Abstract
In the present study, we evaluated the relative abundance of angiotensin type 2 receptor (AT2R) protein in various tissues of adult rats. We found that pancreatic islets expressed the highest AT2R protein compared with all other tissues. Accordingly, we then determined the functional significance of AT2R in the endocrine pancreas in in vivo and in vitro experiments by using angiotensin II (ANG II) alone, losartan (Los; AT1R antagonist), compound 21 (C21; AT2R agonist), and PD-123319 (PD; AT2R antagonist). Experiments carried out in rats indicated that, 1) ANG II treatment significantly increased plasma insulin concentration (1.51 ± 0.20 vs. 0.82 ± 0.14 ng/ml, n = 7, P < 0.05) in the fed state. This insulinotropic effect was further augmented by combined treatment with ANG II + Los (2.31 ± 0.25 ng/ml, n = 7, P < 0.01). C21 also elevated insulin levels (2.13 ± 0.20 ng/ml, n = 7, P < 0.01), which was completely abolished by PD. 2) ANG II impaired glucose tolerance, whereas ANG II + Los or C21 improved this function. 3) All treated rats displayed an enhanced insulin secretory response to a glucose challenge. 4) All treated rats displayed upregulated proinsulin 2 mRNA and insulin protein expression in the pancreas. In in vitro experiments using INS-1E cells and isolated rat islets, we found that AT2R activation significantly improved insulin biosynthesis and secretion. These results suggest that the AT2R functions as an insulinotropic mediator. AT2R and its downstream signaling pathways may be potential therapeutic targets for diabetes.
Keywords: compound 21, insulin production, INS-1E cells
as one of the primary angiotensin II (ANG II) receptor subtypes, the angiotensin type 2 receptor (AT2R) was pharmacologically identified (10, 41) and molecularly cloned (19, 27) around 20 years ago. However, a complete understanding of the function of the AT2R is still incomplete. Evidence implies that the AT2R exerts numerous effects such as vasodilatation, tissue protection, and regeneration (21). These biological actions, however, are rarely observed in intact animals because of the relatively lower expression level of AT2R compared with its antagonistic twin, the angiotensin type 1 receptor (AT1R), and the absence of an exclusive native ligand to the AT2R (13).
Another reason that AT2R actions are not well appreciated comes from the prevailing concept concerning its ontogeny (13). The AT2R has long been considered to express at high levels in the fetus and then dramatically decline within 24 h after birth. As a consequence, the AT2R in the adult animal has been believed to be a retrogressive receptor and play a minor regulatory role (5). Recent data from our laboratory, however, documented that this dogma may not be true (42). We found that adult rat (43) and mouse (15) expressed significantly higher AT2R protein compared with the fetus and neonate. We believe that these results suggest an important function for the AT2R in adulthood (16, 17).
ANG II has been recently assumed to play a role in the regulation of pancreatic endocrine function (40). Chu and Leung (11) demonstrated that the activated AT1R in pancreatic islet contributes to the progressive β-cell failure via oxidative mechanism in the type 2 diabetic model, the db/db mouse. Given that ANG II stimulated both AT1R and AT2R, the influence of selective AT2R activation in the pancreas has not been previously investigated.
In the current study, we first compared the relative abundance of AT2R in various tissues of adult rats. We found that the pancreas uniquely expressed the highest AT2R protein compared with all other tissues, with equal distribution in both islet and acinar components. Accordingly, we then evaluated the functional significance of AT2R in the endocrine pancreas. We postulated that the AT2R might represent a novel signaling pathway within β-cells to regulate insulin production and secretion. We believe that an identification of a novel pathway like this will provide an insight into the islet biology as well as a new therapeutic option to diabetes. In this experiment, the influence of AT2R activation or blockade on insulin biosynthesis or release was examined in vivo by using male adult rats and in vitro by using INS-1E and dissociated islets from neonatal rats.
RESEARCH DESIGN AND METHODS
Animals
Seventy one adult male (320–360 g) and 5 pregnant female (17–18th day gestation) Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) were used in the current experiments. The adult male rats were group housed, and pregnant female rats were housed individually with standard rat food and tap water ad libitum. All experiments were approved by the Institutional Animal Care and Use Committee of the University of Nebraska Medical Center and were carried out under the guidelines of the American Physiological Society and the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Rats for tissue distribution and ontogeny of AT2R.
Eight adult male and 5 pregnant female rats were used in this experiment. The adult male rats were killed by CO2. The tissues were removed and immediately frozen on dry ice, and then stored at −80°C. The pregnant female rats were allowed to give birth to and care for pups until the pups' brain, pancreas, and testicles were collected at the different stages of development: at 1 day, 3 days, and 1–6 wk after birth.
Rats for functional experiments.
Sixty three adult male rats were used in this experiment, which were assigned to five groups to receive the following treatments: vehicle control (saline), ANG II (Sigma), ANG II + losartan (Los; Merck), compound 21 (C21; Vicore Pharma), and C21 + PD-123319 (PD; Tocris). ANG II activates both AT1R and AT2Rs. Los is an AT1R antagonist, and therefore primarily the AT2R was stimulated following the combination of ANG II + Los. C21 is a novel nonpeptide AT2R agonist (39) that has high affinity and selectivity for the AT2R and was employed to directly activate AT2R (38). PD is a selective AT2R antagonist. These reagents were continuously delivered for 7 days by using subcutaneous osmotic minipumps (Alzet 2001, DURECT; ALZET Osmotic Pumps, Cupertino, CA). The reagent doses in nanograms per kilogram per minute were 300 for ANG II and C21 and 900 for Los and PD at an infusion rate of 1 μl/h. After 7 days of treatment, one-half of the rats was killed to collect blood for serum insulin measurements and the pancreas for proinsulin 2 mRNA and insulin protein examination in the fed state. The other one-half was used for oral glucose tolerance testing to evaluate insulin secretion and an intraperitoneal insulin tolerance test to examine insulin sensitivity.
Measurement of Blood Pressure and Heart Rate
Under isoflurane anaesthesia, rats were implanted with radiotelemetry units (model TA11PA-C40; Data Science International, St. Paul, MN) that were secured in the inguinal area. The catheter connected to the unit was inserted in the descending aorta via the right femoral artery against blood flow for the measurement of pulsatile and mean arterial blood pressure. Heart rate was derived from the pressure pulse. The blood pressure and heart rate were recorded daily for 10 min at 10:00 A.M. in the conscious state.
Oral Glucose Tolerance Test, Intraperitoneal Insulin Tolerance Test, Glucose Concentration, and Insulin Concentration
An oral glucose tolerance test was performed after a 16-h overnight fast. Glucose solution (50% in water) was administered orally (2 g/kg body wt) by gavage, and a small amount (1–3 μl) of blood was obtained from the tail vein at 0, 15, 30, 60, 120, and 240 min after glucose loading for serum insulin measurement. For the insulin tolerance test, rats were given an intraperitoneal injection of a 0.5 U/kg insulin solution after 4 h of fasting, and the blood glucose was measured at 0, 15, 30, 60, 90, and 120 min after insulin administration. Blood glucose concentration was evaluated using test strips (ReliOn; ARKRAY, Minneapolis, MN) based on the glucose oxidase method. Serum insulin concentration was measured with an enzyme-linked immunosolvent assay (ELISA) kit (ALPCO Diagnostics, Salem, NH).
INS-1E Cell Culture, Calcium Imaging, and Culture Medium Collection
INS-1E cells (24) generously provided by Dr. P. Maechler (Geneva, Switzerland) were plated at a density of 5 × 105 cells/ml and grown in RPMI 1640 medium with 5% heat-inactivated fetal calf serum, 1 mM sodium pyruvate, 50 μM 2-mercaptoethanol, 2 mM glutamine, 10 mM HEPES, 100 U/ml penicillin, and 100 μg/ml streptomycin.
To evaluate the effects of C21 on calcium influx, INS-1E cells were mounted on a perfusion chamber (cultured overnight) and were preincubated for 2 h in glucose-free culture medium. The calcium-sensitive flourochrome fluo 3 (2 μM; Molecular Probes, Eugene, OR) was then loaded in the cells and incubated for 30 min at 37°C in glucose-free Kreb-Ringer bicarbonate HEPES buffer (KRBH). The cells were then rinsed two times with KRBH buffer and scanned every 10 s to obtain calcium images (×40 objective, yellow filter) by a laser confocal microscope (Leica TSC STED) at room temperature. After the baseline calcium level was stable, C21 was added to the chamber (final concentration: 100 nM), and the intracellular calcium level was again continuously monitored.
To determine the effects of C21 on the insulin secretory response, the INS-1E cells were precultured for 2 h in glucose-free culture medium and then switched to KRBH buffer containing 1.7 mmol/l glucose for 1 h. The medium was then removed and replaced with KRBH containing 16.7 mmol/l glucose, with or without C21 (100 nM) and incubated for another hour. The supernatant was then collected for insulin assay using the same ELISA kit as that used for the serum insulin measurements.
Islet Isolation, Culture, and Treatment
The pancreases obtained from neonatal rats (3–4 days) were placed in sterile ice-cold HBSS (pH 7.4) containing penicillin (100 U/ml), streptomycin (0.1 mg/ml), and fungizone (0.25 mg/ml; Life Technologies). This HBSS was then replaced with another HBSS containing 1 mg/ml collagenase (type V; Sigma) and shaken (200 cycles/min) in a water bath (37°C) for 8–10 min to digest pancreatic tissue. The enzymatic action was terminated by adding 15 ml ice-cold HBSS. The digested pancreatic tissue was then gently dispersed by pipetting, collected by centrifugation, and resuspended in 5 ml RPMI 1640 (pH 7.4, 11.1 mM glucose and 25 mM HEPES; Life Technologies) supplemented with antibiotics (100 U/ml penicillin and 0.1 mg/ml streptomycin), fungizone (0.25 mg/ml), and 10% heat-inactivated, virus-free and mycoplasma-free FBS (Life Technologies). The digested pancreatic tissue with medium was distributed in 1-ml aliquots on petri dishes (100 mm; Falcon, Lincoln Park, NJ) containing 10 ml medium, followed by incubation at 37°C (O2-CO2, 95:5) for 24 h to obtain pure islets. The cultured islets were then resuspended with a 5-ml pipette, collected by centrifugation, counted by visual inspection under a dissecting microscope, and then transferred to Falcon 24-well culture plates (50 islets/well). After overnight incubation, the medium was removed, and islets were washed with fresh medium containing 0.25 ml Krebs-Ringer bicarbonate buffer (KRBB) supplemented with 10 mM HEPES and 2 mg/ml of bovine serum albumin. The islets were first incubated for 1 h in a medium containing 1.7 mM glucose and then incubated for additional 1 h in a medium containing 0.25 ml KRBB and 16.7 mM glucose. The islets of the control group were continuously kept in the medium with 1.7 mM glucose. During the second 1-h incubation, the islets were treated with ANG II (100 nM), ANG II (100 nM) + Los (1 μM), C21 (1 μM), and C21 (1 μM) + PD (1 μM). The Los and PD were added 10 min before ANG II and C21 were added. After incubation, the supernatant medium was collected for the measurement of insulin concentration, and the islets were continuously cultured for an additional 23 h by adding fresh medium with all the reagents. After 24 h, the islets were harvested for the evaluation of proinsulin 2 mRNA and insulin protein expression by using real-time RT-PCR and Western blotting analysis, respectively.
Quantitative Real-Time RT-PCR
The AT2R, AT1aR, and proinsulin 2 mRNA expressions were evaluated by real-time RT-PCR. Total RNA was extracted from the pancreas with TRIZOL Reagent (Invitrogen), which was then reversely transcribed into double-stranded cDNA using an iScript cDNA Synthesis Kit (Bio-Rad). Templates (50 ng cDNA) were subjected in triplicate to real-time PCR using a thermocycler (PTC-200 Peltier Thermal Cycler with CHROMO 4 Continuous Fluorescence Detector; Bio-Rad) and Brilliant III Ultra-Fast QPCR Master Mix (Agilent Technologies, Santa Clara, CA). Gene-specific primers and probes of rat AT2R (Rn.PT.49a.9182868.g), AT1aR (Rn.PT.49a.6344391), proinsulin 2 (Rn.PT.51.18628503.g), and GAPDH (Rn.PT.39a.11180736.g) from the Integrated DNA Technologies (Coralville, IA) were used for relative quantification of mRNA expressions. Table 1 shows the detailed sequences of primers and probes. The comparative CT method (ΔΔCT) was used to quantify the results obtained by real-time RT-PCR. The target gene mRNA was first normalized against GAPDH mRNA as ΔCT and then the ΔΔCT was calculated, where ΔΔCT = ΔCT (test sample) − ΔCT (control sample). The reported relative mRNA expression in the test sample compared with the control sample was calculated as 2ΔΔCT.
Table 1.
Gene-specific primers and probes for real-time PCR (from Integrated DNA Technologies)
| Name of Genes | Forward Primers | Reverse Primers | Probes |
|---|---|---|---|
| AT2R | CCCTTCCTGTATTGTTTCGTTG | GACATAGTCTCTCTCTTGCCTTG | 56-FAM/AGCTCCGTA/ZEN/GTGTGTTTAGAGTTCCCA/3IABkFQ |
| AT1aR | TCCTGTTCCACCCGATCA | GCCAGCCATTTTATACCAATCTC | 56-FAM/CAGCTCTGC/ZEN/CACATTCCCTGAGT/3IABkFQ |
| Proinsulin 2 | CAAGCAGGTCATTGTTCCAAC | GTAGAGAGCTTCCACCAAGTG | 56-FAM/CCAGAGGAT/ZEN/GAGCAGGGCCAG/3IABkFQ |
| GAPDH | AACCCATCACCATCTTCCAG | CCAGTAGACTCCACGACATAC | 56-FAM/CAGCACCAG/ZEN/CATCACCCCATTTG/3IABkFQ |
AT2R, angiotensin type 2 receptor; AT1aR, angiotensin type 1a receptor.
Western Blot Analysis
The protein abundance of AT2R, AT1R, and insulin was examined by Western blot. Briefly, the tissues were homogenized in RIPA buffer, and total protein was extracted from the homogenates. Protein concentration was measured using a protein assay kit (Pierce BCA Protein Assay Kit; Thermo Scientific) and then adjusted by adding 2× 4% SDS sample buffer to obtain equal concentrations among these samples. The samples were then loaded on a 10% SDS-PAGE gel (30 μg protein/well) and subjected to electrophoresis. The fractionized protein on the gel was electrically transferred to a PVDF membrane. The membrane was first incubated with blocking solution (5% defatted milk in TBST) for 30 min at room temperature to prevent nonspecific antibody binding and then probed with AT2R primary antibody (rabbit polyclonal to AT2R, ab78747, 1:1,000 dilution; Abcam), AT1R primary antibody (AT1R rabbit polyclonal IgG, sc-1173, 1:500; Santa Cruz Biotechnology), or insulin primary antibody (rabbit polyclonal antibody, sc-9168, 1:1,000 dilution; Santa Cruz Biotechnology). After incubation by primary antibodies, the membranes were probed with secondary antibodies followed by the treatment with enhanced chemiluminescence substrate (Pierce, Rockford, IL). The bands on the membrane were visualized and analyzed using a UVP BioImaging System. After the target gene blot density was obtained, the membrane was then treated with Restore Western Blot Stripping Buffer (Thermo Scientific) to remove the target gene band. The membrane was then probed with GAPDH primary antibody (GAPDH mouse monoclonal IgG, sc-32233, 1:1,000; Santa Cruz Biotechnology) followed by the same process to the target gene. The final reported data are the normalized AT2R, AT1R, or insulin band densities normalized to GAPDH.
Immunofluorescence Staining
The localization of AT2R and insulin in rat pancreatic islets and INS-1E cells was detected using immunofluorescence staining. For the pancreas preparation, the rat was killed by CO2, and the pancreas was rapidly removed, frozen, and sectioned on a cryostat to obtain 30-μm free-floating sections. For the INS-1E preparation, the cells were grown on a cover slip. The samples were fixed with 4% paraformaldehyde in PBS for 30 min at room temperature and then permeabilized with a solution containing 0.3% Triton X-100 dissolved in PBS. The samples were then blocked by using blocking solution containing 10% normal goat serum (NGS) and 0.3% Triton X-100 in PBS at room temperature for 2 h. The samples were then incubated with primary antibodies (rabbit anti-AT2R: 1:500, sc-9040; goat anti-insulin A: 1:500, sc-7839; Santa Cruz Biotechnology) in 10% NGS and 0.3% Triton X-100 in PBS at 4°C overnight. Following three washes with PBS, the samples were incubated for 2 h with secondary fluorescent antibodies (donkey anti-rabbit, A21206, 1:500; Donkey anti-goat, A11058, 1:500; Invitrogen). The samples were mounted with antifade reagent with DAPI (UltraCruzTM Mounting Medium, sc-24941; Santa Cruz Biotechnology) at room temperature. The slides were examined with a laser confocal microscope (objective: ×40, Leica TSC STED).
Statistical Analyses
All data are expressed as means ± SE. Significant differences between brain stem and other tissues, developmental stages, or control and various drug treatments were determined by one-way ANOVA followed post hoc by the Bonferroni test. Values of P < 0.05 were considered as statistically significant.
RESULTS
Tissue Distribution and Ontogeny of AT2R Protein
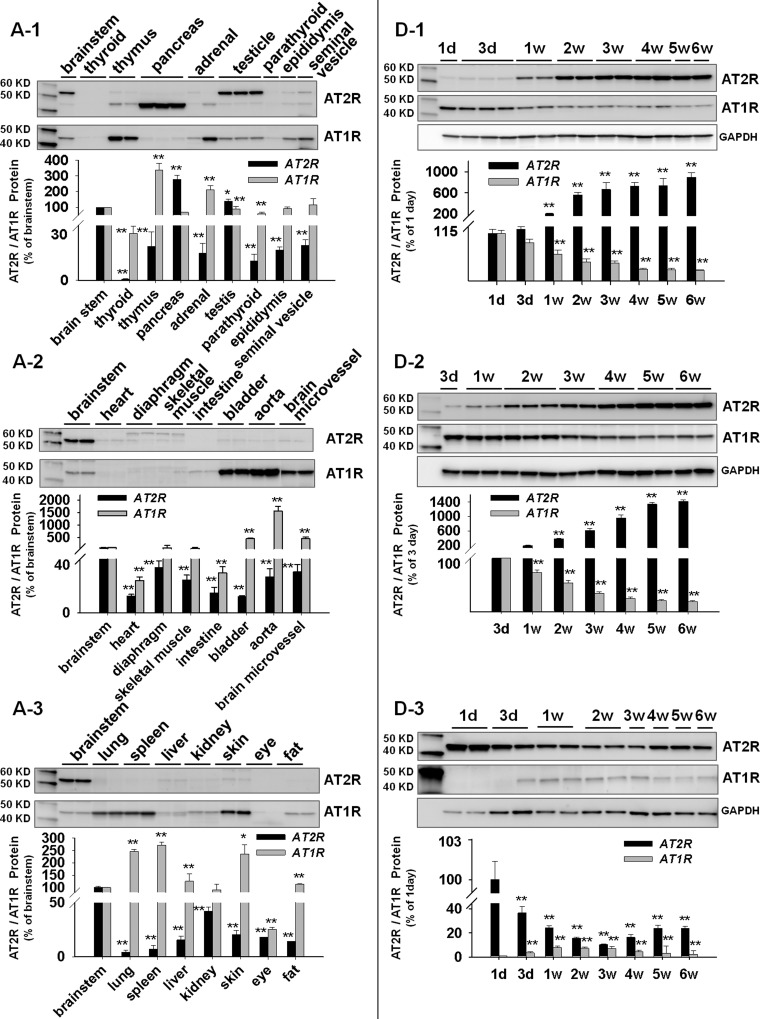
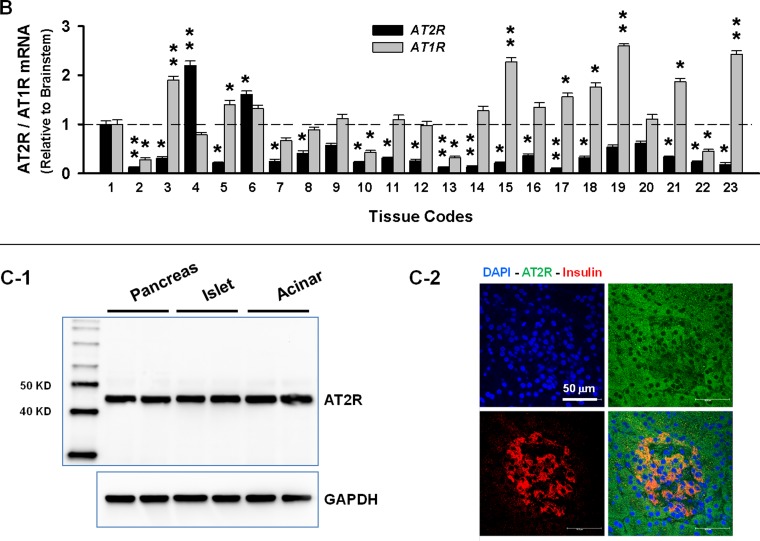
The first aim of this study was to evaluate the relative levels of AT2R protein expression in all available tissues of male adult rats. At the same time, the AT1R was also examined because of our previous finding showing that these two receptors displayed a strong negative correlation in expression pattern (15) during maturation. These data are shown in Fig. 1, A1, A2, and A3, in which the tissues were simply assigned to three groups. Group 1 represents glandular tissues, including thyroid, thymus, pancreas, adrenal, testicle, parathyroid, epididymis, and seminal vesicle (Fig. 1A1). Group 2 are muscular tissues or organs primarily constructed of muscle, including heart, diaphragm, skeletal muscle, intestine, bladder, aorta, and the brain microvessels isolated from the left cerebral hemisphere according to the techniques described by Goldstein et al. (18) (Fig. 1A2). The tissues that could not be placed in the above two groups were assigned as group 3, general tissues. These include lung, spleen, liver, kidney, skin, eyeball, and fat (Fig. 1A3). Because AT2R protein has been demonstrated to be stably expressed in brain stem in our previous studies (15, 42, 43), the brain stem therefore was used to normalize AT2R expression in other tissues to obtain comparable expression levels. From Fig. 1 it can be seen that the pancreas expresses the highest density of AT2R protein, followed by testicle and brain stem in adult rats. On the other hand, the highest AT1R protein expression was found in aorta, followed by bladder, thymus, microvessels, skin, spleen, and lung. Figure 1B shows the mRNA expressions showing higher AT2R mRNA in pancreas, testicle, and brain stem, a similar expression pattern to that of the proteins. In addition, we also confirmed the higher AT1R mRNA in thymus, aorta, liver, and fat.
Fig. 1.
Angiotensin type 2 receptor (AT2R) and angiotensin type 1 receptor (AT1R) protein and mRNA expressions. A: relative levels of AT2R and AT1R proteins in various tissues of adult male rats. Brain stem was employed as a standard to normalize the expression level. A1, glandular tissues; A2, muscular tissues; A3, general tissues. *P < 0.05 and **P < 0.01 compared with brain stem; n = 6 animals/group. B: relative expression levels of AT2R and AT1R mRNA in various tissues compared with the brain stem in adult male rats. Tissue Codes: 1, brain stem; 2, thyroid; 3, thymus; 4, pancreas; 5, adrenal; 6, testicle; 7, parathyroid; 8, epididymis; 9, seminal vesicle; 10, heart; 11, diaphragm; 12, skeletal muscle; 13, intestine; 14, bladder; 15, aorta; 16, brain microvessel; 17, lung; 18, spleen; 19, liver; 20, kidney; 21, skin; 22, eye; and 23, fat. *P < 0.05 and **P < 0.01 compared with brain stem; n = 4/group. C: AT2R protein in pancreas. B1, Western blot showing AT2R expression in the extract of whole pancreas, islet, and acinar. B2, immunofluorescence staining showing localization of AT2R protein in islets and acinar cells. D: ontogeny of AT2R and AT1R proteins in brain stem (D1), testicle (D2), and pancreas (D3). **P < 0.01 compared with day 1 or day 3; n = 5/group. d, day; w, wk after birth.
After identifying that the pancreas expresses the highest AT2R, we then determined the distribution of AT2R protein within this organ. From Fig. 1C1 it can seen that there were no apparent differences in AT2R expression between total pancreas, islet, and acinar, suggesting that the high AT2R protein is expressed in both endocrine and exocrine components of pancreas. This phenomenon was also confirmed by the immunofluorescence staining shown in Fig. 1C2.
In a previous study, we demonstrated the ontogeny of AT2R in mice with higher expression in adult and lower in fetus (15). In the current experiment, we therefore examined the developmental changes of AT2R protein in the brain stem, testicle, and pancreas, the three organs exhibiting abundant AT2R in adult rats. Figure 1B shows that AT2R protein expression is gradually increased in both brain stem (Fig. 1D1) and testicle (Fig. 1D2) of developing rats from 1 day to 6 wk of age. This expression pattern is similar to that reported previously in the mouse brain stem (15, 42). Interestingly, in the pancreas (Fig. 1D3), we observed a “U-shape curve” in AT2R expression during development, with the lowest point at 3 wk after birth and the highest expression in day 1. On the other hand, AT1R displayed an opposite expression pattern to AT2R in these three organs during development.
Effect of AT2R Activation on Serum Insulin, Blood Glucose, Blood Pressure, and Heart Rate
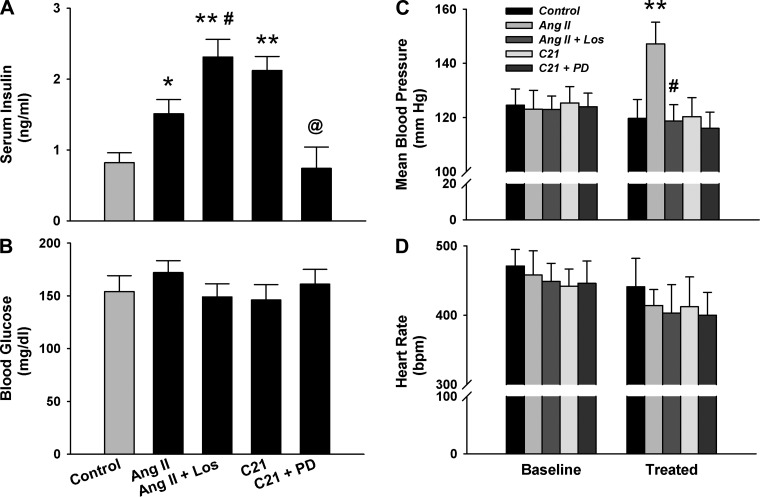
To evaluate the functional significance of AT2R in the endocrine pancreas, we examined the influence of ANG II, ANG II + Los, C21, and C21 + PD infusion for 7 days on the levels of serum insulin and blood glucose in the fed state. Figure 2A shows that ANG II significantly elevated serum insulin concentration compared with the control. This effect was further augmented by Los, an AT1R blocker. In addition, C21, a nonpeptide AT2R agonist, also significantly increased serum insulin level. This effect was completely abolished by PD, an AT2R antagonist. These results suggest an insulinotropic effect induced by the AT2R and a contrasting effect by the AT1R. However, none of these treatments significantly altered the blood glucose concentration in the fed state (Fig. 2B). As expected, ANG II treatment significantly increased the blood pressure, which was abolished by Los. C21 displayed no effect on blood pressure (Fig. 2C). None of these treatments significantly altered heart rate (Fig. 2D).
Fig. 2.
Serum insulin (A), blood glucose (B), mean blood pressure (C), and heart rate (D) in the rats receiving a 7-day treatment of angiotensin II (ANG II), ANG II + losartan (Los), compound 21 (C21), and C21 + PD-123319 (PD). *P < 0.05 and **P < 0.01 compared with control; #P < 0.05 compared with ANG II; @P < 0.05 compared with C21. n = 7/group.
Effect of AT2R Activation on Glucose Tolerance and Insulin Sensitivity
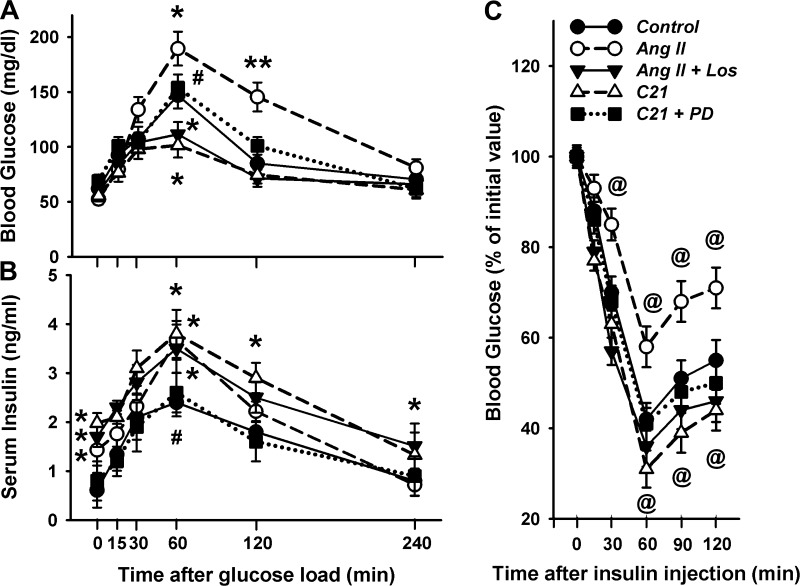
The basal blood glucose level after 16 h of fasting did not differ between groups (Fig. 3A). The peak glucose evoked by the glucose load was significantly lower in the C21 group compared with control. This effect was completely abolished by PD, suggesting that AT2R activation improved glucose tolerance. On the other hand, the ANG II-treated rats displayed significantly higher peak glucose levels, whereas ANG II + Los-treated rats exhibited significantly lower glucose compared with control. This result implies that ANG II impaired glucose tolerance by stimulating the AT1R. However, when the AT1R was occupied by Los, more ANG II binds to the AT2R to improve glucose tolerance. Accordingly, Los reversed not merely abolished the ANG II-evoked effect.
Fig. 3.
Oral glucose tolerance test (OGTT) and intraperitoneal insulin tolerance test (ipITT) in the rats receiving a 7-day treatment of ANG II, ANG II + Los, C21, and C21 + PD. A: blood glucose. B: serum insulin in OGTT. C: glucose concentration in ipITT. OGTT and ipITT were performed after 16 and 4 h of fasting, respectively. *P < 0.05 and **P < 0.01, ANG II, ANG II + Los, and C21 vs. control; #P < 0.05, C21 + PD vs. C21; @P < 0.05, ANG II and C21 vs. control. n = 7/group.
The insulin level during fasting conditions and after the glucose challenge was significantly higher in the C21-treated rats compared with control, which was completely abolished by PD (Fig. 3B). These data suggest that the above-described improvement of glucose tolerance was mediated by an AT2R-induced insulinotropic effect. Interestingly, ANG II-treated rats also displayed a higher peak insulin concentration compared with control, which was not altered by Los, suggesting that AT1R was not involved in this process.
Figure 3C shows the insulin tolerance test. After insulin administration, ANG II-treated rats displayed a blunted drop in blood glucose that was abolished by Los. On the other hand, C21-treated rats exhibited an enhanced insulin action. These results suggested that AT2R and AT1R also exerted an opposite effect on insulin sensitivity.
Effect of AT2R Activation on Expressions of Proinsulin 2 mRNA and Insulin Protein in the Rat Pancreas
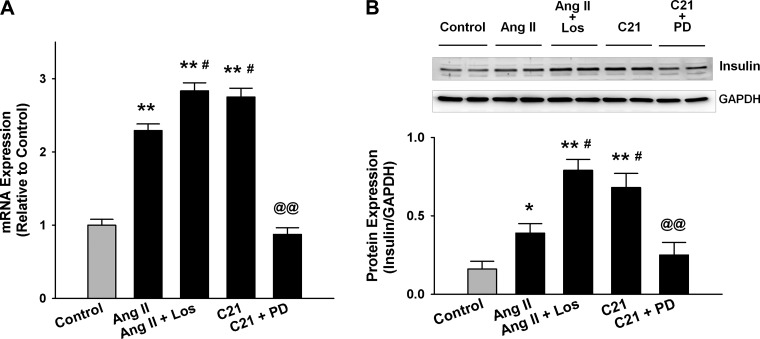
To determine a potential molecular mechanism underlying the elevation of serum insulin levels evoked by AT2R activation, we evaluated the expressions of proinsulin 2 mRNA and insulin protein in the rat pancreas from the above-described in vivo experiment. We found that both mRNA (Fig. 4A) and protein (Fig. 4B) levels in the pancreatic extract of C21-treated rats were significantly higher compared with control. This effect was completely abolished by PD, suggesting that the upregulated insulin gene transcription and translation contributed to the AT2R-induced elevation of blood insulin level. The ANG II-treated rats also displayed higher proinsulin 2 mRNA and insulin protein levels compared with the control, which was further upregulated by Los. These data suggest AT2R as the predominant angiotensin receptor subtype in pancreatic islets, and AT1R exhibits an opposite effect to AT2R.
Fig. 4.
Proinsulin 2 mRNA level by real-time RT-PCR (A) and insulin protein level by Western blot (B) in the whole pancreatic extract of the rats receiving a 7-day treatment of ANG II, ANG II + Los, C21, and C21 + PD. *P < 0.05 and **P < 0.01 compared with control; #P < 0.05 compared with ANG II; @@P < 0.01 compared with C21. n = 7/group.
Effects of AT2R Activation on Insulin Biosynthesis and Insulin Secretion in INS-1E Cells and Isolated Islets
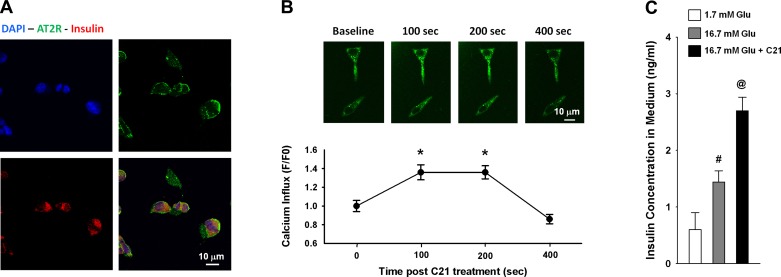
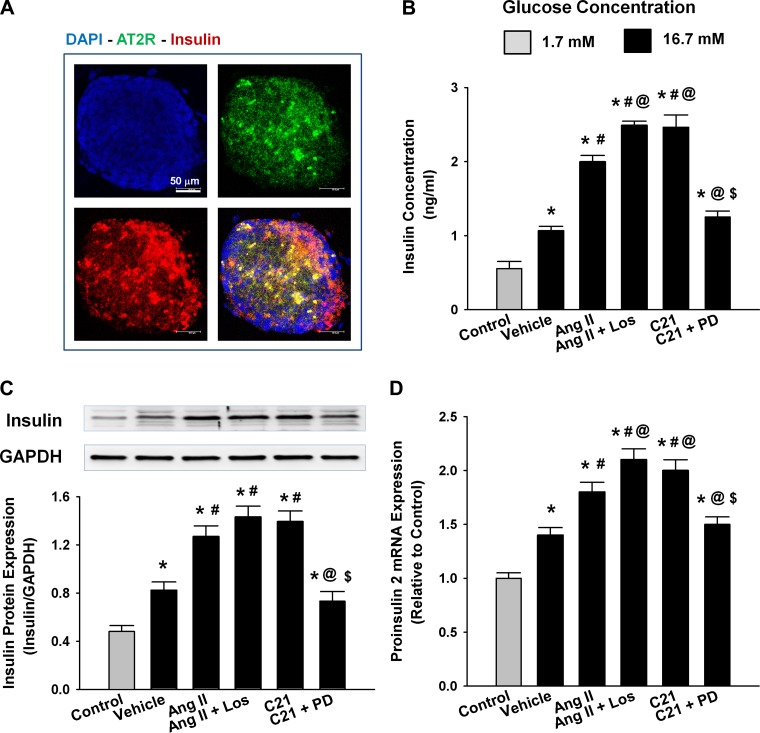
To determine if AT2R stimulation directly influences β-cells and islet function, we performed two in vitro experiments. Figure 5A shows the colocalization of positive immunoreactivity to AT2R and insulin proteins in individual INS-1E cells in which the AT2R was concentrated in the cellular membrane and insulin was primarily cytoplasmic, confirming that INS-1E is an insulin secretory cell line with AT2R. Figure 5B shows an increase in intracellular calcium of INS-1E as detected by increased green fluorescence at 100–200 s after addition of C21 in the medium. However, the intracellular calcium at 400 s after C21 treatment tended to be lower than the basal level, which could be because of the long duration of exposure to nonphysiological environmental conditions (e.g., room temperature). Figure 5C shows the elevation of insulin concentration in the medium of INS-1E after C21 treatment for 1 h. These results demonstrate that AT2R activation directly stimulates insulin secretion from β-cells. Figure 6 shows the increased insulin concentration in the culture medium of the isolated islets and upregulated insulin protein and proinsulin 2 mRNA expressions in the isolated islets by ANG II and C21 treatment. This insulinotropic effect of C21 was abolished by PD, and the ANG II's effect was augmented by Los.
Fig. 5.
Effect of C21 on calcium influx in INS-1E cells. A: colocalization of insulin and AT2R by immunofluorescence staining. B: calcium influx evoked by acute C21 treatment. C: insulin secretion induced by 1 h C21 treatment. *P < 0.05 compared with the baseline (0 min), n = 22 cells. #P < 0.05 compared with 1.7 mM glucose group; @P < 0.05 compared with 16.7 mM glucose group, n = 5/group.
Fig. 6.
Effect of C21 on insulin biosynthesis and secretion in dissociated islets from neonatal rats. A: colocalization of insulin and AT2R by immunofluorescence staining. B: insulin concentration in the medium. C and D: insulin protein expression and proinsulin 2 mRNA expression in the cultured islets. *P < 0.05 compared with control; #P < 0.05 compared with vehicle; @P < 0.05 compared with ANG II; $P < 0.05 compared with C21. n = 4/group.
DISCUSSION
A series of experiments from our laboratory have demonstrated a higher AT2R expression in the adult rat and mouse compared with the fetus and neonate (15, 42, 43). These results imply a potential functional significance of this ANG II receptor subtype in mature animals, contrary to the concept that currently prevails (5, 13). In the present study, we expanded this notion and found that the adult pancreas expressed the highest AT2R protein, which was equally distributed in both pancreatic islets and acinar cells. These results strongly suggest that the AT2R is potentially involved in both endocrine and exocrine functions of the pancreas. This idea was supported by an observation in larger animals employing autoradiography by Chappell et al. (7, 8). These investigators found that the pancreas of adult canine and primate expressed significantly higher AT2R than AT1R. Accordingly, they assumed that the AT2R was the predominant ANG II receptor subtype in the pancreas, a unique phenomenon opposite to that in other organs where the AT1R is predominant. Based on our current data and that of Chappell et al. (7, 8) we speculated that, in the mature animal, the pancreatic islets express the highest AT2R, which functions as the predominant ANG II receptor subtype. Taken together, these data strongly suggest that the net biological influence of ANG II on the endocrine pancreas of adult rats is primarily mediated by the AT2R rather than AT1R.
In the current study, we further evaluated the functional consequence of AT2R activation on the endocrine function of pancreas by subcutaneous infusion of ANG II, ANG II + Los, C21, and C21 + PD in adult rats. We found that C21 significantly increased plasma insulin concentration, and this effect was completely abolished by PD, strongly suggesting an insulinotropic effect of the AT2R. Interestingly, the rats receiving ANG II also displayed a significantly higher insulin level compared with the control rats, with this effect being further augmented by combined treatment with ANG II + Los. These results provide functional evidence supporting our hypothesis that the AT2R functions as the predominant ANG II receptor subtype in pancreatic islets of adult rats because ANG II evoked the same insulinotropic effect as C21. On the other hand, the data that Los further potentiated the ANG II-induced elevation of insulin implies an inhibitory effect of AT1R on the endocrine function of the pancreas offsetting the effects of AT2R. Moreover, this positive influence of Los on insulin secretion may also represent an enhanced angiotensin-converting enzyme (ACE) 2-ANG-(1—7)- Mas signaling pathway, which has recently been demonstrated to improve glycemia in ANG II-infused mice (9). We cannot rule out that ANG II is converted to ANG-(1— 7) by the action of ACE2. However, the C21 data suggest that the AT2R is at least one of the potent pathways that mediate this insulinotropic response. We acknowledge that the elevation of fasting insulin levels by AT2R stimulation might not be of benefit following C21 therapy. On the other hand, the enhanced insulin secretory response to glucose loading in the C21-treated rats implies a potential therapeutic benefit of this compound.
Data obtained from rat, mouse, and human experiments clearly corroborate the present finding that ANG II exerts an insulinotropic effect. For example, in rats, Ran et al. (33) found that 2 wk infusion of ANG II significantly increased plasma insulin level and insulin secretion induced by a glucose tolerance test (the insulin concentration was elevated during fasting and after glucose loading). This phenomenon was also observed in an ANG II-infused mouse model (9, 25). On the other hand, in a perfusion preparation of rat pancreas-duodenum, Carlsson et al. (6) observed an elevated insulin concentration in the effluent during acute ANG II perfusion. In cultured human islets (32), MIN6 cell clusters (32), and INS-1 cells (37), ANG II treatment also significantly increased insulin secretion, suggesting a direct insulinotropic effect in β-cells. In healthy humans, Buchanan et al. (4) have reported that an acute ANG II infusion caused dose-dependent elevations of plasma insulin. Because the insulin concentration was only an incidental observation, mechanisms were not explored in these experiments. It has been postulated that the increased plasma insulin concentration during ANG II administration reflects a reduction in whole body insulin clearance that may have been mediated by reduced renal blood flow in response to ANG II (4). Another explanation is that the insulin present in the islets was washed out and the effluent insulin concentration was momentarily increased because of ANG II-induced vasoconstriction (6). In addition, AT1R in β-cells was also assumed to mediate ANG II-evoked insulin secretion through elevations in intracellular calcium (32). Based on the data in the current study, however, we believe that it is the AT2R in pancreatic islets that directly contributes to the elevation of plasma insulin induced by ANG II treatment.
In the present study, we also found that the expressions of proinsulin 2 mRNA and insulin protein in the pancreas of rats receiving ANG II, ANG II + Los, and C21 treatment were significantly upregulated compared with the control rats. The effects of C21 were completely abolished by PD. These results suggest that AT2R-evoked elevation of plasma insulin concentration was mediated by the upregulation of proinsulin 2 gene transcription and insulin protein translation in the pancreas. Indeed, by employing the INS-1E cells and dissociated islets, we found that AT2R activation significantly elevated insulin level in the medium and upregulated the expressions of proinsulin 2 mRNA and insulin protein. In addition, we also observed the C21-evoked increase of intracellular Ca2+ concentration in the INS-1E cells, suggesting an improvement of insulin secretion by AT2R activation. At least four types of calcium channels exist: the L-type, T-type, P/Q-type, and N-type have been found in the INS-1E cells (31). We believe that both L-type and T-type channels probably contribute to the C21-evoked calcium influx because these two channels have been demonstrated to participate in insulin secretion of INS-1 cells (2, 12).
Consistent with the AT2R's insulinotropic effect, we also found that C21 treatment significantly attenuated the elevation of blood glucose level following a glucose challenge, concomitant with an increase in plasma insulin concentration. This result indicated that AT2R activation improved glucose tolerance in adult rats. Indeed, a more rapid clearance of glucose after a glucose load has been reported in C21-treated mice, and this effect was attributed to the enhancement of adipocyte differentiation by AT2R activation (30). However, incongruent with the observation that ANG II elevated plasma insulin level, we found that the ANG II-infused rats displayed an impaired glucose tolerance compared with control rats, a phenomenon similar to the observation by several other laboratories (9, 25). Although the exact mechanisms are still unclear, the potential reasons may include the reduced insulin sensitivity (34), vasoconstriction (6), impairment of intracellular insulin signaling (26), or suppression of glucose transporter translocation (29). We speculated that AT2Rs in pancreatic islets contribute to the ANG II-induced insulinotropic effect, whereas the AT1R in insulin-dependent tissues such as adipose tissue, skeletal muscle, and liver might be responsible for the ANG II-evoked impairment of glucose tolerance. Indeed, by using an insulin tolerance test, we found a significantly lower insulin sensitivity in ANG II-treated rats that was abolished by Los. Interestingly, we also observed an increase in insulin sensitivity in C21-treated rats. This finding implies that C21 not only stimulates pancreatic islets to improve insulin production but also acts on the insulin target tissues, probably in adipose tissue, skeleton muscle, and liver, to enhance insulin action. Indeed, several laboratories have recently reported an improvement of insulin resistance by C21 in diabetic rats and mice via decreasing the size of large adipocytes and increasing the number of small adipocytes (30, 36). Therefore, C21 might be employed as a potent antidiabetic therapy because of its dual effects on insulin.
Another interesting finding from this study is the ontogeny of AT2R protein expression in pancreas. When we characterized the tissue distribution of AT2R in adult rats, we found that, in addition to the pancreas, the brain stem and testicle also expressed high levels of AT2R protein. Although no data are available to associate AT2R with testicular function, the brain AT2R has been demonstrated to participate in the regulation of sympathetic tone by facilitating potassium channel activity (17) and exerts neuroprotective effects by suppressing apoptosis (28). In a previous study, we have demonstrated an age-related upregulation of AT2R protein expression in mouse brain stem (15). It is of interest to examine the developmental changes of AT2R in rat pancreas, brain stem, and testicle. Consistent with our findings in mouse brain stem (15), AT2R protein expression in rat brain stem and testicle was gradually upregulated during development, with higher AT2R in adult and lower AT2R in the neonate. However, rat pancreas exhibited a “U-shape” developmental change in AT2R expression from 1 day to 6 wk of age with the lowest expression around 3 wk after birth and highest expression in day 1. This unexpected finding is temporally coincidental with the time course of β-cell apoptosis during rat pancreatic development, in which a transient burst of β-cell apoptosis has been observed around 3 wk of age (14, 35), the period of weaning in rats. This phenomenon has also been confirmed in piglets (3) and humans (22) and was suggested to be associated with islet remodeling and/or changes in β-cell maturation (1). Given the antiapoptotic effect of AT2R observed in neurons (28) and myocardial cells (20), the transient downregulation of AT2R expression in the pancreas at 3 wk after birth may be responsible for the transient burst of β-cell apoptosis during the period of weaning. The antiapoptotic effect of the AT2R in β-cells should be an attractive area for therapy. In addition, the highest AT2R density observed in the 1-day neonate pancreas suggests a potential involvement of this receptor in islet growth and development. Indeed, the AT2R has been recently demonstrated to play a crucial role in the development of fetal pancreatic progenitor cells into islet-like cell clusters in the human embryo (23).
Finally, in the current experiment, we employed Western blotting analysis to determine the expression levels of AT2R, AT1R, and insulin proteins, which raises a concern of the antibody specificity. However, additional analyses using qPCR revealed a similar tendency of mRNA levels to change in the same direction as the protein, at least partially corroborating the results of Western blot analysis.
In conclusion, the data from the current study indicate that adult rat pancreas expresses the highest AT2R density of organs sampled. The AT2R was identified as a novel insulinotropic mediator. AT2R activation elevated blood insulin level and improved glucose tolerance via promoting proinsulin gene expression, insulin protein biosynthesis, insulin secretion, and insulin action. Application of C21, the first nonpeptide highly selective AT2R agonist, may be a promising therapeutic option for diabetes mellitus.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant RO1-HL-093028.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: C.H.S., I.H.Z., and L.G. conception and design of research; C.H.S. and L.G. performed experiments; C.H.S., I.H.Z., and L.G. analyzed data; C.H.S., I.H.Z., and L.G. interpreted results of experiments; C.H.S. and L.G. prepared figures; C.H.S., I.H.Z., and L.G. edited and revised manuscript; I.H.Z. and L.G. approved final version of manuscript; L.G. drafted manuscript.
ACKNOWLEDGMENTS
Dr. Pierre Maechler in the University of Geneva generously provided us the INS-1E cell line. Compound 21 used in this study was a gift from Vicore Pharma. The authors acknowledge the expert technical assistance of Li Yu.
REFERENCES
- 1. Ackermann AM, Gannon M. Molecular regulation of pancreatic beta-cell mass development, maintenance, and expansion. J Mol Endocrinol 38: 193–206, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Bhattacharjee A, Whitehurst RM, Jr, Zhang M, Wang L, Li M. T-type calcium channels facilitate insulin secretion by enhancing general excitability in the insulin-secreting beta-cell line, INS-1. Endocrinology 138: 3735–3740, 1997 [DOI] [PubMed] [Google Scholar]
- 3. Bock T, Kyhnel A, Pakkenberg B, Buschard K. The postnatal growth of the beta-cell mass in pigs. J Endocrinol 179: 245–252, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Buchanan TA, Thawani H, Kades W, Modrall JG, Weaver FA, Laurel C, Poppiti R, Xiang A, Hsueh W. Angiotensin II increases glucose utilization during acute hyperinsulinemia via a hemodynamic mechanism. J Clin Invest 92: 720–726, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carey RM, Wang ZQ, Siragy HM. Role of the angiotensin type 2 receptor in the regulation of blood pressure and renal function. Hypertension 35: 155–163, 2000 [DOI] [PubMed] [Google Scholar]
- 6. Carlsson PO, Berne C, Jansson L. Angiotensin II and the endocrine pancreas: effects on islet blood flow and insulin secretion in rats. Diabetologia 41: 127–133, 1998 [DOI] [PubMed] [Google Scholar]
- 7. Chappell MC, Bosch SM, Hansen BC, Ferrario CM, Diz DI. Differential expression of AT2 angiotensin II receptors in the primate pancreas (Abstract). J Hypertension 12: S181, 1994 [Google Scholar]
- 8. Chappell MC, Diz DI, Jacobsen DW. Pharmacological characterization of angiotensin II binding sites in the canine pancreas. Peptides 13: 313–318, 1992 [DOI] [PubMed] [Google Scholar]
- 9. Chhabra KH, Xia H, Pedersen KB, Speth RC, Lazartigues E. Pancreatic angiotensin-converting enzyme 2 improves glycemia in angiotensin II-infused mice. Am J Physiol Endocrinol Metab 304: E874–E884, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chiu AT, Herblin WF, McCall DE, Ardecky RJ, Carini DJ, Duncia JV, Pease LJ, Wong PC, Wexler RR, Johnson AL. Identification of angiotensin II receptor subtypes. Biochem Biophys Res Commun 165: 196–203, 1989 [DOI] [PubMed] [Google Scholar]
- 11. Chu KY, Leung PS. Angiotensin II type 1 receptor antagonism mediates uncoupling protein 2-driven oxidative stress and ameliorates pancreatic islet beta-cell function in young Type 2 diabetic mice. Antioxid Redox Signal 9: 869–878, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Cui X, Yang G, Pan M, Zhang XN, Yang SN. Akt signals upstream of L-type calcium channels to optimize insulin secretion. Pancreas 41: 15–21, 2012 [DOI] [PubMed] [Google Scholar]
- 13. de GM, Catt KJ, Inagami T, Wright JW, Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev 52: 415–472, 2000 [PubMed] [Google Scholar]
- 14. Finegood DT, Scaglia L, Bonner-Weir S. Dynamics of beta-cell mass in the growing rat pancreas. Estimation with a simple mathematical model. Diabetes 44: 249–256, 1995 [DOI] [PubMed] [Google Scholar]
- 15. Gao J, Chao J, Parbhu KJ, Yu L, Xiao L, Gao F, Gao L. Ontogeny of angiotensin type 2 and type 1 receptor expression in mice. J Renin Angiotensin Aldosterone Syst 13: 341–352, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gao L, Wang WZ, Wang W, Zucker IH. Imbalance of angiotensin type 1 receptor and angiotensin II type 2 receptor in the rostral ventrolateral medulla: potential mechanism for sympathetic overactivity in heart failure. Hypertension 52: 708–714, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gao L, Zucker IH. AT2 receptor signaling and sympathetic regulation. Curr Opin Pharmacol 11: 124–130, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goldstein GW, Wolinsky JS, Csejtey J, Diamond I. Isolation of metabolically active capillaries from rat brain. J Neurochem 25: 715–717, 1975 [DOI] [PubMed] [Google Scholar]
- 19. Kambayashi Y, Bardhan S, Takahashi K, Tsuzuki S, Inui H, Hamakubo T, Inagami T. Molecular cloning of a novel angiotensin II receptor isoform involved in phosphotyrosine phosphatase inhibition. J Biol Chem 268: 24543–24546, 1993 [PubMed] [Google Scholar]
- 20. Kaschina E, Grzesiak A, Li J, Foryst-Ludwig A, Timm M, Rompe F, Sommerfeld M, Kemnitz UR, Curato C, Namsolleck P, Tschope C, Hallberg A, Alterman M, Hucko T, Paetsch I, Dietrich T, Schnackenburg B, Graf K, Dahlof B, Kintscher U, Unger T, Steckelings UM. Angiotensin II type 2 receptor stimulation: a novel option of therapeutic interference with the renin-angiotensin system in myocardial infarction? Circulation 118: 2523–2532, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Kaschina E, Unger T. Angiotensin AT1/AT2 receptors: regulation, signalling and function. Blood Press 12: 70–88, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Kassem SA, Ariel I, Thornton PS, Scheimberg I, Glaser B. Beta-cell proliferation and apoptosis in the developing normal human pancreas and in hyperinsulinism of infancy. Diabetes 49: 1325–1333, 2000 [DOI] [PubMed] [Google Scholar]
- 23. Leung KK, Liang J, Ma MT, Leung PS. Angiotensin II type 2 receptor is critical for the development of human fetal pancreatic progenitor cells into islet-like cell clusters and their potential for transplantation. Stem Cells 30: 525–536, 2012 [DOI] [PubMed] [Google Scholar]
- 24. Merglen A, Theander S, Rubi B, Chaffard G, Wollheim CB, Maechler P. Glucose sensitivity and metabolism-secretion coupling studied during two-year continuous culture in INS-1E insulinoma cells. Endocrinology 145: 667–678, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Mitsuishi M, Miyashita K, Muraki A, Itoh H. Angiotensin II reduces mitochondrial content in skeletal muscle and affects glycemic control. Diabetes 58: 710–717, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Motley ED, Eguchi K, Gardner C, Hicks AL, Reynolds CM, Frank GD, Mifune M, Ohba M, Eguchi S. Insulin-induced Akt activation is inhibited by angiotensin II in the vasculature through protein kinase C-alpha. Hypertension 41: 775–780, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Mukoyama M, Nakajima M, Horiuchi M, Sasamura H, Pratt RE, Dzau VJ. Expression cloning of type 2 angiotensin II receptor reveals a unique class of seven-transmembrane receptors. J Biol Chem 268: 24539–24542, 1993 [PubMed] [Google Scholar]
- 28. Namsolleck P, Boato F, Schwengel K, Paulis L, Matho KS, Geurts N, Thone-Reineke C, Lucht K, Seidel K, Hallberg A, Dahlof B, Unger T, Hendrix S, Steckelings UM. AT2-receptor stimulation enhances axonal plasticity after spinal cord injury by upregulating BDNF expression. Neurobiol Dis 51: 177–191, 2013 [DOI] [PubMed] [Google Scholar]
- 29. Ogihara T, Asano T, Ando K, Chiba Y, Sakoda H, Anai M, Shojima N, Ono H, Onishi Y, Fujishiro M, Katagiri H, Fukushima Y, Kikuchi M, Noguchi N, Aburatani H, Komuro I, Fujita T. Angiotensin II-induced insulin resistance is associated with enhanced insulin signaling. Hypertension 40: 872–879, 2002 [DOI] [PubMed] [Google Scholar]
- 30. Ohshima K, Mogi M, Jing F, Iwanami J, Tsukuda K, Min LJ, Ogimoto A, Dahlof B, Steckelings UM, Unger T, Higaki J, Horiuchi M. Direct angiotensin II type 2 receptor stimulation ameliorates insulin resistance in type 2 diabetes mice with PPARgamma activation. PLoS One 7: e48387, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Orecna M, Hafko R, Toporcerova V, Strbak V, Bacova Z. Cell swelling-induced insulin secretion from INS-1E cells is inhibited by extracellular Ca2+ and is tetanus toxin resistant. Cell Physiol Biochem 26: 197–208, 2010 [DOI] [PubMed] [Google Scholar]
- 32. Ramracheya RD, Muller DS, Wu Y, Whitehouse BJ, Huang GC, Amiel SA, Karalliedde J, Viberti G, Jones PM, Persaud SJ. Direct regulation of insulin secretion by angiotensin II in human islets of Langerhans. Diabetologia 49: 321–331, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Ran J, Hirano T, Adachi M. Chronic ANG II infusion increases plasma triglyceride level by stimulating hepatic triglyceride production in rats. Am J Physiol Endocrinol Metab 287: E955–E961, 2004 [DOI] [PubMed] [Google Scholar]
- 34. Rao RH. Pressor doses of angiotensin II increase hepatic glucose output and decrease insulin sensitivity in rats. J Endocrinol 148: 311–318, 1996 [DOI] [PubMed] [Google Scholar]
- 35. Scaglia L, Cahill CJ, Finegood DT, Bonner-Weir S. Apoptosis participates in the remodeling of the endocrine pancreas in the neonatal rat. Endocrinology 138: 1736–1741, 1997 [DOI] [PubMed] [Google Scholar]
- 36. Shum M, Pinard S, Guimond MO, Labbe SM, Roberge C, Baillargeon JP, Langlois MF, Alterman M, Wallinder C, Hallberg A, Carpentier AC, Gallo-Payet N. Angiotensin II type 2 receptor promotes adipocyte differentiation and restores adipocyte size in high-fat/high-fructose diet-induced insulin resistance in rats. Am J Physiol Endocrinol Metab 304: E197–E210, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Siebelmann M, Wensing J, Verspohl EJ. The impact of ANG II and IV on INS-1 cells and on blood glucose and plasma insulin. J Recept Signal Transduct Res 30: 234–245, 2010 [DOI] [PubMed] [Google Scholar]
- 38. Unger T, Dahlof B. Compound 21, the first orally active, selective agonist of the angiotensin type 2 receptor (AT2): implications for AT2 receptor research and therapeutic potential. J Renin Angiotensin Aldosterone Syst 11: 75–77, 2010 [DOI] [PubMed] [Google Scholar]
- 39. Wan Y, Wallinder C, Plouffe B, Beaudry H, Mahalingam AK, Wu X, Johansson B, Holm M, Botoros M, Karlen A, Pettersson A, Nyberg F, Fandriks L, Gallo-Payet N, Hallberg A, Alterman M. Design, synthesis, and biological evaluation of the first selective nonpeptide AT2 receptor agonist. J Med Chem 47: 5995–6008, 2004 [DOI] [PubMed] [Google Scholar]
- 40. Wang L, Leung PS. The role of renin-angiotensin system in cellular differentiation: implications in pancreatic islet cell development and islet transplantation. Mol Cell Endocrinol 2013; PMID: 23994025 [DOI] [PubMed] [Google Scholar]
- 41. Whitebread S, Mele M, Kamber B, de GM. Preliminary biochemical characterization of two angiotensin II receptor subtypes. Biochem Biophys Res Commun 163: 284–291, 1989 [DOI] [PubMed] [Google Scholar]
- 42. Yu L, Shao C, Gao L. Developmental expression patterns for angiotensin receptors in mouse skin and brain. J Renin Angiotensin Aldosterone Syst 2012; PMID: 22526820 [DOI] [PubMed] [Google Scholar]
- 43. Yu L, Zheng M, Wang W, Rozanski GJ, Zucker IH, Gao L. Developmental changes in AT1 and AT2 receptor-protein expression in rats. J Renin Angiotensin Aldosterone Syst 11: 214–221, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]