Abstract
Intestinal fat absorption is known to be, overall, a highly efficient process, but much less is known about the efficiency with which individual dietary fatty acids (FA) are absorbed by the adult small intestine. We therefore measured the absorption efficiency of the major dietary FA using sucrose polybehenate (SPB) as a nonabsorbable marker and analyzed how it is modulated by acyl chain physicochemical properties and polymorphisms of proteins involved in chylomicron assembly. Dietary FA absorption efficiency was measured in 44 healthy subjects fed a standard diet containing 35% fat and 5% SPB. FA and behenic acid (BA) were measured in homogenized diets and stool samples by gas chromatography-mass spectroscopy, and coefficients of absorption for each FA were calculated as 1 − [(FA/BA)feces/(FA/BA)diet]. Absorption coefficients for saturated FA decreased with increasing chain length and hydrophobicity (mean ± SE) and ranged from 0.95 ± 0.02 for myristate (14:0), 0.80 ± 0.03 for stearate (18:0), to 0.26 ± 0.02 for arachidate (20:0). Absorption coefficients for unsaturated FA increased with increasing desaturation from 0.79 ± 0.03 for elaidic acid (18:1t), 0.96 ± 0.01 for linoleate (18:2), to near complete absorption for eicosapentaenoic (20:5) and docosahexaenoic (22:6) acids. Of several common genetic polymorphisms in key proteins involved in the chylomicron assembly pathway, only the intestinal fatty acid-binding protein-2 A54T allele (rs1799883) had any impact on FA absorption. We conclude that acyl chain length, saturation, and hydrophobicity are the major determinants of the efficiency with which dietary FA are absorbed by the adult small intestine.
Keywords: intestinal lipid absorption, sucrose polybehenate, mixed micelles, chylomicron assembly, genetic polymorphisms
the human diet contains fats that incorporate a broad spectrum of fatty acids (FA) of varying chain length and degree of desaturation (50). Decades of epidemiological and clinical studies have established that individual FA can have dramatically different effects on human lipid metabolism and disease. For example, dietary saturated FA, especially myristic (14:0), palmitic (16:0), and elaidic (18:1trans) acids, raise plasma low density lipoprotein cholesterol levels and increase the risk for developing atherosclerotic cardiovascular disease (66), whereas increased intake of oleic acid (18:1) and polyunsaturated FA, such as linoleic (18:2) and linolenic (18:3) acids, has the opposite effects (16). The long-chain, highly unsaturated ω3 FA eicosahexaenoic acid (EPA, 20:5) and docosahexaenoic acid (DHA, 22:6) lower plasma triglycerides (TG), mediate a number of salutary metabolic and anti-inflammatory effects (4, 32, 36), and accelerate neonatal neural development (23, 24). Thus, the efficiency with which different dietary FA are absorbed by the intestine is relevant to assessing their impact on human health.
Dietary fat absorption has classically been measured by fat balance studies (12, 50). The method is conceptually simple: subjects eat a standardized diet containing a defined amount of fat; daily dietary intake is carefully recorded; and the entire fecal output over several days is collected, homogenized, and analyzed for TG. However, in practice, fat balance studies are burdened by esthetic, logistical, and technical issues that can introduce considerable inaccuracy. Thus, although total dietary TG absorption is acknowledged to be, overall, a highly efficient process (1, 12, 25, 50), much less is known about the efficiency with which individual nutritionally important FA are absorbed by the healthy adult gut.
A novel solution to these problems was described by Jandacek et al., who showed that sucrose polybehenate (SBP), a component of the Food and Drug Administration (FDA)-approved food additive Olestra, in which multiple behenic acid (BA, 22:0) chains are esterified to sucrose, can serve as a useful nonabsorbable marker in fat balance studies (26). Because SPB is not hydrolyzed by intestinal lipases, it passes unchanged into the stool; thereafter, chemical hydrolysis of diet and stool samples liberates BA, and determination of the FA-to-BA ratios in both diet and stool yields the absorption efficiency of each dietary FA with a single analysis. With this elegant ratio method there is no need to collect the total fecal output over several days (very small daily samples suffice), and the accuracy of the calculated absorption coefficients is less sensitive to subject-to-subject variation in dietary intake or stool frequency.
In the present study, we used the SPB method to measure coefficients of absorption for the full spectrum of nutritionally significant dietary FA in a cohort of healthy adult subjects and analyzed how FA absorption efficiency is modulated by acyl chain physicochemical properties. In addition, we also examined how several functional polymorphisms in key proteins involved in chylomicron assembly impact intestinal FA absorption. Our data establish that acyl chain length, degree of saturation and hydrophobicity, but not common genetic polymorphisms, are the major determinants of the efficiency with which dietary FA are absorbed by the adult small intestine, in keeping with the central role of bile salt-mixed micelles in solubilizing dietary FA in the intestinal lumen (12, 50). Comparison of these data with ongoing studies examining the impact of bariatric surgical procedures on the absorption of individual dietary FA may further elucidate the mechanisms by which these procedures exert their salutary metabolic effects.
MATERIALS AND METHODS
Subjects.
This research was conducted in accord with the ethical principles of The Declaration of Helsinki. The research protocol and informed consent forms used in the study were reviewed and approved by the Wake Forest Health Sciences Institutional Review Board (IRB00001070). Healthy adult subjects were recruited by posted notices. The sole inclusion criteria were age ≥18 yr and body mass index (BMI) between 19 and 35. Exclusion criteria were: 1) a history of gastrointestinal disease, including chronic constipation, chronic diarrhea, inflammatory bowel disease, chronic pancreatitis, or liver disease; 2) a history of gastrointestinal surgery, including cholecystectomy, gastric bypass, or bowel resection; 3) a history of hyperlipidemia; hypertension; diabetes; thyroid, renal, or cardiovascular disease; 4) use of insulin, oral hypoglycemic medications, or medications that can alter lipid absorption or metabolism; 5) known intolerance to fish oil or Olestra; and 6) pregnancy or breast feeding. The purpose and procedures of the study were reviewed with each eligible respondent; subjects interested in participating then provided written informed consent.
Study design.
The study was conducted in the Wake Forest School of Medicine Clinical Research Unit (CRU), which provided a dedicated team of research nurses, a metabolic kitchen directed by a research dietician, and an on-site specimen processing lab. Every morning for 4 days, subjects came to the CRU to pick up a cooler containing all of their meals for the day, which had been previously prepared by the CRU metabolic kitchen. Subjects were instructed to eat and drink only the foods provided by the metabolic kitchen during the study, with the exception of water or noncaloric beverages. On days 3 and 4, subjects collected a small sample of feces in sterile plastic containers, froze them immediately, and returned them to the CRU the following day. Stool samples were stored at −20°C until FA analysis was conducted.
Study diet.
The CRU research dietician created menus for two full days of nutrient-controlled meals that provided 15% of calories as protein, 50% as carbohydrate, and 35% as fat, and 360 mg cholesterol/day. The 2-day meal cycle was repeated once during the study. To assure that the diet contained sufficient ω3 FA to accurately measure EPA (20:5) and DHA (22:6) absorption, the subjects took a 1,000-mg fish oil capsule with lunch and dinner. SPB (SEFOSE 2275), a component of the FDA-approved food additive Olestra, was obtained from Proctor & Gamble Chemicals (Cincinnati, OH). SPB was ground to a powder and incorporated into baked items (e.g., muffins, breads, cookies) eaten at each meal such that the daily SPB intake was 5% of total fat intake. Thus, for example, a 2,000 kcal/day diet with a 35% fat content contained 3.9 grams of SBP, the same amount contained in a half ounce serving of “low-fat” potato chips (∼8 chips) (52). For analysis, all the foods in the 2-day diet cycle, plus the fish oil capsules, were combined, homogenized, and stored at −20°C. Compliance with the diet was monitored by having the subjects complete daily checklists of which foods they did/did not consume, and thereafter, in the analysis phase of the study, by monitoring the appearance of BA in the fecal samples.
FA analysis.
Weighed samples of the homogenized diet and the subjects' stools were saponified with methanolic NaOH, extracted with hexane, and analyzed in duplicate by gas chromatography-mass spectroscopy (6) to quantitate the concentration of BA (22:0) and the major dietary FA: myristic acid (14:0), myristoleic acid (14:1), palmitic acid (16:0), palmitoleic acid (16:1), stearic acid (18:0), oleic acid (18:1), elaidic acid (18:1trans), linoleic acid (18:2), α-linolenic acid (18:3ω3), γ-linolenic acid (18:3ω6), arachidic acid (20:0), gadoleic acid (20:1), eicosadienoic acid (20:2), arachidonic acid (ARA, 20:4ω6), eicosapentaenoic acid (EPA, 20:5ω3), and DHA (22:6ω3). The fractional absorption for each FA was calculated as: 1 − [(FA/BA)feces/(FA/BA)diet]. Total dietary TG absorption was similarly calculated by summing all the FA present in the diet and the stool samples from each subject. FA melting points (44) and reverse-phase HPLC retention times (9, 38) were taken from literature values.
Genetic polymorphism analysis.
Buffy coat DNA was collected from each subject and analyzed in the CRU Molecular Genetics Core Laboratory to identify several single nucleotide polymorphisms (SNP) in the genes for key proteins involved in chylomicron assembly that have been previously reported to alter postprandial plasma TG levels. Table 1 lists these SNP, their allele frequencies, and their reported impact on postprandial plasma TG. DNA sequencing reactions were performed as previously described (19). Sequencing products were analyzed on an ABI 3730 XL DNA Analyzer (Applied Bioystems, Foster City, CA). Sequence alignment and polymorphism identification were performed with Sequencher 4.2 (Gene Codes, Ann Arbor, MI). A genotype for each SNP was successfully assigned for most of the subjects.
Table 1.
Functional polymorphisms in key genes involved with chylomicron assembly
| Gene | Ref. SNP No. | Substitution | Reported Frequency | Observed Frequency | Effect on Postprandial Plasma TG | Reference No. |
|---|---|---|---|---|---|---|
| APOA4 | Rs675 | T347S | 0.20 | 0.30 | Decrease | 21,46 |
| APOB | Rs693 | 2488C>T | 0.47 | 0.52 | Decrease | 40 |
| APOB | rs679899 | V591A | 0.36 | 0.42 | Decrease | 54 |
| MTTP | rs1800591 | −493G>T | 0.25 | 0.23 | Increase | 43 |
| FABP2 | rs1799883 | A54T | 0.28 | 0.34 | Increase | 2 |
APO, apolipoprotein; MTTP, microsomal triglyceride transfer protein; FABP2, fatty acid-binding protein-2.
Statistical analysis.
Data were analyzed using Sigma Stat 3.00 (Systat Software, San Jose, CA). Parametric data are expressed as means ± SE. The significance of differences in the mean coefficients of absorption among individual FA was determined by analysis of variance with Holm-Sidak or Dunn's multiple-comparison post hoc testing. Correlations among total dietary TG absorption efficiency, BMI, and body surface area were examined by Pearson product moment analysis. The impacts of gender and genotype on individual FA absorption efficiency were analyzed by unpaired Student's t-tests. The significance of differences in the frequency of variant alleles between male and female subjects was determined by Chi-square analysis. Differences were considered significant at P < 0.05.
RESULTS
A total of 44 subjects (36 women and 8 men) participated in the study. Subject age ranged from 21 to 59 yr; the mean age of the entire cohort was 34.3 ± 1.6 yr (Table 2). BMI ranged from 18.5 to 33.9 kg/m2, with a mean of 25.0 ± 0.6 kg/m2; only six subjects had a BMI >30 kg/m2. Subject body surface area, calculated using the Dubois formula (11), ranged from 1.30 to 2.45 m2, with a mean of 1.80 ± 0.04 m2. No subject experienced any gastrointestinal side effects from the study diet. The coefficient of absorption for total dietary TG for the entire cohort was 0.92 ± 0.01; the coefficient of variation was 7.4%. Gender had no effect on total dietary TG absorption efficiency. There was no significant correlation between total dietary TG absorption efficiency and either BMI or body surface area in either gender.
Table 2.
Subject characteristics
| All Subjects | Women | Men | |
|---|---|---|---|
| n | 44 | 36 | 8 |
| Age, yr | 34.3 ± 1.6 | 35.3 ± 1.8 | 29.9 ± 3.2 |
| Body mass index, kg/m2 | 25.0 ± 0.6 | 24.4 ± 0.7 | 26.7 ± 1.3 |
| Body surface area, m2 | 1.80 ± 0.04 | 1.72 ± 0.03a | 2.13 ± 0.06 |
| Total fat absorption | 0.92 ± 0.01 | 0.91 ± 0.01 | 0.94 ± 0.01 |
Values are means ± SE; n, no. of subjects.
P < 0.001, females vs. males by Student's t-test.
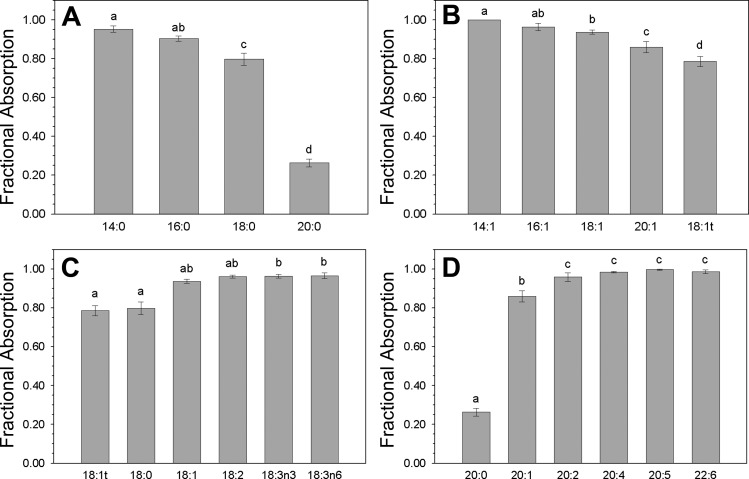
The efficiency with which individual FA were absorbed was influenced by both acyl chain length and saturation. For saturated FA, absorption efficiency decreased with increasing chain length from 0.953 ± 0.017 for myristic acid (14:0) to 0.798 ± 0.032 for stearic acid (18:0), and fell sharply to 0.264 ± 0.02 for arachic acid (20:0) (Fig. 1A). A similar pattern was observed for the monounsaturated FA: absorption efficiency decreased from 1.000 ± 0.000 for myristoleic acid (14:1) to 0.860 ± 0.029 for eiscosenoic acid (20:1) (Fig. 1B). Absorption efficiency increased for C18 FA with the number of double bonds, from 0.798 ± 0.032 for stearic acid (18:0) to 0.963 ± 0.009 for γ-linolenic acid (18:3) (Fig. 1C) and similarly for C20 and 22 FA, from 0.264 ± 0.02 for arachic acid (20:0) to 0.998 ± 0.001 for EPA (20:5) (Fig. 1D). Gender had no effect on the mean absorption efficiency of any FA; there was no correlation between individual FA absorption efficiency and either BMI or body surface area.
Fig. 1.
Fractional absorption of dietary fatty acids. Data are means ± SE. The difference in the mean values among the group of fatty acids displayed in each panel was statistically different at P < 0.001 by ANOVA; bars for individual fatty acids labeled with different letters are significantly different from one another at P < 0.05. A: saturated fatty acids: myristic acid (14:0), palmitic acid (16:0), stearic acid (18:0), and arachidic acid (20:0). B: monounsaturated fatty acids: myristoleic acid (14:1), palmitoleic acid (16:1), oleic acid (18:1), elaidic acid (18:1t), and gadoleic acid (20:1). C: C18 fatty acids: elaidic acid (18:1t), stearic acid (18:0), oleic acid (18:1), linoleic acid (18:2), α-linolenic acid (18:3ω3), and γ-linolenic acid (18:3ω6). D: C20 and C22 fatty acids: arachidic acid (20:0), gadoleic acid (20:1), eicosadienoic acid (20:2), arachidonic acid (20:4), eicosapentaenoic acid (20:5), and docosahexaenoic acid (22:6).
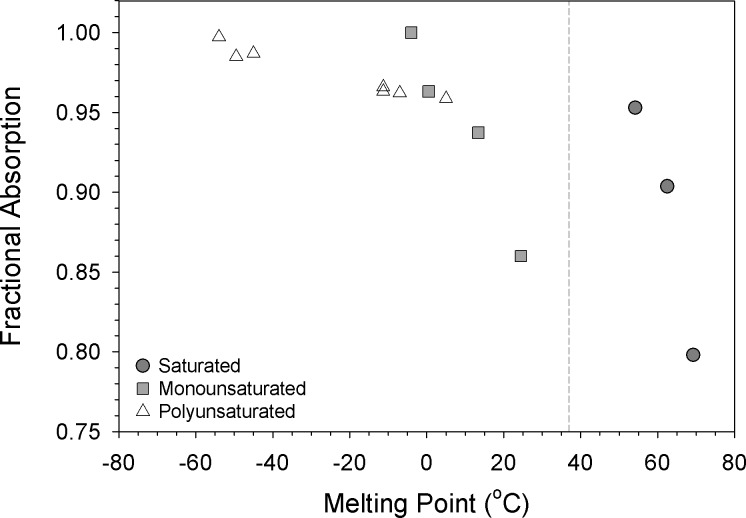
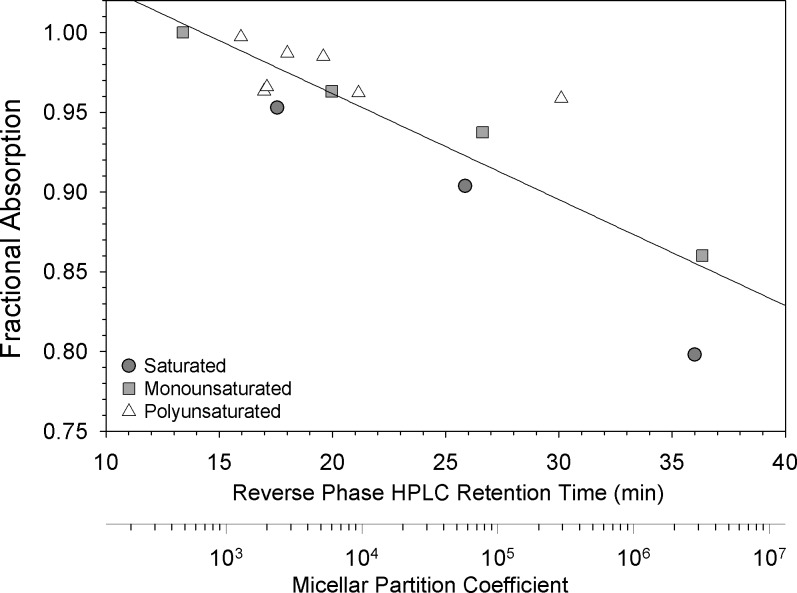
Because acyl chain length and saturation are the major determinants of FA physicochemical properties, we examined the relationship between FA absorption efficiency, melting point, and hydrophobicity. The highly unsaturated FA ARA, EPA, and DHA displayed the lowest melting points and highest fractional absorption (Fig. 2). In general, FA absorption efficiency was inversely related to melting point, although the slope of the curve for polyunsaturated FA was much shallower than that for monounsaturated or saturated FA; moreover, the saturated FA, which are solid at body temperature, displayed a curve that was discontinuously shifted to the right. Examination of the relationship between FA absorption efficiency and hydrophobicity (expressed as reverse-phase HPLC column retention time or micellar partition coefficient) yielded a much more uniform set of inverse relationships among all classes of FA (Fig. 3), most likely because hydrophobic partitioning of FA between mixed micelles and the unstirred water layer is a critical determinant of FA uptake by the enterocyte brush border (12).
Fig. 2.
Dietary fatty acid fractional absorption vs. melting point. The mean fractional absorption of each individual fatty acid, classified as either saturated (dark shaded circle), monounsaturated (light shaded square), or polyunsaturated (open triangle), was plotted vs. its literature melting point (44). The vertical dashed line marks body temperature (37°C).
Fig. 3.
Dietary fatty acid fractional absorption vs. hydrophobicity. The mean fractional absorption of each individual fatty acid, saturated (dark shaded circle), monounsaturated (light shaded square), or polyunsaturated (open triangle), was plotted vs. its reverse-phase HPLC column retention time and micellar partition coefficient, two measures that reflect fatty acid hydrophobicity. Retention times were taken from the literature (9, 38); micellar partition coefficients were taken directly from the literature (55) or extrapolated using data from Refs. 9, 38, and 55. The line is a linear regression for the values of all fatty acid classes, y = 1.095 − 0.00665x, R = 0.869, P < 0.001.
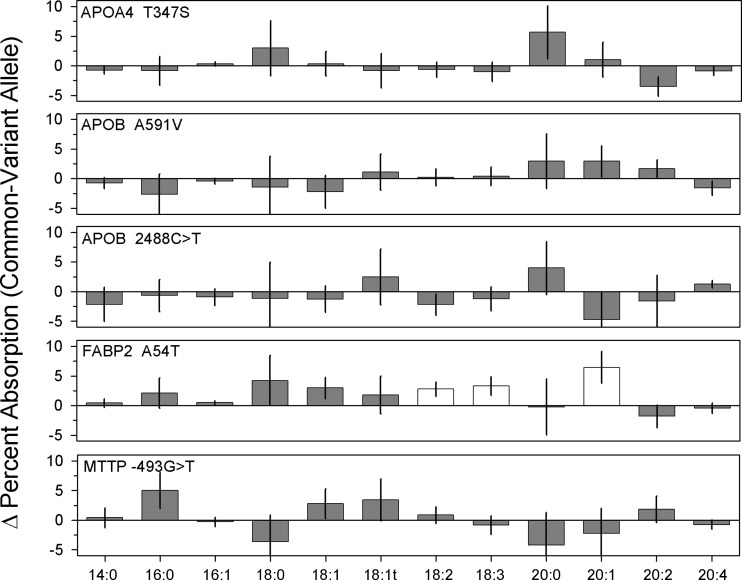
The final step in intestinal fat absorption is chylomicron assembly and secretion (1, 25). Of the key proteins involved in this process, several contain SNP reported to impact postprandial plasma TG levels (2, 21, 40, 43, 46, 54) (Table 1), which suggests that they might modulate intestinal TG absorption. The frequency of these variant alleles in the entire cohort was similar to that reported in the literature; there was no significant difference in variant allele frequency between men and women. Analysis of the impact of these alleles on FA absorption revealed that, with the exception of a small but significant effect of the fatty acid-binding protein-2 (FABP2) 54T-allele on linoleic (18:2), linolenic (18:2), and eiscosenoic (20:1) acid absorption, none of the variant alleles had a significant impact on the absorption efficiency of individual dietary FA (Fig. 4).
Fig. 4.
Impact of selected genetic polymorphisms on fatty acid absorption. Complete genotype data for the indicated single nucleotide polymorphism was available for 42 subjects. For each fatty acid, bars indicate the difference ± SD in mean percent absorption between subjects homozygous for the common allele and subjects carrying one or two copies of the variant allele. Thus, positive values indicate that carriers of a variant allele absorbed that fatty acid less efficiently than subjects homozygous for the common allele; negative values indicate that variant allele carriers absorbed that fatty acid more efficiently. Open bars indicate that the difference between genotype groups was significant at P < 0.05; all other differences were not statistically significant. APO, apolipoprotein; FABP2, fatty acid-binding protein-2; MTTP, microsomal triglyceride transfer protein.
DISCUSSION
Over the course of evolution, TG emerged as a major form of energy storage because of their high energy density and low metabolic cost of storage (18). Efficient TG absorption is thus a biological imperative, especially in mammals, whose metabolic paradigm requires that sufficient energy stores are available for thermogenesis, internal gestation, and lactation (53). Nonetheless, the nutritional advantages of TG are offset by a significant biophysical problem: they are insoluble in water. Thus, dietary TG absorption requires a complex sequence of intraluminal and intracellular processes: TG are first emulsified in the stomach and hydrolyzed by gastric and pancreatic lipases; liberated FA are solubilized in bile acid mixed micelles and transported through the brush border in the enterocyte, where they are bound by caveolin-1 (58) and FABP2; and finally, short-chain FA (i.e., those with <12 carbons) pass directly into the portal circulation, whereas long-chain FA (i.e., those with >12 carbons) are reesterified to TG and enter the chylomicron assembly pathway, where they are packaged into nascent lipoproteins and secreted into the mesenteric lymphatics (12, 50). Given the complexity of these processes, it would seem likely that multiple biophysical, biological, and genetic factors could affect the overall efficiency of FA absorption.
Although differences in the efficiency of dietary FA absorption have been described in animals (33, 34), the majority of human studies that have examined the intestinal absorption of individual FA have been conducted in hospitalized premature infants and neonates (7, 13, 17, 27, 39, 47, 48, 63) not only because fat balance studies are easier to perform in this setting (i.e., milk or formula intake can be precisely measured, and obtaining a complete fecal collection is a simple matter of saving diapers) but also because this information was directly relevant to optimizing the FA composition of enteral formulas used to treat critically ill infants at nutritional risk (7, 49). Most of these studies noted that palmitic and stearic acid are absorbed less efficiently than the other major dietary FA; absorption of the longer, highly unsaturated FA was either not determined, or, unlike our present observations, found to be the least efficient (7, 13, 49), possibly because of the impaired lipase and bile acid secretion that characterize the immature digestive tract (39). The few studies that have measured individual FA absorption in adults have similarly noted that saturated FA, particularly stearic acid, are absorbed with lower efficiency (5, 15, 29). However, to date, quantitative data on the absorption of the full spectrum of dietary FA in adults have not been reported.
Our findings reveal that there is considerable variability in the efficiency with which individual FA are absorbed by the healthy adult gut. Specifically, we observed that absorption of saturated FA is less efficient than mono- or polyunsaturated FA and that the absorption efficiency is inversely related to the number of carbons in the fatty acyl chains but is increased with the number of double bonds. The observed effects of acyl chain length and saturation on the efficiency of dietary FA absorption cannot be explained by a differential substrate specificity of pancreatic lipase, since chain length has only a weak impact on its rate of hydrolysis, and higher degrees of fatty acyl desaturation actually inhibit its activity (41, 68). Rather, the inverse relationship between FA hydrophobicity and absorption efficiency is evidence of the central importance of physicochemical phenomenon in intestinal FA absorption, specifically solubilization into bile acid mixed micelles in the lumen, micellar diffusion through the unstirred water layer, and uptake by the enterocyte brush-border membrane (12, 50). In this regard, acyl chain length is a critical factor determining micellar partitioning (8, 55), which, in turn, was shown to be a major determinant of mucosal FA uptake in an inverted gut sac model of intestinal transport (56).
Several common SNP in key proteins in the chylomicron assembly pathway are reported to alter the postprandial plasma TG response to a lipid meal (Table 1), which suggests that they act by either modulating the efficiency of TG absorption or by altering postabsorptive processes such as intravascular chylomicron lipolysis or hepatic uptake. Apolipoprotein (Apo) B and microsomal triglyceride transfer protein (MTTP) are ancient intracellular lipid transfer proteins that are required for the assembly and secretion of TG-rich lipoproteins (57). Mutations that generate truncated forms of apo B or dysfunctional MTTP result in impaired (hypobetalipoproteinemia) or absent (abetalipoproteinemia) intestinal TG absorption, respectively (61). Two common apo B SNP, V591A and 2488C>T, are associated with lower postprandial TG levels (40, 54), whereas a −493G/T nucleotide substitution in the MTTP promoter is associated with a higher postprandial TG area under the curve (43). Apo A-IV is a 46-kDa glycoprotein synthesized by the intestinal enterocytes during TG absorption (59). In vitro studies have shown that mutations near the apo A-IV COOH-terminus can dramatically alter intracellular chylomicron assembly and TG transport (42), although recent studies in apo A-IV knockout mice found no impact on FA absorption (31). In this regard, individuals carrying an allele for the most common apo A-IV SNP, which encodes a T347S substitution, display lower postprandial TG area under the curve (21, 46) and have a higher BMI and adiposity (65). However, despite these suggestive observations, we found that apo B, MTTP, or apo A-IV alleles had no impact on total dietary TG or individual FA absorption.
FABP2, a member of a large family of intracellular lipid transport proteins (60), is highly expressed in the enterocytes of the small intestine, where it binds intracellular free FA with high affinity. An A54T substitution in the FABP2 gene increases chylomicron secretion in intestinal explants (37) and is associated with increased postprandial TG levels (2), insulin resistance (3), and obesity (3, 35). However, contrary to the anticipated positive impact of this allele on FA absorption, 54T carriers in our cohort displayed significant, albeit very small, decreases in the absorption efficiency of linoleic (18:2), linolenic (18:3), and gadoleic (20:1) acids (Fig. 4). Given the small magnitude of the 54T effect, and the fact that mice bearing a disrupted FABP2 gene did not display any metabolic evidence of intestinal fat malabsorption (62), these data suggest that FABP2 plays a modulating rather than obligatory role in lipid absorption and may exert its peripheral metabolic effects by other mechanisms.
Why did we fail to observe an impact of these polymorphisms on FA absorption? One possibility is that, although polymorphisms such as FABP2 rs1799883 and MTTP rs1800591 may increase the rate of chylomicron synthesis and/or TG transport on a cellular level, the absorptive redundancy of the small intestine may mask any effect on bulk TG transport efficiency at the organ level (51). Another possibility is that postprandial TG area under the curve can be a poor surrogate for intestinal TG absorption efficiency, since it reflects a balance between intestinal lipoprotein production rate and intravascular clearance (67). In this regard, apo B and apo A-IV are both secreted on the surface of chylomicrons, and their impact on postprandial TG levels may be mediated primarily by apolipoprotein-dependent intravascular clearance mechanisms (21, 67). Finally, the limited numbers of SNP we examined in our relatively small subject cohort may not have been sufficient to detect very small allelic effects on FA absorption.
These data raise the question as to how digestive disorders that interfere with different steps in TG digestion might affect the differential absorption of specific FA classes. For example, we predict that conditions that impair TG emulsification [e.g., gastric resection (64) or dysmotility (30)] or inhibit TG lipolysis (e.g., pancreatic insufficiency) might be expected to indiscriminately decrease the absorption of all FA, whereas conditions that alter physiological bile acid recycling (e.g., ileal resection, cholecystectomy, use of bile acid binders) might be expected to selectively decrease absorption of the most hydrophobic FA by interfering with their solubilization in mixed micelles. The impact of bariatric procedures on selective FA absorption may be of particular interest, since the G protein-coupled receptors on the surface of L cells in the distal gut that mediate secretion of GLP-1 and PYY exhibit distinct FA ligand specificities (10, 20, 22) that could be amenable to dietary and pharmacological manipulation.
A caveat to this study is that we did not compare the SBP method with traditional fat balance techniques. However, our goal was not to measure total TG absorption, but rather to examine the differential absorption of individual FA, for which the SBP technique is uniquely suited. In this regard, Dorsey et al. reported a significant correlation between total TG absorption efficiency measured using SPB vs. fat balance methods in patients with cystic fibrosis (14), although the correlation coefficient was only 0.219, and the values determined by the SPB method were lower than those found by the fat balance method and exhibited considerable variability. However, it is important to note that their study design was significantly different from our study in several critical aspects: 1) the severity of pancreatic insufficiency and the dosing of pancreatic enzyme replacement was not standardized in their subject cohort; 2) the TG content of their subjects' diets was not rigorously controlled by a research kitchen, nor was it directly assayed; 3) their subjects ate only a single small test meal containing SPB, rather than a 3-day diet that incorporated SPB into foods eaten at each meal; and 4) the lauric acid-to-BA ratios in the test meal and feces were used as a surrogate for total dietary TG absorption. Together, these factors likely contributed to the wide variability in TG absorption values they observed with both methods, with coefficients of variation ranging from 20.1 to 43.9%, and the low correlation coefficient between the two. On the other hand, the approach used herein yielded coefficients of variation for total dietary TG absorption of 7.4% in the present study and 9.4% in a recent study of orlistat-induced dietary fat malabsorption (Weinberg, unpublished data). Finally, it is important to acknowledge that FA that are less efficiently absorbed, e.g., stearic acid, may undergo chain shortening and other modifications by the action of colonic bacteria, and hence their absorption efficiency may be underestimated by nonisotopic techniques that cannot correct for this phenomenon (28).
In summary, using SBP as a nonabsorbable marker we have observed that acyl chain length, saturation, and hydrophobicity, but not common genetic polymorphisms in key proteins involved in chylomicrons assembly, are the major determinants of the efficiency with which the major dietary FA are absorbed by the small intestine in healthy adults. Companion studies are currently in progress to delineate the impact of bariatric surgical procedures and luminally active agents that inhibit TG lipolysis and BA absorption on the absorption of individual dietary FA.
GRANTS
This work was supported by National Institutes of Health Grants R01-HL-30897 and M01-RR-07122.
DISCLOSURES
None of the authors have any conflicts (financial, professional, or personal) to disclose that are relevant to the conduct of this research or publication of this manuscript.
AUTHOR CONTRIBUTIONS
Author contributions: R.L.M. and L.E. performed experiments; R.L.M. and R.B.W. analyzed data; R.L.M. and R.B.W. edited and revised manuscript; R.L.M., L.E., and R.B.W. approved final version of manuscript; R.B.W. conception and design of research; R.B.W. interpreted results of experiments; R.B.W. prepared figures; R.B.W. drafted manuscript.
ACKNOWLEDGMENTS
Present address for R. L. McKimmie: Piedmont Gastroenterology Specialists, Winston Salem, NC.
Glossary
- ARA
Arachidonic acid
- BA
Behenic acid
- BMI
Body mass index
- CRU
Clinical Research Unit
- DHA
Docosahexaenoic acid
- EPA
Eicosahexaenoic acid
- FA
Fatty acid
- FABP2
Fatty acid binding protein-2
- GLP-1
Glucagon-like peptide-1
- LDL
Low density lipoproteins
- MTTP
Microsomal triglyceride transfer protein
- PYY
Polypeptide YY
- SBP
Sucrose polybehenate
- SNP
Single nucleotide polymorphism
- TG
Triglycerides
REFERENCES
- 1.Abumrad NA, Davidson NO. Role of the gut in lipid homeostasis. Physiol Rev 92: 1061–1085, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agren JJ, Vidgren HM, Valve RS, Laakso M, Uusitupa M. Postprandial responses of individual fatty acids in subjects homozygous for the threonine- or alanine-encoding allele in codon 54 of the intestinal fatty acid binding protein 2 gene. Am J Clin Nutr 73: 31–35, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Albala C, Santos JL, Cifuentes M, Villarroel AC, Lera L, Liberman C, Angel B, Pérez-Bravo F. Intestinal FABP2 A54T polymorphism: association with insulin resistance and obesity in women. Obes Res 12: 340–345, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Albert CM, Campos H, Stampfer MJ, Ridker PM, Manson JE, Willett WC, Ma J. Blood levels of long-chain n-3 fatty acids and the risk of sudden death. N Engl J Med 346: 1113–1138, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Baer DJ, Judd JT, Kris-Etherton PM, Zhao G, Emken EA. Stearic acid absorption and its metabolizable energy value are minimally lower than those of other fatty acids in healthy men fed mixed diets. J Nutr 133: 4129–4134, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Berquin IM, Min Y, Wu R, Wu J, Perry D, Cline JM, Thomas MJ, Thornburg T, Kulik G, Smith A, Edwards IJ, D'Agostino R, Zhang H, Wu H, Kang JX, Chen TQ. Modulation of prostate cancer genetic risk by omega-3 and omega-6 fatty acids. J Clin Invest 117: 1866–1875, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boehm G, Muller H, Kohn G, Moro G, Minoli I, Böhles HJ. Docosahexaenoic and arachidonic acid absorption in preterm infants fed LCP-free or LCP-supplemented formula in comparison to infants fed fortified breast milk. Ann Nutr Metab 41: 235–241, 1997 [DOI] [PubMed] [Google Scholar]
- 8.Borgström B. Partition of lipids between emulsified oil and micellar phases of glyceride-bile salt dispersions. J Lipid Res 8: 598–608, 1967 [PubMed] [Google Scholar]
- 9.Bravi E, Perretti G, Montanari L. Fatty acids by high-performance liquid chromatography and evaporative light-scattering detector. J Chromatogr A 1134: 210–214, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Briscoe CP, Tadayyon M, Andrews JL, Benson WG, Chambers JK, Eilert MM, Ellis C, Elshourbagy NA, Goetz AS, Minnick DT, Murdock PR, Sauls HR, Shabon U, Spinage LD, Strum JC, Szekeres PG, Tan KB, Way JM, Ignar DM, Wilson S, Muir AI. The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. J Biol Chem 278: 11303–11311, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Burton RF. Estimating body surface area from mass and height: theory and the formula of Du Bois and Du Bois. Ann Hum Biol 35: 170–184, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Carey MC, Small DM. Lipid digestion and absorption. Ann Rev Physiol 45: 651–677, 1983 [DOI] [PubMed] [Google Scholar]
- 13.Carnielli VP, Verlato G, Pederzini F, Luijendijk I, Boerlage A, Pedrotti D, Sauer PJ. Intestinal absorption of long-chain polyunsaturated fatty acids in preterm infants fed breast milk or formula. Am J Clin Nutr 67: 97–103, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Dorsey J, Buckley D, Summer S, Jandacek RJ, Rider T, Tso P, Narkewicz MR, Heubi JE. Fat malabsorption in cystic fibrosis: comparison of quantitative fat assay and a novel assay using fecal lauric/behenic acid. J Pediatr Gastroenterol Nutr 50: 441–446, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dougherty RM, Allman MA, Iacono JM. Effects of diets containing high or low amounts of stearic acid on plasma lipoprotein fractions and fecal fatty acid excretion of men. Am J Clin Nutr 61: 1120–1128, 1995 [DOI] [PubMed] [Google Scholar]
- 16.Erkkilä A, de Mello VD, Risérus U, Laaksonen DE. Dietary fatty acids and cardiovascular disease: an epidemiological approach. Prog Lipid Res 47: 172–187, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Finley AJ, Davidson M. Bile acid excretion and patterns of fatty acid absorption in formula-fed premature infants. Pediatrics 65: 132–138, 1980 [PubMed] [Google Scholar]
- 18.Flatt JP. Use and storage of carbohydrate and fat. Am J Clin Nutr 61, Suppl 4: 1952S–959S, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Hawkins GA, Amelung PJ, Smith RS, Jongepier H, Howard TD, Koppelman GH, Meyers DA, Bleecker ER, Postma DS. Identification of polymorphisms in the human glucocorticoid receptor gene (nr3c1) in a multi-racial asthma case and control screening. DNA Seq 15: 167–173, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Hirasawa A, Tsumaya K, Awaji T, Katsuma S, Adachi T, Yamada M, Sugimoto Y, Miyazaki S, Tsujimoto G. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med 11: 90–99, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Hockey KJ, Anderson RA, Hantgan RR, Weinberg RB. Effect of the apolipoprotein A-IV Q360H polymorphism on post-prandial plasma triglyceride clearance. J Lipid Res 42: 211–217, 2001 [PubMed] [Google Scholar]
- 22.Iakoubov R, Izzo A, Yeung A, Whiteside CI, Brubaker PL. Protein kinase Czeta is required for oleic acid-induced secretion of glucagon-like peptide-1 by intestinal endocrine L cells. Endocrinology 148: 1089–1098, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Innis SM. The role of dietary n-6 and n-3 fatty acids in the developing brain. Dev Neurosci 22: 474–80, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Innis SM. Polyunsaturated fatty acids in human milk: an essential role in infant development. Adv Exp Med Biol 554: 27–43, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Iqbal J, Hussain MM. Intestinal lipid absorption. Am J Physiol Endocrinol Metab 296: E1183–E1194, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jandacek RJ, Heubi JE, Tso P. A novel, noninvasive method for the measurement of intestinal fat absorption. Gastroenterology 127: 139–144, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Jensen C, Buist NRM, Wilson T. Absorption of individual fatty acids from long chain or medium chain triglycerides in very small infants. Am J Clin Nutr 43: 745–751, 1986 [DOI] [PubMed] [Google Scholar]
- 28.Jones AE, Stolinski M, Smith RD, Murphy JL, Wootton SA. Effect of fatty acid chain length and saturation on the gastrointestinal handling and metabolic disposal of dietary fatty acids in women. Br J Nutr 81: 37–43, 1999 [PubMed] [Google Scholar]
- 29.Jones PJ, Pencharz PB, Clandinin MT. Absorption of 13C-labeled stearic, oleic, and linoleic acids in humans: application to breath tests. J Lab Clin Med 105: 647–652, 1985 [PubMed] [Google Scholar]
- 30.Kalvaria I, Clain JE. Diabetic diarrhoea and steatorrhoea. A case report and review of the literature. S Afr Med J 55: 562–564, 1979 [PubMed] [Google Scholar]
- 31.Kohan AB, Wang F, Li X, Vandersall AE, Huesman S, Xu M, Yang Q, Lou D, Tso P. Is apolipoprotein A-IV rate limiting in the intestinal transport and absorption of triglyceride? Am J Physiol Gastrointest Liver Physiol 304: G1128–G1135, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Arterioscler Thromb Vasc Biol 23: e20–e30, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Kritchevsky D. Stearic acid metabolism and atherogenesis: history. Am J Clin Nutr 60: 997S–1001S, 1994 [DOI] [PubMed] [Google Scholar]
- 34.Labonté ED, Camarota LM, Rojas JC, Jandacek RJ, Gilham DE, Davies JP, Ioannou YA, Tso P, Hui DY, Howles PN. Reduced absorption of saturated fatty acids and resistance to diet-induced obesity and diabetes by ezetimibe-treated and Npc1l1−/− mice. Am J Physiol Gastrointest Liver Physiol 295: G776–G783, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lara-Castro C, Hunter GR, Lovejoy JC, Gower BA, Fernández JR. Association of the intestinal fatty acid-binding protein Ala54Thr polymorphism and abdominal adipose tissue in African-American and Caucasian women. J Clin Endocrinol Metab 90: 1196–1201, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Lee KW, Hamaad A, MacFadyen RJ, Lip GY. Effects of dietary fat intake in sudden death: reduction of death with omega-3 fatty acids. Curr Cardiol Rep 6: 371–378, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Levy E, Menard D, Delvin E, Stan S, Mitchell G, Lambert M, Ziv E, Feoli-Fonseca JC, Seidman E. The polymorphism at codon 54 of the FABP2 gene increases fat absorption in human intestinal explants. J Biol Chem 276: 39679–39684, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Lin JT, McKeon TA, Stafford AE. Gradient reversed-phase high-performance liquid chromatography of saturated, unsaturated, and oxygenated free fatty acids and their methyl esters. J Chromatogr A 699: 85–91, 1995 [Google Scholar]
- 39.Lindquist S, Hernell O. Lipid digestion and absorption in early life: an update. Curr Opin Clin Nutr Metab Care 13: 314–320, 2010 [DOI] [PubMed] [Google Scholar]
- 40.Lopez-Miranda J, Ordovas JM, Ostos MA, Marin C, Jansen S, Salas J, Blanco-Molina A, Jimenez-Pereperez JA, Lopez-Segura F, Perez-Jimenez F. Dietary fat clearance in normal subjects is modulated by genetic variation at the apolipoprotein B gene locus. Arterioscler Thromb Vasc Biol 17: 1765–1773, 1997 [DOI] [PubMed] [Google Scholar]
- 41.Lowe ME. Molecular mechanisms of rat and human pancreatic lipases. J Nutr 127: 549–557, 1997 [DOI] [PubMed] [Google Scholar]
- 42.Lu S, Yao Y, Cheng X, Mitchell S, Leng S, Meng S, Gallagher JW, Shelness GS, Morris GS, Mahan J, Frase S, Mansbach CM, Weinberg RB, Black DD. Overexpression of apolipoprotein A-IV enhances lipid secretion in IPEC-1 cells by increasing chylomicron size. J Biol Chem 281: 3473–3483, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Lundahl B, Hamsten A, Karpe F. Postprandial plasma apoB48 levels are influenced by a polymorphism in the promoter of the microsomal triglyceride transfer protein gene. Arterioscler Thromb Vasc Biol 22: 289–293, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Lundblad RL, MacDonald FM, eds. Handbook of Biochemistry and Molecular Biology (4th ed.). Boca Raton, FL: CRC, 2010, p. 189–190 [Google Scholar]
- 45.Lust M, Nandurkar S, Gibson PR. Measurement of faecal fat excretion: an evaluation of attitudes and practices of Australian gastroenterologists. Intern Med J 36: 77–85, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Mata P, Ordovas JM, Lopez-Miranda J, Lichtenstein AH, Clevidence B, Judd JT, Schaefer EJ. Apo A-IV phenotype affects diet-induced plasma LDL cholesterol lowering. Arterioscler Thromb 14: 884–891, 1994 [DOI] [PubMed] [Google Scholar]
- 47.Morgan C, Davies L, Corcoran F, Stammers J, Colley J, Spencer SA, Hull D. Fatty acid balance studies in term infants fed formula milk containing long-chain polyunsaturated fatty acids. Acta Paediatr 87: 136–142, 1998 [DOI] [PubMed] [Google Scholar]
- 48.Morgan C, Stammers J, Colley J, Spencer SA, Hull D. Fatty acid balance studies in preterm infants fed formula milk containing long-chain polyunsaturated fatty acids (LCP) II. Acta Paediatr 87: 318–324, 1998 [DOI] [PubMed] [Google Scholar]
- 49.Moya M, Cortes E, Juste M, De Dios JG, Vera A. Fatty acid absorption in preterms on formulas with and without long-chain polyunsaturated fatty acids and in terms on formulas without these added. Eur J Clin Nutr 55: 755–762, 2001 [DOI] [PubMed] [Google Scholar]
- 50.Mu H, Høy CE. The digestion of dietary triacylglycerols. Prog Lipid Res 43: 105–133, 2004 [DOI] [PubMed] [Google Scholar]
- 51.Nassir F, Wilson B, Han X, Gross RW, Abumrad NA. CD36 is important for fatty acid and cholesterol uptake by the proximal but not distal intestine. J Biol Chem 282: 19493–19501, 2007 [DOI] [PubMed] [Google Scholar]
- 52.Neuhouser ML, Rock CL, Kristal AR, Patterson RE, Neumark-Sztainer D, Cheskin LJ, Thornquist MD. Olestra is associated with slight reductions in serum carotenoids but does not markedly influence serum fat-soluble vitamin concentrations. Am J Clin Nutr 83: 624–631, 2006 [DOI] [PubMed] [Google Scholar]
- 53.Ottaviani E, Malagoli D, Franceschi C. The evolution of the adipose tissue: a neglected enigma. Gen Comp Endocrinol 174: 1–4, 2011 [DOI] [PubMed] [Google Scholar]
- 54.Peacock RE, Karpe F, Talmud PJ, Hamsten A, Humphries SE. Common variation in the gene for apolipoprotein B modulates postprandial lipoprotein metabolism: a hypothesis generating study. Atherosclerosis 116: 135–145, 1995 [DOI] [PubMed] [Google Scholar]
- 55.Sallee VL. Determinants of fatty acid and alcohol monomer activities in mixed micellar solutions. J Lipid Res 19: 207–214, 1978 [PubMed] [Google Scholar]
- 56.Sallee VL. Permeation of long-chain fatty acids and alcohols in rat intestine. Am J Physiol Endocrinol Metab Gastrointest Physiol 236: E721–E727, 1979 [DOI] [PubMed] [Google Scholar]
- 57.Shelness GS, Ledford AS. Evolution and mechanism of apolipoprotein B-containing lipoprotein assembly. Curr Opin Lipidol 16: 325–332, 2005 [DOI] [PubMed] [Google Scholar]
- 58.Siddiqi S, Sheth A, Patel F, Barnes M, Mansbach2nd CM. Intestinal caveolin-1 is important for dietary fatty acid absorption. Biochim Biophys Acta 1831: 1311–1321, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stan S, Delvin E, Lambert M, Seidman E, Levy E. Apo A-IV: an update on regulation and physiologic functions. Biochim Biophys Acta 1631: 177–187, 2003 [DOI] [PubMed] [Google Scholar]
- 60.Storch J, Corsico B. The emerging functions and mechanisms of mammalian fatty acid-binding proteins. Annu Rev Nutr 28: 73–95, 2008 [DOI] [PubMed] [Google Scholar]
- 61.Tarugi P, Averna A. Hypobetalipoproteinemia: genetics, biochemistry, and clinical spectrum. Adv Clin Chem 54: 81–107, 2011 [PubMed] [Google Scholar]
- 62.Vassileva G, Huwyler L, Poirier K, Agellon LB, Toth MJ. The intestinal fatty acid binding protein is not essential for dietary fat absorption in mice. FAEB J 14: 2040–2046, 2000 [DOI] [PubMed] [Google Scholar]
- 63.Verkade HJ, Hoving EB, Muskiet FA, Martini IA, Jansen G, Okken A, Vonk RJ, Bijleveld CM. Fat absorption in neonates: comparison of long-chain-fatty-acid and triglyceride compositions of formula, feces, and blood. Am J Clin Nutr 53: 643–651, 1991 [DOI] [PubMed] [Google Scholar]
- 64.Walther B, Clementsson C, Vallgren S, Ihse I, Akesson B. Fat malabsorption in patients before and after total gastrectomy, studied by the triolein breath test. Scand J Gastroenterol 24: 309–314, 1989 [DOI] [PubMed] [Google Scholar]
- 65.Weinberg RB. Apolipoprotein A-IV and diet-gene interactions. Curr Opin Lipidol 13: 125–134, 2002 [DOI] [PubMed] [Google Scholar]
- 66.Woodside JV, McKinley MC, Young IS. Saturated and trans fatty acids and coronary heart disease. Curr Atheroscler Rep 10: 460–466, 2008 [DOI] [PubMed] [Google Scholar]
- 67.Xiao C, Hsieh J, Adeli K, Lewis GF. Gut-liver interaction in triglyceride-rich lipoprotein metabolism. Am J Physiol Endocrinol Metab 301: E429–E446, 2011 [DOI] [PubMed] [Google Scholar]
- 68.Yang LY, Kuksis A, Myher JJ. Lipolysis of menhaden oil triacylglycerols and the corresponding fatty acid alkyl esters by pancreatic lipase in vitro: a reexamination. J Lipid Res 31: 137–147, 1990 [PubMed] [Google Scholar]