Abstract
The ligand-gated channels transient receptor potential vanilloid 1 (TRPV1) and P2X3 have been reported to facilitate colorectal afferent neuron sensitization, thus contributing to organ hypersensitivity and pain. In the present study, we hypothesized that TRPV1 and P2X3 cooperate to modulate colorectal nociception and afferent sensitivity. To test this hypothesis, we employed TRPV1-P2X3 double knockout (TPDKO) mice and channel-selective pharmacological antagonists and evaluated combined channel contributions to behavioral responses to colorectal distension (CRD) and afferent fiber responses to colorectal stretch. Baseline responses to CRD were unexpectedly greater in TPDKO compared with control mice, but zymosan-produced CRD hypersensitivity was absent in TPDKO mice. Relative to control mice, proportions of mechanosensitive and -insensitive pelvic nerve afferent classes were not different in TPDKO mice. Responses of mucosal and serosal class afferents to mechanical probing were unaffected, whereas responses of muscular (but not muscular/mucosal) afferents to stretch were significantly attenuated in TPDKO mice; sensitization of both muscular and muscular/mucosal afferents by inflammatory soup was also significantly attenuated. In pharmacological studies, the TRPV1 antagonist A889425 and P2X3 antagonist TNP-ATP, alone and in combination, applied onto stretch-sensitive afferent endings attenuated responses to stretch; combined antagonism produced greater attenuation. In the aggregate, these observations suggest that 1) genetic manipulation of TRPV1 and P2X3 leads to reduction in colorectal mechanosensation peripherally and compensatory changes and/or disinhibition of other channels centrally, 2) combined pharmacological antagonism produces more robust attenuation of mechanosensation peripherally than does antagonism of either channel alone, and 3) the relative importance of these channels appears to be enhanced in colorectal hypersensitivity.
Keywords: pelvic nerve, purinergic receptor, single fiber, transient receptor potential, visceral pain
chronic abdominal pain is a key feature of irritable bowel syndrome (IBS), which is prevalent, costly, and difficult to manage. An important contributor to pain in IBS is heightened perception of mechanical events in the bowel (i.e., hypersensitivity). Indeed, patients with IBS typically report greater pain and/or reduced response thresholds to rectal balloon distension than control subjects (3, 24, 28, 34, 46). Although central processes contribute to colorectal hypersensitivity, the driving force is increased afferent mechanosensitivity (i.e., sensitization). For example, intrarectal lidocaine reduces pain evoked by rectal distension in healthy subjects as well as ongoing pain and both visceral and somatic (referred) hypersensitivity in patients with IBS (24, 32, 49, 50). Accordingly, reversing or moderating afferent sensitization and hyperexcitability is a clinically important goal, and developing drugs that selectively target colorectal mechanosensation and/or sensitization would improve management of IBS pain. Targets include ion channels expressed in colorectal afferents, two of which are transient receptor potential vanilloid 1 (TRPV1) and P2X3.
TRPV1 is a capsaicin-, heat- and proton-gated ion channel expressed in the majority of colorectal afferents (8, 35). Expression of TRPV1 in colorectal afferents is increased in patients with IBS, and the magnitude of channel expression often positively correlates with the severity of sensory symptoms (1). TRPV1 knockout (KO) mice exhibit reduced responses of stretch-sensitive afferents (20) and decreased behavioral responses to colorectal distension (CRD) (19) in both naive and hypersensitive states. TRPV1 antagonists similarly decrease afferent and behavioral responses to CRD (9, 29, 31, 52).
P2X3 exists in homomeric P2X3 and heteromeric P2X2/3 configurations and is expressed in ∼20% of colorectal afferents (6, 40). Both configurations are activated via pressure-dependent mucosal release of ATP (39, 54), which facilitates distension-evoked mechanosensation (54). In agreement, we found that genetic deletion of P2X3 attenuates colorectal mechanosensation and sensitization (39). Furthermore, the expression and function of P2X3 is enhanced in rodent models of IBS (40, 56).
We hypothesized that combined inhibition of these two ligand-gated channels should produce greater attenuation of mechanosensation than inhibition of either channel alone. To test this hypothesis, we utilized TRPV1-P2X3 double knockout (TPDKO) mice together with selective pharmacological antagonists to evaluate 1) channel contributions to colorectal nociception and hypersensitivity in vivo and 2) afferent mechanosensation and sensitization in vitro. Portions of these data have been previously reported in abstract form (22).
MATERIALS AND METHODS
Animals.
Adult male mice (20–30 g) of the following strains were used: C57BL/6 wild-type control (Taconic, Germantown, NY) and global, nonconditional TPDKO. TPDKO mice were generated by crossing TRPV1 and P2X3 single knockout mice. All knockout mice were backcrossed onto a Taconic C57BL/6 genetic background for >10 generations. TRPV1 single knockout mice were provided by Dr. H. R. Koerber, the University of Pittsburgh. P2X3 single knockout mice were obtained from The Jackson Laboratory (Bar Harbor, ME) with the permission of Dr. D. A. Cockayne, Roche Bioscience, Palo Alto, CA. Genotypes were confirmed by PCR and Southern blot analysis. We used only homozygous TPDKO mice. Although we did not test for functional deletion of ion channels in double knockout mice, previous single-fiber and patch-clamp recordings in colorectal afferents from the two parent genotypes TRPV1−/− and P2X3−/− revealed complete insensitivity to the respective channel agonists capsaicin (20) and α,β-meATP (39). All protocols were approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
Labeling, single-cell reverse transcriptase PCR, and calcium imaging of colorectal DRG neurons.
Dorsal root ganglion (DRG) sensory neurons innervating the colorectum (c-DRGs) were labeled, collected, and processed as previously detailed (37, 40). Briefly, a laparotomy was performed on mice anesthetized with isofluorane (Hospira, Lake Forest, IL) to expose the distal colon. Three to six boluses (2–3 μl) of 2% by weight 1,1′-dioctadecyl-3,3,3′,3-tetramethylindocarbocyanine methanesulfonate (DiI; Molecular Probes, Eugene, OR) dissolved in DMSO were injected into the colon wall ∼1.5 cm rostral to the anal verge. Mice were allowed 2 wk for postsurgical recovery and transport of DiI to DRG somata.
Subsequently, mice were overdosed with isofluorane followed by bilateral removal of L6-S2 (LS) DRGs, which principally contribute to the pelvic nerve (PN). DRGs were incubated at 37°C for 10 min in Hanks' Balanced Salt solution (Sigma-Aldrich, St. Louis, MO) containing l-cysteine (5.5 mM), papain (60 Units; Worthington Biochemical, Lakewood, NJ), and saturated NaHCO3 (2 mM). Collagenase II (4,320 U; Worthington) and dispase II (14 U; Roche Diagnostics, Indianapolis, IN) were added and cells incubated for an additional 20 min before quenching enzyme activity with 10% fetal bovine serum (Sigma) dissolved in advanced Dulbecco's modified eagle medium/F12 containing 1% penicillin/streptomycin (Invitrogen, Carlsbad, CA). The cell mixture was gently triturated, and cells were plated on poly-d-lysine-coated glass coverslips (Becton Dickinson Labware, Bedford, MA) and incubated (37°C, 95% O2) for 16–18 h before beginning experiments. No additional growth factors were added to culture media. Two to three mice were used per preparation.
Dissociated and cultured LS c-DRGs were identified by DiI content, collected individually with an ∼50-μm diameter glass pipette, and expelled into a microcentrifuge tube containing reverse transcriptase (RT) mix (SuperScript II; Invitrogen) (30). Cell lysis and RT reactions consisted of incubation with 20 U SuperScript II (Invitrogen) at 65°C for 1.5 min, at room temperature for 2 min, at 37°C for 20 min, and finally at 65°C for 10 min. Negative controls were performed by omitting RT or using a cell-free bath aspirate as the template. The first-strand cDNA from a c-DRG neuron was used as the template for amplification of TRPV1 and P2X3 transcripts using a nested, multiplex PCR strategy with external and internal primer sequences listed in Table 1. First-round multiplex PCR was performed in a 25-μl solution containing 2 μl cDNA, 5× GoTaq reaction buffer (Promega, Madison, WI), 20 μM each of forward and reverse external primers for both channels, 2.5 mM dNTPs, and 5 U/μl GoTaq DNA polymerase (Promega). Reactions were incubated at 95°C for 10 min and then cycled 35 times at 94°C/30 s, 52°C/30 s, and 72°C/30 s before a final extension step at 72°C for 10 min. Each initial PCR product served as a template in a subsequent PCR reaction using a nested internal primer pair for each channel separately. This second-round PCR product was electrophoresed onto a 2% agarose-ethidium bromide gel, digitally photographed (LAS 3000 imaging system; Fujifilm, Tokyo, Japan), and analyzed using ImageJ (version 1.42q, NIH). Only neurons producing detectable amplification of a housekeeping gene (GAPDH) were analyzed further.
Table 1.
Primer pairs for PCR amplification of TRPV1 and P2X3 mRNA
| Gene (expected size) | External Primers | Internal Primers | Genebank Number |
|---|---|---|---|
| TRPV1 | GGGAAGAATAACTCACTGCCTGTG | GGCGAGACTGTCAACAAGATTGC | NM_001001445.1 |
| (486, 191 bp) | TGGGTCCTCGTTGATGATGC | TCATCCACCCTGAAGCACCAC | |
| P2X3 | GCTCCGTAGAAGAAGATGGAGA | TGTCCTAAGAGGATCCTGTACC | NM_145526 |
| (251, 141 bp) | CTGTGTGACCATGTTAGGGATG | GGCATCTAGCACATAGAAGTGG |
Single-cell PCR was performed in dissociated colorectal afferent somata from L6-S2 dorsal root ganglions (LS DRGs). A nested PCR strategy with external and internal cDNA primer pairs was used to amplify reverse-transcribed transient receptor potential vanilloid 1 (TRPV1) and P2X3 mRNA. Sequences for cDNA primers are indicated with the 5′ end on the left. The Genebank reference used for designing primers is indicated at the right. bp, base pairs.
Other DiI-labeled c-DRGs were loaded with 2.5 μM of the fluorescent Ca2+ indicator Fura 2-AM and 0.025% pluronic acid (both from TEF Laboratories, Austin, TX) as detailed previously (25). Treated cells were transferred to a recording chamber continuously perfused with bath solution containing (in mM): 130 NaCl, 3 KCl, 2.5 CaCl2, 0.6 Mg Cl2, 10 HEPES, and 10 glucose and pH adjusted with Tris base to 7.4 and osmolarity with sucrose to 325 mOsmol/l. Ca2+ transients were measured as the ratio of fluorescence emission (510 nm) in response to 340/380 nm excitation controlled by a λ 10–2 filter changer (Sutter Instruments, Novato, CA). Data were acquired using Metafluor software (Molecular Devices, Sunnyvale, CA) and a CCD camera (Model RTE/CCD 1300; Roper Scientific, Trenton, NJ). DiI-positive c-DRGs were stimulated in the following sequence: KCl (30 mM KCl in normal bath with 100 mM NaCl to maintain osmolarity), the P2X3 agonist α,β-meATP (100 μM in normal bath; Sigma), and the TRPV1 agonist capsaicin (500 nM in normal bath and <0.01% ethanol; Sigma). All drugs were delivered via a fast-step superfusion system (Model DAD-12; ALA Scientific Instruments, Westbury, NY).
CRD.
The visceromotor response (VMR) to CRD was measured to assess colorectal nociception and hypersensitivity (7). Briefly, mice were anesthetized (isofluorane), and a pair of electrodes was implanted into the abdominal musculature and exteriorized at the back of the neck for subsequent electromyographic recording of muscle activity in unanesthetized mice. Contraction in response to CRD was quantified with Spike2 software (Cambridge Electronic Design, Cambridge, UK) as electromyographic activity during distension minus predistension resting activity. Polyethylene distension balloons (1.5-cm length, 0.9-cm diameter) were inserted transanally 1 cm beyond the anal verge under isofluorane sedation. Mice were placed inside darkened, sound-attenuated plastic cylinders to minimize movement and stress. A 45-min postisofluorane recovery period preceded CRD. Distension balloons were inflated for 10-s with pressurized nitrogen to 15, 30, 45, or 60 mmHg. Each pressure was tested three times with 4 min between distensions, starting at 15 mmHg (nonnoxious) and ending at 60 mmHg (noxious). Baseline VMRs were recorded 4 days after surgery (day 0), after which intracolonic treatment with either 0.1 ml of normal saline vehicle or zymosan (30 mg/ml; Sigma) was given and repeated daily for two additional consecutive days; VMRs to CRD were recorded again 1 day after the third intracolonic treatment (i.e., day 3) (39). Some TPDKO mice were killed after day 3 CRD for single fiber electrophysiology (described below).
Single-fiber electrophysiology.
Mice were killed by CO2 inhalation, and the distal 2–3 cm of the colorectum was dissected out with the PN innervation intact (12). Dissection was performed in ice-cold oxygenated Krebs solution containing 4 μM nifedipine (L-type Ca2+ channel blocker to inhibit spontaneous muscle contraction; Sigma) and 3 μM indomethacin (to inhibit cyclooxygenase; Sigma). The dissected colon-nerve preparation was isolated and continually perfused with 31–33°C Krebs solution. The colorectum was opened longitudinally along the antimesenteric border and pinned mucosal side up. The PN was threaded into a separate oil-filled recording chamber and progressively teased apart into 6–10 bundles (∼10 μm thick) to isolate single fibers (>3:1 signal-to-noise ratio). Recordings were made by laying bundles atop a platinum-iridium wire extracellular electrode ∼100 μm in diameter. Neural activity was amplified (10,000-fold; DAM80; World Precision Instruments, New Haven, CT), filtered (0.3–10 kHz), and sampled (20 kHz) using a 1401 interface (CED) and Spike2.
An electrical search strategy was used for unbiased detection of all excitable afferent receptive endings (REs) and measurement of their electrical activation thresholds (12). All REs were tested for mechanosensitivity as follows: mucosal stroking with a fine brush producing ∼0.1 mN of perpendicular force; blunt perpendicular probing (1–80 mN; 5-s duration) and uniform circumferential stretch [0–170 mN, equivalent to 45 mmHg CRD (11, 12), applied as a ramp (5 mN/s, 34 s) or fast step (to 80 or 170 mN in 0.2 s)]. Probing and stretching were performed using a servo-controlled force actuator (Aurora Scientific, Toronto, ON, Canada). Colorectal PN afferents were classified as previously described (12). Briefly, all REs responded to blunt probing except mechanically insensitive afferents (MIAs). Muscular afferents also responded to stretch, mucosal afferents also to stroking, and muscular/mucosal afferents also to stretch and stroking. Serosal afferents responded only to probing. On average, 1–6 fibers were studied per mouse.
Agonists, antagonists, and a sensitizing inflammatory soup (IS) were applied directly atop isolated REs (11). IS was composed of bradykinin, prostaglandin E2, serotonin, and histamine (all at 10 μM) with pH adjusted to 6.0 (20). IS was applied for 3 min followed by mechanical testing 3 min later and then every 5 min until washout (i.e., return to baseline). Channel antagonists were applied for 5 min followed immediately by mechanical testing repeated every 5 min until washout. In our hands, a 5-min intertest interval is sufficient for full recovery of fiber response to mechanical stimulation. To inhibit TRPV1, we utilized A889425 (Abbott Laboratories, Abbott Park, IL), a highly selective competitive antagonist for the capsaicin-binding site with an IC50 of ∼300 nM (5, 27). A889425 was dissolved in 1-methyl-2-pyrrolidinone (1M2P) and diluted to a final concentration in Krebs solution. Solvent control experiments revealed no significant effect of 1% 1M2P on fiber mechanosensitivity (see Fig. 6 for reference). Consequently, this vehicle concentration was chosen for all further single and combined antagonist experiments. P2X3 was inhibited with TNP-ATP (Sigma), a competitive antagonist for P2X1, P2X3, and P2X2/3 with an IC50 of ∼30 nM (15). The TRPV1 agonist capsaicin (3 μM in Krebs and <0.01% ethanol) and P2X agonist α,β-meATP (1 mM in Krebs) (6, 12) were applied for 2 min after complete washout of antagonists as determined by the return of the baseline response magnitude of an afferent. Agonist application was followed immediately by mechanical testing as described above.
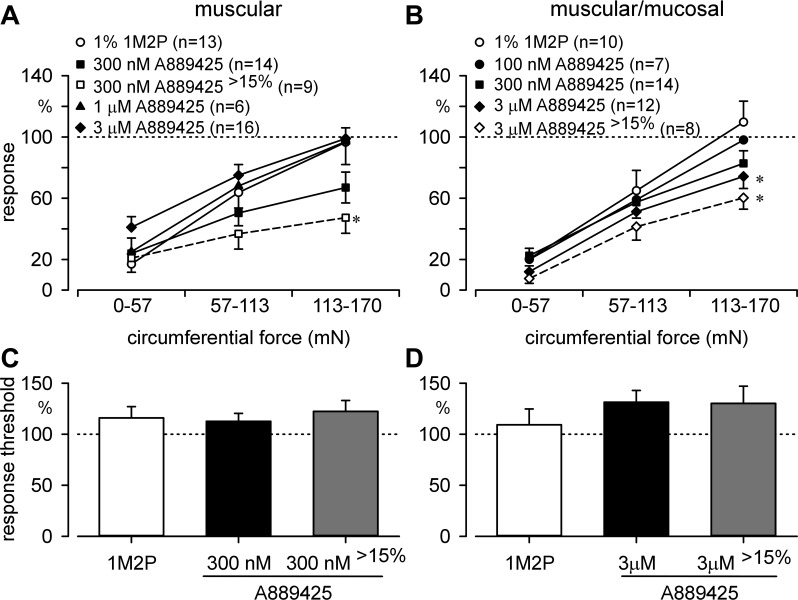
Fig. 6.
Antagonism of TRPV1 attenuated mechanosensitivity of stretch-sensitive colorectal afferents. A: relative to vehicle (1% 1-methyl-2-pyrrolidinone, 1M2P), A889425 did not significantly decrease stretch-response functions of muscular afferents at any concentration tested although 300 nM reduced responses to ∼60%. Application of an effect criterion of ≥15% posttreatment reduction of stretch-responsive functions (9/14 fibers; ∼65% of total) revealed significant attenuation by 300 nM A889425 of response to stretch (F3,120 = 10.5, *P < 0.001). B: 3 μM A889425 significantly attenuated muscular/mucosal stretch-response functions relative to vehicle (F3,102 = 2.9, *P < 0.05). Application of the effect criterion as above (8/12 fibers; ∼65%) similarly yielded significant attenuation by 3 μM A889425 of response to stretch (F3,90 = 4.8, P < 0.01). C and D: response thresholds of muscular and muscular/mucosal afferents, with or without application of the effect criterion, were unaffected by A889425. For statistical analyses, responses post-A889425 were compared against responses post-1M2P, which were compared against their respective predrug baselines. n = number of fibers/group. >15% denotes application of response criterion.
Statistical analyses.
Data are presented as means ± SE. Responses to CRD, probing, and ramped stretch are presented as stimulus-response functions. For ramped stretch, stimulus-response functions are presented as binned counts during stretch (0–53, 53–113, and 113–170 mN). To compare CRD and single-fiber stimulus-response functions before and after treatment, responses at each pressure or force were normalized to the pretreatment response at the final pressure (60 mmHg) or force (113–170 mN). Data were analyzed using one- or two-way ANOVAs (Holm-Sidak post hoc tests), paired or unpaired Student's t-tests, or Fischer's exact test (SigmaPlot 9; Systat Software, San Jose, CA) as appropriate. Single-fiber spontaneous activity was rare and, when present, was subtracted from stimulus-evoked activity. P < 0.05 was considered statistically significant.
RESULTS
Coexpression of TRPV1 and P2X3.
As detailed in Table 2, the majority of c-DRGs from C57BL/6 control mice coexpressed mRNA for TRPV1 and P2X3. The proportion of c-DRGs that expressed TRPV1 mRNA closely paralleled the proportion of neurons that responded to capsaicin (500 nM). About a third of these capsaicin-sensitive neurons also responded to α,β-meATP (100 μM). In contrast, only a minority of c-DRGs responded to α,β-meATP, but the majority of these neurons also responded to capsaicin.
Table 2.
Expression of TRPV1 and P2X3 mRNA and response to channel agonists in lumbosacral colon sensory neurons
| mRNA Expression |
Response to Channel Agonists |
|||
|---|---|---|---|---|
| % | n | % | n | |
| TRPV1 only | 77% | (10/13) | 74% | (23/31) |
| P2X3 in TRPV1 positive neurons | 80% | (8/10) | 30% | (7/23) |
| P2X3 only | 85% | (11/13) | 35% | (11/31) |
| TRPV1 in P2X3 positive neurons | 67% | (8/11) | 64% | (7/11) |
A total of 14 LS DRG sensory neurons innervating the colorectum (c-DRG) including 1 control from 1 preparation were tested for mRNA expression. A total of 31 LS c-DRGs from 2 preparations was examined for Ca2+-transient responses to α,β-meATP (100 μM) followed by capsaicin (500 nM). For Ca2+ imaging, only cells that initially responded to 30 mM KCl were treated with agonists.
Response to CRD.
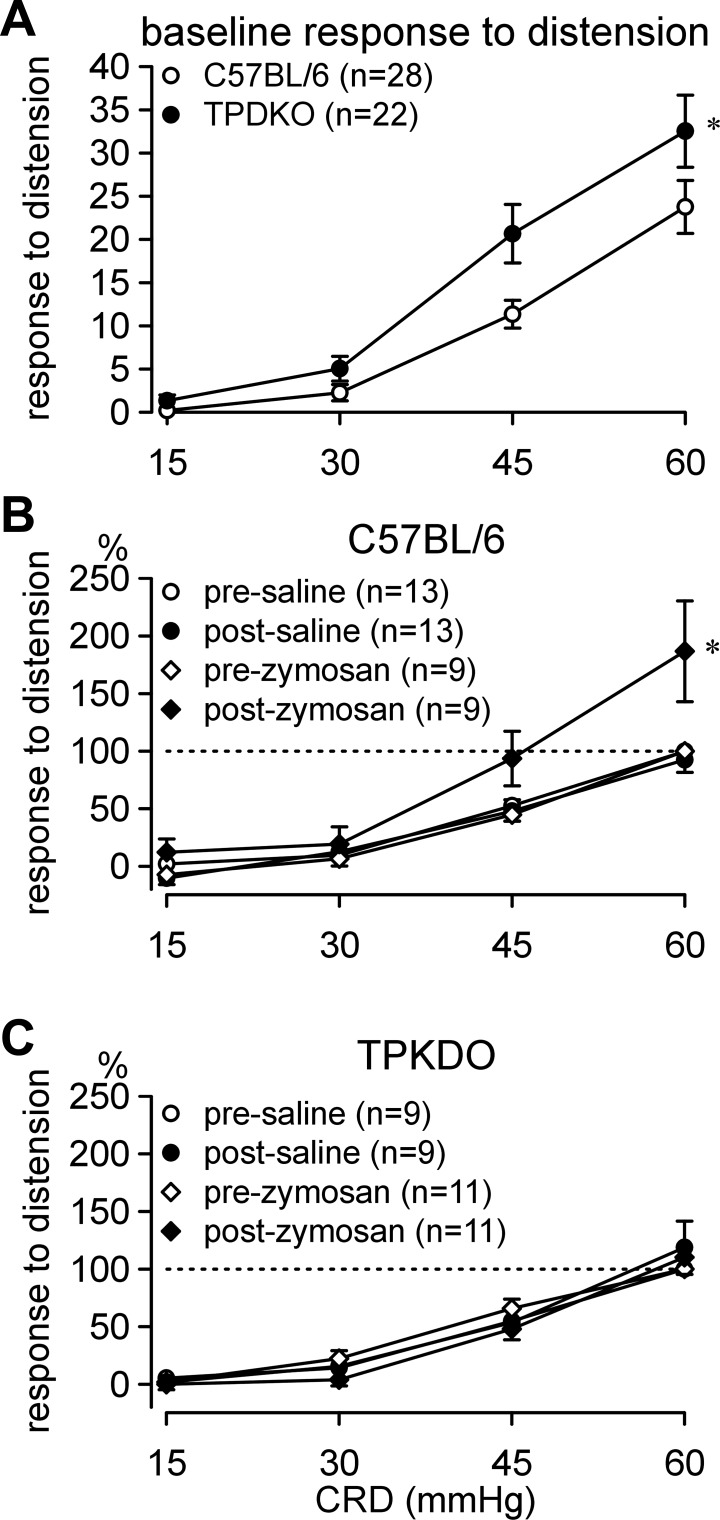
C57BL/6 and TPDKO mice responded similarly to CRD (Fig. 1A). However, responses were statistically greater in TPDKO mice at noxious intensities of CRD (i.e., 45 and 60 mmHg). Intracolonic treatment with saline had no effect on responses to CRD in either genotype, whereas treatment with zymosan elicited significant colorectal hypersensitivity in C57BL/6 (as previously reported; e.g., Ref. 14) but not TPDKO mice (Fig. 1, B and C). Statistical details are in the figure legend.
Fig. 1.
Visceromotor responses to colorectal distension (CRD). A: relative to C57BL/6 controls, transient receptor potential vanilloid 1 (TRPV1)-P2X3 double knockout (TPDKO) mice exhibited greater baseline responses to CRD (F1/144 = 5.8, *P < 0.05; Holms-Sidak post hoc tests, P < 0.01 at 45 and 60 mmHg CRD). B: intracolonic zymosan, but not saline, produced colorectal hypersensitivity in C57BL/6 mice (F1/24 = 6.4, P < 0.05), but not in TPDKO mice. C: visceromotor responses (not normalized) were used to generate stimulus-response curves in A; normalized responses were used for B and C. n = number of mice/group.
Characterization of colorectal afferents.
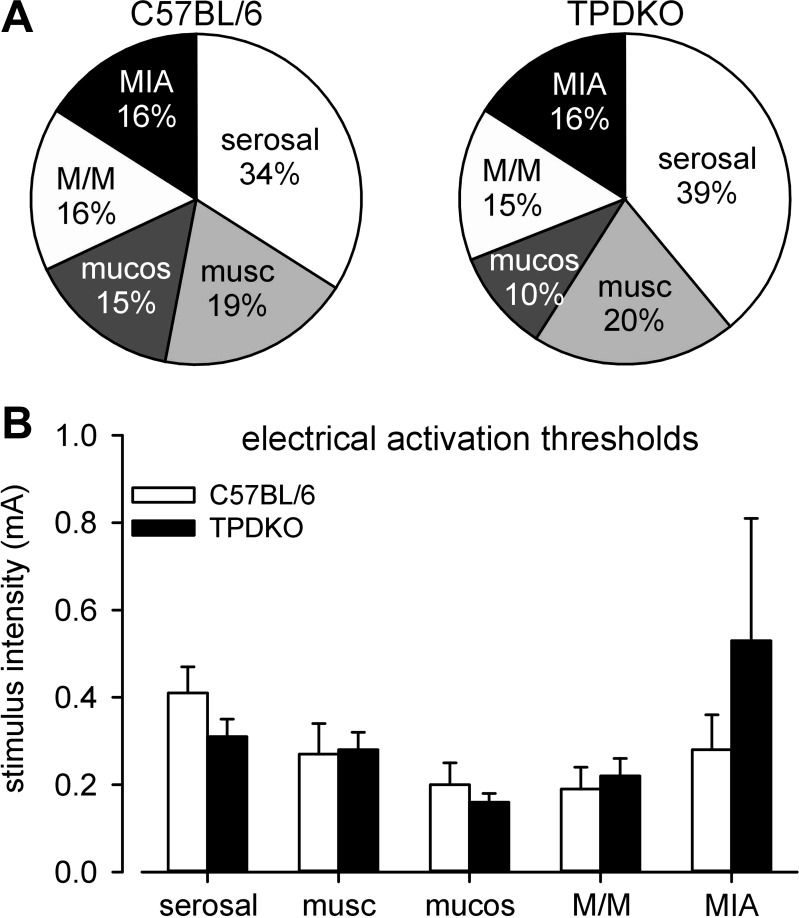
To explore the contributions of PN afferents to the CRD phenotype and to study in detail the effects of combined ablation of TRPV1 and P2X3 on colorectal afferent mechanosensation, we compared classes of colorectal PN afferents in C57BL/6 and TPDKO mice. Neither the proportions nor the electrical activation thresholds of the five classes of PN afferents differed between the two genotypes (Fig. 2). However, relative to C57BL/6 mice, there was a tendency in TPDKO mice for mucosal and muscular/mucosal afferent REs to be topographically shifted to and concentrated in the distal 1 cm of the colorectum (10/18 mucosal and 15/32 muscular/mucosal REs in C57BL/6 mice vs. 7/10 mucosal and 13/17 muscular/mucosal REs in TPDKO mice). There were no other differences in topographical distributions of PN afferents between genotypes.
Fig. 2.
Codeletion of TRPV1 and P2X3 (i.e., TPDKO) had no effect on proportions (A) or electrical activation thresholds (B) of pelvic nerve colorectal afferents. A total 116 fibers was studied from 23 C57BL/6 mice and 102 fibers from 24 TPDKO mice. musc, muscular; mucos, mucosal; M/M, muscular/mucosal; MIA, mechanically insensitive afferent.
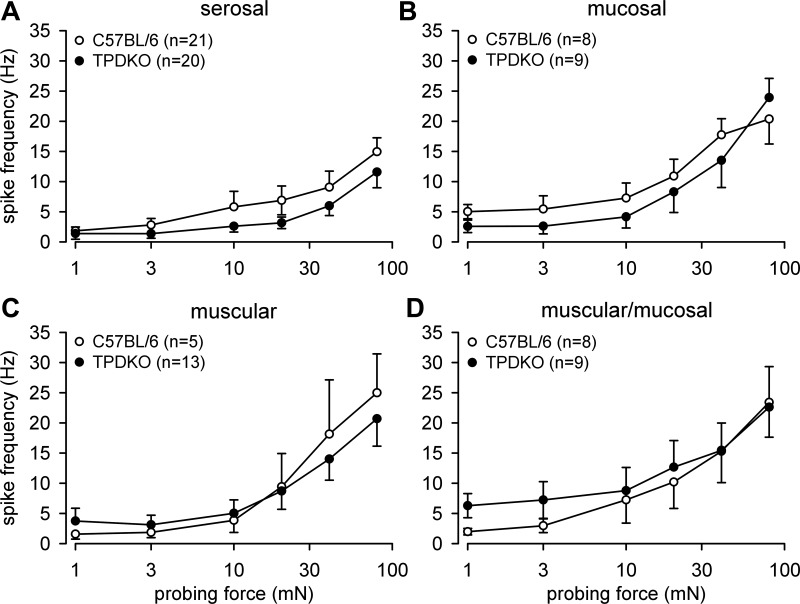
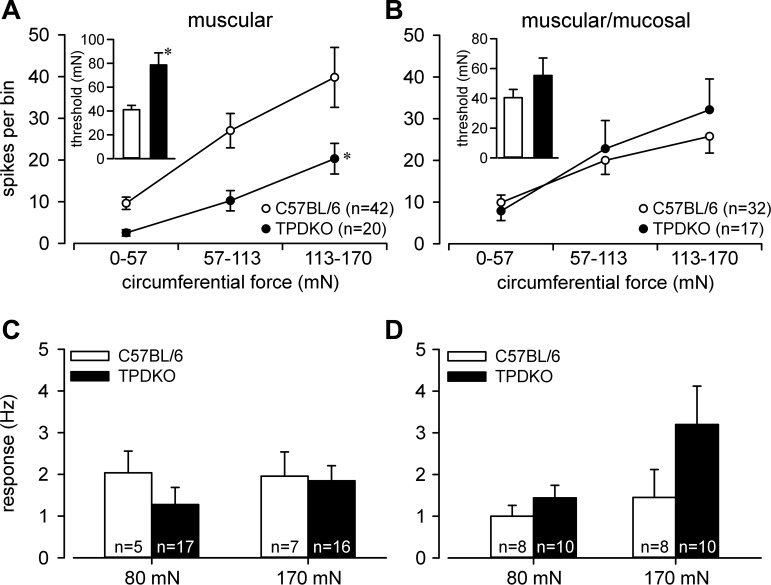
Serosal and mucosal REs in both genotypes gave similar responses to probing (Fig. 3, A and B). Likewise, there was no difference in response to probing between stretch-sensitive muscular and muscular/mucosal afferents (Fig. 3, C and D). As noted previously (e.g., Ref. 12), responses of muscular afferents to stretch in C57BL/6 mice were generally more robust than responses of their muscular/mucosal afferent counterparts. In TPDKO mice, responses of muscular (Fig. 4A), but not muscular/mucosal afferents (Fig. 4B), to stretch were significantly attenuated relative to C57BL/6 mice. Responses to the fast-step stretch protocol did not reveal any genotype differences between muscular and muscular/mucosal afferents (Fig. 4, C and D).
Fig. 3.
Codeletion of TRPV1 and P2X3 (i.e., TPDKO) had no effect on colorectal afferent responses to blunt probing of receptive endings. Stimulus-response curves are presented for serosal (A), mucosal (B), muscular (C), and muscular/mucosal (D) afferents. n = number of fibers/group.
Fig. 4.
Responses of muscular (A), but not muscular/mucosal (B), afferents to circumferential stretch (0–170 mN, 5 mN/s) were reduced in TPDKO relative to C57BL/6 mice (F1/180 = 11.8, *P < 0.001). Insets: response thresholds were increased in muscular (t = 4.3, P < 0.001), but not muscular/mucosal, afferents. C and D: responses of muscular and muscular/mucosal afferents to stepped stretch (0.2-s rise time) did not differ between genotypes. n = number of fibers/group.
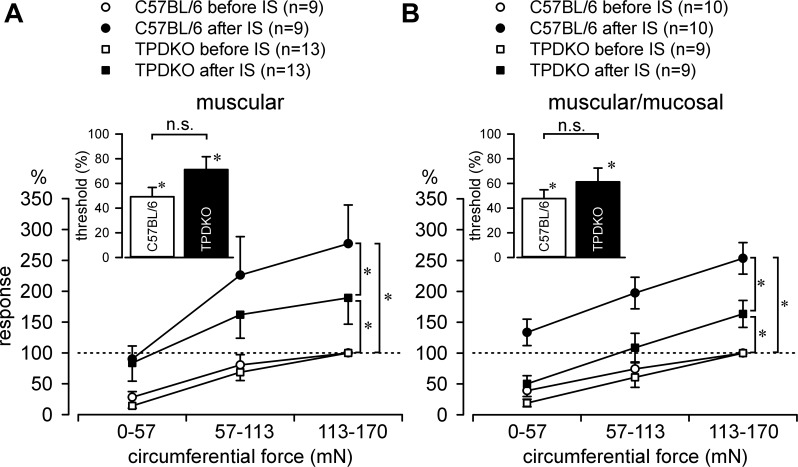
We next examined IS-induced sensitization of stretch-sensitive afferents (stretch-insensitive mucosal and serosal afferents do not sensitize). IS significantly increased stretch-response functions and decreased response thresholds (i.e., sensitized) of muscular as well as muscular/mucosal afferents in both C57BL/6 and TPDKO mice (Fig. 5). However, both afferent classes sensitized to a significantly lesser degree in TPDKO mice. All fibers tested, regardless of genotype, were also activated by IS, revealing RE chemosensitivity. Recovery (washout) was typically complete in both genotypes within ∼20 min after IS. Single-fiber recordings were also performed in TPDKO mice treated intracolonically with saline (36 fibers from 4 mice) or zymosan (26 fibers from 5 mice). Importantly, none of these mice showed any hypersensitivity in CRD experiments. There were no significant differences between saline- and zymosan-treated mice with respect to either proportions of colorectal afferent classes or the response thresholds or stretch-response functions of stretch-sensitive afferents. This is consistent with the absence of colorectal hypersensitivity in these mice (Fig. 1C) as well as the decreased IS-induced afferent sensitization in naïve TPDKO mice (Fig. 5).
Fig. 5.
Codeletion of TRPV1 and P2X3 (i.e., TPDKO) attenuated inflammatory soup (IS)-induced sensitization of stretch-sensitive colorectal afferents. A: IS sensitized stretch-response functions of muscular afferents from C57BL/6 (F1/16 = 9.5, *P < 0.05) and TPDKO mice (F1/24 = 6.6, *P < 0.05). IS also decreased response thresholds (inset: n.s., not significant) in C57BL/6 [paired t-test comparing post-IS thresholds normalized to pre-IS thresholds (threshold, %): t = 6.7, *P < 0.001] and TPDKO mice (t = 2.7, *P < 0.05). B: IS sensitized muscular/mucosal afferents in both C57BL/6 (F1/18 = 37.0, *P < 0.001) and TPDKO mice (F1/16 = 8.6, *P < 0.05). Afferent response thresholds were equally decreased after IS (inset) in both C57BL/6 (t = 7.3, *P < 0.001) and TPDKO mice (t = 3.5, *P < 0.05). n = number of fibers/group. The magnitude of sensitization was significantly less in TPDKO mice in both muscular (A; P < 0.05) and muscular/mucosal (B; P < 0.001) afferents relative to the sensitization produced in afferents recorded from C57BL/6 mice.
Pharmacological antagonism of TRPV1 and P2X3.
To pharmacologically validate the TPDKO phenotype and better interpret channel interaction, we applied TRPV1 and/or P2X3 antagonists onto colorectal afferent REs in C57BL/6 mice, focusing on afferent responses to stretch, the stimulus most affected in TPDKO mice and most relevant for colorectal mechanosensation in vivo. Responses to stretch of 34 muscular and 28 muscular/mucosal afferents were examined before (baseline) and after vehicle (Krebs for TNP-ATP and 1M2P in concentrations of 0.01, 0.1, 1.0, and 10.0% for A889425) and incremental antagonist concentrations around their reported IC50s (100 nM and 300 nM and 1, 3, and 10 μM for A889425; 30 nM and 300 nM and 1 and 3 μM for TNP-ATP) to determine effective concentrations. Most REs were exposed to both vehicles and to both antagonists during testing, but not to all concentrations of vehicles or antagonists.
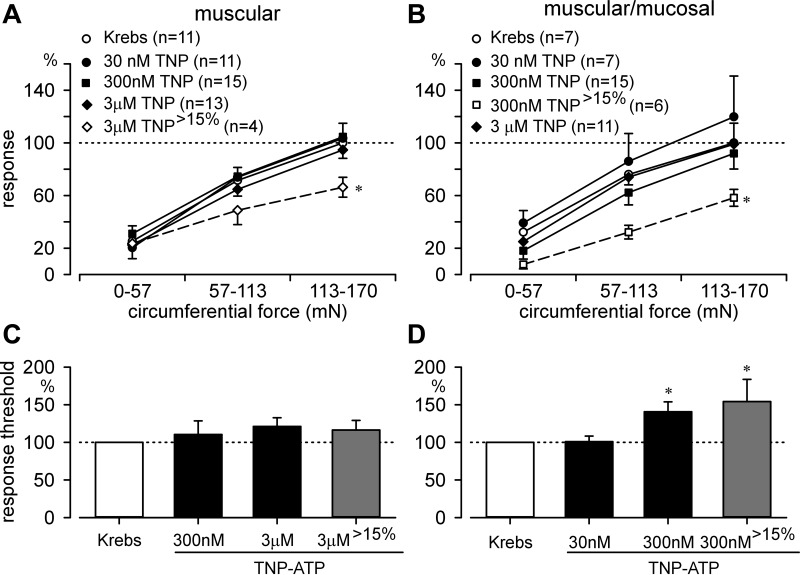
The efficacy of the TRPV1 antagonist A889425 varied considerably and its effects on responses of stretch-sensitive afferents were not apparent at all concentrations tested; 300 nM was most effective in reducing responses of muscular afferents to stretch, whereas 3 μM was most effective in reducing responses of muscular/mucosal afferents (Fig. 6, A and B). Because of intrinsic variability among colorectal afferents and consequent variability in their responses to stretch after exposure to vehicle and because TRPV1 is not expressed in all colorectal afferents (Table 2), we set as an effect criterion a ≥15% posttreatment reduction in afferent response to stretch. Using this criterion (denoted by >15 in Figs. 6 and 7), ∼65% of both muscular (9/14) and muscular/mucosal (8/12) afferents exhibited a significant reduction in their response to stretch after treatment with 300 nM and 3 μM A889425, respectively. To pharmacologically probe TRPV1 expression in these experiments, we applied capsaicin (3 μM) to eight of the above stretch-sensitive afferents after multiple exposures to A889425. Although washout after A889425 was apparent within ∼10 min (i.e., responses to stretch returned to baseline) in the majority of afferents tested, application of capsaicin to REs excited only 2/8 afferents tested (both muscular); however, capsaicin increased response threshold in 6/8 afferents (1/2 muscular/mucosal and 5/6 muscular). In contrast, response thresholds were unaffected by A889425, with or without the effect criterion invoked (Fig. 6, C and D).
Fig. 7.
Antagonism of P2X3 attenuated mechanosensitivity in stretch-sensitive colorectal afferents. A: relative to pretreatment baseline, TNP-ATP had no effect on stretch-response functions in muscular afferents; application of the ≥15% effect criterion (4/13 fibers; ∼31%) revealed a significant effect at the 3 μM concentration (F3,111 = 2.9, *P < 0.05, post hoc comparison, P < 0.05). B: TNP-ATP did not affect muscular/mucosal afferent stretch-response functions, but application of the effect criterion (6/15 fibers; 40%) revealed a significant effect of 300 nM TNP-ATP (F3,81 = 5.5, *P < 0.01, post hoc comparison, P < 0.05). TNP-ATP did not affect response thresholds of muscular afferents (C) but did increase response thresholds of muscular/mucosal afferents without (F2,34 = 6.3, P < 0.01, post hoc comparison, P < 0.01) as well as with (F2,21 = 4.8, *P < 0.05, post hoc comparison, P < 0.05) the effect criterion invoked (D). n = number of fibers/group. TNP, abbreviation for TNP-ATP. >15% denotes application of response criterion.
Compared with vehicle (Krebs solution), 30–300 nM, 1 and 3 μM concentrations of the P2X antagonist TNP-ATP did not attenuate responses of grouped muscular or muscular/mucosal afferents to stretch (Fig. 7, A and B). Applying the same effect criterion as above, ∼35% of both muscular and muscular/mucosal afferents demonstrated a ≥15% decrease in their stimulus-response functions after exposure to 3 μM and 300 nM TNP-ATP, respectively. At these concentrations, TNP-ATP significantly attenuated responses to stretch in both muscular and muscular/mucosal afferents. As above with capsaicin, we attempted to pharmacologically establish P2X3 and P2X2/3 expression by applying α,β-meATP to REs after multiple exposures to TNP-ATP; 3/10 REs tested were excited (1/5 muscular/mucosal and 2/5 muscular), whereas 5/10 REs exhibited an increase in response to stretch (1/5 muscular/mucosal and 4/5 muscular). In contrast to the TRPV1 antagonist, TNP-ATP significantly increased response thresholds of muscular/mucosal afferents (Fig. 7D). Antagonist washout was apparent within ∼10 min in all muscular and most muscular/mucosal afferents tested.
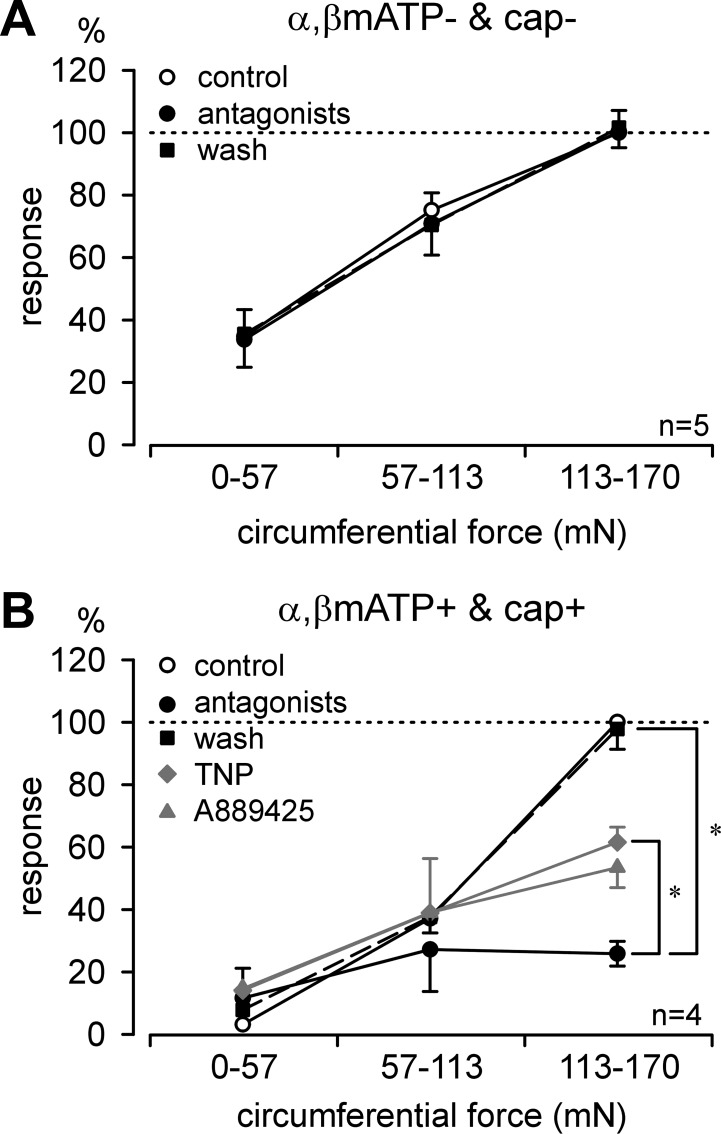
In another group of 11 stretch-sensitive afferent endings, we tested combined pharmacological antagonism with A889425 plus ATP-TNP at the most effective concentrations determined above (300 nM and 3 μM) and also applied the TRPV1 and P2X3 agonists capsaicin (3 μM) and α,β-meATP (3 mM), respectively. Four out of eleven afferents responded to both agonists (3/6 muscular/mucosal and 1/5 muscular afferents). Of those four afferents, two were activated directly by capsaicin; the other two exhibited increased responses to stretch by 137 ± 109%. Similarly, α,β-meATP activated two afferents directly and sensitized the other two (51 ± 13% increase in response to stretch). Five of the eleven afferents responded to neither agonist; responses to stretch were not changed after application of capsaicin (−3.8 ± 5.1%) or α,β-meATP (−4.0 ± 6.1%). The remaining 2/11 responded only to α,β-meATP (1/6 muscular/mucosal and 1/5 muscular afferents). Importantly, no afferents responded to capsaicin alone, indicating 100% coexpression of TRPV1 with P2X3 and/or P2X2/3. Interestingly, afferents responding to both capsaicin and α,β-meATP had significantly higher response thresholds to stretch than those responding to neither agonist (71.5 ± 9.1 vs. 21.1 ± 1.6 mN, t-test, P < 0.001). Figure 8 illustrates that combined antagonist application did not affect responses to stretch in those five afferents that did not respond to either agonist. Co-antagonism with A889425 and TNP-ATP, however, significantly attenuated responses to stretch in those four afferents that responded to both agonists.
Fig. 8.
Combined antagonism of TRPV1 and P2X3 attenuated mechanosensitivity of stretch-sensitive muscular and muscular/mucosal colorectal afferents. Combined A889425 and TNP-ATP application to afferent endings that did not respond to either capsaicin (cap) or α,β-methylene ATP (α,βmATP) was without effect on responses to stretch (A), whereas combined A889425 and TNP-ATP application to afferent endings that responded to both cap and α,βmATP significantly attenuated responses to stretch (F2,27 = 7.1, *P = 0.003, post hoc comparison, P = 0.007 vs. control) (B). For purposes of comparison, muscular and muscular/mucosal stretch-sensitive afferents that met the response criterion (i.e., >15%) were pooled from Figs. 6, A and B (A889425), and 7, A and B (TNP-ATP), and illustrated here in half-tone. Combined antagonism produced a significantly greater attenuation in response than TNP-ATP alone (F1,36 = 7.7, P < 0.01) and a lower response than A889425 alone (borderline significant, F1,57 = 3.6, P = 0.06).
DISCUSSION
In agreement with previous studies in rat lumbar DRG neurons (16, 41, 48), the present work demonstrates that a substantial proportion of mouse PN colorectal afferent somata coexpress TRPV1 with P2X3. Previously, we reported deficits in colorectal mechanosensation and sensitization with single deletion of either TRPV1 or P2X3 (19, 20, 39). Given the coexpression reported here and elsewhere, we expected similar, if not greater, effects in TRPV1-P2X3 double knockout mice. As expected, colorectal hypersensitivity (i.e., enhanced VMR to balloon distension) did not develop in TPDKO mice. Unexpectedly, basal VMRs to CRD were greater in TPDKO than C57BL/6 mice despite significantly reduced responses of muscular afferents to stretch and unchanged responses of other afferent classes in TPDKO mice.
There are a number of possible explanations for the greater basal VMRs to CRD noted in TPDKO relative to C57BL/6 mice. One possibility is disinhibition or compensatory overexpression of ion channels such as TRPV4, TRPA1, or homomeric P2X2 in the peripheral and/or central terminals of primary afferents or in second-order spinal neurons. Of the two channels studied, P2X3 may be most contributory to enhanced VMRs in TPDKO mice because P2X3 single knockout mice have been reported to exhibit pronociceptive behaviors, including enhanced avoidance of noxious thermal stimuli (38). An alternate explanation for increased basal VMR is the redistribution we noted in TPDKO mice of mucosal and muscular/mucosal afferent REs to the distal 1 cm of the colorectum. In our experience, greater VMRs to CRD are evoked with more distal positioning of the distension balloon. This is due, in part, to greater relative excitability of mouse PN afferent endings in the more muscular, distal ∼1 cm of the colorectum (11), a finding also noted in guinea pig rectum (26). Because the proportions of each class of afferent were the same in both genotypes, this caudal transposition in TPDKO mice was unlikely a result of conversion of one class of afferent into another. In support, there were no significant differences between genotypes with respect to probing-response functions of any type of afferent. In other words, the same length, equally positioned CRD balloon in TPDKO mice may stimulate a greater number and proportion of lower threshold and/or more excitable distal PN afferent endings, which may allow central summation. This, of course, in no way discounts greater VMRs and increased inhibitory rectovesicular reflexes (53) with longer-length CRD balloons.
Codeletion of TRPV1 and P2X3 reduced responses of muscular afferents to stretch but had no effect on muscular/mucosal afferents. Responses of muscular and muscular/mucosal afferents to stretch in P2X3-null mice did not differ from responses in C57BL/6 mice (39). In contrast, responses of muscular/mucosal afferents to stretch in mice lacking TRPV1 were significantly attenuated relative to C57BL/6 mice; responses of muscular afferents were unexpectedly approximately twice those of control although not statistically significant (20). Collectively, it appears that deletion of either TRPV1 or P2X3 alone does not alter net peripheral input from PN afferents in response to stretch, whereas codeletion of both channels results in a net decrease in peripheral input from stretch-sensitive afferents.
The TRPV1 antagonist A889425 significantly attenuated responses of muscular and muscular/mucosal afferents to stretch in C57BL/6 mice. Consistent with previous (8) and current (Table 2) reports of TRPV1 expression in 60–70% of mouse colorectal afferents, A889425 was effective in ∼65% of stretch-sensitive afferents, and capsaicin treatment increased response thresholds (i.e., desensitized) in ∼75% of this same afferent population. The latter capsaicin-induced desensitization is a well-recognized phenomenon noted in both human patients and rodents (2, 6). It is unclear why capsaicin reduced responses to stretch in some fibers while it mechanically sensitized or activated others. This could be speculated to be dependent on different pretreatment cellular contents and states of individual afferents. It is also unclear why the dose-response curves for A889425 differed between muscular and muscular/mucosal afferents. It is certainly possible that expression of TRPV1 is more robust in one class of afferent than it is in the other. It is difficult to quantify this given the inability to distinguish muscular vs. muscular/mucosal afferents with more quantitative techniques such as patch-clamp electrophysiology in dissociated colorectal afferent somata.
TNP-ATP was not as broadly effective as A889425 on stretch-sensitive afferents, consistent with the expression of P2X channels in c-DRG somata. Fewer afferent responses met the effect criterion (≥15% reduction) when exposed to TNP-ATP, but those that did (33% of the afferents tested) exhibited attenuated responses to stretch. This correlates well with the proportions of c-DRG somata expressing P2X3 reported here (Table 2) and elsewhere (6, 40). TNP-ATP additionally increased response thresholds in muscular/mucosal afferents, supporting the view that P2X3 and/or P2X2/3 are important primarily for the initiation of nociception (18) and suggesting a greater contribution to mechanosensation by these channels in muscular/mucosal vs. muscular afferents. It should be appreciated that TNP-ATP is not selective for P2X3 and can act as a competitive antagonist at P2X1, P2X3, and P2X2/3 receptors. In previous work (40), we found evidence for P2X3 homomeric (33%) and P2X2/3 heteromeric (20%) expression in c-DRG neurons; <3% of all c-DRG neurons exhibited P2X2 homomeric expression. These data were supported by electrophysiological (whole cell patch clamp) evidence; no P2X1-type inward currents were noted. Thus, although TNP-ATP is not P2X3 selective, the virtual absence of P2X1 and P2X2 homomeric receptors in mouse c-DRG neurons limits the activity of TNP-ATP to c-DRG neurons expressing P2X3 receptors, either in homomeric or heteromeric configurations.
With regard to the effect criterion used to define treatment effect, we chose a ≥15% change from pretreatment response or threshold because in our hands this was greater than the inherent response variability with the same, repeated stimulus in the same fiber. This percent change was also greater than the inherent variability noted after application of vehicle. Supporting use of this criterion as well as antagonist selectivity, in single antagonist/agonist experiments, of the 6/8 stretch-sensitive fibers demonstrating a ≥15% capsaicin-induced increase in response threshold, five of the six fibers exhibited a ≥15% A889425-induced decrease in response to stretch. Likewise, of the 5/10 stretch-sensitive fibers that showed a ≥15% α,β-meATP-induced increase in stimulus response, 2/5 exhibited a ≥15% TNP-ATP-induced decrease in stimulus response or a ≥15% increase in response threshold. The reasons for incomplete association between agonist- and antagonist-induced changes in afferent responses to stretch are unclear but may reflect inadequate washout of antagonists before application of agonists.
Combined antagonism of TRPV1 and P2X3 significantly attenuated responses to stretch in colorectal afferents that responded to both capsaicin and α,β-meATP but had no effect in afferents that did not respond to either agonist. The results suggest an additive, if not greater than additive, attenuation of afferent response to stretch by simultaneous antagonism of both channels. This may be secondary to a combined effect of two independent processes, or it may indicate an interaction that could be mediated through shared Ca2+-dependent signaling cascades (10, 21, 33, 36, 47, 55) or modulatory conformational spread (4) via direct physical association of TRPV1 and P2X3 as demonstrated previously (41). Importantly, this combined antagonist/agonist experiment additionally confers pharmacological validity to the selectivity of the drugs used (e.g., TNP-ATP has effects only on REs that respond to α,β-meATP). Why both antagonists alone or in combination attenuated muscular/mucosal as well as muscular afferents while combined genetic deletion of TRPV1 and P2X3 affected only muscular fibers is unclear. This may be secondary to disinhibition, recruitment, or compensatory overexpression of other ion channels as discussed above. It is not uncommon for discrepancies to exist between knockout and pharmacological models, and, for this reason, the effects of single vs. combined channel manipulation are compared within rather than across experimental models.
To further explore the contributions of TRPV1 and P2X3 to colorectal afferent mechanosensation, we evaluated in TPDKO mice acute sensitization of stretch-sensitive afferents by IS. In both TRPV1- and P2X3-null mice, IS sensitized muscular/mucosal but not muscular afferent endings (20, 39). In TPDKO mice, however, both afferent classes were sensitized by IS although the magnitude of sensitization was significantly reduced relative to sensitization in C57BL/6 mice. Differences in outcomes between single and double knockout mice may be explained by compensatory changes in protein expression as noted above. In muscular/mucosal afferents, sensitization may cause upregulation or enhanced functioning of TRPV1 and P2X3 (17) as well as other ion channels. Therefore, if only one channel is deleted, functioning of the remaining channels may be sufficiently enhanced when sensitized such that no loss-of-function phenotype is apparent. Only when both channels are eliminated is a reduction in afferent responses to stretch evident, and even then some sensitization is still present. In muscular afferents, on the other hand, deletion of either or both TRPV1 and P2X3 is sufficient to produce a loss-of-function phenotype, suggesting that both channels are critical and/or redundant for mechanical sensitization in this afferent class. Although we collected only limited single-fiber data in zymosan-treated TPDKO mice, there was no significant sensitization of stretch-sensitive afferents, consistent with the absence of CRD hypersensitivity and attenuated IS-induced sensitization. This is in contrast to C57BL/6 mice, which exhibit significant zymosan-induced CRD hypersensitivity and sensitization of muscular/mucosal afferents (14). Moreover, consistent with previous work in C57BL/6 mice (13, 14, 43), IS treatment in TPDKO mice produced a greater magnitude of sensitization of stretch-sensitive afferents than did zymosan treatment. This may be explained by the concentration of several inflammatory mediators in IS, which was designed to maximally sensitize afferents. Therefore, the persistence of IS-induced afferent sensitization in TPDKO mice is not inconsistent with the absence of behavioral and afferent sensitization in these same mice with milder, more physiological zymosan sensitization.
We made several additional notable observations. First, responses of muscular afferents to ramped stretch were significantly attenuated in TPDKO mice, whereas responses to stepped stretch or probing were not. This suggests a stimulus-specific contribution of TRPV1 and/or P2X3 or P2X2/3 to afferent mechanosensation. It is possible that only slow ramps or repeated steps of increasing stimulus intensity [as performed in our previous studies (20, 39)] are sufficient to recruit these channels because these stimuli allow sufficient time for biochemical generation of ATP and endogenous lipid ligands of TRPV1. Second, TRPV1 and P2X3 appear to be more important for mechanosensation/sensitization in muscular/mucosal afferents than in other colorectal afferent classes. Coincidently, zymosan-induced colorectal hypersensitivity to CRD is associated with sensitization of muscular/mucosal afferents (14). Thus TRPV1 and P2X3 may be important in the development and/or maintenance of zymosan-induced colorectal hypersensitivity and, by extension, IBS pain. Third, deletion of both channels abolished zymosan-induced recruitment of MIAs, which also has been observed in C57BL/6 mice (14). Considering that very few colorectal PN MIAs respond to capsaicin (12), our observation in TPDKO mice suggests that P2X3 may be important for mechanical sensitization of MIAs.
In summary, the present study confirms the importance of TRPV1 and P2X3 for colorectal mechanosensation and hypersensitivity at the levels of the primary afferent as well as the whole organism. By evaluating inhibition of both channels simultaneously, this work also contributes to the growing appreciation of the interaction and cooperation between distinct ion channels, which is not uncommon [e.g., TRPV1-TRPA1 (42) and P2X3-GABAA (44)] and may prove important for guiding drug development. With regard to limitations, the single-fiber work focused exclusively on PN colorectal afferents to build on previous studies in this same afferent population (19, 20, 39). Although the PN is necessary and sufficient for mediating colorectal nociception (23), the other source of extrinsic colorectal innervation, the lumbar splanchnic nerve (LuSN), was not evaluated here. Because the LuSN pathway may be relevant for chemosensation (6) and/or central sensitization (45), a TRPV1-P2X3 interaction in LuSN afferents or thoracolumbar spinal cord may reveal unexpected contributions to mechanosensation. For example, it has been reported that activity in PN colorectal afferents actively modulates thoracolumbar dorsal horn neuron processing of the same distending colorectal stimulus through a supraspinal loop (51). Indeed, we previously concluded that P2X3 contributed to colorectal hypersensitivity at both peripheral and central sites (39). Because increased afferent mechanosensitivity is considered key to IBS, we focused here on the TRPV1-P2X3 interaction in PN afferents, but afferent input has obvious central implications that were not experimentally addressed here. The results in TPDKO mice are generally supportive of previous studies in single knockout mice as well as pharmacological antagonism. Differences in outcomes between single and double knockout mice could be due to functional redundancy of TRPV1 and P2X3 in some afferents and compensation in others. The relative importance of these channels appears to be enhanced in hypersensitivity, suggesting their importance in colorectal mechanosensation and potential combined pharmacological antagonism as a strategy for improving treatment for IBS pain and hypersensitivity.
GRANTS
This work was supported by NIH awards R01 DK093525 (G. Gebhart) and T32 NS007433 (M. Kiyatkin).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: M.E.K., E.S.S., and G.F.G. conception and design of research; M.E.K. and B.F. performed experiments; M.E.K., B.F., and G.F.G. analyzed data; M.E.K., B.F., E.S.S., and G.F.G. interpreted results of experiments; M.E.K. and B.F. prepared figures; M.E.K. drafted manuscript; M.E.K., B.F., E.S.S., and G.F.G. edited and revised manuscript; M.E.K., B.F., E.S.S., and G.F.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Michael Burcham for assistance in preparation of figures, Jun-Ho La, Nicole Scheff, and Michael S. Gold for assistance with single-cell PCR and Ca2+ imaging, and Abbott Laboratories for their generous gift of the TRPV1 antagonist A889425.
REFERENCES
- 1.Akbar A, Yiangou Y, Facer P, Walters JR, Anand P, Ghosh S. Increased capsaicin receptor TRPV1-expressing sensory fibres in irritable bowel syndrome and their correlation with abdominal pain. Gut 57: 923–929, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bortolotti M, Coccia G, Grossi G, Miglioli M. The treatment of functional dyspepsia with red pepper. Aliment Pharmacol Ther 16: 1075–1082, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Bouin M, Plourde V, Boivin M, Riberdy M, Lupien F, Laganiere M, Verrier P, Poitras P. Rectal distention testing in patients with irritable bowel syndrome: sensitivity, specificity, and predictive values of pain sensory thresholds. Gastroenterology 122: 1771–1777, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Bray D, Duke T. Conformational spread: the propagation of allosteric states in large multiprotein complexes. Annu Rev Biophys Biomol Struct 33: 53–73, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Brederson JD, Chu KL, Reilly RM, Brown BS, Kym PR, Jarvis MF, McGaraughty S. TRPV1 antagonist, A-889425, inhibits mechanotransmission in a subclass of rat primary afferent neurons following peripheral inflammation. Synapse 66: 187–195, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Brierley SM, Carter R, Jones W, 3rd, Xu L, Robinson DR, Hicks GA, Gebhart GF, Blackshaw LA. Differential chemosensory function and receptor expression of splanchnic and pelvic colonic afferents in mice. J Physiol 567: 267–281, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christianson JA, Gebhart GF. Assessment of colon sensitivity by luminal distension in mice. Nat Protoc 2: 2624–2631, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Christianson JA, Traub RJ, Davis BM. Differences in spinal distribution and neurochemical phenotype of colonic afferents in mouse and rat. J Comp Neurol 494: 246–259, 2006 [DOI] [PubMed] [Google Scholar]
- 9.De Schepper HU, De Winter BY, Van Nassauw L, Timmermans JP, Herman AG, Pelckmans PA, De Man JG. TRPV1 receptors on unmyelinated C-fibres mediate colitis-induced sensitization of pelvic afferent nerve fibres in rats. J Physiol 586: 5247–5258, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Docherty RJ, Yeats JC, Bevan S, Boddeke HW. Inhibition of calcineurin inhibits the desensitization of capsaicin-evoked currents in cultured dorsal root ganglion neurones from adult rats. Pflügers Arch 431: 828–837, 1996 [DOI] [PubMed] [Google Scholar]
- 11.Feng B, Brumovsky PR, Gebhart GF. Differential roles of stretch-sensitive pelvic nerve afferents innervating mouse distal colon and rectum. Am J Physiol Gastrointest Liver Physiol 298: G402–G409, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng B, Gebhart GF. Characterization of silent afferents in the pelvic and splanchnic innervations of the mouse colorectum. Am J Physiol Gastrointest Liver Physiol 300: G170–G180, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng B, Kiyatkin ME, La JH, Ge P, Solinga R, Silos-Santiago I, Gebhart GF. Activation of guanylate cyclase-C attenuates stretch responses and sensitization of mouse colorectal afferents. J Neurosci 33: 9831–9839, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng B, La JH, Schwartz ES, Tanaka T, McMurray TP, Gebhart GF. Long-term sensitization of mechanosensitive and -insensitive afferents in mice with persistent colorectal hypersensitivity. Am J Physiol Gastrointest Liver Physiol 302: G676–G683, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gever JR, Cockayne DA, Dillon MP, Burnstock G, Ford AP. Pharmacology of P2X channels. Pflügers Arch 452: 513–537, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Guo A, Vulchanova L, Wang J, Li X, Elde R. Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur J Neurosci 11: 946–958, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Hamilton SG, Wade A, McMahon SB. The effects of inflammation and inflammatory mediators on nociceptive behaviour induced by ATP analogues in the rat. Br J Pharmacol 126: 326–332, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamilton SG, Warburton J, Bhattacharjee A, Ward J, McMahon SB. ATP in human skin elicits a dose-related pain response which is potentiated under conditions of hyperalgesia. Brain 123: 1238–1246, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Jones RC, 3rd, Otsuka E, Wagstrom E, Jensen CS, Price MP, Gebhart GF. Short-term sensitization of colon mechanoreceptors is associated with long-term hypersensitivity to colon distention in the mouse. Gastroenterology 133: 184–194, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Jones RC, 3rd, Xu L, Gebhart GF. The mechanosensitivity of mouse colon afferent fibers and their sensitization by inflammatory mediators require transient receptor potential vanilloid 1 and acid-sensing ion channel 3. J Neurosci 25: 10981–10989, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.King B, Chen CC, Akopian AN, Burnstock G, Wood JN. A role for calcineurin in the desensitization of the P2X3 receptor. Neuroreport 8: 1099–1102, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Kiyatkin M, Gebhart G. Role of TRPV1 and P2X3 in mechanosensation in colorectal primary afferents. In: Program No 47305 2012 Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience, 2012 [Google Scholar]
- 23.Kyloh M, Nicholas S, Zagorodnyuk VP, Brookes SJ, Spencer NJ. Identification of the visceral pain pathway activated by noxious colorectal distension in mice. Front Neurosci 5: 16, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lembo T, Munakata J, Mertz H, Niazi N, Kodner A, Nikas V, Mayer EA. Evidence for the hypersensitivity of lumbar splanchnic afferents in irritable bowel syndrome. Gastroenterology 107: 1686–1696, 1994 [DOI] [PubMed] [Google Scholar]
- 25.Lu SG, Zhang X, Gold MS. Intracellular calcium regulation among subpopulations of rat dorsal root ganglion neurons. J Physiol 577: 169–190, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lynn PA, Olsson C, Zagorodnyuk V, Costa M, Brookes SJ. Rectal intraganglionic laminar endings are transduction sites of extrinsic mechanoreceptors in the guinea pig rectum. Gastroenterology 125: 786–794, 2003 [DOI] [PubMed] [Google Scholar]
- 27.McGaraughty S, Chu KL, Brown BS, Zhu CZ, Zhong C, Joshi SK, Honore P, Faltynek CR, Jarvis MF. Contributions of central and peripheral TRPV1 receptors to mechanically evoked and spontaneous firing of spinal neurons in inflamed rats. J Neurophysiol 100: 3158–3166, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Mertz H, Naliboff B, Munakata J, Niazi N, Mayer EA. Altered rectal perception is a biological marker of patients with irritable bowel syndrome. Gastroenterology 109: 40–52, 1995 [DOI] [PubMed] [Google Scholar]
- 29.Miranda A, Nordstrom E, Mannem A, Smith C, Banerjee B, Sengupta JN. The role of transient receptor potential vanilloid 1 in mechanical and chemical visceral hyperalgesia following experimental colitis. Neuroscience 148: 1021–1032, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nealen ML, Gold MS, Thut PD, Caterina MJ. TRPM8 mRNA is expressed in a subset of cold-responsive trigeminal neurons from rat. J Neurophysiol 90: 515–520, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Phillis BD, Martin CM, Kang D, Larsson H, Lindstrom EA, Martinez V, Blackshaw LA. Role of TRPV1 in high-threshold rat colonic splanchnic afferents is revealed by inflammation. Neurosci Lett 459: 57–61, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Plourde V, Lembo T, Shui Z, Parker J, Mertz H, Tache Y, Sytnik B, Mayer E. Effects of the somatostatin analogue octreotide on rectal afferent nerves in humans. Am J Physiol Gastrointest Liver Physiol 265: G742–G751, 1993 [DOI] [PubMed] [Google Scholar]
- 33.Price TJ, Jeske NA, Flores CM, Hargreaves KM. Pharmacological interactions between calcium/calmodulin-dependent kinase II alpha and TRPV1 receptors in rat trigeminal sensory neurons. Neurosci Lett 389: 94–98, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ritchie J. Pain from distension of the pelvic colon by inflating a balloon in the irritable colon syndrome. Gut 14: 125–132, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robinson DR, McNaughton PA, Evans ML, Hicks GA. Characterization of the primary spinal afferent innervation of the mouse colon using retrograde labelling. Neurogastroenterol Motil 16: 113–124, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Rosenbaum T, Gordon-Shaag A, Munari M, Gordon SE. Ca2+/calmodulin modulates TRPV1 activation by capsaicin. J Gen Physiol 123: 53–62, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwartz ES, Christianson JA, Chen X, La JH, Davis BM, Albers KM, Gebhart GF. Synergistic role of TRPV1 and TRPA1 in pancreatic pain and inflammation. Gastroenterology 140: 1283–1291; e1281–1282, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shimizu I, Iida T, Guan Y, Zhao C, Raja SN, Jarvis MF, Cockayne DA, Caterina MJ. Enhanced thermal avoidance in mice lacking the ATP receptor P2X3. Pain 116: 96–108, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Shinoda M, Feng B, Gebhart GF. Peripheral and central P2X receptor contributions to colon mechanosensitivity and hypersensitivity in the mouse. Gastroenterology 137: 2096–2104, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shinoda M, La JH, Bielefeldt K, Gebhart GF. Altered purinergic signaling in colorectal dorsal root ganglion neurons contributes to colorectal hypersensitivity. J Neurophysiol 104: 3113–3123, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stanchev D, Blosa M, Milius D, Gerevich Z, Rubini P, Schmalzing G, Eschrich K, Schaefer M, Wirkner K, Illes P. Cross-inhibition between native and recombinant TRPV1 and P2X(3) receptors. Pain 143: 26–36, 2009 [DOI] [PubMed] [Google Scholar]
- 42.Staruschenko A, Jeske NA, Akopian AN. Contribution of TRPV1-TRPA1 interaction to the single channel properties of the TRPA1 channel. J Biol Chem 285: 15167–15177, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tanaka T, Shinoda M, Feng B, Albers KM, Gebhart GF. Modulation of visceral hypersensitivity by glial cell line-derived neurotrophic factor family receptor α-3 in colorectal afferents. Am J Physiol Gastrointest Liver Physiol 300: G418–G424, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Toulme E, Blais D, Leger C, Landry M, Garret M, Seguela P, Boue-Grabot E. An intracellular motif of P2X(3) receptors is required for functional cross-talk with GABA(A) receptors in nociceptive DRG neurons. J Neurochem 102: 1357–1368, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Traub RJ. Evidence for thoracolumbar spinal cord processing of inflammatory, but not acute colonic pain. Neuroreport 11: 2113–2116, 2000 [DOI] [PubMed] [Google Scholar]
- 46.Trimble KC, Farouk R, Pryde A, Douglas S, Heading RC. Heightened visceral sensation in functional gastrointestinal disease is not site-specific. Evidence for a generalized disorder of gut sensitivity. Dig Dis Sci 40: 1607–1613, 1995 [DOI] [PubMed] [Google Scholar]
- 47.Tsuda M, Hasegawa S, Inoue K. P2X receptors-mediated cytosolic phospholipase A2 activation in primary afferent sensory neurons contributes to neuropathic pain. J Neurochem 103: 1408–1416, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Ueno S, Tsuda M, Iwanaga T, Inoue K. Cell type-specific ATP-activated responses in rat dorsal root ganglion neurons. Br J Pharmacol 126: 429–436, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Verne GN, Robinson ME, Vase L, Price DD. Reversal of visceral and cutaneous hyperalgesia by local rectal anesthesia in irritable bowel syndrome (IBS) patients. Pain 105: 223–230, 2003 [DOI] [PubMed] [Google Scholar]
- 50.Verne GN, Sen A, Price DD. Intrarectal lidocaine is an effective treatment for abdominal pain associated with diarrhea-predominant irritable bowel syndrome. J Pain 6: 493–496, 2005 [DOI] [PubMed] [Google Scholar]
- 51.Wang G, Tang B, Traub RJ. Pelvic nerve input mediates descending modulation of homovisceral processing in the thoracolumbar spinal cord of the rat. Gastroenterology 133: 1544–1553, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wiskur BJ, Tyler K, Campbell-Dittmeyer K, Chaplan SR, Wickenden AD, Greenwood-Van Meerveld B. A novel TRPV1 receptor antagonist JNJ-17203212 attenuates colonic hypersensitivity in rats. Methods Find Exp Clin Pharmacol 32: 557–564, 2010 [DOI] [PubMed] [Google Scholar]
- 53.Wyndaele M, De Wachter S, De Man J, Minagawa T, Wyndaele JJ, Pelckmans PA, De Winter BY. Mechanisms of pelvic organ crosstalk: 1. Peripheral modulation of bladder inhibition by colorectal distention in rats. J Urol 190: 765–771, 2013 [DOI] [PubMed] [Google Scholar]
- 54.Wynn G, Rong W, Xiang Z, Burnstock G. Purinergic mechanisms contribute to mechanosensory transduction in the rat colorectum. Gastroenterology 125: 1398–1409, 2003 [DOI] [PubMed] [Google Scholar]
- 55.Xu GY, Huang LY. Ca2+/calmodulin-dependent protein kinase II potentiates ATP responses by promoting trafficking of P2X receptors. Proc Natl Acad Sci USA 101: 11868–11873, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu GY, Shenoy M, Winston JH, Mittal S, Pasricha PJ. P2X receptor-mediated visceral hyperalgesia in a rat model of chronic visceral hypersensitivity. Gut 57: 1230–1237, 2008 [DOI] [PubMed] [Google Scholar]