander M, Yu-Lee L. Prolactin activation of IRF-1 transcription involves changes in histone acetylation. FEBS Lett 488: 91–94, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Boucher TJ, Okuse K, Bennett DL, Munson JB, Wood JN, McMahon SB. Potent analgesic effects of GDNF in neuropathic pain states. Science 290: 124–127, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Bradshaw H, Miller J, Ling Q, Malsnee K, Ruda MA. Sex differences and phases of the estrous cycle alter the response of spinal cord dynorphin neurons to peripheral inflammation and hyperalgesia. Pain 85: 93–99, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Butelman ER, Harris TJ, Perez A, Kreek MJ. Effects of systemically administered dynorphin A(1–17) in rhesus monkeys. J Pharmacol Exp Ther 290: 678–686, 1999 [PubMed] [Google Scholar]
- 17.Caligioni CS. Assessing reproductive status/stages in mice. Curr Protoc Neurosci Appendix 4: Appendix 4I, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288: 306–313, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Ceyhan GO, Schafer KH, Kerscher AG, Rauch U, Demir IE, Kadihasanoglu M, Bohm C, Muller MW, Buchler MW, Giese NA, Erkan M, Friess H. Nerve growth factor and artemin are paracrine mediators of pancreatic neuropathy in pancreatic adenocarcinoma. Ann Surg 251: 923–931, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Clement-Lacroix P, Ormandy C, Lepescheux L, Ammann P, Damotte D, Goffin V, Bouchard B, Amling M, Gaillard-Kelly M, Binart N, Baron R, Kelly PA. Osteoblasts are a new target for prolactin: analysis of bone formation in prolactin receptor knockout mice. Endocrinology 140: 96–105, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Clevenger CV, Kline JB. Prolactin receptor signal transduction. Lupus 10: 706–718, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Colburn RW, Lubin ML, Stone DJ, Jr, Wang Y, Lawrence D, D'Andrea MR, Brandt MR, Liu Y, Flores CM, Qin N. Attenuated cold sensitivity in TRPM8 null mice. Neuron 54: 379–386, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Cook CD, Nickerson MD. Nociceptive sensitivity and opioid antinociception and antihyperalgesia in Freund's adjuvant-induced arthritic male and female rats. J Pharmacol Exp Ther 313: 449–459, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Corbacho AM, Valacchi G, Kubala L, Olano-Martin E, Schock BC, Kenny TP, Cross CE. Tissue-specific gene expression of prolactin receptor in the acute-phase response induced by lipopolysaccharides. Am J Physiol Endocrinol Metab 287: E750–E757, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Dai Y, Wang S, Tominaga M, Yamamoto S, Fukuoka T, Higashi T, Kobayashi K, Obata K, Yamanaka H, Noguchi K. Sensitization of TRPA1 by PAR2 contributes to the sensation of inflammatory pain. J Clin Invest 117: 1979–1987, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Bellis A, Bizzarro A, Pivonello R, Lombardi G, Bellastella A. Prolactin and autoimmunity. Pituitary 8: 25–30, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Diogenes A, Patwardhan AM, Jeske NA, Ruparel NB, Goffin V, Akopian AN, Hargreaves KM. Prolactin modulates TRPV1 in female rat trigeminal sensory neurons. J Neurosci 26: 8126–8136, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eyal O, Jomain JB, Kessler C, Goffin V, Handwerger S. Autocrine prolactin inhibits human uterine decidualization: a novel role for prolactin. Biol Reprod 76: 777–783, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL., 3rd Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain 10: 447–485, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gardell LR, Wang R, Ehrenfels C, Ossipov MH, Rossomando AJ, Miller S, Buckley C, Cai AK, Tse A, Foley SF, Gong B, Walus L, Carmillo P, Worley D, Huang C, Engber T, Pepinsky B, Cate RL, Vanderah TW, Lai J, Sah DW, Porreca F. Multiple actions of systemic artemin in experimental neuropathy. Nat Med 9: 1383–1389, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Giraldo E, Hinchado MD, Garcia JJ, Ortega E. Influence of gender and oral contraceptives intake on innate and inflammatory response. Role of neuroendocrine factors. Mol Cell Biochem 313: 147–153, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Gregus AM, Doolen S, Dumlao DS, Buczynski MW, Takasusuki T, Fitzsimmons BL, Hua XY, Taylor BK, Dennis EA, Yaksh TL. Spinal 12-lipoxygenase-derived hepoxilin A3 contributes to inflammatory hyperalgesia via activation of TRPV1 and TRPA1 receptors. Proc Natl Acad Sci USA 109: 6721–6726, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grimaldi G, Christian M, Steel JH, Henriet P, Poutanen M, Brosens JJ. Down-regulation of the histone methyltransferase EZH2 contributes to the epigenetic programming of decidualizing human endometrial stromal cells. Mol Endocrinol 25: 1892–1903, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33a.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 32: 77–88, 1988 [DOI] [PubMed] [Google Scholar]
- 34.Hilty C, Bruhlmann P, Sprott H, Gay RE, Michel BA, Gay S, Neidhart M. Altered diurnal rhythm of prolactin in systemic sclerosis. J Rheumatol 27: 2160–2165, 2000 [PubMed] [Google Scholar]
- 35.Horseman ND, Zhao W, Montecino-Rodriguez E, Tanaka M, Nakashima K, Engle SJ, Smith F, Markoff E, Dorshkind K. Defective mammopoiesis, but normal hematopoiesis, in mice with a targeted disruption of the prolactin gene. EMBO J 16: 6926–6935, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jeske NA, Patwardhan AM, Gamper N, Price TJ, Akopian AN, Hargreaves KM. Cannabinoid WIN 55,212–2 regulates TRPV1 phosphorylation in sensory neurons. J Biol Chem 281: 32879–32890, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jimena P, Aguirre MA, Lopez-Curbelo A, de Andres M, Garcia-Courtay C, Cuadrado MJ. Prolactin levels in patients with systemic lupus erythematosus: a case controlled study. Lupus 7: 383–386, 1998 [DOI] [PubMed] [Google Scholar]
- 38.Khan AA, Diogenes A, Jeske NA, Henry MA, Akopian A, Hargreaves KM. Tumor necrosis factor alpha enhances the sensitivity of rat trigeminal neurons to capsaicin. Neuroscience 155: 503–509, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, Corey DP. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron 50: 277–289, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Lin SH, Leslie FM, Civelli O. Neurochemical properties of the prolactin releasing peptide (PrRP) receptor expressing neurons: evidence for a role of PrRP as a regulator of stress and nociception. Brain Res 952: 15–30, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Lyons DJ, Hellysaz A, Broberger C. Prolactin regulates tuberoinfundibular dopamine neuron discharge pattern: novel feedback control mechanisms in the lactotrophic axis. J Neurosci 32: 8074–8083, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Madaan V, Bestha DP, Kolli V, Jauhari S, Burket RC. Clinical utility of the risperidone formulations in the management of schizophrenia. Neuropsychiatr Dis Treat 7: 611–620, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Malin SA, Molliver DC, Koerber HR, Cornuet P, Frye R, Albers KM, Davis BM. Glial cell line-derived neurotrophic factor family members sensitize nociceptors in vitro and produce thermal hyperalgesia in vivo. J Neurosci 26: 8588–8599, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mateo L, Nolla JM, Bonnin MR, Navarro MA, Roig-Escofet D. High serum prolactin levels in men with rheumatoid arthritis. J Rheumatol 25: 2077–2082, 1998 [PubMed] [Google Scholar]
- 45.Matera L. Endocrine, paracrine and autocrine actions of prolactin on immune cells. Life Sci 59: 599–614, 1996 [DOI] [PubMed] [Google Scholar]
- 46.McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 416: 52–58, 2002 [DOI] [PubMed] [Google Scholar]
- 47.Mena F, Gonzalez-Hernandez A, Navarro N, Castilla A, Morales T, Rojas-Piloni G, Martinez-Lorenzana G, Condes-Lara M. Prolactin fractions from lactating rats elicit effects upon sensory spinal cord cells of male rats. Neuroscience 248C: 552–561, 2013 [DOI] [PubMed] [Google Scholar]
- 48.Milano W, D'Acunto CW, De Rosa M, Festa M, Milano L, Petrella C, Capasso A. Recent clinical aspects of hyperprolactinemia induced by antipsychotics. Rev Recent Clin Trials 6: 52–63, 2012 [DOI] [PubMed] [Google Scholar]
- 49.Millan MJ, Czlonkowski A, Pilcher CW, Almeida OF, Millan MH, Colpaert FC, Herz A. A model of chronic pain in the rat: functional correlates of alterations in the activity of opioid systems. J Neurosci 7: 77–87, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moderscheim TA, Gorba T, Pathipati P, Kokay IC, Grattan DR, Williams CE, Scheepens A. Prolactin is involved in glial responses following a focal injury to the juvenile rat brain. Neuroscience 145: 963–973, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Neidhart M, Fluckiger EW. Hyperprolactinaemia in hypophysectomized or intact male rats and the development of adjuvant arthritis. Immunology 77: 449–455, 1992 [PMC free article] [PubMed] [Google Scholar]
- 52.Neziri AY, Curatolo M, Nuesch E, Scaramozzino P, Andersen OK, Arendt-Nielsen L, Juni P. Factor analysis of responses to thermal, electrical, and mechanical painful stimuli supports the importance of multi-modal pain assessment. Pain 152: 1146–1155, 2011 [DOI] [PubMed] [Google Scholar]
- 53.Neziri AY, Scaramozzino P, Andersen OK, Dickenson AH, Arendt-Nielsen L, Curatolo M. Reference values of mechanical and thermal pain tests in a pain-free population. Eur J Pain 15: 376–383, 2011 [DOI] [PubMed] [Google Scholar]
- 54.Obreja O, Rathee PK, Lips KS, Distler C, Kress M. IL-1 beta potentiates heat-activated currents in rat sensory neurons: involvement of IL-1RI, tyrosine kinase, and protein kinase C. FASEB J 16: 1497–1503, 2002 [DOI] [PubMed] [Google Scholar]
- 55.Ochoa-Amaya JE, Malucelli BE, Cruz-Casallas PE, Nasello AG, Felicio LF, Carvalho-Freitas MI. Dual effects of hyperprolactinemia on carrageenan-induced inflammatory paw edema in rats. Neuroimmunomodulation 18: 245–253, 2011 [DOI] [PubMed] [Google Scholar]
- 56.Ormandy CJ, Camus A, Barra J, Damotte D, Lucas B, Buteau H, Edery M, Brousse N, Babinet C, Binart N, Kelly PA. Null mutation of the prolactin receptor gene produces multiple reproductive defects in the mouse. Genes Dev 11: 167–178, 1997 [DOI] [PubMed] [Google Scholar]
- 57.Patwardhan AM, Scotland PE, Akopian AN, Hargreaves KM. Activation of TRPV1 in the spinal cord by oxidized linoleic acid metabolites contributes to inflammatory hyperalgesia. Proc Natl Acad Sci USA 106: 18820–18824, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pi X, Voogt JL, Grattan DR. Detection of prolactin receptor mRNA in the corpus striatum and substantia nigra of the rat. J Neurosci Res 67: 551–558, 2002 [DOI] [PubMed] [Google Scholar]
- 59.Qazi AM, Tsai-Morris CH, Dufau ML. Ligand-independent homo- and heterodimerization of human prolactin receptor variants: inhibitory action of the short forms by heterodimerization. Mol Endocrinol 20: 1912–1923, 2006 [DOI] [PubMed] [Google Scholar]
- 60.Ren K, Blass EM, Zhou Q, Dubner R. Suckling and sucrose ingestion suppress persistent hyperalgesia and spinal Fos expression after forepaw inflammation in infant rats. Proc Natl Acad Sci USA 94: 1471–1475, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reuwer AQ, van Eijk M, Houttuijn-Bloemendaal FM, van der Loos CM, Claessen N, Teeling P, Kastelein JJ, Hamann J, Goffin V, von der Thusen JH, Twickler MT, Aten J. The prolactin receptor is expressed in macrophages within human carotid atherosclerotic plaques: a role for prolactin in atherogenesis? J Endocrinol 208: 107–117, 2011 [DOI] [PubMed] [Google Scholar]
- 62.Ruparel S, Henry MA, Akopian A, Patil M, Zeldin DC, Roman L, Hargreaves KM. Plasticity of cytochrome P450 isozyme expression in rat trigeminal ganglia neurons during inflammation. Pain 153: 2031–2039, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rushen J, Foxcroft G, De Passille AM. Nursing-induced changes in pain sensitivity, prolactin, and somatotropin in the pig. Physiol Behav 53: 265–270, 1993 [DOI] [PubMed] [Google Scholar]
- 64.Scotland PE, Patil M, Belugin S, Henry MA, Goffin V, Hargreaves KM, Akopian AN. Endogenous prolactin generated during peripheral inflammation contributes to thermal hyperalgesia. Eur J Neurosci 34: 745–754, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sessle BJ. Acute and chronic craniofacial pain: brainstem mechanisms of nociceptive transmission and neuroplasticity, and their clinical correlates. Crit Rev Oral Biol Med 11: 57–91, 2000 [DOI] [PubMed] [Google Scholar]
- 66.Sessle BJ. Peripheral and central mechanisms of orofacial inflammatory pain. Int Rev Neurobiol 97: 179–206, 2011 [DOI] [PubMed] [Google Scholar]
- 67.Shin J, Cho H, Hwang SW, Jung J, Shin CY, Lee SY, Kim SH, Lee MG, Choi YH, Kim J, Haber NA, Reichling DB, Khasar S, Levine JD, Oh U. Bradykinin-12-lipoxygenase-VR1 signaling pathway for inflammatory hyperalgesia. Proc Natl Acad Sci USA 99: 10150–10155, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Straub RH, Georgi J, Helmke K, Vaith P, Lang B. In polymyalgia rheumatica serum prolactin is positively correlated with the number of typical symptoms but not with typical inflammatory markers. Rheumatology (Oxford) 41: 423–429, 2002 [DOI] [PubMed] [Google Scholar]
- 69.Straub RH, Zeuner M, Lock G, Scholmerich J, Lang B. High prolactin and low dehydroepiandrosterone sulphate serum levels in patients with severe systemic sclerosis. Br J Rheumatol 36: 426–432, 1997 [DOI] [PubMed] [Google Scholar]
- 70.Tamura I, Asada H, Maekawa R, Tanabe M, Lee L, Taketani T, Yamagata Y, Tamura H, Sugino N. Induction of IGFBP-1 expression by cAMP is associated with histone acetylation status of the promoter region in human endometrial stromal cells. Endocrinology 153: 5612–5621, 2012 [DOI] [PubMed] [Google Scholar]
- 71.Trott JF, Hovey RC, Koduri S, Vonderhaar BK. Alternative splicing to exon 11 of human prolactin receptor gene results in multiple isoforms including a secreted prolactin-binding protein. J Mol Endocrinol 30: 31–47, 2003 [DOI] [PubMed] [Google Scholar]
- 72.Tseng YH, Kessler MA, Schuler LA. Regulation of interleukin (IL)-1alpha, IL-1beta, and IL-6 expression by growth hormone and prolactin in bovine thymic stromal cells. Mol Cell Endocrinol 128: 117–127, 1997 [DOI] [PubMed] [Google Scholar]
- 73.Verschuren JE, Enzlin P, Dijkstra PU, Geertzen JH, Dekker R. Chronic disease and sexuality: a generic conceptual framework. J Sex Res 47: 153–170, 2010 [DOI] [PubMed] [Google Scholar]
- 74.Walker SE. Modulation of hormones in the treatment of lupus. Am J Manag Care 7: S486–S489, 2001 [PubMed] [Google Scholar]
- 75.Walker SE, Allen SH, McMurray RW. Prolactin and autoimmune disease. Trends Endocrinol Metab 4: 147–151, 1993 [DOI] [PubMed] [Google Scholar]
- 76.Xie YL, Hassan SA, Qazi AM, Tsai-Morris CH, Dufau ML. Intramolecular disulfide bonds of the prolactin receptor short form are required for its inhibitory action on the function of the long form of the receptor. Mol Cell Biol 29: 2546–2555, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]