Abstract
Obesity-related kidney disease occurs as a result of complex interactions between metabolic and hemodynamic effects. Changes in microvascular perfusion may play a major role in kidney disease; however, these changes are difficult to assess in vivo. Here, we used perfusion ultrasound imaging to evaluate cortical blood flow in a mouse model of high-fat diet-induced kidney disease. C57BL/6J mice were randomized to a standard diet (STD) or a high-fat diet (HFD) for 30 wk and then treated either with losartan or a placebo for an additional 6 wk. Noninvasive ultrasound perfusion imaging of the kidney was performed during infusion of a microbubble contrast agent. Blood flow within the microvasculature of the renal cortex and medulla was derived from imaging data. An increase in the time required to achieve full cortical perfusion was observed for HFD mice relative to STD. This was reversed following treatment with losartan. These data were concurrent with an increased glomerular filtration rate in HFD mice compared with STD- or HFD-losartan-treated mice. Losartan treatment also abrogated fibro-inflammatory disease, assessed by markers at the protein and messenger level. Finally, a reduction in capillary density was found in HFD mice, and this was reversed upon losartan treatment. This suggests that alterations in vascular density may be responsible for the elevated perfusion time observed by imaging. These data demonstrate that ultrasound contrast imaging is a robust and sensitive method for evaluating changes in renal microvascular perfusion and that cortical perfusion time may be a useful parameter for evaluating obesity-related renal disease.
Keywords: ultrasound contrast imaging, obesity-related kidney disease, renal perfusion
the growing epidemic of obesity is a serious health and economic burden throughout the world. The consequence of obesity over time is characterized by insulin resistance, hyperglycemia, atherosclerosis, dyslipidemia, and hypertension. This cluster of risk factors referred to as metabolic syndrome occurs with 50% prevalence in patients 50- to 60-yr-old (4) and contributes to cardiovascular disease. Obesity is a major risk factor for diabetes and hypertension, which together account for ∼70% of all cases of end-stage renal disease (6). However, there is growing evidence suggesting that obesity by itself may be an independent risk factor for the development of vascular dysfunction and renal disease (21, 44, 66). Obesity-related kidney disease occurs as a result of complex interactions between metabolic and hemodynamic factors and is characterized by changes of renal perfusion, vascular dysfunction, as well as albuminuria, glomerulosclerosis, and tubulointerstitial fibrosis (20, 37, 48, 50). Alterations in blood flow within the cortex or the medulla may therefore be likely to occur in obesity-related kidney disease. However, techniques for directly measuring perfusion of the renal microvasculature, either in small animal models or in clinical medicine, are limited.
Ultrasound perfusion imaging using nontargeted vascular contrast agents provides a real-time and noninvasive method to assess microvascular perfusion in living subjects (7). Contrast agents for ultrasound perfusion imaging are gas-encapsulated microbubbles stabilized by a shell of lipid, protein, or polymer (8). Microbubble contrast agents are generally between 1 and 8 μm in diameter, allowing uninterrupted passage through capillaries. Previous studies have demonstrated that the rheology of microbubble agents is similar to that of erythrocytes (22, 23, 34) and behave as purely intravascular flow tracers. Unlike iodinated contrast agents and chelates used for MRI, microbubble agents are not filtered by the kidney. Thus the contrast images obtained using this technique represent true vascular perfusion.
Several microbubble-based imaging agents have been approved for clinical cardiac and radiological indications in the United States, Europe, China, and elsewhere. Clinical applications in the kidney are generally limited to characterization of overt vascular anomalies (such as stenosis and segmental infarction) and qualitative evaluation of perfusion abnormalities. Here, we sought to test whether this technique has the sensitivity to detect subtle changes in renal cortical blood flow that may occur in the context of glomerular fibrosis, and subsequent therapy, in a model of obesity-related kidney disease.
MATERIALS AND METHODS
Animals and interventional study.
Male C57BL/6 mice were purchased from Jackson Laboratory (Bar Harbor, ME). All animal procedures were approved by the Institutional Animal Care and Use Committee of University of California, San Diego. Starting at 6 wk of age, mice were fed a high-fat diet (HFD, n = 14) in which 60% of total calories were from fat, 20% from protein, and 20% from carbohydrate (Research Diets, New Brunswick, NJ) or standard diet (STD, n = 7), consisting of 5% fat and 24.5% protein for 30 wk. Mice in the HFD group were further randomized into two groups of six. The first group (n = 8) was treated with losartan (Sigma), an ANG II receptor 1 antagonist, at 5 mg·kg−1·day−1 in drinking water for 6 wk starting after 30 wk on HFD. The second group (n = 6) served as a placebo control. It has been previously demonstrated that there are not a significant antihypertensive effects caused by this dose of losartan (5). The concentration of compounds was adjusted once every other day based on water consumption and body weight The food intake as well as the body temperature was also measured.
All mice were imaged at week 36 and then euthanized. Mice were placed in metabolic cages for 24-h urine collection before treatment and on the last day of the treatment period. After euthanasia, blood samples were collected and portions of both kidneys were snap-frozen in liquid nitrogen for RNA isolation. An additional portion was frozen in OCT or fixed in 4% PAF for immunostaining.
Ultrasound contrast agent.
This study was performed using a perfusion ultrasound contrast agent (Targestar P; Targeson) commercially available for life science research. This agent is a lipid encapsulated perfluorocarbon microbubble incorporating a coating of poly(ethyleneglycol). The mean diameter of the microbubble particles is 2.2 um.
Ultrasound imaging.
All ultrasound imaging was performed on a Siemens Sequoia 512 (Siemens Medical Solutions), using a high-frequency clinical transducer (15L-8). All contrast images were acquired at a dynamic range of 70 dB, mechanical index of 0.19–0.24, and gain of 0 dB. No persistence or edge enhancement was applied.
On the final study day, mice were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg) and placed in the left lateral decubitus position on a heated stage. The skin along the flank was first shaved with an electric razor and then depilitated with a commercial crème product. A cannula consisting of a 10-cm length of PE-10 tubing connected to a 27-G needle bent into an L shape was inserted into the retro-orbital sinus and secured to the stage with tape. A mound of heated ultrasound coupling gel was placed on the right flank, and the ultrasound transducer was fixed in place with a flexible clamp ∼1 inch above the skin surface. Images of the right kidney were first acquired in B-mode at a center frequency of 14 MHz, and the transducer position was adjusted so as to obtain a uniform view of the kidney in long axis. The same field of view was then acquired in contrast imaging mode (Cadence CPS), and the contrast gain was adjusted such that the noise was just slightly visible. Contrast agents were diluted 1:10 by volume in sterile 0.9% saline and infused at a rate of 60 μl/min using a syringe pump (Harvard Apparatus). Entry of the contrast agent into the kidney was apparent on ultrasound imaging within several seconds of beginning the infusion. After a 1-min period of infusion to allow the vascular concentration of contrast agent to reach steady state, a high mechanical index burst was applied (MI = 0.7, duration 2.0 s). This disrupted all contrast agents within the field of view, creating a “negative bolus” effect. A clip consisting of ∼2 s before the burst and 20 s after was digitally recorded at the maximum frame rate possible (14–18 Hz). Complete reperfusion of all segments of the kidney was found to reproducibly occur within 4–6 s of the destructive burst.
Ultrasound image analysis.
Data analysis was performed in ImageJ (v 1.46r; National Institutes of Health). The first 20 s of digital video after the burst were analyzed for each animal. Polygonal regions of interest encompassing the renal medulla or cortex were defined, being careful to avoid the interlobular arteries. Ultrasound imaging data are typically presented with color mapping (here, in shades of orange) and compression to highlight relevant aspects of the image during the scan; this can confound quantitative analysis of imaging data by the introduction of nonlinearity in the relationship between the image intensity and the true microbubble concentration. Therefore, the nonlinear color map and postprocessing settings were reversed to yield linearized intensity data. The linearized intensity data were baseline subtracted and normalized by peak intensity. Time-to-peak (TTP; measured in seconds) was defined as the time required for the normalized intensity to reach 95% of the peak intensity. This was computed from a single time-intensity curve for each animal.
Glomerular filtration rate.
Twenty minutes after the infusion of ultrasound contrast agents was stopped, FITC-inulin was injected in bolus to measure the glomerular filtration rate (GFR) based on published methods (12, 47, 56, 62). 5% inulin was dissolved in normal saline and dialyzed 24 h to remove unbound inulin. This solution was sterile filtered and then injected (2 μl·−1·body wt−1) retro-orbitally to anesthetized mice. Twenty microliters of blood were collected from the tail vein at 3, 5, 7, 10, 15, 30, 60, and 75 min into a Na+-heparinized microcap. The samples were spun down, and 1 μl of plasma was reconstituted in HEPES buffer. The fluorescence was then determined using a Nanodrop ND-3300 fluorospectrometer (NanodropTechnologies). GFR was calculated using a two-phase exponential decay principle using Graph Pad (Prism 5) software.
Urine and plasma analysis.
The urine albumin and creatinine were measured with a mouse Albuwell ELISA kit and a Creatinine Companion kit (Exocell). As an index of oxidative stress, urine samples were also analyzed for hydrogen peroxide by Amplex red assay (Invitrogen) following the manufacturer's protocol.
Quantitative real-time PCR.
Frozen kidneys (−80°C) samples were homogenized and total RNA was then extracted. The mRNA quantification was performed using a two-step real-time RT-PCR. Real-time PCR was performed on kidney using the primers for angiotensinogen, renin, monocyte chemotattractant protein-1 (MCP-1), α1-type I collagen, α1-type IV collagen, and β-actin as a housekeeping gene. Relative gene expressions were calculated using the 2−ΔΔCt method.
Immunohistochemistry.
Immunostainings of macrophages and endothelial cells on paraffin-embedded mouse kidneys were performed using rat anti-CD43 antibody (BD Biosciences) and rat anti-mouse CD31 primary antibody (AbD Serotec), respectively. Quantitation of CD43-positive cells was evaluated by a semiquantitative analysis as described previously (9). Briefly, the distribution of positive cells in the different histological structures of the renal tissue was performed on one section per experimental animal. For each section, 10 square fields (0.084 mm2/field) were observed at ×400 magnification. The relative area occupied by CD31 in renal tissue was evaluated by a computer-assisted morphometric approach. Images were obtained with a DeltaPix digital camera (model DP200) and further analyzed using the National Institute of Health ImageJ software to quantify the percentage of stained area. The sections were analyzed by scanning 10 fields at ×200 magnification.
Statistical analysis.
Results are presented as means values ± SE. The level for statistical significance was defined as P < 0.05. Analyses were carried out using Graph Pad Prism Software version 4.03. Difference between data groups were evaluated for significance using, one-way ANOVA and Newman-Keuls post hoc test for multiple comparisons.
RESULTS
Model characterization and metabolic data.
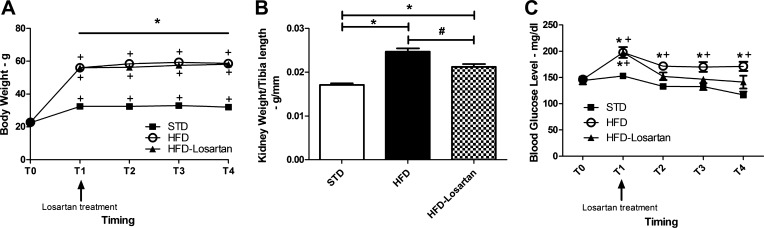
The metabolic data of mice fed a normal or HFD with or without losartan treatment are shown in Fig. 1. The temporal evolution of the body weight presented in Fig. 1A showed a significant increase in all groups from T0, corresponding to the day 0, to T4 which corresponds to the end of the experimental protocol (week 36). However, the body weights of mice fed a HFD or HFD + losartan were significantly higher than these of STD mice. At week 36, the body weight reached 58.53 ± 1.08 g (P < 0.001) in HFD group, 58.18 ± 0.78 g (P < 0.001) in the HFD + losartan group, and 31.86 ± 0.74 g in STD mice. Losartan treatment did not prevent body weight gain. Kidney weights of HFD mice were significantly higher than these of STD mice (0.0246 ± 0.0007 g/mm of tibia length vs. 0.0171 ± 0.0003 g/mm of tibia length; P < 0.001). Kidney hypertrophy was significantly attenuated with losartan compared with HFD mice treated with placebo (0.0210 ± 0.0006 g/mm of tibia length vs. 0.0246 ± 0.0007 g/mm of tibia length, respectively; P = 0.001). Nevertheless, the kidney weight was still higher compared with STD mice (P < 0.001). Blood glucose was significantly higher in HFD mice throughout the experimental protocol (T0 to T4). Moreover, at week 36, the level of blood glucose was 171.2 ± 8.5 mg/dl (P = 0.045) compared with 122.6 ± 3.8 for the STD mice. Treatment with losartan tended to reduce the blood glucose although results were not significant compared with HFD alone.
Fig. 1.
A: temporal evolution of body weight in mice fed a standard diet (STD), a high-fat diet (HFD), or HFD-losartan. B: changes in kidney weight between mice fed a STD, a HFD, or HFD-losartan. C: temporal evolution of blood glucose in mice fed a STD, a HFD, or HFD-losartan. T0 corresponds to day 0; T1 corresponds to week 30, just before starting losartan or placebo treatment; T2 corresponds to week 32; T3 and T4 correspond to weeks 34 and 36, respectively. A: values are means ± SE; n = 6–8 in each group. Statistical analyses were performed by two-way ANOVA followed by Newman-Keuls: *P ≤ 0.05 HFD and HFD-losartan vs. mice on STD and +P ≤ 0.05 vs. T0. B: values are means ± SE; n = 6–8 in each group. Statistical analyses were performed by one-way ANOVA followed by Newman-Keuls: *P ≤ 0.05 vs. mice on STD and #P ≤ 0.05 vs. mice on HFD. C: values are means ± SE; n = 6–8 in each group. Statistical analyses were performed by two-way ANOVA followed by Newman-Keuls: *P ≤ 0.05 HFD or HFD + losartan vs. mice on STD and +P ≤ 0.05 vs. T0.
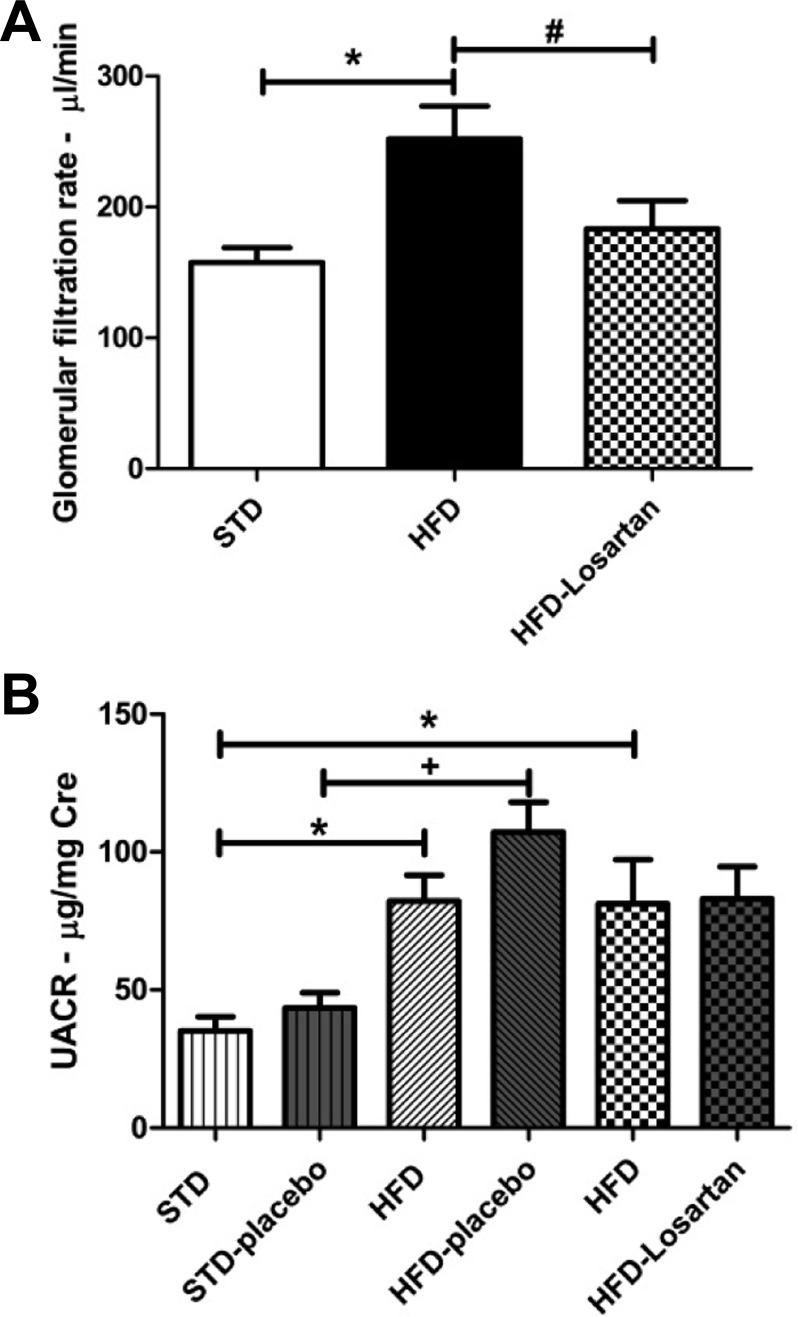
As illustrated in Fig. 2, the GFR was significantly higher in mice fed a HFD (252.2 ± 24.8 μl/min; P = 0.0043) compared with the mice fed a STD (157.7 ± 11.2 μl/min). Moreover, increased GFR was significantly attenuated by losartan treatment (183.4 ± 21.5 μl/min; P < 0.05; Fig. 2A). In addition, HFD induced a significant elevation of albuminuria as measured at week 30 [82.23 ± 9.45 μg/mg Cre HFD-before placebo (P = 0.021) and 81.16 ± 16.13 μg/mg Cre HFD-before losartan (P = 0.014) vs. 35.14 ± 5.22 μg/mg Cre STD-before placebo], which was further enhanced at week 36 in HFD mice receiving the placebo (107.20 ± 10.77 μg/mg Cre; P = 0.002). Interestingly, HFD mice treated with losartan did not present a further increase in albuminuria (83.04 ± 11.65 μg/mg Cre), showing a beneficial effect of losartan on renal function (Fig. 2B).
Fig. 2.
Determination of the glomerular filtration rate and albuminuria in mice fed a STD, a HFD, or HFD-losartan. A: quantitative glomerular filtration rate determined by the renal FITC-inulin clearance expressed in μl/min. Values are means ± SE; n = 6–8 in each group. Statistical analyses were performed by one-way ANOVA followed by Newman-Keuls: *P ≤ 0.05 vs. mice on STD and #P ≤ 0.05 vs. mice on HFD. B: quantitative urine albumin/creatinine ratio (UACR) before and after placebo or losartan treatment in mice on STD or HFD at 30 wk (before) and 36 wk (after treatment). Values are means ± SE; n = 6–8 in each group. Statistical analyses were performed by one-way ANOVA followed by Newman-Keuls: *P ≤ 0.05 vs. mice on STD and +P ≤ 0.05 vs. mice on STD-placebo.
Perfusion imaging.
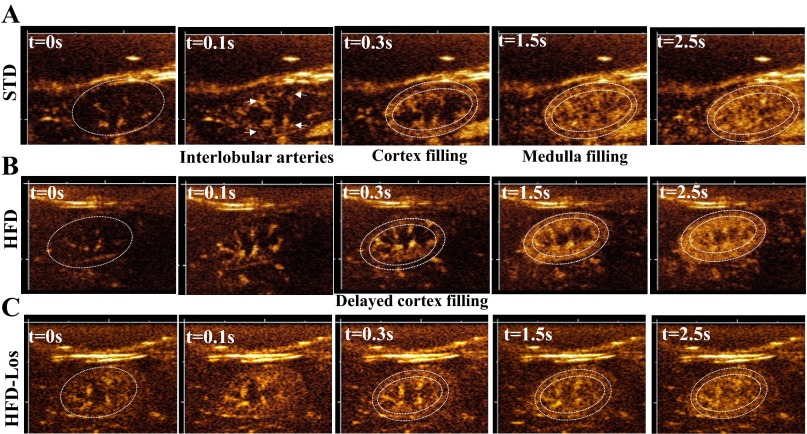
As it has been demonstrated elsewhere (41, 68), microbubble contrast agents exhibit a triphasic enhancement pattern within the intact kidney. Enhancement of the renal artery and interlobular arteries is visualized first, followed by enhancement of the renal cortex, and then of the renal medulla. Each of the three phases was detectable in all mice with the imaging system described here (Fig. 3, A, STD, B, HFD, and C, HFD + losartan). Out of 21 kidneys imaged, data from one scan was discarded due to excessive imaging artifact in the signal.
Fig. 3.
Sequential filling of cortex and medulla in mice fed a STD, a HFD, or a HFD + losartan. Representative ultrasound images showing sequential filling of cortex and medulla in kidneys of mice on STD (A), HFD (B), and HFD + losartan (C). Location of kidney is delineated by dotted white line in t = 0 panel; location of cortex delineated in t = 0.3-s pane.
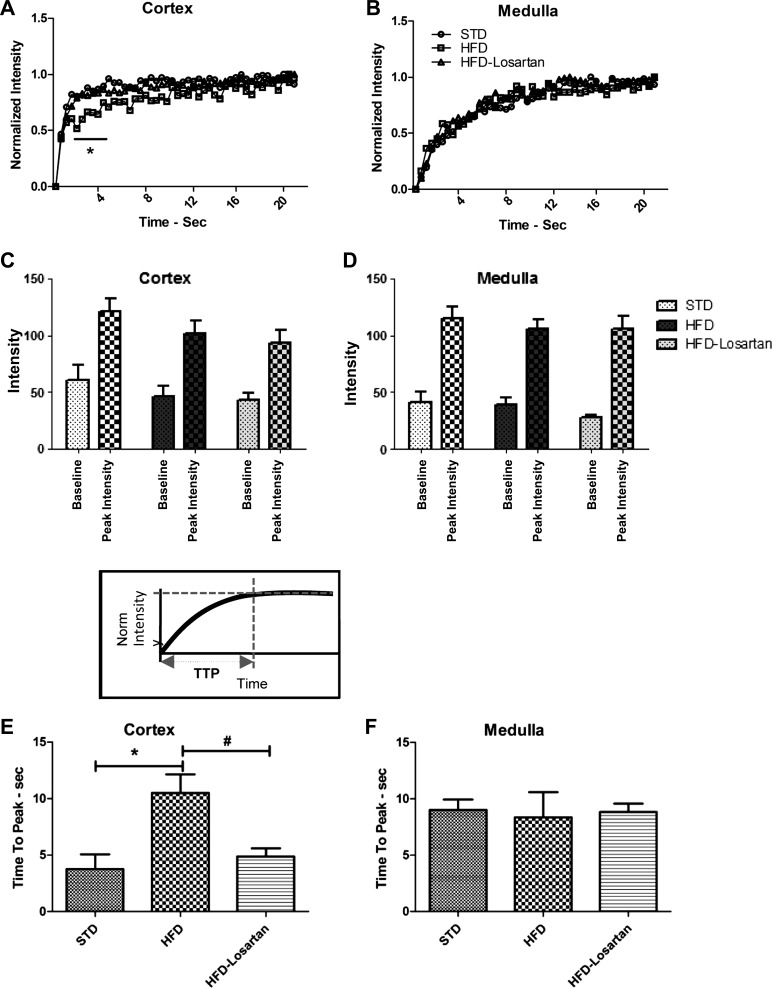
In mice fed a STD, time-intensity analysis showed a very rapid entry of contrast into the cortex, while the medulla took several seconds longer to fully perfuse. This was appreciable from the time-intensity curves (Fig. 4A). In HFD mice, the time-intensity curve for the cortex manifested a rightward shift, representing a longer duration of time required for the cortex to fully perfuse (Fig. 4B). Finally, the third row illustrating the sequence taken from a HFD treated with losartan revealed that losartan alleviated the changes observed in the cortical perfusion in HFD (Fig. 4C). The delay observed in the HFD mice was quantified in the TTP calculation, and a significant increase in TTP was found for HFD mice relative to STD (Fig. 4F). The observed delay in cortical perfusion was reversed in HFD mice treated with losartan (Fig. 4C), and the TTP for these mice was not statistically different to that of STD mice (Fig. 4F). No change in TTP among cohorts was found for the medulla (Fig. 4G).
Fig. 4.
Time-intensity analysis. Representative normalized time-intensity curves from renal cortex (A) and medulla (B) in mice fed a STD (○), HFD (), or HFD after losartan treatment (△). Raw intensity data without baseline subtraction nor normalization for each mouse cohort is shown for the cortex (C) and medulla (D). Quantitative analysis of the time-to-peak (TTP) of cortex (E) and medulla (F) in STD, HFD, or HFD-losartan. Values are means ± SE; n = 6–8 in each group. Statistical analyses were performed by two-way ANOVA followed by Newman-Keuls: *P ≤ 0.05 vs. STD and HFD + losartan (A and B) and a one-way ANOVA followed by Newman-Keuls: *P ≤ 0.05 vs. mice on STD and #P ≤ 0.05 vs. mice on HFD (E and F).
There was no difference in the baseline or peak enhancement among any of the cohorts (Fig. 4, D and E), suggesting that the total volume of contrast agent entering the kidneys was similar.
Microvascular density.
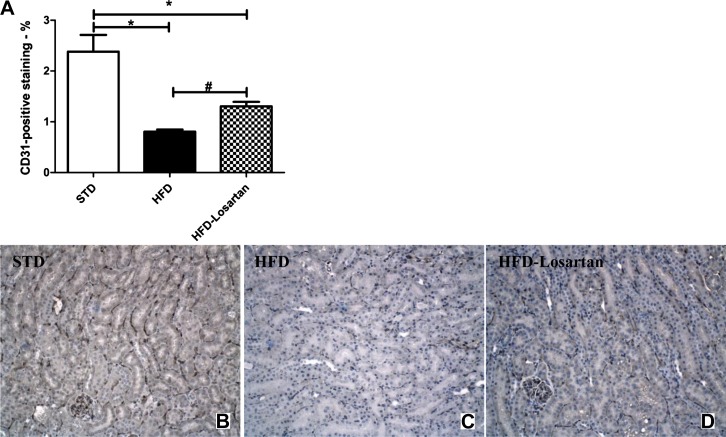
Immunostaining of CD31, a marker of endothelial cells, was performed on paraffin-embedded kidney sections in mice fed a STD, a HFD, or a HFD-losartan (Fig. 5, A–D). The density of cortical microvessels, assessed by CD31, was reduced in the HFD cohort. Treatment with losartan mediated a partial recovery (Fig. 5, A–D), although vessel density was still slightly less than that of mice on STD.
Fig. 5.
Determination of peritubular capillaries in mice fed a STD, a HFD, or a HFD-losartan. Semiquantitative analysis of CD31-positive staining in mice fed a STD, a HFD, or a HFD-losartan at week 36 (A). Representative photomicrographs of CD31 immunostaining (×200) in mice fed a STD (B), a HFD (C), or a HFD-losartan (D). Values are means ± SE; n = 6–8 in each group. Statistical analyses were performed by one-way ANOVA followed by Newman-Keuls: *P ≤ 0.05 vs. mice on STD and #P ≤ 0.05 vs. mice on HFD.
Determination of inflammatory and profibrotic markers.
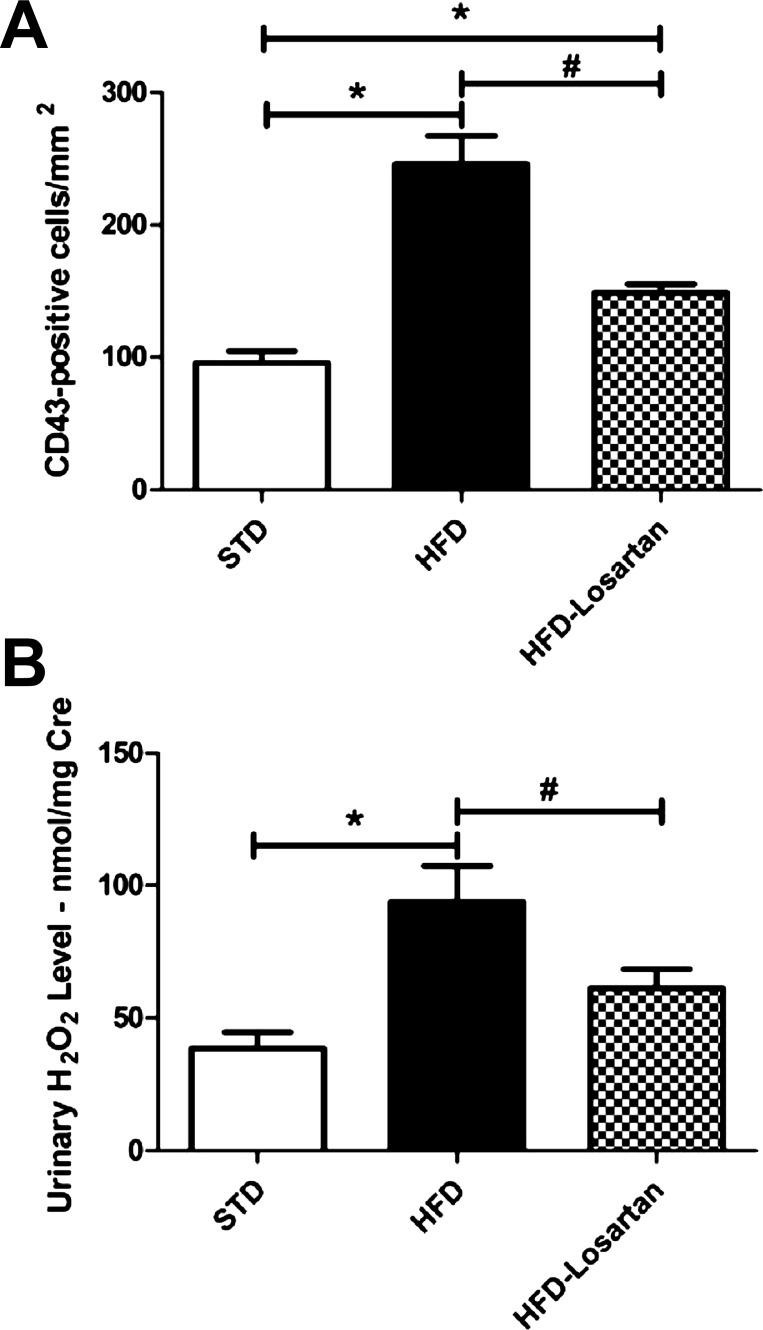
The data in Table 1 illustrate the mRNA level of several markers involved in renin-angiotensin pathway, fibrosis, and inflammation. As observed, the angiotensinogen and renin mRNA level did not differ between groups. Collagen type I and IV mRNAs were elevated with HFD and reduced to baseline levels with losartan treatment. MCP-1, a specific marker of inflammation, showed a significantly higher mRNA level in mice fed a HFD. The increase in MCP-1 was clearly attenuated by losartan (Table. 1). Moreover, the significant increase of macrophages (CD43-positive cells) with HFD was significantly attenuated with losartan (Fig. 6A). Finally, the urinary hydrogen peroxide level, also considered as a marker of renal inflammation, was significantly higher after HFD and was reduced with losartan (Fig. 6B).
Table 1.
Determination of inflammatory and profibrotic markers in mice fed a STD, a HFD, or a HFD-Losartan at week 36
| STD | HFD | HFD-Losartan | |
|---|---|---|---|
| Angiotensinogen | 1.00 ± 0.14 | 0.94 ± 0.16 | 1.10 ± 0.19 |
| Renin | 1.00 ± 0.15 | 0.82 ± 0.10 | 0.92 ± 0.12 |
| Type I collagen | 1.00 ± 0.11 | 1.43 ± 0.18 | 0.97 ± 0.12 |
| Type IV collagen | 1.00 ± 0.09 | 1.42 ± 0.14* | 1.04 ± 0.12 |
| MCP-1 | 1.00 ± 0.18 | 4.16 ± 1.01* | 2.73 ± 0.71 |
Values are means ± SE; n = 6–8 in each group.
HFD, high-fat diet; STD, standard diet; MCP-1, monocyte chemotattractant protein-1.
Quantitative real-time PCR was performed with kidney from all groups each normalized against β-actin. Statistical analyses were performed by one-way ANOVA followed by Newman-Keuls:
P ≤ 0.05 vs. mice on STD.
Fig. 6.
Determination of inflammatory markers in mice fed a STD, a HFD, or HFD-losartan. Semi-quantitative analysis of CD43-positive cells in renal tissue (A), and quantitative urine hydrogen peroxide level in mice on STD, HFD, or HFD-losartan (B). Values are means ± SE; n = 6–8 in each group. Statistical analyses were performed by one-way ANOVA followed by Newman-Keuls: *P ≤ 0.05 vs. mice on STD and #P ≤ 0.05 vs. mice on HFD.
DISCUSSION
This study demonstrates the use of perfusion ultrasound imaging as a fast and practical technique to measure regional kidney blood flow in mice. Here, C57bl/6 male mice were fed a HFD. Feeding a HFD to mice is known to induce metabolic alterations (10), including increased body weight, kidney hypertrophy, and hyperglycemia (as observed in Fig. 1). A continuous infusion of microbubble-contrast agent was administered intravenously to anesthetized mice fed a STD or a HFD. In addition, to better demonstrate the use of ultrasound-contrast agents in monitoring modulations in renal blood perfusion, losartan was administered with HFD concurrently for 6 wk. Losartan is a specific ANG II receptor 1 antagonist. ANG II is a crucial mediator in the progression of obesity and diabetes related kidney disease (1, 33, 60, 61). ANG II participates to the hyperfiltration and glomerulosclerosis through hemodynamic and nonhemodynamic effects (16, 35, 39, 40, 43, 45).
The use of nontargeted microbubble contrast agent in a mouse model of obesity-related kidney disease demonstrated that in mice fed a STD the cortex was observed to fully perfuse rapidly, with perfusion of the medulla occurring several seconds later. A significant delay in the cortical perfusion time was observed in mice on HFD, and this behavior was quantified using the TTP intensity calculation. The increase in cortical TTP was abrogated upon treatment with losartan. Similar differences between cohorts were found for inflammatory markers at the protein and messenger level. Additionally, a rarefaction in cortical capillaries was found in HFD-treated mice and reversed with losartan, suggesting that alterations in blood vessel density may be responsible for our imaging findings.
To link the imaging data to pathological features of obesity-related kidney disease, GFR and albuminuria were determined. We found that HFD significantly increased GFR and albuminuria; this increase was abrogated by losartan treatment. These data might result from the vasodilation of renal afferent arteriole and vasoconstriction of efferent arteriole in response to circulating vasoactive substance. This leads to an increase of the hydrostatic pressure in the glomerular capillaries, which contributes to an elevated GFR and albuminuria. However, it has been shown that ANG II can exaggerate the vasoconstriction response of the efferent arteriole in obese rats, favoring glomerular dysfunction (49). In our study, since losartan, an AT1 angiotensin receptor antagonist, prevents the increased GFR and restores the cortical blood flow, we might hypothesize that ANG II acts through the AT1 receptor, which has been demonstrated to be distributed on renal efferent arteriole (17, 29).
The delay in the cortical perfusion time observed in mice on HFD might result from the interplay between hemodynamic and nonhemodynamic mechanisms. It is known that impaired vascular regulation is associated with inflammatory and fibrotic cytokines as well as albuminuria and may result in glomerular hyperfiltration (53, 70). In our study, HFD mice showed an increase in inflammatory (MCP-1 mRNA level, hydrogen peroxide, and CD43+ macrophages) and fibrotic (collagen IV mRNA level) markers while losartan treatment reversed this. Therefore, the partial resolution to the proinflammatory state in losartan-treated HFD mice can be related to the prevention of the cortical perfusion. It has been shown that angiotensin receptor antagonist has a beneficial effect on endothelial dysfunction as well as in insulin resistance (15, 25, 65). Moreover, a recent study demonstrated that losartan treatment could restore the AMPK activation in the kidney in a similar HFD model (11). AMPK is considered as a cellular energy sensor, which plays a crucial role in glucose metabolism and has been demonstrated to reduce renal inflammation with HFD feeding (3, 10, 38, 52).
Finally, we assessed the peritubular capillary density by CD31 expression. Our data indicated a loss of peritubular capillaries with HFD and partial resolution upon losartan treatment. This data suggest that changes in vascular density may be responsible for our imaging observations.
There is a paucity of quantitative methods for noninvasively assessing renal perfusion. In the clinical context, computerized tomography has been the preferred modality for renal imaging, although recent concerns about toxicity of some computerized tomography contrast agents have largely diminished its use in patients with renal disease. Contrast MRI has shown utility, although the cost, relatively low throughput, and concerns about contrast agent nephrotoxicity have limited its use in clinical and research settings. Doppler ultrasound imaging is able to depict blood flow in large vessels but generally does not have the sensitivity to depict flow at the microvascular level. In small animals, the gold standard for imaging perfusion remains laser Doppler flowmetry. This technique provides accurate measurement of erythrocyte flux, although it has limited depth penetration and requires surgical manipulation for use in the kidney. The use of microbubble contrast agents with a nondestructive contrast imaging mode (such as that implemented on most modern ultrasound scanners) enables the noninvasive use of ultrasound imaging for detection of blood flow at the capillary level. This technique can be readily scaled for use in small animals by selection of a high-frequency probe, does not require any surgical procedures, and can be accomplished in a matter of minutes.
Animal studies have demonstrated a strong correlation between blood flow derived from perfusion ultrasound imaging and flow probes (19, 31, 68) and radiolabeled microspheres (58) with various microbubble formulations, suggesting that perfusion ultrasound accurately reflects blood flow. Subsequent studies have been performed in rat (13, 31, 41), rabbit (59), dog (63, 64, 68), swine (24, 69), mouse (55, 57), and isolated organs (54). Contrast ultrasound imaging of the kidney is not in widespread clinical use, although studies have demonstrated potential utility for evaluation of focal abnormalities (46) and in identifying rejection following renal transplant (14, 27, 28). Of particular note, Kleinert et al. (30) showed that TTP correlated to GFR in a small study of scleroderma patients, and Kalantarinia et al. (26) demonstrated an increase cortical blood velocity in healthy subjects following a high-protein meal. These collective data demonstrate the perfusion ultrasound as an accurate, reproducible, and translatable method for noninvasively assessing renal blood flow.
Much of the work in validating perfusion ultrasound imaging in the kidney has been performed using surgically (19, 58, 68) or pharmacologically (13, 31, 41, 57) induced changes in renal blood flow but not in models of common clinically relevant diseases. To our knowledge, the data presented here are the first evidence that this technique can detect chronic kidney disease and its response to treatment. Moreover, the relatively simple procedure utilized here provided sufficient robustness without the need for extensive image processing or analysis. This may be of benefit to researchers utilizing small animal models and also as a clinical tool for routine evaluation of renal perfusion in patients with metabolic disease.
Our study has several limitations. Kidneys were evaluated in only one imaging plane, which may introduce variability due to undersampling (13). Volumetric imaging may therefore be required to achieve the greatest sensitivity, although at the expense of increased procedure time and complexity. Imaging in our study was performed using a clinical ultrasound scanner operating at 14 MHz, offering a spatial resolution of ∼250 μm. Ultra-high-frequency ultrasound, operating at 40 MHz and with a spatial resolution of ∼100 um, can be used with microbubble contrast agents (51). However, as noted by Sullivan et al. (57), the sensitivity to contrast agents using ultra-high ultrasound scanners is generally limited. We found that use of a clinical scanner, operating at an imaging frequency close to the microbubble natural resonant frequency, offered a suitable balance between spatial resolution and contrast sensitivity for imaging the murine kidney. Finally, we used a relatively simple analysis method based on TTP intensity. Previous studies have extracted quantitative parameters relating to blood volume and velocity based on curve fitting of time-intensity data. The most common analysis utilizes a shifted exponential function (26, 31, 57, 67, 68). This method relies upon the a priori assumption that the time-intensity curve follows the mathematical model used, an assumption about which there is some disagreement in the field (2, 18, 32, 36, 42). To our surprise, we found that our simple TTP analysis, which requires no assumptions regarding the mathematical shape of the time-intensity curve, offered sufficient robustness to detect changes in cortical blood flow in our animal model.
In summary, our study demonstrates that the kidney responds to the challenge of high-fat feeding, exhibiting renal function impairment, inflammation, and fibrotic response at the gene expression, histologic, and functional level. Losartan treatment improved the overall kidney function status by preventing kidney hypertrophy, reducing renal inflammation, preventing hyperfiltration, and preventing further increased albuminuria. In our study, losartan was also used to validate the use of ultrasound-contrast agents in monitoring modulations in renal blood perfusion. Hence, ultrasound-contrast agent technique was proven to be sensitive enough to monitor the prevention of the cortical perfusion in losartan-treated HFD mice. Therefore, we demonstrate that the use of perfusion ultrasound imaging may be an useful tool for noninvasively assessing the extent, and monitoring the resolution, of obesity-related kidney disease.
GRANTS
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R43-DK-3083142 and R44-DK-083142 (to J. J. Rychak and K. Sharma). Additional funding was provided from National Institute of Diabetes and Digestive and Kidney Diseases Grant DP3-1DP3-DK-094352 and Veterans Affairs Merit Award 5101BX000277 (to K. Sharma). A. E. Declèves was a research fellow supported by the Belgian fund “Wallonie-Bruxelles International.”
DISCLOSURES
J. J. Rychak and D. J. Smith are employees and own stock in Targeson, Inc.
AUTHOR CONTRIBUTIONS
Author contributions: A.-E.D., J.J.R., and K.S. conception and design of research; A.-E.D. and J.J.R. performed experiments; A.-E.D. analyzed data; A.-E.D., J.J.R., and K.S. interpreted results of experiments; A.-E.D. prepared figures; A.-E.D. drafted manuscript; A.-E.D., J.J.R., D.J.S., and K.S. edited and revised manuscript; A.-E.D., J.J.R., D.J.S., and K.S. approved final version of manuscript.
ACKNOWLEDGMENTS
Technical assistance from Ismayil Guracar (Siemens Medical Solutions) is gratefully acknowledged. We thank N. Caron of UNamur (Belgium) for helpful comments; these have helped improve this paper.
REFERENCES
- 1.Amann K, Benz K. Structural renal changes in obesity and diabetes. Semin Nephrol 33: 23–33, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Arditi M, Frinking PJ, Zhou X, Rognin NG. A new formalism for the quantification of tissue perfusion by the destruction-replenishment method in contrast ultrasound imaging. IEEE Trans Ultrason Ferroelectr Freq Control 53: 1118–1129, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Bijland S, Mancini SJ, Salt IP. Role of AMP-activated protein kinase in adipose tissue metabolism and inflammation. Clin Sci 124: 491–507, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Bruce KD, Byrne CD. The metabolic syndrome: common origins of a multifactorial disorder. Postgrad Med J 85: 614–621, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Chagnac A, Weinstein T, Korzets A, Ramadan E, Hirsch J, Gafter U. Glomerular hemodynamics in severe obesity. Am J Physiol Renal Physiol 278: F817–F822, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Collins AJ, Foley RN, Chavers B, Gilbertson D, Herzog C, Johansen K, Kasiske B, Kutner N, Liu J, St Peter W, Guo H, Gustafson S, Heubner B, Lamb K, Li S, Peng Y, Qiu Y, Roberts T, Skeans M, Snyder J, Solid C, Thompson B, Wang C, Weinhandl E, Zaun D, Arko C, Chen SC, Daniels F, Ebben J, Frazier E, Hanzlik C, Johnson R, Sheets D, Wang X, Forrest B, Constantini E, Everson S, Eggers P, Agodoa L. United States Renal Data System 2011 Annual Data Report: atlas of chronic kidney disease & end-stage renal disease in the United States. Am J Kidney Dis 59: A7, e1–420, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Cosgrove D, Lassau N. Imaging of perfusion using ultrasound. Eur J Nucl Med 1: S65–85, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Dayton PA, Rychak JJ. Molecular ultrasound imaging using microbubble contrast agents. Front Biosci 12: 5124–5142, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Decleves AE, Caron N, Nonclercq D, Legrand A, Toubeau G, Kramp R, Flamion B. Dynamics of hyaluronan, CD44, and inflammatory cells in the rat kidney after ischemia/reperfusion injury. Intl J Mol Med 18: 83–94, 2006 [PubMed] [Google Scholar]
- 10.Decleves AE, Mathew AV, Cunard R, Sharma K. AMPK mediates the initiation of kidney disease induced by a high-fat diet. J Am Soc Nephrol 22: 1846–1855, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deji N, Kume S, Araki S, Isshiki K, Araki H, Chin-Kanasaki M, Tanaka Y, Nishiyama A, Koya D, Haneda M, Kashiwagi A, Maegawa H, Uzu T. Role of angiotensin II-mediated AMPK inactivation on obesity-related salt-sensitive hypertension. Biochem Biophys Res Commun 418: 559–564, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Eisner C, Faulhaber-Walter R, Wang Y, Leelahavanichkul A, Yuen PS, Mizel D, Star RA, Briggs JP, Levine M, Schnermann J. Major contribution of tubular secretion to creatinine clearance in mice. Kidney Int 77: 519–526, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feingold S, Gessner R, Guracar IM, Dayton PA. Quantitative volumetric perfusion mapping of the microvasculature using contrast ultrasound. Invest Radiol 45: 669–674, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischer T, Filimonow S, Dieckhofer J, Slowinski T, Muhler M, Lembcke A, Budde K, Neumayer HH, Ebeling V, Giessing M, Thomas A, Morgera S. Improved diagnosis of early kidney allograft dysfunction by ultrasound with echo enhancer–a new method for the diagnosis of renal perfusion. Nephrol Dial Transplant 21: 2921–2929, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Flammer AJ, Hermann F, Wiesli P, Schwegler B, Chenevard R, Hurlimann D, Sudano I, Gay S, Neidhart M, Riesen W, Ruschitzka F, Luscher TF, Noll G, Lehmann R. Effect of losartan, compared with atenolol, on endothelial function and oxidative stress in patients with type 2 diabetes and hypertension. J Hypertens 25: 785–791, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Gupte M, Boustany-Kari CM, Bharadwaj K, Police S, Thatcher S, Gong MC, English VL, Cassis LA. ACE2 is expressed in mouse adipocytes and regulated by a high-fat diet. Am J Physiol Regul Integr Comp Physiol 295: R781–R788, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Helou CM, Imbert-Teboul M, Doucet A, Rajerison R, Chollet C, Alhenc-Gelas F, Marchetti J. Angiotensin receptor subtypes in thin and muscular juxtamedullary efferent arterioles of rat kidney. Am J Physiol Renal Physiol 285: F507–F514, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Hudson JM, Karshafian R, Burns PN. Quantification of flow using ultrasound and microbubbles: a disruption replenishment model based on physical principles. Ultrasound Med Biol 35: 2007–2020, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Hudson JM, Leung K, Burns PN. The lognormal perfusion model for disruption replenishment measurements of blood flow: in vivo validation. Ultrasound Med Biol 37: 1571–1578, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Iacobini C, Menini S, Ricci C, Scipioni A, Sansoni V, Mazzitelli G, Cordone S, Pesce C, Pugliese F, Pricci F, Pugliese G. Advanced lipoxidation end-products mediate lipid-induced glomerular injury: role of receptor-mediated mechanisms. J Pathol 218: 360–369, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Imig JD. Eicosanoids and renal damage in cardiometabolic syndrome. Expert Opin Drug Metab Toxicol 4: 165–174, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ismail S, Jayaweera AR, Camarano G, Gimple LW, Powers ER, Kaul S. Relation between air-filled albumin microbubble and red blood cell rheology in the human myocardium. Influence of echocardiographic systems and chest wall attenuation. Circulation 94: 445–451, 1996 [DOI] [PubMed] [Google Scholar]
- 23.Jayaweera AR, Edwards N, Glasheen WP, Villanueva FS, Abbott RD, Kaul S. In vivo myocardial kinetics of air-filled albumin microbubbles during myocardial contrast echocardiography. Comparison with radiolabeled red blood cells. Circ Res 74: 1157–1165, 1994 [DOI] [PubMed] [Google Scholar]
- 24.Jimenez C, de Gracia R, Aguilera A, Alonso S, Cirugeda A, Benito J, Regojo RM, Aguilar R, Warlters A, Gomez R, Largo C, Selgas R. In situ kidney insonation with microbubble contrast agents does not cause renal tissue damage in a porcine model. J Ultrasound Med 27: 1607–1615, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Jin HM, Pan Y. Angiotensin type-1 receptor blockade with losartan increases insulin sensitivity and improves glucose homeostasis in subjects with type 2 diabetes and nephropathy. Nephrol Dial Transplant 22: 1943–1949, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Kalantarinia K, Belcik JT, Patrie JT, Wei K. Real-time measurement of renal blood flow in healthy subjects using contrast-enhanced ultrasound. Am J Physiol Renal Physiol 297: F1129–F1134, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kay DH, Mazonakis M, Geddes C, Baxter G. Ultrasonic microbubble contrast agents and the transplant kidney. Clin Radiol 64: 1081–1087, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Kim JH, Eun HW, Lee HJ, Goo DE, Choi DL. Clinical use of renal perfusion imaging by means of harmonic sonography with a microbubble contrast agent in patients after renal transplantation: preliminary study. J Ultrasound Med 24: 755–762, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Kimura K, Inokuchi S, Sugaya T, Suzuki N, Yoneda H, Shirato I, Mise N, Oba S, Miyashita K, Tojo A, Hirata Y, Goto A, Sakai T, Murakami K, Omata M. Location and action of angiotensin II type 1 receptor in the renal microcirculation. Kidney Int Suppl 63: S201–204, 1997 [PubMed] [Google Scholar]
- 30.Kleinert S, Roll P, Baumgaertner C, Himsel A, Mueller A, Fleck M, Feuchtenberger M, Jenett M, Tony HP. Renal perfusion in scleroderma patients assessed by microbubble-based contrast-enhanced ultrasound. Open Rheumatol J 6: 50–53, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kogan P, Johnson KA, Feingold S, Garrett N, Guracar I, Arendshorst WJ, Dayton PA. Validation of dynamic contrast-enhanced ultrasound in rodent kidneys as an absolute quantitative method for measuring blood perfusion. Ultrasound Med Biol 37: 900–908, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krix M, Kiessling F, Farhan N, Schmidt K, Hoffend J, Delorme S. A multivessel model describing replenishment kinetics of ultrasound contrast agent for quantification of tissue perfusion. Ultrasound Med Biol 29: 1421–1430, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Leehey DJ, Singh AK, Alavi N, Singh R. Role of angiotensin II in diabetic nephropathy. Kidney Int Suppl 77: S93–98, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Lindner JR, Song J, Jayaweera AR, Sklenar J, Kaul S. Microvascular rheology of Definity microbubbles after intra-arterial and intravenous administration. J Am Soc Echocardiogr 15: 396–403, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Lu H, Boustany-Kari CM, Daugherty A, Cassis LA. Angiotensin II increases adipose angiotensinogen expression. Am J Physiol Endocrinol Metab 292: E1280–E1287, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Lucidarme O, Franchi-Abella S, Correas JM, Bridal SL, Kurtisovski E, Berger G. Blood flow quantification with contrast-enhanced US: “entrance in the section” phenomenon-phantom and rabbit study. Radiology 228: 473–479, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Mathew AV, Okada S, Sharma K. Obesity related kidney disease. Curr Diabetes Rev 7: 41–49, 2011 [DOI] [PubMed] [Google Scholar]
- 38.O'Neill HM, Holloway GP, Steinberg GR. AMPK regulation of fatty acid metabolism and mitochondrial biogenesis: implications for obesity. Mol Cell Endocrinol 366: 135–151, 2013 [DOI] [PubMed] [Google Scholar]
- 39.Ogihara T, Asano T, Ando K, Chiba Y, Sakoda H, Anai M, Shojima N, Ono H, Onishi Y, Fujishiro M, Katagiri H, Fukushima Y, Kikuchi M, Noguchi N, Aburatani H, Komuro I, Fujita T. Angiotensin II-induced insulin resistance is associated with enhanced insulin signaling. Hypertension 40: 872–879, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Olivares-Reyes JA, Arellano-Plancarte A, Castillo-Hernandez JR. Angiotensin II and the development of insulin resistance: implications for diabetes. Mol Cell Endocrinol 302: 128–139, 2009 [DOI] [PubMed] [Google Scholar]
- 41.Pollard RE, Dayton PA, Watson KD, Hu X, Guracar IM, Ferrara KW. Motion corrected cadence CPS ultrasound for quantifying response to vasoactive drugs in a rat kidney model. Urology 74: 675–681, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Potdevin TC, Fowlkes JB, Moskalik AP, Carson PL. Analysis of refill curve shape in ultrasound contrast agent studies. Med Phys 31: 623–632, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Praga M, Hernandez E, Morales E, Campos AP, Valero MA, Martinez MA, Leon M. Clinical features and long-term outcome of obesity-associated focal segmental glomerulosclerosis. Nephrol Dial Transplant 16: 1790–1798, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Praga M, Morales E. Obesity, proteinuria and progression of renal failure. Curr Opin Nephrol Hypertens 15: 481–486, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Prasannarong M, Santos FR, Henriksen EJ. ANG-(1–7) reduces ANG II-induced insulin resistance by enhancing Akt phosphorylation via a Mas receptor-dependent mechanism in rat skeletal muscle. Biochem Biophys Res Commun 426: 369–373, 2012 [DOI] [PubMed] [Google Scholar]
- 46.Puls R, Hosten N, Lemke M, Teichgraber UK, Steinkamp HK, Felix R. Perfusion abnormalities of kidney parenchyma: microvascular imaging with contrast-enhanced color and power Doppler ultrasonography–preliminary results. J Ultrasound Med 19: 817–821, 2000 [DOI] [PubMed] [Google Scholar]
- 47.Qi Z, Whitt I, Mehta A, Jin J, Zhao M, Harris RC, Fogo AB, Breyer MD. Serial determination of glomerular filtration rate in conscious mice using FITC-inulin clearance. Am J Physiol Renal Physiol 286: F590–F596, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Ritz E, Koleganova N, Piecha G. Is there an obesity-metabolic syndrome related glomerulopathy? Curr Opin Nephrol Hypertens 20: 44–49, 2011 [DOI] [PubMed] [Google Scholar]
- 49.Roos MH, Eringa EC, van Rodijnen WF, van Lambalgen TA, Ter Wee PM, Tangelder GJ. Preglomerular and postglomerular basal diameter changes and reactivity to angiotensin II in obese rats. Diabetes Obes Metab 10: 898–905, 2008 [DOI] [PubMed] [Google Scholar]
- 50.Ruggiero C, Ehrenshaft M, Cleland E, Stadler K. High-fat diet induces an initial adaptation of mitochondrial bioenergetics in the kidney despite evident oxidative stress and mitochondrial ROS production. Am J Physiol Endocrinol Metab 300: E1047–E1058, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rychak JJ, Lindner JR, Ley K, Klibanov AL. Deformable gas-filled microbubbles targeted to P-selectin. J Control Release 114: 288–299, 2006 [DOI] [PubMed] [Google Scholar]
- 52.Saha AK, Xu XJ, Balon TW, Brandon A, Kraegen EW, Ruderman NB. Insulin resistance due to nutrient excess: is it a consequence of AMPK downregulation? Cell Cycle 10: 3447–3451, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sasson AN, Cherney DZ. Renal hyperfiltration related to diabetes mellitus and obesity in human disease. World J Diabetes 3: 1–6, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schlosser T, Pohl C, Veltmann C, Lohmaier S, Goenechea J, Ehlgen A, Koster J, Bimmel D, Kuntz-Hehner S, Becher H, Tiemann K. Feasibility of the flash-replenishment concept in renal tissue: which parameters affect the assessment of the contrast replenishment? Ultrasound Med Biol 27: 937–944, 2001 [DOI] [PubMed] [Google Scholar]
- 55.Sirsi S, Feshitan J, Kwan J, Homma S, Borden M. Effect of microbubble size on fundamental mode high frequency ultrasound imaging in mice. Ultrasound Med Biol 36: 935–948, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sturgeon C, Sam AD, 2nd, Law WR. Rapid determination of glomerular filtration rate by single-bolus inulin: a comparison of estimation analyses. J Appl Physiol 84: 2154–2162, 1998 [DOI] [PubMed] [Google Scholar]
- 57.Sullivan JC, Wang B, Boesen EI, D'Angelo G, Pollock JS, Pollock DM. Novel use of ultrasound to examine regional blood flow in the mouse kidney. Am J Physiol Renal Physiol 297: F228–F235, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taylor GA, Barnewolt CE, Adler BH, Dunning PS. Renal cortical ischemia in rabbits revealed by contrast-enhanced power Doppler sonography. AJR Am J Roentgenol 170: 417–422, 1998 [DOI] [PubMed] [Google Scholar]
- 59.Taylor GA, Ecklund K, Dunning PS. Renal cortical perfusion in rabbits: visualization with color amplitude imaging and an experimental microbubble-based US contrast agent. Radiology 201: 125–129, 1996 [DOI] [PubMed] [Google Scholar]
- 60.Thethi T, Kamiyama M, Kobori H. The link between the renin-angiotensin-aldosterone system and renal injury in obesity and the metabolic syndrome. Curr Hypertens Rep 14: 160–169, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tuck ML, Sowers J, Dornfeld L, Kledzik G, Maxwell M. The effect of weight reduction on blood pressure, plasma renin activity, and plasma aldosterone levels in obese patients. N Engl J Med 304: 930–933, 1981 [DOI] [PubMed] [Google Scholar]
- 62.Vallon V, Schroth J, Satriano J, Blantz RC, Thomson SC, Rieg T. Adenosine A(1) receptors determine glomerular hyperfiltration and the salt paradox in early streptozotocin diabetes mellitus. Nephron Physiol 111: p30–38, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Verbeek XA, Willigers JM, Prinzen FW, Peschar M, Ledoux LA, Hoeks AP. High-resolution functional imaging with ultrasound contrast agents based on RF processing in an in vivo kidney experiment. Ultrasound Med Biol 27: 223–233, 2001 [DOI] [PubMed] [Google Scholar]
- 64.Waller KR, O'Brien RT, Zagzebski JA. Quantitative contrast ultrasound analysis of renal perfusion in normal dogs. Vet Radiol Ultrasound 48: 373–377, 2007 [DOI] [PubMed] [Google Scholar]
- 65.Wang N, Chai W, Zhao L, Tao L, Cao W, Liu Z. Losartan increases muscle insulin delivery and rescues insulin's metabolic action during lipid infusion via microvascular recruitment. Am J Physiol Endocrinol Metab 304: E538–E545, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Y, Chen X, Song Y, Caballero B, Cheskin LJ. Association between obesity and kidney disease: a systematic review and meta-analysis. Kidney Int 73: 19–33, 2008 [DOI] [PubMed] [Google Scholar]
- 67.Wei K, Jayaweera AR, Firoozan S, Linka A, Skyba DM, Kaul S. Quantification of myocardial blood flow with ultrasound-induced destruction of microbubbles administered as a constant venous infusion. Circulation 97: 473–483, 1998 [DOI] [PubMed] [Google Scholar]
- 68.Wei K, Le E, Bin JP, Coggins M, Thorpe J, Kaul S. Quantification of renal blood flow with contrast-enhanced ultrasound. J Am Coll Cardiol 37: 1135–1140, 2001 [DOI] [PubMed] [Google Scholar]
- 69.Yi K, Ji S, Kim J, Yoon J, Choi M. Contrast-enhanced ultrasound analysis of renal perfusion in normal micropigs. J Vet Sci 13: 311–314, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zatz R, Meyer TW, Rennke HG, Brenner BM. Predominance of hemodynamic rather than metabolic factors in the pathogenesis of diabetic glomerulopathy. Proc Natl Acad Sci USA 82: 5963–5967, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]