Abstract
It is unclear whether regular exercise alone (no caloric restriction) is a useful strategy to reduce adiposity and obesity-related metabolic risk factors in obese girls. We examined the effects of aerobic (AE) vs. resistance exercise (RE) alone on visceral adipose tissue (VAT), intrahepatic lipid, and insulin sensitivity in obese girls. Forty-four obese adolescent girls (BMI ≥95th percentile, 12–18 yr) with abdominal obesity (waist circumference 106.5 ± 11.1 cm) were randomized to 3 mo of 180 min/wk AE (n = 16) or RE (n = 16) or a nonexercising control group (n = 12). Total fat and VAT were assessed by MRI and intrahepatic lipid by proton magnetic resonance spectroscopy. Intermuscular AT (IMAT) was measured by CT. Insulin sensitivity was evaluated by a 3-h hyperinsulinemic (80 mU·m2·min−1) euglycemic clamp. Compared with controls (0.13 ± 1.10 kg), body weight did not change (P > 0.1) in the AE (−1.31 ± 1.43 kg) and RE (−0.31 ± 1.38 kg) groups. Despite the absence of weight loss, total body fat (%) and IMAT decreased (P < 0.05) in both exercise groups compared with control. Compared with control, significant (P < 0.05) reductions in VAT (Δ−15.68 ± 7.64 cm2) and intrahepatic lipid (Δ−1.70 ± 0.74%) and improvement in insulin sensitivity (Δ0.92 ± 0.27 mg·kg−1·min−1 per μU/ml) were observed in the AE group but not the RE group. Improvements in insulin sensitivity in the AE group were associated with the reductions in total AT mass (r = −0.65, P = 0.02). In obese adolescent girls, AE but not RE is effective in reducing liver fat and visceral adiposity and improving insulin sensitivity independent of weight loss or calorie restriction.
Keywords: insulin sensitivity, intrahepatic lipid, visceral fat, exercise, adolescents
the epidemic rate of childhood obesity is a major health concern in the US, as overweight and obese youth are at increased risk of developing comorbidities such as nonalcoholic fatty liver disease (35), type 2 diabetes (33), and metabolic syndrome (21, 41), once considered diseases of adulthood. Although both diet and physical activity are considered to be the first lines of approach to treat obese youth (9), we recently reported that, in obese adolescent boys, increasing physical activity alone, independent of calorie restriction, is beneficial to reduce total fat, visceral adiposity, and intrahepatic lipid and improves cardiorespiratory fitness (CRF) (22). In obese adolescent girls, the utility of exercise alone as a strategy for reducing obesity-related metabolic risk factors is currently unclear. Given the lower physical activity levels in girls than in boys (14) and the fact that physical activity declines substantially in girls during adolescence (17), we conducted a randomized controlled trial to examine the role of regular exercise alone (i.e., no calorie restriction) in reducing obesity-related risk factors in previously sedentary obese adolescent girls. Specifically, we compared the effects of aerobic (AE) vs. resistance exercise (RE) on insulin sensitivity, visceral adipose tissue (VAT), and ectopic fat depositions in the liver and skeletal muscle.
MATERIALS AND METHODS
Subjects.
The study (ClinicalTrials.gov identifier: NCT01323088) was conducted from August 2010 through October 2012 at Children's Hospital of Pittsburgh (CHP). Obese [BMI ≥ 95th percentile (30)] black and white girls were recruited via flyers posted in the city public transportation system and posters placed on campus and from the Weight Management and Wellness Center at CHP. Inclusion criteria included that the subjects be 12–18 yr of age, pubertal (Tanner stages III–V), nonsmokers, nondiabetic, and physically inactive (no participation in structured physical activity for the past 3 mo, except for school physical education classes). Exclusion criteria included recent significant weight change (BMI >2–3 kg/m2), musculoskeletal injuries, endocrine disorders (e.g., polycystic ovary syndrome, type 2 diabetes), syndromic obesity, pregnancy, psychiatric disorders, and use of chronic medications that are known to influence glucose metabolism or body composition. Girls with oral or injectable contraceptives were also excluded. Participants self-identified as black or white. A complete medical history, physical examination, and pubertal development were assessed according to Tanner criteria (37) by a certified nurse practitioner. The investigation was approved by the University of Pittsburgh Institutional Review Board. Parental informed consent and child assent were obtained from all participants before participation. All participants underwent routine hematological and biochemical tests at the Pediatric Clinical and Translational Research Center (PCTRC) at CHP.
Randomization.
Randomization was performed after baseline evaluation was completed. Similarly to our previous study (22), random assignment to one of three interventions, AE, RE, or a nonexercising control group, was performed by lottery using a completely randomized design and cell sizes of 16.
Exercise regimen.
The exercise groups exercised at either the downtown Pittsburgh YMCA exercise facility or the exercise laboratory at CHP for 3 mo. All exercise sessions were by appointment and supervised by exercise physiology graduate students. Participants in the AE group exercised three times per week for 60 min/session (including a 5-min warmup and 5-min cooldown) using treadmills and/or ellipticals. AE programs progressively increased in duration and intensity beginning at 40 min at ∼50% of V̇o2peak and increased up to 60 min at 60–75% of V̇o2peak by week 2. Participants wore a heart rate monitor (Polar Oy, Kempele, Finland) during the exercise sessions to ensure achievement of the target heart rate. The heart rate range associated with 60–75% of V̇o2peak was determined from the baseline maximal oxygen uptake test for each subject. Energy expenditure was estimated using the heart rate-V̇o2 relationship observed during the V̇o2peak test.
The RE group performed a series of 10 whole body exercises, three times per week for 60 min/session. Each training session included leg press, leg extension, leg flexion, chest press, latissimus pulldown, seated row, bicep curl, and tricep extension using weight machines. In addition, a single set of pushups and situps was performed. For the first 4 wk, participants performed one to two sets of eight to 12 repetitions at 60% of baseline one-repetition maximum (1RM) with proper lifting techniques. During weeks 4–13, subjects performed two sets of eight to 12 repetitions to fatigue.
Control subjects were asked not to participate in structured physical activities throughout the study. To maintain adherence, participants were given the opportunity to participate in exercise sessions following the completion of postintervention evaluations.
Dietary regimen.
All participants were asked to follow a weight maintenance diet (55–60% carbohydrate, 15–20% protein, and 25–30% fat) throughout the study to be able to assess the effects of regular exercise alone on insulin sensitivity and fat distribution. Daily energy requirements to maintain baseline body weight were determined at baseline by estimating resting energy expenditure and multiplying the obtained value by a factor of 1.2 (12).
Anthropometrics.
Body weight was measured to the nearest 0.1 kg, and height was measured to the nearest 0.1 cm. Waist circumference was measured at the top of the iliac crest, and the average of two measurements was used in the analyses.
Oral glucose tolerance test.
Participants reported to the PCTRC after an overnight fast for a 2-h oral glucose tolerance test (OGTT; 1.75 g/kg, maximum of 75 g). Blood samples were obtained at −15, 0, 15, 30, 60, 90, and 120 min for determination of glucose and insulin levels. Glucose and insulin area under the curve (AUC) were determined using a trapezoid model (2). Participants remained in the PCTRC and stayed overnight at the CHP to undergo the euglycemic clamp test the next morning.
Measurement of insulin sensitivity.
Fasting endogenous glucose production was measured with a primed (2.2 μmol/kg), constant-rate infusion of [6,6-2H2]glucose (Isotech, Miamisburg, OH) from 0730 to 0930, as shown by us previously (4). Blood was sampled at the start of the stable isotope infusion (−120 min) and every 10 min from −30 to 0 min (basal period) for determination of plasma glucose, insulin, and isotopic enrichment of glucose. Fasting hepatic glucose production (HGP) was calculated during the last 30 min (−30 to time zero) of the basal 2-h infusion period. Fasting hepatic insulin sensitivity was calculated as the inverse of the product of hepatic glucose production and fasting plasma insulin concentration (1,000/HGP × fasting plasma inulin), as shown previously (4). After the 2-h baseline isotope infusion period, insulin-mediated glucose uptake and insulin sensitivity were measured during a 3-h hyperinsulinemic euglycemic clamp from 0930 to 1230. Intravenous crystalline insulin (Humulin; Eli Lilly, Indianapolis, IN) was infused at a constant rate of 80 mU·m2·min−1, and plasma glucose was clamped at 5.6 mmol/l with a variable-rate infusion of 20% dextrose based on arterialized plasma glucose determinations every 5 min. Peripheral insulin sensitivity was calculated by dividing insulin-stimulated glucose disposal rate by the steady-state plasma insulin concentration during the last 30 min of the clamp. In the exercise groups, postexercise clamp test was performed 48–72 h post-exercise session to control for the effects of acute exercise on glucose uptake (31). One subject in the aerobic group did not complete the postintervention clamp due to difficulty with IV access. One control subject's postintervention clamp test ended early due to IV issues, and the subject's glucose disposal rate at 80 min was used to calculate insulin sensitivity. Among study completers (n = 36), 14 and 19 subjects were examined in the luteal and follicular phases, respectively, at baseline and 17 and 16 subjects were examined in the luteal and follicular phases, respectively, at followup. The phase of the menstrual cycle was not determined in three subjects who had irregular menstrual cycle at both time points.
Biochemical measurements.
Plasma glucose was measured by the glucose oxidase method with a glucose analyzer (YSI, Yellow Springs, OH), and the insulin concentration was determined by radioimmunoassay (3).
Total adipose tissue, skeletal muscle, and abdominal adipose tissue.
Whole body magnetic resonance imaging (MRI) was obtained with a 3.0 Tesla magnet (Siemens Medical Systems, Erlangen, Germany), using our standard protocol (25). One subject in the control group (post-measurement) and in the AE group (both pre- and post-measurement) did not complete MRI or 1H-magnetic resonance spectroscopy (MRS) due to claustrophobia.
Intrahepatic lipid by proton 1H-MRS.
1H-MRS was performed with a 3.0 Tesla MR system (Siemens, Tim Trio, Erlangen, Germany), using a body matrix coil and a spine matrix (Siemens, Erlangen, Germany), using our standard protocol (22). A voxel (30 × 30 × 20 mm3) was placed, avoiding blood vessels and intrahepatic bile ducts, using the following parameters (repetition time = 4,000 ms, echo time = 30 ms). Eight acquisitions were recorded in a measuring time of 32 s without water suppression, and the average of eight spectra was used for intrahepatic lipid (%) calculation, as shown below.
Spectra were fitted using the AMARES algorithm in the Java-based magnetic resonance user interface (jMRUI) software package (28). Absolute concentrations of intrahepatic lipid (CH2) were obtained from the AUC of the methylene signals of lipids at 1.3 ppm, using tissue water content as an internal reference. One subject's (AE) baseline data were excluded due to motion artifact.
Intermuscular adipose tissue by computed tomography.
Mid-thigh computed tomography (CT) images were obtained on a GE CTI-Helical Scanner (GE Medical Systems, Milwaukee, WI) using 170 mA, 120 kV, a 512 × 512 matrix, and a 48-cm field of view using our standard protocol (23). Intermuscular adipose tissue (IMAT) area was defined as adipose tissue (AT) area beneath the fascia lata surrounding skeletal muscle and AT area between muscle bundles, as shown previously (11).
Cardiorespiratory fitness and muscular strength.
Cardiorespiratory fitness (CRF) was determined using a graded treadmill test with the use of standard open-circuit spirometry techniques (AEI Technologies, Pittsburgh, PA) until volitional fatigue, using our standard protocol (22). Muscular strength was assessed with a 1RM test for the supine chest press and seated leg press using weight stack equipment (Life fitness, Schiller Park, IL). Muscular strength index was calculated as the sum of the 1RM scores for the chest and leg press expressed per killigram of body weight (16).
Statistical analysis.
A one-way analysis of variance (ANOVA) and analysis of covariance (ANCOVA) adjusting for BMI and race were performed to examine group differences at baseline. When the ANOVA P value was <0.05, Tukey's post hoc comparison test was used to locate group differences. We examined the effect of the intervention using an intent-to-treat analysis for only randomized subjects with baseline data. Missing followup data values were estimated using the multiple-imputations procedure (Proc MI) with 100 imputations (27). Repeated-measures ANCOVA was used to determine treatment change differences for each variable, using the imputed data with adjustment for baseline values for that variable. We also examined the effect of the exercise intervention using as-treated analyses in participants who had complete baseline and followup data. Least-squared means difference post hoc tests were used to determine differences between the control and intervention groups. The relationships between changes in total and abdominal fat and insulin sensitivity were evaluated by Pearson correlations coefficients.
P values of <0.05 were accepted to indicate statistical significance. All analyses were performed using commercially available software (SAS, version 9.2; SAS Institute, Cary, NC). Unless otherwise indicated, data are expressed as means (SE).
RESULTS
Baseline characteristics.
Baseline subject characteristics are shown in Table 1. Fasting insulin, insulin AUC, insulin sensitivity, and V̇o2peak were lower (P < 0.05) in the RE group compared with the AE group. However, these differences did not remain significant (P > 0.05) after adjusting for BMI and race.
Table 1.
Subject characteristics at baseline
| Control (n = 12) | Aerobic Exercise (n = 16) | Resistance Exercise (n = 16) | P Value | P Value (ANCOVA Adjusted for BMI and Race) | |
|---|---|---|---|---|---|
| Black/white (n) | 9/3 | 9/7 | 12/4 | 0.438 | |
| Puberty, III/IV/V (n) | 0/1/11 | 0/3/13 | 0/2/14 | 0.719 | |
| Anthropometric | |||||
| Age, yr | 15.0 ± 2.2 | 14.6 ± 1.9 | 14.8 ± 1.9 | 0.819 | |
| Body weight, kg | 93.3 ± 13.1 | 88.9 ± 16.3 | 97.1 ± 16.0 | 0.326 | |
| BMI, kg/m2 | 35.3 ± 4.0 | 32.9 ± 3.8 | 36.4 ± 3.8† | 0.049 | |
| Waist, cm | 110.9 ± 8.4 | 106.5 ± 11.1 | 115.3 ± 11.7 | 0.079 | |
| MRI (n = 15 in the aerobic group) | |||||
| Total AT, kg | 48.1 ± 9.4 | 43.3 ± 11.0 | 50.4 ± 11.5 | 0.186 | 0.760 |
| Total body fat, % | 51.3 ± 3.5 | 47.8 ± 4.2 | 51.5 ± 4.7† | 0.035 | 0.298 |
| Skeletal muscle, kg | 22.7 ± 2.7 | 23.0 ± 4.2 | 23.2 ± 3.1 | 0.928 | 0.206 |
| VAT, cm2 | 58.6 ± 23.7 | 51.8 ± 23.3 | 63.2 ± 23.6 | 0.407 | 0.576 |
| ASAT, cm2 | 538.2 ± 117.9 | 444.5 ± 155.0 | 536.8 ± 127.9 | 0.114 | 0.545 |
| Intrahepatic lipid, % | 3.0 ± 5.4 | 2.2 ± 3.3 | 2.0 ± 1.3 | 0.738 | 0.530 |
| CT at midthigh | |||||
| IMAT, cm2 | 59.3 ± 13.3 | 53.8 ± 24.7 | 58.3 ± 18.9 | 0.736 | 0.376 |
| Muscle attenuation, HU | 52.0 ± 1.6 | 52.2 ± 2.5 | 50.7 ± 3.4 | 0.231 | 0.526 |
| Metabolic | |||||
| Fasting glucose, mg/dl | 97.0 ± 6.7 | 93.2 ± 5.9 | 94.4 ± 6.8 | 0.309 | 0.410 |
| Fasting insulin, μU/ml | 31.1 ± 15.3 | 28.6 ± 16.5 | 45.8 ± 22.0† | 0.026 | 0.082 |
| Fasting HGP, mg·kg−1·min−1 | 1.89 ± 0.38 | 1.87 ± 0.29 | 1.79 ± 0.44 | 0.165 | 0.206 |
| Fasting hepatic insulin sensitivity, mg·kg−1·min−1 per μU/ml−1 | 22.9 ± 14.4 | 24.2 ± 12.2 | 16.5 ± 10.5 | 0.188 | 0.101 |
| Glucose at 2 h, mg/dl | 121.7 ± 28.5 | 120.7 ± 19.8 | 117.7 ± 15.5 | 0.865 | 0.797 |
| Insulin at 2 h, μU/ml | 104.1 ± 102.8 | 153.6 ± 143.7 | 207.0 ± 170.8 | 0.188 | 0.171 |
| Glucose AUC, mg·min−1·dl | 15,283.9 ± 3,044.2 | 14,818.1 ± 2,363.7 | 14,748.0 ± 1,334.9 | 0.807 | 0.564 |
| Insulin AUC, μU·min−1·ml | 15,781.8 ± 8,121.4 | 17,038.0 ± 14,281.2 | 28,605.9 ± 17,643.8‡ | 0.035 | 0.077 |
| Insulin sensitivity, mg·kg−1·min−1 per μU/ml | 2.7 ± 1.3 | 2.8 ± 1.3 | 1.8 ± 0.8† | 0.034 | 0.101 |
| Insulin sensitivity, mg·kg FFM−1·min−1 per μU/ml | 5.1 ± 2.5 | 5.1 ± 2.4 | 3.4 ± 1.6 | 0.060 | 0.100 |
| Fitness | |||||
| V̇o2peak, ml·kg−1·min−1 | 23.9 ± 3.0 | 28.5 ± 3.8 | 24.3 ± 4.3† | 0.004 | 0.090 |
| Muscular strength index | 1.0 ± 0.2 | 1.1 ± 0.2 | 1.0 ± 0.2 | 0.897 | 0.906 |
Values are means (SD). AT, adipose tissue; VAT, visceral AT; ASAT, abdominal subcutaneous AT; CT, computed tomography; IMAT, intermuscular AT; HU, Hounsfield units; HGP, hepatic glucose production; AUC, area under the curve; FFM, fat-free mass.
Different from the aerobic exercise group (P < 0.05);
different from the control group (P = 0.06).
Adherence to the exercise programs.
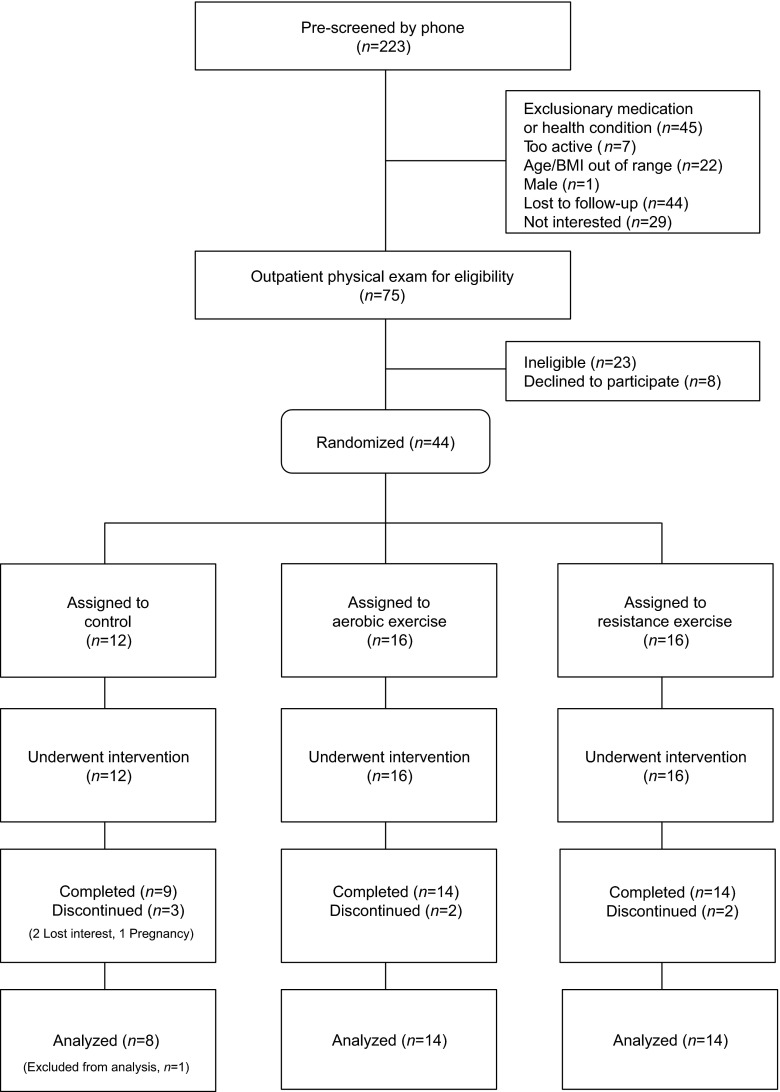
Of the 44 obese girls randomized, 37 completed their assigned treatment (Fig. 1). We excluded one control subject from data analyses who was dissatisfied with the group assignment and was intentionally reducing calorie intake during the study. Average (± SD) attendance at the exercise sessions was 95% (± 4.3%) in the AE and 97% (± 2.8%) in the RE groups, and average exercise duration was similar between the AE (56.0 ± 1.1 min/session) and RE (57.0 ± 0.7 min/session) group. In the AE group, the average heart rate was 153.0 ± 6.6 beats/min, and energy expenditure was 536.6 ± 72.9 kcal/session.
Fig. 1.
Participant flow diagram. All subjects assigned to each group (including subjects who discontinued the study) were included in intent-to-treat analyses.
Changes in CRF and muscular strength.
Compared with the nonexercising control group, CRF increased (P < 0.05) by 17% in the AE group but not in the RE group (Table 2). Muscular strength increased (P < 0.05) in the RE group (45%) only in comparison with controls.
Table 2.
Absolute changes in total and regional fat distribution and fitness after 3 mo
| Aerobic Exercise (n = 14/ITT =16) |
Resistance Exercise (n = 14/ITT =16) |
||||
|---|---|---|---|---|---|
| Control (n = 8/ITT =12) (Means ± SE) | Means ± SE | P value | Means ± SE | P value | |
| ITT analysis (n = 44) | |||||
| Body weight, kg | 0.13 ± 1.10 | −1.31 ± 1.43 | 0.360 | −0.31 ± 1.38 | 0.822 |
| BMI, kg/m2 | −0.03 ± 0.44 | −0.46 ± 0.58 | 0.430 | −0.28 ± 0.54 | 0.608 |
| Waist, cm | −0.25 ± 1.30 | −2.48 ± 1.67 | 0.137 | −1.82 ± 1.66 | 0.271 |
| Total AT, kg | 0.70 ± 1.01 | −2.38 ± 1.25 | 0.058 | −2.23 ± 1.21 | 0.065 |
| Total body fat, % | 0.10 ± 0.66 | −1.70 ± 0.85 | 0.046 | −1.63 ± 0.78 | 0.035 |
| Skeletal muscle, kg | 0.21 ± 0.51 | 0.13 ± 0.63 | 0.834 | 0.61 ± 0.61 | 0.317 |
| VAT, cm2 | 5.87 ± 6.17 | −15.68 ± 7.64 | 0.041 | −4.52 ± 7.23 | 0.532 |
| ASAT, cm2 | −2.93 ± 16.3 | −7.78 ± 20.42 | 0.703 | −14.36 ± 19.61 | 0.464 |
| Intrahepatic lipid, % | 0.75 ± 0.57 | −1.70 ± 0.74 | 0.022 | −0.70 ± 0.69 | 0.308 |
| IMAT, cm2 | 1.1 ± 3.4 | −13.5 ± 4.2 | 0.001 | −10.9 ± 4.2 | 0.010 |
| Muscle attenuation, HU | 0.41 ± 0.42 | 0.13 ± 0.53 | 0.811 | 0.23 ± 0.54 | 0.678 |
| V̇o2peak, ml·kg−1·min−1 | −0.21 ± 1.42 | 4.91 ± 1.82 | 0.007 | 2.87 ± 1.71 | 0.095 |
| Muscular strength index | 0.07 ± 0.09 | 0.08 ± 0.11 | 0.481 | 0.45 ± 0.11 | <0.0001 |
| Per-protocol analysis (n = 36) | |||||
| Body weight, kg | 0.28 ± 1.1 | −1.44 ± 0.84 | 0.307 | −0.44 ± 0.83 | 0.748 |
| BMI, kg/m2 | 0.02 ± 0.44 | −0.52 ± 0.57 | 0.369 | −0.34 ± 0.54 | 0.540 |
| Waist, cm | −0.37 ± 1.22 | −2.39 ± 1.56 | 0.135 | −1.72 ± 1.57 | 0.281 |
| Total AT, kg | 0.67 ± 0.96 | −2.33 ± 1.21 | 0.063 | −2.20 ± 1.18 | 0.072 |
| Total body fat, % | 0.08 ± 0.63 | −1.66 ± 0.81 | 0.050 | −1.61 ± 0.76 | 0.041 |
| Skeletal muscle, kg | 0.20 ± 0.49 | 0.16 ± 0.61 | 0.798 | 0.63 ± 0.60 | 0.297 |
| VAT, cm2 | 4.91 ± 5.79 | −15.40 ± 7.29 | 0.043 | −4.28 ± 7.03 | 0.547 |
| ASAT, cm2 | −3.2 ± 15.70 | −6.92 ± 19.77 | 0.729 | −13.69 ± 19.14 | 0.480 |
| Intrahepatic lipid, % | 0.55 ± 0.56 | −1.59 ± 0.70 | 0.031 | −0.59 ± 0.69 | 0.398 |
| IMAT, cm2 | 0.8 ± 3.3 | −13.3 ± 4.13 | 0.003 | −10.9 ± 4.2 | 0.014 |
| Muscle attenuation, HU | 0.39 ± 0.42 | 0.15 ± 0.52 | 0.766 | 0.22 ± 0.53 | 0.682 |
| V̇o2peak, ml·kg−1·min−1 | −0.43 ± 1.36 | 5.17 ± 1.78 | 0.007 | 3.10 ± 1.69 | 0.077 |
| Muscular strength index | 0.07 ± 0.09 | 0.08 ± 0.11 | 0.459 | 0.45 ± 0.11 | 0.0003 |
Values are imputed means (SE). ITT, intent to treat. Change values for the intervention groups are the difference compared with control, with adjustment for baseline values assessed using ANCOVA. P values compared with the control group.
Changes in total adiposity and skeletal muscle.
Body weight, BMI, and waist circumference did not change (P > 0.1) in either exercise group (Table 2). Compared with controls, a significant reduction (P < 0.05) in percent body fat was observed within the AE (−1.70 ± 0.85%) and RE (−1.63 ± 0.78%) groups. Skeletal muscle mass did not change within any of the exercise groups (P > 0.05).
Changes in VAT, intrahepatic lipid, and IMAT.
Compared with controls, significant (P < 0.05) reductions in VAT (Δ−15.68 ± 7.64 cm2) and intrahepatic lipid (Δ−1.70 ± 0.74%) were observed in the AE but not in the RE group (Table 2). In both the AE (Δ−13.5 ± 4.2 cm2) and RE (Δ−10.9 ± 4.2 cm2) groups, there were reductions (P < 0.05) in IMAT compared with controls.
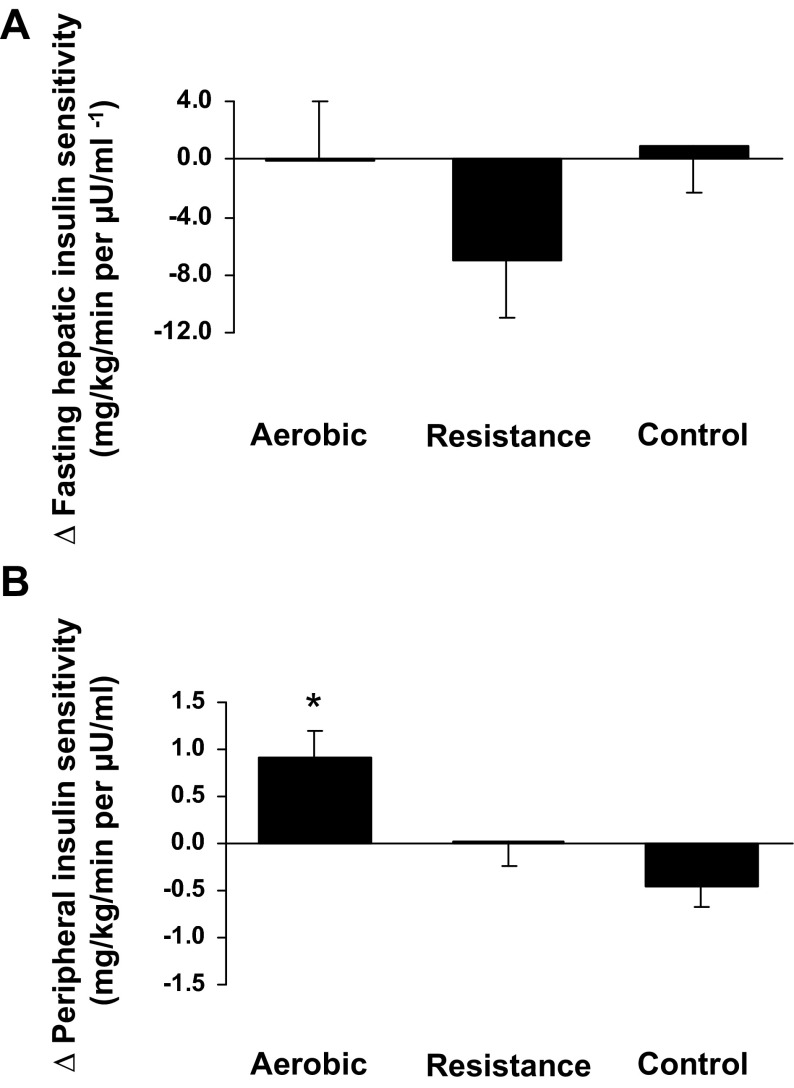
Changes in insulin sensitivity.
Compared with controls, fasting glucose production and hepatic insulin sensitivity did not change significantly in the AE or the RE group (Table 3). Peripheral insulin sensitivity improved significantly in the AE group (0.92 ± 0.27 mg·kg−1·min−1 per μU/ml, P = 0.0007; Fig. 2B), even when insulin sensitivity was expressed per unit of FFM [Δ1.43 ± 0.44 mg·kg fat-free mass (FFM)−1·min−1 per μU/ml, P = 0.001] compared with controls (Δ−0.79 ± 0.35 mg·kg FFM−1·min−1 per μU/ml). These observations remained unchanged when analyses were repeated, excluding a control subject whose postintervention clamp ended early due to IV issues. The improvement in peripheral insulin sensitivity in the AE group was significantly associated with the loss in total AT mass (r = −0.65, P = 0.02) but not VAT or intrahepatic lipid (P > 0.05). No significant changes in OGTT parameters (such as glucose and insulin levels at 2 h and glucose and insulin AUCs) were observed in any groups.
Table 3.
Absolute changes in metabolic variables after 3 mo
| Aerobic Exercise (n = 14/ITT =16) |
Resistance Exercise (n = 14/ITT =16) |
||||
|---|---|---|---|---|---|
| Control (n = 8/ITT =12) Means ± SE | Means ± SE | P Value | Means ± SE | P Value | |
| ITT analysis (n = 44) | |||||
| Fasting glucose, mg/dl | 1.35 ± 2.03 | −2.11 ± 2.64 | 0.424 | 0.61 ± 2.54 | 0.811 |
| Fasting insulin, μU/ml | 0.07 ± 3.34 | −7.62 ± 4.33 | 0.079 | −1.29 ± 4.37 | 0.768 |
| Fasting HGP, mg·kg−1·min−1 | −0.09 ± 0.16 | 0.32 ± 0.20 | 0.120 | 0.14 ± 0.20 | 0.491 |
| Hepatic insulin sensitivity, mg·kg−1·min−1 per μU/ml−1 | 0.87 ± 3.17 | −0.04 ± 4.11 | 0.992 | −6.96 ± 3.97 | 0.080 |
| Glucose at 2 h, mg/dl | 5.47 ± 6.39 | −3.01 ± 8.0 | 0.706 | −2.01 ± 8.12 | 0.805 |
| Insulin at 2 h, μU/ml | 15.17 ± 39.48 | −60.66 ± 49.62 | 0.222 | 9.25 ± 50.53 | 0.855 |
| Glucose AUC, mg·min−1·dl | 12.95 ± 490.55 | 62.69 ± 630.72 | 0.921 | −126.12 ± 613.69 | 0.837 |
| Insulin AUC, μU·min−1·ml | −837.00 ± 2,927.61 | −4,087.57 ± 3,715.96 | 0.272 | −2,441.82 ± 3,834.03 | 0.524 |
| Insulin sensitivity, mg·kg−1·min−1 per μU/ml | −0.46 ± 0.21 | 0.92 ± 0.27 | 0.0007 | 0.03 ± 0.27 | 0.902 |
| Insulin sensitivity, mg·kg FFM−1·min−1 per μU/ml | −0.79 ± 0.35 | 1.43 ± 0.44 | 0.0011 | −0.13 ± 0.45 | 0.767 |
| Per-protocol analysis (n = 36) | |||||
| Fasting glucose, mg/dl | 1.60 ± 2.01 | −2.35 ± 2.53 | 0.361 | 0.38 ± 2.52 | 0.882 |
| Fasting insulin, μU/ml | −0.05 ± 3.34 | −8.03 ± 4.19 | 0.064 | −1.65 ± 4.3 | 0.707 |
| Fasting HGP, mg·kg−1·min−1 | −0.09 ± 0.16 | 2.92 ± 0.20 | 0.146 | 0.12 ± 1.96 | 0.556 |
| Hepatic insulin sensitivity, mg·kg−1·min−1 per μU/ml−1 | 1.39 ± 3.12 | −0.25 ± 3.96 | 0.951 | −7.22 ± 3.95 | 0.077 |
| Glucose at 2 h, mg/dl | 4.74 ± 6.24 | −2.56 ± 7.80 | 0.745 | −2.00 ± 7.97 | 0.803 |
| Insulin at 2 h, μU/ml | 10.8 ± 38.8 | −57.70 ± 48.42 | 0.242 | 9.49 ± 49.74 | 0.850 |
| Glucose AUC, mg·min−1·dl | 43.44 ± 485.72 | 7.47 ± 608.45 | 0.990 | −180.29 ± 609.69 | 0.769 |
| Insulin AUC, μU·min−1·ml | −739.76 ± 2,921.24 | −4,418.58 ± 3,590.20 | 0.227 | −2,764.87 ± 3,820.03 | 0.475 |
| Insulin sensitivity, mg·kg−1·min−1 per μU/ml | −0.49 ± 0.21 | 1.10 ± 0.07 | 0.0002 | 0.07 ± 0.26 | 0.793 |
| Insulin sensitivity, mg·kg FFM−1·min−1 per μU/ml | −0.78 ± 0.34 | 1.44 ± 0.43 | 0.0021 | −0.13 ± 0.44 | 0.778 |
Values are imputed means (SE). Change values for the intervention groups are the difference compared with control, with adjustment for baseline values assessed using ANCOVA. P values compared with the control group.
Fig. 2.
Absolute change in hepatic insulin sensitivity and peripheral insulin sensitivity for each intervention group. Values for the control group are imputed means (SE). Change values for the intervention groups are the difference compared with control, with adjustment for baseline values as assessed using ANCOVA. *P < 0.001 compared with the control group.
DISCUSSION
The present investigation reveals that in obese adolescent girls, despite the absence of weight loss, significant reductions in percent body fat and IMAT were achieved after 3 mo (3 days/wk) of AE and RE programs. Moreover, AE but not RE was associated with significant reductions in visceral adiposity and intrahepatic lipid and improvements in insulin sensitivity and CRF. These findings suggest, for the first time, that AE may be a better mode of exercise than RE in obese adolescent girls to reduce abdominal adiposity and liver fat and improve insulin resistance.
Although adult studies report the beneficial effects of exercise alone on insulin action in women (8, 32, 34), little information is available regarding the independent role of regular exercise on insulin resistance in adolescent girls. Treuth et al. (38) reported no significant changes in fasting glucose and insulin or glucose and insulin AUC in response to a 5-mo strength training (3 days/wk, 20 min/session) in obese prepubertal girls (n = 9). By contrast, Nassis et al. (29) demonstrated that 12 wk of AE without weight loss (3 days/wk, 40 min/session) resulted in a significant reduction in insulin AUC (23%) in overweight and obese girls (9–15 yr; n = 19). Using a randomized controlled trial, our finding that AE without calorie restriction and weight loss resulted in significant improvements in insulin sensitivity (33%), assessed by a 3-h hyperinsulinemic euglycemic clamp technique, extends previous observations (29, 38) using surrogate measurements of insulin sensitivity (OGTT) and provides evidence that engaging in AE alone is an effective means of improving insulin sensitivity in these high-risk obese adolescent girls. Additionally, the use of whole body MRI, 1H-MRS, and computed tomography in our study allowed direct assessments of changes in whole body adipose tissue distribution in response to AE vs. RE.
Our finding that AE without calorie restriction is associated with significant reductions in intrahepatic lipid and VAT in obese adolescent girls is consistent with van der Heijden et al. (39), who reported that a 12-wk AE without calorie restriction (2 days/wk, 30 min/session) was associated with reductions in intrahepatic lipid (∼37%) and VAT (∼9.3%) in a mixed sample of obese Hispanic boys and girls (n = 15). The current findings with respect to AE are paralleled with our previous observations in obese adolescent boys (22), who showed significant reductions in VAT (7%) and intrahepatic lipid content (40%). However, with respect to RE, the two sexes responded differently. Unlike the obese adolescent boys (22), obese girls did not have significant reductions in intrahepatic lipid and VAT in response to RE. Furthermore, unlike the obese adolescent boys (22), we did not find a significant increase in skeletal muscle mass in obese adolescent girls in response to RE. We are unclear about this observed sex difference in response to RE, as the exercise training regimens and the methodologies (1H-MRS and MRI) were identical in both studies. Perhaps testosterone in adolescent boys may enhance the benefits of RE on skeletal muscle mass.
It is well established that visceral fat is a strong risk factor for obesity-related comorbidities in youth (24, 40). Although the underlying mechanisms by which visceral fat is associated with metabolic abnormalities are unclear, it has been hypothesized that excess free fatty acids released from the visceral adipocytes drain directly into the liver via the portal vein, resulting in intrahepatic lipid accumulation, VLDL production, and reduced insulin clearance in the liver (“the portal theory”) (5). However, in this study, visceral fat and intrahepatic lipid were not associated with both hepatic and peripheral insulin sensitivity in obese adolescent girls. These are different from our previous findings in obese adolescent boys (22) demonstrating that the change in insulin sensitivity was significantly correlated with the corresponding changes in visceral fat (r = −0.47, P < 0.05). Perhaps sex differences in the amount of visceral fat (lower visceral fat in obese girls vs. obese boys) may explain the strength of the relationships between visceral fat and insulin sensitivity in obese boys vs. obese girls.
There were also sex differences with respect to the change in peripheral insulin sensitivity after the exercise training program. Although we observed significant improvements in insulin sensitivity in response to RE in obese adolescent boys (22), the identical RE intervention did not result in improvements in insulin sensitivity in obese adolescent girls. Theoretically, one would expect that cardiometabolic and diabetes risk factors would improve after resistance training. Previously, Kirwan et al. (19) showed that eccentric exercise resulted in transient decreases in insulin sensitivity (−37%) in healthy individuals that persists for ∼48 h after the exercise bout. It has been suggested that the reductions in insulin sensitivity after eccentric exercise are mediated by increased inflammatory markers related to exercise-induced muscle damage (18). However, because we acquired insulin sensitivity measurements with identical protocols in boys and girls, this is unlikely to explain the observed contrast in sex differences in insulin sensitivity following the two different exercise regimens. Alternatively, others report substantial interindividual variability in the ability to improve health outcomes in response to regular exercise. For example, Bouchard et al. (6) reported that among study completers (n = 1,687) from six exercise intervention trials (HERITAGE family study, DREW, INFLAME, STRRIDE, MARYLAND, and JYVASKYLA), 8.4% had adverse changes in fasting insulin, along with 13.3% for HDL cholesterol and 12.2% for systolic blood pressure after AE independent of age and CRF. It is unknown the degree to which this interindividual variation in RE response occurs. Furthermore, the possibility that the two sexes may respond differently to various exercise regimens points to the need to individualize the exercise training to gain the most health benefit.
Our finding that both AE and RE are associated with reductions in IMAT in obese adolescent girls is of importance given that IMAT is inversely associated with insulin sensitivity in adolescents (23). That regular exercise is effective in reducing IMAT in obese girls is consistent with adult studies (13, 26) demonstrating the beneficial effects of exercise in reducing skeletal muscle lipid content measured by CT. However, these observations differ from studies employing 1H-MRS, which report no significant changes in intramyocellular lipid (IMCL) in response to regular exercise in obese adolescents (22, 39) and obese adults (15). Although both CT and 1H-MRS methods have been used in clinical research for assessing skeletal muscle lipid in vivo, it is important to note that IMAT measured by CT and IMCL measured by 1H-MRS do not equate. Although CT is unable to differentiate between IMCL and extramyocellular lipids (EMCL), it measures a larger muscle group, and muscle attenuation measured by CT as an overall lipid marker is more reproducible than EMCL or IMCL measured separately by 1H-MRS (20).
Similarly to our previous study in obese boys (22), obese adolescent girls complied well with the prescribed exercise training regimen, resulting in high attendance rates. However, anecdotally, the girls in the RE group did not enjoy the treatment intervention as much as the AE group. Interestingly, this was the opposite sentiment given by obese boys. Therefore, given the superior improvements in metabolic health with AE and the enjoyment factor, we propose that AE may be a better mode of exercise for adolescent girls of this age group.
The current physical activity guidelines from the US Department of Health and Human Services (2008) suggest that youth should engage in both aerobic and muscle strengthening exercise to improve overall health (1). Indeed, randomized controlled studies in adults demonstrate that the combination of AE and RE is a better exercise strategy than either exercise modality alone to improve glycemic control (7, 36) or insulin sensitivity (10). However, in children and adolescents, it is currently unknown whether a combined AE and RE program would be associated with greater improvements in insulin sensitivity than either exercise alone or whether the response would be similar in boys and girls. Further investigations should shed light on this.
Limitations of this study warrant mention. Given the set length of intervention and the acute effects of exercise on insulin sensitivity, we were unable to measure insulin sensitivity during the same menstrual cycle before and after the intervention, which was true for all three groups. Our findings are limited to obese healthy black and white adolescent girls. Whether our findings would remain true in other racial groups, prepubertal girls and girls with oral or injectable contraceptives, or girls with type 2 diabetes is unknown. Although we randomly assigned participants to intervention groups, this does not always result in similar characteristics between groups. Indeed, at randomization, subjects in the RE group tended to have higher percent body fat and lower insulin sensitivity compared with those in the AE group. Because treatment changes are often related to the baseline value (i.e., poorer baseline values allow for a potentially larger improvement), we adjusted all analyses examining treatment effects for that corresponding baseline value. However, because of the small sample size in this study, we did not simultaneously adjust for all group baseline differences, as this may limit our power and potentially be an overadjustment since many of the health and obesity markers are intercorrelated. Although participants were asked to log their energy intake during the study, this was completed by very few participants and was generally done poorly.
In summary, the results of this study suggest that in previously sedentary obese adolescent girls, both AE and RE (3 days/wk, ∼180 min/wk), without calorie restriction and weight loss, are associated with reductions in total fat and IMAT. However, only AE and not RE is associated with reductions in visceral adiposity and liver fat and improvement in insulin sensitivity, a major risk factor for type 2 diabetes in youth.
GRANTS
This research was supported by National Institutes of Health Grants 1-R21-DK-083654-01A1, UL1-RR-024153, and 2K24-HD-01357, the Cochrane-Weber Foundation, the Renziehausen Fund, and the Richard L. Day Endowed Chair.
DISCLOSURES
The authors have no conflicts of interest, financial or otherwise, to declare.
AUTHOR CONTRIBUTIONS
S.L. obtained the funding and contributed to the conception and design of the research; S.L., A.R.D., D.W., Y.K., I.L., M.R.-V., and S.A. performed the experiments; S.L., A.R.D., D.W., Y.K., I.L., M.R.-V., and J.L.K. analyzed the data; S.L., J.L.K., S.S., C.B., and S.A. interpreted the results of the experiments; S.L. prepared the figures; S.L. drafted the manuscript; S.L., A.R.D., D.W., Y.K., I.L., M.R.-V., J.L.K., S.S., C.B., and S.A. edited and revised the manuscript; S.L., A.R.D., D.W., Y.K., I.L., M.R.-V., J.L.K., S.S., C.B., and S.A. approved the final version of the manuscript. S.L. is the guarantor of this work, had full access to all of the data, and takes full responsibility for the integrity of data and the accuracy of the data analysis.
ACKNOWLEDGMENTS
We express our gratitude to the study participants and their parents and to Nancy Guerra (certified registered nurse practitioner), Jacqueline Washington (research coordinator), Resa Stauffer (laboratory research technician), the PCTRC nursing staff, and the YMCA of Greater Pittsburgh for donating their exercise facilities.
REFERENCES
- 1.US Department of Health and Human Services 2008 Physical Activity Guidelines for Americans (available at www.health.gov/paguidelines). Washington, DC: ODPHP, publication no. U0036, 2008 [Google Scholar]
- 2. Allison DB, Paultre F, Maggio C, Mezzitis N, Pi-Sunyer FX. The use of areas under curves in diabetes research. Diabetes Care 18: 245–250, 1995 [DOI] [PubMed] [Google Scholar]
- 3. Arslanian SA, Saad R, Lewy V, Danadian K, Janosky J. Hyperinsulinemia in african-american children: decreased insulin clearance and increased insulin secretion and its relationship to insulin sensitivity. Diabetes 51: 3014–3019, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Bacha F, Saad R, Gungor N, Arslanian SA. Adiponectin in youth: relationship to visceral adiposity, insulin sensitivity, and beta-cell function. Diabetes Care 27: 547–552, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Björntorp P. “Portal” adipose tissue as a generator of risk factors for cardiovascular disease and diabetes. Arteriosclerosis 10: 493–496, 1990 [PubMed] [Google Scholar]
- 6. Bouchard C, Blair SN, Church TS, Earnest CP, Hagberg JM, Hakkinen K, Jenkins NT, Karavirta L, Kraus WE, Leon AS, Rao DC, Sarzynski MA, Skinner JS, Slentz CA, Rankinen T. Adverse metabolic response to regular exercise: is it a rare or common occurrence? PLoS One 7: e37887, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Church TS, Blair SN, Cocreham S, Johannsen N, Johnson W, Kramer K, Mikus CR, Myers V, Nauta M, Rodarte RQ, Sparks L, Thompson A, Earnest CP. Effects of aerobic and resistance training on hemoglobin A1c levels in patients with type 2 diabetes: a randomized controlled trial. JAMA 304: 2253–2262, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cuff DJ, Meneilly GS, Martin A, Ignaszewski A, Tildesley HD, Frohlich JJ. Effective exercise modality to reduce insulin resistance in women with type 2 diabetes. Diabetes Care 26: 2977–2982, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Daniels SR, Jacobson MS, McCrindle BW, Eckel RH, Sanner BM. American Heart Association Childhood Obesity Research Summit: executive summary. Circulation 119: 2114–2123, 2009 [DOI] [PubMed] [Google Scholar]
- 10. Davidson LE, Hudson R, Kilpatrick K, Kuk JL, McMillan K, Janiszewski PM, Lee S, Lam M, Ross R. Effects of exercise modality on insulin resistance and functional limitation in older adults: a randomized controlled trial. Arch Intern Med 169: 122–131, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Goodpaster BH, Thaete FL, Kelley DE. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr 71: 885–892, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Harris JA, Benedict FF. A Biometric Study of Basal Metabolism in Man. Washington, DC: Carnegie Institution of Washington, 1919 [Google Scholar]
- 13. Hutchison SK, Teede HJ, Rachon D, Harrison CL, Strauss BJ, Stepto NK. Effect of exercise training on insulin sensitivity, mitochondria and computed tomography muscle attenuation in overweight women with and without polycystic ovary syndrome. Diabetologia 55: 1424–1434, 2012 [DOI] [PubMed] [Google Scholar]
- 14. Imperatore G, Cheng YJ, Williams DE, Fulton J, Gregg EW. Physical activity, cardiovascular fitness, and insulin sensitivity among U.S. adolescents: the National Health and Nutrition Examination Survey, 1999–2002. Diabetes Care 29: 1567–1572, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Johnson NA, Sachinwalla T, Walton DW, Smith K, Armstrong A, Thompson MW, George J. Aerobic exercise training reduces hepatic and visceral lipids in obese individuals without weight loss. Hepatology 50: 1105–1112, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Jurca R, Lamonte MJ, Church TS, Earnest CP, Fitzgerald SJ, Barlow CE, Jordan AN, Kampert JB, Blair SN. Associations of muscle strength and fitness with metabolic syndrome in men. Med Sci Sports Exerc 36: 1301–1307, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Kimm SY, Glynn NW, Kriska AM, Barton BA, Kronsberg SS, Daniels SR, Crawford PB, Sabry ZI, Liu K. Decline in physical activity in black girls and white girls during adolescence. N Engl J Med 347: 709–715, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Kirwan JP, del Aguila LF. Insulin signalling, exercise and cellular integrity. Biochem Soc Trans 31: 1281–1285, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Kirwan JP, Hickner RC, Yarasheski KE, Kohrt WM, Wiethop BV, Holloszy JO. Eccentric exercise induces transient insulin resistance in healthy individuals. J Appl Physiol 72: 2197–2202, 1992 [DOI] [PubMed] [Google Scholar]
- 20. Larson-Meyer DE, Smith SR, Heilbronn LK, Kelley DE, Ravussin E, Newcomer BR. Muscle-associated triglyceride measured by computed tomography and magnetic resonance spectroscopy. Obesity (Silver Spring) 14: 73–87, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee S, Bacha F, Gungor N, Arslanian S. Comparison of different definitions of pediatric metabolic syndrome: relation to abdominal adiposity, insulin resistance, adiponectin, and inflammatory biomarkers. J Pediatr 152: 177–184, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Lee S, Bacha F, Hannon T, Kuk JL, Boesch C, Arslanian S. Effects of aerobic versus resistance exercise without caloric restriction on abdominal fat, intrahepatic lipid, and insulin sensitivity in obese adolescent boys: a randomized, controlled trial. Diabetes 61: 2787–2795, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee S, Guerra N, Arslanian S. Skeletal muscle lipid content and insulin sensitivity in black versus white obese adolescents: is there a race differential? J Clin Endocrinol Metab 95: 2426–2432, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee S, Gungor N, Bacha F, Arslanian S. Insulin resistance: link to the components of the metabolic syndrome and biomarkers of endothelial dysfunction in youth. Diabetes Care 30: 2091–2097, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Lee S, Kim Y, Kuk JL, Boada FE, Arslanian S. Whole-body MRI and ethnic differences in adipose tissue and skeletal muscle distribution in overweight black and white adolescent boys. J Obes 2011: 159373, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee S, Kuk JL, Davidson LE, Hudson R, Kilpatrick K, Graham TE, Ross R. Exercise without weight loss is an effective strategy for obesity reduction in obese individuals with and without Type 2 diabetes. J Appl Physiol 99: 1220–1225, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Ratitch B, O'Kelly M. Implementation of Pattern-Mixture Models Using Standard SAS/STAT Procedures. PharmaSUG, Paper-SP04, 2011 [Google Scholar]
- 28. Naressi A, Couturier C, Devos JM, Janssen M, Mangeat C, de Beer R, Graveron-Demilly D. Java-based graphical user interface for the MRUI quantitation package. MAGMA 12: 141–152, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Nassis GP, Papantakou K, Skenderi K, Triandafillopoulou M, Kavouras SA, Yannakoulia M, Chrousos GP, Sidossis LS. Aerobic exercise training improves insulin sensitivity without changes in body weight, body fat, adiponectin, and inflammatory markers in overweight and obese girls. Metabolism 54: 1472–1479, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, Grummer-Strawn LM, Curtin LR, Roche AF, Johnson CL. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics 109: 45–60, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Perseghin G, Price TB, Petersen KF, Roden M, Cline GW, Gerow K, Rothman DL, Shulman GI. Increased glucose transport-phosphorylation and muscle glycogen synthesis after exercise training in insulin-resistant subjects. N Engl J Med 335: 1357–1362, 1996 [DOI] [PubMed] [Google Scholar]
- 32. Poehlman ET, Dvorak RV, DeNino WF, Brochu M, Ades PA. Effects of resistance training and endurance training on insulin sensitivity in nonobese, young women: a controlled randomized trial. J Clin Endocrinol Metab 85: 2463–2468, 2000 [DOI] [PubMed] [Google Scholar]
- 33. Rosenbloom AL, Joe JR, Young RS, Winter WE. Emerging epidemic of type 2 diabetes in youth. Diabetes Care 22: 345–354, 1999 [DOI] [PubMed] [Google Scholar]
- 34. Ryan AS, Pratley RE, Goldberg AP, Elahi D. Resistive training increases insulin action in postmenopausal women. J Gerontol A Biol Sci Med Sci 51: M199–M205, 1996 [DOI] [PubMed] [Google Scholar]
- 35. Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics 118: 1388–1393, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Sigal RJ, Kenny GP, Boule NG, Wells GA, Prud'homme D, Fortier M, Reid RD, Tulloch H, Coyle D, Phillips P, Jennings A, Jaffey J. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Ann Intern Med 147: 357–369, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Tanner JM. Growth and maturation during adolescence. Nutr Rev 39: 43–55, 1981 [DOI] [PubMed] [Google Scholar]
- 38. Treuth MS, Hunter GR, Figueroa-Colon R, Goran MI. Effects of strength training on intra-abdominal adipose tissue in obese prepubertal girls. Med Sci Sports Exerc 30: 1738–1743, 1998 [DOI] [PubMed] [Google Scholar]
- 39. van der Heijden GJ, Wang ZJ, Chu ZD, Sauer PJ, Haymond MW, Rodriguez LM, Sunehag AL. A 12-week aerobic exercise program reduces hepatic fat accumulation and insulin resistance in obese, Hispanic adolescents. Obesity (Silver Spring) 18: 384–390, 2010 [DOI] [PubMed] [Google Scholar]
- 40. Weiss R, Dufour S, Taksali SE, Tamborlane WV, Petersen KF, Bonadonna RC, Boselli L, Barbetta G, Allen K, Rife F, Savoye M, Dziura J, Sherwin R, Shulman GI, Caprio S. Prediabetes in obese youth: a syndrome of impaired glucose tolerance, severe insulin resistance, and altered myocellular and abdominal fat partitioning. Lancet 362: 951–957, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, Allen K, Lopes M, Savoye M, Morrison J, Sherwin RS, Caprio S. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med 350: 2362–2374, 2004 [DOI] [PubMed] [Google Scholar]