Abstract
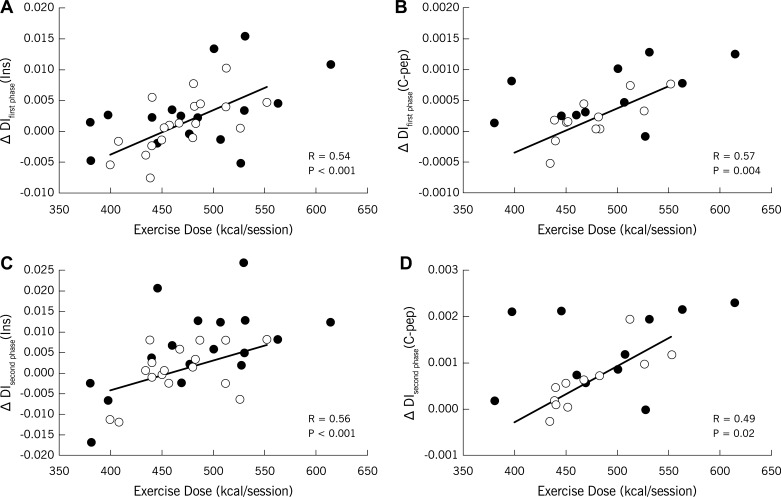
Although some studies suggest that a linear dose-response relationship exists between exercise and insulin sensitivity, the exercise dose required to enhance pancreatic β-cell function is unknown. Thirty-five older obese adults with prediabetes underwent a progressive 12-wk supervised exercise intervention (5 days/wk for 60 min at ∼85% HRmax). Insulin and C-peptide responses to an OGTT were used to define the first- and second-phase disposition index (DI; β-cell function = glucose-stimulated insulin secretion × clamp-derived insulin sensitivity). Maximum oxygen consumption (V̇o2max) and body composition (dual-energy X-ray absorptiometry and computed tomography) were also measured before and after the intervention. Exercise dose was computed using V̇o2/heart-rate derived linear regression equations. Subjects expended 474.5 ± 8.8 kcal/session (2,372.5 ± 44.1 kcal/wk) during the intervention and lost ∼8% body weight. Exercise increased first- and second-phase DI (P < 0.05), and these changes in DI were linearly related to exercise dose (DIfirst phase: r = 0.54, P < 0.001; DIsecond phase: r = 0.56, P = 0.0005). Enhanced DI was also associated with increased V̇o2max (DIfirst phase: r = 0.36, P = 0.04; DIsecond phase: r = 0.41, P < 0.02) but not lower body fat (DIfirst phase: r = −0.21, P = 0.25; DIsecond phase: r = −0.30, P = 0.10) after training. Low baseline DI predicted an increase in DI after the intervention (DIfirst phase: r = −0.37; DIsecond phase: r = −0.41, each P < 0.04). Thus, exercise training plus weight loss increased pancreatic β-cell function in a linear dose-response manner in adults with prediabetes. Our data suggest that higher exercise doses (i.e., >2,000 kcal/wk) are necessary to enhance β-cell function in adults with poor insulin secretion capacity.
Keywords: aging, obesity, insulin resistance, glucose intolerance, type 2 diabetes
approximately 79 million men and women in the US are characterized as having prediabetes, of which up to 30% may develop type 2 diabetes in the next 10 years (1, 3). Although insulin sensitivity is considered a key etiological factor in the progression from prediabetes to type 2 diabetes, many insulin-resistant men and women maintain normoglycemia due to compensatory rises in insulin secretion (17). Thus, preservation of pancreatic β-cell function in response to glucose is fundamental to preventing type 2 diabetes, particularly in obese individuals with impaired glucose tolerance that have lost ∼50–70% of their β-cell function (7). Unfortunately, the optimal exercise prescription for enhancing pancreatic β-cell function is unknown.
Exercise training decreases glucose-stimulated insulin secretion in young (19), middle-aged (9, 33) and older men and women (4, 21). However, the amount of insulin needed to maintain normoglycemia is influenced by the prevailing level of insulin sensitivity. As such, the product of glucose-stimulated insulin secretion and insulin sensitivity [i.e., disposition index (DI)] may provide a more accurate view of pancreatic β-cell function (16). In fact, the DI is considered a better predictor of diabetes development than insulin sensitivity alone (2, 37). β-Cell function is viewed as a two-pool model that is characterized by the readily available pool of insulin that is released upon initial glucose stimulation (1st phase) and the synthesis of new insulin following fluctuations in postprandial glucose (2nd phase) (5). Contrasting studies suggest that moderate- (33) to high-volume (6) exercise augments first-phase pancreatic β-cell function. However, these studies did not characterize second-phase pancreatic function, thereby limiting our understanding of the efficacy of exercise dose on β-cell function across the postprandial period in men and women (6, 33). It has been suggested that, in addition to adequate first-phase pancreatic β-cell function, second-phase pancreatic β-cell function is clinically relevant for maintaining glycemic control (18). Consequently, there is a need to better understand the link between exercise dose and pancreatic β-cell function across the postprandial glucose time frame so that optimal lifestyle programs may be designed for type 2 diabetes prevention. Animal work suggests that exercise enhances GLUT2 transporter content (20), improves Akt signaling and glucokinase activity (10, 20, 22), and raises mitochondrial respiration in pancreatic tissue (10). Despite reports emphasizing that high doses of physical activity are needed to improve insulin sensitivity (6, 11, 24) and cardiometabolic biomarkers (e.g., total cholesterol and/or blood pressure) (13), no intervention has determined the interaction between exercise dose and β-cell function. Therefore, the purpose of this study was to determine the relationship between exercise dose and pancreatic β-cell function in men and women with prediabetes. We hypothesized that higher exercise doses would correlate with increased first- and second-phase β-cell function.
METHODS
Subjects.
Thirty-five older (66.8 ± 0.8 yr) obese (BMI = 35.1 ± 0.7 kg/m2) men (n = 16) and women (n = 19) who were previously involved in obesity-related lifestyle modification studies were included in this study (31). Subjects were recruited via advertisements in the Cleveland, OH, area. All subjects underwent health screenings that included a resting and exercise stress test with 12-lead electrocardiogram, medical history, and physical examination as well as blood and urine chemistry analysis. Subjects were excluded if they smoked, were physically active (>60 min/wk), were weight unstable (>2 kg within the previous 6 mo), had known chronic disease (i.e., renal, liver, heart, etc.), and/or were taking medications known to affect glucose homeostasis. All subjects provided written signed and verbal informed consent, and the Cleveland Clinic Institutional Review Board approved the study.
Metabolic control period.
Subjects underwent a 3-day inpatient stay in our Clinical Research Unit prior to metabolic assessments. Subjects were provided weight maintenance meals [resting metabolic rate × 1.2; ∼55% carbohydrate (CHO), 30% fat, and 15% protein] and refrained from strenuous activity before testing. Respiratory gases (V̇o2 and V̇co2) were analyzed by indirect calorimetry for determination of resting metabolic rate and substrate oxidation (Vmax Encore, Viasys, Yorba Linda, CA) before and after training, as described previously (35).
Body composition and cardiometabolic risk factors.
Weight was assessed on a digital platform with minimal clothing, and height was recorded on a stadiometer. Computerized tomography (Siemens Medical Solutions, Malvern, PA) was used to determine total abdominal fat and visceral adipose tissue (31). Dual X-ray absorptiometry (Lunar Prodigy, Madison, WI) was used to quantify total body fat and fat-free mass. Blood pressure was obtained after a 10-min rest period, and fasting blood triglyceride and cholesterol levels were obtained. Maximal oxygen consumption (V̇o2max) was determined (Jaeger Oxygcon Pro; Viasys) during a treadmill exercise test according to standard criteria (35), and maximal heart rate (HRmax) was used to prescribe exercise intensity.
Exercise training.
Subjects participated in a supervised aerobic exercise program 5 days/wk at 60–65% of HRmax for the first 4 wk. Thereafter, the exercise intensity was increased and maintained at 80–85% HRmax. Subjects exercised for 50–60 min, with a 10-min warmup and cooldown. V̇o2max was repeated at weeks 4 and 8 to ensure that the appropriate exercise intensity was maintained throughout training. Subjects also met weekly with a dietitian to assess proper nutrient intake (i.e., ∼55% CHO, 30% fat, and 15% protein). Food records were averaged over a 3-day period before and during the last week of the intervention to determine total caloric and macronutrient intake.
Exercise dose.
Linear regression equations of V̇o2 as a function of HR were performed at four distinct time points using week 0, 4, 8, and 12 HRmax tests. These regression equations allowed us to compute the average V̇o2 in liters per minute for each training session based on the average HR obtained at that particular session. The average oxygen uptake was then converted into energy units (1 liter of O2 = 5 kcal) to obtain the energy expended per minute for each session (27). This energy expenditure value was summed for each exercise session performed over the 12-wk program and then averaged, and the product of average kilocalories per minute and the duration of the exercise bout enabled us to calculate the kilocalories expended per session (i.e., exercise dose). These were then summed to obtain the caloric expenditure per week (i.e., exercise volume). To account for changes in energy expenditure as a function of improved cardiovascular fitness, we applied week 0, 4, 8, and 12 V̇o2max regression equations to weeks 1–3, 4–7, 8–10, and 11–12, respectively. This approached allowed us to account for individual differences in response to exercise. The exercise dose difference between individuals is thus due to their initial fitness and training-induced improvements in fitness.
Insulin sensitivity.
Subjects underwent a 120-min euglycemic hyperinsulinemic clamp after an overnight fast. A primed, constant infusion (40 mU·m2·min−1) of insulin was administered via catheters placed in an antecubital vein. Glucose (20%) was infused at a variable rate to maintain plasma glucose at 90 mg/dl (34). Insulin sensitivity was defined as the average glucose infusion rate during the final 30 min of the clamp divided by ambient insulin concentrations.
Pancreatic β-cell function.
After an overnight fast, a 75-g oral glucose tolerance test (OGTT) was performed. Blood samples were obtained from an antecubital vein at 0, 30, 60, 90, and 120 min for the determination of glucose, insulin, and C-peptide concentrations. Area under the curve during the OGTT was calculated using the trapezoidal method. First- and second-phase glucose-stimulated insulin secretion (GSIS) was calculated by dividing plasma insulin by glucose area under the curve during the first 30 and last 60 min of the OGTT. The first- and second-phase DI was used to characterize pancreatic β-cell function and calculated as GSIS × insulin sensitivity. C-peptide was also used in place of insulin to more accurately reflect prehepatic insulin secretion. Because of limited sample volume and available sample with aprotonin, C-peptide analysis was performed in 23 subjects. Hepatic extraction was also estimated as insulin divided by C-peptide area under the curve during the OGTT (15).
Biochemical analysis.
All samples were centrifuged at 1,000 rpm for 10 min at 4°C and stored at −80°C until analysis. Plasma glucose was collected in a lithium-heparin vacutainer, and samples were measured using a glucose oxidase assay (YSI 2300 STAT Plus; Yellow Springs Instruments, Yellow Springs, OH). Plasma insulin and C-peptide were collected in vacutainers containing EDTA and the protease inhibitor aprotonin, and samples were analyzed using a radioimmunoassay (Millipore, Billerica, MA). Plasma triglycerides and cholesterol were analyzed using enzymatic methods with an automated platform (Roche Modular Diagnostics, Indianapolis, IN).
Statistical analysis.
Data were analyzed using the statistical program R (Leopard build 64-bit; The R Foundation, Vienna, Austria). Pre- and postintervention data were compared using paired two-tailed t-tests. As part of secondary analysis to generalize our findings, we report male and female responses to exercise training. Differences (post − pre) between men and women after training were compared using unpaired two-tailed t-tests. Bivariate linear regression analysis was used to determine associations between study outcomes. Insulin sensitivity and GSIS were also used as covariates using multivariate regression analysis to adjust for their influence on the association between exercise dose and DI. Significance was accepted as P < 0.05, and data are reported as means ± SE.
RESULTS
Subject and exercise characteristics.
Prior to the intervention, men and women were on average obese, dyslipidemic, prehypertensive, and glucose intolerant (Table 1). Subjects reduced total food intake (1,868.2 ± 59.0 vs. 1,676.7 ± 61.3 kcal/day, P < 0.02), which was attributable mainly to lower carbohydrate (254.9 ± 7.4 vs. 234.9 ± 9.2 g/day, P < 0.01) and fat (61.0 ± 2.2 vs. 52.7 ± 2.7 g/day, P < 0.02), since protein did not change statistically (76.7 ± 2.6 vs. 71.7 ± 2.8 g/day, P = 0.06). Subjects expended ∼474.5 ± 8.8 kcal/session (men = 481.6 ± 16.2 vs. women = 468.4 ± 9.0, P = 0.48) during the intervention. The average exercise intensity was 7.9 ± 0.1 kcal/min (men = 8.0 ± 0.3 vs. women = 7.8 ± 0.2; P = 0.48), with 2,372.5 ± 44.1 kcal expended over the 5-day training week (men = 2,408.3 ± 81.1 vs. women = 2,342.3 ± 45.1, P = 0.48).
Table 1.
Metabolic characteristics before and after exercise training
| Variable | Group Baseline (n = 35) | Group, Δ | Male Baseline (n = 16) | Males, Δ | Female Baseline (n = 19) | Females, Δ |
|---|---|---|---|---|---|---|
| Age, yr | 66.8 ± 0.8 | 65.0 ± 1.9 | 65.9 ± 0.9 | |||
| Body weight, kg | 99.0 ± 2.4 | −8.1 ± 0.7** | 96.3 ± 3.4 | −7.9 ± 0.7** | 93.7 ± 3.0 | −5.9 ± 0.6**^ |
| BMI, kg/m2 | 35.1 ± 0.7 | −2.8 ± 0.2** | 34.2 ± 1.1 | −2.7 ± 0.2** | 35.8 ± 1.1 | −2.3 ± 0.2**^ |
| Body fat, % | 43.0 ± 1.3 | −3.8 ± 0.6** | 42.0 ± 1.7 | −3.6 ± 0.6** | 48.2 ± 1.4^ | −2.1 ± 0.5**^ |
| Fat mass, kg | 42.5 ± 1.6 | −6.7 ± 0.7** | 41.5 ± 1.9 | −6.3 ± 0.7** | 45.2 ± 2.3 | −4.7 ± 0.7**^ |
| FFM, kg | 56.4 ± 2.0 | −1.2 ± 0.4** | 54.8 ± 2.5 | −1.2 ± 0.4* | 48.1 ± 1.9^ | −1.2 ± 0.4* |
| Total Abdominal fat, cm3 | 612.6 ± 22.7 | −109.7 ± 12.4** | 596.8 ± 27.1 | −105.7 ± 12.2 | 646.2 ± 27.5 | −97.1 ± 19.2 |
| Visceral fat, cm3 | 151.4 ± 14.0 | −30.6 ± 6.5** | 147.0 ± 13.7 | −29.3 ± 6.2** | 132.8 ± 3.9 | −20.9 ± 3.9** |
| V̇o2max, ml/min | 2,080.8 ± 85.9 | 263.6 ± 44.5** | 2,319.2 ± 99.9 | 255.7 ± 42.5** | 1,761.9 ± 70.9^ | 209.9 ± 48.5** |
| V̇o2max, ml·kg FFM−1·min−1 | 37.8 ± 1.6 | 5.0 ± 1.0** | 34.5 ± 1.9 | 5.4 ± 0.8** | 34.8 ± 2.7 | 5.3 ± 1.4** |
| Total cholesterol, mg/dl | 204.7 ± 4.8 | −25.0 ± 3.8** | 204.6 ± 6.7 | −36.3 ± 5.3** | 204.8 ± 7.2 | −15.0 ± 4.4**^ |
| Triglycerides, mg/dl | 160.6 ± 12.0 | −43.7 ± 9.4** | 166.8 ± 18.1 | −62.7 ± 15.3** | 155.1 ± 16.3 | −26.8 ± 10.4** |
| HDL cholesterol, mg/dl | 46.3 ± 2.5 | −1.4 ± 0.8 | 43.0 ± 2.9 | 0.8 ± 1.8 | 50.3 ± 4.1 | −5.6 ± 0.8**^ |
| Systolic BP, mmHg | 133.0 ± 3.2 | −13.1 ± 2.5** | 134.8 ± 4.8 | −14.7 ± 3.6** | 131.0 ± 4.9 | −14.4 ± 11.4** |
| Diastolic BP, mmHg | 79.3 ± 2.1 | −6.5 ± 1.9** | 79.6 ± 3.1 | −6.7 ± 2.7* | 78.9 ± 2.8 | −6.9 ± 2.8* |
Data are reported as means ± SE. BMI, body mass index; FFM, fat-free mass; BP, blood pressure. Δ = change between post- and pretraining. Clamp-derived insulin sensitivity was defined as GIR/I. To convert total cholesterol and HDL mg/dl to mM, divide by 38.67. To convert triglyceride mg/dl to mM, divide by 88.57.
P < 0.05 and
P < 0.01 compared with baseline;
P < 0.05 compared with males.
Responses to exercise.
Exercise training reduced total body fat and weight by ∼6–8 kg (P < 0.001; Table 1) and increased cardiovascular fitness by 12% in men and women (P < 0.0001). There was also an increase in basal fat oxidation (0.77 ± 0.08 vs. 0.99 ± 0.09 mg·kg fat-free mass−1·min−1, P = 0.02) despite no change in resting metabolic rate after the intervention (1,548 ± 52.7 vs. 1,549.6 ± 44.1 kcal/day, P = 0.96). There was no difference between men and women in fat oxidation or resting metabolic rate following the intervention (data not shown).
OGTT and pancreatic β-cell function.
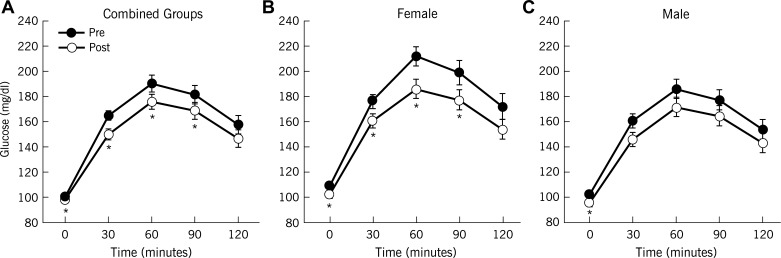
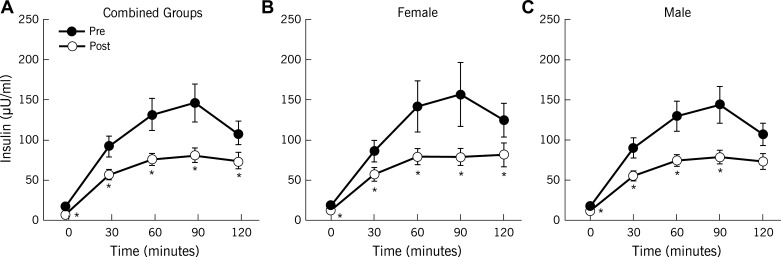
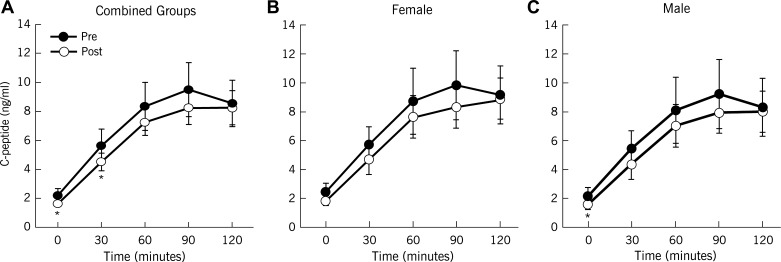
Exercise training enhanced clamp-derived insulin sensitivity (P < 0.0001; Table 2), and reduced plasma glucose in response to the OGTT (Fig. 1). Although exercise decreased circulating insulin after exercise training (Fig. 2), C-peptides showed only subtle reductions, particularly during the first 30 min of the OGTT (Fig. 3). Overall, there were no sex differences in response to exercise training for blood glucose, insulin, or C-peptide (Table 2). Hepatic extraction was higher in response to the OGTT after the intervention (1st phase: 0.07 ± 0.01 vs. 0.09 ± 0.01 ng·ml−1·μU−1·ml−1, 30 min; 2nd phase: 0.08 ± 0.01 vs. 0.11 ± 0.01 ng·ml−1·μU−1·ml−1, 60 min; P < 0.05) for combined groups. There were no differences in hepatic extraction between men and women (data not shown). Nevertheless, in general, exercise decreased GSIS (Table 2). When GSIS was corrected for changes in ambient insulin sensitivity, first- and second-phase DI (i.e., β-cell function) were elevated after training (P < 0.05; Table 2).
Table 2.
Glucose homeostasis before and after exercise training
| Variable | Group Baseline | Group, Δ | Male Baseline | Males, Δ | Female Baseline | Females, Δ |
|---|---|---|---|---|---|---|
| Glucose AUC0–30 | −327.5 ± 76.5 | −327 ± 76.5** | 3,759.8 ± 70.7 | −231.1 ± 101.1* | 4,294.1 ± 94.8̂ | −408.7 ± 111.4* |
| Glucose AUC60–120 | 10,659.9 ± 402.6 | −766.4 ± 310.3** | 9,380.7 ± 475.6 | −301.3 ± 393.6 | 11,737.1 ± 514.0̂ | −1,158.1 ± 455.7* |
| Glucose AUC0–120 | 20,036.5 ± 591.8 | −1,535.9 ± 446.0** | 17,854.3 ± 651.6 | −668.2 ± 556.2 | 21,874.1 ± 714.4̂ | −2,266.5 ± 640.5* |
| Insulin AUC0–30 | 1,642.5 ± 210.9 | −614.6 ± 0.6** | 1,735.8 ± 384.5 | −726.5 ± 232.7̂̂ | 1,564.0 ± 224.4 | −520.4 ± 183.5* |
| Insulin AUC60–120 | 8,008.4 ± 1,186.0 | −3,324.8 ± 905.2** | 7,184.5 ± 1,225.2 | −2,620.0 ± 737.0̂̂ | 8,702.3 ± 1,944.3 | −3,918.4 ± 1,533.0*^ |
| Insulin AUC0–120 | 13,018.3 ± 1,720.0 | −5,315.0 ± 1,201.3** | 1,226.0 ± 1,993.7 | −4,726.9 ± 1,024.3̂̂ | 13,685.4 ± 2,729.6 | −5,810.2 ± 2,062.8* |
| C-peptide AUC0–30 | 117.5 ± 16.6 | −25.0 ± 10.4** | 112.4 ± 20.5 | −26.4 ± 14.8 | 122.5 ± 27.0 | −23.7 ± 15.7 |
| C-peptide AUC60–120 | 538.5 ± 72.6 | −60.5 ± 53.4 | 515.1 ± 68.0 | −52.0 ± 65.2 | 561.9 ± 131.9 | −69.1 ± 87.9 |
| C-peptide AUC0–120 | 1,078.03 ± 72.6 | −127.0 ± 97.5 | 1,032.4 ± 136.8 | −132.4 ± 123.2 | 1123.5 ± 252.6 | −121.6 ± 157.4 |
| First-phase INS0–30/GLC0–30 | 0.41 ± 0.05 | −0.14 ± 0.04** | 0.46 ± 0.10 | −0.18 ± 0.06* | 0.37 ± 0.05 | −0.10 ± 0.05* |
| Second-phase INS60–120/GLC60–120 | 0.77 ± 0.11 | −0.30 ± 0.08** | 0.77 ± 0.13 | −0.28 ± 0.09* | 0.78 ± 0.18 | −0.30 ± 0.13* |
| First-phase C-peptide0–30/GLC0–30 | 0.030 ± 0.004 | 0.03 ± 0.04 | 0.03 ± 0.01 | −1.58 ± 0.08 | 0.03 ± 0.01 | −1.64 ± 0.10 |
| Second-phase C-peptide60–120/GLC60–120 | 0.056 ± 0.007 | −0.006 ± 0.005 | 0.06 ± 0.01 | −0.007 ± 0.006 | 0.05 ± 0.01 | −0.003 ± 0.007 |
| First-phase DI (INS) | 0.008 ± 0.001 | 0.002 ± 0.001** | 0.008 ± 0.001 | 0.002 ± 0.001* | 0.008 ± 0.001 | 0.001 ± 0.001 |
| Second-phase DI (INS) | 0.015 ± 0.002 | 0.003 ± 0.001** | 0.014 ± 0.001 | 0.003 ± 0.001* | 0.013 ± 0.002 | 0.002 ± 0.002 |
| First-phase DI (C-peptide) | 0.001 ± 0.000 | 0.0004 ± 0.0001** | 0.001 ± 0.001 | 0.0004 ± 0.0001* | 0.002 ± 0.001 | 0.0002 ± 0.0001†^ |
| Second-phase DI (C-peptide) | 0.001 ± 0.000 | 0.001 ± 0.000** | 0.001 ± 0.001 | 0.002 ± 0.001** | 0.001 ± 0.001 | 0.002 ± 0.003*‡ |
| Insulin sensitivity | 0.026 ± 0.002 | 0.019 ± 0.002** | 0.025 ± 0.004 | 0.025 ± 0.004** | 0.026 ± 0.003 | 0.014 ± 0.002**^ |
Data are reported as means ± SE. AUC, area under the curve; INS, insulin; GLC, glucose; DI, disposition index. First- and second-phase glucose-stimulated insulin secretion (GSIS) calculations were derived from a 75-g oral glucose tolerance test. GSIS derived from AUC insulin [μU·ml−1·mg−1·dl, 30 (or 60) min] and C-peptide [ng·ml−1·mg−1·dl, 30 (or 60) min] blood measurements. Insulin sensitivity was defined as GIR/I from the euglycemic hyperinsulinemic clamp (mg·kg−1·min−1 per μU/ml). To convert glucose mg/dl to mM, divide by 18. To convert insulin μU/ml to pM, multiply by 6. To convert C-peptide ng/ml to nM, multiply by 0.333. β-Cell function was determined using GSIS and C-peptide secretion multiplied by clamp-derived insulin sensitivity (i.e., DI). †P = 0.07,
P < 0.05, and
P < 0.01 compared with baseline
(P = 0.07); ^P < 0.05 and ^^P < 0.01 compared with males.
Fig. 1.
Effect of exercise training with weight loss on plasma glucose responses. Data are means ± SE. Pre, pretraining; post, posttraining. *P < 0.05 compared with pretraining.
Fig. 2.
Effect of exercise training with weight loss on plasma insulin responses. Data are means ± SE. *P < 0.05 compared with pretraining.
Fig. 3.
Effect of exercise training with weight loss on plasma C-peptide responses. Data are means ± SE. Note that C-peptide analysis included 23 of the 35 subjects. *P < 0.05 compared with pretraining.
Correlational analysis.
First- and second-phase DI were both linearly associated with exercise dose (Fig. 4). The insulin- and C-peptide-derived calculations of DI were correlated at baseline (DIfirst phase: r = 0.68, P < 0.001; DIsecond phase: r = 0.61, P < 0.001, respectively) and after the intervention (DIfirst phase: r = 0.78, P < 0.00001; DIsecond phase: r = 0.68, P = 0.0006, respectively). Thus, all remaining correlations were analyzed using the insulin-derived DI to minimize type 1 and type 2 statistical errors. Baseline DI was inversely correlated with enhanced DI after exercise (DIfirst phase: r = −0.37, P = 0.04; DIsecond phase: r = −0.41, P = 0.02). Increased DI was also positively correlated with increased V̇o2max after training (DIfirst phase: r = 0.36, P = 0.04; DIsecond phase: r = 0.41, P = 0.02). However, the change in DI did not correlate with reductions in body fat (DIfirst phase: r = −0.21, P = 0.25; DIsecond phase: r = −0.30, P = 0.10) or visceral adiposity (DIfirst phase: r = 0.06, P = 0.73; DIsecond phase: r = 0.12, P = 0.50) after the intervention. Lower fasting glucose after training was associated with elevated first-phase DI (r = −0.39, P = 0.02) and increased fat oxidation (r = 0.41, P = 0.01) but not insulin sensitivity (r = −0.16, P = 0.33) or second-phase DI (r = −0.17, P = 0.33) after training. Multiple linear regression analysis for first-phase DI indicated that insulin sensitivity (estimate = 1.6 × 10−1; SE = 4.6 × 10−2; t-value = 3.5, P = 0.001), GSIS (1st phase: estimate = 9.4 × 10−3, SE = 2.6 × 10−3, t-value = 3.5, P = 0.001), and exercise dose (estimate = 2.9 × 10−5, SE = 1.3 × 10−5, t-value = 2.2, P = 0.03) were all significant predictors of β-cell function. For second-phase DI, the results indicated that insulin sensitivity (estimate = 2.0 × 10−1, SE = 7.7 × 10−2, t-value = 2.6, P = 0.01) and GSIS (2nd phase: estimate = 1.2 × 10−2, SE = 3.3 × 10−3, t-value = 3.6, P = 0.001) were significant predictors of β-cell function, whereas exercise dose trended toward significance (estimate = 3.6 × 10−5, SE = 2.2 × 10−5, t-value = 1.6, P = 0.10).
Fig. 4.
Correlations between the change (Δ) in 1st- and 2nd-phase disposition index (DI; or β-cell function) derived from insulin (A and C) and C-peptide (B and D) with exercise energy expenditure (kcal/session). Average kcal/min during each exercise session was averaged over the 12-wk intervention and was used to calculate the kcal expended/session (i.e., exercise dose). ○, Women; ●, men.
DISCUSSION
The major finding from this study is that exercise training with modest energy reduction increased both first- and second-phase pancreatic β-cell function, calculated using both plasma insulin and C-peptide, in a linear dose-response manner in men and women with prediabetes. Our data are consistent with Davis et al. (6), who reported that high-dose compared with low-dose exercise enhanced first-phase β-cell function in overweight children. However, Slentz et al. (33) reported increased first-phase β-cell function after moderate compared with high-volume exercise in middle-aged, overweight, normoglycemic adults. The inconsistency in the literature regarding exercise dose and pancreatic function may be related to methodology. Our data and the report by Davis et al. (6) used the OGTT to characterize β-cell function, whereas the study by Slentz et al. (33) used the intravenous glucose tolerance test. Pancreatic function derived from intravenous glucose tolerance test is dependent on β-cell glucose responsiveness and the readily available release of insulin, whereas the OGTT additionally characterizes the processing and synthesis of new insulin as well as evoking incretin and neural-mediated effects on the pancreas (19). Thus, these different methodological approaches are likely to have contributed to the somewhat different quantitative insulin responses and hence, contrasting conclusions regarding the effectiveness of lifestyle modification programs, including exercise, on β-cell function.
High fasting and postprandial glucose concentrations are linked to the deterioration of first- and second-phase β-cell function, respectively (18). Consequently, improving both first- and second-phase β-cell function may be necessary to optimally manage hyperglycemia. Exercise increases β-cell function in adults with type 2 diabetes, whereas it reduces compensatory insulin secretion in normal glucose-tolerant individuals (8, 34). However, there are limited data examining the impact of exercise on pancreatic β-cell function in adults with prediabetes (4). We observed a significant inverse association between low-baseline β-cell function and a rise in β-cell function after our intervention. These data are consistent with the view that individuals with poor insulin secretion at the start of a lifestyle intervention are likely to respond by increasing β-cell function. Circulating insulin is influenced by whole body insulin sensitivity and the capacity to secrete insulin. Thus, it is not possible to properly evaluate GSIS from circulating insulin during an OGTT without taking insulin sensitivity into account. The DI was developed (2, 5, 16) as the product of both GSIS and insulin sensitivity and serves as an index of β-cell function that reflects the integrated capacity for whole body glucose disposal. In our study, although insulin sensitivity likely contributed to improvements in β-cell function, neither insulin sensitivity nor GSIS alone correlated with reductions in circulating glucose levels. Thus, the improvement in DI may be a more clinically relevant determinant of glycemic control when exercise is used as an intervention (2, 37). Moreover, the improvement in β-cell function is specific to the postprandial period, as there were clear reductions in fasting insulin and C-peptide concentrations (Fig. 2 and 3). Taken together, our findings suggest that some exercise is better than none for glycemic control, and increasing doses of exercise appear to be important for enhancing β-cell function in adults with poor insulin secretion capacity (11).
The relationship between exercise dose and pancreatic function may be linked to obesity (17, 28). It is reasonable to expect that individuals expending more kilocalories during exercise would have greater reductions in body fat, which in turn, would lead to lower blood lipids and/or inflammatory markers that impair β-cell function (36, 38). However, we did not observe a direct association between reductions in body fat and β-cell function, suggesting that the improvement in β-cell function after exercise and a modest reduction in calorie intake may be independent of changes in fat mass. Alternatively, higher exercise doses were linked to increased V̇o2max, and this enhanced fitness was directly associated with β-cell function. We did not design this study to determine the exact mechanism by which V̇o2max contributes to higher β-cell function, but previous work in rodents suggests that exercise enhances GLUT2 transporter content (20), improves Akt signaling and glucokinase activity (10, 20, 22), and raises mitochondrial respiration (10). Consistent with improved mitochondrial capacity, we did observe a significant increase in fat oxidation after our intervention. Thus, we speculate that the greater energy expenditure from fat after exercise reduces lipotoxicity in the pancreas and improves glucose regulation (28, 30, 32). It is worth noting, however, that we detected a significant correlation between improvements in first-phase β-cell function and reductions in fasting hyperglycemia. This association is consistent with work highlighting that glucotoxcity is a factor impairing insulin secretion (32). Thus, it is possible that improvements in glucolipotoxicity contributed to improvements in β-cell function. Finally, we recognize that insulin-resistant muscle may secrete myokines that affect β-cell mass and/or secretion (12, 26). As a result, it remains possible that exercise training altered the release of an unknown myokine and improved the cross-talk between skeletal muscle and the pancreas to elevate β-cell function (14). Whether other organs such as the liver or adipose tissue secrete hormones that modulate pancreatic function awaits further investigation.
Hepatic extraction is a potential factor influencing circulating insulin (15, 23, 29). In our study, exercise reduced postprandial plasma insulin to a larger extent than C-peptide concentrations. This difference is likely related to hepatic extraction and suggests that our insulin-derived β-cell function calculations may underestimate insulin secretion. However, plasma insulin was significantly associated with C-peptide-derived calculations of β-cell function. Thus, our findings suggest that plasma insulin provides a reasonable estimate of β-cell function. Another factor to consider is that the number of kilocalories expended during exercise in this study was within a narrow range (i.e., ∼400–600 kcal/session). Thus, it is possible that expending more or less kilocalories (e.g., 900 or 200 kcal/session) during each exercise session could yield different results (24). Although subjects were counseled to maintain nutritional intake throughout the study, we observed less food intake during the intervention. Therefore, caloric restriction in addition to energy expenditure may have contributed to improvements in β-cell function. However, we did not detect any relationship between changes in body fat and β-cell function, and this suggests that weight loss via energy deficit is unlikely to be the primary mechanism leading to improved β-cell function after lifestyle modification. Taken together, our data suggest that future work is needed to determine the most time-efficient exercise strategy to maximize energy expenditure, as this appears to be an important factor for improving β-cell function.
In conclusion, our findings suggest that exercise interventions expending >2,000 kcal/wk increase pancreatic β-cell function in a linear dose-response manner in men and women. This observation is clinically relevant since current health guidelines recommend that men and women must expend between 1,000 and 2,000 kcal/wk to receive cardiometabolic benefit. Because glucose-intolerant adults appear to have blunted responses to exercise interventions (25), it would seem appropriate to tailor exercise intensity, duration, and mode to maximize energy expenditure for optimal glucoregulatory effects.
GRANTS
This research was supported by RO1-AG-12834 (to J. P. Kirwan). S. K. Malin was supported by NIH-T32-DK-007319.
DISCLOSURES
The authors report no conflicts of interest, financial or otherwise.
AUTHOR CONTRIBUTIONS
S.K.M. and J.P.K. contributed to the conception and design of the research; S.K.M., T.P.S., A.B., S.F., J.F., and J.P.K. performed the experiments; S.K.M., T.P.S., A.B., and S.F. analyzed the data; S.K.M., T.P.S., A.B., S.F., and J.P.K. interpreted the results of the experiments; S.K.M. prepared the figures; S.K.M. drafted the manuscript; S.K.M., T.P.S., A.B., S.F., J.F., and J.P.K. edited and revised the manuscript; S.K.M., T.P.S., A.B., S.F., J.F., and J.P.K. approved the final version of the manuscript.
REFERENCES
- 1.No authors listed National Diabetes Fact Sheet, 2011 (Online). Atlanta, GA: Centers for Disease Control and Prevention; www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf [2011]. [Google Scholar]
- 2. Abdul-Ghani MA, Williams K, DeFronzo RA, Stern M. What is the best predictor of future type 2 diabetes? Diabetes Care 30: 1544–1548, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Blake DR, Meigs JB, Muller DC, Najjar SS, Andres R, Nathan DM. Impaired glucose tolerance, but not impaired fasting glucose, is associated with increased levels of coronary heart disease risk factors: results from the Baltimore Longitudinal Study on Aging. Diabetes 53: 2095–2100, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Bloem CJ, Chang AM. Short-term exercise improves beta-cell function and insulin resistance in older people with impaired glucose tolerance. J Clin Endocrinol Metab 93: 387–392, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cobelli C, Toffolo GM, Dalla Man C, Campioni M, Denti P, Caumo A, Butler P, Rizza R. Assessment of β-cell function in humans, simultaneously with insulin sensitivity and hepatic extraction, from intravenous and oral glucose tests. Am J Physiol Endocrinol Metab 293: E1–E15, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Davis CL, Pollock NK, Waller JL, Allison JD, Dennis BA, Bassali R, Melendez A, Boyle C, Gower B. Exercise dose and diabetes risk in overweight and obese children: a randomized controlled trial. JAMA 308: 1103–1112, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. DeFronzo RA, Abdul Ghani MA. Preservation of β-cell function: the key to diabetes prevention. J Clin Endocrinol Metab 96: 2354–2366, 2011 [DOI] [PubMed] [Google Scholar]
- 8. Dela F, Stallknecht B. Effect of physical training on insulin secretion and action in skeletal muscle and adipose tissue of first-degree relatives of type 2 diabetic patients. Am J Physiol Endocrinol Metab 299: E80–E91, 2010 [DOI] [PubMed] [Google Scholar]
- 9. Dela F, von Linstow M, Mikines K, Galbo H. Physical training may enhance β-cell function in type 2 diabetes. Am J Physiol Endocrinol Metab 287: E1024–E1031, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Delghingaro Augusto V, Decary S, Peyot ML, Latour M, Lamontagne J, Paradis Isler N, Lacharite-Lemieux M, Akakpo H, Birot O, Nolan CJ, Prentki M, Bergeron R. Voluntary running exercise prevents β-cell failure in susceptible islets of the Zucker diabetic fatty rat. Am J Physiol Endocrinol Metab 302: E254–E264, 2012 [DOI] [PubMed] [Google Scholar]
- 11. Dube JJ, Allison KF, Rousson VH, Goodpaster B, Amati F. Exercise dose and insulin sensitivity: relevance for diabetes prevention. Med Sci Sports Exerc 44: 793–799, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Flier SN, Kulkarni RN, Kahn CR. Evidence for a circulating islet cell growth factor in insulin-resistant states. Proc Natl Acad Sci USA 98: 7475–7480, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hamer M, Stamatakis E. Low-dose physical activity attenuates cardiovascular disease mortality in men and women with clustered metabolic risk factors. Circ Cardiovasc Qual Outcomes 5: 494–499, 2012 [DOI] [PubMed] [Google Scholar]
- 14. Handschin C, Choi CS, Chin S, Kim S, Kawamori D, Kurpad AJ, Neubauer N, Hu J, Mootha VK, Kim YB, Kulkarni RN, Shulman GI, Spiegelman BM. Abnormal glucose homeostasis in skeletal muscle-specific PGC-1alpha knockout mice reveals skeletal muscle-pancreatic beta cell crosstalk. J Clin Invest 117: 3463–3474, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Henry RR, Brechtel G, Griver K. Secretion and hepatic extraction of insulin after weight loss in obese noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 66: 979–986, 1988 [DOI] [PubMed] [Google Scholar]
- 16. Kahn SE, Prigeon RL, McCulloch DK, Boyko EJ, Bergman RN, Schwartz MW, Neifing JL, Ward WK, Beard JC, Palmer JP. Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects. Evidence for a hyperbolic function. Diabetes 42: 1663–1672, 1993 [DOI] [PubMed] [Google Scholar]
- 17. Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444: 840–846, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Kanat M, Norton L, Winnier D, Jenkinson C, DeFronzo RA, Abdul Ghani MA. Impaired early- but not late-phase insulin secretion in subjects with impaired fasting glucose. Acta Diabetol 48: 209–217, 2011 [DOI] [PubMed] [Google Scholar]
- 19. King DS, Staten MA, Kohrt WM, Dalsky GP, Elahi D, Holloszy JO. Insulin secretory capacity in endurance-trained and untrained young men. Am J Physiol Endocrinol Metab 259: E155–E161, 1990 [DOI] [PubMed] [Google Scholar]
- 20. Kiraly MA, Bates HE, Kaniuk NA, Yue JT, Brumell JH, Matthews SG, Riddell M, Vranic M. Swim training prevents hyperglycemia in ZDF rats: mechanisms involved in the partial maintenance of β-cell function. Am J Physiol Endocrinol Metab 294: E271–E283, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Kirwan JP, Kohrt WM, Wojta DM, Bourey RE, Holloszy JO. Endurance exercise training reduces glucose-stimulated insulin levels in 60- to 70-year-old men and women. J Gerontol 48: M84–M90, 1993 [DOI] [PubMed] [Google Scholar]
- 22. Koranyi LI, Bourey RE, Slentz CA, Holloszy JO, Permutt MA. Coordinate reduction of rat pancreatic islet glucokinase and proinsulin mRNA by exercise training. Diabetes 40: 401–404, 1991 [DOI] [PubMed] [Google Scholar]
- 23. Lorenzo C, Hanley AJ, Wagenknecht LE, Rewers MJ, Stefanovski D, Goodarzi M, Haffner SM. Relationship of insulin sensitivity, insulin secretion, and adiposity with insulin clearance in a multiethnic population: the insulin resistance atherosclerosis study. Diabetes Care 36: 101–103, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Magkos F, Tsekouras Y, Kavouras SA, Mittendorfer B, Sidossis LS. Improved insulin sensitivity after a single bout of exercise is curvilinearly related to exercise energy expenditure. Clin Sci (Lond) 114: 59–64, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Malin SK, Kirwan JP. Fasting hyperglycaemia blunts the reversal of impaired glucose tolerance after exercise training in obese older adults. Diabetes Obes Metab 14: 835–841, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mauvais-Jarvis F, Virkamaki A, Michael MD, Winnay JN, Zisman A, Kulkarni RN, Kahn CR. A model to explore the interaction between muscle insulin resistance and beta-cell dysfunction in the development of type 2 diabetes. Diabetes 49: 2126–2134, 2000 [DOI] [PubMed] [Google Scholar]
- 27. McArdle WD, Katch FI, Katch VL. Exercise Physiology: Energy, Nutrition, and Human Performance. Philadelphia, PA: Lippincott Williams & Wilkins, 2007, p. 184–194 [Google Scholar]
- 28. McGarry JD. Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes 51: 7–18, 2002 [DOI] [PubMed] [Google Scholar]
- 29. Meier JJ, Veldhuis JD, Butler PC. Pulsatile insulin secretion dictates systemic insulin delivery by regulating hepatic insulin extraction in humans. Diabetes 54: 1649–1656, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Muzumdar R, Ma X, Atzmon G, Vuguin P, Yang X, Barzilai N. Decrease in glucose-stimulated insulin secretion with aging is independent of insulin action. Diabetes 53: 441–446, 2004 [DOI] [PubMed] [Google Scholar]
- 31. O'Leary VB, Marchetti CM, Krishnan RK, Stetzer BP, Gonzalez F, Kirwan JP. Exercise-induced reversal of insulin resistance in obese elderly is associated with reduced visceral fat. J Appl Physiol 100: 1584–1589, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Poitout V, Robertson RP. Minireview: Secondary beta-cell failure in type 2 diabetes—a convergence of glucotoxicity and lipotoxicity. Endocrinology 143: 339–342, 2002 [DOI] [PubMed] [Google Scholar]
- 33. Slentz CA, Tanner CJ, Bateman LA, Durheim MT, Huffman KM, Houmard JA, Kraus WE. Effects of exercise training intensity on pancreatic beta-cell function. Diabetes Care 32: 1807–1811, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Solomon TP, Haus JM, Kelly KR, Rocco M, Kashyap SR, Kirwan JP. Improved pancreatic beta-cell function in type 2 diabetic patients after lifestyle-induced weight loss is related to glucose-dependent insulinotropic polypeptide. Diabetes Care 33: 1561–1566, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Solomon TP, Haus JM, Kelly KR, Cook M, Filion J, Rocco M, Kashyap SR, Watanabe R, Barkoukis H, Kirwan JP. A low-glycemic index diet combined with exercise reduces insulin resistance, postprandial hyperinsulinemia, and glucose-dependent insulinotropic polypeptide responses in obese, prediabetic humans. Am J Clin Nutr 92: 1359–1368, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Utzschneider KM, Carr DB, Hull RL, Kodama K, Shofer JB, Retzlaff BM, Knopp RH, Kahn SE. Impact of intra-abdominal fat and age on insulin sensitivity and beta-cell function. Diabetes 53: 2867–2872, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Utzschneider KM, Prigeon RL, Faulenbach MV, Tong J, Carr DB, Boyko EJ, Leonetti DL, McNeely MJ, Fujimoto WY, Kahn SE. Oral disposition index predicts the development of future diabetes above and beyond fasting and 2-h glucose levels. Diabetes Care 32: 335–341, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Walton C, Godsland IF, Proudler AJ, Felton CV, Wynn V. Effect of body mass index and fat distribution on insulin sensitivity, secretion, and clearance in nonobese healthy men. J Clin Endocrinol Metab 75: 170–175, 1992 [DOI] [PubMed] [Google Scholar]