Abstract
Gastric infection by Helicobacter pylori is the most common cause of ulcer disease and gastric cancer. The mechanism of progression from gastritis and inflammation to ulcers and cancer in a fraction of those infected is not definitively known. Significant acidity is unique to the gastric environment and is required for ulcer development. The interplay between gastric acidity and H. pylori pathogenesis is important in progression to advanced disease. The aim of this study was to characterize the impact of acid on gastric epithelial integrity and cytokine release and how H. pylori infection alters these responses. Human gastric epithelial (HGE-20) cells were grown on porous inserts, and survival, barrier function, and cytokine release were studied at various apical pH levels in the presence and absence of H. pylori. With apical acidity, gastric epithelial cells demonstrate increased barrier function, as evidenced by increased transepithelial electrical resistance (TEER) and decreased paracellular permeability. This effect is reduced in the presence of wild-type, but not urease knockout, H. pylori. The epithelial inflammatory response is also modulated by acidity and H. pylori infection. Without H. pylori, epithelial IL-8 release decreases in acid, while IL-6 release increases. In the presence of H. pylori, acidic pH diminishes the magnitude of the previously reported increase in IL-8 and IL-6 release. H. pylori interferes with the gastric epithelial response to acid, contributing to altered barrier function and inflammatory response. H. pylori diminishes acid-induced tightening of cell junctions in a urease-dependent manner, suggesting that local pH elevation promotes barrier compromise and progression to mucosal damage.
Keywords: Helicobacter pylori, gastric epithelium, apical acidity
the normal acid-secreting stomach of ∼50% of the world's population is colonized by Helicobacter pylori, leading to gastritis, peptic and duodenal ulcers, gastric carcinoma, and MALT (mucosa-associated lymphoid tissue) lymphoma (9, 47, 51, 52, 65). Initial infection often occurs early in life and typically persists throughout life without treatment (37). Infection always causes inflammation, in the form of a chronic, active gastritis, which can be asymptomatic (44). A small percentage of those infected develop more advanced disease, associated with significant morbidity and mortality. H. pylori infection is the most common cause of ulcer disease and gastric cancer (21, 65). Gastric cancer is the fourth most common cancer and the second most common cause of cancer death (25). The mechanism of progression from bacterial infection to advanced disease is not definitively known, underscoring the importance of studying the interactions between bacteria and host.
H. pylori, a neutralophile, is able to survive and grow in the acidic gastric environment via a novel mechanism of acid acclimation (42), in which the periplasmic pH is maintained near neutrality. This uniquely allows for maintenance of cytoplasmic pH at a level appropriate for survival and growth of a neutralophile without the need for large-scale pH change of the environment (42, 57, 70, 71). The gastric lumen in normal acid-secreting humans has a median daily pH of 1.4, with elevations to pH ∼4.0 after meals due to the buffering action of food (66). Despite this extreme acidity, it was assumed that the gastric surface pH is closer to neutral due to a pH gradient or barrier through the mucous layer (15). More recent work suggests that the site of H. pylori infection is actually acidic. In a mouse model, H. pylori infection resulted in a thinner mucous layer, inhibited the accumulation of mucus, inhibited the increase in mucosal blood flow in response to acid, and abolished the putative pH gradient at the mucous layer. Inhibition of bicarbonate transport may play a role in these infection-associated changes (30). Studies using pH-sensitive fluorescent probes and confocal microscopy showed a surface pH of ∼4.0 in the mouse stomach (6). Analysis of the transcriptome of H. pylori infecting the gerbil stomach showed that the organism lives in an acidic environment, since most of the acid acclimation genes are upregulated to a greater extent than in vitro at pH 4.5 (58). Comparison of the in vivo (gerbil) and in vitro transcriptome data suggests a pH of ≤4.5 at the site of infection and is consistent with the above-mentioned measurements of gastric surface pH, either with fluorescent probes or pH microelectrodes in the infected mouse stomach (6, 30). Gastric acidity is clearly a critical component of the gastric environment and H. pylori pathogenesis.
The gastric epithelium is designed to withstand acidity. Gastric ulcers do not develop spontaneously in the normal acid-secreting stomach; acidity is required, but not sufficient, for ulcer development (68). A breech in the gastric epithelial barrier can lead to exposure of the underlying serosa to acid, causing tissue damage and ulceration (68). The epithelial barrier is maintained by the adherens junctions, which mechanically link neighboring cells, and the tight junctions, which impede paracellular permeability/diffusion of solutes (28). The degree of epithelial “tightness” depends on the physiological role of a particular tissue or organ. Within the gastrointestinal tract, low paracellular permeability is seen in the distal colon, which allows for NaCl and water reabsorption against a concentration gradient. Conversely, higher paracellular permeability in the small intestinal epithelium allows bulk movement of fluids (2). The esophageal epithelium, which is not normally exposed to low pH, is injured by acid exposure. Epithelial integrity is compromised in esophageal epithelium exposed to varying degrees of acidity (24), suggesting that the normal gastric epithelium would need appropriate defense mechanisms to prevent the effects of acid. The gastric epithelium is considered a “tight” epithelium, with relatively low permeability to water and solutes (4), which fits with the need to protect underlying tissue from proton leak. Measurable resistance across gastric cell layers is more accurate and yields higher values when measurements are adjusted to account for the true extensive apical surface area (19).
Cell junctions are dynamic structures, with changes possible at the protein level, a property important for regulation of permeability and signaling (63). It has been suggested that H. pylori impacts epithelial integrity (39), although the mechanism is unclear, and there is no prior documentation of an in vitro model system using true gastric epithelial cells, physiological pH values, and physiological urea concentrations. H. pylori tends to cluster at cell junctions (29, 64), suggesting a potential mechanism for epithelial disruption via interference with the link between cells. In nongastric cell models, tight junction proteins redistribute and traffic to sites of bacterial attachment (1). The H. pylori virulence factor CagA has been shown to interfere with the cell junctions via interaction with zonula occludens-1 (ZO-1) and junctional adhesion molecule (1), but other bacterial factors could also be involved, since cagA-negative strains are also highly correlated with gastric ulcers and cancer in certain parts of the world (22).
Commonly used gastric cell lines are derived from gastric cancers and do not possess the characteristics of a true epithelium. For example, AGS cells will form a monolayer in culture but do not form tight junctions (3, 5, 34). This correlates well with the observation that many gastric cancers are associated with decreased cell junction formation (54). NCI-N87 cells, derived from a human differentiated gastric carcinoma, have shown promise as a tissue culture model of a true gastric epithelium, with uniform expression of E-cadherin and ZO-1 at confluence (5, 11, 32). This cell line has been used in coculture experiments with H. pylori, although published studies do not reference concurrent exposure to acidity (8, 16, 26, 33, 36, 56, 60). A problem with the NCI-N87 cell line is the formation of distinct cellular phenotypes within single cultures. The cell line has been successfully subcloned, leading to the development of human gastric epithelial (HGE) cells, including the HGE-20 cell line (11). This cell line expresses ZO-1 at the edges of all cells, even prior to confluence, generates a transepithelial electrical resistance (TEER) once confluent, and expresses gastric zymogens and mucins at levels and in combinations consistent with in vivo studies of gastric cells (11). They respond to growth factors associated with cell proliferation and migration (TGFα/EGF) and demonstrate wound-healing properties (11). On the basis of these properties, the HGE-20 cell line was chosen as the model system for this study.
The aim of this study was to determine the effect of apical acid and H. pylori infection on epithelial integrity and cytokine release using a gastric-specific in vitro model system. The hypothesis was that H. pylori infection impairs a protective response of gastric epithelial cells to apical acidity. This study shows that the HGE-20 cell monolayer responds to acidic pH with increased TEER and decreased paracellular permeability. The ability of the cell layer to mount this response to acidity is compromised by the addition of H. pylori in coculture, an effect that is partially dependent on urease activity. Acidic pH and H. pylori coculture impact the epithelial inflammatory response by altering the levels of IL-6 and IL-8 release. Determination of the interplay between H. pylori and the gastric epithelium in the physiological setting of apical acidity may provide insight into advanced disease caused by H. pylori infection.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
H. pylori strain ATCC 43504 was used for most experiments, unless indicated otherwise. This strain is vacA- and cagA-positive and has an intact Cag pathogenicity island, with expression of all the components needed for type IV secretion of the CagA protein (41, 53, 72). A nonpolar ATCC 43504 ureB (HP0072) deletion mutant (ΔureB) was constructed by allelic exchange, as summarized below. Bacteria were grown under microaerobic (5% O2-10% CO2-85% N2) conditions on trypticase soy agar plates supplemented with 5% sheep blood (GIBCO) or in brain heart infusion medium (Difco) supplemented with 7% horse serum (GIBCO) and 0.25% yeast extract (Difco). Bacteria in medium were grown in the presence of Dent selective supplement (Oxoid), and the ΔureB was grown with 20 μg/ml kanamycin (Sigma). H. pylori strain 69a containing green fluorescent protein (GFP) was obtained from Dr. Reiner Haas (31) and grown in the presence of 20 μg/ml chloramphenicol. The GFP gene in this strain is located on a plasmid, not inserted into the chromosome.
Construction of the ΔureB strain.
A genomic knockout of ureB was constructed by homologous recombination, with replacement of the ureB gene by the kanamycin cassette. pBluescript (Stratagene) containing a kanamycin resistance gene in the multicloning site flanked by SalI (5′) and BglII (3′) was used to generate the knockout plasmid. Primers were designed to flank the regions ∼600 bp upstream from the 5′ end of the gene and 400 bp downstream from the 3′ end. The 600-bp upstream segment was amplified with a 5′ primer containing a site for digestion by XbaI (5′-gcttaactatctagaagcggtagctttgattagtgc-3′) and a 3′ primer containing a site for digestion by SalI (5′-ctgctaatcgtcgacatttcttactccttaattg-3′). The 400-bp downstream segment was amplified with a 5′ primer containing a site for digestion by BglII (5′-gcattttctaagatcttttaggagcaacgctccttaaatcc-3′) and a 3′ primer containing a site for digestion by Acc65I (5′-ctagtcaaatggtaccatacttgagcaatatcttcagcac-3′). The purified PCR products were sequentially ligated into pBluescript around the kanamycin resistance gene. The construct was introduced into H. pylori strain ATCC 43504 by natural transformation, and colonies were selected in the presence of 40 μg/ml kanamycin. The higher kanamycin concentration was used only for selection; subsequent culture conditions were as described above. Knockouts were confirmed by a series of PCRs, and presence/expression of ureA, ureI, and ureE was confirmed by RT-PCR and Western blotting.
Construction of E. coli-expressing GFP.
E. coli strain Top10 (Invitrogen) was transformed with pH-sensitive GFP (pEGFP, Clontech) using electroporation (800 Ω, 1.5 kV, 25 μF) and selected in the presence of 100 μg/ml ampicillin. Expression and function of fluorescence were confirmed with a fluorometer (Fluorolog, Jobin Yvon Hariba) and by confocal microscopy.
Cell culture.
HGE-20 cells, a derivative of the NCI-N87 gastric carcinoma cell line (11), were provided by Dr. Daniel Mènard. Cells were grown in 50:50 DMEM (CellGro Mediatech)-Ham's F-12 medium (Invitrogen) with 10% FBS (Altana) and 100 U/ml penicillin-0.1 mg/ml streptomycin (Sigma) in tissue culture flasks until subconfluent. Cells were then plated on 24-mm-diameter, 0.4-μm pore size Transwell inserts in six-well plates (Corning Costar). Once confluent, TEER was checked using an EndOhm chamber and a voltohmmeter (World Precision Instruments). Survival of cells in hypoxic conditions required for H. pylori was confirmed by LIVE/DEAD assay (Invitrogen; see Confocal microscopy), by resistance measurements over several days, and by direct comparison with cells grown under standard tissue culture conditions.
Measurement of TEER.
TEER was measured using an EndOhm chamber that accommodates the Transwell inserts and a voltohmmeter (World Precision Instruments). Cells were used 1 wk after reaching confluence, and resistance was measured prior to starting experiments to ensure consistency and cell viability. Cells were washed with medium without antibiotics, and some wells were infected with H. pylori wild-type or ΔureB at multiplicity of infection >100:1 to ensure adherence. After 14 h, nonadherent bacteria were washed off, and appropriate medium (50:50 DMEM-Ham's F-12 medium, no antibiotics) was added to the apical chamber. Medium pH was adjusted with HCl to the desired pH and then filter-sterilized. Urea (5 mM) was added to the basolateral side to mimic physiological exposure of H. pylori to urea from the blood. EndOhm chambers were placed in the microaerobic incubator with cords accessible from the outside, so the incubator door was not opened during the experiments. TEER was measured every 15 min for 2 h. Apical and basolateral pH levels were measured at the conclusion of the experiment. Time course was expanded in some experiments to determine limits of experimental effect under the conditions studied. For some experiments, apical pH of 2.5–4.5 was used. For experiments with bacteria, pH 4.5 was chosen, because at this pH, the bacteria are displaying their acid acclimation responses (57, 71) and regulation of acid acclimation genes is seen (45, 58, 72). Apical pH was fixed at 4.5 for some experiments with bacteria using 50 mM Homo-PIPES (Fluka). Control experiments in the absence of bacteria included incubation with basolateral acidity, incubation in a standard tissue culture incubator, and replacement of neutral pH in the apical chamber with monitoring for viability and maintenance of TEER from several hours to several days.
Measurement of paracellular permeability.
Paracellular permeability was measured as previously described (67, 69). HGE-20 cell monolayers grown for 1 wk after becoming confluent on Transwell porous inserts were incubated in varying apical conditions, pH 4.5 or 7.4 and with or without wild-type or ΔureB H. pylori. Addition of bacteria was completed as stated above (see Measurement of TEER). Urea (5 mM) was added to the basolateral side. The fluorescent membrane-impermeable dye 2,7-bis(2-carboxyethyl)-5(6)-carboxyfluorescein (BCECF) free acid (10 μM) was added to the lower chamber. For determination of dye accumulation in the upper chamber, 50-μl aliquots were diluted in 3 ml of PBS, pH 7.2, and fluorescence intensity was measured in a fluorometer (Fluorolog) every 15 min for 2 h. Accumulation of BCECF free acid in the upper chamber reflects paracellular flux of the dye through the monolayer, because this dye is membrane-impermeable and, therefore, can penetrate the monolayer only between the cells. The fluorescence intensity in the upper chamber was plotted vs. incubation time. Values are means ± SE, and statistical significance was determined by t-test.
Confocal microscopy.
Confocal microscopic images were obtaining using a Zeiss LSM 510 laser scanning confocal microscope and LSM 510 software. At the conclusion of the TEER experiments, cell viability was confirmed using the eukaryotic BacLight LIVE/DEAD kit (Invitrogen), where live cells fluoresce in the green spectrum and dead cells in the red spectrum. For study of localization of bacteria, cells were incubated with GFP-expressing H. pylori or E. coli and nonadherent bacteria were removed by washing. Acidic or neutral medium without antibiotics was added to the apical chamber (neutral only for E. coli experiments), and 5 mM urea was added to the basolateral chamber. Cells were stained with 3,3′-dipropylthiadicarbocyanine iodide [DiSC3(5)] for visibility. Localization of fluorescent bacteria was determined using confocal microscopy.
Measurement of cytokine release.
HGE-20 cell monolayers grown for 1 wk after becoming confluent on Transwell porous inserts were incubated in varying apical conditions, pH 4.5 or 7.4 and with or without wild-type or ΔureB H. pylori strains. Bacteria were added to the monolayers as described above (see Measurement of TEER). Urea (5 mM) was added to the basolateral side. After 2 h of incubation in microaerobic conditions, the apical and basolateral media were collected and frozen. IL-6 and IL-8 release in the culture supernates was measured by ELISA (Aushon, Billerica, MA). Data (means ± SE) are expressed as pg/ml, and difference between groups was evaluated by t-test.
RESULTS
HGE-20 cells form an epithelial layer in culture and tolerate microaerobic conditions.
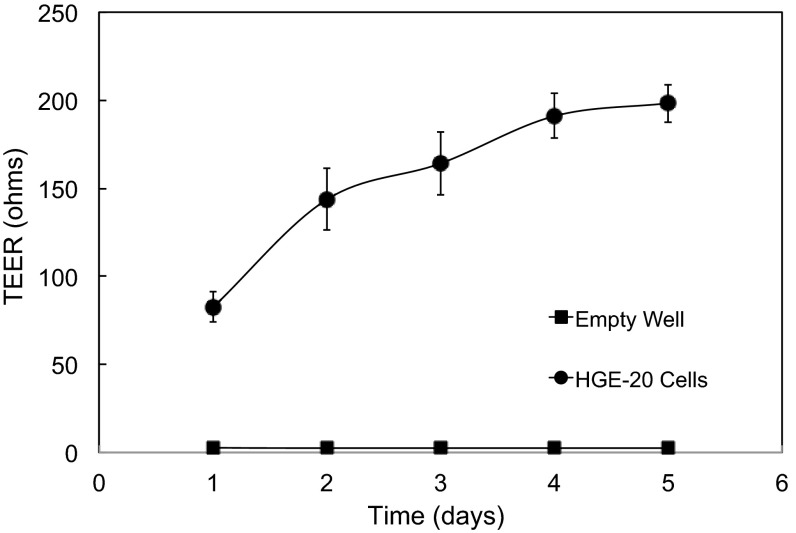
Cells were plated on porous Transwell inserts and maintained in a microaerobic (5% O2-10% CO2-85% N2) environment from the time of passage. Control cells were maintained concurrently under standard tissue culture conditions (5% CO2-95% O2). Once cells were visibly confluent, resistance was checked daily. Resistance of an empty well was 1–2 Ω and was insignificant compared with final values. There was no difference in peak resistance between the two incubators. The resistance peaked by day 5 and remained consistent at 200–250 Ω (× 4.52 cm2 = 900–1,100 ohm·cm2; Fig. 1), demonstrating that this cell line is able to form a monolayer with tight junctions. Cells were incubated in apical pH 4.5 medium for 2 h under microaerobic conditions and then stained with a eukaryotic LIVE/DEAD kit and examined by confocal microscopy. The cells were mainly alive at the conclusion of the incubation (data not shown), indicating that this culture system is appropriate for the experiments to follow.
Fig. 1.
Human gastric epithelial (HGE-20) cells form a polarized epithelium on porous inserts. HGE-20 cells were grown on porous inserts. Transepithelial electrical resistance (TEER) was measured every day starting when the cells appeared confluent. Measurements peaked by 4–5 days. TEER was stable over the course of 1–2 wk (data not shown). Values are means ± SE; n = 8. TEER of empty wells was 1–3 Ω.
TEER increases with apical acidity.
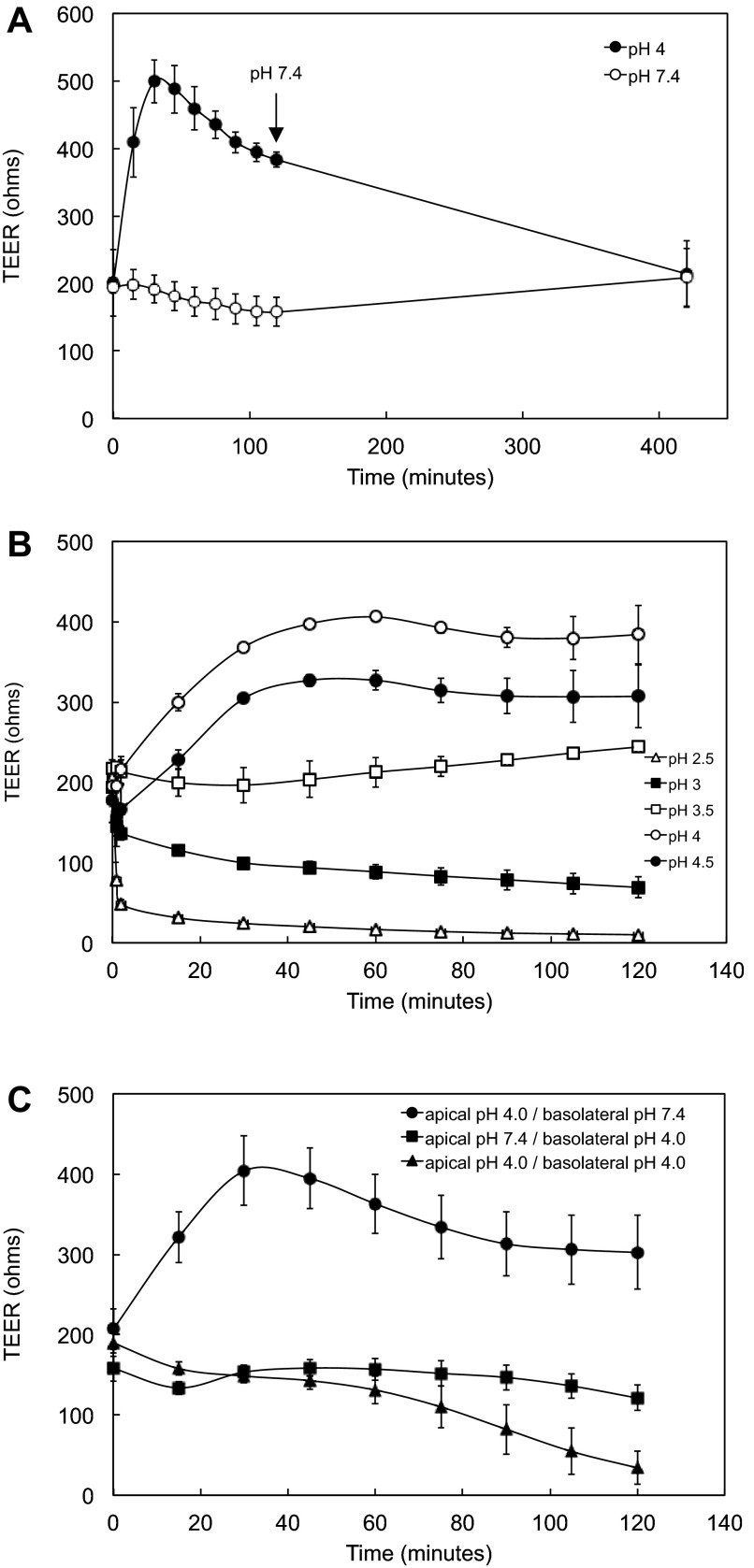
Apical pH 4 or 4.5 medium was added to HGE-20 cells plated on porous inserts, and resistance was measured every 15 min. In apical pH 7.4 medium, the resistance was unchanged over time. With apical acidity, the resistance quickly increased over the initial time points, with a peak and stabilization at 45–75 min. The elevation in resistance was stable throughout the 2-h time course of the experiments and, when monitored over an extended duration, was maintained for ∼4 h, then gradually decayed. The pH gradient between apical and basolateral sides was maintained over 2 h. At the conclusion of some experiments, pH 4 or 4.5 apical medium was replaced with pH 7.4 medium, and TEER was measured over time. Resistance was stable at baseline levels typically seen at pH 7.4 after 7 h and remained stable over 5 days, indicating that exposure to apical acidity was not lethal to the cells (Fig. 2A, 5-day data point not shown). Experiments were repeated using a pH range of 2.5–4.5 in 0.5-unit increments compared with pH 7.4. The cell layer exhibited maximal TEER at pH 4, consistent with approximations of gastric surface pH (6, 58). TEER was stable at pH 3.5, decreased slightly at pH 3, and dropped noticeably at pH 2.5 over the 2-h time course (Fig. 2B). The pH gradient was maintained at 2 h, even at pH 2.5. Resistance dropped when cells were exposed to basolateral acidity, consistent with the idea that polarized cell junctions protect against acidity (Fig. 2C).
Fig. 2.
HGE-20 cell layers become tighter in response to apical acidity. A: TEER increases with apical acidity. Replacement of apical medium with pH 7.4 medium at the conclusion of experiments carried out in pH 4 medium (at 120 min, arrow) led to maintenance of TEER at the baseline seen in the pH 7.4 graph after 7 h (420 min), indicating that apical acid exposure did not lead to cell death. TEER remained stable at 5 days after the experiment (data not shown). B: HGE-20 cells were exposed to apical acidity (pH 2.5–4.5) for 2 h, and TEER was measured every 15 min. Apical and basolateral pH was recorded at the conclusion of the experiments. The cell layer became tighter, with higher TEER, most significantly at pH 4 and 4.5. C: exposure to basolateral acidity led to dissipation of TEER. Values are means ± SE; n = 3.
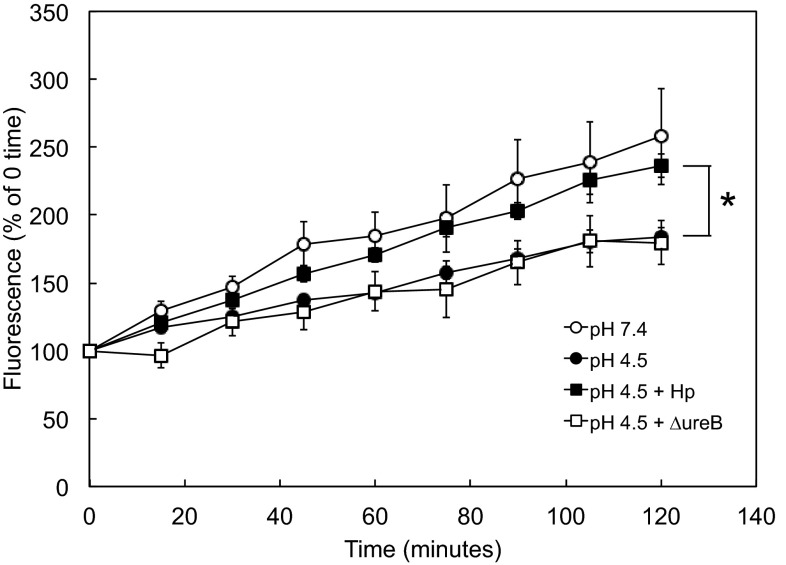
Fig. 5.
Paracellular permeability of HGE-20 monolayers decreases at acidic pH, and this response is impeded by H. pylori. Some cells were incubated with H. pylori for 14 h at MOI >100:1 prior to the start of the experiment. 2,7-Bis(2-carboxyethyl)-5(6)-carboxyfluorescein (BCECF) free acid and urea were added to the basolateral chamber, and acidic or neutral pH medium was added to the apical chamber. Aliquots were removed from the apical chamber every 15 min, and fluorescence was measured. Permeability was decreased at acidic pH, localizing the acid-induced change to the cell junction. The presence of H. pylori interfered with the acid-induced decrease in permeability, and this change was, at least in part, urease-dependent. Values are means ± SE; n = 6–9 replicates. *P < 0.01, pH 4.5 with H. pylori vs. pH 4.5 without H. pylori at 120 min (by t-test).
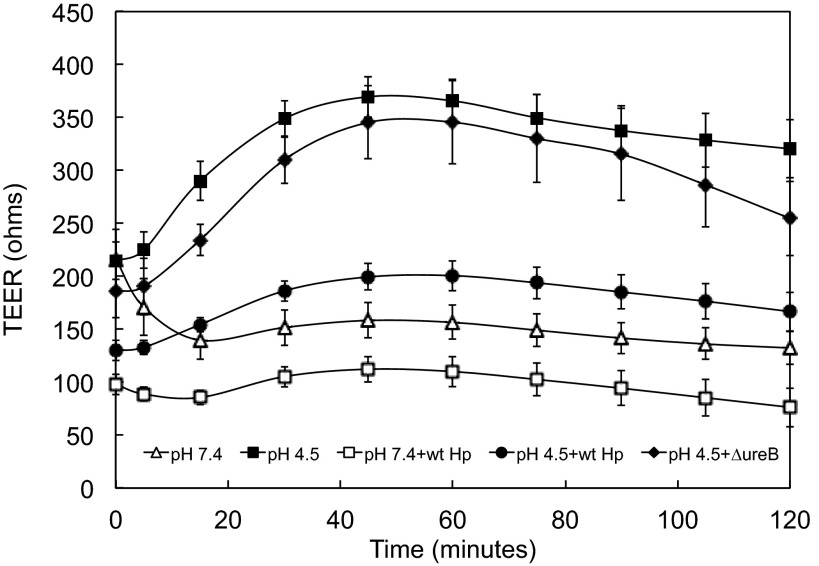
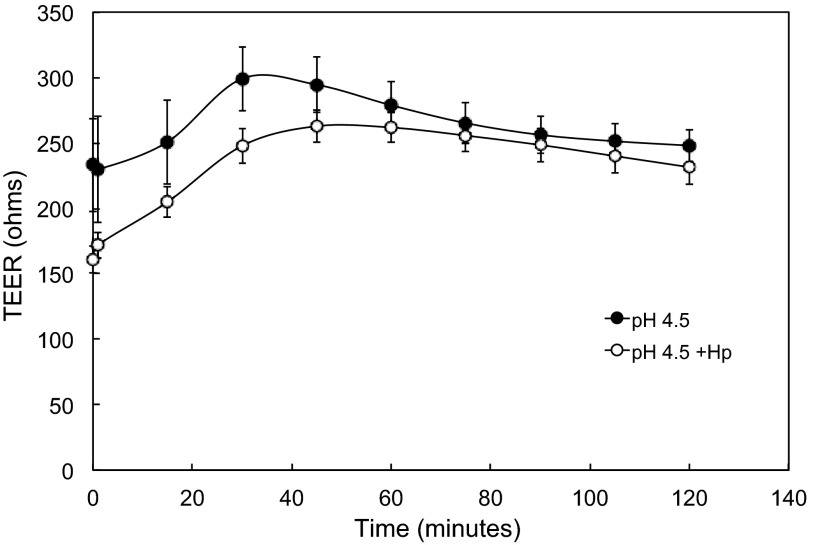
Coculture with H. pylori diminishes magnitude of acid-induced increase in TEER.
It was previously documented that, at neutral pH, gastric cells in prolonged coculture with H. pylori have a decreased TEER (73). Similar results were seen in the HGE-20 cell line after coculture with H. pylori for 14 h, as shown at time 0 in Fig. 3. Once attachment of bacteria was ensured, nonadherent organisms were removed and the pH 7.4 apical medium was replaced with pH 4.5 medium; pH 4.5 was chosen, since significant changes in acid acclimation genes of H. pylori are seen at this pH (58, 72). Urea (5 mM) was present in the basolateral chamber for all experiments to mimic physiological conditions. At pH 4.5, with urea present, in microaerobic conditions, it is well documented that H. pylori are able to survive (41, 46). The bacteria were still mainly intact bacilli when viewed by confocal microscopy in the LIVE/DEAD experiments described above. Given the doubling time of 6 h, growth of the bacteria was not expected in the time frame of these experiments. In the presence of wild-type H. pylori, the ability of the cell layer to tighten in response to apical acidity was diminished (Fig. 3). This suggests interference with the mechanism underlying the acid-induced increase in TEER by the bacteria. The TEER response for the ΔureB strain with apical acidity was almost identical to the curve with no bacteria, showing that the effect of the bacteria is at least in part urease-dependent. This was further investigated by using strong buffer in the apical medium to ensure no pH change during the course of the experiments.
Fig. 3.
Coculture of HGE-20 cells with H. pylori (Hp) impedes the acid-induced increase in TEER. Cells were incubated with H. pylori for 14 h at multiplicity of infection (MOI) >100:1 and washed; then acidic or neutral medium was added to the apical chamber. As expected, the baseline was lower after incubation with H. pylori. With apical acidity and basolateral urea, the ability of the cell layer to tighten or increase resistance in response to acidity was impaired by H. pylori, and this effect was nearly abolished in the absence of urease activity. Values are means ± SE; n = 4–12 replicates.
Effect of H. pylori on TEER is partially urease-dependent.
Cells were grown on porous inserts, and H. pylori was added to some wells at neutral pH, 14 h prior to experiments, as described above. Homo-PIPES (50 mM) was added to the apical medium, with final pH 4.5, and TEER was measured with or without H. pylori for 2 h. With strongly buffered apical medium, the resistance curve with bacteria approached that without bacteria (Fig. 4). Apical and basolateral pH were unchanged in the conditions with or without bacteria. This suggests that local pH change generated by urease activity interferes with the acid-induced tightening of the epithelial layer.
Fig. 4.
Effect of H. pylori on epithelial tightening is minimal in the presence of strong buffer. Cells were incubated with H. pylori for 14 h at MOI >100:1 and washed; then medium at pH 4.5 with 50 mM Homo-PIPES was added to the apical chamber and 5 mM urea was added to the basolateral chamber. Control experiments were done without bacteria. As expected, the baseline was lower after incubation with H. pylori. Over the 2-h time course, H. pylori did not impact tightening of the cell layer, suggesting that the effect is dependent on local pH change. Values are means ± SE; n = 3.
Apical acidity and H. pylori impact paracellular permeability.
HGE-20 cells on porous inserts were incubated in pH 4.5 or 7.4 apical medium. The membrane-impermeant fluorescent dye BCECF free acid was added to the basolateral chamber. Apical fluorescence, reflective of flux of BCECF and paracellular permeability, decreased in acid compared with neutral pH (Fig. 5). This parallels TEER data and also localizes the effect to the cell junction. The presence of H. pylori adherent to the cells, with apical acidity, leads to an increase in permeability, with the curve more closely resembling that at pH 7.4. This effect appears to be partially urease-dependent (Fig. 5).
H. pylori clusters at HGE-20 cell junctions in acid.
E. coli- or H. pylori-expressing GFP was cocultured with confluent monolayers of HGE-20 cells and then examined using confocal microscopy, with HGE-20 cells counterstained with DiSC3(5). For experiments with H. pylori, images were obtained after incubation with apical acidity and basolateral urea. E. coli were randomly distributed, while H. pylori were seen predominantly at cell junctions (data not shown). This was confirmed by images taken in the z direction. There was no clear difference in distribution of H. pylori at acidic or neutral pH, but the bacteria were already attached when acid exposure was initiated. This suggests that H. pylori is able to attain a survival advantage at the cell junction that is not relevant for E. coli, which are able to transit the stomach using an acid-tolerance response but are not able to colonize (10, 40, 49). Since urea moves paracellularly from the blood into the gastric lumen (48), easy access to the urea required for H. pylori survival in acid likely plays a role. Qualitatively similar results were seen with the ATCC 43504 strain, as observed in the LIVE/DEAD experiments described above, but since imaging was more accurate with the GFP-expressing strain, especially compared with E. coli, this strain was used for the above experiments.
IL-8 release from HGE-20 cells is impacted by apical acidity and H. pylori.
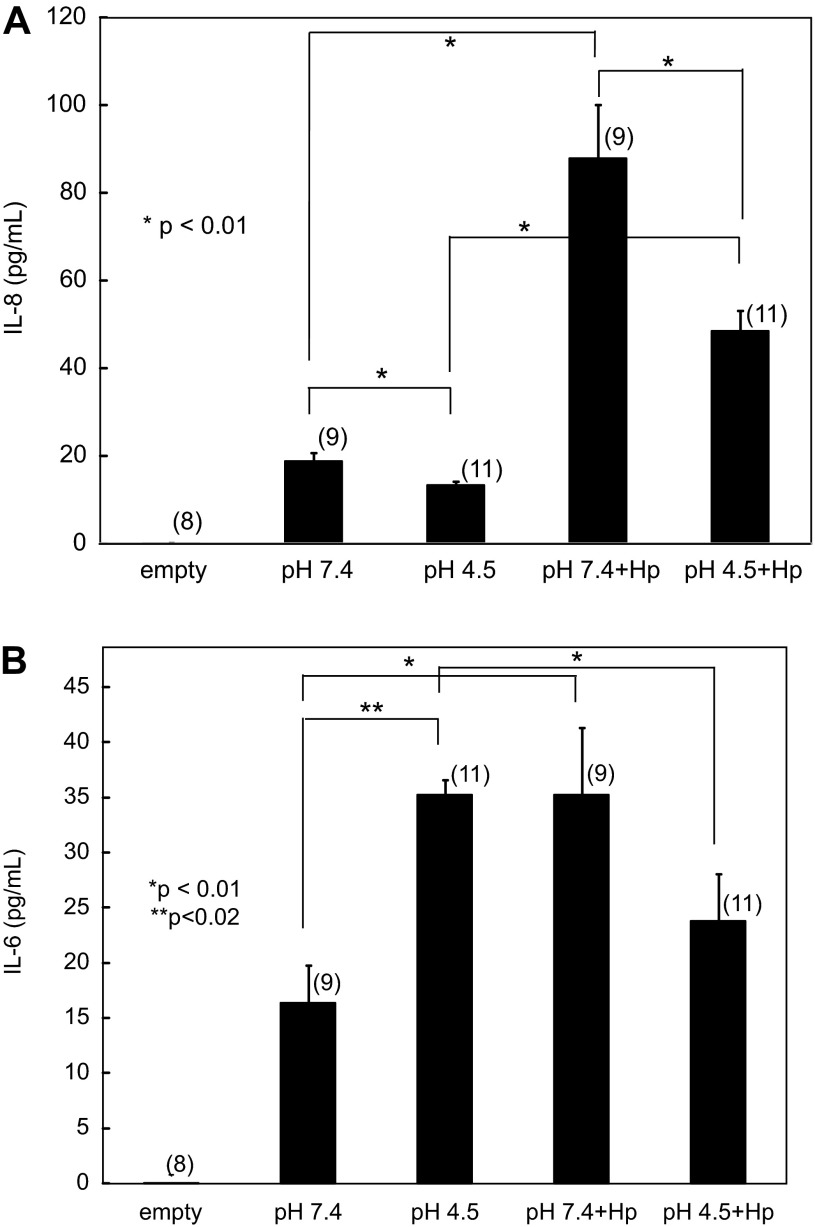
IL-8 release was measured in culture supernates by ELISA after 2 h of incubation in pH 4.5 or 7.4 medium with or without adherent H. pylori. As has been well documented previously in other model systems (17), IL-8 release increases significantly in this cell line in the presence of H. pylori at neutral pH. There was also a significant increase from baseline with apical acidity when H. pylori was present. IL-8 release was lower with apical acidity in the presence and absence of H. pylori. For all comparisons, P < 0.01 (Fig. 6A). Apical and basolateral supernates showed similar levels of IL-8 for all conditions; apical data are shown due to a larger number of replicates. This effect was independent of urease activity. The attenuated increase in inflammatory response in the presence of acidity may aid in immune evasion.
Fig. 6.
Apical acidity and H. pylori impact cytokine release from HGE-20 cells. HGE-20 cells with or without H. pylori were incubated for 2 h with apical pH 4.5 or 7.4 medium and basolateral urea. Culture supernates from the apical and basolateral chambers were collected, and IL-8 (A) and IL-6 (B) release was measured by ELISA. Apical data are shown. Epithelial IL-8 release is decreased at acidic pH, while IL-6 release is increased. The presence of H. pylori with apical acidity leads to decreased release of both cytokines compared with neutral pH. Values are means ± SE of number of replicates in parentheses. Comparisons between conditions were done by t-test.
IL-6 release from HGE-20 cells is impacted by apical acidity and H. pylori.
IL-6 release was measured in culture supernates by ELISA after 2 h of incubation in pH 4.5 or 7.4 medium with or without adherent H. pylori. Baseline IL-6 release was higher in acidic medium (P < 0.02). IL-6 release at neutral pH was higher in the presence of H. pylori (P < 0.01) but lower with apical acidity and H. pylori than with apical acidity alone (P < 0.01; Fig. 6B). This suggests that gastric acidity plays a role in the immune response to H. pylori infection.
DISCUSSION
HGE-20 cells provide a viable, uniform, and gastric-specific in vitro model system that forms a true epithelium in culture. This model system has allowed for study of the reaction of the gastric epithelium to physiological acidity and the role of H. pylori in potentially interfering with this response. The HGE-20 cell layer becomes tighter in response to apical acidity, most significantly at pH 4–4.5, an effect that can be localized to the cell junction due to parallel effects on TEER and paracellular permeability. The apical junctional complex regulates cell-cell contact, polarity, and paracellular permeability (28). Breakdown of integrity of epithelial cell contacts is an early event in ulcer formation. The apical junctional complex, located on the apical-lateral margin of epithelial cells, provides barrier function and defines epithelial cell polarity (23). The adherens junction initiates and maintains cell-cell contacts (27, 28). The tight junction segregates apical and basolateral membrane proteins and regulates paracellular permeability (28). The adherens and tight junctions have integral membrane proteins and associated cytoplasmic proteins that link membrane proteins to the cytoskeleton and trigger various signaling pathways. It follows that changes in this region could allow proton infiltration and contribute to ulcer disease. Defense mechanisms against acid in this study are still viable at least to pH 2.5, as evidenced by maintenance of the pH barrier in the short term, suggesting that the cell layer can survive temporary excursions of pH below 4–4.5, as would be expected over the course of a 24-h period in the stomach. Apical acid is not toxic to the cell layer, as evidenced by the postexperiment survival of the cells and maintenance of TEER at baseline up to 5 days after replacement of the apical medium with neutral pH. In contrast, TEER dissipated with exposure to basolateral acidity, consistent with cell polarity, basolateral intolerance to acidic pH, and the separation of the apical and basolateral chambers by cell junctions.
The most common cause of gastric ulceration is infection by H. pylori (65). The data presented here suggest that H. pylori adherence in the region of the cell junction could interfere with mucosal defenses against acidity and allow epithelial breakdown. As movement of urea into the gastric lumen is via the paracellular route (48), attachment of the bacteria at the cell junction would allow the most efficient access to urea. The bacteria are able to traffic acid acclimation proteins to the inner membrane to increase efficiency of periplasmic alkalization (43, 59), so adaptation at the site of colonization to further facilitate this response fits with the model of gastric colonization. Once situated at the site of contact between cells, H. pylori is in an ideal position to compromise epithelial integrity. If the bacteria are able to limit the ability of the epithelial layer to withstand acidity, presumably at least in part by interfering with acid-induced tightening of the cell junctions, as suggested here, then acid-related damage to underlying tissues and ulceration will follow.
An in vitro model system cannot perfectly mimic the in vivo environment. This study was limited by the use of a small-volume, closed system. For this reason, time of acid exposure was relatively brief, with experiments done over 2 h, and infection had to be established at neutral pH. In the stomach, there is a constant pH flux, within a tight range, depending on time of day and time from meals (66). Any metabolic waste products generated will be flushed out by bulk fluid flow. However, this system, within the time course of the experiments, does approximate physiological conditions to the closest degree possible, in a manner that has not previously been reported, and provides a framework for study of H. pylori pathogenesis and virulence and gastric epithelial response to acid.
Multiple bacterial factors play a role in interfering with barrier function. The well-studied virulence factor CagA, when injected into epithelial cells via a type IV secretion system, interferes with the junctional proteins ZO-1 and junctional adhesion molecule, altering structure and function of the cell junction in a Madin-Darby canine kidney cell model at neutral pH (1). Purified VacA or vaculating toxin preincubated at acidic pH before application to nongastric cell monolayers decreased TEER and increased paracellular permeability, while non-acid-activated protein had a significantly attenuated effect (50). In these studies, barrier function was modulated but not lost, as a baseline TEER was consistently maintained in all experiments across several different cell lines. Studies done on gastric cells at neutral pH had a decreased TEER in coculture with H. pylori, which was unaffected by knockout of cagA or vacA but was attenuated by knockout of ureB or incubation with NH4Cl, functionally equivalent to the pH elevation generated by urease activity in a closed system (73). Urease activity should be minimal at neutral pH, with the urea channel UreI closed, limiting access of urea to intrabacterial urease, and in the absence of physiological urea concentrations (57). Local pH elevation in the region of the tight junction may lead to decreased epithelial integrity (TEER), as seen at pH 7.4 in our in vitro studies, or the products of urea hydrolysis may have a direct effect on the junction. The present study has confirmed that the effect of H. pylori on cell junctions at acidic pH, as measured by TEER and paracellular permeability, is, at least in part, urease-dependent. The urease dependence of this response and its disappearance in the presence of strongly buffered acidic medium suggest that local pH in the region of the junction is important in infection-induced epithelial damage. Urease activity by H. pylori does not raise the pH of the bulk gastric juice or the bulk epithelial surface pH, but local changes in the microenvironment where bacteria are attached at the cell junctions are possible (3). Local pH increase at the tight junction prevents the tightening response, allowing the bulk luminal and surface acidity to cause submucosal damage. In further support of this mechanism, recent work on Cryptococcus neoformans revealed that urease activity plays a role in disruption of the blood-brain barrier, leading to invasive disease (62). Multiple additional virulence factors are likely to be involved, with progression to advanced gastric disease, especially those genes upregulated in a gerbil infection model and in physiological acidity (58), and induced changes in inflammatory response must also be considered.
Many cell-specific factors likely also play a role in maintenance of barrier function in acid. The alterations to the cell junctions in response to acid are too rapid to occur at the level of gene expression, so protein composition, trafficking, or signaling changes are likely involved. Tightening of an epithelial barrier is an established reaction to physiological stress encountered by a variety of different cell types. For example, airway epithelial cells tighten in response to shear stress, which is important for normal respiratory function and protection of underlying tissue from the external environment (61). The mechanism for this tightening has been localized to the cell junction and interactions between septin-2 and actin (61). Dispersed canine oxyntic mucosa reformed monolayers and increased barrier function in response to apical acidity (12, 13). The results reported here with the HGE-20 cell line parallel the results of the reconstituted primary cell model, suggesting that cells normally exposed to changes in acidity are able to adjust to their physiological stressor. This work confirms that this response is dependent on functional tight junctions and is apical-specific and that the system can be overwhelmed beyond a certain level of stress in the absence of bulk fluid flow. The gastric pathogen H. pylori appears to interfere with this protective mechanism, providing insight into ulcer development. Nongastric epithelia, such as in the esophagus, are not normally exposed to acid and have impaired barrier function in response to decreased pH (24), underscoring the specificity of adaptation of different epithelial cell types to their natural environment.
Physiological acidity is relevant in the modulation of inflammation. Signaling cascades can differ when exposed to acidic pH, as has been shown with NF-κB activation (7). IL-8 release has been studied in AGS cells, which do not form a true epithelium, in the presence of mild acidity (pH 5–6.8). Despite the physiological limitations of this work, IL-8 release increased in response to mild acidity with H. pylori infection (but not to mild acidity alone), and activated signaling pathways that were pH-dependent or -independent were identified (14). These findings underscore the importance of delineating the impact of the unique and harsh gastric environment on H. pylori pathogenesis and immune evasion. In this work, without bacteria present, epithelial IL-6 release is increased at acidic pH, while IL-8 release is decreased. Release of both cytokines is increased in response to H. pylori infection, with a diminished inflammatory response at acidic pH compared with neutral pH. The differential response to acid reflects the disparate roles of the two cytokines in signaling. IL-6 is pleiotropic, with a range of different functions (35). One role is resistance to apoptosis, which promotes cell viability (74) and could explain increased release from the epithelium in response to acid. IL-6 also stimulates the T cell response (35), a function more likely to be invoked in the presence of a pathogen. IL-8 is a chemokine with a specific role in neutrophil chemotaxis (55), and the trend, although not the magnitude, of the response to acid is the same with or without bacteria. In the presence of bacteria, there is an increase in release of IL-6 and IL-8, but the increase is significantly less at acidic pH. If there is a diminished epithelial inflammatory response to H. pylori at acidic pH, this could in part explain why it is so difficult for the immune system to clear the infection. Clinically, acid suppression changes the pattern of H. pylori-associated gastritis from a predominantly antral to a fundic gastritis (38), which supports the concept that the bacteria are adapted to live in a specific niche (58). With the focus of inflammation shifted from the antrum to the corpus, the risk for duodenal ulcer decreases and the risk for cancer increases (18, 20). Gastric epithelial cells are able to adapt to the primary physiological stressor in their environment, acidic pH, and H. pylori infection interferes with this adaptive mechanism, providing insight into the pathogenesis of advanced infection-associated disease.
GRANTS
This work was supported by National Institutes of Health Grants K12 HD-034610 (E. A. Marcus), P30 DK-41301 (E. A. Marcus), and DK-053642 (G. Sachs) and Department of Veterans Affairs Grant 1I01BX001006 (G. Sachs).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
E.A.M., O.V., E.T., G.S., and D.R.S. are responsible for conception and design of the research; E.A.M., O.V., E.T., and D.R.S. performed the experiments; E.A.M., O.V., and D.R.S. analyzed the data; E.A.M., O.V., E.T., G.S., and D.R.S. interpreted the results of the experiments; E.A.M., O.V., and D.R.S. prepared the figures; E.A.M. and D.R.S. drafted the manuscript; E.A.M., O.V., G.S., and D.R.S. edited and revised the manuscript; E.A.M., O.V., E.T., G.S., and D.R.S. approved the final version of the manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Daniel Mènard for allowing us to use the HGE-20 cell line for this work.
REFERENCES
- 1.Amieva MR, Vogelmann R, Covacci A, Tompkins LS, Nelson WJ, Falkow S. Disruption of the epithelial apical-junctional complex by Helicobacter pylori CagA. Science 300: 1430–1434, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson JM, Van Itallie CM. Physiology and function of the tight junction. Cold Spring Harbor Perspect Biol 1: a002584, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Athmann C, Zeng N, Kang T, Marcus EA, Scott DR, Rektorschek M, Buhmann A, Melchers K, Sachs G. Local pH elevation mediated by the intrabacterial urease of Helicobacter pylori cocultured with gastric cells. J Clin Invest 106: 339–347, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ballard ST, Hunter JH, Taylor AE. Regulation of tight-junction permeability during nutrient absorption across the intestinal epithelium. Annu Rev Nutr 15: 35–55, 1995 [DOI] [PubMed] [Google Scholar]
- 5.Basque JR, Chenard M, Chailler P, Menard D. Gastric cancer cell lines as models to study human digestive functions. J Cell Biochem 81: 241–251, 2001 [PubMed] [Google Scholar]
- 6.Baumgartner HK, Montrose MH. Regulated alkali secretion acts in tandem with unstirred layers to regulate mouse gastric surface pH. Gastroenterology 126: 774–783, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Bellocq A, Suberville S, Philippe C, Bertrand F, Perez J, Fouqueray B, Cherqui G, Baud L. Low environmental pH is responsible for the induction of nitric-oxide synthase in macrophages. Evidence for involvement of nuclear factor-κB activation. J Biol Chem 273: 5086–5092, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Bland DA, Suarez G, Beswick EJ, Sierra JC, Reyes VE. H. pylori receptor MHC class II contributes to the dynamic gastric epithelial apoptotic response. World J Gastroenterol 12: 4689–4693, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blaser MJ. Hypotheses on the pathogenesis and natural history of Helicobacter pylori-induced inflammation. Gastroenterology 102: 720–727, 1992 [DOI] [PubMed] [Google Scholar]
- 10.Booth IR. Regulation of cytoplasmic pH in bacteria. Microbiol Rev 49: 359–378, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chailler P, Menard D. Establishment of human gastric epithelial (HGE) cell lines exhibiting barrier function, progenitor, and prezymogenic characteristics. J Cell Physiol 202: 263–274, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Chen MC, Chang A, Buhl T, Tanner M, Soll AH. Apical acidification induces paracellular injury in canine gastric mucosal monolayers. Am J Physiol Gastrointest Liver Physiol 267: G1012–G1020, 1994 [DOI] [PubMed] [Google Scholar]
- 13.Chen MC, Solomon TE, Kui R, Soll AH. Apical EGF receptors regulate epithelial barrier to gastric acid: endogenous TGF-α is an essential facilitator. Am J Physiol Gastrointest Liver Physiol 283: G1098–G1106, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Choi IJ, Fujimoto S, Yamauchi K, Graham DY, Yamaoka Y. Helicobacter pylori environmental interactions: effect of acidic conditions on H. pylori-induced gastric mucosal interleukin-8 production. Cell Microbiol 9: 2457–2469, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Code CF. Defense mechanisms of the gastric mucosa. Scand J Gastroenterol Suppl 67: 201–204, 1981 [PubMed] [Google Scholar]
- 16.Conlin VS, Curtis SB, Zhao Y, Moore ED, Smith VC, Meloche RM, Finlay BB, Buchan AM. Helicobacter pylori infection targets adherens junction regulatory proteins and results in increased rates of migration in human gastric epithelial cells. Infect Immun 72: 5181–5192, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crabtree JE, Farmery SM, Lindley IJ, Figura N, Peichl P, Tompkins DS. CagA/cytotoxic strains of Helicobacter pylori and interleukin-8 in gastric epithelial cell lines. J Clin Pathol 47: 945–950, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Vries AC, van Grieken NC, Looman CW, Casparie MK, de Vries E, Meijer GA, Kuipers EJ. Gastric cancer risk in patients with premalignant gastric lesions: a nationwide cohort study in the Netherlands. Gastroenterology 134: 945–952, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Diamond JM, Machen TE. Impedance analysis in epithelia and the problem of gastric acid secretion. J Membr Biol 72: 17–41, 1983 [DOI] [PubMed] [Google Scholar]
- 20.El-Omar EM, Oien K, El-Nujumi A, Gillen D, Wirz A, Dahill S, Williams C, Ardill JE, McColl KE. Helicobacter pylori infection and chronic gastric acid hyposecretion. Gastroenterology 113: 15–24, 1997 [DOI] [PubMed] [Google Scholar]
- 21.Ernst PB, Gold BD. The disease spectrum of Helicobacter pylori: the immunopathogenesis of gastroduodenal ulcer and gastric cancer. Annu Rev Microbiol 54: 615–640, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Evans DJ, Jr, Evans DG. Helicobacter pylori CagA: analysis of sequence diversity in relation to phosphorylation motifs and implications for the role of CagA as a virulence factor. Helicobacter 6: 187–198, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Fanning AS, Van Itallie CM, Anderson JM. Zonula occludens-1 and -2 regulate apical cell structure and the zonula adherens cytoskeleton in polarized epithelia. Mol Biol Cell 23: 577–590, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farre R, van Malenstein H, De Vos R, Geboes K, Depoortere I, Vanden Berghe P, Fornari F, Blondeau K, Mertens V, Tack J, Sifrim D. Short exposure of oesophageal mucosa to bile acids, both in acidic and weakly acidic conditions, can impair mucosal integrity and provoke dilated intercellular spaces. Gut 57: 1366–1374, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127: 2893–2917, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Gnad T, Feoktistova M, Leverkus M, Lendeckel U, Naumann M. Helicobacter pylori-induced activation of β-catenin involves low density lipoprotein receptor-related protein 6 and Dishevelled. Mol Cancer 9: 31, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris TJ, Tepass U. Adherens junctions: from molecules to morphogenesis. Nat Rev Mol Cell Biol 11: 502–514, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Hartsock A, Nelson WJ. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta 1778: 660–669, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hazell SL, Lee A, Brady L, Hennessy W. Campylobacter pyloridis and gastritis: association with intercellular spaces and adaptation to an environment of mucus as important factors in colonization of the gastric epithelium. J Infect Dis 153: 658–663, 1986 [DOI] [PubMed] [Google Scholar]
- 30.Henriksnas J, Phillipson M, Storm M, Engstrand L, Soleimani M, Holm L. Impaired mucus-bicarbonate barrier in Helicobacter pylori-infected mice. Am J Physiol Gastrointest Liver Physiol 291: G396–G403, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Heuermann D, Haas R. A stable shuttle vector system for efficient genetic complementation of Helicobacter pylori strains by transformation and conjugation. Mol Gen Genet 257: 519–528, 1998 [DOI] [PubMed] [Google Scholar]
- 32.Ji J, Chen X, Leung SY, Chi JT, Chu KM, Yuen ST, Li R, Chan AS, Li J, Dunphy N, So S. Comprehensive analysis of the gene expression profiles in human gastric cancer cell lines. Oncogene 21: 6549–6556, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Jung YJ, Lee KL, Kim BK, Kim JW, Jeong JB, Kim SG, Kim JS, Jung HC, Song IS. [Usefulness of NCI-N87 cell lines in Helicobacter pylori infected gastric mucosa model]. Korean J Gastroenterol 47: 357–362, 2006 [PubMed] [Google Scholar]
- 34.Kim N, Marcus EA, Wen Y, Weeks DL, Scott DR, Jung HC, Song IS, Sachs G. Genes of Helicobacter pylori regulated by attachment to AGS cells. Infect Immun 72: 2358–2368, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kishimoto T, Akira S, Narazaki M, Taga T. Interleukin-6 family of cytokines and gp130. Blood 86: 1243–1254, 1995 [PubMed] [Google Scholar]
- 36.Krueger S, Kuester D, Bernhardt A, Wex T, Roessner A. Regulation of cathepsin X overexpression in H. pylori-infected gastric epithelial cells and macrophages. J Pathol 217: 581–588, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Lehours P, Yilmaz O. Epidemiology of Helicobacter pylori infection. Helicobacter 12 Suppl 1: 1–3, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Lundell L, Havu N, Miettinen P, Myrvold HE, Wallin L, Julkunen R, Levander K, Hatlebakk JG, Liedman B, Lamm M, Malm A, Walan A. Changes of gastric mucosal architecture during long-term omeprazole therapy: results of a randomized clinical trial. Aliment Pharmacol Ther 23: 639–647, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Lytton SD, Fischer W, Nagel W, Haas R, Beck FX. Production of ammonium by Helicobacter pylori mediates occludin processing and disruption of tight junctions in Caco-2 cells. Microbiology 151: 3267–3276, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Ma Z, Gong S, Richard H, Tucker DL, Conway T, Foster JW. GadE (YhiE) activates glutamate decarboxylase-dependent acid resistance in Escherichia coli K-12. Mol Microbiol 49: 1309–1320, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Marcus EA, Inatomi N, Nagami GT, Sachs G, Scott DR. The effects of varying acidity on Helicobacter pylori growth and the bactericidal efficacy of ampicillin. Aliment Pharmacol Ther 36: 972–979, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marcus EA, Moshfegh AP, Sachs G, Scott DR. The periplasmic α-carbonic anhydrase activity of Helicobacter pylori is essential for acid acclimation. J Bacteriol 187: 729–738, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marcus EA, Sachs G, Wen Y, Feng J, Scott DR. Role of the Helicobacter pylori sensor kinase ArsS in protein trafficking and acid acclimation. J Bacteriol 194: 5545–5551, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1: 1311–1315, 1984 [DOI] [PubMed] [Google Scholar]
- 45.Merrell DS, Goodrich ML, Otto G, Tompkins LS, Falkow S. pH-regulated gene expression of the gastric pathogen Helicobacter pylori. Infect Immun 71: 3529–3539, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meyer-Rosberg K, Scott DR, Rex D, Melchers K, Sachs G. The effect of environmental pH on the proton motive force of Helicobacter pylori. Gastroenterology 111: 886–900, 1996 [DOI] [PubMed] [Google Scholar]
- 47.Nomura A, Stemmermann GN, Chyou PH, Kato I, Perez-Perez GI, Blaser MJ. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N Engl J Med 325: 1132–1136, 1991 [DOI] [PubMed] [Google Scholar]
- 48.Orbach E, Finkelstein A. The nonelectrolyte permeability of planar lipid bilayer membranes. J Gen Physiol 75: 427–436, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Padan E, Zilberstein D, Schuldiner S. pH homeostasis in bacteria. Biochim Biophys Acta 650: 151–166, 1981 [DOI] [PubMed] [Google Scholar]
- 50.Papini E, Satin B, Norais N, de Bernard M, Telford JL, Rappuoli R, Montecucco C. Selective increase of the permeability of polarized epithelial cell monolayers by Helicobacter pylori vacuolating toxin. J Clin Invest 102: 813–820, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parsonnet J. Gastric adenocarcinoma and Helicobacter pylori infection. West J Med 161: 60, 1994 [PMC free article] [PubMed] [Google Scholar]
- 52.Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, Sibley RK. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med 325: 1127–1131, 1991 [DOI] [PubMed] [Google Scholar]
- 53.Quaglia NC, Normanno G, Dambrosio A, Celano GV, Parisi A, Firinu A, Buonavoglia C. Multiplex-touchdown PCR assay for the detection and genotyping of Helicobacter pylori from artificially contaminated sheep milk. J Food Protect 68: 2136–2139, 2005 [DOI] [PubMed] [Google Scholar]
- 54.Ramesh S, Nash J, McCulloch PG. Reduction in membranous expression of β-catenin and increased cytoplasmic E-cadherin expression predict poor survival in gastric cancer. Br J Cancer 81: 1392–1397, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Remick DG. Interleukin-8. Crit Care Med 33: S466–467, 2005 [DOI] [PubMed] [Google Scholar]
- 56.Schirrmeister W, Gnad T, Wex T, Higashiyama S, Wolke C, Naumann M, Lendeckel U. Ectodomain shedding of E-cadherin and c-Met is induced by Helicobacter pylori infection. Exp Cell Res 315: 3500–3508, 2009 [DOI] [PubMed] [Google Scholar]
- 57.Scott DR, Marcus EA, Weeks DL, Lee A, Melchers K, Sachs G. Expression of the Helicobacter pylori ureI gene is required for acidic pH activation of cytoplasmic urease. Infect Immun 68: 470–477, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scott DR, Marcus EA, Wen Y, Oh J, Sachs G. Gene expression in vivo shows that Helicobacter pylori colonizes an acidic niche on the gastric surface. Proc Natl Acad Sci USA 104: 7235–7240, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scott DR, Marcus EA, Wen Y, Singh S, Feng J, Sachs G. Cytoplasmic histidine kinase (HP0244)-regulated assembly of urease with UreI, a channel for urea and its metabolites, CO2, NH3, and NH4+, is necessary for acid survival of Helicobacter pylori. J Bacteriol 192: 94–103, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sekiguchi H, Irie K, Murakami A. Suppression of CD74 expression and Helicobacter pylori adhesion by auraptene targeting serum starvation-activated ERK1/2 in NCI-N87 gastric carcinoma cells. Biosci Biotechnol Biochem 74: 1018–1024, 2010 [DOI] [PubMed] [Google Scholar]
- 61.Sidhaye VK, Chau E, Breysse PN, King LS. Septin-2 mediates airway epithelial barrier function in physiologic and pathologic conditions. Am J Respir Cell Mol Biol 45: 120–126, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Singh A, Panting RJ, Varma A, Saijo T, Waldron KJ, Jong A, Ngamskulrungroj P, Chang YC, Rutherford JC, Kwon-Chung KJ. Factors required for activation of urease as a virulence determinant in Cryptococcus neoformans. mBio 4: e00220–00213, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Steed E, Balda MS, Matter K. Dynamics and functions of tight junctions. Trends Cell Biol 20: 142–149, 2010 [DOI] [PubMed] [Google Scholar]
- 64.Steer HW. Surface morphology of the gastroduodenal mucosa in duodenal ulceration. Gut 25: 1203–1210, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med 347: 1175–1186, 2002 [DOI] [PubMed] [Google Scholar]
- 66.Teyssen S, Chari ST, Scheid J, Singer MV. Effect of repeated boluses of intravenous omeprazole and primed infusions of ranitidine on 24-hour intragastric pH in healthy human subjects. Dig Dis Sci 40: 247–255, 1995 [DOI] [PubMed] [Google Scholar]
- 67.Tokhtaeva E, Sachs G, Souda P, Bassilian S, Whitelegge JP, Shoshani L, Vagin O. Epithelial junctions depend on intercellular trans-interactions between the Na,K-ATPase β1 subunits. J Biol Chem 286: 25801–25812, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tytgat GN. Etiopathogenetic principles and peptic ulcer disease classification. Dig Dis 29: 454–458, 2011 [DOI] [PubMed] [Google Scholar]
- 69.Vagin O, Tokhtaeva E, Yakubov I, Shevchenko E, Sachs G. Inverse correlation between the extent of N-glycan branching and intercellular adhesion in epithelia. Contribution of the Na,K-ATPase β1 subunit. J Biol Chem 283: 2192–2202, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Voland P, Weeks DL, Marcus EA, Prinz C, Sachs G, Scott D. Interactions among the seven Helicobacter pylori proteins encoded by the urease gene cluster. Am J Physiol Gastrointest Liver Physiol 284: G96–G106, 2003 [DOI] [PubMed] [Google Scholar]
- 71.Weeks DL, Eskandari S, Scott DR, Sachs G. A H+-gated urea channel: the link between Helicobacter pylori urease and gastric colonization. Science 287: 482–485, 2000 [DOI] [PubMed] [Google Scholar]
- 72.Wen Y, Marcus EA, Matrubutham U, Gleeson MA, Scott DR, Sachs G. Acid-adaptive genes of Helicobacter pylori. Infect Immun 71: 5921–5939, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wroblewski LE, Shen L, Ogden S, Romero-Gallo J, Lapierre LA, Israel DA, Turner JR, Peek RM., Jr Helicobacter pylori dysregulation of gastric epithelial tight junctions by urease-mediated myosin II activation. Gastroenterology 136: 236–246, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yao Z, Fenoglio S, Gao DC, Camiolo M, Stiles B, Lindsted T, Schlederer M, Johns C, Altorki N, Mittal V, Kenner L, Sordella R. TGF-β IL-6 axis mediates selective and adaptive mechanisms of resistance to molecular targeted therapy in lung cancer. Proc Natl Acad Sci USA 107: 15535–15540, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]