Abstract
Clinical trials utilizing bone marrow-derived mesenchymal stem cell (BM-MSC) therapies show promise for treating a variety of pathologic conditions. Paramount to optimization of such cell-based therapies is a thorough understanding of MSC biology. Despite the tremendous potential that exists for the clinical use of canine BM-MSCs in veterinary medicine, as well as in preclinical studies for human medicine, relatively little information exists regarding basic biological properties of the cells. In this study, we compared the importance of donor characteristics (age and harvest site) and ex vivo expansion on canine BM-MSC frequency (CFU-f) and differentiation potential. Advancing age was found to have a negative effect on CFU-f as well as osteogenic potential. Site of harvest was also found to have significant effects on MSC properties. MSCs obtained from the humerus were found at the lowest frequency and were least osteogenic compared to those harvested from the tibia, femur, and ilium. Osteogenic potential diminished significantly by the third passage. These results suggest important donor parameters and culture effects to consider in translational studies examining MSC-based regenerative medical strategies.
Keywords: Mesenchymal stem cell (MSC), Regenerative medicine, Veterinary cell therapy, Canine, Tissue engineering, Aging
INTRODUCTION
Adult stem cell therapies represent a promising treatment modality in the age of regenerative medicine and tissue engineering in both human and veterinary medicine. In particular, mesenchymal stem cells (MSCs) have shown tremendous promise in numerous preclinical and clinical trials. Although initial MSC-based therapies focused on orthopedic applications, MSCs have been shown in preclinical studies and clinical trials to be efficacious in treating dysfunction in many organ and tissue types, including cardiac, pulmonary, hepatic, renal, neuro logic, chronic wound, and immune system dis orders (19,23,24,44,47,51). The ability of MSCs to ameliorate clinical signs associated with pathology in such a wide variety of organ systems includes their capacity to contribute directly to reparative cell populations and the formation of an extracellular matrix, as well as their ability to indirectly promote healing through the production of trophic factors and chemokines to influence native cells involved in repair, inflammatory, or immune responses. MSCs have been isolated from a wide variety of tissue types including bone marrow, adipose, peripheral blood, umbilical cord, and placenta, among others (16,45,49). MSCs can be isolated and culture-expanded to provide a more enriched cell source of progenitors compared to that found in donor tissue. Furthermore, MSCs can be differentiated in vitro, which can accelerate healing and reduce the expense of administering costly purified growth factors to the entire surgical site. Several studies suggest that allogeneic MSCs do not induce adverse immune responses (3,15,42), thus allowing for the use of culture-expanded MSCs, collected from a single donor, to treat multiple patients. Finally, MSCs retain their growth kinetics, self-renewal, and differentiation potential after cryopreservation (9), allowing for centralized processing and storage with timely distribution for an “off-the-shelf ” therapy to surgeons in academic or private practice settings, similar to what is currently available for blood products. Recent preclinical and clinical studies have shown promise for MSC-based therapeutic strategies for treating both local defects using directly applied cells or transplanted MSC-engineered tissues as well as generalized diseases through systemic transplantation.
The use of the dog as a reliable preclinical model in developing cellular transplantation therapies dates back over half a century with the first successful canine bone marrow-derived stem cell transplantation (41). Refinement of techniques for optimizing stem cell transplantation using the canine model has been critical to the success of hematopoietic reconstitution in people, with posttransplantation outcome assessments in dogs accurately predicting outcome in human patients (17). Unlike laboratory rodents, dogs have a relatively longer life, are outbred, and, in a nonlaboratory setting, are exposed to external and environmental factors that are implicated in various disease states, such as cancer, obesity, and traumatic injuries. Not only do canine biochemical and physiological processes more closely resemble those in humans, compared to rodents, but the clinical presentation and progression of various human pathologies are also often better represented by this species (30). The size of the dog coupled with clinical advances in companion animal care allows for unique treatment options, imaging, and repeated biological sampling that are difficult or impossible in rodent models. These conditions may also allow for an increased sensitivity in detection of untoward side effects of novel therapies that would minimize risk to humans. The increase in demand for sophisticated, stateof-the-art care for canine companions has led to a large increase in clinical trials in canine patients, which will provide a unique opportunity for assessing both efficacy and safety of adult stem cell therapies that can be translated to human medicine (8). Given the value of the canine model for translational studies to advance human medicine, as well as the obvious impact on cutting edge veterinary therapies, defining donor characteristics and ex vivo expansion strategies that optimize therapeutic potential for canine MSCs is critical for further advancement of MSC-based regenerative medicine/tissue engineering.
Unfortunately, many key questions remain unanswered regarding the basic biology of canine MSCs, which may directly impact success of MSC-based therapies in veterinary medicine and therefore have an indirect impact on translated therapies in human patients. Herein, we define donor age and skeletal site of harvest as having a significant effect on MSC properties such as colony-forming efficiency and differentiation potential. In addition, we provide evidence that passage has a significant detrimental impact on osteogenic capacity of these cells. Given the clear advantages in using the dog as a preclinical model for refinement of MSC-based therapies, establishing superior donor characteristics of canine MSCs and limiting practices that would diminish their therapeutic efficacy promises to have a significant impact on future clinical trials in dogs and humans.
MATERIALS AND METHODS
Donor Populations
Eighteen mixed-breed dogs, produced and raised in an in-house breeding colony at the University of Pennsylvania, were the subjects of unrelated research projects. No research animals were used exclusively for this study. Individual donors for the described study were healthy, retired breeding animals or untreated control animals from which nonskeletal tissues were being collected, and therefore, their previous inclusion in another study had no impact on our study. All research dogs were raised and cared for in accordance with National Institutes of Health and USDA guidelines for the care and use of animals in research. The protocol for procuring bone marrow and care and use of all dogs was approved by the Institutional Animal Care and Use Committee at the University of Pennsylvania. Bone marrow-derived MSCs (BM-MSCs) were obtained from two defined age groups: skeletally immature dogs (n = 10; mean age ± SD: 4.9 ± 1.9 months) and aged skeletally mature dogs (n = 8; 89.5 ± 20.9 months). Both age groups were consisted of sexually intact dogs of mixed gender with 5 of the 10 young dogs being females, while 3 of the 8 aged dogs were females.
Isolation and Culture of MSCs
Immediately after euthanasia, long bones (humeri, femurs, and tibias) and ilia were harvested under aseptic conditions. The ends of the long bones and cortical rim of the ilia were removed to allow flushing of marrow contents from the bones using sterile saline. Each marrow sample was suspended in Dulbecco’s modified Eagle’s medium containing high glucose (DMEM, Invitrogen, Carlsbad, CA, USA) and centrifuged to remove the adipose fraction. Marrow cells were layered on a hydrophilic polysaccharide gradient (Ficoll-Paque Plus, GE Healthcare Biosciences, Piscataway, NJ, USA) and centrifuged for 30 min at 1,900 × g to concentrate nucleated cells at the interface, as previously described (46). The mononuclear cell fraction was collected and washed twice with DMEM, and the cell number was determined by use of a hemocytometer.
Primary marrow cultures were established at a density of 1.8 × 105 cells/cm2 in DMEM supplemented with 10% fetal bovine serum (FBS, Atlanta Biologicals, Inc., Lawrenceville, GA, USA), 100 U/ml penicillin, and 100 µg/ml streptomycin (Sigma, St. Louis, MO, USA). The cells were incubated in this growth media at 37°C in a humidified 5% CO2 atmosphere. After 24 h, the nonadherent cells were washed away with phosphatebuffered saline (PBS) and fresh media were added to the dishes, with subsequent media changes occurring every 2–3 days. At 12–14 days, prior to confluence, cells were lifted from the dishes using 0.5% trypsin (Invitrogen) and either replated under experimental conditions or replated for further expansion at a density of 3.6 × 104/cm2.
Colony-Forming Unit-Fibroblastic (CFU-f) Assay and Proliferative Index Estimation
CFU-f assay was performed as previously described (33), using fresh bone marrow mononuclear cells (BMMCs) isolated from young and aged dogs (n = 8 and 6, respectively). Cells were cultured in growth media as described above prior to fixation of cell colonies with methanol and subsequent staining with Giemsa Stain solution (Acros, Somerville, NJ, USA) for 15 min. The number of colonies (defined by ≥50 cells) was counted. An increase in cell number (proliferation index) was assessed in MSC cultures by measuring the number of viable cells present in cultures 6 days after plating equivalent numbers of cells per well using a tetrazolium (3-[4,5-dimethylthizol-2-yl]-5-[3carboxymethoxyphynyl]-2-[4-sulfophenul]-2H-tetrazolium inner salt [MTS])-based assay (Promega Corp., Madison, WI, USA) as previously described (46).
Osteogenic Differentiation
Alkaline phosphatase (AP) activity assays were performed as previously described (46). Briefly, cells were seeded in growth media at an initial plating density of 1 × 104 cells/cm2. After 24 h, growth media were replaced with fresh media with or without 100 ng/ml of bone morphogenetic protein 6 (BMP6, R&D Systems, Minneapolis, MN, USA) and 100 µg/ml of L-ascorbic acid 2-phosphate (Asc, Sigma, St. Louis, MO, USA). The media were changed once on day 3 after induction. Six days after induction, cultures were harvested for AP activity and normalized per number of viable cells per well as determined by the use of the MTS assay as previously described. The average activity of triplicate wells for individual dogs/ sites was utilized for statistical analysis.
For longer-term osteogenic cultures, cells were plated at a slightly lower initial density (0.54 × 104 cells/cm2) and osteogenesis was induced using 100 nM dexamethasone (Dex), 10 mM β-glycerol-phosphate (BGP), and 100 µg/ml of Asc (all obtained from Sigma). The initial media change occurred at 24 h and then every 2–3 days until cell culture staining with Alizarin red S on days 7, 14, and 21 after induction. Parallel cultures (days 14 and 21) were established for analysis of osteogenic gene expression, as described below. For staining, cells were washed with PBS once, rinsed with 50% EtOH for 3 min, stained with 1% w/v Alizarin red S in 0.01% ammonium hydroxide for 10 min at room temperature, and washed with deionized water three times. Images of control and osteogenic cultures were obtained prior to semiquantitative analysis of mineralization as described previously (36). Briefly, Alizarin red S was released from the cell matrix by incubating with 10% cetylpyridinium chloride in 10 mM sodium phosphate (pH 7.0) for 30 min (Sigma). The concentration of released Alizarin red S was determined by measuring the absorbance at 562 nm with Varioskan Flash (Thermo, West Palm Beach, FL, USA). As noted by other investigators (21), occasional lifting of cells occurred in osteogenic cultures of canine MSCs at longer time points (particularly day 21). Because this would negatively impact quantitative measurements of Alizarin red per well, wells with significant loss of cells and associated matrix were excluded from analysis. The extent of Alizarin red S staining increases linearly over time from days 7 to 14 to 21. Quantitative data comparisons were performed on cultures at the specified time point as indicated.
Adipogenic Differentiation
Adipogenic differentiation was performed in monolayer cultures of cells seeded at an initial plating density of 1.0 × 104/cm2. After 3 days, growth medium was replaced with fresh media or adipogenic media consisting of DMEM supplemented with 5% FBS and 5% rabbit serum (Atlanta Biological, Laurenceville, GA, USA), 5 µg/ml of insulin (Sigma), 1 µM Dex, and 5 µg/ml of Rosiglitazone (RZD, Cayman; Ann Arbor, MI, USA). Culture media were changed every 2–3 days. Lipid droplets were identified by staining with Oil Red O 21 days postinduction, as previously described (31). Duplicate wells were plated for harvesting RNA at that time point.
Chondrogenic Differentiation
Cells were plated at 0.7 × 105/cm2 in growth medium for chondrogenic and control cultures. After 3 days, medium was changed to DMEM supplemented by 1% human recombinant insulin, human transferrin, selenous acid, bovine serum albumin (BSA), and linoleic acid (ITS+Premix) from BD Biosciences (Bedford, MA, USA) with or without the inducing reagents 1 µg/ml of Asc and 100 ng/ml of BMP6. Changes to the medium occurred every 2–3 days during the 21-day induction period, at which time, the cells were harvested for RNA and Alcian blue staining. For staining, cells were washed with PBS, stained with 1% w/v Alcian blue in 3% acetic acid for 30 min at room temperature, washed three times for 2 min with 3% acetic acid, and rinsed in double distilled water.
Isolation of RNA and Real-Time Quantitative Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Total RNA was isolated and quantitated as previously described (46). Total cellular RNA (1 µg) was subjected to reverse transcription using the reverse transcription system from Eppendorf (Hamburg, Germany). The resulting cDNA was ready to serve as a template for PCR amplification. Real-time PCR was performed with 7500 Fast Real-Time PCR system from Applied Biosystems (Foster City, CA, USA), by use of the SYBR Green Master Mix (Applied Biosystems). The specific oligonucleotide primers (27) used are listed in Table 1. The oligonucleotide primers were synthesized by Integrated DNA Technologies, Inc. (Coralville, IA, USA). The gene expression levels were calculated for samples after normalization to the housekeeping gene [β2-microglobulin (B2M)] (46).
Table 1.
Primer Sequences Used in Real-Time RT-PCR Analysis
| Forward (5′–3′) |
|
|---|---|
| Genes | Reverse (5′–3′) |
| BSP | For-TTGCTCAGCATTTTGGGAATGG |
| Rev-AACGTGGCCGATACTTAAAGAC | |
| Osteocalcin | For-GAGGGCAGCGAGGTGGTGAG |
| Rev-TCAGCCAGCTCGTCACAGTTGG | |
| Col2A | For-GAAACTCTGCCACCCTGAATG |
| Rev-GCTCCACCAGTTCTTCTTGG | |
| Aggrecan | For-ATCAACAGTGCTTACCAAGACA |
| Rev-ATAACCTCACAGCGATAGATCC | |
| SOX9 | For-GCTCGCAGTACGACTACACTGAC |
| Rev-GTTCATGTAGGTGAAGTGGAG | |
| PPARg | For-ACACGATGCTGGCGTCCTTGATG |
| Rev-TGGCTCCATGAAGTCACCAAAGG | |
| FABP4 | For-ATCAGTGTAAACGGGGATGTG |
| Rev-GACTTTTCTGTCATCCGCAGTA | |
| LPL | For-ACACATTCACAAGAGGGTCACC |
| Rev-CTCTGCAATCACACGGATGGC | |
| β2-microglobulin | For-TCTACATTGGGCACTGTGTCAC |
| Rev-TGAAGAGTTCAGGTCTGACCAAG |
Data Analysis
CFU-f analysis represents single cultures for each site from individual donors. All real-time RT-PCR assays were performed in duplicate and all other assays performed in triplicate for each site for individual donors. Data from duplicate or triplicate samples were averaged for each site from individual donors prior to subsequent use in statistical analysis. Normality was determined using the Shapiro–Wilk test. Paired or unpaired Student’s t tests or the Wilcoxon rank sum test was utilized to determine statistical significance between two groups of normally distributed or nonnormally distributed data, respectively. Values of p < 0.05 were considered statistically significant. When multiple comparisons were performed, significance between groups was assessed by two-way analysis of variance (ANOVA). A Holm–Sidak post hoc test was then performed to determine significance between each group. Unadjusted p values less than or equal to the critical level set by the post hoc test after multiple comparisons were made were considered significant. Values are expressed as mean ± standard error of the mean (SEM) in the text and figures, unless otherwise stated. Study groups were compared utilizing SigmaPlot software (Systat, Inc., Chicago, IL, USA).
RESULTS
Characterization of Canine Bone Marrow-Derived MSCs (BM-MSCs)
As anticipated, colonies developed from single adherent cells in cultures after the nonadherent BMMC population was removed. These colonies expanded, and 12–14 days later, prior to confluence, cells were passaged. These isolated first passage (P1) BM-MSCs, when replated, attached and grew uniformly over the culture surface, assuming a typical polymorphic, fibroblast-like morphology in monolayer culture under growth (nondifferentiating) conditions (Fig. 1A). Multipotentiality of these cells was confirmed by their ability to differentiate along osteogenic, adipogenic, and chondrogenic lineages under appropriate conditions. Differentiation assays also confirm the absence of any significant lineage-restricted cells in these populations by the lack of induction of differentiation markers in cells cultured under noninducing conditions.
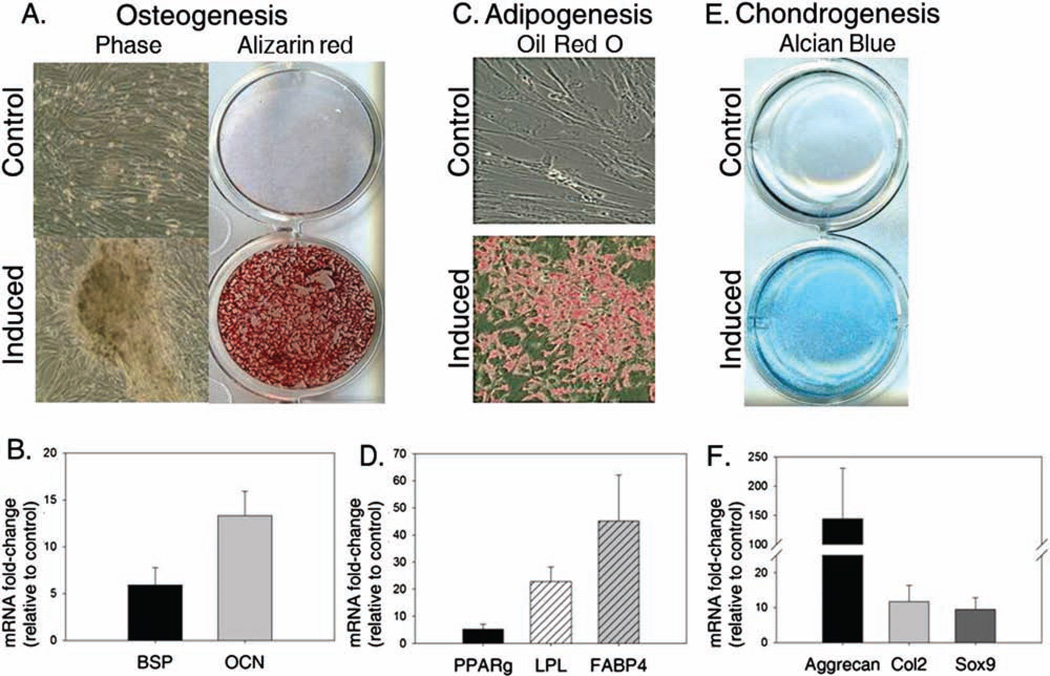
Figure 1.
Multipotential differentiation of canine bone marrow-derived mesenchymal stem cells. Osteogenesis. (A) Bone marrow mesenchymal stem cells (BM-MSCs) maintained a typical fibroblastic phenotype in control medium and showed no Alizarin red S staining. In contrast, cells cultured under osteogenic conditions (Induced) formed nodules and deposited a calcium-rich extracellular matrix as evidenced by Alizarin red S staining. (B) In addition, osteogenic cultures showed induction of bone sialoprotein (BSP) and osteocalcin (OCN) relative to control cultures (day 21 postinduction). Adipogenesis. (C) Oil Red O staining revealed cytoplasmic lipid inclusions in cells cultured in the presence of adipogenic inducers (Induced), as compared to cells cultured under control conditions (day 21). (D) mRNA expression analysis for adipogenic markers at the corresponding time point shows induction of peroxisome proliferator-activated receptor γ (PPARγ), lipoprotein lipase (LPL), and fatty acid binding protein-4 (FABP4) in induced cultures relative to control cultures. Chondrogenesis. (E, F) Alcian blue staining reveals a proteoglycan-rich matrix is secreted by BM-MSCs cultured under chondrogenic conditions (day 21) compared to control growth medium (E) with a corresponding induction of the chondrogenic markers, Aggrecan, type II collagen (Col2) and sex-determining region Y box 9 (Sox9) (F).
Compared to cells in growth medium, which maintained a fibroblastic morphology, cell size appeared to diminish under osteogenic conditions, with cells forming a slightly more polygonal appearance and forming nodules throughout the cultures. Cultures grown under osteogenic, but not under control conditions, deposited a mineralized matrix as evidenced by positive staining with Alizarin red S (Fig. 1A). To complement mineralization assays, bone sialoprotein (BSP) and osteocalcin (OCN) mRNA levels were assessed by real-time RT-PCR in cells cultured under control and osteogenic conditions. This analysis confirmed a greater than fivefold induction of these osteogenic markers in cells cultured in the presence of inducers compared to control cultures (Fig. 1B).
Small vacuoles appeared in MSCs of monolayer cultures treated with adipogenic induction medium within 7–14 days compared to their relative absence in untreated control cultures. Oil Red O staining of day 21 cultures confirmed the presence of neutral lipid positive vacuoles, consistent with an adipocyte phenotype (Fig. 1C). Corresponding molecular analysis for adipogenic genes, peroxisome proliferator-activated receptor γ (PPARγ), lipoprotein lipase (LPL), and fatty acid binding protein-4 (FABP4) revealed induction of all three markers in cells grown under adipogenic conditions relative to those cultured in growth medium (Fig. 1D).
Chondrogenesis was induced under appropriate conditions as evidenced by Alcian blue staining of cultures grown in differentiation media (Fig. 1E). In contrast, glycosaminoglycans (GAG) failed to accumulate in any MSC cultures under control conditions, as evidenced by the absence of Alcian blue staining. Chondrogenic treatment also resulted in induction of all markers assessed compared to control cultures (Fig. 1F). Both type II collagen (Col2) and sex-determining region Y box 9 (Sox9) expression were increased approximately 10-fold relative to that found in corresponding control cultures. A more dramatic upregulation of aggrecan (>100-fold) was found in cultures of MSCs grown under chondrogenic conditions relative to those grown under noninducing conditions.
Assessment of MSC Frequency and Proliferation
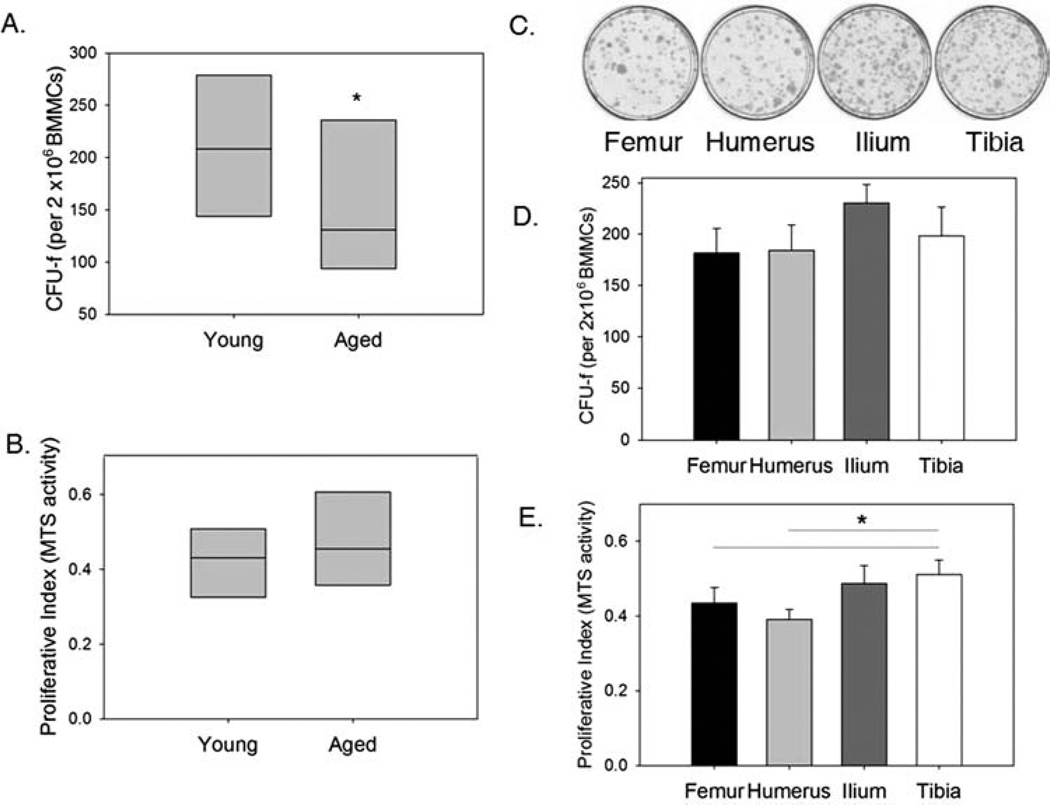
The mean CFU-f for all BM-MSC samples analyzed regardless of age or site of harvest was 192.9 ± 11.59 per 2 × 106 BMMCs (n = 52). Increased age of the donor was found to have a significant (p ≤ 0.01) negative effect on MSC frequency (Fig. 2A). Nearly a 40% reduction in median CFU-f was noted in samples obtained from aged dogs relative to those harvested from young dogs. In contrast, age did not appear to affect proliferation of first passage (P1) MSCs (Fig. 2B). A second donor parameter, site of harvest, was also shown to have a significant effect (p < 0.05) on MSC frequency in BMMC samples. CFU-f was highest in bone marrow samples obtained from the ilium and lowest from the femur or humerus (Fig. 2C, D). Similarly, iliac samples had the highest CFU-f of bone marrow harvested from these four sites in aged dogs (data not shown). Analysis of all sites from aged dogs revealed a decline in number of CFU-f compared to the corresponding site in young dogs. Samples from the tibia were found to have a significantly increased proliferative rate compared to samples harvested from both the humerus and femur in young dogs (Fig. 2E).
Figure 2.
Effect of donor age and site of harvest on CFU-f and proliferation of BM-MSCs. (A) MSC frequency per 2 × 106 of bone marrow mononuclear cells (BMMCs) in bone marrow samples [as determined by Colony forming unit-fibroblastic assay (CFU-f)] was significantly reduced in samples harvested from aged compared to young dogs (*p ≤0.01). Data from all sites were pooled for analysis of the effect of age on CFU-f, such that n = 31 and 21 for young and aged dogs, respectively. Each box represents the interquartile range (25th to 75th percentiles). The horizontal line within each box represents the median. (B) In contrast, donor age had no effect on canine MSC proliferation (data from all sites were collected and pooled where n = 40 and 25 for young and aged dog samples). Data are presented as MTS activity in day 6 cultures of MSCs obtained from young and aged dogs (plated at identical initial plating density). (C) Representative images of MSC cultures stained with giemsa stain for CFU-f assays, showing differences in CFU-f frequency in BMMCs harvested from the femur, humerus, ilium, and tibia of a young dog. (D) Quantitative analysis of CFU-f by harvest site shows an increase in frequency of MSCs harvested from iliac samples compared to the other sites. Two-way ANOVA confirms that site of harvest has a significant impact on CFU-f numbers (p < 0.05). (E) Proliferation in MSC cultures obtained from the tibia is significantly increased relative to that in cultures harvested from either the femur or humerus (*p≤0.01). Data are presented as mean MTS activity in day 6 cultures of MSCs obtained from the femur, humerus, ilium, and tibia in young dogs (plated at identical initial plating density).
Increasing Age Diminishes Osteogenic Capacity of Canine BM-MSCs
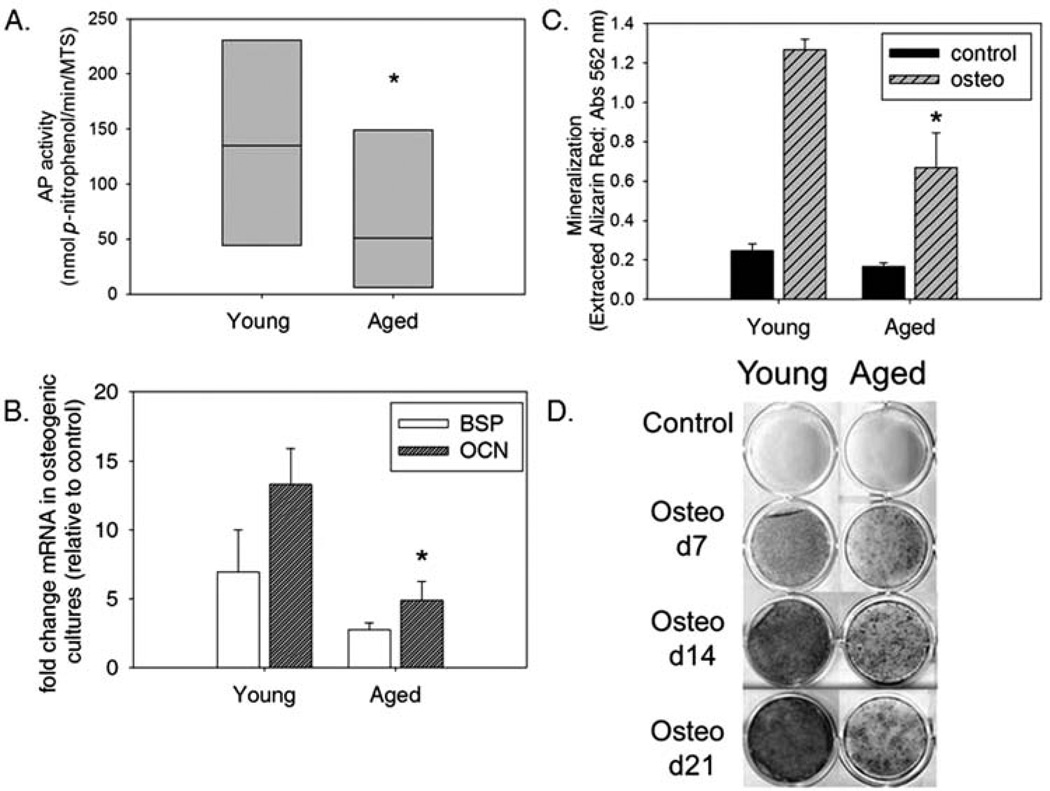
To determine if donor age influences the differentiation capacity of MSCs, induction of early stage osteogenesis was assessed by AP activity in cellular extracts from triplicate cultures of BM-MSC samples from young and aged dogs after 6 days of osteoinduction or maintenance growth (control). Mean AP activity in cultures of BM-MSC from both young and aged dogs grown under noninducing conditions is negligible (0.20 and 0.17 nmol p-nitrophenol/ min/MTS, respectively). Osteogenic treatment results in a substantial induction of AP activity in cultured cells obtained from both young and aged dogs; however, a significant (p < 0.05) reduction in AP activity is found in aged dog samples relative to those from young dogs, suggesting diminished (or delayed) capacity for osteogenesis in MSCs harvested from aged individuals (Fig. 3A). This finding is supported by decreased expression of osteogenic markers BSP and OCN in cultures of MSCs harvested from aged dogs compared to those from the same site in young dogs (Fig. 3B). MSCs from both young and aged dogs are capable of producing a calcium-rich matrix; however, the degree of mineralization occurring in cultures of young dog MSCs is significantly greater (p < 0.05) than that in cultures from aged dogs (Fig. 3C, D). Considered in combination with the AP activity and marker expression data, we conclude that, although MSCs from both young and aged dogs are capable of osteogenesis, those harvested from young individuals are significantly more efficient in their ability to differentiate.
Figure 3.
Osteogenesis is diminished in MSCs harvested from aged compared to young dogs. First passage cultures of MSCs obtained from bone marrow of young and aged donors were cultured in control and osteogenic media. The effect of age on MSC response to osteoinduction was assessed by: quantitating alkaline phosphatase (AP) activity, osteogenic marker induction and mineralization. (A) Following 6 days of osteogenic induction, AP activity was determined and normalized on the basis of cell number. Each box represents the interquartile range (25th to 75th percentiles). The horizontal line within each box represents the median. Comparison of AP activity normalized to cell number revealed a significant decrease of AP in aged dog samples cultured under osteogenic conditions relative to those obtained from young dogs (*p < 0.05). (B) MSCs harvested from the femoral BM cavity of young and aged donors cultured in osteogenic conditions showed mRNA induction of both bone sialoprotein (BSP) and osteocalcin (OCN) compared to control cultures (14 and 21 days after induction, respectively), although cultures of MSCs from aged donors had a diminished response to osteo inducers relative to those harvested from young donors. Fold induction of OCN expression is significantly diminished in MSCs harvested from aged dogs relative to young dogs (*p < 0.05). (C) Analysis of quantified Alizarin red S extracted from the matrix of femoral bone marrow MSC cultures from young and aged donors cultured under either control or osteogenic conditions for 14 days reveals a significant effect of age on matrix mineralization. MSCs from both young and aged dogs respond to osteoinducers but those from aged donors are significantly less responsive than those from young donors (*p < 0.05). (D) Alizarin red S staining of mineralized matrix in representative cultures from a young and an aged donor showing increasing deposition of a richly mineralized matrix in the young donor cells as compared to aged donor cells at 7, 14, and 21 days. Mineralization also increases over time in aged dog MSC cultures, albeit at a diminished amount relative to young dogs at corresponding time points.
Harvest Site Influences BM-MSCs Osteogenic Capacity
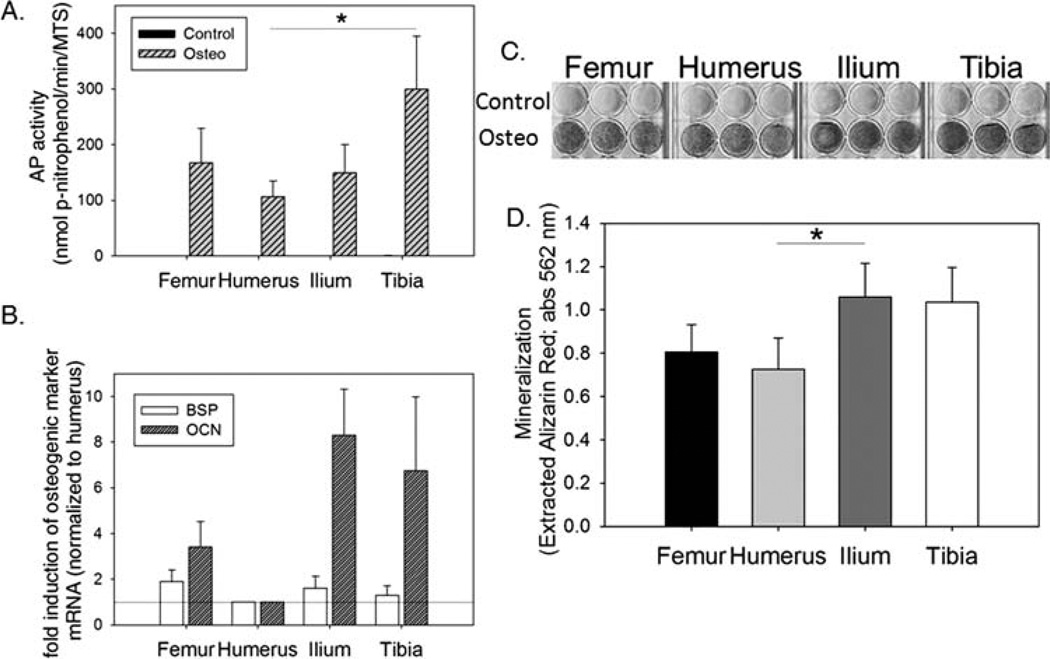
Bone marrow was harvested from three commonly used aspiration sites in the dog (humerus, ilium, and tibia) as well as the femur to assess whether the site of harvest influenced osteogenic response of isolated MSCs. Based on several markers for early and late stage osteogenesis, iliac and tibial samples are superior in their ability to differentiate along the osteogenic lineage compared to humeral samples (Fig. 4). Minimal or no AP activity was detected in cells from any of the four sites cultured under noninducing conditions, suggesting that contamination with a significant number of differentiated progenitors from a particular site did not occur (Fig. 4A). AP activity normalized to cell number in MSCs harvested from the four sites suggests that those obtained from the humerus are inferior in osteogenic capacity. AP activity is significantly greater in induced tibial-derived MSC cultures compared to those harvested from the humerus (*p ≤ 0.01). This pattern is conserved in MSCs harvested from the femur, humerus, and ilium from aged dogs (data not shown), supporting humeral-derived canine BM-MSCs to be least osteogenic, regardless of age. Accordingly, iliac and tibial samples show increased expression of osteogenic markers, BSP and OCN, relative to humeral and femoral samples (Fig. 4B). Finally, mineralization as evidenced by Alizarin red S staining is subjectively greater in iliac and tibial-derived MSCs compared to those from the humerus or femur (Fig. 4C). This is confirmed when Alizarin red S was extracted and quantitated (Fig. 4D). The amount of calcium-bound Alizarin red S is significantly increased in iliac MSC cultures compared to those obtained from the humerus. Taken together, these data suggest that site of origin influences the differentiation capacity of cells and that iliac crest or tibia contain donor cells with enhanced osteogenic capacity relative to the humerus.
Figure 4.
Site of harvest influences osteogenic potential of canine MSCs. A comparison of osteogenic potential of MSCs harvested from the femur, humerus, ilium, and tibia of young dogs reveals that humeral-derived MSCs are least responsive to osteoinducers. (A) AP activity is significantly elevated in osteogenic-induced cultures of MSCs harvested from the tibia relative to those from the humerus at day 6 postinduction (*p ≤ 0.01). Corresponding controls are shown to confirm minimal to no AP activity in these cultures and therefore the absence of differentiated cells under noninducing conditions. (B) Effect of site on induction of BSP and OCN mRNA expression in response to osteoinducers was assessed in MSC cultures at day 14. Values are set relative to BSP and OCN expression in humerus-derived MSC cultures, highlighting consistent increases in osteogenic marker expression in other harvest sites relative to the humerus. (C) Alizarin red S staining showing typical induction of matrix mineralization in MSC cultures (day 7) from a representative young donor. An increase in intensity of Alizarin red S stain is seen in cultures of MSCs harvested from the ilium and tibia relative to the femur and humerus. (D) Site of harvest was found to have a significant effect on mineralization of MSC cultures (p < 0.05). Cultures of MSCs harvested from the ilium and tibia were found to have the greatest mineralization while those from the humerus had the least mineralized matrix deposited. Multiple comparison analysis reveals a significant elevation in extracted Alizarin red S in cultures of MSCs from the ilium relative to the humerus.
Osteogenic Capacity of Canine BMMSC Is Not Sustained With Increasing Passage
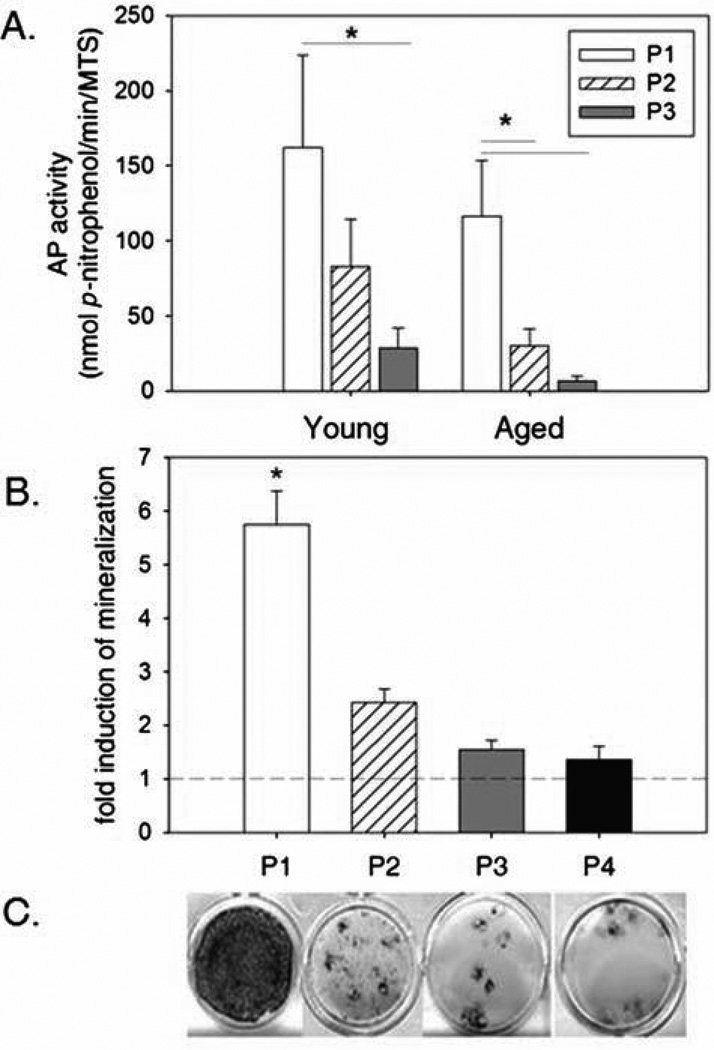
MSCs are found in a relatively low frequency in the bone marrow, so expansion of cells through ex vivo cultivation and passage is often cited as a requirement to generate clinically relevant numbers of cells. Analysis of AP activity in osteogenic cultures suggests that increasing passage of BM-MSCs from young and aged dogs diminishes osteogenic capacity. In young dogs, a significant reduction in AP activity is evident by the third passage, whereas a significant reduction occurs by the second passage in MSCs obtained from aged dogs with a further reduction occurring again in third passage cultures (Fig. 5A). MTS activity was not found to diminish in cultures of passaged cells (data not shown), suggesting that diminished proliferative potential does not contribute to this effect. This data suggest that aged dog MSCs are both less responsive to osteoinducers and are more susceptible to losing their osteogenic capacity with passage compared to MSCs harvested from young individuals. Despite a dramatic reduction in AP activity in P3 MSCs from young and aged dogs, it should be noted that the fold induction of mean AP activity under osteogenic relative to control conditions is still ≥95-fold in both age groups. Extent of mineralization was also found to diminish with increasing passage (Fig. 5B). Although occasional individual nodules throughout P4 cultures would develop into mineralized foci, the overall level of mineralization was found to decrease with each successive passage.
Figure 5.
Osteogenic differentiation is diminished with increasing passage in canine MSC cultures. Increasing passage was found to have a significant negative effect on AP activity within and mineralization of canine MSC cultures (p ≤ 0.01 for both). (A) Analysis of AP activity in day 6 osteogenic cultures of MSCs harvested from the femoral bone marrow of both young and aged dogs reveals diminishing AP activity with increasing passage. AP activity in aged dog MSC cultures are diminished in comparison to corresponding cultures from young dogs at each passage. A significant decrease in AP activity occurs by the third passage in young dogs and by the second passage in aged dogs (*p < 0.05). (B) Quantitation of Alizarin red S staining in day 7 cultures of MSCs harvested from femoral bone marrow of young dogs reveals diminished capacity for mineralization with increasing passage number. Mineral deposition in Passage 1 (P1) cultures is significantly greater (*) than in all later passages examined (P1 vs. P2–P4; *p ≤ 0.01). (C) Images of MSC cultures (day 21) stained with Alizarin red S from an individual young donor reveals that mineralization occurs throughout P1 cultures of MSCs but is restricted to smaller nodules in higher passage cultures (from left to right, P1 > P2 > P3 ~ P4).
DISCUSSION
MSC-based therapy has shown promise in preclinical models for treatment of a variety of human diseases including repair of damaged cartilage (13,48), tendons (4,50), myocardium (35,44), and spinal cord (20). Additionally, MSCs have been used as cellular vehicles for gene therapy (25). Recently, MSCs have also shown promise for the treatment of hepatic and renal diseases, as well as diseases such as diabetes and graft-versushost disease (11,28,32,43). Currently, 105 clinical trials are recruiting patients or are ongoing for MSC therapies in humans (www.clinicaltrials.gov), including the use of autologous and allogeneic MSCs to treat a variety of orthopedic, nervous system, cardiac, intestinal, hepatic, periodontal, and immune-mediated diseases. It is clear that MSC-based therapy has many important clinical applications for human and veterinary medicine. The dog has served as an accurate model for assessing the ability of MSCs to improve the healing of a wide variety of tissues, including bone, myocardium, spinal cord, cartilage defects, and osteoarthritis (3,5,10,35). Additionally, the dog has been a reliable model in which to perform studies to examine efficacy and safety of autologous versus allogeneic MSC therapy (3,10,20,44).
A major challenge for cell-based tissue repair strategies is the elucidation of predelivery strategies that optimize therapeutic efficacy. Prior to this report, the effects of donor characteristics, such as age and site of harvest, as well as postharvesting processing, such as passage during cellular expansion, on canine MSCs have received little to no attention. We report herein that both age and site of harvest have significant effects on CFU-f as well as differentiation capacity of canine BM-MSCs. In addition, culture expansion through passage of cells was also found to negatively impact the osteogenic differentiation capacity of cells. These findings have important implications for the design of clinical trials examining efficacy of canine MSCs in this valuable large animal model.
Despite the fact that a significant target population for MSC-based therapies are geriatric patients suffering from degenerative conditions or from impaired healing associated with aging and comorbidities, both in vitro and in vivo studies examining MSC biology and therapies have almost exclusively utilized MSC harvested from (and introduced into) juvenile individuals. We describe a significant negative effect of age on both MSC frequency in the bone marrow of dogs based on CFU-f analysis as well as their ability to differentiate along the osteogenic lineage. The frequency of MSCs per BMMC is consistent with what has been previously described in the dog (21,22); however, this is the first study to examine the effect of age on CFU-f in the dog. Diminished MSC frequency associated with age has been reported in other species, including humans, mice, rabbits, and rats (7,18,37,38). Multiple studies have also shown diminished differentiation capacity of MSCs from aged individuals compared to those harvested from young individuals in noncanine species (29,34,38,52). This may be particularly important for their therapeutic potential to repair bone, as diminished osteogenic capacity of MSCs harvested from aged donors has been confirmed in several in vivo studies, while one study has shown no difference between MSCs harvested from young versus aged individuals in their ability to repair tendon lesions (12). Similar to our finding that canine MSCs from aged individuals are capable of undergoing mineralization, albeit to a diminished extent relative to young donors, Mendes et al. also found that MSCs derived from donors of every age group were capable of forming bone in vivo although the frequency with which cells from aged individuals could do so significantly decreased with increasing donor age (26). By defining optimal donor age, rational decisions can be made as to whether autologous or allogeneic MSC therapy is warranted. Most studies examining efficacy of MSCs for cell-based therapies have utilized cells obtained from young donors; however, if autologous cells are to be used for clinical trials, additional studies are needed to determine if MSCs from older individuals, who are most likely to be affected by these diseases or impaired healing, will be efficacious. It will be impossible to interpret the potential for success of MSC-based therapies if the optimal cell preparations are not identified.
In addition to defining differences in osteogenic potential with increasing donor age, determining if optimal site(s) of MSC harvest exist will also have an important impact on therapies. MSCs can be isolated from a wide variety of tissues, including but not limited to bone marrow, adipose tissue, umbilical cord blood, and peripheral blood. In fact, data from multiple laboratories suggest that the tissue of origin may influence MSC properties both in vitro and in vivo (1,2,16,18). Previous studies focusing on bone marrow-derived as well as adipose-derived MSCs suggest that MSC frequency and differentiation capacity may also be influenced by the specific site of tissue harvest (27,29,33). We examined four harvest sites to represent three common sites for bone marrow harvest in the dog (humerus, ilium, and tibia) as well as that from the femoral cavity. Bone marrow aspirated from the femoral cavity during total hip replacement procedures is typically discarded but could provide a large source of MSCs for ex vivo expansion and subsequent use in multiple dogs without causing additional morbidity to donors. A significant advantage of using the canine model for examining harvest site-specific differences in MSCs, compared to small animal (rodent) models, is the dog’s size, which allows for direct comparison of MSC properties (including CFU-f and differentiation) within an individual. Our data suggest that, of the sites examined, humerus contains the lowest MSC frequency and that humerus-derived MSCs have significantly diminished osteogenic capacity compared with those from ilium and tibia. Although not examined in this study, two studies have found a significant increase in osteogenic response from BM-MSCs derived from the orofacial region compared to those harvested from the iliac crest (2,29). This skeletal site specificity of BM-MSCs has been attributed to differences in embryological origins (i.e., orofacial bones arise from neural crest while appendicular and axial bones arise from mesoderm) of these bones (29). Of bones derived from mesoderm, effects of harvest site on MSC properties have also been previously noted. Risbud et al. found a higher CFU-f frequency in bone marrow obtained from the vertebral body compared to the iliac crest, although no differences in osteogenic response between vertebral body and iliac crest-derived MSCs was detected (33). Differences in other stem cell populations within marrow cavities of bones of the appendicular and axial skeleton have been described (40), which may alter the in vivo MSC niche of different skeletal bones and therefore may influence MSC differentiation capacity even when removed from that environment.
Finally, ex vivo expansion is a necessary step in the production of relevant numbers of BM-MSCs for clinical applications. Human MSCs have been shown to maintain their response to osteogenic supplements for over 10 passages (9), in contrast to rat MSCs in which osteogenic potential diminished by the third passage (39). Our finding that osteogenic capacity of canine MSCs diminishes with increasing passage supports loss of AP activity in canine MSC cultures with sequential passage as previously reported by Kadiyala et al. (21). Similar to their findings, we also found a significant increase in AP activity present in third passage osteogenic cultures from young and aged donors relative to corresponding control cultures but that values are significantly less than earlier passage cells. Although we did not find an association between this loss in differentiation capacity and proliferation within cultures, senescence of canine MSCs as a consequence of aging or ex vivo expansion was not specifically examined. Shortening of MSC telomere length, one of the mechanisms underlying cell senescence in other cell types has been associated with advancing donor age and in vitro expansion and may provide explanation for diminished differentiation capacity seen in canine MSCs in this study (6,14).
The results presented here demonstrate significant effects of both donor age and harvest site as well as ex vivo expansion on canine MSC properties such as frequency and differentiation capacity. As with any in vitro analysis of MSC potential, these effects must be examined in vivo. Although in vitro osteogenic capacity has been shown to be predictive of in vivo capacity of MSCs, the effects of donor age and harvest site on the ability of canine MSCs to repair individual tissues in vivo is at this time undetermined. How donor age, site of harvest and ex vivo expansion modulate trophic factor expression and differentiation toward alternative lineages has yet to be explored. In addition, the ability of MSCs to repair tissue will also be determined by the particular niche at the site of interest. These advances on the biology of canine MSCs, described herein, represent an important step to directing translational research in this valuable large animal model.
ACKNOWLEDGMENTS
This work was supported by Grants to SWV from the Canine Health Foundation (CHF00970) and the National Institutes of Health (K08AR053945). We thank Mark Haskins, V.M.D., Ph.D, for providing a source of canine bone marrow (NIH grant RR02512), Dr. Ken Drobatz for assistance with statistical analysis, and Dr. Paula Henthorn for assistance with sequencing and homology alignment. We would also like to thank Patty O’Donnell and Lauren Hakkinen for technical assistance.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1.Ahren BJ, Schaer TP, Terkhorn SP, Jackson KV, Mason NJ, Hankenson KD. Evaluation of equine peripheral blood apheresis product, bone marrow, and adipose tissue as sources of mesenchymal stem cells and their differentiation potential. Am. J. Vet. Res. 2011;72:127–133. doi: 10.2460/ajvr.72.1.127. [DOI] [PubMed] [Google Scholar]
- 2.Akintoye SO, Lam T, Shi S, Brahim J, Collins MT, Robey PG. Skeletal site-specific characterization of orofacial and iliac crest human bone marrow stromal cells in same individuals. Bone. 2006;38:758–768. doi: 10.1016/j.bone.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 3.Arinzeh T, Peter S, Archambault M, van den Bos C, Gordon S, Kraus K, Smith A, Kadiyala S. Allogeneic mesenchymal stem cells regenerate bone in a critical-sized canine segmental defect. J. Bone Joint Surg. Am. 2003;85-A:1927–1935. doi: 10.2106/00004623-200310000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Awad HA, Boivin GP, Dressler MR, Smith FNL, Young RG, Butler DL. Repair of patellar tendon injuries using a cell-collagen composite. J. Orthop. Res. 2006;21:420–431. doi: 10.1016/S0736-0266(02)00163-8. [DOI] [PubMed] [Google Scholar]
- 5.Bartunek J, Croissant JD, Wijns W, Gofflot S, de Lavareille A, Vanderheyden M, Kaluzhny Y, Mazouz N, Willemsen P, Penicka M, Mathieu M, Homsy C, De Bruyne B, McEntee K, Lee IW, Heyndrickx GR. Pretreatment of adult bone marrow mesenchymal stem cells with cardiomyogenic growth factors and repair of the chronically infarcted myocardium. Am. J. Physiol. Heart Circ. Physiol. 2007;292:1095–1104. doi: 10.1152/ajpheart.01009.2005. [DOI] [PubMed] [Google Scholar]
- 6.Baxter MA, Wynn RF, Jowitt SN, Wraith JE, Fairbairn LJ, Bellantuono I. Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem Cells. 2004;22:675–682. doi: 10.1634/stemcells.22-5-675. [DOI] [PubMed] [Google Scholar]
- 7.Bergman RJ, Gazit D, Kahn AJ, Gruber S, McDougall S, Hahn TJ. Age-related changes in osteogenic stem cells in mice. J. Bone Miner. Res. 1996;11:568–577. doi: 10.1002/jbmr.5650110504. [DOI] [PubMed] [Google Scholar]
- 8.Brown DC. Control of selection bias in parallel-group controlled clinical trials in dogs and cats: 97 trials (2000–2005) J. Am. Vet. Med. Assoc. 2006;229:990–993. doi: 10.2460/javma.229.6.990. [DOI] [PubMed] [Google Scholar]
- 9.Bruder S, Jaiswal N, Haynesworth S. Growth kinetics, self-renewal and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J. Cell. Biochem. 1997;64:278–294. doi: 10.1002/(sici)1097-4644(199702)64:2<278::aid-jcb11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 10.Bruder S, Kraus K, Goldberg V, Kadiyala S. The effect of implants loaded with autologous mesenchymal stem cells on the healing of canine segmental bone defects. J. Bone Joint Surg. Am. 1998;80:985–996. doi: 10.2106/00004623-199807000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Chen L-B, Jiang X-B, Yang L. Differentiation of rat marrow mesenchymal stem cells into islet beta-cells. World J. Gastroenterol. 2004;10:3016–3020. doi: 10.3748/wjg.v10.i20.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dressler MR, Butler DL, Boivin GP. Effects of age on the repair ability of mesenchymal stem cells in rabbit tendon. J Orthop. Res. 2005;23:287–293. doi: 10.1016/j.orthres.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 13.Fuchs JR, Hannouche D, Terada S, Vacanti JP, Fauza DO. Fetal tracheal augmentation with cartilage engineered from bone marrow-derived mesenchymal progenitor cells. J. Pediatr. Surg. 2003;38:984–987. doi: 10.1016/s0022-3468(03)00139-8. [DOI] [PubMed] [Google Scholar]
- 14.Guillot PV, Gotherstrom C, Chan J, Kurata H, Fisk NM. Human first trimester fetal mesenchymal stem cells (MSC) express pluripotency markers, grow faster, and have longer telomeres compared to adult MSC. Stem Cells. 2007;25:646–654. doi: 10.1634/stemcells.2006-0208. [DOI] [PubMed] [Google Scholar]
- 15.Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, Gerstenblith G, DeMaria AN, Denktas AE, Gammon RS, Hermiller JB, Jr., Reisman MA, Schaer GL, Sherman W. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (Prochymal) after acute myocardial infarction. J. Am. Coll. Cardiol. 2009;54:2277–2286. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hass R, Kasper C, Böhm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun. Signal. 2011;9:2–14. doi: 10.1186/1478-811X-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayes B, Fagerlie SR, Ramakrishnan A, Baran S, Harkey M, Graf L, Bar M, Bendoraite A, Tewari M, Torok-Storb B. Derivation, characterization, and in vitro differentiation of canine embryonic stem cells. Stem Cells. 2008;26:465–473. doi: 10.1634/stemcells.2007-0640. [DOI] [PubMed] [Google Scholar]
- 18.Huibregtse BA, Johnstone B, Goldberg VM, Caplan AI. Effect of age and sampling site on the chondro-osteogenic potential of rabbit marrow-derived mesenchymal progenitor cells. J. Orthop. Res. 2000;18:18–24. doi: 10.1002/jor.1100180104. [DOI] [PubMed] [Google Scholar]
- 19.Jengebluth P, Luedde M, Ferrer E, Luedde T, Vucur M, Peinado VI, Go T, Schreiber C, Richthofen MV, Bader A, Haag J, Darsow KH, Bartel SJ, Lange HA, Furlani D, Steinhoff G, Macchiarini P. Mesenchymal stem cells restore lung function by recruiting resident and non-resident proteins. Cell Transplant. 2011;20:1561–1574. doi: 10.3727/096368910X557254. [DOI] [PubMed] [Google Scholar]
- 20.Jung DL, Ha J, Kang BT, Kim J-W, Quan F-S, Lee J-H, Woo E-J, Park H-M. A comparison of autologous and allogeneic bone marrow-derived mesenchymal stem cell transplantation in canine spinal cord injury. J. Neurol. Sci. 2009;285:67–77. doi: 10.1016/j.jns.2009.05.027. [DOI] [PubMed] [Google Scholar]
- 21.Kadiyala S, Young RG, Thiede MA, Bruder SP. Culture expanded canine mesenchymal stem cells possess osteochondrogenic potential in vivo and in vitro. Cell Transplant. 1997;6:125–134. doi: 10.1177/096368979700600206. [DOI] [PubMed] [Google Scholar]
- 22.Kamishina H, Farese JP, Storm JA, Cheeseman JA, Clemmons RM. The frequency, growth kinetics, and osteogenic/adipogenic differentiation properties of canine bone marrow stromal cells. In Vitro Cell Dev. Biol. Anim. 2008;44:472–479. doi: 10.1007/s11626-008-9137-6. [DOI] [PubMed] [Google Scholar]
- 23.Kanazawa H, Fujimoto Y, Teratani T, Iwasaki J, Kasahara N, Negishi K, Tsuruyama T, Uemoto S, Kobayashi E. Bone marrow-derived mesenchymal stem cells ameliorate hepatic ischemia reperfusion injury in a rat model. PLoS One. 2011;6:e19195. doi: 10.1371/journal.pone.0019195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Blanc K, Frassoni F, Ball F, Roelofs H, lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M, Dini G, Egeler RM, Bacigalupo A, Fibbe W, Ringdén O. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: A phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 25.Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, Dzau VJ. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat. Med. 2003;9:1195–1201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 26.Mendes SC, Tibbe JM, Veenhof M, Bakker K, Both S, Platenburg PP, Oner FC, de Bruijn JD, Van Blitterswijk CA. Bone tissue-engineered implants using human bone marrow stromal cells: Effects of culture conditions and donor age. Tissue Eng. 2002;8:911–920. doi: 10.1089/107632702320934010. [DOI] [PubMed] [Google Scholar]
- 27.Neupane M, Chang C-C, Kiupel M, Yuzbasiyan-Gurkan V. Isolation and characterization of canine adipose-derived mesenchymal stem cells. Tissue Eng. 2008;14:1007–1015. doi: 10.1089/ten.tea.2007.0207. [DOI] [PubMed] [Google Scholar]
- 28.Ong S-Y, Dai H, Leong KW. Inducing hepatic differentiation of human mesenchymal stem cells in pellet culture. Biomaterials. 2006;27:4087–4097. doi: 10.1016/j.biomaterials.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 29.Osyczka AM, Damek-Poprawa M, Wojtowicz A, Akintoye SO. Age and skeletal sites affect BMP-2 responsiveness of human bone marrow stromal cells. Connect. Tissue Res. 2009;50:270–277. doi: 10.1080/03008200902846262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parker HG, Shearin AL, Ostrander EA. Man’s best friend becomes biology’s best in show: genome analyses in the domestic dog. Annu. Rev. Genet. 2010;44:309–336. doi: 10.1146/annurev-genet-102808-115200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramirez-Zacarias Jl, Castro-Munozledo F, Castro-Munozledo F. Quantitation of adipose conversion and triglycerides by staining intracytoplasmic lipids with Oil Red O. Histochemistry. 1992;97:493–497. doi: 10.1007/BF00316069. [DOI] [PubMed] [Google Scholar]
- 32.Ringden O, Uzunel M, Rasmusson I, Remberger M, Sundberg B, Lonnies H, Marschall H-U, Dlugosz A, Szakos A, Hassan Z, Omazic B, Aschan J, Barholt L, Le Banc K. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006;81:1390–1397. doi: 10.1097/01.tp.0000214462.63943.14. [DOI] [PubMed] [Google Scholar]
- 33.Risbud MV, Shapiro IM, Guttapalli A, Di Martino A, Danielson KG, Beiner JM, Hillibrand A, Albert TJ, Anderson DG, Vaccaro AR. Osteogenic potential of adult human stem cells of the lumbar vertebral body and iliac crest. Spine. 2006;31:83–89. doi: 10.1097/01.brs.0000193891.71672.e4. [DOI] [PubMed] [Google Scholar]
- 34.Roura S, Farré J, Soler-Botija C, LLach A, Hove-Madsen L, Cairó JJ, gòdia F, Cinca J, Bayes-Genis A. Effects of aging on the pluripotential capacity of human CD105+ mesenchymal stem cells. Eur. J. Heart Fail. 2006;8:555–563. doi: 10.1016/j.ejheart.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 35.Silva GV, Litovsky S, Assad JAR, Sousa ALS, Martin BJ, Vela D, Coulter SC, Lin J, Ober J, Vaughn WK, Branco RVC, Oliveria EM, He R, Geng Y-J, Willerson JT, Perin EC. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation. 2005;111:156. doi: 10.1161/01.CIR.0000151812.86142.45. [DOI] [PubMed] [Google Scholar]
- 36.Spinella-Jaegle S, Roman-Roman S, Faucheu C, Dunn F-W, Kawai S, galléa S, Stiot V, Blanchet AM, Courtois B, Baron R, Rawadi G. Opposite effects of bone morphogenetic protein-2 and transforming growth factor-β1 on osteoblast differentiation. Bone. 2001;29:323–330. doi: 10.1016/s8756-3282(01)00580-4. [DOI] [PubMed] [Google Scholar]
- 37.Stolzing A, Jones E, McGonagle D, Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: Consequences for cell therapies. Mech. Ageing Dev. 2008;129:163–173. doi: 10.1016/j.mad.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 38.Stolzing A, Scutt A. Age-related impairment of mesenchymal progenitor cell function. Aging Cell. 2006;5:213–224. doi: 10.1111/j.1474-9726.2006.00213.x. [DOI] [PubMed] [Google Scholar]
- 39.Sugiura F, Kitoh H, Ishiguro N. Osteogenic potential of rat mesenchymal stem cells after several passages. Biochem. Biophys. Res. Comm. 2004;316:233–239. doi: 10.1016/j.bbrc.2004.02.038. [DOI] [PubMed] [Google Scholar]
- 40.Suter SE, Gouthro TA, McSweeney PA, Nash RA, Haskins ME, Felsburg PJ, Henthorn PS. Isolation and characterization of pediatric canine bone marrow CD34+ cells. Vet. Immunol. Immunopath. 2004;101:31–47. doi: 10.1016/j.vetimm.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 41.Thomas ED, Storb R. The development of the scientific foundation of hematopoietic cell transplantation based on animal and human studies. In: Thomas ED, Blume KG, Forman SJ, editors. Hematopoietic cell transplantation. 2nd edition. Boston, MA: USA Blackwell Science; 1999. pp. 1–11. [Google Scholar]
- 42.Togel F, Cohen A, Zhang P, Yang Y, Hu Z, Westenfelder C. Autologous and allogeneic marrow stromal cells are safe and effective for the treatment of acute kidney injury. Stem Cells Dev. 2009;18:475–486. doi: 10.1089/scd.2008.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Togel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through diffentiation- independent mechanisms. Am. J. Physiol. 2005;289:31–42. doi: 10.1152/ajprenal.00007.2005. [DOI] [PubMed] [Google Scholar]
- 44.Vela DC, Silva GV, Assad JAR, Sousa ALS, Coulter S, Fernandes MR, Perin EC, Willerson JT, Buja LM. Histopathological study of healing after allogenic mesenchymal stem cell delivery in myocardial infarction in dogs. J. Histochem. Cytochem. 2009;57:167–176. doi: 10.1369/jhc.2008.952507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Viera NM, Brandalise V, Zucconi E, Secco M, Strauss BE, Zatz M. Isolation, characterization and differentiation potential of canine adipose-derived stem cells. Cell Transplant. 2010;19:279–289. doi: 10.3727/096368909X481764. [DOI] [PubMed] [Google Scholar]
- 46.Volk SW, Diefenderfer DL, Christopher SA, Haskins ME, Leboy PS. Effects of osteogenic inducers on cultures of canine mesenchymal stem cells. Am. J. Vet. Res. 2005;66:1729–1737. doi: 10.2460/ajvr.2005.66.1729. [DOI] [PubMed] [Google Scholar]
- 47.Volk SW, Radu A, Zhang L, Liechty KW. Stromal progenitor cell therapy corrects the wound healing defect in the ischemic rabbit ear model of chronic wound repair. Wound Rep. Regen. 2007;15:736–747. doi: 10.1111/j.1524-475X.2007.00277.x. [DOI] [PubMed] [Google Scholar]
- 48.Wakitani S, Imoto K, Yamamoto T, Saito M, Murata N, Yoneda M. Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthritis Cartilage. 2002;10:199–206. doi: 10.1053/joca.2001.0504. [DOI] [PubMed] [Google Scholar]
- 49.Yamada Y, Ito K, Nakamura S, Ueda M, Nagasaka T. Promising cell-based therapy for bone regeneration using stem cells from deciduous teeth, dental pulp, and bone marrow. Cell Transplant. 2011;20:1003–1013. doi: 10.3727/096368910X539128. [DOI] [PubMed] [Google Scholar]
- 50.Young RG, Butler DL, Weber W, Caplan AI, Gordon SL, Fink DJ. Use of mesenchymal stem cells in a collagen matrix for Achilles tendon repair. J. Orthop. Res. 1998;16:406–413. doi: 10.1002/jor.1100160403. [DOI] [PubMed] [Google Scholar]
- 51.Zeng X, Zeng YS, Ma YH, Du BL, Zhang W, Li Y, Chan WY. Bone marrow mesenchymal stem cells in a three dimensional gelatin sponge scaffold attenuate inflammation, promote angiogenesis and reduce cavity formation in experimental spinal cord injury. Cell Transplant. 2011;20:1881–1899. doi: 10.3727/096368911X566181. [DOI] [PubMed] [Google Scholar]
- 52.Zheng H, Martin JA, Duwayri Y, Falcon G, Buckwalter JA. Impact of aging on rat bone marrow-derived stem cell chondrogenesis. J. Gerontol. A Biol. Sci. Med. Sci. 2007;62A:136–148. doi: 10.1093/gerona/62.2.136. [DOI] [PubMed] [Google Scholar]