Abstract
Chronic inflammation and enteric infections are frequently associated with epithelial Na+/H+ exchange (NHE) inhibition. Alterations in electrolyte transport and in mucosal pH associated with inflammation may represent a key mechanism leading to changes in the intestinal microbial composition. NHE3 expression is essential for the maintenance of the epithelial barrier function. NHE3−/− mice develop spontaneous distal chronic colitis and are highly susceptible to dextran sulfate (DSS)-induced mucosal injury. Spontaneous colitis is reduced with broad-spectrum antibiotics treatment, thus highlighting the importance of the microbiota composition in NHE3 deficiency-mediated colitis. We herein characterized the colonic microbiome of wild-type (WT) and NHE3−/− mice housed in a conventional environment using 454 pyrosequencing. We demonstrated a significant decrease in the phylogenetic diversity of the luminal and mucosal microbiota of conventional NHE3−/− mice compared with WT. Rederivation of NHE3−/− mice from conventional to a barrier facility eliminated the signs of colitis and decreased DSS susceptibility. Reintroduction of the conventional microflora into WT and NHE3−/− mice from the barrier facility resulted in the restoration of the symptoms initially described in the conventional environment. Interestingly, qPCR analysis of the microbiota composition in mice kept in the barrier facility compared with reconventionalized mice showed a significant reduction of Clostridia classes IV and XIVa. Therefore, the gut microbiome plays a prominent role in the pathogenesis of colitis in NHE3−/− mice, and, reciprocally, NHE3 also plays a critical role in shaping the gut microbiota. NHE3 deficiency may be a critical contributor to dysbiosis observed in patients with inflammatory bowel disease.
Keywords: slc9a3, bacterial diversity, colonic microbiota, high-throughput sequencing, mucosal immunology
commensal intestinal microbiota is essential for the normal development and functions of the intestine and participates in the regulation of the intestinal development, maturation and education of the immune system, maintenance of intestinal pH, and metabolism of xenobiotics and hormones (30). The intestinal microbiome of healthy individuals is dominated by four major bacterial phyla: Firmicutes, Bacteroidetes, and to a lesser degree Proteobacteria and Actinobacteria. Investigation of the microbiota in patients with inflammatory bowel diseases (IBD) is a growing field and has already generated exciting, though not always consistent, insights. Crohn's disease (CD) and ulcerative colitis (UC) occur preferentially in the gastrointestinal tract areas, which achieve the highest intestinal bacterial concentration, and genetically susceptible germ-free mice do not spontaneously develop colitis (22). Reduced biodiversity (18), a lower proportion of Firmicutes (including clostridia of groups IV and XIVa, the main butyrate-producing bacteria in the gut), and an increase in Bacteroidetes, Proteobacteria, and Actinobacteria (7, 26) have been particularly consistent observations among patients with IBD.
However, despite the recent explosion of the field of gut microbial ecology thanks to novel genomic and metabolomic tools, the causative relationship between intestinal inflammation and altered gut microbiota is not entirely clear. Chronic idiopathic inflammatory conditions and enteric infections are frequently associated with inhibition of intestinal brush border electroneutral Na+/H+ exchange (NHE). This intestinal transport mechanism, mediated primarily by NHE3, plays a critical role in the intestinal sodium and water absorption, regulation of intracellular pH, as well as the acidity of the apical microenvironment. NHE3 expression and/or activity is inhibited by proinflammatory cytokines (3, 19) and enteropathogenic bacteria (9), whereas commensal Lactobacillus acidophilus upregulates intestinal NHE3 expression and function (24). Inhibition of NHE3 expression and/or activity has been described in several experimental models of colitis, including IL-2−/− mice (3), IL-10−/− mice (17), and in dextran sodium sulfate (DSS)- and trinitrobenzene sulfonic acid-induced colitis (27). Sullivan et al. (27) showed that NHE3 was downregulated in sigmoid mucosal biopsies from most cases of active UC and/or CD, in ileal mucosal biopsies of active CD, as well as in ∼50% of sigmoid biopsies from inactive UC or CD. In a study by Siddique et al. (23), NHE3 protein and activity were reduced in both the untreated and treated patients with CD and UC. NHE3 mRNA was reduced only in CD but not in patients with UC. Two recent studies by Yeruva et al. (34) and Farkas et al. (6) demonstrated significant inhibition of NHE3 activity in patients with UC despite preserved protein expression and cellular localization.
The loss of NHE3 activity and mucosal pH regulation secondary to inflammation may represent the key mechanism leading to the altered microbial ecology and potentially affect the extent and outcome of intestinal inflammation. In agreement with those clinical observations, our group described the role of NHE3 in the modulation of inflammatory processes and the maintenance of mucosal integrity. NHE3-deficient mice spontaneously develop colitis (16) and are highly susceptible to DSS-induced intestinal injury (12). More recently, we demonstrated the importance of NHE3 in the maintenance of intestinal barrier integrity and in modulating the inflammatory process in IL-10-deficient mice (15). Our studies suggest that NHE3 participates in mucosal responses to epithelial damage, acting as a modifier gene determining the extent of the inflammatory responses in the face of intestinal injury. More importantly, spontaneous colitis developed by NHE3−/− mice was associated with enhanced bacteria adhesion and translocation in the distal colon (11, 16), which could be significantly reduced by oral treatment with broad-spectrum antibiotics (16), thus highlighting the importance of microbiota in the pathogenesis of inflammation precipitated by the loss of NHE3 activity.
Based on the presented findings, we hypothesized that loss of NHE3 activity leads to changes in gut microbial ecology, which precipitates the development of distal colitis. To this end, we characterized colonic microbiota of NHE3−/− mice and their wild-type (WT) littermates housed in conventional environment by 454 pyrosequencing of 16S rRNA gene fragment libraries and demonstrated significant changes, in many respects resembling those described in patients with IBD. We also rederived NHE3−/− mice through embryo transfer into an ultraclean barrier facility (barrier) and demonstrated significantly reduced symptoms of colitis and reduced susceptibility to DSS. Reintroduction of the conventional microflora into WT and NHE3−/− mice from the barrier facility resulted in the restoration of the symptoms initially described in the conventional environment, distal colitis and increased susceptibility to DSS. Clostridia clusters IV and XIVa and Firmicutes in general were significantly reduced in reconventionalized NHE3 knockout (KO) mice compared with WT, a phenomenon not observed in NHE3−/− mice housed in the barrier facility.
Our recent findings point to the critical role of NHE3 in mucosal homeostasis and imply that the loss of NHE3 activity contributes to dysbiosis, therefore regulating the ultimate degree of inflammation, disease progression, and outcome in IBD.
MATERIALS AND METHODS
Experimental animals.
Slc9a3-deficient mice (NHE3−/−) were obtained from Dr. Gary E. Shull and were maintained on the original mixed (129/Black Swiss) genetic background. Mice were bred as heterozygotes and NHE3+/+, and NHE3−/− littermates were used in the study at 6–8 wk of age. NHE3 colony was established by embryo transfer and maintained in a Helicobater sp-free barrier facility at the University of Arizona Bio-5 Institute. All mice used were from colonies confirmed by PCR to be murine norovirus (MNV)-free.
Mice from the barrier facility were reintroduced to the conventional facility and colonized via oral gavage with fecal microbiota from Helicobacter sp.-positive sentinel WT mice. Briefly, at 3 wk of age, mice received fecal contents from donor BALB/c sentinel mice and were transferred to cages containing soiled bedding from sentinel mice. Fecal pellets were solubilized in sterile phosphate-buffered saline (PBS) and administered to each recipient mouse by oral gavage (25 μl/mice). This procedure was repeated three times during a 3-wk period. Although donor mice were Helicobacter sp. positive, all WT and NHE3−/− mice included in this study were individually identified as Helicobacter sp. negative, using qPCR analysis of fecal samples. In all three environments—conventional, barrier, and reconventionalized—water (autoclaved) and irradiated food were provided ad libitum. Breeders (heterozygous) were maintained on the Teklad Global 2019 (19% protein extruded) diet, and experimental mice were maintained on the NIH-31 modified open formula mouse/rat Diet (7013). Mice are maintained in individually ventilated cages changed biweekly. The barrier facility used in this study provides a nonsterile environment with highly controlled procedures, including sterilization of all supplies and equipment entering the barrier and personnel working in barrier facilities required to scrub their hands, shower before entry, and wear gloves and sterilized clothing, including scrubs, shoes, caps, and masks. Personnel working in the conventional facility were required to wear lab coats, caps, and masks.
Sentinel mice were routinely monitored and determined as free from common murine pathogens (MHV, MPV, MVM, TMEV, Mycoplasma pulmonis, Sendai, EDIM, MNV, ectoparasites, and endoparasites). All animal protocols and procedures were approved by the University of Arizona Animal Care and Use Committee.
Colonic microbiota analysis by pyrosequencing.
16S rRNA gene fragment amplicon libraries from V3-V4 region were created from DNA isolated from fecal pellets removed from distal colon and mucosal scrapings obtained at the time of death. WT and NHE3−/− mice used for microbiota analysis were derived from three litters of two-breeding, heterozygous pairs. Before the scraping of the mucosa, distal colons were opened lengthwise and washed individually in sterile ice-cold (pH 7.4) three times for 10 min. DNA was isolated using FAST DNA SPIN Kit for Soil (MP Biomedicals) and diluted to 10 ng/μl. Amplification was performed with 357F (5′ CCT ACG GGA GGC AGC AG 3′) (14, 31) primer and a modified 786R (5′ AC CAG GGT ATC TAA WCC 3′) primer. The forward primer carried the A pyrosequencing adaptor and a MID sequence, whereas the reverse one was extended with the B adaptor sequence. The reaction mix contained (per 20 μl): 1 ng of template DNA, 10 pmol primer, 2 μl of 2 mM dNTP, 1.5 mM MgCl2, 0.2 U of Taq polymerase, and the appropriate 1× buffer (Fermentas). The cycling conditions were as follows: 95°C for 5 min; 30 cycles of 95°C, 52°C, 72°C (30 s each step); and finally 72°C for 2 min. The reactions were carried out in MasterCycler thermocycler. Eight independent reactions were performed for each sample. The amplicons were gel purified and DNA quantified spectrophotometrically using NanoDrop ND-1000. Purified PCR products were pooled in equimolar amounts and sequenced on GS-FLX machine with the use of Titanium chemistry (Roche).
Bioinformatic and statistical analyses were performed using MOTHUR v. 1.20.3 and custom Perl and Bash scripts on a 24-core, 256 GB RAM machine running Fedora 14 operating system. Briefly, reads were divided into individual samples basing on the MID sequences, quality trimmed, dereplicated, aligned against SILVA template alignment, and screened for those covering the desired region of the alignment. Chimeras were then identified with UCHIME algorithm (5) and removed. High-quality, nonchimeric sequences from all samples were placed in one file, aligned again, and screened, and gap-only and terminal gap-containing columns were filtered out of the alignment. Noise removal via single-linkage preclustering (10) was the final step of sequence preparation. Distance matrix was then calculated based on the final alignment, and operational taxonomic units (OTUs) were constructed via average neighbor clustering. Taxonomic position was assigned to degapped final reads with naïve Bayesian classifier trained on a subset of SILVA 16S rRNA gene sequences database. Rarefaction curves were calculated based on constructed OTUs, whereas species richness was assessed with the Abundance Coverage Estimator (ACE index) (4). Significance of species richness differences between WT and NHE3−/− mice was assessed with Student's t-test.
DSS-induced mucosal injury model.
Six- to eight-week-old male KO mice on mixed background and their age-matched WT littermates were left untreated or given drinking water supplemented with 4% DSS (USB) for 7 days. Mortality and body weight were monitored daily.
Histology and scoring.
Proximal and distal colons from WT and NHE3−/− mice were harvested and fixed in 10% neutral buffered formalin (Fisher Scientific, Tustin, CA). Fixed tissues were then embedded in paraffin, and 5-μm-thick tissue cuts were stained with hematoxylin and eosin (H&E) for light microscopic examination. Sections were graded by a veterinary pathologist blinded to the study design according to previously published criteria (13).
Real-time RT-PCR.
Real-time RT-PCR was used to evaluate mucosal expression of intracellular nitric oxide synthase (iNOS), tumor necrosis factor (TNF), interleukin (IL)-1β, interferon (IFN)-γ, and matrix metalloproteinase (MMP)-8 mRNA. Total RNA was isolated from mouse distal colon using TRIzol reagent (Invitrogen) or Qiagen mRNAeasy kit for the DSS-treated mice (Qiagen). A sample (250 ng) of total RNA was reverse transcribed using iScript cDNA synthesis kit (Bio-Rad). Subsequently, 20 μl of the PCR reactions were set up in 96-well plates containing 10 μl of 2× IQ Supermix (Bio-Rad), 1 μl TaqMan primer/probe set (ABI), 2 μl of the cDNA synthesis reaction (10% of RT reaction), and 7 μl of nuclease-free water. Reactions were run and analyzed on a Bio-Rad iCycler iQ real-time PCR detection system. Data were analyzed by using the comparative Ct method as means of relative quantification, normalized to an endogenous reference (GAPDH) and relative to a calibrator (normalized Ct value obtained from control mice), and expressed as 2−ΔΔCt (Applied Biosystems User Bulletin #2: Rev B “Relative Quantification of Gene Expression”).
Real-time PCR microbiota analysis in barrier vs. reconventionalized mice.
DNA was extracted from stool samples as for pyrosequencing. Quantitative PCR was performed in sealed 96-well plates using a CFX96 thermal cycler (Bio-Rad) and analyzed using CFX Manager (Bio-Rad). Each reaction included 2× PerfeCta SYBR Green FastMix (Quanta Biotech) or phylum- or class-specific primer pairs at 0.2 μM (2) (Table 1) and 10 ng DNA template in a final volume of 20 μl. Optimal annealing temperature for each primer pair was determined using a temperature gradient (Table 1). The following PCR protocol was run: 95°C (30 s), then 95°C (5 s), variable annealing temperature (15 s), and 72°C (10 s) for 30 cycles. Each sample was run in duplicate, and the mean Ct value was used to calculate ΔΔCt values. P values were calculated using the two-tailed Student's t-test.
Table 1.
Primer sequences for real-time PCR analysis
| Target Group | Primer Name | Forward Primer | Annealing Temperature, °C |
|---|---|---|---|
| Universal | 926F | AAACTCAAAKGAATTGACGG | 59 |
| 1062R | CTCACRRCACGAGCTGAC | ||
| Bacteroidetes | 798cfbF | CRAACAGGATTAGATACCCT | 55.7 |
| Cfb967R | GGTAAGGTTCTTCGCGTAT | ||
| Firmicutes | 928F-Firm | TGAAACTYAAAGGAATTGACG | 55.7 |
| 1040FirmR | ACCATGCACCACCTGTC | ||
| Clostridium leptum (IV) | CL-IV-F | CCTTCCGTGCCGSAGTTA | 52.8 |
| CL-IV-R | GAATTAAACCACATACTCCACTGCTT | ||
| Clostridium coccoides (XIVa) | CL-XIVa-F | AAATGACGGTACCTGACTAA | 57.0 |
| CL-XIVa-R | CTTTGAGTTTCATTCTTGCGAA | ||
| Actinobacteria | Act920F3 | TACGGCCGCAAGGCTA | 52.8 |
| Act1200R | TCRTCCCCACCTTCCTCCG | ||
| α-Proteobacteria | A682F | CIAGTGTAGAGGTGAAATT | 52.8 |
| 908aR | CCCCGTCAATTCCTTTGAGTT | ||
| γ-Proteobacteria | 1080 gF | TCGTCAGCTCGTGTYGTGA | 52.8 |
| G1202R | CGTAAGGGCCATGATG |
Primer sequences for real-time PCR analysis of colonic microbiota were adapted from Bacchetti De Gregoris et al. (2). Annealing temperatures were optimized individually for each primer set based on gradient PCR and melting curve analysis.
Statistical analysis.
Statistical significance (for nonsequencing data) was determined by the analysis of variance (ANOVA) followed by Fisher's protected least significant difference post hoc test with StatView software package v.4.53 (SAS Institute). Data are expressed as means ± SE.
RESULTS
Luminal and mucosal microbiota of conventional NHE3−/− mice: changes in colonic microbial ecology.
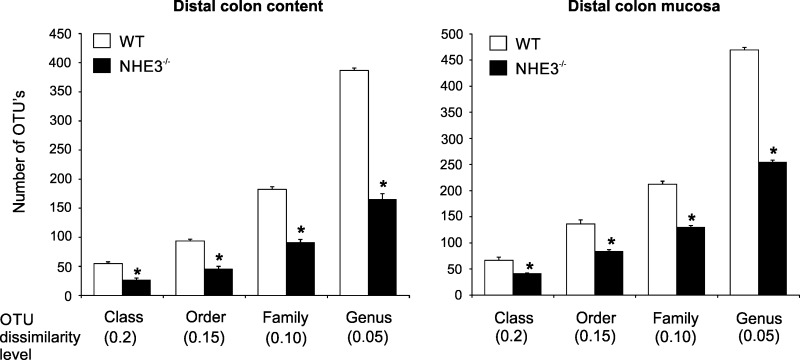
Community profiling of the adherent (mucosal) and luminal (fecal) microbiota in WT mice and NHE3−/− littermates was performed with 454-based 16S rRNA amplicon library sequencing. Species richness assessed with the ACE index was significantly different among WT and NHE3−/− mice in the colonic lumen as well as among the adherent mucosal microbiota at the level of class, order, family, and genus (Fig. 1), with phylogenetic diversity consistently reduced in NHE3-deficient mice compared with WT.
Fig. 1.
Intestinal microbiota in conventional wild-type (WT) and Na+/H+ exchanger 3 (NHE3) knockout (KO) mice. Differences in phylogenetic diversity between microbial luminal and mucosal (adherent) populations in WT and NHE3−/− mice at varying operational taxonomic unit (OTU) dissimilarity levels (species through classes) in the distal colon [means + SE and Student's t-test P values are presented; *difference between WT (n = 4) and NHE3 KO (n = 6) groups].
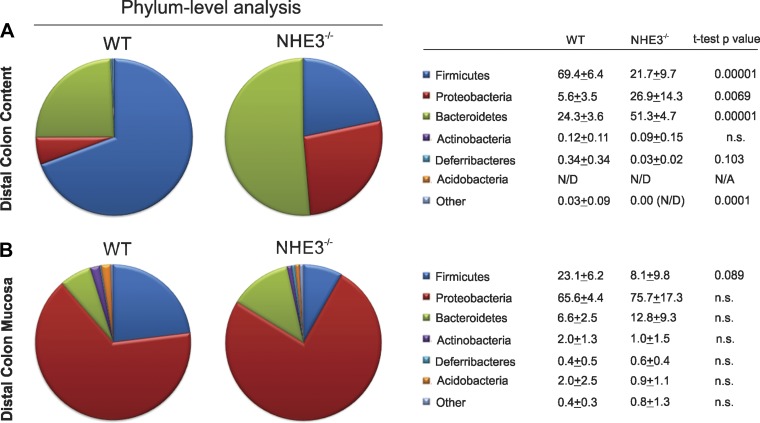
Significant shifts in the major microbial taxonomic groups were seen among both fecal and mucosal bacteria. Phylum-level analysis indicated significant reduction in Firmicutes with concomitant expansion of Proteobacteria and Bacteriodetes phyla in the colonic fecal content of NHE3−/− mice (Fig. 2A). Deferribacteres phylum also expanded in the colonic content of NHE3−/− mice although without reaching statistical significance (P = 0.103; Fig. 2A). Changes among the mucosal microbiota were less pronounced, with most significant decrease in Firmicutes (P = 0.089; Fig. 2B).
Fig. 2.
Phylum and class analysis in conventional WT and NHE3 KO. Distribution of the major phyla in the colonic content (A) and mucosa (B), and classes in WT and NHE3−/− mice house in a conventional facility [means + SE and Student's t-test P values are presented; *difference between WT (n = 4) and NHE3 KO (n = 6) groups].
Genus-level analysis of fecal microbiota showed significant changes among the three most affected phyla: Firmicutes (Marvinbryantia, Anaerotruncus, Oscillibacter, Coprococcus, Moryella, Dorea, Proteocatella, Oribacterium Acetivibrio, Lactonifactor, Coprobacillus, Lactobacillus, Roseburia, Sporacetigenium, and Acetitomaculum), Proteobacteria (Parasutterella and Desulfovibrio), and Bacteroidetes (Parabacteroides, Bacteroides, Haliscomenobacter, Parasegetibacter, Anaerophaga). More detailed list with relative contribution of the genera sorted by the P value is presented in Table 2.
Table 2.
Selected differentially displayed genera in the distal colonic content of WT and NHE3−/− mice
| WT |
NHE3−/− |
t-Test |
||||
|---|---|---|---|---|---|---|
| Phylum | Genus | Mean, % | SE | Mean, % | SE | P Value |
| Firmicutes | Marvinbryantia | 0.51 | 0.07 | 0.02 | 0.01 | 0.000 |
| Anaerotruncus | 0.63 | 0.09 | 0.04 | 0.02 | 0.000 | |
| Oscillibacter | 4.69 | 0.85 | 0.50 | 0.17 | 0.001 | |
| Coprococcus | 16.54 | 2.73 | 3.11 | 0.52 | 0.001 | |
| Moryella | 0.83 | 0.12 | 0.18 | 0.07 | 0.002 | |
| Dorea | 1.28 | 0.22 | 0.19 | 0.07 | 0.002 | |
| Proteocatella | 4.15 | 1.02 | 0.21 | 0.12 | 0.006 | |
| Oribacterium | 13.52 | 3.76 | 0.12 | 0.05 | 0.008 | |
| Acetivibrio | 0.37 | 0.10 | 0.03 | 0.02 | 0.009 | |
| Lactonifactor | 0.19 | 0.05 | 0.00 | 0.00 | 0.010 | |
| Coprobacillus | 0.00 | 0.00 | 0.09 | 0.03 | 0.016 | |
| Lactobacillus | 17.19 | 4.14 | 4.81 | 1.26 | 0.023 | |
| Roseburia | 2.96 | 0.97 | 0.24 | 0.17 | 0.026 | |
| Sporacetigenium | 0.03 | 0.02 | 0.72 | 0.26 | 0.026 | |
| Acetitomaculum | 0.10 | 0.04 | 0.00 | 0.00 | 0.050 | |
| Sporobacter | 0.06 | 0.02 | 0.00 | 0.00 | 0.052 | |
| Butyricicoccus | 0.10 | 0.04 | 0.01 | 0.01 | 0.058 | |
| Butyrivibrio | 0.09 | 0.04 | 0.00 | 0.00 | 0.063 | |
| Peptococcus | 0.03 | 0.01 | 0.00 | 0.00 | 0.066 | |
| Enterococcus | 0.00 | 0.00 | 0.07 | 0.03 | 0.069 | |
| Anaerobacter | 0.73 | 0.27 | 4.55 | 1.96 | 0.081 | |
| Hespellia | 0.38 | 0.20 | 0.00 | 0.00 | 0.097 | |
| Proteobacteria | Parasutterella | 0.05 | 0.03 | 0.49 | 0.10 | 0.002 |
| Desulfovibrio | 0.05 | 0.01 | 0.00 | 0.00 | 0.013 | |
| Actinobacillus | 0.00 | 0.00 | 0.06 | 0.03 | 0.056 | |
| Bdellovibrio | 0.03 | 0.02 | 0.00 | 0.00 | 0.072 | |
| Helicobacter | 4.63 | 1.62 | 18.87 | 7.07 | 0.078 | |
| Thioreductor | 0.02 | 0.01 | 2.15 | 1.16 | 0.092 | |
| Bacteroidetes | Parabacteroides | 0.50 | 0.12 | 17.01 | 3.37 | 0.001 |
| Bacteroides | 1.50 | 0.30 | 11.10 | 2.30 | 0.003 | |
| Haliscomenobacter | 0.77 | 0.15 | 0.15 | 0.09 | 0.007 | |
| Parasegetibacter | 0.13 | 0.04 | 0.01 | 0.01 | 0.016 | |
| Anaerophaga | 1.84 | 0.66 | 4.94 | 1.02 | 0.031 | |
Genera were selected based on t-test P < 0.1 and sorted by increasing P value within each phylum. Mean and SE for each genotype is indicated (n = 4 for WT and n = 6 for NHE3−/− mice). NHE, Na+/H+ exchange.
Rederivation of NHE3−/− mice into barrier facility reduces the symptoms of distal colitis.
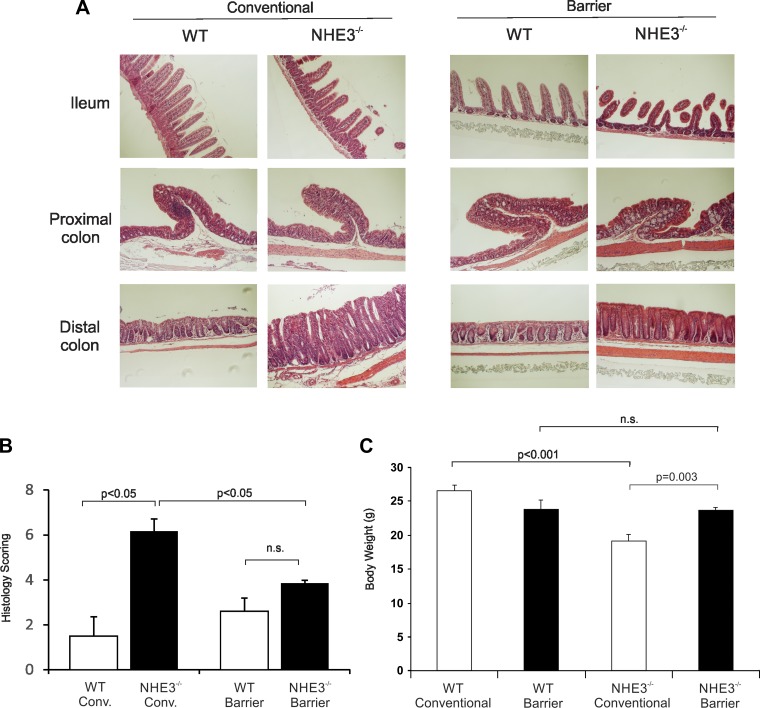
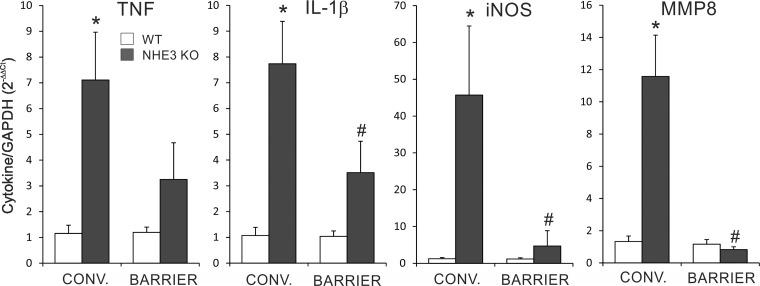
To determine the influence of the microbiota composition on spontaneous distal colitis in NHE3-deficient mice, we rederived NHE3+/− mice from a conventional facility into a barrier Helicobater sp-free facility through embryo transfer. Analysis of rederived WT and NHE3−/− mice housed in the barrier facility showed significant reduction of the histological signs of distal colitis in NHE3−/− mice compared with conventional NHE3-deficient mice, with a residual hyperplasia and reduced, but still noticeable, granulocytic infiltration (Fig. 3, A and B). Consistent with our previously published observations (16), there was a significant difference in body weight of WT and NHE3−/− mice housed in conventional environment, whereas no significant differences were observed among WT and NHE3−/− mice housed in the barrier facility (Fig. 3C). Mucosal mRNA expression of major proinflammatory markers TNF, IL-1β, iNOS, and MMP8 (the latter as a surrogate marker of neutrophil infiltration) was significantly reduced in NHE3−/− mice housed in the barrier facility compared with mice housed in the conventional facility (Fig. 4). Mucosal expression of IFN-γ, described originally in the small intestine of NHE3−/− mice by Woo et al. (33) and by our group (12), did not change in the distal colon of NHE3-deficient mice in either conventional or barrier environment (data not shown). Collectively, these results suggested, not only that NHE3 deficiency not only affected the colonic microbiota composition, but also that the intestinal microbiota modulates the severity of distal colitis in NHE3−/− mice in a manner similar to broad-spectrum antibiotics (16).
Fig. 3.
Evaluation of colitis in WT and NHE3 KO housed in the barrier facility. A: histological evaluation of ileum, proximal, and distal colon from WT and NHE3−/− mice housed in the conventional or barrier facility. Magnifications ×100. B: sections were graded by scoring based on the degree of lamina propria mononuclear cell infiltration, crypt hyperplasia, and architectural distortion by an unbiased pathologist. C: observed differences in body weight between WT and NHE3−/− mice housed in the conventional or barrier facility. Statistical analysis was performed with ANOVA followed by Fisher protected least significant difference (PLSD) post hoc test (conventionalized WT n = 22, NHE3−/− n = 19; barrier WT n = 4, NHE3−/− n = 15 all male).
Fig. 4.
Proinflammatory mRNA expression in WT and NHE3 KO housed in the conventional and ultra-clean facilities. RT-PCR analysis of tumor necrosis factor (TNF), interleukin (IL)-1β, inducible nitric oxide synthase (iNOS), and matrix metalloproteinase (MMP)8 expression in the distal colon of NHE3−/− mice and their WT littermates housed in conventional (CONV) and barrier facilities. *Differences between WT and NHE3 KO mice, #differences between NHE3 KO mice in conventional vs. barrier facilities. Statistical analysis was performed with ANOVA followed by Fisher PLSD post hoc test with P ≤ 0.05 considered significant (conventional, WT n = 6, NHE3−/− n = 9; barrier WT n = 6, NHE3−/− n = 12).
Restoration of spontaneous distal colitis in reconventionalized NHE3−/− mice.
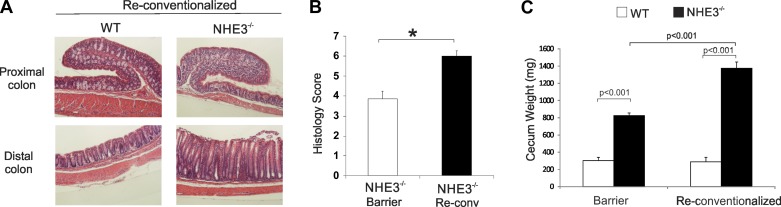
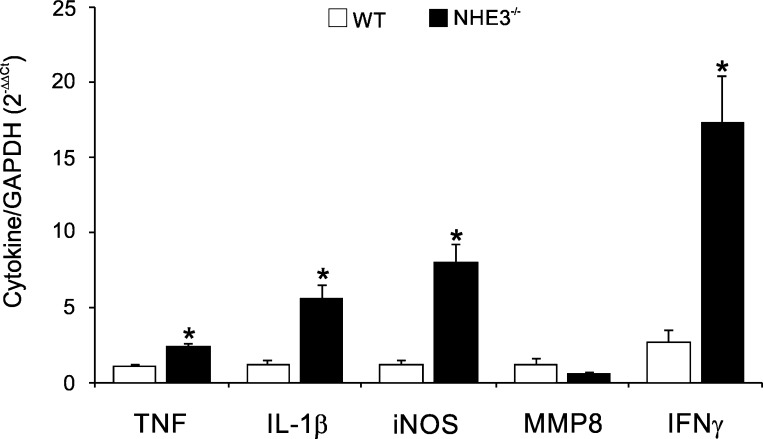
WT and NHE3−/− from the barrier facility were reintroduced to the conventional facility (reconventionalized) and colonized via oral gavage with the fecal microbiota from Helicobacter sp.-positive sentinel WT mice. Subsequent generations relied on maternal transmission of microbiota. For all subsequent generations, we did not detect Helicobacter sp. in the reconventionalized mice, thus indicating that Helicobacter failed to colonize the recipient mice. Despite this fact, colonic morphology from reconventionalized NHE3−/− mice showed restoration of distal colitis, with significant increase in the distal colon histological score, associated primarily by hyperplasia, leukocytic infiltration, and loss of goblet cells (Fig. 5, A and B). Cecum weight, an indicator of small intestinal absorptive defect, was significantly greater in reconventionalized NHE3−/− mice compared with the barrier-housed animals, suggesting that microbiota also modulate the intestinal Na+ and water-absorptive defect (Fig. 5C). Interestingly, compared with WT mice and with barrier-housed NHE3−/− mice, changes in histological appearance were not reflected in higher mucosal expression of MMP8, suggesting that increased neutrophilic infiltration was not the major driver of inflammation in reconventionalized NHE3−/− mice (Fig. 6). TNF, IL-1β, and iNOS were significantly elevated in the distal colon of the reconventionalized NHE3−/− mice although not significantly above the levels observed in the barrier facility (Figs. 4 and 6). Surprisingly, IFN-γ expression showed a significant increase in the distal colon of reconventionalized NHE3 KO mice compared with WT in the barrier environment (Fig. 6).
Fig. 5.
Evaluation of spontaneous colitis in barrier WT and NHE3 KO rederived in the conventional environment (reconventionalized). WT and NHE3 KO mice from the barrier facility were reintroduced to the conventional facility and colonized via oral gavage with fecal content from Helicobacter sp.-positive sentinel WT mice. Subsequent generations relied on maternal transmission of microbiota. A: hematoxylin and eosin (H&E) staining of proximal and distal colon of reconventionalized WT and NHE3−/− mice. B: histological analysis of colonic morphology in NHE3−/− mice housed in the barrier facility or rederived in the conventional environment, respectively. Sections were graded by scoring based on the degree of lamina propria mononuclear cell infiltration, crypt hyperplasia, and architectural distortion by an unbiased pathologist. C: cecum weight of barrier and reconventionalized WT and NHE3 KO mice. Statistical analysis was performed with ANOVA followed by Fisher's PLSD post hoc test with P ≤ 0.05 considered significant. *Significant difference between WT and NHE3 KO mice (barrier WT n = 20, NHE3−/− n = 17; reconventionalized WT n = 9, NHE3−/− n = 12).
Fig. 6.
Proinflammatory mRNA expression in reconventionalized WT and NHE3 KO. Real-time PCR analysis of TNF, IL-1β, iNOS, MMP8, and interferon (IFN)-γ mRNA expression in the distal colon of reconventionalized WT and NHE3 KO mice. Data were normalized to GAPDH mRNA as an internal control and calculated using ΔΔCt method. Statistical analysis was performed with ANOVA followed by Fisher's PLSD post hoc test with P ≤ 0.05 considered significant (reconventionalized WT n = 5 and NHE3−/− n = 7).
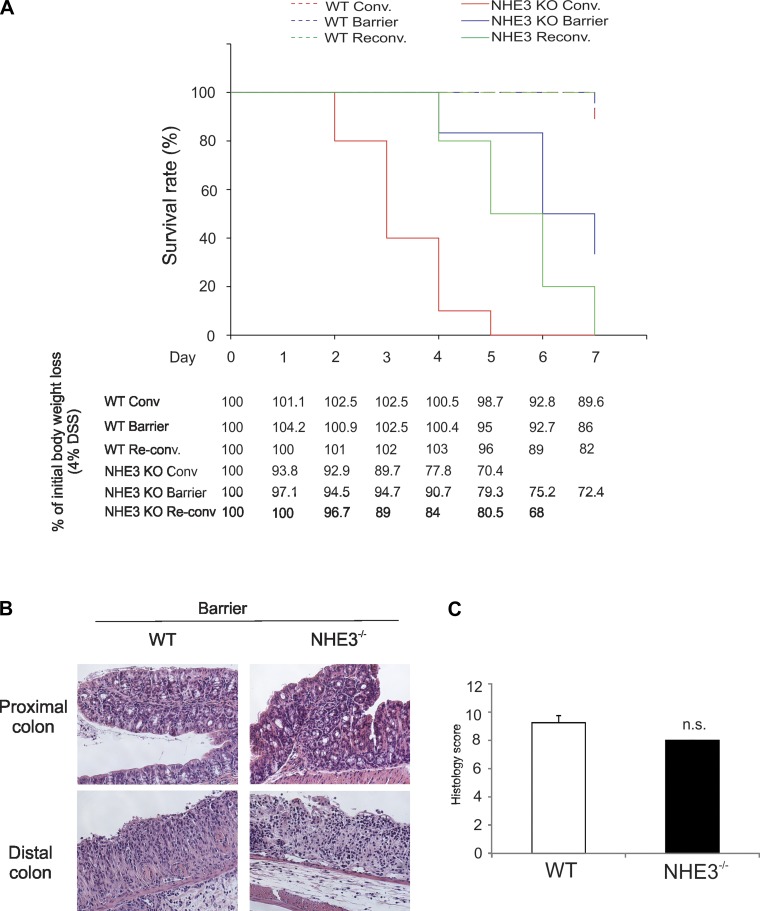
Susceptibility to DSS-induced mucosal injury in NHE3−/− mice housed in the barrier facility and after reconventionalization.
NHE3−/− mice housed in conventional facility are highly susceptible to the cytotoxic effects of DSS (12). To determine whether changes in the microbial environment modulate the DSS-induced morbidity and mortality, we compared the effects of 4% DSS in drinking water in conventional, barrier, and reconventionalized WT and NHE3−/− mice. No significant differences were observed among WT mice treated with DSS with 95% surviving until day 7 regardless of the environment (Fig. 7A and data not shown). Consistent with our earlier observations (12), all conventional NHE3−/− mice died within 5 days with intestinal bleeding, dramatically lower erythrocyte count, hemoglobin, and hematocrit (Fig. 7A and data not shown). NHE3−/− mice housed in the barrier facility only displayed a 20% mortality rate after 5 days, with progressively increased death rate, which reached 65% by day 7 (Fig. 7A). This delayed mortality was accompanied with slower body weight loss (Fig. 7A). However, in mice surviving until day 7, a similar degree of mucosal inflammation was observed (Fig. 7B), with no difference in the mean histological score (Fig. 7C) and no difference in the penetrance of colitis, as determined by the number of mice with a histological score in distal colon exceeding an arbitrary threshold of two similar in both genotypes (data not shown).
Fig. 7.
Evaluation of dextran sodium sulfate (DSS)-induced colitis in WT and NHE3 KO maintained in the conventional environment, barrier facility, or reconventionalized. A: mortality of WT and NHE3−/− mice housed in conventional environment, barrier facilities, or reconventionalized in response to 4% DSS. Table depicts body weight loss in WT and NHE3−/− mice treated with 4% DSS. B: histological analysis (H&E staining) of colonic morphology after treatment with 4% DSS in WT and NHE3−/− mice housed in barrier facility (magnification ×200). Sections were graded by scoring based on the degree of lamina propria mononuclear cell infiltration, crypt hyperplasia, and architectural distortion by an unbiased pathologist. C: cumulative (proximal + distal colon) histology score in barrier-housed WT and NHE3−/− mice treated with 4% DSS for 7 days. Statistical analysis was performed with ANOVA followed by Fisher's PLSD post hoc test with P ≤ 0.05 considered significant (conventionalized WT n = 9, NHE3−/− n = 9; barrier WT n = 19, NHE3−/− n = 15; reconventionalized WT n = 5, NHE3−/− n = 8) (n.s., not significant).
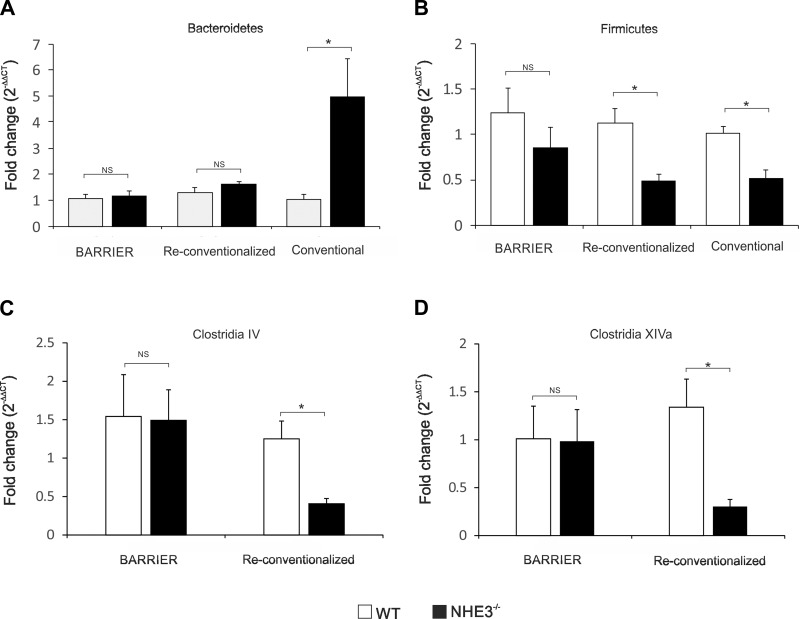
qPCR analysis and comparison of selected taxonomic groups of bacteria in the fecal content of barrier, conventional, and reconventionalized WT and NHE3−/− mice.
Based on the earlier pyrosequencing results, we performed targeted analysis of selected taxonomic groups of bacteria in WT and NHE3−/− mice housed in the conventional barrier facility (attenuated colitis) and reconventionalized mice (restored distal colitis) by real-time PCR. Of the two phyla most significantly changed in the colonic contents of conventional NHE3−/− mice, relative contribution of Bacteroidetes did not change in the barrier facility or after reconventionalization (Fig. 8A). Interestingly, Firmicutes remained unaltered in the barrier mice but significantly decreased among conventional and reconventionalized NHE3−/− mice with more pronounced distal colitis (Fig. 8B). Among Firmicutes, we observed a significant decrease in the relative abundance of butyrate-producing, highly oxygen-sensitive Clostridia belonging to the clostridial clusters IV and XIVa in reconventionalized NHE3−/− mice but not in NHE3−/− mice housed in the barrier facility (Fig. 8, C and D).
Fig. 8.
Comparative analysis of the microflora by qPCR in conventional, barrier, and reconventionalized WT and NHE3−/− mice. 16S qPCR analysis of Bacteriodetes (A), Firmicutes (B) phyla and Clostridia IV (C) and XIVa (D) clusters in the lumen of the distal colon of WT and NHE3-deficient mice housed in the conventional or barrier facility or reconventionalized. Statistical analysis was performed with Student's t-test (WT vs. NHE3 KO mice) with P ≤ 0.05 considered significant (barrier WT n = 7, NHE3−/− n = 12; reconventionalized WT n = 11, NHE3−/− n = 15, conventional WT n = 4, NHE3−/− n = 6).
DISCUSSION
Studies with Slc9a3-deficient mice originally generated in Gary Shull's laboratory (20) continue to yield new insights into the role of intestinal and renal NHE3 in epithelial transport homeostasis. Our group was the first to describe that conventionally housed NHE3−/− mice maintained on the original mixed genetic background develop bacterially mediated, spontaneous distal colitis, which could be ameliorated by the oral administration of broad-spectrum antibiotics (16), and display remarkably high susceptibility to DSS-induced mucosal injury (12). We described increased bacterial adhesion by in situ hybridization and bacterial translocation by tissue gram staining in the distal colon of NHE3−/− mice, and our collaborators recently described changes in the mucous structure and bacterial penetrance (11, 16). Interestingly, a divergence of symptoms has developed between NHE3-deficient mice in various labs. NHE3−/− mice bred into pure FVB/N genetic background at the University of Cincinnati display diarrhea-predominant phenotype (Shull and Worrell; personal communications), whereas mice on the original mixed 129/Black Swiss background housed at the University of Arizona develop very mild or no diarrhea but clear symptoms of distal colitis (12, 15, 16). This discrepancy in symptoms may be strain related; crossing NHE3−/− mice into C57BL/6 background increases diarrhea and spontaneous mortality (Kiela at al., unpublished observations), whereas crossing for over 10 generations into pure 129/SvEv background retains inflammatory phenotype (15). However, a possibility of distinct microbial gut ecologies, which vary among different animal facilities and serve as modulators of Na+ and water absorption as well as colonic inflammation, cannot be dismissed.
Here, we hypothesized that the microbiota composition plays an essential role in NHE3 deficiency-mediated colitis and that, reciprocally, NHE3 deficiency might alter the microbial composition of the gastrointestinal tract. To address this hypothesis, we compared distal colitis symptoms and susceptibility to DSS-induced mucosal damages in correlation with the composition of the colonic microbiota in conventional mice, mice rederived through embryo transfer into a barrier facility and in reconventionalized mice. Comparative analysis of fecal and mucosal microbiota of conventional mice demonstrated a significant decrease in bacterial diversity and profound changes in the overall microbial ecology of the distal large intestine NHE3−/− mice compared with their WT littermates. Interestingly, NHE3−/− mice re-derived into a barrier facility exhibit no inflammatory phenotype which highlighted a reciprocal relationship between NHE3 activity and the gut microbial composition. Reintroduction of conventional microflora in barrier mice restored spontaneous distal colitis in NHE3−/− mice. Barrier-housed NHE3−/− mice exhibited delayed mortality in response to 4% DSS compared with the conventional colony, and reintroduction of conventional microflora to barrier-reared mice partially restored the high susceptibility to DSS-induced injury. This effect was reminiscent of the effects of broad-spectrum antibiotics as reported by our group earlier (12). It appears, however, that restoration of colitis in reconventionalized mice, admittedly through association with different microbiota than that of the original conventional colony, had different immunological basis, as it was not associated with complete restoration of the high levels of mucosal TNF, iNOS, IL-1β, or MMP8, but rather with elevated levels of colonic expression of IFN-γ. Conventional NHE3−/− mice overexpressing IFN-γ only in the small intestinal mucosa were the primary source of the cytokine CD8+ memory T cells (12). Whether the source of colonic IFN-γ production in reconventionalized NHE3−/− is cells of the adaptive or innate immune system (e.g., macrophages) remains to be determined.
Interestingly, restoration of distal colitis in reconventionalized mice was accompanied with decreased contribution of Firmicutes but no change in Bacteroidetes. Two Clostridium clusters, IV (C. leptum) and XIVa (C. coccoides), were significantly reduced in reconventionalized NHE3−/− mice compared with their WT littermates. Decreases in members of these two clusters of butyrate-producing clostridia have been reproducibly reported in the gut of patients with IBD (25, 28), and some of the species from these cluster are considered thus probiotic candidates for the treatment of IBD. A defined mix of Clostridium strains from these two clusters has also been recently shown to promote regulatory T cell accumulation in the colonic mucosa and oral inoculation of Clostridium during the early life of conventionally reared mice, resulting in resistance to colitis and systemic immunoglobulin E responses (1). These species represent the main producers of butyrate, which, not only serves as the major energy source for colonic epithelial cells (8), but also inhibits NF-κB signaling (21) and reinforces the mucosal barrier function by increasing the antimicrobial peptide and mucin production and by regulating the expression of major tight junction proteins (32). Very little is known about the optimum growth conditions for Clostridium leptum (cluster IV) and Clostridium coccoides (cluster XIVa) groups, as members of these clusters are highly oxygen sensitive and very difficult to culture. Because NHE3 plays a critical role in the regulation of the acidity of the luminal and apical environment, the simplest explanation for the decrease in these Firmicutes is the shift in colonic pH toward more alkaline (20). Alternatively or in conjunction with this effect, expansion of Proteobacteria and Bacteroidetes may reduce the niche available to Firmicutes. It is tempting to hypothesize that some of the proinflammatory effects of NHE3 deficiency in the distal colon are mediated secondarily through insufficient Treg conversion or recruitment, or insufficient butyrate production, although these questions will have to be experimentally addressed.
In summary, we provide new evidence for the critical role of NHE3 and the consequences of the loss of NHE3 activity, which extend beyond the pHi regulation and transepithelial Na+ transport. Under physiological condition, NHE3, a brush border NHE, plays an essential role in the regulation of the acidity of the apical microenvironment (29). In the face of genetic loss of Slc9a3 in a mouse model (11, 12, 15, 16), or significant inhibition of NHE3 expression and/or activity during the IBD progression (17, 23, 27, 34), the resulting changes in the microbiome composition may contribute to the degree of colitis developed. Conversely, susceptibility of NHE3−/− mice to DSS-induced mucosal injury and changes in the tight and adherens junctions (12), as well as increased epithelial cell apoptosis in NHE3/IL-10 double KO mice (15), suggest another, potentially intrinsic, epithelial cell defect, which is subject to modulation by colonic microbiota. Such mechanism could explain the effects of antibiotics or rederivation. In conclusion, we provide the first description of profound changes in the colonic microbial ecology in the absence of NHE3, suggesting that the functional inhibition of NHE3 activity in patients with IBD may be a critical intermediary in the development of dysbiosis and potentially perpetuate a development of a more “proinflammatory enterotype”.
GRANTS
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases Grant 2R01 DK-041274 (to F. Ghishan and P. Kiela) and by the EU European Regional Development Fund, the Operational Program Innovative Economy 2007-2013 (Agreement POIG.01.01.02-14-054/09-00, to L. Lipinski).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: C.B.L., D.L., F.M.H., K.W.S., L.L., M.T.M.-K., R.-M.T.M., R.R., K.A.H., and M.G. performed experiments; C.B.L., D.L., F.M.H., K.W.S., R.-M.T.M., R.R., M.G., D.G.B., and P.R.K. analyzed data; C.B.L., D.L., F.M.H., D.G.B., and P.R.K. interpreted results of experiments; C.B.L., K.W.S., and P.R.K. prepared figures; C.B.L. and P.R.K. drafted manuscript; C.B.L., D.L., K.W.S., L.L., M.G., F.K.G., and P.R.K. edited and revised manuscript; C.B.L., D.L., L.L., M.G., F.K.G., and P.R.K. approved final version of manuscript; F.K.G. and P.R.K. conception and design of research.
ACKNOWLEDGMENTS
We thank Mrs. Dorota Tokarska for technical expertise in the preparation of amplicon libraries for pyrosequencing and Dr. Leszek Lipinski for supervision and coordination of the metagenomic analysis, as well as Dr. Marcin Golebiewski for the bioinformatics support.
REFERENCES
- 1.Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, Taniguchi T, Takeda K, Hori S, Ivanov II, Umesaki Y, Itoh K, Honda K. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 331: 337–341, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bacchetti De Gregoris T, Aldred N, Clare AS, Burgess JG. Improvement of phylum- and class-specific primers for real-time PCR quantification of bacterial taxa. J Microbiol Methods 86: 351–356, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Barmeyer C, Harren M, Schmitz H, Heinzel-Pleines U, Mankertz J, Seidler U, Horak I, Wiedenmann B, Fromm M, Schulzke JD. Mechanisms of diarrhea in the interleukin-2-deficient mouse model of colonic inflammation. Am J Physiol Gastrointest Liver Physiol 286: G244–G252, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Chao A, Lee SM. Estimating the Number of Classes via Sample Coverage. J Am Stat Assoc 87: 210–217, 1992 [Google Scholar]
- 5.Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27: 2194–2200, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farkas K, Yeruva S, Rakonczay Z, Jr, Ludolph L, Molnar T, Nagy F, Szepes Z, Schnur A, Wittmann T, Hubricht J, Riederer B, Venglovecz V, Lazar G, Kiraly M, Zsembery A, Varga G, Seidler U, Hegyi P. New therapeutic targets in ulcerative colitis: the importance of ion transporters in the human colon. Inflamm Bowel Dis 17: 884–898, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Fava F, Danese S. Intestinal microbiota in inflammatory bowel disease: friend of foe? World J Gastroenterol 17: 557–566, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fleming LL, Floch MH. Digestion and absorption of fiber carbohydrate in the colon. Am J Gastroenterol 81: 507–511, 1986 [PubMed] [Google Scholar]
- 9.Hecht G, Hodges K, Gill RK, Kear F, Tyagi S, Malakooti J, Ramaswamy K, Dudeja PK. Differential regulation of Na+/H+ exchange isoform activities by enteropathogenic E. coli in human intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 287: G370–G378, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Huse SM, Huber JA, Morrison HG, Sogin ML, Welch DM. Accuracy and quality of massively parallel DNA pyrosequencing. Genome Biol 8: R143, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johansson ME, Gustafsson JK, Holmen-Larsson J, Jabbar KS, Xia L, Xu H, Ghishan FK, Carvalho FA, Gewirtz AT, Sjovall H, Hansson GC. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut. 2013. Feb 20 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiela PR, Laubitz D, Larmonier CB, Midura-Kiela MT, Lipko MA, Janikashvili N, Bai A, Thurston R, Ghishan FK. Changes in mucosal homeostasis predispose NHE3 knockout mice to increased susceptibility to DSS-induced epithelial injury. Gastroenterology 137: 965–975; e961–910, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kiela PR, Midura AJ, Kuscuoglu N, Jolad SD, Solyom AM, Besselsen DG, Timmermann BN, Ghishan FK. Effects of Boswellia serrata in mouse models of chemically induced colitis. Am J Physiol Gastrointest Liver Physiol 288: G798–G808, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Lane DJ. 16S/23S rRNA sequencing. In: Nucleic Acid Techniques in Bacterial Systematics, edited by Stackebrandt E, Goodfellow M. New York, NY: John Wiley and Sons, 1991, pp. 115–175 [Google Scholar]
- 15.Larmonier CB, Laubitz D, Thurston RD, Bucknam AL, Hill FM, Midura-Kiela M, Ramalingam R, Kiela PR, Ghishan FK. NHE3 modulates the severity of colitis in IL-10-deficient mice. Am J Physiol Gastrointest Liver Physiol 300: G998–G1009, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laubitz D, Larmonier CB, Bai A, Midura-Kiela MT, Lipko MA, Thurston RD, Kiela PR, Ghishan FK. Colonic gene expression profile in NHE3-deficient mice: evidence for spontaneous distal colitis. Am J Physiol Gastrointest Liver Physiol 295: G63–G77, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lenzen H, Lunnemann M, Bleich A, Manns MP, Seidler U, Jorns A. Downregulation of the NHE3-binding PDZ-adaptor protein PDZK1 expression during cytokine-induced inflammation in interleukin-10-deficient mice. PloS One 7: e40657, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ott SJ, Musfeldt M, Wenderoth DF, Hampe J, Brant O, Folsch UR, Timmis KN, Schreiber S. Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut 53: 685–693, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rocha F, Musch MW, Lishanskiy L, Bookstein C, Sugi K, Xie Y, Chang EB. IFN-gamma downregulates expression of Na(+)/H(+) exchangers NHE2 and NHE3 in rat intestine and human Caco-2/bbe cells. Am J Physiol Cell Physiol 280: C1224–C1232, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Schultheis PJ, Clarke LL, Meneton P, Miller ML, Soleimani M, Gawenis LR, Riddle TM, Duffy JJ, Doetschman T, Wang T, Giebisch G, Aronson PS, Lorenz JN, Shull GE. Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nat Genet 19: 282–285, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Segain JP, Raingeard de la Bletiere D, Bourreille A, Leray V, Gervois N, Rosales C, Ferrier L, Bonnet C, Blottiere HM, Galmiche JP. Butyrate inhibits inflammatory responses through NFkappaB inhibition: implications for Crohn's disease. Gut 47: 397–403, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sellon RK, Tonkonogy S, Schultz M, Dieleman LA, Grenther W, Balish E, Rennick DM, Sartor RB. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun 66: 5224–5231, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siddique I, Hasan F, Khan I. Suppression of Na+/H+ exchanger isoform-3 in human inflammatory bowel disease: lack of reversal by 5′-aminosalicylate treatment. Scand J Gastroenterol 44: 56–64, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Singh V, Raheja G, Borthakur A, Kumar A, Gill RK, Alakkam A, Malakooti J, Dudeja PK. Lactobacillus acidophilus upregulates intestinal NHE3 expression and function. Am J Physiol Gastrointest Liver Physiol 303: G1393–G1401, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermudez-Humaran LG, Gratadoux JJ, Blugeon S, Bridonneau C, Furet JP, Corthier G, Grangette C, Vasquez N, Pochart P, Trugnan G, Thomas G, Blottiere HM, Dore J, Marteau P, Seksik P, Langella P. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci USA 105: 16731–16736, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sokol H, Seksik P. The intestinal microbiota in inflammatory bowel diseases: time to connect with the host. Curr Opin Gastroenterol 26: 327–331, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Sullivan S, Alex P, Dassopoulos T, Zachos NC, Iacobuzio-Donahue C, Donowitz M, Brant SR, Cuffari C, Harris ML, Datta LW, Conklin L, Chen Y, Li X. Downregulation of sodium transporters and NHERF proteins in IBD patients and mouse colitis models: potential contributors to IBD-associated diarrhea. Inflamm Bowel Dis 15: 261–274, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takaishi H, Matsuki T, Nakazawa A, Takada T, Kado S, Asahara T, Kamada N, Sakuraba A, Yajima T, Higuchi H, Inoue N, Ogata H, Iwao Y, Nomoto K, Tanaka R, Hibi T. Imbalance in intestinal microflora constitution could be involved in the pathogenesis of inflammatory bowel disease. Int J Med Microbiol 298: 463–472, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Thwaites DT, Hirst BH, Simmons NL. Substrate specificity of the di/tripeptide transporter in human intestinal epithelia (Caco-2): identification of substrates that undergo H(+)-coupled absorption. Br J Pharmacol 113: 1050–1056, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tlaskalova-Hogenova H, Stepankova R, Hudcovic T, Tuckova L, Cukrowska B, Lodinova-Zadnikova R, Kozakova H, Rossmann P, Bartova J, Sokol D, Funda DP, Borovska D, Rehakova Z, Sinkora J, Hofman J, Drastich P, Kokesova A. Commensal bacteria (normal microflora), mucosal immunity and chronic inflammatory and autoimmune diseases. Immunol Lett 93: 97–108, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Turner S, Pryer KM, Miao VP, Palmer JD. Investigating deep phylogenetic relationships among cyanobacteria and plastids by small subunit rRNA sequence analysis. J Eukaryot Microbiol 46: 327–338, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Vanhoutvin SA, Troost FJ, Hamer HM, Lindsey PJ, Koek GH, Jonkers DM, Kodde A, Venema K, Brummer RJ. Butyrate-induced transcriptional changes in human colonic mucosa. PloS One 4: e6759, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woo AL, Gildea LA, Tack LM, Miller ML, Spicer Z, Millhorn DE, Finkelman FD, Hassett DJ, Shull GE. In vivo evidence for interferon-gamma-mediated homeostatic mechanisms in small intestine of the NHE3 Na+/H+ exchanger knockout model of congenital diarrhea. J Biol Chem 277: 49036–49046, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Yeruva S, Farkas K, Hubricht J, Rode K, Riederer B, Bachmann O, Cinar A, Rakonczay Z, Molnar T, Nagy F, Wedemeyer J, Manns M, Raddatz D, Musch MW, Chang EB, Hegyi P, Seidler U. Preserved Na(+)/H(+) exchanger isoform 3 expression and localization, but decreased NHE3 function indicate regulatory sodium transport defect in ulcerative colitis. Inflamm Bowel Dis 16: 1149–1161, 2010 [DOI] [PubMed] [Google Scholar]