Abstract
Accumulation of visceral fat, more so than subcutaneous fat, is strongly associated with severe metabolic complications. However, the factors regulating depot-specific adipogenesis are poorly understood. In this study, we show differential expression of pregnancy-associated plasma protein-A (PAPP-A), a secreted regulator of local insulin-like growth factor (IGF) action, in adipose tissue of mice. PAPP-A mRNA expression was fivefold higher in visceral (mesenteric) fat compared with subcutaneous (inguinal, subscapular), perirenal, and brown fat of mice. To investigate the possible role of depot-specific PAPP-A expression in fat accumulation, wild-type (WT) and PAPP-A knockout (KO) mice were fed a high-fat diet (HFD) for up to 20 wk. Adipocyte size increased in subcutaneous and perirenal depots similarly in WT and PAPP-A KO mice. However, fat cell size and in vivo lipid uptake were significantly reduced in mesenteric fat of PAPP-A KO compared with WT mice. After 20 wk on HFD, phosphorylation of AKT, a downstream signaling intermediate of IGF-I and insulin receptor activation, was significantly decreased by 50% in mesenteric compared with subcutaneous fat in WT mice, but was significantly increased threefold in mesenteric compared with subcutaneous fat in PAPP-A KO mice. This appeared to be because of enhanced insulin-stimulated signaling in mesenteric fat of PAPP-A KO mice. These data establish fat depot-specific expression of PAPP-A and indicate preferential impact of PAPP-A deficiency on visceral fat in the mouse that is associated with enhanced insulin receptor signaling. Thus, PAPP-A may be a potential target for treatment and/or prevention strategies for visceral obesity and related morbidities.
Keywords: adipose tissue, mesenteric fat
the prevalence of obesity is increasing rapidly in Western countries and, indeed, is now considered an epidemic (10). Visceral obesity, in particular, is associated with increased risk of developing insulin resistance and severe metabolic complications, including cardiovascular disease and diabetes (18, 23, 34). There are new and important indications that adipose tissue from different regions is inherently distinct, which likely contributes to differential fat distribution and function (27, 28). However, the factors regulating depot-specific adipogenesis are poorly understood.
Different fat depots of both humans and experimental animals vary in function, responses to diet, and gene expression (reviewed in Ref. 29). Genomewide expression profiles of primary preadipocytes isolated from human visceral fat depots and abdominal subcutaneous fat depots indicate differences in regard to gene signature (28). Pregnancy-associated plasma protein-A (PAPP-A) is one of the most distinctive genes expressed, with levels in visceral preadipocytes exceeding those in subcutaneous preadipocytes. Similarly, microarray data from Gealekman et al. (16) show fivefold higher PAPP-A expression in human preadipocytes from visceral fat compared with subcutaneous fat. The same regional differences in PAPP-A expression have been reported for fetal baboon preadipocytes (30).
PAPP-A is a secreted zinc metalloprotease that can associate with the surface of cells enabling autocrine/paracrine action. The major known function of PAPP-A is to enhance local insulin-like growth factor (IGF) signaling through its ability to degrade inhibitory IGF-binding proteins (IGFBPs), particularly IGFBP-4, in the pericellular environment (reviewed in Ref. 11). The IGF system is involved in adipose tissue development, although its roles remain to be defined (reviewed in Refs. 3 and 6). IGF-I is expressed both in the stromal-vascular portion, which contains adipocyte precursors, as well as in mature adipocytes. Adipose tissue-derived IGF-I appears to act primarily in an autocrine/paracrine manner to regulate adipose tissue development, and undifferentiated preadipocytes have especially high levels of IGF-I receptor. In in vitro systems, differentiation is initiated by exposure to IGF-I or high concentrations of insulin, the latter acting as a surrogate IGF-I receptor activator. In addition, IGF-I can influence insulin signaling through the insulin receptor (2, 22). Therefore, regulation of IGF action by PAPP-A in a depot-specific manner could be highly relevant for adipose tissue function. To begin to address this issue, we determined 1) fat depot-specific expression of PAPP-A in the mouse, 2) the impact of PAPP-A gene deletion on adipocyte size and number in the various fat depots in mice on a high-fat diet (HFD), and 3) the effect of changes in adipocyte phenotype on insulin sensitivity.
A better understanding of the factors regulating fat tissue development in the different adipose tissue depots could have major implications for potential treatment and/or prevention strategies for obesity and obesity-related morbidities.
MATERIALS AND METHODS
Mice.
Wild-type (WT) and PAPP-A knockout (KO) mice on a mixed C57Bl/6, 129 background were obtained from heterozygous breeding as described previously (13). Littermates were fed a HFD [21% by weight (42% of calories) fat and 0.15% by weight cholesterol] obtained from Harland Tekland (South Easton, MA) starting at 1 wk postweaning and continuing for 3, 10, and 20 wk. Another two groups of WT and PAPP-A KO mice were maintained on standard chow diet for 10 wk.
All procedures involving animals complied with the standards stated in the Guide for the Care and Use of Laboratory Animals and were approved by Mayo Clinic's Institutional Animal Care and Use Committee.
Fat cell size and number.
At harvest, adipose tissue depots (inguinal, subscapular, perigonadal, perirenal, mesenteric, brown, and pericardial) were individually excised and weighed. A portion of each depot was snap-frozen for RNA analysis, fixed [in PBS containing 2.0% formaldehyde, 0.2% glutaraldehyde, 10 min, room temperature] for imaging, and prepared for cell size determination. Adipocyte area was measured as previously described (31). Fat tissues were washed two times in PBS and gently compressed between two glass slides, and photographs from at least five random fields were taken at an original magnification of ×20 using an inverted Nikon microscope (Eclipse Ti200–300; Melville, NY) equipped with a CCD. The cell area of every clearly visible adipocyte within each photograph was measured using Nikon NIS software. The number of fat cells in adipose tissue was obtained by dividing total depot weight by mean fat cell volume times the density of triolein (33).
RNA isolation and real-time PCR.
Adipose tissue depots were rapidly isolated, minced in Trizol (Life Technologies, Carlsbad, CA), and frozen at −80°C. Once thawed, samples were homogenized by passing fat tissue through a 21-gauge needle several times. Total RNA was then isolated from each fat depot, reverse transcribed with the SuperScript III First-Strand Synthesis System (Life Technologies), and evaluated by quantitative real-time PCR using the iCycler iQ5 Detection System with iQ SYBR green PCR Master Mix (Bio-Rad, Hercules, CA). Amplification plots were analyzed with iQ5 Optical System Software version 2.1 (Bio-Rad). Relative quantification, fold changes, and statistical significance of gene expression were determined by the Pfaffl method using the relative expression software tool (REST) 2009 (QIAGEN, Valencia, CA). Primer sequences used for measurement of mRNA expression of mouse IGF system components (PAPP-A, IGF-I, IGF-I-receptor, IGFBP-4), macrophages (F4/80), cytokines [interleukin (IL)-10, IL-6, tumor necrosis factor-α (TNFα)], adiponectin (AdipoQ), fatty acid synthase, and reference genes (TATA box-binding protein, ribosomal protein L22) are listed in Table 1.
Table 1.
Primer sequences for real-time PCR
| Gene | NCBI Accession No. | Forward (5′ to 3′) | Reverse (5′ to 3′) | Size |
|---|---|---|---|---|
| PAPP-A | NM_021362.1 | gccgtgggagcaatatc | gatggcacactctgaccctat | 164 |
| IGF-I | NM_010512.4 | acctcttcccacgtagctca | ttgctcttaaggaggccaaa | 119 |
| IGF-IR | NM_010513.2 | gcctcctgtacctcagtgga | aagggttcgggtaaaggaaa | 134 |
| IGFBP-4 | NM_010517.3 | ccccattctggtccctattt | tctggcttgcctggatagat | 173 |
| F4/80 | NM_010130.4 | ctttggctatgggcttccagtc | gcaaggaggacagagtttatcgtg | 165 |
| IL-6 | NM_031168.1 | cccaacagacctgtctatacca | ggcaaatttcctgattatatcc | 208 |
| IL-10 | NM_010548.2 | gctcttactgactggcatgag | cgcagctctaggagcatgtg | 105 |
| TNF-α | NM_013693.2 | ttgcctcctcttttgcttatg | gggagcagaggttcagtgat | 110 |
| AdipoQ | NM_009605.4 | tgttcctcttaatcctgccca | ccaacctgcacaagttccctt | 104 |
| FASn | NM_007988.3 | tgagcgcacctttgatgacatcgt | atgccgtcaggtttcagtccca | 104 |
| TBP | NM_013684.3 | ctcagttacaggtggcagca | cagcacagagcaagcaactc | 120 |
| RPL22 | NM_009079.2 | ggcggaggagtcgtgacc | ctctctttgctgttggcgaca | 158 |
PAPP-A, pregnancy-associated plasma protein-A; IGF-I, insulin-like growth factor-I; IGF-IR, insulin-like growth factor-I receptor; IGFBP, insulin-like growth factor-binding protein; F4/80, macrophages; IL, interleukin; TNF-α, tumor necrosis factor-α; AdipoQ, adiponectin; FASn, fatty acid synthase; TBP, TATA box-binding protein; RPL22, ribosomal protein L22.
Lipid uptake.
After 5 wk on HFD, mice were administered [3H]triolein (Perkin-Elmer, Waltham, MA) by gavage (0.05 μCi in olive oil), and 8 h later blood was collected, and fat depots, liver, and intestine were harvested and weighed. Lipid was extracted, dried, and weighed as previously described (24), and radioactivity was assessed by scintillation counting.
Glucose tolerance and insulin sensitivity.
Glucose tolerance was assessed following a 6-h fast by measuring blood glucose concentrations before (time 0) and 15, 30, 60, 90, and 120 min after an intraperitoneal bolus of glucose (0.5–1.5 g/kg). Following a recovery period of at least 7 days, insulin sensitivity was assessed following a 4-h fast by measuring glucose concentrations before (time 0) and 15, 30, 60, 90, and 120 min after an intraperitoneal bolus of insulin (0.50–0.75 mU/g). In another study, 6-h fasted mice were given an intraperitoneal bolus of insulin (0.75 mU/g), and after 15 min mesenteric and inguinal fat were harvested and put into M-Per protein extraction buffer (Thermo Scientific, Rockford, IL) with freshly added protease inhibitors (complete miniprotease inhibitor cocktail tablet; Roche Diagnostics, Indianapolis, IN) and phosphatase inhibitors (2 mM sodium orthovanadate, 10 mM pyrophosphate, 40 mM β-glycerol phosphate, 10 mM sodium fluoride; Sigma, St. Louis, MO). Tissues were minced on ice and stored frozen overnight for subsequent immunoblotting.
Immunoblotting.
Tissue samples were sonicated for 5 s and then centrifuged at 14,000 rpm for 10 min at 40C. Forty micrograms of protein were separated by 10% SDS-PAGE and transferred to PVFD. Filters were preblocked with 5% milk/Tris-buffered saline, 0.1% Tween 20, and then incubated overnight at 40C with primary antibody for total AKT (Cell Signaling Technology, Danvers, MA,) or AKT phosphorylated at Ser473 (Novus Biologicals, Littleton, CO). Secondary antibody was horseradish peroxidase-conjugated goat anti-rabbit (Jackson ImmunoResearch Laboratories, West Grove, PA). Antigen-antibody reactions were visualized using an enhanced chemiluminescent detection system (GE Healthcare, Cleveland, OH). KODAK Image Station 4000R Digital Imaging System (Rochester, NY) was used for capture and analyses.
Statistical evaluation.
Results are presented as means ± SE. ANOVA and post hoc t-tests were performed for multiple comparisons. Unpaired Student's t-tests were used to compare data from WT and PAPP-A KO mice. Statistical significance was set at P < 0.05.
RESULTS
PAPP-A expression differs among mouse fat depots.
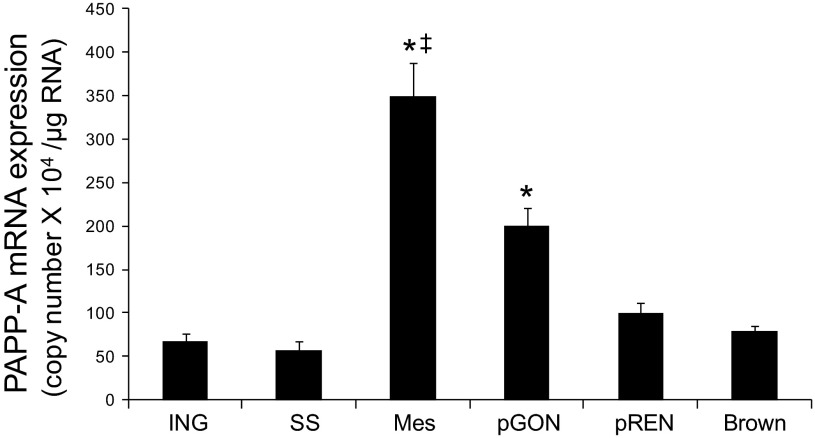
To determine if fat depot PAPP-A expression varies among fat depots in mice, as in humans, we assessed PAPP-A mRNA expression in six fat depots (inguinal, subscapular, perirenal, perigonadal, mesenteric, brown) in chow-fed WT mice. Inguinal and subscapular fat are the main subcutaneous fat depots in mice. Perirenal fat is intra-abdominal but extraperitoneal. Mesenteric is the true visceral fat depot in mice, i.e., intraperitoneal with portal vein drainage; perigonadal fat is also intraperitoneal (29). Brown adipose tissue was included to compare with the white adipose tissues. As shown in Fig. 1, PAPP-A was most highly expressed in the mesenteric fat depot of the mouse; it was fivefold higher in mesenteric fat than in subcutaneous, perirenal, or brown fat. PAPP-A expression in perigonadal fat was also increased, although not to the same extent as in mesenteric fat. Thus, PAPP-A shows fat depot-specific expression in mice, with the highest level of expression in visceral adipose tissue, consistent with the pattern in humans.
Fig. 1.
Pregnancy-associated plasma protein-A (PAPP-A) expression in fat depots of mice. PAPP-A expression was assessed by real-time PCR as described in materials and methods. Results are means ± SE; n = 7–16 mice. ING, inguinal; SS, subscapular; Mes, mesenteric; pGON, perigonadal; pREN, perirenal. *Significantly different from ING, P < 0.05. ‡Significant difference between Mes and pGON, P < 0.05.
Effect of HFD on whole fat depot weights, adipocyte cell size, and adipocyte number.
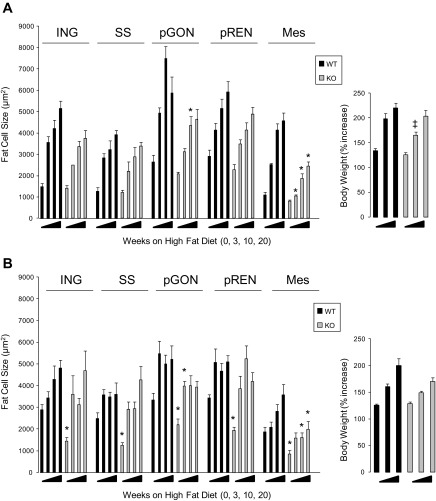
To define the impact of PAPP-A deletion on fat depot weights and fat cell sizes and numbers in response to high-fat feeding, we studied male and female KO and WT mice on a HFD or normal chow. At 7 wk of age, WT and PAPP-A KO mice were switched to a HFD for 3, 10, or 20 wk or maintained on normal chow diet for 10 wk. Both WT and PAPP-A KO mice gained weight on HFD. Only in males at 10 wk of HFD was there a significant difference between groups (Fig. 2). Data for total fat depot weight (adipocytes and vascular-stroma), expressed as percentage of body weight, are shown in Table 2. In male mice, there were no significant differences in depot weights between WT and PAPP-A KO male mice on chow diet (0 wk on HFD), except for the perigonadal fat. There was an increase in depot weight with HFD to different extents in the different depots. The greatest weight gain was in the inguinal depot, with little or no increase in brown fat. Interestingly, there was a significant decrease in mesenteric and pericardial fat in PAPP-A KO compared with WT mice fed HFD for 20 wk. In general, fat depots in female WT and PAPP-A KO mice showed similar responses to HFD as male mice, especially in regard to suppressed weight gain in mesenteric and pericardial depots at 20 wk. However, female PAPP-A KO mice had decreased white adipose depot weights on chow diet compared with WT.
Fig. 2.
Time course of body weight (right) and depot-specific fat cell size changes (left) in male (A) and female (B) wild-type (WT) and PAPP-A knockout (KO) mice on high-fat diet (HFD). Results are means ± SE; n = 8–12. *Significantly different from 10 wk chow diet (0 wk HFD), P < 0.05. ‡Significant difference between WT and PAPP-A KO.
Table 2.
Fat depot weights of WT and PAPP-A KO Mice
| Depot Weight as %Body Wt |
||||
|---|---|---|---|---|
| Males |
Females |
|||
| Weeks on HFD | WT | KO | WT | KO |
| ING | ||||
| 0 | 1.1 ± 0.16 | 1.2 ± 0.13 | 2.6 ± 0.19 | 1.5 ± 0.17* |
| 10 | 4.2 ± 0.25 | 3.7 ± 0.26 | 3.7 ± 0.14 | 3.9 ± 0.24 |
| 20 | 5.2 ± 0.28 | 4.6 ± 0.25 | 4.3 ± 0.23 | 4.1 ± 0.34 |
| SS | ||||
| 0 | 0.8 ± 0.12 | 0.8 ± 0.13 | 1.9 ± 0.26 | 1.0 ± 0.23* |
| 10 | 2.3 ± 0.16 | 2.6 ± 0.25 | 2.3 ± 0.13 | 2.5 ± 0.28 |
| 20 | 2.5 ± 0.27 | 3.0 ± 0.18 | 2.3 ± 0.12 | 2.9 ± 0.26 |
| pGON | ||||
| 0 | 2.4 ± 0.26 | 1.6 ± 0.17* | 4.1 ± 0.38 | 1.6 ± 0.23* |
| 10 | 6.1 ± 0.31 | 5.5 ± 0.34 | 6.3 ± 0.53 | 4.1 ± 0.16* |
| 20 | 6.4 ± 0.52 | 6.4 ± 0.29 | 9.9 ± 0.81 | 5.7 ± 0.77* |
| pREN | ||||
| 0 | 0.7 ± 0.85 | 0.5 ± 0.04 | 1.7 ± 0.41 | 0.7 ± 0.20 |
| 10 | 1.4 ± 0.12 | 1.5 ± 0.09 | 1.3 ± 0.08 | 1.3 ± 0.12 |
| 20 | 1.7 ± 0.16 | 1.7 ± 0.10 | 1.5 ± 0.10 | 1.5 ± 0.17 |
| Mes | ||||
| 0 | 1.1 ± 0.14 | 1.1 ± 0.07 | 1.8 ± 0.30 | 1.0 ± 0.17* |
| 10 | 2.6 ± 0.19 | 2.2 ± 0.12 | 1.9 ± 0.12 | 1.8 ± 0.08 |
| 20 | 2.5 ± 0.12 | 1.9 ± 0.22* | 2.3 ± 0.17 | 1.8 ± 0.14* |
| Brown | ||||
| 0 | 0.5 ± 0.04 | 0.5 ± 0.03 | 0.4 ± 0.04 | 0.5 ± 0.04 |
| 10 | 1.0 ± 0.13 | 0.8 ± 0.07 | 0.6 ± 0.03 | 0.6 ± 0.04 |
| 20 | 0.8 ± 0.05 | 0.8 ± 0.07 | 0.5 ± 0.03 | 0.5 ± 0.06 |
| pCard | ||||
| 0 | ||||
| 10 | ||||
| 20 | 0.3 ± 0.03 | 0.1 ± 0.01* | 0.2 ± 0.02 | 0.1 ± 0.0* |
Depot weights, expressed as %body wt, are presented as means ± SE; n = 8–12 mice. WT, wild type; KO, knockout; ING, inguinal; SS, subscapular; pGON, perigonadal; pREN, perirenal; Mes, mesenteric; pCard, pericardial. Mice at 7 wk of age were fed a high-fat diet (HFD) for 10 and 20 wk. Mice of the same age and fed a chow diet for 10 wk are referred to as 0 wk on HFD.
Significant difference between WT and PAPP-A KO, P < 0.05.
Adipocyte size and number were determined for each of the five depots (brown fat and pericardial fat were not assessed beyond total depot weight). In male mice (Fig. 2A), there were no significant differences in fat cell size in any of the depots between WT and PAPP-A KO mice on chow diet. Fat cell size increased in each depot in WT mice fed a HFD (compared with chow diet). This increase in fat cell size in inguinal, subscapular, and perirenal depots was similar in WT and PAPP-A KO mice. However, the diet-induced increase in fat cell size was significantly blunted in mesenteric fat of PAPP-A KO compared with WT mice at each time point. There were also statistically significant differences in perigonadal fat cell size between male WT and PAPP-A KO mice after 10 wk on a HFD. Similar effects of HFD were seen in female mice (Fig. 2B). However, there were significant differences in fat cell size between female WT and PAPP-A KO mice on chow diet, suggesting sexual dimorphism in PAPP-A's effect on adipose tissue development. Nonetheless, there was no difference in sizes of adipocytes from inguinal, subscapular, or perirenal depots between WT and PAPP-A KO mice on HFD. The increase in fat cell size was inhibited in mesenteric fat at 10 and 20 wk and in perigonadal fat at 3 wk on HFD. Adipocyte number in mice on the chow diet varied, with the lowest number being in the perirenal depot and the highest in the mesenteric depot. This was true for WT and PAPP-A KO mice and both males and females. There were no significant differences in adipocyte number between WT and PAPP-A KO mice on HFD in any of the depots (Table 3). However, there were significant differences in adipocyte number in perigonadal depots between WT and PAPP-A KO mice on chow diet (and in perirenal depots in female mice), which may contribute to the differences observed in total depot weight (see Table 2). The formula employed for estimating fat cell number (total depot weight divided by mean fat cell volume corrected for lipid density) may not apply to mesenteric fat, where the stromal-vascular compartment contributes significantly to depot weight, and this may be disproportionate in mesenteric fat of PAPP-A KO mice.
Table 3.
Adipocyte number in WT and PAPP-A KO mice
| Cell Number (×105) |
||||
|---|---|---|---|---|
| Males |
Females |
|||
| Weeks on HFD | WT | KO | WT | KO |
| ING | ||||
| 0 | 100 ± 8 | 80 ± 7 | 85 ± 9 | 78 ± 9 |
| 10 | 121 ± 14 | 82 ± 13 | 77 ± 9 | 63 ± 9 |
| 20 | 109 ± 11 | 115 ± 18 | 76 ± 9 | 63 ± 10 |
| SS | ||||
| 0 | 79 ± 10 | 69 ± 8 | 89 ± 6 | 80 ± 12 |
| 10 | 95 ± 11 | 76 ± 11 | 50 ± 6 | 58 ± 8 |
| 20 | 82 ± 7 | 72 ± 9 | 77 ± 12 | 51 ± 5 |
| pGON | ||||
| 0 | 83 ± 15 | 48 ± 4* | 96 ± 16 | 43 ± 9* |
| 10 | 73 ± 11 | 95 ± 28 | 97 ± 18 | 58 ± 6 |
| 20 | 99 ± 9 | 119 ± 24 | 127 ± 10 | 105 ± 26 |
| pREN | ||||
| 0 | 18 ± 1 | 14 ± 2 | 24 ± 3 | 13 ± 3* |
| 10 | 29 ± 3 | 24 ± 3 | 21 ± 3 | 15 ± 2 |
| 20 | 33 ± 7 | 25 ± 4 | 26 ± 3 | 24 ± 5 |
| Mes | ||||
| 0 | 134 ± 28 | 131 ± 15 | 127 ± 24 | 122 ± 18 |
| 10 | 79 ± 12 | 113 ± 11 | 85 ± 7 | 101 ± 14 |
| 20 | 68 ± 6 | 89 ± 8 | 79 ± 21 | 106 ± 16 |
Adipocyte nos., calculated as described in materials and methods, are presented as means ± SE of n = 8–12 mice. Mice at 7 wk of age were fed a HFD for 10 and 20 wk. Mice of the same age and fed a chow diet for 10 wk are referred to as 0 wk on HFD.
Significant difference between WT and PAPP-A KO, P < 0.05.
To assess depot-specific fat cell size further, taking into account the variability within each mouse as well as between WT and PAPP-A KO mice, we analyzed the frequency distribution of fat cell size. Data from male WT and PAPP-A KO mice after 10 wk on chow or HFD are shown in Fig. 3. On chow diet, fat cell size distribution in all the depots was similar between WT and PAPP-A KO mice. Peak adipocyte size frequency for inguinal, subscapular, mesenteric, and perigonadal fat appeared at ∼1,500, 1,500, 1,000, and 2,000 μm2, respectively. Fat cell size was increased with HFD equivalently in inguinal and subscapular depots from WT and PAPP-A KO mice with peak frequency at 3,000–3,500 and 2,000–2,500 μm2, respectively (Fig. 3, A–D). In the mesenteric depot, increases in fat cell size were blunted in the PAPP-A KO mice compared with WT (Fig. 3, E and F). Thus, peak frequency in WT mesenteric fat occurred at ∼4,000 μm2 and in PAPP-A KO mesenteric fat at 2,000 μm2. There was greater variability in size distribution in gonadal fat, but there also appeared to be suppression of diet-induced fat cell enlargement in this depot (Fig. 3, G and H).
Fig. 3.
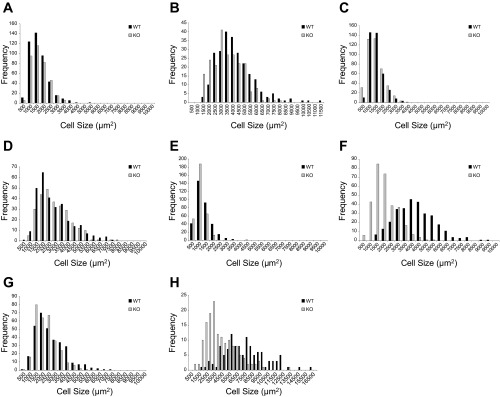
Fat cell size distribution and frequency. Fat cell size in ING (A and B), SS (C and D), Mes (E and F), and pGON (G and H) depots from male WT and PAPP-A KO mice on 10 wk of chow (A, C, E, and G) or HFD (B, D, F, and H).
Thus, there appears to be a preferential impact of PAPP-A deficiency on visceral fat cell size in mice on a HFD.
Gene expression.
To test if expression of IGF system components, macrophages, cytokines, or adipokines varies among inguinal, perigonadal, and mesenteric fat, mRNA levels were assayed in these fat depots from male mice that had been on a HFD for 10 wk (Table 4). There were no significant differences in IGF-I, IGF-I receptor, or IGFBP-4 expression levels between WT and PAPP-A KO mice in any of the depots. Expression of a macrophage marker (F4/80), as well as IL-6 and IL-10, also showed no significant differences. Furthermore, there were no obvious differences in macrophage immunostaining intensities or patterns between PAPP-A and WT mice (data not shown). TNF-α mRNA in perigonadal tissue was significantly suppressed in PAPP-A KO compared with WT mice on HFD. Adiponectin mRNA was significantly increased in perigonadal and mesenteric fat of PAPP-A KO mice on HFD. In addition, there was no significant difference in fatty acid synthase expression in fat depots from WT and PAPP-A KO mice.
Table 4.
Gene expression in adipose tissue from WT and PAPP-A KO mice 10 wk on HFD
| PAPP-A KO/WT |
|||
|---|---|---|---|
| ING | pGON | Mes | |
| IGF System | |||
| IGF-I | 0.9 | 0.8 | 1.0 |
| IGF-IR | 1.1 | 1.1 | 1.1 |
| IGFBP-4 | 0.9 | 0.9 | 1.1 |
| Macrophages/cytokines | |||
| F4/80 | 0.9 | 0.8 | 1.6 |
| IL-6 | 1.1 | 0.7 | 1.1 |
| IL-10 | 1.0 | 1.0 | 0.6 |
| TNFα | 0.8 | 0.7* | 0.7 |
| Metabolism | |||
| Adiponectin | 1.1 | 1.6* | 1.5* |
| FASn | 1.0 | 0.4 | 0.8 |
Results are presented as the ratio PAPP-A KO/WT, n = 7 mice. Gene expression was assessed by real-time PCR as described in materials and methods.
Significant difference between WT and PAPP-A KO as determined by the Pfaff1 method, P < 0.05.
Lipid uptake.
Besides de novo lipogenesis, changes in fat cell size can result from changes in lipid uptake and/or lipolysis. To determine depot-specific lipid uptake, we administered [3H]triolein by gavage to WT and PAPP-A KO mice. After 8 h, inguinal, subscapular, perirenal, perigonadal, and mesenteric fat depots were harvested, and lipid was extracted from the tissues. Tritium counts in these extracts were measured and expressed as percent uptake (Table 5). Uptake was similar in inguinal, subscapular, perirenal, and perigonadal fat depots from WT and PAPP-A KO mice. However, there was a significant decrease in lipid uptake in mesenteric fat from PAPP-A KO compared with WT mice.
Table 5.
Lipid uptake
| Percent Uptake |
||
|---|---|---|
| WT | PAPP-A KO | |
| ING | 18 ± 0.8 | 17 ± 2.5 |
| SS | 16 ± 1.1 | 16 ± 1.7 |
| pREN | 19 ± 0.5 | 21 ± 2.4 |
| pGON | 11 ± 0.8 | 10 ± 1.2 |
| Mes | 10 ± 0.4 | 7 ± 0.5* |
Values are means ± SE; n = 5 mice. Male WT and PAPP-A KO mice 5 wk on HFD were administered [3H]triolein by gavage. Eight hours later, tissues were harvested, lipid was extracted, and radioactivity was assessed as described in materials and methods.
Significant difference between WT and PAPP-A KO, P = 0.002.
Glucose tolerance and insulin sensitivity.
To determine the effect of PAPP-A deletion on insulin sensitivity, glucose tolerance and insulin sensitivity tests were performed in WT and PAPP-A KO mice after 20 wk on HFD (Fig. 4). Although there appeared to be a slight nonsignificant increase in whole body glucose tolerance in PAPP-A KO mice, there was no significant difference in insulin sensitivity between the two groups of mice.
Fig. 4.
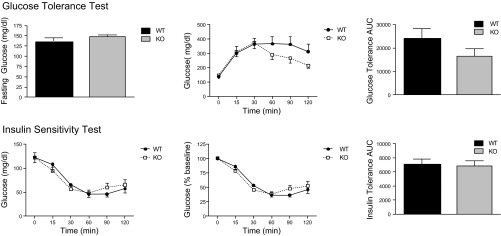
Glucose tolerance and insulin sensitivity testing in male WT and PAPP-A KO mice, 20 wk on HFD. See materials and methods for details about the tests. Results are means ± SE; n = 8.
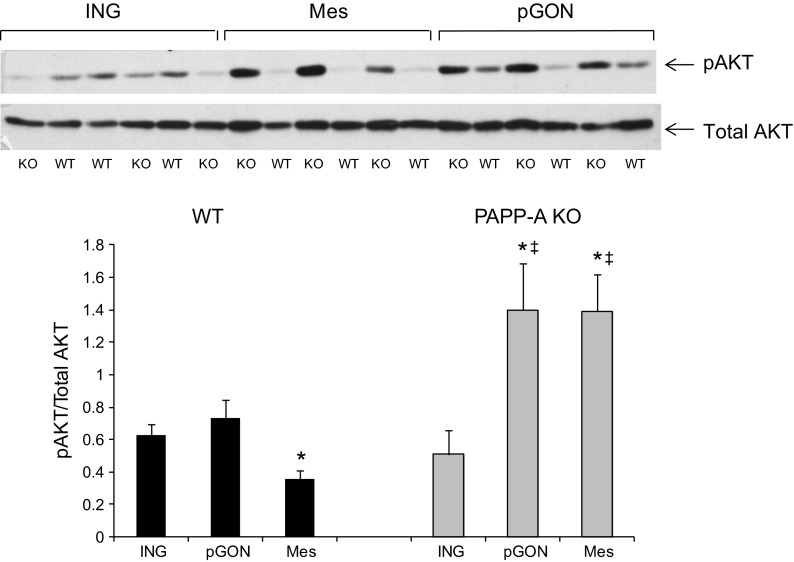
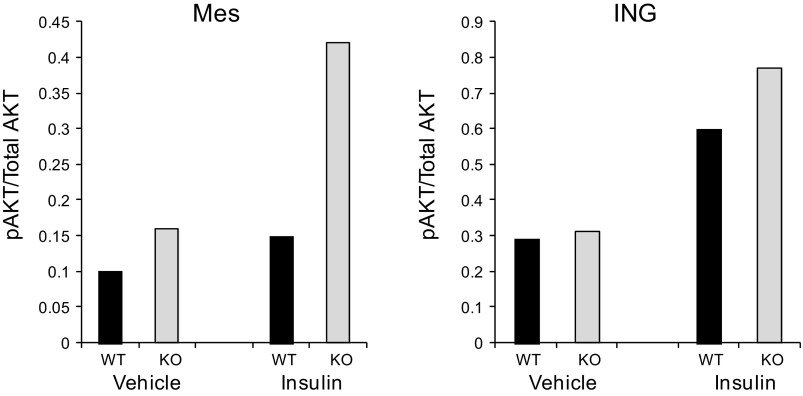
Because these whole body glucose tolerance and insulin sensitivity tests reflect overall contributions of skeletal muscle, liver, and brain as well as all adipose tissue, we looked at fat depot-specific IGF-I/insulin receptor signaling via phosphorylation of AKT on Ser47 (8, 11). After 20 wk on HFD, basal phosphorylated AKT (pAKT) was twofold greater in inguinal vs. mesenteric fat of WT mice. There was no difference between inguinal and perigonadal fat. In contrast, basal pAKT was threefold higher in mesenteric vs. inguinal fat in PAPP-A KO mice (Fig. 5). AKT phosphorylation was significantly greater in mesenteric and perigonadal from PAPP-A KO compared with WT mice. Furthermore, insulin-stimulated pAKT in mesenteric adipose tissue appeared to be greater in PAPP-A KO than in WT mice; there was no apparent difference in insulin response in inguinal fat from the same mice (Fig. 6).
Fig. 5.
AKT phosphorylation in fat depots from WT and PAPP-A KO mice. Fats from ING, pGON, and Mes depots were immunoblotted for phosphorylated (p) and total AKT, as described in materials and methods. Results, presented as a ratio of pAKT/total AKT, are means ± SE; n = 3. *Significantly different from ING, P < 0.05. Significant difference between WT and PAPP-A KO.
Fig. 6.
Insulin-stimulated AKT phosphorylation in WT and PAPP-A KO mice. Fifteen minutes after ip insulin administration, fats from Mes and ING depots were harvested for immunoblotting. Results, presented as a ratio of pAKT/total AKT, are the averages of two mice.
DISCUSSION
There were three major findings of this study. First, PAPP-A was differentially expressed in the various adipose tissue depots of mice, with the highest expression found in visceral adipose tissue. This pattern of expression is similar to what has been reported for preadipocytes from human and baboon fat depots (16, 28, 30) and human adipose tissues (our unpublished data), supporting the mouse as a suitable mammalian model for investigating the role of depot-specific PAPP-A expression. Second, PAPP-A deficiency (PAPP-A KO mice) preferentially restrained enlargement of visceral fat cells in response to a HFD. This may be clinically relevant because increased visceral fat is more strongly associated with diabetes, cardiovascular disease, and metabolic disease than increased subcutaneous fat or general obesity (18, 23, 29, 34). Indeed, subcutaneous fat has been shown to have positive health benefits (32). Third, reduced visceral fat cell enlargement in PAPP-A KO mice on a HFD was associated with enhanced local insulin-sensitive signaling.
A strong point of this study was the evaluation of multiple fat depots of the mouse, which included the mesenteric fat depot. Few studies in rodents specifically look at mesenteric fat; rather they use one or two more readily accessible fat depots. For PAPP-A-deficient mice, most of the impact was on mesenteric fat, and changes in mesenteric fat were sometimes, but not always, reflected in intra-abdominal perigonadal fat and were very different from subcutaneous fat. Berryman et al. (5) emphasized the importance of comparing the impact of growth hormone on multiple fat pads, including mesenteric, in mice. Catalano et al. (8) also found major differences in the contribution of the mesenteric depot vs. the epididymal depot in the development of insulin resistance in rats.
Increased fat cell size accounts for increases in fat mass in mild obesity, whereas severe obesity also increases the number of fat cells. Extensive prolonged weight gain is required before increases in fat cell number are observed. In this study, up to 20 wk of HFD increased adipocyte cell size but not number. The HFD in this study was 42% of calories as fat, which significantly affected fat depot weight gain and adipocyte enlargement. It also produced a low-grade inflammatory environment in the fat depots (i.e., increased TNF-α mRNA expression, data not shown). However, there did not appear to be major differences between WT and PAPP-A KO mice in any of the fat depots with regard to macrophage accumulation or macrophage “phenotype switching,” which would be associated with changes in F4/80, IL-6, and IL-10 expression (21). Thus, we might propose this to be an appropriate experimental animal model for humans at risk of developing obesity-related disorders if weight gain is not better controlled. On this diet, PAPP-A KO mice had blunted fat cell enlargement in visceral fat depots, but not in any of the subcutaneous fat depots. Also, adiponectin expression was increased in the visceral fat depots. Adiponectin has been shown to have anti-inflammatory properties and to be negatively associated with obesity and insulin sensitivity (35).
What might be the mechanism(s) underlying the depot-specific effect of PAPP-A deficiency to reduce fat cell size? Fat cell enlargement can be the result of increased de novo lipogenesis and/or increased uptake and storage of dietary lipid. The lack of differences in fatty acid synthase expression between WT and PAPP-A KO mice in any of the depots examined suggests that altered lipogenesis is unlikely to explain differences in fat cell size in these experimental animals. On the other hand, in vivo [3H]triolein uptake was significantly reduced in mesenteric fat of PAPP-A KO mice, and not in any of the other depots. Thus, the reduced enlargement of mesenteric fat cell size in PAPP-A KO mice on HFD is due, at least in part, to reduced lipid uptake. It is of interest that Tang et al. (26) showed that PAPP-A decreased cholesterol efflux in activated macrophages and that this effect was IGF mediated. In many respects, preadipocytes are similar to macrophages (29). Additional effects of PAPP-A deficiency to increase lipid release and/or lipolysis, particularly in mesenteric fat, may also contribute to differential effects on adipocyte size. However, any depot-specific study of PAPP-A and lipolysis would have to be approached using in vitro systems, which may not represent the interactive in vivo setting.
There were two additional observations of note. In the initial studies of depot-specific PAPP-A expression, brown fat was included in the analysis to compare with white adipose tissue. Expression was low, similar to subcutaneous fat. High-fat feeding and PAPP-A deficiency had little or no effect on the brown fat depot in the mouse. Thus, PAPP-A does not appear to have a major role in the thermogenesis/metabolism of brown fat (25). However, an interesting and potentially clinically relevant observation that warrants follow-up was that PAPP-A KO mice 20 wk on HFD had significantly decreased pericardial fat. This fat has been associated with cardiovascular disease (15). Increased PAPP-A is also associated with cardiovascular disease (4, 7), and a mouse model prone to development of atherosclerosis was resistant to atherosclerotic plaque progression in the absence of PAPP-A (17). In a recent study, elevated serum PAPP-A correlated with abnormal glucose metabolism, upper body obesity, and increased cardiovascular risk factors (20).
There was no significant effect of PAPP-A deficiency on whole body glucose tolerance and insulin sensitivity with high-fat feeding in this study, as was also seen in our previous study with chow-fed mice (14). However, skeletal muscle is the primary site of insulin-mediated glucose disposal, and the liver too plays a major role. Thus, fat depot-specific contributions are likely to be masked using these methodologies unless there is severe obesity and insulin resistance. We used an alternative in vivo approach evaluating depot-specific AKT phosphorylation on Ser473, a measure of insulin/IGF-I receptor signaling (9, 12). Basal levels of pAKT were significantly greater (2-fold) in inguinal vs. mesenteric fat in WT mice. This is consistent with several cell culture studies indicating that subcutaneous preadipocytes have greater capacity for proliferation, differentiation, and survival than visceral preadipocytes and that IGF-I is a critical mediator of these adipogenic pathways (6, 29). Cleveland-Donovan et al. (9) showed that IGF-I-stimulated pAKT Ser473 was reduced in preadipocytes from visceral fat compared with subcutaneous fat. However, depot-specific AKT phosphorylation was just the opposite in PAPP-A KO mice, with basal levels of pAKT being significantly (3-fold) greater in mesenteric compared with inguinal fat. These findings might at first seem counterintuitive since PAPP-A deficiency should reduce local IGF signaling without any changes in gene expression of ligand, receptor, or inhibitory IGFBP (11). However, insulin receptors mediating metabolic effects are more prominent than IGF-I receptors on mature adipocytes (1, 2, 6). PAPP-A modulation of IGF bioavailability may regulate visceral adipose tissue response to insulin. It can be postulated that IGFs produced at high levels in adipose tissue can blunt insulin signaling through binding site competition (19, 22). PAPP-A deficiency would reduce locally available IGF and thereby reduce the competition. Indeed, insulin-stimulated AKT phosphorylation was greater in mesenteric fat from PAPP-A KO than WT mice in vivo. Improved insulin response to caloric restriction in obese rats was associated with decreased mesenteric fat cell size, and was not seen in the other fat depots (8). Further in vivo studies are necessary to explore this possible interplay.
Agents targeted to specific fat depots would be particularly valuable for the treatment/prevention of obesity. Based on the findings of this study, drugs that are able to selectively inhibit PAPP-A expression or activity may offer a novel form of therapy limiting visceral fat accumulation with favorable effects on metabolic health.
GRANTS
This work was supported by National Institutes of Health Grants R01-AG-028141 (to C. A. Conover) and 1PO1-AG-31736 (Project 4 to J. L. Kirkland) and by the Minnesota Obesity Center Grant DK-50456.
DISCLOSURES
The authors have nothing to disclose.
AUTHOR CONTRIBUTIONS
Author contributions: C.A.C. and S.L.H. conception and design of research; C.A.C. and S.L.H. performed experiments; C.A.C. and S.L.H. analyzed data; C.A.C. interpreted results of experiments; C.A.C. prepared figures; C.A.C., T.T., and J.L.K. drafted manuscript; C.A.C., S.L.H., T.T., and J.L.K. edited and revised manuscript; C.A.C., S.L.H., T.T., and J.L.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Laurie Bale, Jacquelyn Grell, Suban Chakraborty, Sally West, and Tamar Pirtskhalava for excellent technical support.
REFERENCES
- 1.Back K, Arnqvist HJ. Changes in insulin and IGF-I receptor expression during differentiation of human preadipocytes. Growth Horm IGF Res 19: 101–111, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Back K, Brannmark C, Stralfors P, Arnqvist HJ. Differential effects of IGF-I, IGF-II and insulin in human preadipocytes and adipocytes: role of insulin and IGF-I receptors. Mol Cell Endocrinol 339: 130–135, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Baxter RC, Twigg SM. Actions of IGF binding proteins and related proteins in adipose tissue. Trends Endocrinol Metab 20: 49–505, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Bayes-Genis A, Conover CA, Overgaard MT, Bailey KR, Christiansen M, Holmes DR, Jr, Virmani R, Oxvig C, Schwartz RS. Pregnancy-associated plasma protein A as a marker of acute coronary syndromes. N Engl J Med 345: 1022–1029, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Berryman DE, List EO, Palmer AJ, Chung MU, Wright-Piekarski J, Lubbers E, O'Connor P, Okada S, Kopchick JJ. Two-year body composition analyses of long-lived GHR null mice. J Gerontol Biol Sci 65A: 31–40, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bluher S, Kratzsch J, Kiess W. Insulin-like growth factor I, growth hormone and insulin in white adipose tissue. Best Pract Res Clin Endocrinol Metab 19: 577–587, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Bonaca MP, Scirica BM, Sabatine MS, Jarolim P, Murphy SA, Chamberlin JS, Rhodes DW, Southwick PC, Braunwald E, Morrow DA. Prospective evaluation of pregnancy-associated plasma protein-A and outcomes in patients with acute coronary syndromes. J Am Coll Cardiol 60: 332–338, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Catalano KJ, Stefanovski D, Bergman RN. Critical role of the mesenteric depot versus other intra-abdominal adipose depots in the development of insulin resistance in young rats. Diabetes 59: 1416–1423, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cleveland-Donovan K, Maile LA, Tsiaras WG, Tchkonia T, Kirkland JL, Boney CM. IGF-I activation of the AKT pathway is impaired in visceral but not subcutaneous preadipocytes from obese subjects. Endocrinology 151: 3752–3763, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins FS. A view of the U.S. obesity epidemic. NIH Director's Blog November 7, 2012 [Google Scholar]
- 11.Conover CA. Key questions and answers about pregnancy-associated plasma protein-A. Trends Endocrinol Metab 23: 242–249, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conover CA, Bale LK, Durham SK, Powell DR. Insulin-like growth factor (IGF) binding protein-3 potentiation of IGF action is mediated through the phosphatidylinositol-3-kinase pathway and is associated with alteration in protein kinase B/AKT sensitivity. Endocrinology 141: 3098–3103, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Conover CA, Bale LK, Overgaard MT, Johnstone EW, Laursen UH, Fuchtbauer EM, Oxvig C, van Deursen J. Metalloproteinase pregnancy-associated plasma protein A is a critical growth regulatory factor during fetal development. Development 131: 1187–1194, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Conover CA, Mason MA, Levine JA, Novak CM. Metabolic consequences of pregnancy-associated plasma protein-A deficiency in mice: exploring possible relationship to the longevity phenotype. J Endocrinol 198: 599–605, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding J, Hsu FC, Harris TB, Liu Y, Kritchevsky SB, Szklo M, Ouyang P, Espeland MA, Lohman KK, Criqui MH, Allison M, Bluemke DA, Carr JJ. The association of pericardial fat with incident coronary heart disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr 90: 499–504, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gealekman O, Guseva N, Hartigan C, Apotheker S, Gorgoglione M, Gurav K, Tran KV, Straubhaar J, Nicoloro S, Czech JP, Thompson M, Perugini RA, Corvera S. Depot-specific differences and insufficient subcutaneous adipose tissue angiogenesis in human obesity. Circulation 123: 186–194, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harrington SC, Simari RD, Conover CA. Genetic deletion of pregnancy-associated plasma protein-A is associated with resistance to atherosclerotic lesion development in apolipoprotein E-deficient mice challenged with a high-fat diet. Circ Res 100: 1696–1702, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Jensen MD. Role of body fat distribution and the metabolic complications of obesity. J Clin Endocrinol Metab 93: S57–S63, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiselyov VV, Versteyhe S, Gauguin L, De Meyts P. Harmonic oscillator model of the insulin and IGF1 receptors' allosteric binding and activation. Mol Systems Biol 5: 243–254, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li L, Ren W, Li J, Liu J, Wang L, Zheng X, Liu D, Li S, Souvenir R, Tang J. Increase in serum pregnancy-associated plasma protein-A is correlated with increase in cardiovascular risk factors in adult patients with growth hormone deficiency. Endocrine 42: 375–381, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 117: 175–184, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Rourke RW, Phillips SA, Kuhn S, Purnell JQ, Ma X, Adamo ML, Roberts CT., Jr IGF system expression in human adipose tissue. Presented at Endo 2007. In: The 89th Ann Meet Endocr Soc Program and Abstracts Book. Chevy Chase, MD: Endocrine Society Press, Page 156, Abstract P1–8, 2007 [Google Scholar]
- 23.Perrini S, Leonardini A, Laviola L, Giorgino F. Biological specificity of visceral adipose tissue and therapeutic intervention. Arch Physiol Biochem 114: 277–286, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Romanski SA, Nelson RM, Jensen MD. Meal fatty acid uptake in human adipose tissue: technical and experimental design issues. Am J Physiol Endocrinol Metab 279: E447–E454, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Seale P, Kajimura S, Spiegelman BM. Transcriptional control of brown adipocyte development and physiological function of mice and men. Genes Dev 23: 788–797, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang SL, Chen WJ, Yin K, Zhao GJ, Mo ZC, Lv YC, Ouyang XP, Yu XH, Kuang HJ, Jiang ZS, Fu YC, Tang CK. PAPP-A negatively regulates ABCA1, ABCG1 and SR-B1 expression by inhibiting LXRα through the IGF-I-mediated signaling pathway. Atherosclerosis 222: 344–354, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Tchkonia T, Giorgadze N, Pirtskhalava T, Tchoukalova Y, Karagiannides I, Forse RA, Deponte M, Stevenson M, Guo W, Han J, Waloga G, Lash TL, Jensen MD, Kirkland JL. Fat depot origin affects adipogenesis in primary cultured and cloned human preadipocytes. Am J Physiol Regul Integr Comp Physiol 282: R1286–R1296, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Tchkonia T, Lenburg M, Thomou T, Giorgadze N, Frampton G, Pirtskhalava T, Cartwright A, Cartwright M, Glanagan J, Karagiannides I, Gerry N, Forse A, Tchoukalova Y, Jensen MD, Pothoulakis C, Kirkland JL. Identification of depot-specific human fat cell progenitors through distinct expression profiles and developmental gene patterns. Am J Physiol Endocrinol Metab 292: E298–E307, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Tchkonia T, Thomou T, Zhu Y, Karagiannides I, Pothoulakis C, Jensen MD, Kirkland JL. Mechanisms and metabolic implications of regional differences among fat depots. Cell Metab 17: 644–656, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tchoukalova YD, Nathanielsz PW, Conover CA, Smith S, Ravussin E. Regional variation in adipogenesis and IGF regulatory proteins in the fetal baboon. Biochem Biophys Res Commun 380: 679–683, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tchoukalova YD, Votruba SB, Tchkonia T, Giorgadze N, Kirkland JL, Jensen MD. Regional differences in cellular mechanisms of adipose tissue gain with overfeeding. Proc Natl Acad Sci USA 107: 18226–18231, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tran TT, Yamamoto Y, Gesta S, Kahn CR. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab 7: 410–420, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Villaret A, Galitzky, Decaunes P, Esteve D, Marques MA, Sengenes C, Chiotasso P, Tchkonia T, Lafontan M, Kirkland JL, Bouloumie A. Adipose tissue endothelial cells from obese human subjects: differences among depots in angiogenic, metabolic, and inflammatory gene expression and cellular senescence. Diabetes 59: 2755–2763, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev 21: 697–738, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 7: 941–946, 2001 [DOI] [PubMed] [Google Scholar]