Abstract
Prolactin (PRL) and placental lactogens stimulate β-cell replication and insulin production in pancreatic islets and insulinoma cells through binding to the PRL receptor (PRLR). However, the contribution of PRLR signaling to β-cell ontogeny and function in perinatal life and the effects of the lactogens on adaptive islet growth are poorly understood. We provide evidence that expansion of β-cell mass during both embryogenesis and the postnatal period is impaired in the PRLR−/− mouse model. PRLR−/− newborns display a 30% reduction of β-cell mass, consistent with reduced proliferation index at E18.5. PRL stimulates leucine incorporation and S6 kinase phosphorylation in INS-1 cells, supporting a role for β-cell mTOR signaling in PRL action. Interestingly, a defect in the development of acini is also observed in absence of PRLR signaling, with a sharp decline in cellular size in both endocrine and exocrine compartments. Of note, a decrease in levels of IGF-II, a PRL target, in the Goto-Kakizaki (GK) rat, a spontaneous model of type 2 diabetes, is associated with a lack of PRL-mediated β-cell proliferation in embryonic pancreatic buds. Reduced pancreatic IGF-II expression in both rat and mouse models suggests that this factor may constitute a molecular link between PRL signaling and cell ontogenesis. Together, these results provide evidence that PRL signaling is essential for pancreas ontogenesis during the critical perinatal window responsible for establishing functional β-cell reserve.
Keywords: insulin, diabetes, proliferation, apoptosis, IGF-II, placental lactogens
adult pancreatic β-cells are terminally differentiated and have a low proliferative capacity. However, high rates of β-cell neogenesis and proliferation during perinatal adaptation establish a functional β-cell reserve, which appears to be essential for long-term protection against type 2 diabetes (35).
The factors controlling β-cell development during the perinatal period are unclear, but several lines of evidence suggest a role for the lactogenic hormones of the pituitary (prolactin, PRL) and placenta (placental lactogens, PL). Known for pleiotropic effects on mammary development, immunoregulation, brain function and behavior, and electrolyte and energy homeostasis (4, 7, 20), the lactogens circulate at high concentrations in the late-gestational fetus and newborn infant (18, 35) and stimulate β-cell replication and glucose-stimulated insulin secretion (GSIS) in isolated human and rodent islets and rat and mouse insulinoma cells (2, 3, 9, 15). Overexpression of rat PL in β-cells in vitro increases β-cell replication and glucose-stimulated insulin production (14, 35), while targeted overexpression of mouse PL in transgenic mice increases β-cell proliferation and protects against β-cell death induced by streptozotocin (44). These effects are mediated through Jak2/Stat5 activation and upregulation of antiapoptotic proteins (19). PRL also protects rat insulinoma cells against glucolipotoxicity-induced cell death through similar signaling pathways (26). Conversely, global deletion (knockout/KO) of the PRL receptor (PRLR), which mediates the actions of PL as well as PRL (5, 8, 11), reduces β-cell mass and GSIS in pregnant and nonpregnant mice (16, 23).
While these findings establish a physiological role for the lactogens in islet and β-cell maturation, development, and function in postnatal life, the role of PRL signaling in β-cells during perinatal adaptation remains poorly understood. Also unclear are the molecular mechanisms by which the lactogens promote β-cell expansion. A recent study suggested that menin, an endocrine tumor suppressor and transcriptional regulator and a potential PRL target, participates in the physiological adaptation of β-cell mass during pregnancy (24). Additional investigations highlight the roles of other mediators of PRL action (e.g., serotonin, cell cyclins, and cyclin-dependent kinases/inhibitors) (2, 3, 25). Among PRL-regulated genes, we and others have shown that insulin-like growth factor II (IGF-II) is a PRL target in mammary gland (10) and brown adipocytes (46). PRLR−/− newborns exhibited hypotrophic brown adipose tissue related to a decreased IGF-II content (46). Decreases in pancreatic IGF-II expression and β-cell mass have been also previously demonstrated in the Goto-Kakizaki (GK) rat, a spontaneous model of type 2 diabetes (12, 21). Thus, we postulated that IGF-II might be a mediator of PRL-induced pancreatic development and growth.
The aim of this study was to clarify the physiological role of PRL signaling in pancreatic and β-cell development, growth, and igf2 expression. For this purpose, we analyzed pancreas development using two rodent models (PRLR−/− mice and GK rats) during the perinatal period, which constitutes the critical stage for establishment of β-cell reserve (13, 35). Our findings indicate that PRL signaling and IGF-II act in concert to modulate pancreatic β-cell and acinar growth during perinatal development.
MATERIALS AND METHODS
Animals.
PRLR+/+ and PRLR−/− 129/SvJ mice (36) were kept on a 12:12-h light-dark cycle in a temperature-controlled colony room with ad libitum access to food and water. GK and Wistar (used as control) rats were bred in our local colony (34) and housed with free access to food and water. Pregnancy was confirmed by the presence of a vaginal plug. Pregnant rat females (days 13.5 of gestation) were euthanized with lethal doses of pentobarbital sodium (Ceva Santé Animale, Libourne, France). All procedures were approved by the Ministère de l'Agriculture, France.
Tissues.
Embryos from Wistar and pregnant GK rats at 13.5 days of gestation were removed from the uterus and weighed. The dorsal pancreatic rudiments were dissected and separated from surrounding mesenchyme as previously described (32). Body weights of PRLR+/+ and PRLR−/− mice were recorded at embryonic day 18.5 (E18.5), the day of birth (D0.5), and 6 days later (D6.5). Mice were euthanized and pancreases were collected, weighted, and immediately placed in Tri Reagent solution (Life Technologies, Saint-Aubin, France) or in 4% PFA to explore gene expression and histological analyses, respectively.
Effects of PRL on leucine uptake and phosphorylated p70 S6 kinase in rat insulinoma cells.
Rat insulinoma (INS-1) cells were grown to 80% confluence in RPMI containing 10% fetal bovine serum. The cells were washed three times with PBS and incubated in serum-free RPMI containing 5 mM glucose, 0.1% human serum albumin, 10 μg/ml human transferrin, 50 μM ethanolamine, 0.1 nM triiodothyronine, 50 μM phosphoethanolamine, and 1% antibiotic-antimycotic solution.
To quantify the effects of PRL on leucine uptake, we pretreated the cells with rapamycin (100 nM) or diluent for 30 min and then added rat PRL (20 nM) or diluent to the medium. The cells were incubated for 20 h; [14C]leucine (0.25 μCi/ml) was added 16 h prior to terminating the experiment. After extensive washing, the cellular protein was precipitated with cold 10% TCA, washed, and dissolved in 0.3 N NaOH. [14C]leucine incorporated into TCA-precipitable proteins was normalized to total cellular protein.
We used Western blot analysis to estimate the levels of phosphorylated p70 S6 kinase in PRL- and diluent-treated cells. The cells were treated with rat PRL (20 nM) or diluent in serum-free medium for 2 or 16 h. The cells were then washed in ice-cold PBS and centrifuged at 5,000 g for 1 min. Whole cell lysates (30 μg of protein) were separated on 4–12% Bis-Tris SDS-PAGE (Invitrogen, Carlsbad, CA) and transferred to polyvinylidene difluoride membranes. The membranes were washed in TBS-T, blocked for 60 min in 4% milk buffer (Chemicon) with 3% BSA (Sigma), washed again in TBS-T, and incubated in 1% PVP + TBS solution containing a rabbit polyclonal antibody to phosphorylated p70 S6 kinase (Thr421/Ser424; Santa Cruz Biotechnology, Santa Cruz, CA) or to p70 S6 kinase (Cell Signaling Technology, Danvers, MA). After overnight incubation at 4°C, the blots were washed in TBS-T and incubated with an anti-rabbit IgG (1:10,000; GE Health Care, Piscataway, NJ) for 1 h and washed in TBS-T. After the washing, the membranes were exposed to chemiluminescent substrate (ECL Advance Western blotting detection kit; GE Health Care) and imaged using the VersaDoc model 4000 imaging system (Bio-Rad, Los Angeles, CA). Mouse monoclonal anti-tubulin antibody was used to detect tubulin as an internal control.
Dorsal pancreatic rudiment culture.
Culture of dorsal pancreatic rudiments was performed as previously described (12, 32). Pancreatic rudiments were grown in three-dimensional collagen gels containing RPMI (Sigma-Aldrich, Saint Quentin Fallavier, France), 80% type I rat tail collagen (3 mg/ml; Institut Jacques Boy, Reims, France), and 10% sodium bicarbonate in 0.1 mM NaOH. The culture medium consisted of 500 μl of RPMI 1640 (Cambrex, Emerainville, France) supplemented with 5.5 mM glucose and penicillin-streptomycin (50 U/ml and 50 μg/ml, respectively, Cambrex), HEPES (10 mM, Cambrex), l-glutamine (2 mM, Cambrex), nonessential amino acids 1× (Life Technologies), and 1% heat-inactivated horse serum. Cultures were incubated at 37°C in a humidified atmosphere of 95% O2 and 5% CO2. The culture medium was changed every 2 days, and the media were stored at −20°C until measurement of insulin concentration. In some experiments, ovine PRL (1 μg/ml, Sigma-Aldrich) was added daily. During the last hour of culture, 5 μl of bromodeoxyuridine (BrdU) was added to the medium to label cells in the S phase. At the end of the culture, pancreatic rudiments were fixed for 1 h in aqueous Bouin's solution.
Immunohistochemistry.
Whole pancreases were fixed in aqueous Bouin's solution, paraplast embedded, and sliced in serial sections (5 μm thickness). After rehydration, sections were permeabilized with 0.1% Triton X-100 diluted in PBS for 10 min at room temperature. Sections were rinsed in PBS, incubated in peroxidase blocking solution (3% H2O2 in PBS) for 10 min, rinsed again in PBS, and incubated with blocking solution diluted with antibody diluent (Cell Marque, Rocklin, CA) for 1 h at room temperature. Every 20 sections throughout the length of the pancreas, one section was immunostained for insulin, glucagon, and somatostatin to identify β-, α-, and δ-cells, respectively. Sections were subsequently incubated overnight at 4°C with guinea pig anti-insulin (MP Biomedical, Illkirch, France; diluted 1:200 in blocking solution), rabbit anti-glucagon (Millipore, Molsheim, France; diluted 1:500 in blocking solution), and rabbit anti-somatostatin (Millipore; diluted 1:500 in blocking solution). Sections were rinsed in PBS and incubated with an anti-guinea pig IgG (Dako, Trappes, France; diluted 1:200 in blocking solution) or an anti-rabbit IgG (Abiocode, Agoura Hills, CA; diluted 1:200 in blocking solution). After the PBS washing, sections were incubated in DAB peroxidase substrate solution for 1 min, washed in distilled water, and coverslipped with glycergel (Dako). Determination of β-cell size was calculated (μm2) after quantification of β-cell surface by ImageJ software divided by the total number of nuclei [at least 750 cells counted for each mouse (n = 3–6/group)].
Proliferation assays.
Pregnant and newborn mice and GK rats were injected with BrdU (50 mg/kg body wt) 1 h before pancreas isolation. Paraffin-embedded pancreatic tissue was processed as described above. BrdU detection (Cell proliferation kit; GE Healthcare, Velizy-Villacoublay, France) was performed as previously described (43). Sections were incubated with fast red substrate (Dako) for 2 min, rinsed in distilled water, hematoxylin counterstained, and coverslipped with glycergel (Dako). At least 2,000 cells on six sections per pancreas were counted for each animal (n = 5–7 per group). The percentage of BrdU-positive cells of either insulin-positive cells or acinar cells was calculated at E18.5 and D6.5 for each pancreatic section with the ImageJ software (http://rsbweb.nih.gov/ij/).
β-Cell number calculation.
Total β-cell surface (β-cell surfacetot) and pancreas surface were calculated from insulin-immunostained sections of PRLR+/+ and PRLR−/− mice at E18.5 and D6.5, as described above. β-Cell number was calculated by the following equation: total β-cell surface (β-cell surfacetot in μm2) was divided by the mean of the β-cell surface (β-cell surface in μm2); this value was further normalized to pancreas surface (mm2).
Apoptosis assay.
Apoptosis detection was performed with ApopTag Peroxidase In Situ Apoptosis Detection Kit (Millipore). Insulin detection and hematoxylin counterstaining was performed as described above. The percentage of insulin-positive cells was counted for each genotype at both E18.5 and D6.5 with the ImageJ software.
Gene expression analyses.
Whole pancreases from PRLR+/+ and PRLR−/− were isolated at E18.5, D0.5, and D6.5; total RNA was extracted from each sample by homogenization with Tissuelyser (Qiagen, Courtaboeuf, France). Quantitative real-time PCR was performed as described previously (46). After DNAse I treatment, RNA was reverse-transcribed and used for quantitative RT-PCR (qRT-PCR) using the Power SYBR Green PCR Master Mix (Applied Biosystems, Courtaboeuf, France). Final primer concentrations were 300 nM (primer sequences are available upon request). Reaction parameters were carried out on a StepOne Real-Time PCR System (Applied Biosystems). Relative expression within a given sample was calculated as a ratio (amoles of specific gene/fmoles of 18S). Results are means ± SE.
Statistical analyses.
Data are expressed as means ± SE and were analyzed using a nonparametric Mann-Whitney test with use of the computer software Prism 5 (GraphPad Software, San Diego, CA). Statistical significance is indicated at P values <0.05, <0.01, and <0.001.
RESULTS
PRL signaling is required for pancreas development.
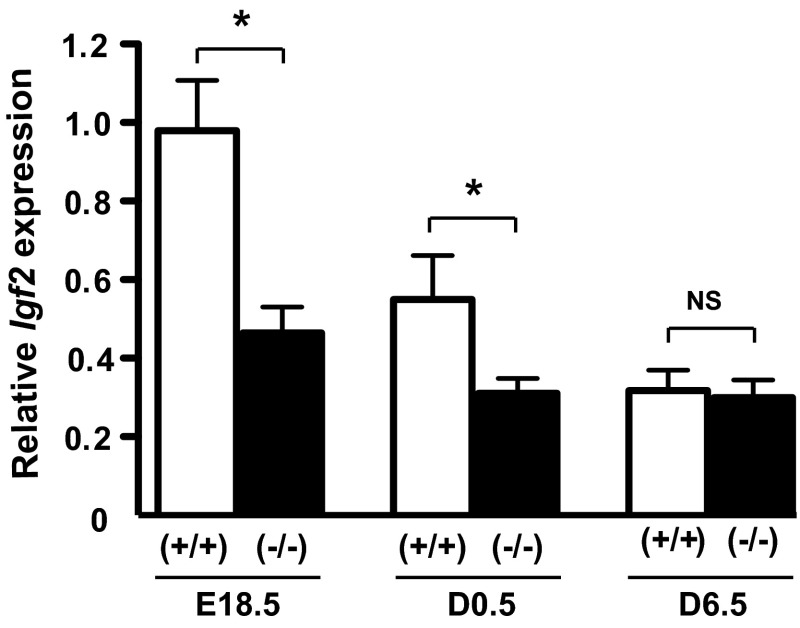
To assess the impact of PRL signaling on pancreas development and the role of PRLR in β-cell ontogenesis, we analyzed a mouse model that is globally deficient in PRLR (36). No differences were detected in body weight or pancreas weight between PRLR+/+ and PRLR−/− mice at E18.5 and D0.5 (Table 1). However, body weight and pancreas weight were ∼25% lower on day 6.5 in the PRLR−/− mice than in wild-type (WT) mice. There was no difference in the ratio of pancreas weight to body weight between the genotypes. Moreover, given that Igf2, a pivotal fetal growth factor, was demonstrated as a PRL target, we measured pancreatic mRNA Igf2 level. As shown in Fig. 1, we observed a developmental stage-dependent reduction of pancreatic Igf2 expression from E18.5 to D6.5 that was more pronounced in PRLR+/+ (P < 0.001) than in PRLR−/− (P < 0.05), consistent with the dramatic fall in IGF-II production in the mouse after birth, already reported in other rodent models (22, 28, 38). More importantly, we showed that PRLR−/− mice display a significant lower pancreatic Igf2 expression at both E18.5 and D0.5 than those of WT animals, such a difference becoming nonsignificant afterward. We thus focused our study at E18.5 and D6.5 to determine whether the lack of PRLR signaling and its subsequent pancreatic Igf2 decrease at birth were associated with defects in endocrine and exocrine pancreatic development during morphogenesis.
Table 1.
Effects of PRLR deficiency on whole body and pancreas weights
| Body Weight, g |
Pancreas Weight, mg |
Pancreas Weight/Body Weight, ‰ |
||||
|---|---|---|---|---|---|---|
| +/+ | −/− | +/+ | −/− | +/+ | −/− | |
| E18.5 (n = 32–47) | 1.08 ± 0.02 | 1.10 ± 0.02 | 5.98 ± 0.17 | 6.10 ± 0.19 | 5.54 ± 0.14 | 5.59 ± 0.18 |
| D0.5 (n = 26–50) | 1.33 ± 0.03 | 1.43 ± 0.02 | 7.73 ± 0.32 | 8.58 ± 0.17 | 5.80 ± 0.21 | 6.01 ± 0.12 |
| D6.5 (n = 37–39) | 3.73 ± 0.09 | 2.80 ± 0.07*** | 13.5 ± 0.58 | 10.54 ± 0.39*** | 3.65 ± 0.16 | 3.71 ± 0.15 |
Data are presented as means ± SE. Evolution of body weight and pancreas weight and their ratio in PRLR+/+ and PRLR−/− mice between embryonic day (E)18.5 and postnatal day (D)6.5. PRLR, prolactin receptor. Statistical analyses were performed between the two genotypes
P < 0,001.
Fig. 1.
Reduction of pancreatic Igf2 expression along perinatal development and in PRLR−/− mice. Igf2 gene expression was determined by qPCR in whole pancreases from PRLR+/+ and PRLR−/− mice at embryonic day €18.5, postnatal day (D)0.5, and D6.5 developmental stages (n = 6–23/group). Relative expression was calculated as a ratio (amoles of Igf2/pmoles of 18S). Data are represented as means ± SE. NS, not significant. Nonparametric statistical analyses were performed between the 2 genotypes *P <0.05.
β-Cell mass and size is controlled by PRL signaling.
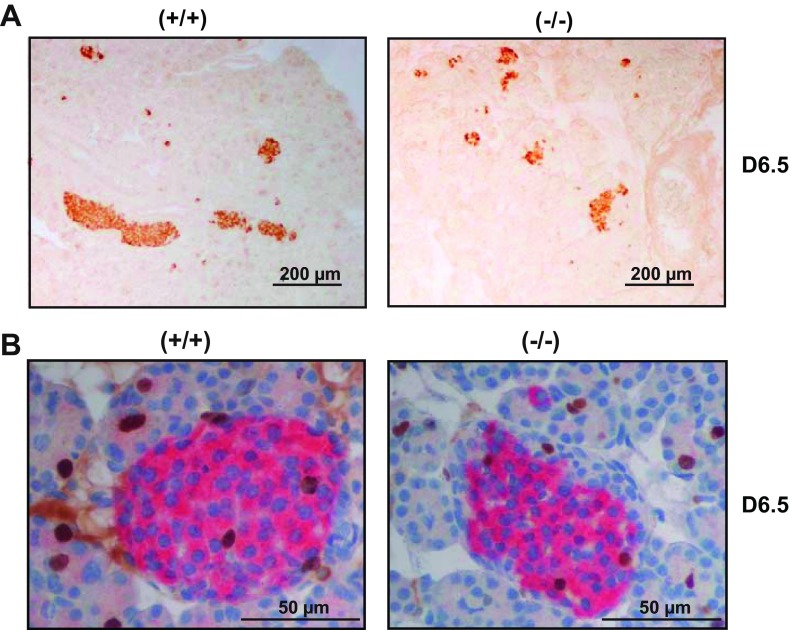
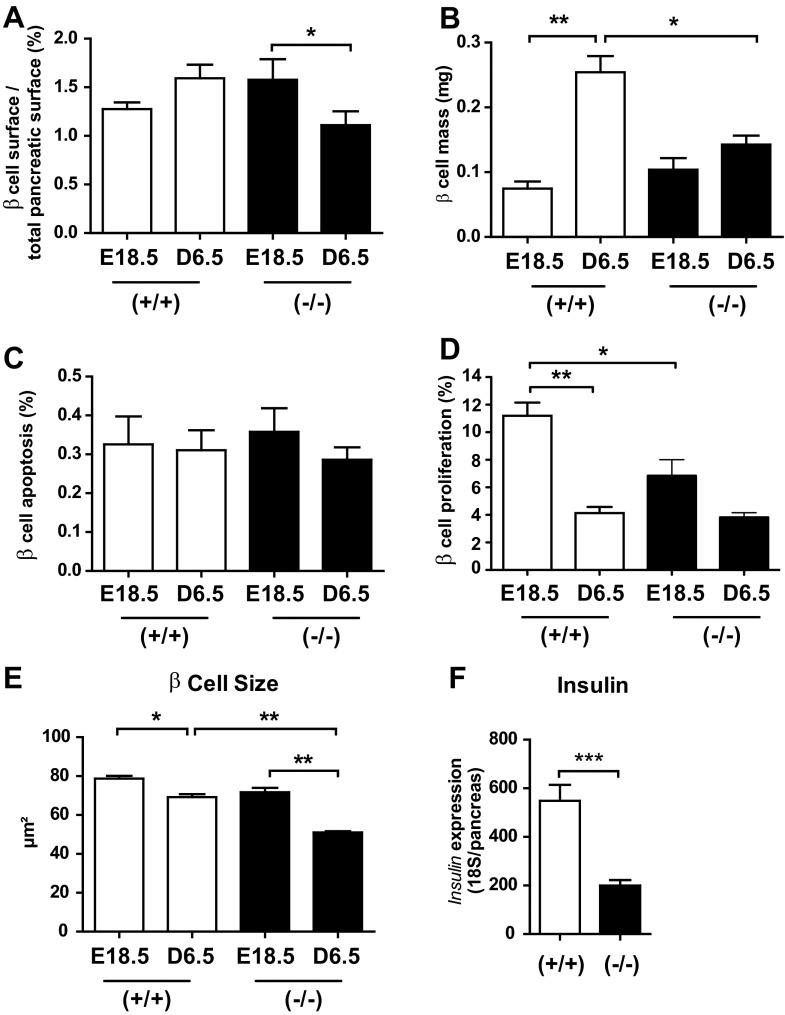
We next evaluated the impact of PRLR inactivation on β-cell ontogenesis. We quantified percentage of β-cell surface in whole pancreas by using insulin immunostaining on pancreas sections at E18.5 and D6.5. Figure 2A demonstrates a reduction of islet size in PRLR−/− mice relative to WT mice at D6.5. We used ImageJ software to quantify percent β-cell surface, total β-cell mass, and β-cell size (Fig. 2B). The percentage of β-cell surface was strongly decreased at D6.5 compared with E18.5 in PRLR−/− mice; in contrast, percent β-cell surface relative to pancreatic surface did not change significantly from E18.5 to D6.5 in WT mice (Fig. 3A). There was a threefold increase in total β-cell mass in WT mice between E18.5 and D6.5 (P < 0.001) but no significant increase in β-cell mass in PRLR−/− mice (P < 0.01 vs. WT controls; Fig. 3B). This is only partly explained by the 25% reduction in pancreatic mass in PRLR−/− mice at D6.5.
Fig. 2.
Lack of prolactin receptor (PRLR) reduces pancreatic β-cell mass during perinatal development. A: pancreas sections from PRLR+/+ and PRLR −/− at D6.5 after insulin immunostaining (brown). Scale bars, 200 μm. B: sections from PRLR+/+ and PRLR −/− pancreases at D6.5 after double BrdU (brown) and insulin (pink) immunostaining. Scale bars, 50 μm.
Fig. 3.
Lack of PRLR reduces total pancreatic β-cell mass, proliferation, and size during perinatal development. A: β-cell surface was normalized to total pancreas surface of PRLR+/+ and PRLR−/− mice at E18.5 and D6.5 (6–8 pancreas sections per animal). Results are means ± SE (n = 3–6/group) and expressed as percentage of total pancreatic surface. *P < 0.05. B: determination of β-cell mass was calculated according to values at left normalized to pancreas weight. *P < 0.05, **P < 0.01. C: percentage of β-cell apoptosis of PRLR+/+ and PRLR−/− pancreases was quantified from insulin-TUNEL assay-immunostained sections and counterstaining with hematoxylin at E18.5 and D6.5. Approximately 3,000 cells were counted for each animal. D: β-cell proliferation was quantified by means of BrdU/insulin-immunostained and hematoxylin-counterstained pancreas sections. At least 2,000 cells were counted for each mouse (n = 5–7). *P < 0.05. E: determination of β-cell size was calculated (μm2) after quantification of total β-cell surface by ImageJ software divided by total number of nuclei [≥750 cells counted for each mouse (n = 3–6/group)]. *P < 0.05, **P < 0.01. F: insulin gene expression was quantified by qPCR in whole pancreases of PRLR+/+ and PRLR−/− mice at D6.5 (n = 10–22/group). ***P < 0.001.
These findings suggested that β-cell proliferation and/or apoptosis rates might be modified in the absence of PRL signaling. Apoptosis did not vary with developmental stage or genotype (Fig. 3C). By contrast, the high β-cell proliferation rate (11%) observed at E18.5 in PRLR+/+ mice significantly decreased at D6.5 (4%), whereas this proliferation index was already significantly low at E18.5 in PRLR−/− mice and remained unchanged at D6.5 (Fig. 3D). Moreover, consistent with the proliferation peak measured in PRLR+/+ mice, the β-cell number significantly increased from 102.3 ± 3.6 at E18.5 to 147.7 ± 13.8/mm2 pancreas at D6.5 (P < 0.05) in PRLR+/+ animals. In sharp contrast, the β-cell number in PRLR−/− E18.5 pancreas was not significantly different from that of PRLR+/+ animals and remained unchanged between E18.5 and D6.5 (142.9 ± 16.4 vs. 157.2 ± 23/mm2 pancreas, P > 0.05). To clarify the underlying processes involved in β-cell mass alterations, we next determined the mean β-cell size using ImageJ analysis of insulin-immunostained sections. A slight but significant reduction of β-cell size was observed in PRLR+/+ mice at D6.5, but the mean β-cell size in PRLR−/− pancreases was reduced by 30% (Fig. 3E). All together, our results showed that, in a developmental dynamic context, the β-cell mass decrease in the PRLR−/− mice on D6.5 resulted from a combination of both a defect in early E18.5 β-cell proliferation and a reduction in cell size.
To determine the functional consequences of β-cell hypoplasia in PRLR−/− mice, we measured insulin mRNA content in whole pancreas by real-time qPCR (Fig. 3F). Insulin mRNA levels were sharply reduced (∼65%) in PRLR−/− pancreas, a finding well correlated with the decreased β-cell mass.
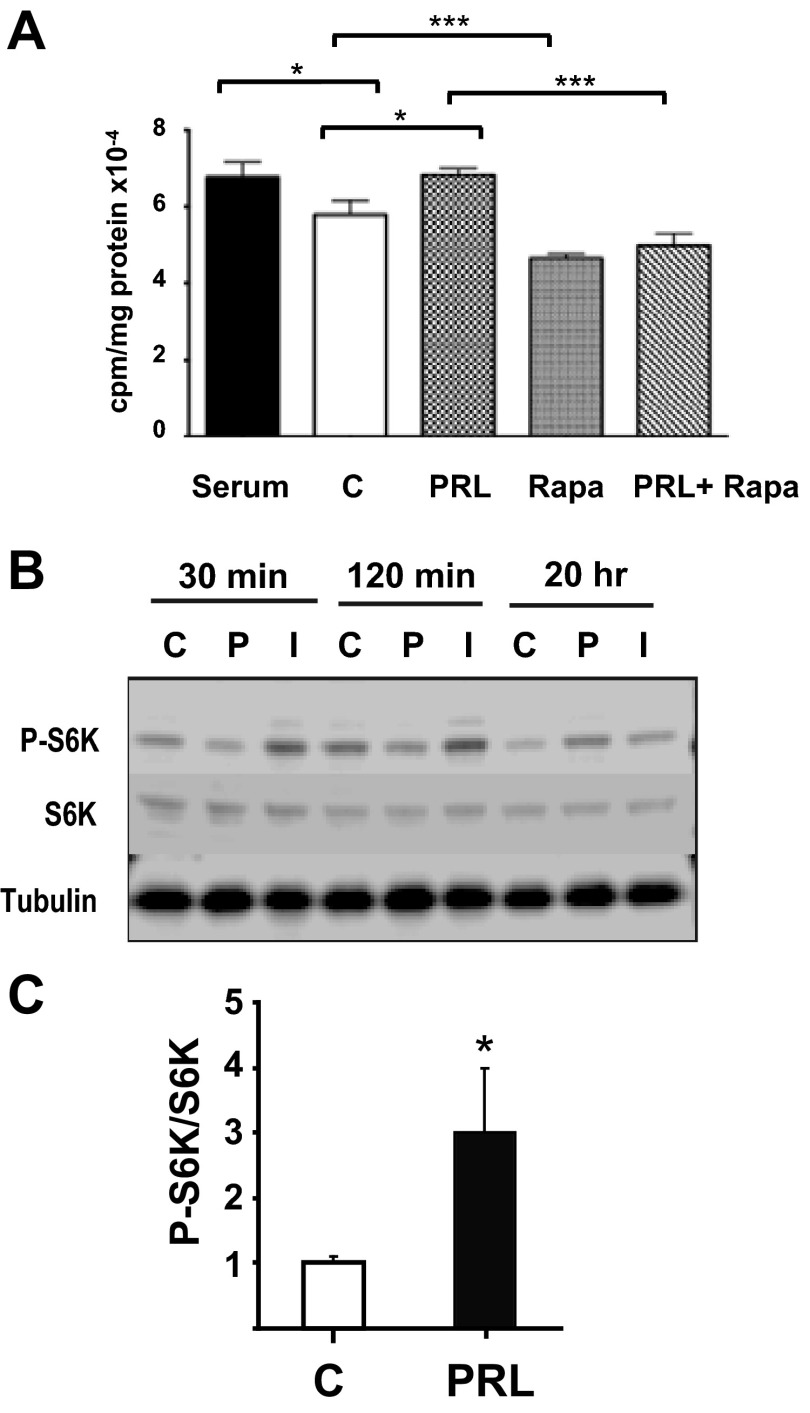
Given that PRL activates the PI3K/Akt pathway (5), it was appropriate to investigate leucine uptake as an index of nutrient uptake and nutrient-dependent phosphorylation of S6 kinase. Pancreatic β-cell size is regulated by nutrient-dependent mTOR signaling and the phosphorylation of p70 S6 kinase (p70S6K) (33, 37). To explore the effects of PRL signaling on β-cell protein synthesis and phospho-p70S6K expression, we measured the uptake and incorporation of radioactive leucine into TCA-precipitable protein and the levels of phosphorylated S6K in rat insulinoma (INS-1) cells incubated with PRL or vehicle in serum-free medium. During a 20-h incubation, PRL stimulated a 16–28% increase in leucine uptake (P < 0.01) into TCA-precipitable protein (Fig. 4A). Rapamycin, an inhibitor of mTOR signaling, reduced leucine uptake by 22% and blocked the effect of PRL. PRL had no effect on the levels of phospho-p70S6K after 30 and 120 min of incubation but increased the levels of phospho-S6K by 20 h (Fig. 4, B and C).
Fig. 4.
PRL stimulates leucine incorporation and p70S6K phosphorylation in rat INS-1 cells. A: [14C]leucine uptake and incorporation into protein was measured in INS-1 cells incubated for 20 h in serum or in serum-free medium containing rat PRL (20 nM), rapamycin (rapa, 100 nM), or a combination of PRL + rapa. Cells treated with diluent represent negative controls (C). [14C]leucine was added for the final 16 h. Similar results (but with lower overall counts) were obtained when [14C]leucine was added for the final hour of incubation; in that case, PRL stimulated a 28% increase in leucine uptake relative to controls. *P < 0.05, ***P < 0.001. B: phosphorylated (p-)p70S6K was estimated by Western analysis of whole cell extracts of INS-1 cells incubated for 30 min, 120 min, or 20 h in RPMI containing 10% FBS or in basal serum-free medium containing diluent (C), rat PRL (20 nM, PRL), or insulin (1 μM, Ins). Immunoblots with anti-S6K and anti-tubulin antibodies were used as loading control. C: ratio of p-S6K to total protein of the densitometric assay is presented. Data are means ± SE. *P < 0.05.
PRLR−/− mice exhibit an alteration of α- and δ-cell mass during perinatal adaptation.
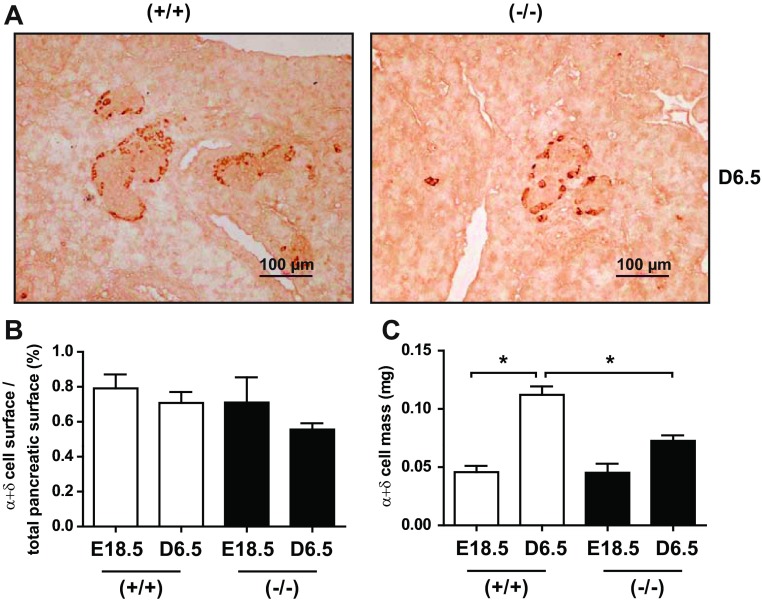
To examine the PRL impact on the other endocrine cell lineages, we performed glucagon and somatostatin labeling on pancreas sections at D6.5 (Fig. 5A). The percent α+δ-cell surface relative to pancreatic mass did not vary according to genotype or developmental stage (Fig. 5B). However, α- and δ-cell mass increased dramatically between E18.5 and D6.5 in WT mice but not in PRLR−/− mice (Fig. 5C). Given the lack of a difference in percent α+δ-cell surface, the decrease in α+δ-cell mass in the PRLR−/− mice may reflect the decrease in overall size of the pancreas rather than a direct effect on α+δ-cell development.
Fig. 5.
PRLR impacts α- and δ-cells ontogenesis. A: pancreas sections from PRLR+/+ and PRLR−/− at D6.5 after glucagon and somatostatin (brown) immunostaining. Scale bars, 100 μm. B: quantification of average α- and δ-cell surface from pancreas sections of PRLR+/+ and PRLR−/− animals. Ppancreas sections (6–8 per mouse) were used to quantify percentage of α- and δ-cells. C: α- and δ-cell mass was calculated after quantification of α- and δ-cell surface by ImageJ, taking into account pancreas weights of PRLR+/+ and PRLR−/− mice. Values represent means ± SE (n = 3–6/group). Statistical differences are indicated: *P < 0.05, **P < 0.01.
Lack of PRL signaling alters exocrine pancreas morphology and function.
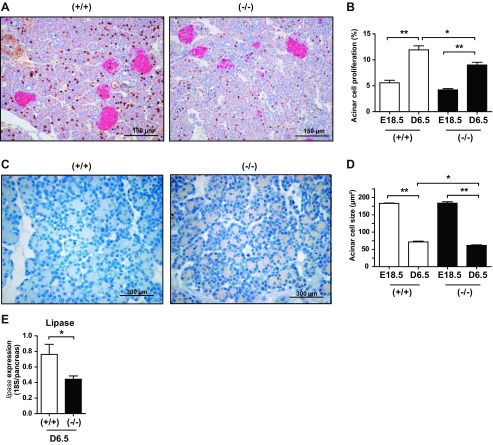
Since the pancreas is composed mainly of acinar cells, we hypothesized that the decrease in pancreatic mass in PRLR−/− mice was caused by a decrease in acinar cell mass. To that end, we performed double BrdU-insulin immunostaining on pancreas sections (Fig. 6A) to delineate the exocrine from the endocrine compartments. Analysis of BrdU staining revealed that acinar cell proliferation was reduced by 25% in PRLR−/− compared with age-matched control mice (Fig. 6B). As illustrated in Fig. 6C, hematoxylin-counterstained pancreas sections revealed an apparent reduction of acinar size in PRLR−/− mice. Measurement of cell size demonstrated a strong reduction of the mean acinar cell surface in both genotypes during pancreas development. In addition, we noted a 15% smaller size of acinar cells in PRLR−/− mice compared with PRLR+/+ mice at D6.5 (Fig. 6D). We quantified lipase mRNA content as an enzymatic marker of exocrine pancreas. Lipase gene expression in whole pancreas of PRLR−/− mice was 50% less than that in PRLR+/+ mice (Fig. 6E). Taken together, the absence of PRLR signaling leads to defects in both endocrine and exocrine pancreatic development during the neonatal adaptive period.
Fig. 6.
Lack of PRLR modifies pancreatic acinar development. A: pancreas sections at D6.5 after double BrdU (brown) and insulin (pink) immunostaining. Scale bars, 150 μm. B: quantification of BrdU-positive cells in acini of PRLR+/+ and PRLR−/− mice at E18.5 and D6.5. Results are expressed as percentage of positive cells vs. total counted cells (≥2,000 cells/animal). *P < 0.05, **P < 0.01. C: hematoxylin-stained pancreas sections from PRLR+/+ and PRLR−/− mice at D6.5. Scale bars, 300 μm. D: determination of acinar cell size of PRLR+/+ and PRLR−/− mice at E18.5 and D6.5 using ImageJ software. Cell surface (mm2) was calculated for ≥750 cells per animal. Results are means ± SE (n = 3–6). *P < 0.05, **P < 0.01. E: lipase gene expression was determined by qPCR in whole pancreases from PRLR+/+ and PRLR−/− mice at D6.5 (n = 3–13/group). *P < 0.05.
Effects of PRL on β-cell replication in pancreatic buds of wistar and GK rats.
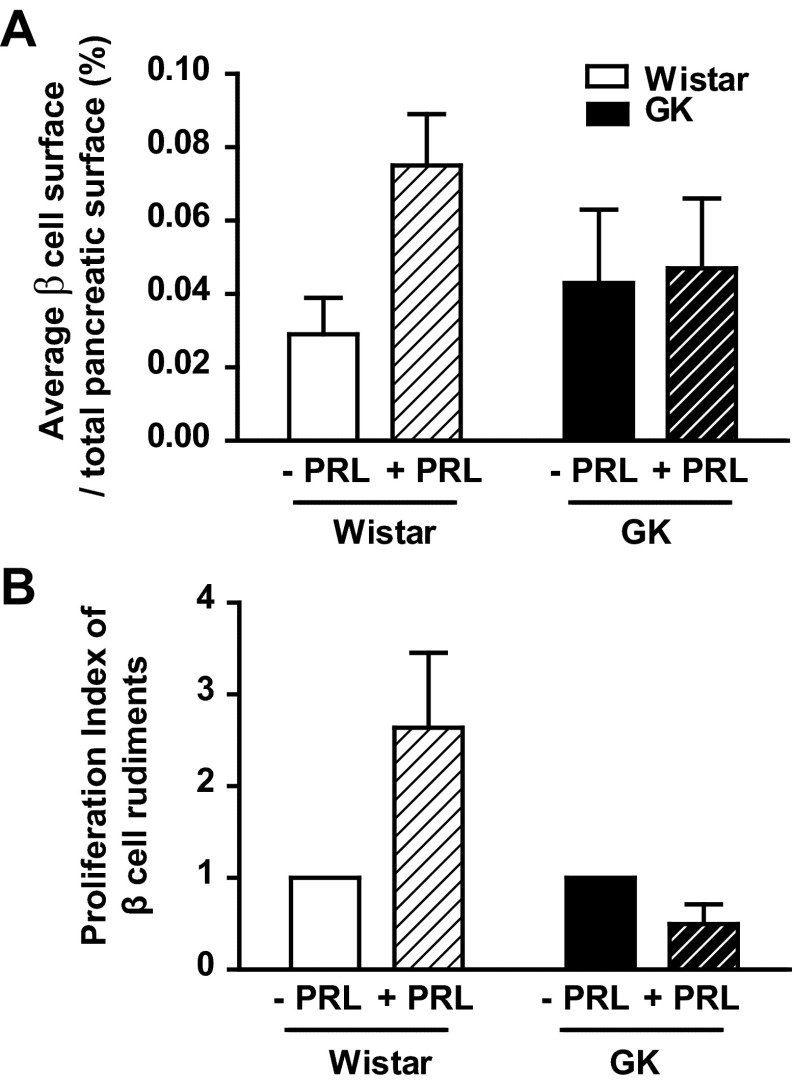
Given the prominent roles of PRL and PL in β-cell expansion during pregnancy (15, 23, 35), we compared the impact of PRL on embryonic pancreas from Wistar rats and GK rats, a model of spontaneous type 2 diabetes. To evaluate the response of fetal β-cells to lactogenic hormones, we treated pancreatic buds of Wistar rats at E13.5 with PRL or diluent for a total of 7 days in culture. PRL treatment induced a threefold increase of β-cell surface in Wistar pancreatic buds but had no effect in GK pancreatic buds (Fig. 7A). Since IGF-II exposure was previously shown to induce β-cell expansion in GK rats (12), the lack of PRL effect on GK bud expansion may be attributed to low Igf2 expression levels. To confirm that IGF-II represents one of the main effectors of PRL action, we showed that 4-h exposure to 20 nM rat PRL on INS-1 cells significantly increased Igf2 mRNA expression, as measured by qPCR from 1 ± 0.06 under standard conditions to 1.3 ± 0.05 (P < 0.05) upon PRL treatment. This finding correlated with the lack of PRL-induced β-cell proliferation in GK buds, as determined by counting the BrdU/insulin positive cells (Fig. 7B). All together, these findings suggest that β-cell PRL signaling is defective in GK rats.
Fig. 7.
Effect of PRL on pancreatic β-cell rudiments from Wistar and GK rats. A: average β-cell surface on total pancreas surface was measured on pancreatic buds from Wistar (white) and GK (black) fetuses at E13.5 incubated in the presence or absence of PRL for 7 days. B: β-cell proliferation index was determined by double insulin-BrdU immunostaining on pancreas buds of GK and Wistar fetus rats after PRL exposure (hatched). Results are expressed as fold increase compared with β-cell proliferation index measured in GK and Wistar buds in the absence of PRL.
DISCUSSION
β-Cell mass and insulin production depend on β-cell expansion during the critical windows of mid-late gestation and the perinatal period (15, 23, 31, 35). Both developmental stages are associated with surges in pituitary PRL and PL, which stimulate β-cell replication and insulin production and increase β-cell survival (2, 9, 23, 26, 35). Here, we show that mice with a global PRLR knockout have reductions in pancreatic and β-cell mass by D6.5. Interestingly, the reductions in pancreatic and β-cell mass in PRLR−/− mice appear to result, at least in part, from reductions in acinar and β-cell size. Nevertheless, the induction by PRL of β-cell replication in embryonic rat pancreatic buds (this study) and newborn rat islets (summarized in Ref. 35) suggests that lactogenic hormones likely increase β-cell mass in the fetal and perinatal periods through increases in β-cell replication as well as β-cell size. It is also possible that lactogens promote β-cell expansion during the perinatal period through induction of neogenesis, but testing of that hypothesis will require further study.
When phenotypes of PRLR−/− vs. PRLR+/+ mice before birth (E18.5) are compared, proliferation rate is the only index that was significantly reduced in the absence of PRL signaling. In contrast, after birth (D6.5), our data suggest that reduced pancreas weight and total β-cell mass observed in PRLR−/− mice may be related to a decreased individual β-cell size while total β-cell surface and β-cell number were not different in the context of a low proliferation rate.
Previous studies from our laboratory showed that IGF-II mediates the growth-promoting effects of PRL on brown adipose tissue in the perinatal period, correlated with the reduced plasma IGF-II levels in the newborn PRLR−/− mice (46). Igf2 gene regulation is very complex and does not rely on a single regulatory event but rather on multiple, entailed, and nonmutually exclusive mechanisms that could be operative in a dynamic and developmental manner.
Here, we demonstrated a reduction in pancreatic Igf2 expression in PRLR−/− newborn (D0.5) mice. This was associated with a reduction in β-cell mass by D6.5. Interestingly, a decrease in pancreatic IGF-II expression in embryonic GK rats is associated with subsequent reductions in β-cell mass. PRL fails to induce β-cell growth and proliferation in GK pancreatic buds (present study), while IGF-II exposure facilitates expansion of the β-cell pool in the same model (12). Thus, the reduction in pancreatic IGF-II expression in PRLR−/− mice may contribute to reductions in pancreatic and/or β-cell mass. It is unclear whether PRL regulates directly or indirectly the expression of IGF-II in pancreatic acinar cells or β-cells; previous studies from our laboratory found no direct effect of PRL on Igf2 mRNA in isolated adult rat islets (2). However, in the present study, we provide evidence that PRL is able to induce Igf2 expression in rat Ins-1 cells. Collectively, our findings suggest that IGF-II may act in concert with PRL signaling to promote β-cell adaptation during the embryonic and perinatal periods. Numerous other factors may be operative, including FoxM1 (47), FOXO1 (1, 3), Pdx1 (27), neurogenin 3 (39), menin (24), and Tph1 (25, 40). It remains to be determined whether PRL signaling modulates the expression of these or other genes in vivo.
It is well known that, in the mouse, IGF-II is highly expressed in a variety of tissues during fetal life but then is dramatically downregulated shortly after birth; the mechanisms responsible for the dramatic postnatal downregulation remain unknown. The well-studied IGF-II imprinting does not seem to be responsible for the sharp postnatal regulation. Recently, the Igf2 expression decline was associated with reduced levels of transcription factor E2f3 that directly binds to Igf2 promoter sequences (29). Thus, this downregulation of E2f3 may account for the postnatal Igf2 downregulation. However, it remains to establish whether the causal relationship occurs in vivo and how PRL signaling may interfere with E2f3 expression before birth.
The reduction in acinar cell size in PRLR−/− mice may seem surprising given that PRLRs are localized to islet β- and (to a lesser extent) α-cells in adult rodents. However, in previous studies we found expression of PRLR mRNA in acinar tissue of human fetuses at 14 wk of gestation (17). Moreover, hyperprolactinemia induced by pituitary graft induced acinar proliferation in mice (30). It is therefore possible that the effects of lactogenic hormones on acinar development and growth are age and stage dependent.
The mechanisms by which lactogenic hormones may regulate acinar cell size are unknown. On the other hand, the reduction in β-cell size in PRLR−/− mice may result from defects in nutrient-dependent mTOR signaling, since PRL stimulates leucine uptake and increases the levels of phospho-p70S6K in rat insulinoma cells. That the effect of PRL on leucine uptake is blocked by rapamycin is additional evidence supporting a role for β-cell mTOR signaling in PRL action. However, cell size also depends on many other factors, including the PI3K/Akt pathway, as described in pioneer studies in Drosophila (6, 45). Overexpression of active Akt1 in mouse β-cells increases cell size (42). Thus, the reduction of β-cell size observed in PRLR−/− pancreases might be related to defects in signaling pathways other than, or in addition to, the mTOR signaling pathway.
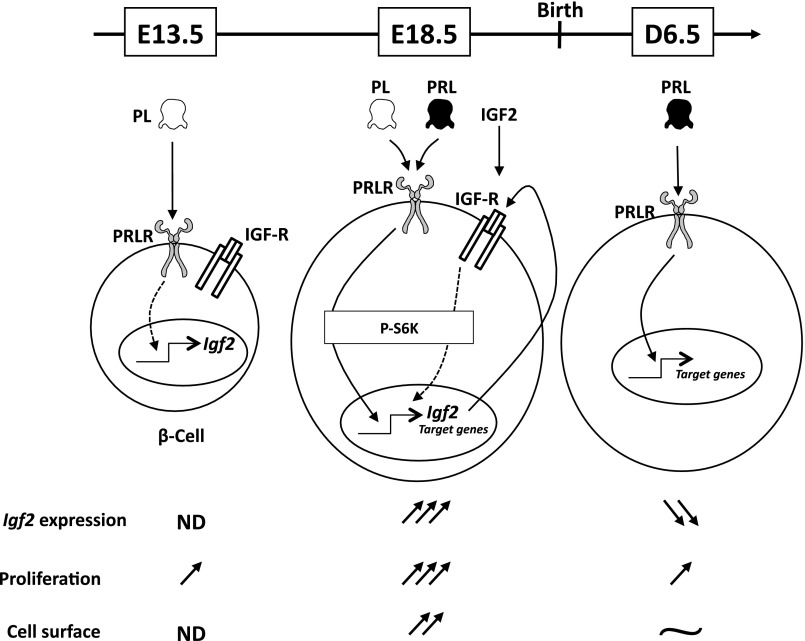
To summarize, both PRL and IGF-II act through separate yet additive pathways to regulate β-cell proliferation and size (Fig. 8). At mid-pregnancy, PRLR is activated only by placental lactogen hormones (PL), since PRL levels are undetectable at this stage. Before birth, precisely at E18.5, PRLR activation leads to a drastic IGF-II production, which may act in an autocrine/paracrine manner through IGF-R signaling. At D6.5, IGF-II is almost undetectable; PRL alone induces other target genes, whereas β-cell proliferation rate is largely reduced, even though a second wave of β-cell proliferation will take place later on (D20) in rodents.
Fig. 8.
Graphic summary of factors regulating β-cell proliferation and size as a function of pancreas developmental stages. At E13.5, PRLR is already expressed in fully differentiated β-cells and only activated by placental lactogen hormones (PL), since PRL levels are undetectable at this stage. However, PRL exposure stimulates embryonic pancreatic rudiment growth and β-cell proliferation (Fig. 7). PRLR activation leads to stimulation of insulin growth factor II (Igf2) expression, notably before birth, and presumably its secretion. At E18.5, PRLR is strongly activated by both PL and PRL inducing at least through S6K pathway (Fig. 4). Drastic IGF-II production was observed, which may act in an autocrine/paracrine manner through IGF receptor (IGF-R) signaling. Other sources of IGF-II could also account for enhanced β-cell proliferation (see Fig. 3D) and increased β-cell size (see Fig. 3E). After birth (D6.5), IGF-II was almost undetectable; PRL alone induces other target genes, whereas proliferation rate is largely reduced. Even though a second wave of β-cell proliferation will take place later (D20) in rodents.
It has been recently proposed that the pathogenesis of β-cell failure is not related to cell apoptosis but rather to a dedifferentiation process converting β-cells into other endocrine cell types (41). Since an increased apoptosis index was excluded in our model, it may be hypothesized that such endocrine cell conversion could contribute to β-cell dysfunction. Given that the β-cell pool in the perinatal period is reduced in PRLR−/− newborns, we suspect that inadequate responses to metabolic stresses (e.g., obesity, pregnancy, glucocorticoid therapy, and aging) could lead to failure of β-cell function, insufficient insulin production, and glucose intolerance. A better understanding of the mechanisms governing the pathogenesis of diabetes may facilitate future developments of relevant preventive and pharmacological strategies.
GRANTS
This work was supported by grants from Agence Nationale de la Recherche (09-BLAN-0246), Institut National de la Santé et de la Recherche Médicale, Université Paris-Sud, the American Diabetes Association, and the US National Institute of Child Health and Development. J. Auffret was the recipient of a doctoral fellowship from the Ministère de l'Education Nationale, de la Recherche et de la Technologie, France.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.A., M.S.F., N.C., Y.M., and C.T.-C. performed experiments; J.A., M.S.F., N.C., C.T.-C., M.L., J.M., and N.B. analyzed data; J.A. and M.L. prepared figures; J.A., M.S.F., N.C., C.T.-C., M.L., J.M., and N.B. approved final version of manuscript; M.S.F., M.L., J.M., and N.B. interpreted results of experiments; M.S.F., M.L., and N.B. drafted manuscript; M.S.F., M.L., J.M., and N.B. edited and revised manuscript; C.T.-C., M.L., J.M., and N.B. conception and design of research.
ACKNOWLEDGMENTS
We thank V. Keo (Inserm U693, Le Kremlin-Bicêtre, France) for technical assistance and S. Berissi (Inserm U845, Paris, France) for the quality of pancreas sections. We thank Drs. Ramamani Arumugam and Eric Horowitz for their assistance with some of the experiments. We are also grateful to S. Viengchareun (Inserm U693, Le Kremlin-Bicêtre, France) for helpful discussions.
REFERENCES
- 1. Al Masri M, Krishnamurthy M, Li J, Fellows GF, Dong HH, Goodyer CG, Wang R. Effect of forkhead box O1 (FOXO1) on β-cell development in the human fetal pancreas. Diabetologia 53: 699–711, 2010 [DOI] [PubMed] [Google Scholar]
- 2. Arumugam R, Fleenor D, Lu D, Freemark M. Differential and complementary effects of glucose and prolactin on islet DNA synthesis and gene expression. Endocrinology 152: 856–868, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arumugam R, Horowitz E, Lu D, Collier JJ, Ronnebaum S, Fleenor D, Freemark M. The interplay of prolactin and the glucocorticoids in the regulation of beta-cell gene expression, fatty acid oxidation, and glucose-stimulated insulin secretion: implications for carbohydrate metabolism in pregnancy. Endocrinology 149: 5401–5414, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ben Jonathan N, Lapensee CR, Lapensee EW. What Can We Learn from Rodents about Prolactin in Humans? Endocr Rev 29: 1–41, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Binart N, Bachelot A, Bouilly J. Impact of prolactin receptor isoforms on reproduction. Trends Endocrinol Metab 21: 362–368, 2010 [DOI] [PubMed] [Google Scholar]
- 6. Bohni R, Riesgo-Escovar J, Oldham S, Brogiolo W, Stocker H, Andruss BF, Beckingham K, Hafen E. Autonomous control of cell and organ size by CHICO, a Drosophila homolog of vertebrate IRS1–4. Cell 97: 865–875, 1999 [DOI] [PubMed] [Google Scholar]
- 7. Bole-Feysot C, Goffin V, Edery M, Binart N, Kelly PA. Prolactin and its receptor: actions, signal transduction pathways and phenotypes observed in prolactin receptor knockout mice. Endocr Rev 19: 225–268, 1998 [DOI] [PubMed] [Google Scholar]
- 8. Bouilly J, Sonigo C, Auffret J, Gibori G, Binart N. Prolactin signaling mechanisms in ovary. Mol Cell Endocrinol 356: 80–87, 2012 [DOI] [PubMed] [Google Scholar]
- 9. Brelje TC, Scharp DW, Lacy PE, Ogren L, Talamantes F, Robertson M, Friesen HG, Sorenson RL. Effect of homologous placental lactogens on islet B-cell division and insulin secretion in rat, mouse, and human islets: implication for placental lactogen regulation of islet function during pregnancy. Endocrinology 132: 879–887, 1993 [DOI] [PubMed] [Google Scholar]
- 10. Brisken C, Ayyannan A, Nguyen C, Heineman A, Reinhardt F, Tan J, Dey SK, Dotto GP, Weinberg RA, Jan T. IGF-2 is a mediator of prolactin-induced morphogenesis in the breast. Dev Cell 3: 877–887, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Brooks CL. Molecular mechanisms of prolactin and its receptor. Endocr Rev 33: 504–525, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Calderari S, Gangnerau MN, Thibault M, Meile MJ, Kassis N, Alvarez C, Portha B, Serradas P. Defective IGF2 and IGF1R protein production in embryonic pancreas precedes β-cell mass anomaly in the Goto-Kakizaki rat model of type 2 diabetes. Diabetologia 50: 1463–1471, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Collombat P, Hecksher-Sorensen J, Serup P, Mansouri A. Specifying pancreatic endocrine cell fates. Mech Dev 123: 501–512, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Fleenor D, Petryk A, Driscoll P, Freemark M. Constitutive expression of placental lactogen in pancreatic β-cells: effects on cell morphology, growth, and gene expression. Pediatr Res 47: 136–142, 2000 [DOI] [PubMed] [Google Scholar]
- 15. Freemark M. Placental hormones and the control of fetal growth. J Clin Endocrinol Metab 95: 2054–2057, 2010 [DOI] [PubMed] [Google Scholar]
- 16. Freemark M, Avril I, Fleenor D, Driscoll P, Petro A, Opara E, Kendall W, Oden JP, Bridges S, Binart N, Breant B, Kelly PA. Targeted deletion of the prolactin receptor: effects on islet development, insulin production, and glucose tolerance. Endocrinology 143: 1378–1385, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Freemark M, Driscoll P, Maaskant R, Petryk A, Kelly PA. Ontogenesis of prolactin receptors in the human fetus in early gestation. Implications for tissue differentiation and development. J Clin Invest 99: 1107–1117, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Freemark M, Kirk K, Pihoker C, Robertson MC, Shiu RC, Driscoll P. Pregnancy lactogens in the rat conceptus and fetus: circulating levels, distribution of binding, and expression of receptor messenger RNA. Endocrinology 133: 1830–1842, 1993 [DOI] [PubMed] [Google Scholar]
- 19. Fujinaka Y, Takane K, Yamashita H, Vasavada RC. Lactogens promote β-cell survival through JAK2/STAT5 activation and Bcl-XL upregulation. J Biol Chem 282: 30707–30717, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Goffin V, Binart N, Touraine P, Kelly PA. Prolactin: the new biology of an old hormone. Annu Rev Physiol 64: 47–67, 2002 [DOI] [PubMed] [Google Scholar]
- 21. Goto Y, Kakizaki M, Masaki N. Production of spontaneous diabetic rats by repetition of selective breeding. Tohoku J Exp Med 119: 85–90, 1976 [DOI] [PubMed] [Google Scholar]
- 22. Graham DE, Rechler MM, Brown AL, Frunzio R, Romanus JA, Bruni CB, Whitfield HJ, Nissley SP, Seelig S, Berry S. Coordinate developmental regulation of high and low molecular weight mRNAs for rat insulin-like growth factor II Proc Natl Acad Sci USA 83: 4519–4523, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huang C, Snider F, Cross JC. Prolactin receptor is required for normal glucose homeostasis and modulation of beta-cell mass during pregnancy. Endocrinology 150: 1618–1626, 2009 [DOI] [PubMed] [Google Scholar]
- 24. Karnik SK, Chen H, McLean GW, Heit JJ, Gu X, Zhang AY, Fontaine M, Yen MH, Kim SK. Menin controls growth of pancreatic beta-cells in pregnant mice and promotes gestational diabetes mellitus. Science 318: 806–809, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Kim H, Toyofuku Y, Lynn FC, Chak E, Uchida T, Mizukami H, Fujitani Y, Kawamori R, Miyatsuka T, Kosaka Y, Yang K, Honig G, van der HM, Kishimoto N, Wang J, Yagihashi S, Tecott LH, Watada H, German MS. Serotonin regulates pancreatic β-cell mass during pregnancy. Nat Med 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kondegowda NG, Mozar A, Chin C, Otero A, Garcia-Ocana A, Vasavada RC. Lactogens protect rodent and human β-cells against glucolipotoxicity-induced cell death through Janus kinase-2 (JAK2)/signal transducer and activator of transcription-5 (STAT5) signalling. Diabetologia 55: 1721–1732, 2012 [DOI] [PubMed] [Google Scholar]
- 27. Kushner JA, Ye J, Schubert M, Burks DJ, Dow MA, Flint CL, Dutta S, Wright CV, Montminy MR, White MF. Pdx1 restores β-cell function in Irs2 knockout mice. J Clin Invest 109: 1193–1201, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Le F, Wang LY, Wang N, Li L, Li lJ, Zheng YM, Lou HY, Liu XZ, Xu XR, Sheng JZ, Huang HF, Jin F. In vitro fertilization alters growth and expression of igf2/h19 and their epigenetic mechanisms in the liver and skeletal muscle of newborn and elder mice. Biol Reprod 88: 75, 2013 [DOI] [PubMed] [Google Scholar]
- 29. Lui JC, Baron J. Evidence that Igf2 down-regulation in postnatal tissues and up-regulation in malignancies is driven by transcription factor E2f3. Proc Natl Acad Sci USA 110: 6181–6186, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Matsuda M, Mori T, Park MK, Yanaihara N, Kawashima S. Enhanced cell proliferation by hyperprolactinemia in both exocrine and endocrine pancreas in mice. Eur J Endocrinol 130: 187–194, 1994 [DOI] [PubMed] [Google Scholar]
- 31. Meier JJ, Butler AE, Saisho Y, Monchamp T, Galasso R, Bhushan A, Rizza RA, Butler PC. Beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans. Diabetes 57: 1584–1594, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Miralles F, Czernichow P, Scharfmann R. Follistatin regulates the relative proportions of endocrine versus exocrine tissue during pancreatic development. Development 125: 1017–1024, 1998 [DOI] [PubMed] [Google Scholar]
- 33. Mori H, Inoki K, Opland D, Munzberg H, Villanueva EC, Faouzi M, Ikenoue T, Kwiatkowski DJ, MacDougald OA, Myers MG, Jr, Guan KL. Critical roles for the TSC-mTOR pathway in β-cell function Am J Physiol Endocrinol Metab 297: E1013–E1022, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Movassat J, Bailbe D, Lubrano-Berthelier C, Picarel-Blanchot F, Bertin E, Mourot J, Portha B. Follow-up of GK rats during prediabetes highlights increased insulin action and fat deposition despite low insulin secretion. Am J Physiol Endocrinol Metab 294: E168–E175, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Newbern D, Freemark M. Placental hormones and the control of maternal metabolism and fetal growth. Curr Opin Endocrinol Diabetes Obes 18: 409–416, 2011 [DOI] [PubMed] [Google Scholar]
- 36. Ormandy CJ, Camus A, Barra J, Damotte D, Lucas BK, Buteau H, Edery M, Brousse N, Babinet C, Binart N, Kelly PA. Null mutation of the prolactin receptor gene produces multiple reproductive defects in the mouse. Genes Dev 11: 167–178, 1997 [DOI] [PubMed] [Google Scholar]
- 37. Pende M, Kozma SC, Jaquet M, Oorschot V, Burcelin R, Marchand-Brustel Y, Klumperman J, Thorens B, Thomas G. Hypoinsulinaemia, glucose intolerance and diminished beta-cell size in S6K1-deficient mice. Nature 408: 994–997, 2000 [DOI] [PubMed] [Google Scholar]
- 38. Roberts CT, Jr,, Brown AL, Graham DE, Seelig S, Berry S, Gabbay KH, Rechler MM. Growth hormone regulates the abundance of insulin-like growth factor I RNA in adult rat liver. J Biol Chem 261: 10025–10028, 1986 [PubMed] [Google Scholar]
- 39. Rukstalis JM, Habener JF. Neurogenin3: a master regulator of pancreatic islet differentiation and regeneration. Islets 1: 177–184, 2009 [DOI] [PubMed] [Google Scholar]
- 40. Schraenen A, Lemaire K, de Faudeur G, Hendrickx N, Granvik M, Van Lommel L, Mallet J, Vodjdani G, Gilon P, Binart N, in't VP, Schuit F. Placental lactogens induce serotonin biosynthesis in a subset of mouse β-cells during pregnancy. Diabetologia 53: 2589–2599, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Talchai C, Xuan S, Lin HV, Sussel L, Accili D. Pancreatic β-cell dedifferentiation as a mechanism of diabetic β-cell failure. Cell 150: 1223–1234, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tuttle RL, Gill NS, Pugh W, Lee JP, Koeberlein B, Furth EE, Polonsky KS, Naji A, Birnbaum MJ. Regulation of pancreatic beta-cell growth and survival by the serine/threonine protein kinase Akt1/PKBalpha. Nat Med 7: 1133–1137, 2001 [DOI] [PubMed] [Google Scholar]
- 43. Uzan B, Figeac F, Portha B, Movassat J. Mechanisms of KGF mediated signaling in pancreatic duct cell proliferation and differentiation. PLoS ONE 4: e4734, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vasavada RC, Garcia-Ocana A, Zawalich WS, Sorenson RL, Dann P, Syed M, Ogren L, Talamantes F, Stewart AF. Targeted expression of placental lactogen in the β-cells of transgenic mice results in β-cell proliferation, islet mass augmentation, and hypoglycemia. J Biol Chem 275: 15399–15406, 2000 [DOI] [PubMed] [Google Scholar]
- 45. Verdu J, Buratovich MA, Wilder EL, Birnbaum MJ. Cell-autonomous regulation of cell and organ growth in Drosophila by Akt/PKB. Nat Cell Biol 1: 500–506, 1999 [DOI] [PubMed] [Google Scholar]
- 46. Viengchareun S, Servel N, Feve B, Freemark M, Lombes M, Binart N. Prolactin receptor signaling is essential for perinatal brown adipocyte function: a role for insulin-like growth factor-2. PLoS ONE 3: e1535, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhang H, Zhang J, Pope CF, Crawford LA, Vasavada RC, Jagasia SM, Gannon M. Gestational diabetes mellitus resulting from impaired beta-cell compensation in the absence of FoxM1, a novel downstream effector of placental lactogen. Diabetes 59: 143–152, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]