Abstract
It is recognized that dopamine promotes natriuresis by inhibiting multiple transporting systems in the proximal tubule. In contrast, less is known about the molecular targets of dopamine actions on water-electrolyte transport in the cortical collecting duct (CCD). Epithelial cells in the CCD are exposed to dopamine, which is synthesized locally or secreted from sympathetic nerve endings. Basolateral K+ channels in the distal renal tubule are critical for K+ recycling and controlling basolateral membrane potential to establish the driving force for Na+ reabsorption. Here, we demonstrate that Kir4.1 and Kir5.1 are highly expressed in the mouse kidney cortex and are localized to the basolateral membrane of the CCD. Using patch-clamp electrophysiology in freshly isolated CCDs, we detected highly abundant 40-pS and scarce 20-pS single channel conductances, most likely representing Kir4.1/5.1 and Kir4.1 channels, respectively. Dopamine reversibly decreased the open probability of both channels, with a relatively greater action on the Kir4.1/5.1 heterodimer. This effect was mediated by D2-like but not D1-like dopamine receptors. PKC blockade abolished the inhibition of basolateral K+ channels by dopamine. Importantly, dopamine significantly decreased the amplitude of Kir4.1/5.1 and Kir4.1 unitary currents. Consistently, dopamine induced an acute depolarization of basolateral membrane potential, as directly monitored using current-clamp mode in isolated CCDs. Therefore, we demonstrate that dopamine inhibits basolateral Kir4.1/5.1 and Kir4.1 channels in CCD cells via stimulation of D2-like receptors and subsequently PKC. This leads to depolarization of the basolateral membrane and a decreased driving force for Na+ reabsorption in the distal renal tubule.
Keywords: basolateral potassium recycling, dopamine receptors, renal potassium channels, distal nephron
it is generally recognized that the kidney is central for the regulation of circulation volume by controlling the water and electrolyte balance (23). Compromised kidney function is tightly linked to disturbances in extracellular fluid volume and to abnormalities in blood pressure (11, 18). The cortical collecting duct (CCD) is the final place where tubular Na+ reabsorption and K+ secretion occur, and, therefore, transport rates at this tubular segment determine urine composition (34). Consistently, several monogenic forms of blood pressure disorders in humans are caused by genetic mutations in the transport proteins of the CCD (31).
Dopamine is a critical regulator of systemic blood pressure affecting fluid and electrolyte balance via the regulation of renal hemodynamics and tubular ion transport (17). In the kidney, dopamine can be synthesized in renal tubular cells and secreted to both apical and basolateral sides (33). Experimental evidence suggests that cultured proximal and distal cells produce and store cathecholamines, including dopamine, in the concentration range of 10 μM, which is sufficient to activate receptors (9). In addition, dopamine is directly released to the kidney cortex from dopaminergic and adrenergic nerve endings (4, 17). Dopamine is an important natriuretic hormone in the kidney (2, 16, 17). Apart from its well-documented effects in renal blood flow and glomerular filtration rate, dopamine suppresses Na+ reabsorption in the renal tubule, resulting in the augmented fractional excretion of Na+ (16). In the proximal tubule, dopamine inhibits activity of apical Na+/H+ exchanger 3, the Na-Pi cotransporter on the apical side, and Na+-K+-ATPase and the Na+-HCO3− cotransporter on the basolateral membrane (for a review, see Ref. 2).
Dopamine exerts its numerous physiological actions by acting on two different subgroups of its receptors: D1-like (D1 and D5) and D2-like (D2–D4) receptors (16, 17). D1-like receptors are coupled to Gs/Go proteins, leading to the activation of adenylyl cyclase and also, in some cases, to stimulation of phospholipase C (17). D2-like receptors are coupled to Gi/Go, inhibiting adenylyl cyclase, but also can activate several signaling cascades, including phospholipase A2 and PKC (17). Genetic deletion of both D1-like and D2-like receptors results in hypertensive phenotype and impaired renal Na+ excretion (3, 13, 35, 37, 39). Each dopamine receptor subtype is abundantly expressed in the kidney vasculature and renal tubule (17, 24). CCD cells express D1-like and D2-like receptors at the apical and basolateral sides (17, 29). In the perfused rabbit CCD, dopamine has been reported to diminish Na+ reabsorption due to depolarization of the basolateral membrane and, to a lesser extent, the apical membrane (29). However, the molecular targets of dopamine actions in the CCD are not defined.
Inward rectifying Kir4.1 and Kir5.1 channels (encoded by Kcnj10 and Kcnj16 genes, respectively) are functionally expressed on the basolateral membrane of distal nephron segments, including the CCD (19, 20). It has become recognized that, in a tandem with Na+-K+-ATPase, these channels perform K+ recycling across the basolateral membrane (12). In addition, Kir4.1, and Kir4.1/5.1 contribute to establishing the resting basolateral membrane potential, providing the driving force for Na+ and Cl− reabsorption (38). When expressed in heterologous systems, Kir5.1 is not functional (7), but this channel heterodimerizes with Kir4.1 to form Kir4.1/5.1 with distinct biophysical properties (36). Loss of function mutations in the gene encoding Kir4.1 results in SeSAME/EAST syndrome in humans, which is associated with multiple neurological (epilepsy, ataxia, and sensorineural deafness) and renal (salt wasting, hypocalciuria, hypomagnesemia, and hypokalemic metabolic alkalosis) symptoms (6, 32). Interestingly, genetic deletion of Kir5.1 produces a renal phenotype in mice that is almost opposite to in SeSAME/EAST syndrome (25). The observed hypokalemia, hypercalciuria, and hypercloremic metabolic acidosis are thought to be due to a switch from highly pH-sensitive and moderately active Kir4.1/5.1 channels to low pH-sensitive and highly active Kir4.1 channels (25). Therefore, both channels are essential for proper water-electrolyte handling by the kidney. It is unclear, though, if endocrine factors, such as dopamine, are capable of modulating activity of Kir4.1/5.1 and Kir4.1 channels to affect tubular Na+ reabsorption.
In the present study, we found that dopamine significantly decreases the activity and open probability (Po) of basolateral Kir4.1/5.1 and Kir4.1 channels in freshly isolated murine CCD cells. The signaling pathway involves the activation of D2-like receptors and subsequent stimulation of PKC. Furthermore, inhibition of Kir4.1/5.1 and Kir4.1 channels by dopamine induces depolarization of the basolateral membrane independently of the activity of Na+-K+-ATPase. Overall, we report here that dopamine reduces the driving force for Na+ reabsorption in the CCD by inhibiting the activity of Kir4.1/5.1 and Kir4.1 channels.
MATERIALS AND METHODS
Reagents and animals.
All chemicals and materials were from Sigma (St. Louis, MO), VWR (Radnor, PA), and Tocris (Ellisville, MO) unless noted otherwise and were at least of reagent grade. Animal use and welfare adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals following protocols reviewed and approved by the Animal Care and Use Committees of the University of Texas Health Science Center and Medical College of Wisconsin. For the experiments, male C57BL/6J mice (6–10 wk old, Charles River Laboratories, Wilmington, MA) were used. Animals were maintained on standard rodent regimen (no. 5001, Purina) and had free access to tap water.
Tissue isolation.
The procedure for isolation of the CCDs suitable for electrophysiology is a modification from previously described protocols (19, 21, 22, 40). Mice were killed by CO2 administration followed by cervical dislocation, and the kidneys were removed immediately. Kidneys were cut into thin slices (<1 mm) with slices placed into ice-cold physiological saline solution (PSS) buffered with HEPES (pH 7.35). Straight cortical-medullary sectors, containing ∼30–50 renal tubules, were isolated by microdissection using watchmaker forceps under a stereomicroscope. Isolated sectors were further incubated in PSS containing 0.8 mg/ml collagenase type I (Alfa Aesar, Ward Hill, MA) and 5 mg/ml of dispase II (Roche Diagnostics, Mannheim, Germany) for 20 min at 37°C followed by extensive washout with an enzyme-free saline solution. Individual CCDs were visually identified by their morphological features (pale color, coarse surface, and, in some cases, bifurcations) and were mechanically isolated from the sectors by microdissection. Isolated CCDs were attached to a 5 × 5-mm coverglass coated with poly-l-lysine. A coverglass-containing CCD was placed in a perfusion chamber mounted on an inverted Nikon Eclipse Ti microscope and perfused with PSS at room temperature. Tubules were used within 1–2 h after isolation.
Immunohistochemistry.
Mouse kidneys were fixed in 10% formalin and processed for paraffin embedding as previously described (15). Kidney sections were cut at 4 μm, dried, and deparaffinized for subsequent labeling by streptavidin-biotin immunohistochemistry. After deparaffinization, slides were treated with a citrate buffer (pH 6) for total of 35 min. Slides were blocked with a perioxidase block (Dako, Coppenhagen, Denmark), avidin block (Vector Laboratories, Burlingame, CA), biotin block (Vector Laboratories), and serum-free protein block (Dako). Tissue sections were incubated for 90 min in 1:1,000 dilutions of rabbit polyclonal antibody to Kir5.1 and goat polyclonal antibody to Kir4.1 (ab74130 and ab105102, respectively, Abcam, Cambridge, MA). Secondary detection was performed with goat anti-goat or anti-rabbit biotinylated IgG (Biocare, Tempe, AZ) followed by streptavidin-horseradish peroxidase (Biocare) and visualized with diaminobenzidine (Dako). All slides were counterstained with Mayer's hematoxylin (Dako), dehydrated, and mounted with permanent mounting medium (Sakura, Torrance, CA).
Single channel recordings.
The single channel activity of Kir4.1/5.1 and Kir4.1 channels in CCD cells was determined in cell-attached patches on the basolateral membrane made under voltage-clamp conditions. Recording pipettes had resistances of 8–10 MΩ. Bath and pipette solutions were (in mM) 150 NaCl, 5 mM KCl, 1 CaCl2, 2 MgCl2, 5 glucose, and 10 HEPES (pH 7.35) and 150 mM KCl, 2 mM MgCl2, and 10 mM HEPES (pH 7.35), respectively. Current-voltage (I-V) relationships were obtained by monitoring channel activity at applied pipette voltages (−Vp) from −100 to +40 mV with a step of 20 mV for at least 180 s.
In the cell-attached configuration, the actual voltage applied to a membrane patch is a sum of the pipette voltage and the resting basolateral membrane potential (which is close to −65 mV; see Fig. 10). Since K+ concentrations in the pipette solution and cytosol are similar, the expected reversal potential (Erev) for K+-selective channels will be when the actual voltage applied to a membrane patch equals 0 mV. This will be achieved when −Vp equals the negative resting basolateral membrane potential, or at approximately +65 mV.
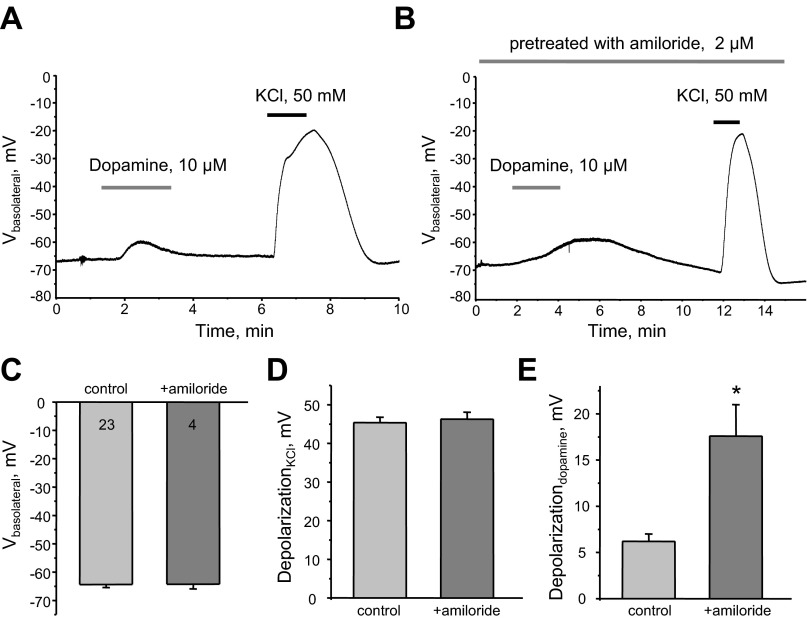
Fig. 10.
Dopamine induces acute depolarization of the basolateral membrane in CCD cells. A and B: representative continuous voltage traces monitoring the basolateral membrane potential (Vbasolateral) in individual cells in the control condition, after application of 10 μM dopamine and 50 mM KCl in the control condition (A), and after pretreatment of isolated CCDs with 2 μM amiloride (B). C: summary graph of resting basolateral membrane potential in the control condition and after amiloride. D and E: summary graphs of KCl-induced (D) and dopamine-induced (E) depolarization of the basolateral membrane in control and amiloride-treated CCDs. *Significant increase vs. control.
For paired patch-clamp experiments, the pipette voltage was −Vp = −40 mV. Current recordings were made in a permanently perfused bath (1.5 ml/min). Drug application times are shown with bars on the top of the representative traces. For each experimental condition, CCDs from at least three different mice were assayed. Gap-free single channel current data from GΩ seals were acquired with an Axopatch 200B (Molecular Devices) patch-clamp amplifier interfaced via a Digidata 1440 (Molecular Devices) to a PC running the pCLAMP 10.3 suite of software (Molecular Devices). Currents were low-pass filtered at 1 kHz with an eight-pole Bessel filter (Warner Instruments). Events were inspected visually before acceptance. In paired experiments, Kir activity was analyzed over a span of 60–120 s for each experimental condition after a new steady-state was reached in response to a treatment. Channel activity and Po were assessed using Clampfit 10.3 software (Molecular Devices). To calculate Po in paired experiments, N was fixed as the greatest number of active channels observed in control or experimental conditions. For representation, current traces were filtered at 200 Hz and corrected for slow baseline drifts as necessary.
Basolateral membrane voltage measurements.
To monitor real-time changes in membrane voltage, CCD cells were studied under current-clamp mode using the perforated-patch technique. Freshly made amphotericin B (400 μM, Enzo life Sciences, Farmingdale, NY) was dissolved in the pipette solution containing 150 mM K-acetate, 5 mM KCl, 2 mM MgCl2, and 10 mM HEPES (pH 7.35) by ultrasonication. Electrical recordings were made once the access resistance from the pipette to the cell interior fell to <15 MΩ, usually 5–10 min after a pipette-to-membrane seal resistance of 5–10 GΩ had been achieved.
Data analysis.
All summarized data are reported as means ± SE. In paired experiments, data from before and after treatment were compared using a paired t-test. Data from unpaired experiments were compared with a Student's (two-tailed) t-test or one-way ANOVA as appropriate. P values of <0.05 were considered significant.
RESULTS
Kir4.1/5.1 and Kir4.1 channels are functionally expressed on the basolateral membrane of CCD cells.
Previous studies (19, 20, 25) have suggested that inward rectifying Kir4.1/5.1 and Kir4.1 channels are the major type of basolateral K+ conductance in the distal parts of renal tubules, including the CCD. Therefore, we first probed the distribution of Kir5.1 and Kir4.1 proteins in kidneys from 8- to 10-wk-old C57BL/6 mice using immunohistochemistry. Figure 1 shows representative images of kidney sections probed for Kir5.1 (Kcnj16) and Kir4.1 (Kcnj10) antibodies. A negative control (stained with secondary antibodies in the absence of primary antibodies) is also shown (Fig. 1A). Most of the staining was identified in the cortex region of the kidney, with close colocalization to around glomeruli structures. The cortex region was identified by the edge of the kidney and glomeruli localization, as demonstrated at lower magnification (×10). Both channels were found predominantly at the basolateral membranes of the CCDs and at the distal convoluted tubules. No staining was observed in the proximal convoluted tubules or glomeruli.
Fig. 1.
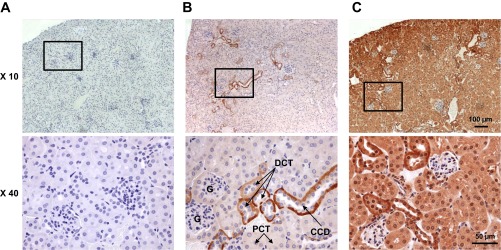
Immunohistochemical staining of Kir5.1 and Kir4.1 channels in the mouse kidney. Representative immunohistochemically stained images of kidney sections of C57BL/6 mice are shown at ×10 and ×40 magnifications. A: negative control tissue stained with secondary antibodies in the absence of primary antibodies. B and C: representative immunohistochemical staining for Kir5.1 (B) and Kir4.1 (C) channels. The boxes at ×10 optical image represent the magnified areas shown at ×40 optical magnification. Scale bars are shown. G, glomerulus; PCT, proximal convoluted tubule; DCT, distal convoluted tubule; CCD, cortical collecting duct.
To define the functional properties of these channels in CCD cells, we next used patch-clamp electrophysiology in a cell-attached configuration. Figure 2 shows a representative micrograph of an enzymatically treated mouse CCD, which allowed us to gain access to the basolateral membrane. Using a patch pipette containing 150 mM KCl, we observed two types of K+ channels with clearly different conductance, Po, and gating kinetics (Figs. 3 and 4).
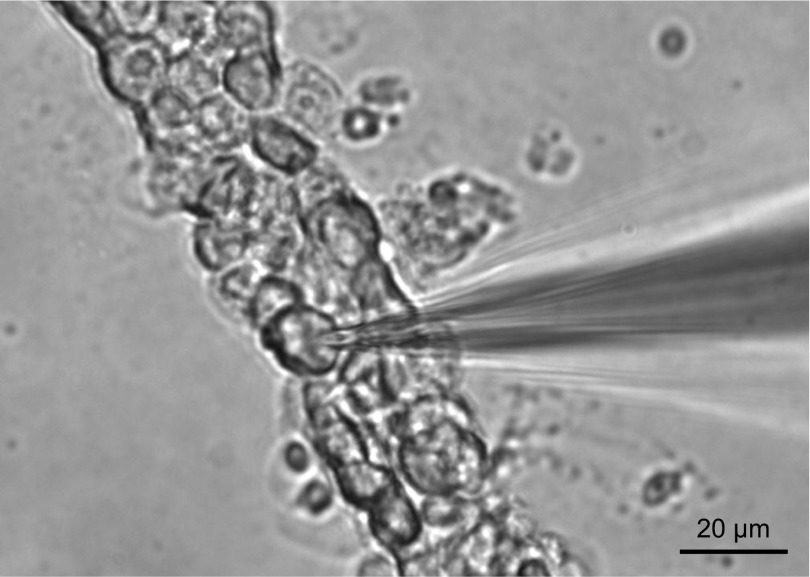
Fig. 2.
Patch-clamp recordings from the basolateral membrane of murine CCD cells. Shown is a representative micrograph of a typical freshly isolated CCD enzymatically treated to expose the basolateral membrane for single channel patch-clamp assessment in the cell-attached configuration and for membrane potential measurements using the current-clamp configuration. A glass pipette is also shown.
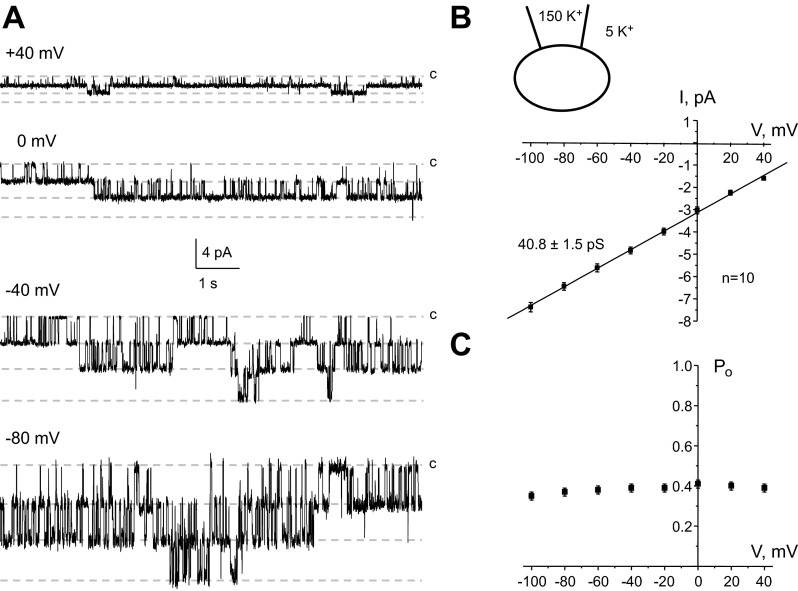
Fig. 3.
Functional properties of Kir4.1/5.1 channels abundantly expressed on the basolateral membrane of CCD cells. A: representative current traces of a 40-pS K+ channel (Kir4.1/5.1) recorded from the same patch at different pipette potentials. The inward K+ currents are downward. c, Closed nonconductant state. B, bottom: average current-voltage (I-V) relationship of the unitary current amplitude for channels similar to that shown in A. Top, K+ concentrations in the recording pipette and bath solution. The number of experiments is also indicated. C: dependence of open probability (Po) of the channel from voltage applied to the recording pipette. For each condition, Po was estimated over a time span of at least 180 s.
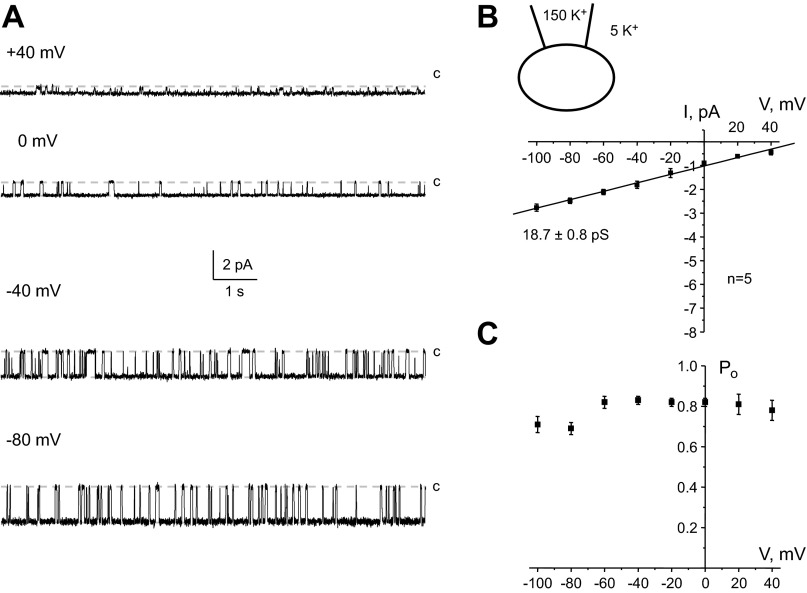
Fig. 4.
Low abundance Kir4.1 channels are functionally expressed on the basolateral membrane of CCD cells. A: representative current traces of a 20-pS K+ channel (Kir4.1) recorded from the same patch at different pipette potentials. The inward K+ currents are downward. B, bottom: average I-V relationship of the unitary current amplitude for channels similar to that shown in A. Top, K+ concentrations in the recording pipette and bath solution. The number of experiments is also indicated. C: dependence of Po of the channel from voltage applied to the recording pipette. For each condition, Po was estimated over a time span of at least 180 s.
Typical patch-clamp recordings at different pipette potentials and the respective I-V relationships of an abundant K+ channel with fast gating kinetics in CCD cells are shown in Fig. 3, A and B, respectively. This channel has a conductance of 40.8 ± 1.5 pS (n = 10) and an estimated Erev around +76 mV under our experimental conditions, indicating a high selectivity for K+ (see materials and methods for more details). Furthermore, Po of the channel demonstrated no apparent voltage dependence, being ∼0.4 at the tested pipette potentials from −100 to +40 mV (Fig. 3C). These properties are similar to those of the previously reported heteromeric Kir4.1/5.1 channel on the basolateral membrane of mouse CCD and distal convoluted tubule cells as demonstrated using pharmacological and genetic approaches (19, 25).
We also occasionally monitored the activity of a smaller K+ channel (Fig. 4A) with a conductance of 18.7 ± 0.8 pS and estimated Erev around +60 mV (Fig. 4B). Despite its scarce occurrence, this channel spent most of the time in its active conducting state interrupted with brief closures (see Fig. 4A). The calculated Po of ∼0.8 was also independent of applied potential (Fig. 4C). Again, these properties are closely reminiscent of those of homomeric Kir4.1 channels expressed on the basolateral membrane of distal nephron cells (19, 25).
We often monitored the activity of both Kir4.1/5.1 and Kir4.1 channels in the same patch. However, these experiments were excluded from analysis due to the complexity of quantification of single channel properties.
Dopamine reversibly inhibits Kir4.1/5.1 and Kir4.1 activity.
Dopamine has been reported to suppress Na+ reabsorption in the proximal tubule and likely in more distal nephron segments, in part by inhibiting transepithelial voltage (17, 29). Kir4.1 and Kir4.1/5.1 channels play an essential role in K+ recycling and establishing basolateral membrane voltage in distal tubular segments (12). Thus, we next tested the hypothesis that dopamine reduces the driving force for Na+ reabsorption by affecting the activity of Kir4.1/5.1 and Kir4.1 channels in situ, in freshly isolated CCDs. Since the Po of the channels is voltage independent (Figs. 3C and 4C), the activity of Kir4.1/5.1 and Kir4.1 channels was further monitored at a fixed pipette potential of −Vp = −40 mV.
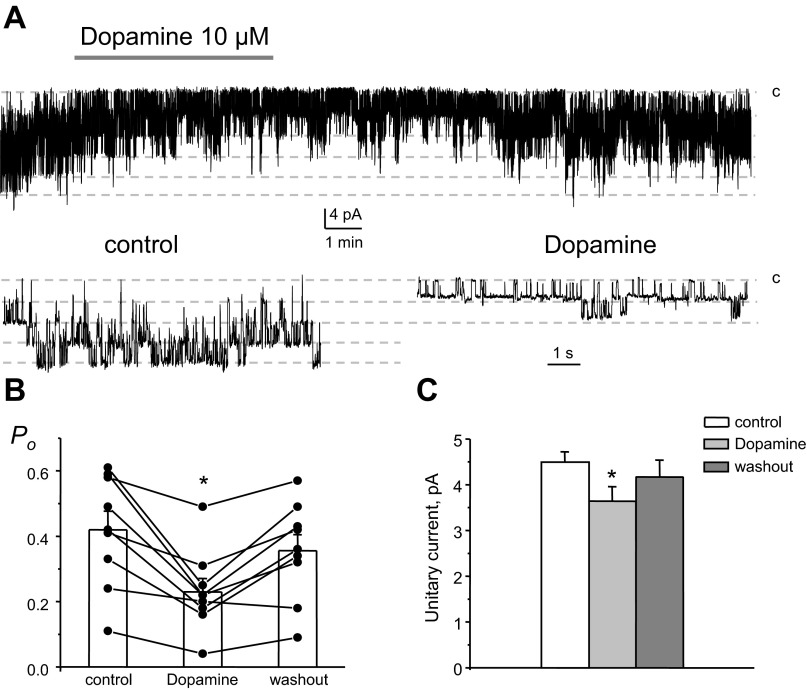
Figure 5A shows a representative current trace from a cell-attached patch monitoring the activity of 40-pS heteromeric Kir4.1/5.1 channels in the control condition, during the application of 10 μM dopamine, and after washout with control media. As can be seen from this paired experiment and the summary graph shown in Fig. 5B, dopamine acutely decreased Kir4.1/5.1 Po in a reversible manner. Mean Po was 0.42 ± 0.05, 0.23 ± 0.04, and 0.36 ± 0.04 in the control condition, during dopamine treatment, and washout, respectively (n = 9). Of importance, dopamine also significantly decreases the amplitude of unitary currents (Fig. 5A, bottom). As shown in Fig. 5C, the mean of the unitary current was 4.5 ± 0.2, 3.6 ± 0.3, and 4.2 ± 0.4 pA in the control condituion, during dopamine treatment, and washout, respectively.
Fig. 5.
Dopamine acutely inhibits Po of basolateral Kir4.1/5.1 channels. A: representative continuous current traces from a cell-attached patch monitoring the activity of basolateral 40-pS Kir4.1/5.1 channels in the control condition, after application of 10 μM dopamine, and after washout with control media. The patch was clamped to an applied pipette voltage (−Vp) of −40 mV. B: summary graph of Kir4.1/5.1 channel Po from paired patch-clamp experiments similar to that shown in A. C: summary graph of dopamine actions on the unitary current amplitude of Kir4.1/5.1 channels from paired patch-clamp experiments similar to that shown in A. *Significant decrease vs. control.
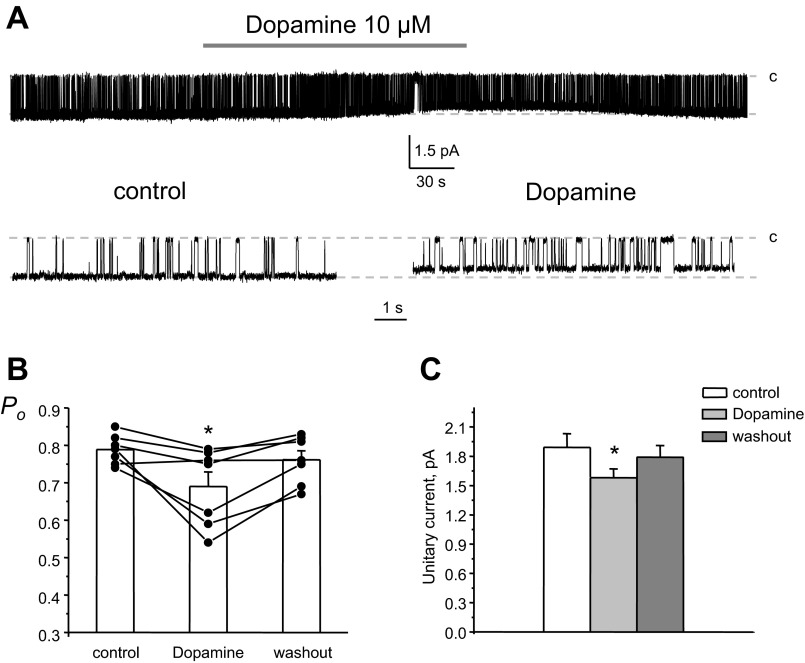
We next quantified changes in the activity of 20-pS monomeric Kir4.1 channels during exogenous dopamine application in native CCD cells. As shown in a representative continuous paired experiment (Fig. 6A), dopamine also significantly decreased channel Po, while the inhibitory effect was considerably smaller. Mean Po was 0.79 ± 0.01, 0.69 ± 0.04, and 0.76 ± 0.03 in the control condition, during dopamine treatment, and washout, respectively (n = 7). Moreover, dopamine significantly reduced the amplitude of unitary currents by ∼20%, which was similar to the observed effects for heteromeric Kir4.1/5.1 channels (see Fig. 5C). The mean of the unitary current was 1.9 ± 0.1, 1.5 ± 0.1, and 1.8 ± 0.1 pA in the control condition, during dopamine treatment, and washout, respectively (Fig. 6C).
Fig. 6.
Dopamine mildly decreases the activity of Kir4.1 channels in the basolateral membrane of CCD cells. A: representative continuous current traces from a cell-attached patch monitoring the activity of a singly 20-pS Kir4.1 basolateral channel in the control condition, after application of 10 μM dopamine, and after washout with control media. The patch was clamped to −Vp = −40 mV. B: summary graph of 20-pS K+ channel Po from paired patch-clamp experiments similar to that shown in A. C: summary graph of dopamine actions on the unitary current amplitude of Kir4.1 channels from paired patch-clamp experiments similar to that shown in A. *Significant decrease vs. control.
We conclude that dopamine inhibits Kir4.1/5.1 and mildly Kir4.1 basolateral K+ channels in murine CCD cells by decreasing Po. Furthermore, dopamine similarly reduces the amplitude of the unitary current of both channels, indicating depolarization of the basolateral membrane potential.
Dopamine inhibits basolateral Kir channels in CCD cells via the D2-like receptor-PKC pathway.
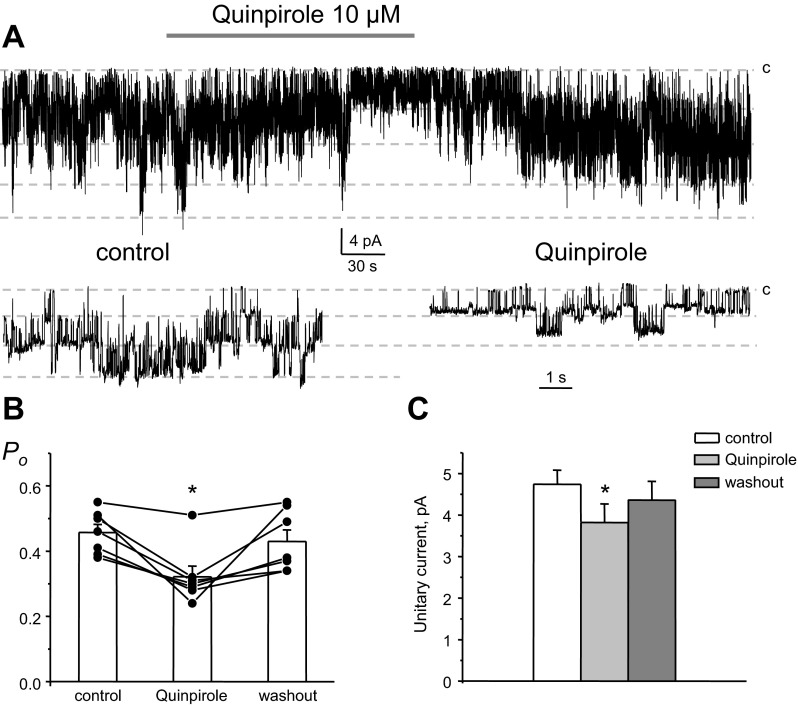
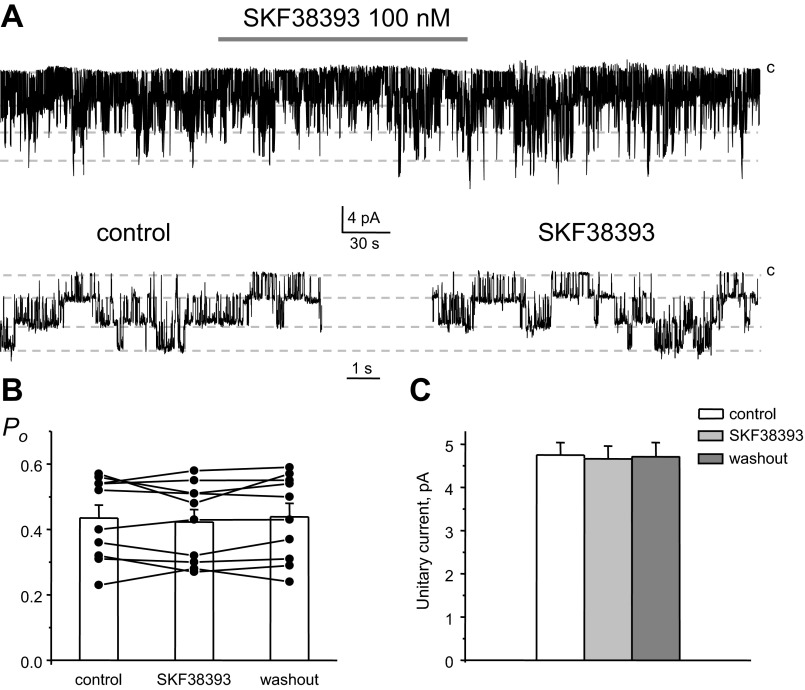
Both D1- and D2-like receptors are abundantly expressed on the basolateral membrane of CCD cells (17, 29). Therefore, we next probed which type of dopamine receptors confers inhibitory actions to basolateral Kir channels. Due to the rare occurrence of homomeric Kir4.1 channels, we focused exclusively on heteromeric Kir4.1/5.1 channels. Application of a selective agonist of D2-like receptors, quinpirole (10 μM), acutely inhibited the activity of 40-pS Kir4.1/5.1 channels (Fig. 7A). A summary graph of changes in Kir4.1/5.1 Po in paired experiments upon stimulation of D2-like receptors is shown in Fig. 7B. Mean Po was 0.46 ± 0.02, 0.31 ± 0.03, and 0.43 ± 0.04 in the control condition, during quinpirole treatment, and washout, respectively (n = 7). Consistently, we also detected a reduction of the amplitude of the unitary current after stimulation of D2-like receptors (Fig. 7C). The mean of the unitary current was 4.7 ± 0.3, 3.8 ± 0.5, and 4.4 ± 0.4 pA in the control condition, during quinpirole treatment, and washout, respectively. In contrast, activation of D1-like receptors with SKF-38393 (100 nM) failed to affect activity (Fig. 8A), Po (Fig. 8B), and unitary current amplitude (Fig. 8C) of Kir4.1/5.1 channels. Mean Po was 0.43 ± 0.03, 0.42 ± 0.03, and 0.43 ± 0.04 in the control condition, during SKF-38393 treatment, and washout, respectively (n = 11). The mean of the unitary current was 4.7 ± 0.3, 4.7 ± 0.3, and 4.7 ± 0.3 pA in the control condition, during SKF-38393 treatment, and washout, respectively. These results support the concept that activation of D2-like but not D1-like receptors recapitulates the inhibitory actions of dopamine on basolateral Kir channels.
Fig. 7.
D2-like receptors mediate dopamine actions on basolateral Kir4.1/5.1 channels. A: representative continuous current traces from a cell-attached patch monitoring the activity of basolateral Kir4.1/5.1 channel activity in the control condition, after application of a D2-like receptor agonist (10 μM quinpirole), and after washout with control media. The patch was clamped to −Vp = −40 mV. B: summary graph of Kir4.1/5.1 channel Po from paired patch-clamp experiments similar to that shown in A. C: summary graph of quinpirole actions on the unitary current amplitude of single 40-pS K+ channels from paired patch-clamp experiments similar to that shown in A. *Significant decrease vs. control.
Fig. 8.
Stimulation of D1-like receptors does not inhibit basolateral Kir4.1/5.1 channels in CCD cells. A: representative continuous current traces from a cell-attached patch monitoring the activity of Kir4.1/5.1 channels in the control condition, after application of a D1-like receptor agonist (100 nM SKF-383930), and after washout with control media. The patch was clamped to −Vp = −40 mV. B: summary graph of Kir4.1/5.1 channel Po from paired patch-clamp experiments similar to that shown in A. C: summary graph of SKF-38393 actions on the unitary current amplitude of Kir4.1/5.1 channels from paired patch-clamp experiments similar to that shown in A.
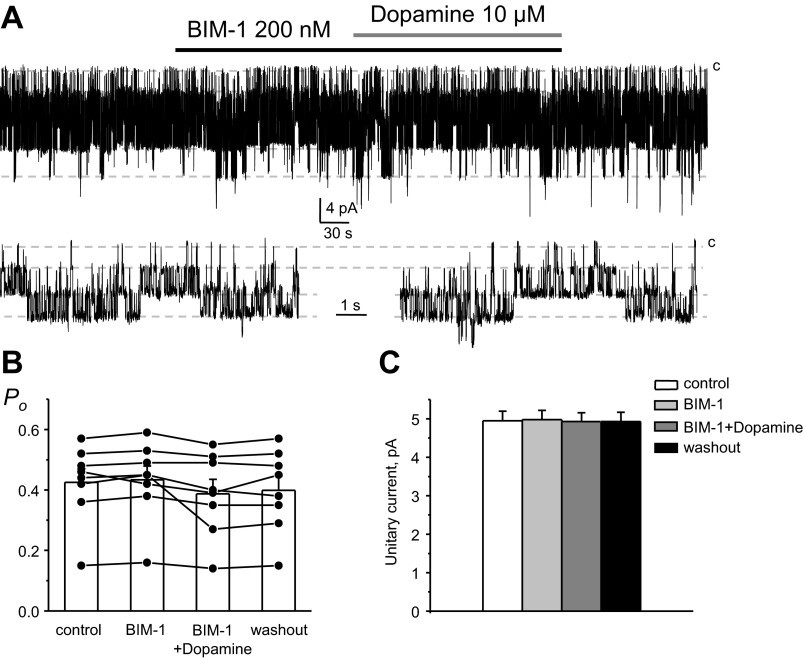
D2-like receptors are typically coupled to Gi/Go proteins, leading to inhibition of adenylyl cyclase, decreases in cAMP levels, and also stimulation of PKC (17). While Kir4.1/5.1 channels have been shown to be insensitive to pharmacological manipulations with cAMP levels in expression systems, they can be inhibited upon direct phosphorylation by PKC (28). Thus, we next probed whether dopamine inhibits the activity of basolateral Kir4.1/5.1 channels in CCD cells in a PKC-dependent manner. As demonstrated in a representative patch-clamp experiment (Fig. 9A), dopamine failed to decrease Po and the unitary current amplitude of 40-pS Kir4.1/5.1 channels when PKC was inhibited with a highly selective cell-permeable antagonist, bisindolylmaleimide I (BIM-1; 200 nM). As shown in Fig. 9B, mean Po was 0.43 ± 0.05, 0.43 ± 0.05, 0.39 ± 0.04, and 0.40 ± 0.05 in the control condition, after BIM-1 treatment, followed by dopamine on the background of BIM-1, and washout, respectively (n = 8). Mean unitary current was 4.9 ± 0.3, 5.0 ± 0.3, 4.9 ± 0.2, and 4.9 ± 0.2 pA in the control condition, after BIM-1, followed by dopamine on the background of BIM-1, and washout, respectively (Fig. 9C).
Fig. 9.
PKC blockade abolishes the inhibitory actions of dopamine on basolateral Kir4.1/5.1 channels in CCD cells. A: representative continuous current traces from a cell-attached patch monitoring the activity of Kir4.1/5.1 channels in the control condition, upon treatment with a PKC inhibitor [200 nM bisindolylmaleimide I (BIM-1)], followed by application with 10 μM dopamine in the continued presence of BIM-1, and after washout with control media. The patch was clamped to −Vp = −40 mV. B: summary graph of Kir4.1/5.1 channel Po from paired patch-clamp experiments similar to that shown in A. C: summary graph of dopamine actions on the unitary current amplitude of Kir4.1/5.1 channels in the presence of PKC blockade from paired patch-clamp experiments similar to that shown in A.
Overall, we conclude that dopamine stimulates D2-like receptors, leading to PKC activation and decreased activity of basolateral Kir channels in CCD cells.
Inhibition of Kir channels by dopamine depolarizes the basolateral membrane.
Our results (Figs. 5–7) demonstrate that dopamine-induced inhibition of Kir4.1/5.1 and Kir4.1 channels is associated with a significant reduction of the amplitude of unitary single channel currents, indicating a decreased basolateral membrane potential. Thus, we next tested whether dopamine depolarized the basolateral plasma membrane by inhibiting Kir channels. For this, we used current-clamp electrophysiology to directly monitor changes in basolateral membrane potential in response to dopamine application in the control condition (Fig. 10A) and after continuous pretreatment with an epithelial Na+ channel (ENaC) blocker, 2 μM amiloride, to inhibit apical conductance and abolish Na+ supplementation for basolateral Na+-K+-ATPase (Fig. 10B). Short applications of 50 mM KCl were used to test viability of cells. As shown in Fig. 10C, resting basolateral membrane voltage was not affected by amiloride (−64 ± 1 and −64 ± 2 mV in the control condition and after amiloride treatment, respectively). We also did not detect changes in the magnitude of KCl-induced depolarization: 45 ± 1 mV in the control condition and 46 ± 2 after treatment (Fig. 10D). Of note, the amplitude of membrane depolarization in response to application of media with high K+ concentration indicates that K+ conductance is the major determinant of basolateral membrane potential in CCD cells. Importantly, the dopamine-induced depolarization was significantly greater in the presence of amiloride (17.6 ± 3.4 mV) than in the control condition (6.2 ± 0.8 mV; Fig. 10E). These results suggest that dopamine induces depolarization of the basolateral membrane by affecting basolateral K+ conductance, most likely mediated by Kir4.1/5.1 and Kir4.1 channels. Furthermore, this effect is augmented when apical conductance is blocked with amiloride and the activity of Na+-K+-ATPase is compromised due to a lack of intracellular Na+ entry.
DISCUSSION
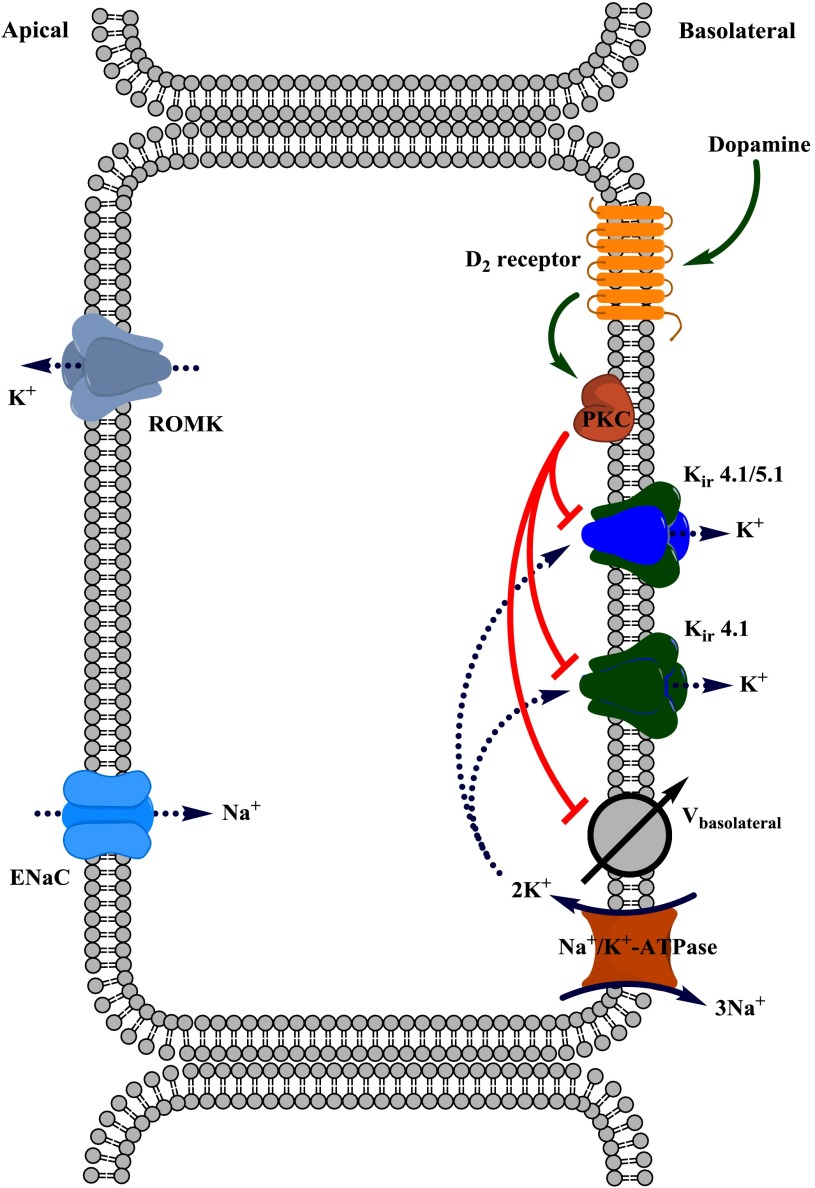
In the present study, we used a physiologically relevant object, freshly isolated mouse CCDs, to identify the molecular targets of dopamine actions in the distal part of the renal tubule (Fig. 11). We report here that dopamine promotes its natriuretic actions at this tubular segment, at least in part, by inhibiting the activity of basolateral K+ channels: heteromeric Kir4.1/5.1 channels and, to a lesser extent, homomeric Kir4.1 channels. The single channel profiles of the recorded basolateral K+ channels, which are nearly identical to those previously reported for Kir4.1/5.1 and Kir4.1 in CCD cells (19, 25), give us confidence to establish their molecular identity. We demonstrate that dopamine-induced inhibition of Kir channels resulted in depolarization of the basolateral membrane and, therefore, a reduction of the driving force for Na+ reabsorption. We also provide evidence showing that this depolarization persists when the activity of Na+-K+-ATPase is compromised. Overall, to our knowledge, this is the first demonstration of a direct regulation of basolateral Kir channels activity in the distal nephron by endocrine factors, such as dopamine.
Fig. 11.
Principal scheme of dopamine actions on Kir4.1/5.1- and Kir4.1-mediated basolateral K+ conductance in murine CCD cells.
We found that low micromolar concentrations of dopamine exert inhibitory actions on basolateral K+ conductance in CCD cells by decreasing single channel Po of Kir4.1/5.1 (Fig. 5) and Kir4.1 (Fig. 6) channels in a reversible manner. It has been shown that renal tubular cells are capable of storing and secreting similar concentrations of dopamine (9), indicating the physiological nature of this regulation. The reduced Kir channel activity translates into a rapid depolarization of basolateral membrane potential during dopamine application (Fig. 10). Similarly, several reports (26, 27) have demonstrated that dopamine regulates excitability and resting membrane potential by affecting Kir channels in medium spiny neurons in the nucleus accumbens, which are thought to be involved in the development of mood behavior and drug abuse. Activation of both D1-like (27) and D2-like (26) receptors depolarizes membrane potential and inhibits Kir channels in these neurons. In the kidney, dopamine is known to regulate transepithelial voltage by inhibiting Na+-K+-ATPase to reduce Na+ reabsorption in the proximal tubule (1, 5). In contrast, we demonstrate that dopamine-induced depolarization of the basolateral membrane was even stronger when the activity of Na+-K+ATPase was disrupted in CCD cells (Fig. 10). This suggests that inhibition of Kir4.1/5.1 and Kir4.1 channels by dopamine is causative for depolarization of the basolateral membrane. However, the regulation of Na+-K+-ATPase activity by dopamine in CCD cells may also contribute (30). In addition, we cannot rule out the possibility that ENaC inhibition with amiloride may affect the functional status of basolateral K+ channels.
We demonstrate that inhibition of Kir4.1/5.1 channels by dopamine was mediated by D2-like receptors (Fig. 7) but not D1-like receptors (Fig. 8). These results are in a good agreement with a previous report (29) showing that similar concentrations of dopamine applied from the basolateral side induced depolarization of transepithelial voltage in perfused rabbit CCDs in a D2-like receptors manner. In our study, we identified Kir4.1/5.1 and Kir4.1 channels as molecular targets for the dopamine-induced depolarization of the basolateral membrane. Indeed, we observed an even greater degree of depolarization of the basolateral membrane when tubules were chronically pretreated with amiloride to inhibit apical conductance and to diminish the Na+ supply for Na+-K+-ATPase (Fig. 10, A and B). We have to admit, though, that the perforated patch clamp gave electrical access not only to the basolateral membrane but also to the apical membrane. However, it has been estimated that the contribution of the apical K+ conductance is minor and that ∼90% of the whole cell K+ conductance arises from the the basolateral membrane in CCD cells (10). Consistently, we did not detect significant changes in the basal membrane potential after treatment with amiloride (Fig. 10C). Therefore, current-clamp electrophysiology in the perforated cell configuration is instrumental for accurate measurements of basolateral membrane potential in freshly isolated CCD cells.
D2-like receptors are typically coupled to Gi/Go heteromeric G proteins, leading to inhibition of adenylyl cyclase and to a reduction of intracellular cAMP levels (17). However, pharmacological manipulation with cAMP levels has little effect on the activity of Kir4.1/5.1 channels overexpressed in Xenopus oocytes (28). This suggests that additional signaling pathways are likely involved in the regulation of Kir4.1/5.1 channels by D2-like receptors. Indeed, we found that inhibition of PKC with BIM-1 disrupted dopamine actions on Kir4.1/5.1 channels (Fig. 9). Activation of D2-like receptors has been shown to stimulate PKC in different cell types (8, 14). Interestingly, PKC has been proposed to directly phosphorylate multiple intracellular residues in heteromeric Kir4.1/5.1 channels in an expression system, leading to its inhibition (28). In contrast, activation of PKC had little effect on the activity of homomeric Kir4.1 channels. In agreement with this study, we found that the inhibitory effect of dopamine was substantially greater for Kir4.1/5.1 than Kir4.1 channels (Figs. 5 vs. 6). It remains to be determined, though, if PKC inhibits the activity of basolateral Kir channels in CCD cells via direct phosphorylation or indirectly by triggering yet unidentified downstream effectors.
In our study, we did not specifically probe if the activity of Kir4.1/5.1 and Kir4.1 channels was different between principal and intercalated cells in the CCD. Among all tested cells that possess functional basolateral Kir channels, we did not detect any apparent heterogeneity or bimodal distribution in the respective Po values. However, we quite often monitored empty patches (∼20% of all patches), potentially indicating that Kir activity might be absent in less abundant intercalated cells (∼25% of total cells in the CCD). Using immunofluorescent microscopy, Lachheb et al. (19) reported that Kir4.1 and Kir5.1 proteins are expressed in aquaporin-2-positive principal cells but not aquaporin-2-negative intercalated cells in the mouse CCD. We also observed CCD cells with apparently lower staining of basolateral Kir channels (Fig. 1). However, we did not perform further discrimination between Kir4.1/5.1 and Kir4.1 expression in principal and intercalated cells, and future studies are granted to probe this.
In summary, this study revealed a mechanism by which dopamine controls Na+ handling in the distal nephron. We demonstrate that dopamine directly inhibited the activity of Kir4.1/5.1 and Kir4.1 channels to depolarize the basolateral plasma membrane and to reduce the driving force for Na+ reabsorption at this site. Therefore, it is conceivable to propose that the salt-sensitive hypertension and augmented renal Na+ retention in mice lacking D2 receptors may be, at least partially, explained by the loss of this negative regulation and, therefore, augmented distal nephron Na+ reabsorption.
GRANTS
This work was supported by National Institutes of Health (NIH) Grant DK-095029 (to O. Pochynyuk), American Heart Association Grant-In-Aid 13GRNT16220002 (to O. Pochynyuk), a S&R Foundation Ryuji Ueno award (to O. Pochynyuk), and NIH Grant HL-108880 (to A. Staruschenko).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: O.Z., M.M., O. Palygin, and N.B. performed experiments; O.Z., M.M., O. Palygin, and N.B. analyzed data; O.Z., M.M., O. Palygin, A.S., and O. Pochynyuk interpreted results of experiments; O.Z., M.M., O. Palygin, A.S., and O. Pochynyuk prepared figures; O.Z., M.M., O. Palygin, N.B., A.S., and O. Pochynyuk edited and revised manuscript; O.Z., M.M., O. Palygin, N.B., A.S., and O. Pochynyuk approved final version of manuscript; A.S. and O. Pochynyuk conception and design of research; A.S. and O. Pochynyuk drafted manuscript.
ACKNOWLEDGMENTS
The authors thank Christine Duris (Medical College of Wisconsin) for assistance with immunohistochemistry experiments.
REFERENCES
- 1.Aperia A, Bertorello A, Seri I. Dopamine causes inhibition of Na+-K+-ATPase activity in rat proximal convoluted tubule segments. Am J Physiol Renal Fluid Electrolyte Physiol 252: F39–F45, 1987 [DOI] [PubMed] [Google Scholar]
- 2.Asghar M, Tayebati SK, Lokhandwala MF, Hussain T. Potential dopamine-1 receptor stimulation in hypertension management. Curr Hypertens Rep 13: 294–302, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Bek MJ, Wang X, Asico LD, Jones JE, Zheng S, Li X, Eisner GM, Grandy DK, Carey RM, Soares-da-Silva P, Jose PA. Angiotensin-II type 1 receptor-mediated hypertension in D4 dopamine receptor-deficient mice. Hypertension 47: 288–295, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Bell C, Sunn N. A functional role for renal dopaminergic nerves in the dog. J Auton Pharmacol 10, Suppl 1: S41–S45, 1990 [DOI] [PubMed] [Google Scholar]
- 5.Bertorello A, Aperia A. Inhibition of proximal tubule Na+-K+-ATPase activity requires simultaneous activation of DA1 and DA2 receptors. Am J Physiol Renal Fluid Electrolyte Physiol 259: F924–F928, 1990 [DOI] [PubMed] [Google Scholar]
- 6.Bockenhauer D, Feather S, Stanescu HC, Bandulik S, Zdebik AA, Reichold M, Tobin J, Lieberer E, Sterner C, Landoure G, Arora R, Sirimanna T, Thompson D, Cross JH, van't Hoff W, Al Masri O, Tullus K, Yeung S, Anikster Y, Klootwijk E, Hubank M, Dillon MJ, Heitzmann D, Arcos-Burgos M, Knepper MA, Dobbie A, Gahl WA, Warth R, Sheridan E, Kleta R. Epilepsy, ataxia, sensorineural deafness, tubulopathy, and KCNJ10 mutations. N Engl J Med 360: 1960–1970, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bond CT, Pessia M, Xia XM, Lagrutta A, Kavanaugh MP, Adelman JP. Cloning and expression of a family of inward rectifier potassium channels. Receptors Channels 2: 183–191, 1994 [PubMed] [Google Scholar]
- 8.Chang HW, Wu VC, Huang CY, Huang HY, Chen YM, Chu TS, Wu KD, Hsieh BS. D4 dopamine receptor enhances angiotensin II-stimulated aldosterone secretion through PKC-ε and calcium signaling. Am J Physiol Endocrinol Metab 294: E622–E629, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Di Marco GS, Vio CP, Dos Santos OF, Schor N, Casarini DE. Catecholamine production along the nephron. Cell Physiol Biochem 20: 919–924, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Gray DA, Frindt G, Zhang YY, Palmer LG. Basolateral K+ conductance in principal cells of rat CCD. Am J Physiol Renal Physiol 288: F493–F504, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Guyton AC. Blood pressure control–special role of the kidneys and body fluids. Science 252: 1813–1816, 1991 [DOI] [PubMed] [Google Scholar]
- 12.Hamilton KL, Devor DC. Basolateral membrane K+ channels in renal epithelial cells. Am J Physiol Renal Physiol 302: F1069–F1081, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hollon TR, Bek MJ, Lachowicz JE, Ariano MA, Mezey E, Ramachandran R, Wersinger SR, Soares-da-Silva P, Liu ZF, Grinberg A, Drago J, Young WS, 3rd, Westphal H, Jose PA, Sibley DR. Mice lacking D5 dopamine receptors have increased sympathetic tone and are hypertensive. J Neurosci 22: 10801–10810, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hozumi Y, Watanabe M, Goto K. Signaling cascade of diacylglycerol kinase beta in the pituitary intermediate lobe: dopamine D2 receptor/phospholipase Cβ4/diacylglycerol kinase β/protein kinase Cα. J Histochem Cytochem 58: 119–129, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ilatovskaya DV, Pavlov TS, Levchenko V, Negulyaev YA, Staruschenko A. Cortical actin binding protein cortactin mediates ENaC activity via Arp2/3 complex. FASEB J 25: 2688–2699, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Jose PA, Asico LD, Eisner GM, Pocchiari F, Semeraro C, Felder RA. Effects of costimulation of dopamine D1- and D2-like receptors on renal function. Am J Physiol Regul Integr Comp Physiol 275: R986–R994, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Jose PA, Raymond JR, Bates MD, Aperia A, Felder RA, Carey RM. The renal dopamine receptors. J Am Soc Nephrol 2: 1265–1278, 1992 [DOI] [PubMed] [Google Scholar]
- 18.Khawaja Z, Wilcox CS. Role of the kidneys in resistant hypertension. Int J Hypertens 2011: 143471, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lachheb S, Cluzeaud F, Bens M, Genete M, Hibino H, Lourdel S, Kurachi Y, Vandewalle A, Teulon J, Paulais M. Kir4.1/Kir5.1 channel forms the major K+ channel in the basolateral membrane of mouse renal collecting duct principal cells. Am J Physiol Renal Physiol 294: F1398–F1407, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Lourdel S, Paulais M, Cluzeaud F, Bens M, Tanemoto M, Kurachi Y, Vandewalle A, Teulon J. An inward rectifier K+ channel at the basolateral membrane of the mouse distal convoluted tubule: similarities with Kir4-Kir5.1 heteromeric channels. J Physiol 538: 391–404, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mamenko M, Zaika O, Doris PA, Pochynyuk O. Salt-dependent inhibition of epithelial Na+ channel-mediated sodium reabsorption in the aldosterone-sensitive distal nephron by bradykinin. Hypertension 60: 1234–1241, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mamenko M, Zaika O, Ilatovskaya DV, Staruschenko A, Pochynyuk O. Angiotensin II increases activity of the epithelial Na+ channel (ENaC) in distal nephron additively to aldosterone. J Biol Chem 287: 660–671, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meneton P, Jeunemaitre X, de Wardener HE, MacGregor GA. Links between dietary salt intake, renal salt handling, blood pressure, and cardiovascular diseases. Physiol Rev 85: 679–715, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev 78: 189–225, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Paulais M, Bloch-Faure M, Picard N, Jacques T, Ramakrishnan SK, Keck M, Sohet F, Eladari D, Houillier P, Lourdel S, Teulon J, Tucker SJ. Renal phenotype in mice lacking the Kir5.1 (Kcnj16) K+ channel subunit contrasts with that observed in SeSAME/EAST syndrome. Proc Natl Acad Sci USA 108: 10361–10366, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perez MF, White FJ, Hu XT. Dopamine D2 receptor modulation of K+ channel activity regulates excitability of nucleus accumbens neurons at different membrane potentials. J Neurophysiol 96: 2217–2228, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Podda MV, Riccardi E, D'Ascenzo M, Azzena GB, Grassi C. Dopamine D1-like receptor activation depolarizes medium spiny neurons of the mouse nucleus accumbens by inhibiting inwardly rectifying K+ currents through a cAMP-dependent protein kinase A-independent mechanism. Neuroscience 167: 678–690, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Rojas A, Cui N, Su J, Yang L, Muhumuza JP, Jiang C. Protein kinase C dependent inhibition of the heteromeric Kir4.1-Kir5.1 channel. Biochim Biophys Acta 1768: 2030–2042, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saito O, Ando Y, Kusano E, Asano Y. Functional characterization of basolateral and luminal dopamine receptors in rabbit CCD. Am J Physiol Renal Physiol 281: F114–F122, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Satoh T, Cohen HT, Katz AI. Different mechanisms of renal Na-K-ATPase regulation by protein kinases in proximal and distal nephron. Am J Physiol Renal Fluid Electrolyte Physiol 265: F399–F405, 1993 [DOI] [PubMed] [Google Scholar]
- 31.Schild L. The ENaC channel as the primary determinant of two human diseases: Liddle syndrome and pseudohypoaldosteronism. Nephrologie 17: 395–400, 1996 [PubMed] [Google Scholar]
- 32.Scholl UI, Choi M, Liu T, Ramaekers VT, Hausler MG, Grimmer J, Tobe SW, Farhi A, Nelson-Williams C, Lifton RP. Seizures, sensorineural deafness, ataxia, mental retardation, and electrolyte imbalance (SeSAME syndrome) caused by mutations in KCNJ10. Proc Natl Acad Sci USA 106: 5842–5847, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soares-da-Silva P, Fernandes MH. Regulation of dopamine synthesis in the rat kidney. J Auton Pharmacol 10, Suppl 1: S25–S30, 1990 [DOI] [PubMed] [Google Scholar]
- 34.Staruschenko A. Regulation of transport in the connecting tubule and cortical collecting duct. Compr Physiol 2: 1541–1584, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Staudacher T, Pech B, Tappe M, Gross G, Muhlbauer B, Luippold G. Arterial blood pressure and renal sodium excretion in dopamine D3 receptor knockout mice. Hypertens Res 30: 93–101, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Tanemoto M, Kittaka N, Inanobe A, Kurachi Y. In vivo formation of a proton-sensitive K+ channel by heteromeric subunit assembly of Kir5.1 with Kir4.1. J Physiol 525: 587–592, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ueda A, Ozono R, Oshima T, Yano A, Kambe M, Teranishi Y, Katsuki M, Chayama K. Disruption of the type 2 dopamine receptor gene causes a sodium-dependent increase in blood pressure in mice. Am J Hypertens 16: 853–858, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Wang WH, Yue P, Sun P, Lin DH. Regulation and function of potassium channels in aldosterone-sensitive distal nephron. Curr Opin Nephrol Hypertens 19: 463–470, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang X, Luo Y, Escano CS, Yang Z, Asico L, Li H, Jones JE, Armando I, Lu Q, Sibley DR, Eisner GM, Jose PA. Upregulation of renal sodium transporters in D5 dopamine receptor-deficient mice. Hypertension 55: 1431–1437, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zaika O, Mamenko M, O'Neil RG, Pochynyuk O. Bradykinin acutely inhibits activity of the epithelial Na+ channel in mammalian aldosterone-sensitive distal nephron. Am J Physiol Renal Physiol 300: F1105–F1115, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]