Abstract
Stem cell-mediated cardiac regeneration is impaired with age. In this study, we identified a novel subpopulation of small juvenile stem cells (SJSCs) isolated from aged bone marrow-derived stem cells (BMSCs) with high proliferation and differentiation potential. SJSCs expressed mesenchymal stem cell markers, CD29+/CD44+/CD59+/CD90+, but were negative for CD45−/CD117− as examined by flow cytometry analysis. SJSCs showed higher proliferation, colony formation, and differentiation abilities compared with BMSCs. We also observed that SJSCs significantly expressed cardiac lineage markers (Gata-4 and myocyte-specific enhancer factor 2C) and pluripotency markers (octamer-binding transcription factor 4, sex-determining region Y box 2, stage-specific embryonic antigen 1, and Nanog) as well as antiaging factors such as telomerase reverse transcriptase and sirtuin 1. Interestingly, SJSCs either from young or aged animals showed significantly longer telomere length as well as lower senescence-associated β-galactosidase expression, suggesting that SJSCs possess antiaging properties, whereas aged BMSCs have limited potential for proliferation and differentiation. Furthermore, transplantation of aged SJSCs into the infarcted rat heart significantly reduced the infarction size and improved left ventricular function, whereas transplantation of aged BMSCs was less effective. Moreover, neovascularization as well as cardiomyogenic differentiation in the peri-infarcted area were significantly increased in the SJSC-transplanted group compared with the BMSC-transplated group, as evaluated by immunohistochemical analysis. Taken together, these findings demonstrate that SJSCs possess characteristics of antiaging, pluripotency, and high proliferation and differentiation rates, and, therefore, these cells offer great therapeutic potential for repair of the injured myocardium.
Keywords: small juvenile stem cell, antiaging, cardiac repair
elderly patients are more likely to have cardiac arrest after myocardial infarction (MI) (13). Myocardial ischemia results in massive death of cardiomyocytes, and acceleration of cell death in the aged myocardium has been proposed as a mechanism for the increased risk of heart failure in the elderly (2). Cumulative loss of cardiomyocytes as a result of aging impairs contractile performance of the heart, and, concurrently, aged cardiomyocytes accumulate cellular senescence markers that typically appear as cells near the end of their useful lifespan (1). Therefore, it is important that therapeutic interventions to suppress heart failure in the elderly should not only prevent cell death but also antagonize the accumulation of senescent cardiomyocytes. Ideally, this could be accomplished by a combination of delayed senescence together with promotion of regeneration of new cardiomyocytes.
A number of studies have indicated that the function of stem cells involved in neural, cardiac, and vascular regeneration is compromised with age (9). Loss of stem cell functions is, at least in part, due to genetic changes by aging. The number and proliferative potential of bone marrow-derived mesenchymal stem cells (BMSCs) declines with age (23–25). In addition, the size of BMSCs from elderly subjects (>40 yr) is much larger than those from young and middle age subjects (7–40 yr) (23). BMSCs were originally isolated by their adherence to tissue culture surfaces, an isolation technique subsequently followed by most investigators (6, 17, 18). Cells were characterized primarily by their ability to generate colonies in culture and to differentiate into osteoblasts, chondrocytes, and adipocytes (18, 20). BMSCs are heterogeneous population consisting of small and large cells (3, 4, 7, 15). It has been reported that the small cell population has a greater potential for proliferation and multipotential differentiation (osteogenesis, chondrogenesis, and adipogenesis differentiation) than large cells (4, 7, 22). Although different cell surface markers have been proposed to prospectively isolate “true” stem cells, there is little agreement on the use of a specific set of markers to isolate stem cells from heterogeneous BMSC populations. Previously, Kucia et al. isolated a rare population (∼0.01%) of Sca-1+/Lin−/CD45− cells from bone marrow mononuclear cells, which they termed as very small embryonic-like stem cells (VSELs) (11). This cell population has been defined by RT-PCR and immunohistochemistry for pluripotent markers such as stage-specific embryonic antigen (SSEA)-1, octamer-binding transcription factor (Oct)-4, Nanog, and Rex-1 as well as Rif-1, a telomerase protein. Transplantation of a relatively small number of VSELs was sufficient to improve left ventricular (LV) function and alleviate myocyte hypertrophy after MI, supporting the potential therapeutic utility of these cells for cardiac repair. However, the number of these VSELs in bone marrow gradually decreases with aging, and their ability to form spheres containing primitive stem cells declines with time.
In the present study, we identified a novel subpopulation of small juvenile stem cells (SJSCs) from aged rat bone marrow and explored their potential for proliferation and differentiation in vitro and in vitro. We examined the expression of markers for pluripotency, cardiac lineage, and antiaging markers in SJSCs. Furthermore, the therapeutic potential for MI in vivo was investigated.
MATERIALS AND METHODS
Isolation of BMSCs and SJSCs from bone marrow.
Our study confirmed with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Pub. No. 85-23, Revised 1985), and the protocol was approved by the Institutional Animal Care and Use Committee of the University of Cincinnati. BMSCs were isolated from bone marrow of Fischer-344 rats (young: 8–12 wk and old: 24–28 mo, respectively) as previously described (8). Briefly, bone marrow cells were seeded onto 100-mm dishes and cultured in low-glucose DMEM (HyClone Laboratories) supplemented with 10% FBS (HyClone Laboratories). Nonadherent hematopoietic cells were discarded during the routine fresh medium replacement. After 6–10 days, the adherent, spindle-shaped cells were expanded for further use in vitro and in vivo experiments. No more than six passages were allowed for the propagation of purified BMSCs in vitro. For the isolation of SJSCs from the BMSC culture, either aged and young BMSCs (3–6 passages) were seeded at a density of 2 × 105 cells/trans (upper) well with 3-μm pores (cell culture insert, BD Bioscience) in complete medium. After incubation at 37°C for 24 h, cells migrating to the lower side (SJSCs) were collected and expanded for another 10–15 days.
Flow cytometry analysis.
SJSCs were trypsinized, centrifuged, and suspended in cold autoMACS Rinsing Solution with 5% BSA (Milteni Biotec, Bergisch Gladbach, Germany) containing propidium iodide (PI; 1 μg/ml, Sigma) on ice until flow cytometry was performed (FACS-Calibur, BD). Cell size plots by flow cytometry were analyzed using forward scatter (FSC; an indirect measurement of size) and side scatter (SSC; a measurement of granularity). Gates were chosen comparing the FSC/SSC diagrams of beads of known size. Debris was excluded by gates in the right angle scatter versus FSC diagrams and by PI fluorescence, which was read using a 610/20-nm band-pass filter. At least 1 × 105 cells/sample were acquired and analyzed. To analyze cell surface markers in SJSCs, the cell suspension was centrifuged and suspended in cold autoMACS Rinsing Solution with 5% BSA (Milteni Biotec). Cells were incubated for 30 min at 4°C with FITC-conjugated antibodies against CD29, CD44, CD59 (BD Pharmingen), CD90 (AbCam), and phycoerythrin-conjugated antibodies against rat CD45 (Beckman Coulter). Labeled cells were analyzed by flow cytometry using isotype-identical antibodies as controls.
Determination of growth curves and doubling time.
Young SJSCs, aged SJSCs, young BMSCs, and aged BMSCs were seeded at a density of 2 × 104 cells/well (1 × 104 cells/cm2) in each well of 24-well plates. After incubation at 37°C for each 24 h, 72 h, and 6–10 days, the number of cells was counted with a hemocytometer. The doubling time was determined by the following formula (5): (t2 − t1)log2/(logN2 − logN1), where t2 is the time of cell harvest at 72 h after cell seeding, t1 is the time of cell harvest at 24 h after cell seeding, N2 is the number of cells at 72 h after seeding, and N1 is the number of cells at 24 h after seeding. Saturation density was expressed as the most number of cells counted in this experiment.
Colony formation assay.
Each cell group was seeded at a density of 200 cells/6-cm diameter dish (10 cells/cm2) and was cultured for 14 days. After being fixed with methanol, cells were stained with Giemsa solution (EM SCIENCE, Gibbstown, NJ). The number of colonies per dish was counted as the colonies including >50 cells under a microscope. The ratio of colony formation was calculated by the following formula: (total number of colonies/number of seeded cells) × 100.
Differentiation assay.
A differentiation assay for SJSCs and BMSCs was performed using the STEMPRO osteogenesis differentiation kit, STEMPRO chondrogenesis differentiation kit, and STEMPRO adipogenesis differentiation kit (Invitrogen). Osteogenic differentiation was estimated by von Kossa staining at 14 days (18). The assessment of chondrogenic differentiation was achieved by toluidine blue staining at 21 days. Evaluation of adipogenic differentiation was performed by oil red O solution at 21 days.
RT-PCR analysis.
Total RNA was isolated from the various treatment groups of cells with an RNeasy Mini kit (Qiagen), and cDNA was prepared using an Omniscript-RT kit (Qiagen) according to the manufacturer's instructions. For PCR amplification, 1 μg cDNA from the reverse transcription reaction was then added to a PCR mix containing the suggested quantity of QIAGEN PCR buffer, Q-Solution, dNTP mix, reverse and forward primers, Taq DNA polymerase, and distilled water. Each PCR was performed with specific primers.
Senescence-associated β-galactosidase staining.
Senescence-associated β-galactosidase (β-gal) was detected by the senescence detection kit (BioVision) per the manufacturer's instructions.
Quantitative fluorescence in situ hybridization for telomere length measurement.
Telomeres were detected by fluorescence in situ hybridization (FISH) with the PNA Telomere Cy3-labbeled probe (F1002; TelC-Cy3, PNA Bio) according to the manufacturer's instructions. Briefly, cells were fixed with 4% paraformaldehyde for 1 h. After being washed with PBS, cells were incubated with 100 ng/μl RNase for 20 min and 0.005% pepsin for 5 min at 37°C. After being dehydrated by incubation with 70%, 85%, and 100% of cold ethanol, cells were incubated with the 200 nM PNA Telomere probe for 10 min at 85°C and incubated for 2 h at room temperature. Cells were then incubated with 2× SSC for 10 min at 60°C. 4′,6-Diamidino-2-phenylindole and Cy3 signals were acquired simultaneously into separate channels using a confocal microscope (Fluoview FV1000, Olympus), and maximum projection from image stacks (10 sections at steps of 1 μm) were generated for image quantification. Telomere length was measured using TFL-TeloV2-2 free software (Vancouver, BC, Canada). With the program, the integrated fluorescence intensity value for each telomere, which is proportional to the number of hybridized probes, is calculated and presented. TFL-Telo is an application program used to estimate the length of telomeres from captured images of metaphases that have been stained for quantitative FISH analysis (19).
Experimental model of acute MI and cell transplantation.
In vivo experiments were performed in a rat heart model of acute MI using young female Fischer-344 rats, as previously described (10). Briefly, rats were anesthetized by an intraperitoneal injection of ketamine-xylazine (24 mg/kg and 4 mg/kg body wt, respectively). After endotracheal intubation and mechanical ventilation using a rodent ventilator (model 683, Harvard Apparatus), the heart was exposed by left-sided minimal thoracotomy. The left anterior descending coronary artery was permanently ligated with prolene no. 6.0 suture. Immediately after ligation, 70 μl of basal DMEM without cells or containing 1 × 106 cells (aged BMSCs and aged SJSCs) were injected intramyocardially in at least three sites in and around the infarct zone. Cells were labeled with PKH26 cell tracker dye to identify and study their fate postengraftment. The chest was closed, and animals were allowed to recover. During postoperation care, buprenex (0.1 mg/kg bid) was administered for 24 h to alleviate pain. For postmortem experiments, animals were euthanized using an overdose of pentobarbitol sodium. Heart tissue was collected and washed with PBS to remove blood traces before use in molecular experiments.
Histological and immunocytochemical analyses.
For measurements of infarction size and area of fibrosis, hearts were arrested in diastole by an intravenous injection of cadmium chloride and fixed in 10% buffered formalin. The heart was excised, cut transversely, and embedded in paraffin for Masson's trichrome staining. Infarct size was defined as sum of the epicardial and endocardial infarct circumference divided by sum of the total LV epicardial and endocardial circumferences using computer-based planimetry with ImageJ analysis software (version 1.6, NIH). Immunostaining was performed using primary antibodies specific for α-sarcomeric actin (Sigma-Aldrich) and von Willebrand factor VIII (vWF; Dako) as previously described (10). Samples were examined using fluorescent microscope (BX41, Olympus). For blood vessel density, blood vessels positive for vWF were counted in both the infarct and peri-infarct areas. At least 12 high-power microscopic fields (×400) each in border zone were randomly selected and counted in each treatment group of animals (n = 10 animals/group). Blood vessel density was expressed as the number of vessels per surface area (0.155 mm2).
Physiological assessment of heart function.
Transthoracic echocardiography was performed to study changes in heart function at 4 wk after the respective treatments using an iE33 equipped with a 7- to 15-MHz broadband transducer (Philips Ultrasound, Bothell, WA). Intraperitoneal anesthesia was administered with 0.1% of ketamine and 0.02% of xylene per gram of body weight for anesthesia. Animals were anesthetized (∼3 min) and placed supine onto the imaging platform. The distal extremities were taped gently to electropads that provided continuous electrocardiographic and heart rate (pulse detection) measurements. The heart was imaged in the two-dimensional mode in the parasternal long-axis and/or parasternal short-axis views, which were subsequently used to position the M-mode cursor perpendicular to the ventricular septum and LV posterior wall, after which M-mode images were obtained. For each animal, measurements were obtained from four to five consecutive heart cycles. Measurements of ventricular anterior thickness, LV internal dimension, and LV posterior wall thickness were made from the two-dimensionally directed M-mode images of the LV in both systole and diastole. The ejection fraction was measured based on the area-length method after measurements of the LV internal area and distance from the apex to basal LV in both systole and diastole. The scoring system was patterned after the American Society of Echocardiography's scoring system used conventionally in interpreting clinical echocardiographic studies.
Statistical analysis.
All experiments were repeated at least three times (n = 3 for in vitro and 6 for in vivo), and values are expressed as means ± SD. Comparison between two mean values was evaluated using an unpaired Student's two-tailed t-test, and comparison between three or more groups was evaluated using one-way ANOVA followed by Bonferroni post hoc analysis. Statistical significance was accepted at P values of <0.05.
RESULTS
Identification of SJSCs in heterogeneous BMSCs and their potential for proliferation and differentiation in vitro.
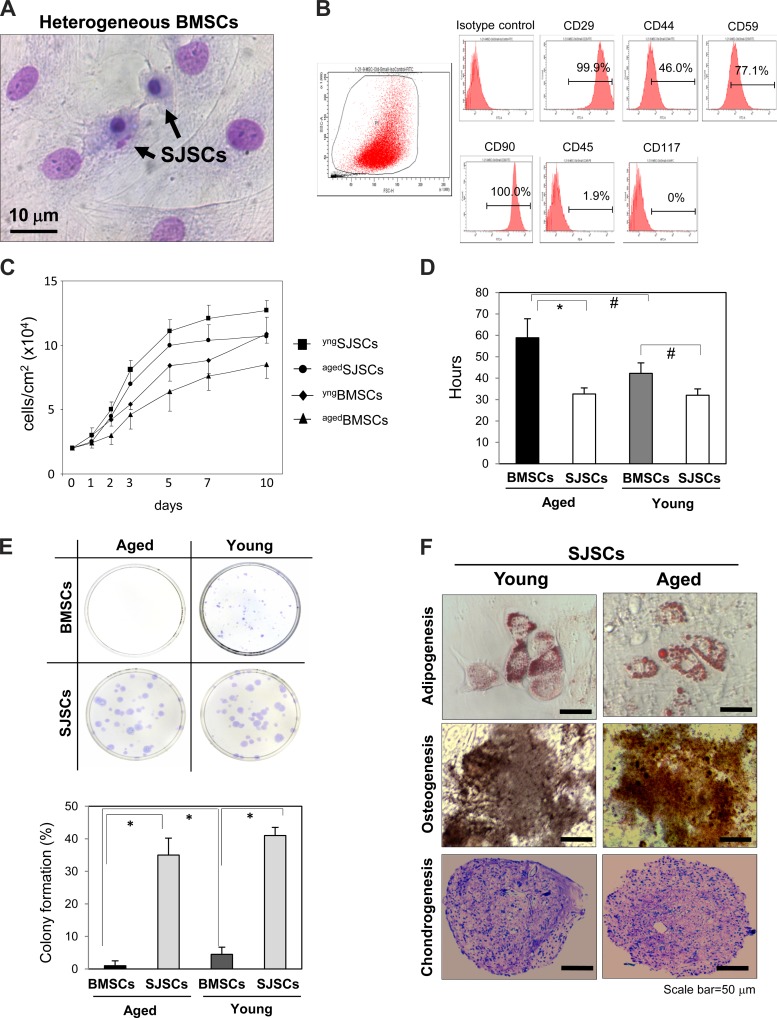
BMSCs are a heterogeneous population including small cells and large cells (Fig. 1A). To isolate the small cell population from heterogeneous BMSCs, BMSCs were filtered using a transwell with 3-μm pores. The number of small cells isolated from heterogeneous BMSCs was ∼0.2% of total BMSCs, and the average nuclei size of SJSCs was 2–4 μm at 24 h after the initial seeding. To analyze the surface markers of the small cell population, we performed FACS analysis for a series of markers commonly used for the identification of mesenchymal stem cells (7, 18). Both aged SJSCs and young SJSCs were positive for the surface markers CD29, CD44, and CD90 but negative for CD45 and CD119 (Fig. 1B). Expression levels of all surface markers were not significantly different between aged SJSCs and young SJSCs. To characterize the unique properties of SJSCs, we performed an in vitro cell proliferation and differentiation assay. SJSCs exhibited a significantly higher proliferation ability compared with that of standard BMSCs (Fig. 1C). More interestingly, unlike other aged stem cells, aged SJSCs still did not lose the high proliferation ability when the growth rate of BMSCs was declined with aging. In addition, the doubling time at 1–3 days from their respective growth curves showed that the proliferation ability of aged SJSCs (32.6 ± 2.9 h) was not different from that of young SJSCs (32.0 ± 3.0 h) but was higher than that of aged BMSCs (61.0 ± 9.2 h), as shown in Fig. 1D. Similarly, the colony formation assay showed that clonogenicity was markedly higher in SJSCs compared with BMSCs (Fig. 1E). Furthermore, there was almost no difference between young SJSCs and aged SJSCs in their differentiation potential to adopt osteogenic (as evaluated by von Kossa staining), chondrogenic (toluidine blue O staining), and adipogenic (oil red O staining) phenotypes when stimulated under specific culture conditions (Fig. 1F), whereas BMSCs from either aged or young animals were less responsive. This suggests that aged SJSCs still possess youthful characteristics, whereas aged BMSCs lack those characteristics.
Fig. 1.
Identification of small juvenile stem cells (SJSCs) from heterogeneous bone marrow-derived stem cells (BMSCs) and their potential for proliferation and differentiation in vitro. A: representative image of a heterogeneous BMSC culture, which contains both large cells and small cells (Giemsa staining). Heterogeneous BMSCs were filtered with 3-μm pores to isolate small cells. B: profile of cell surface markers on aged SJSCs (agedSJSCs) and young SJSCs (yngSJSCs). Both aged and young SJSCs were positive for CD29, CD44, CD59, and CD90 but negative for CD45 and CD117. C: cell growth curves showing that the cell proliferation rate was higher in SJSCs than in BMSCs. agedBMSCs, aged BMSCs; yngBMSCs, young BMSCs. D: SJSCs showed a shorter doubling time measured at 1–3 days compared with BMSCs. There was almost no difference in the doubling time between aged SJSCs and young SJSCs, whereas the doubling time of aged BMSCs was significantly longer than that of young BMSCs. *P < 0.01; #P < 0.05. E, top: representative images of microscopic colonies obtained from aged BMSCs, young BMSCs, aged SJSCs, and young SJSCs. Bottom, clonogenicity was markedly higher in SJSCs compared with BMSCs. *P < 0.01. F: differentiation potential of aged SJSCs was as high as young SJSCs for osteogenic (von Kossa staining), chondrogenic (toluidine blue O staining), and adipogenic (oil red O staining) phenotypes.
Molecular characteristics of SJSCs: pluripotency, cardiac lineage, and telomere length.
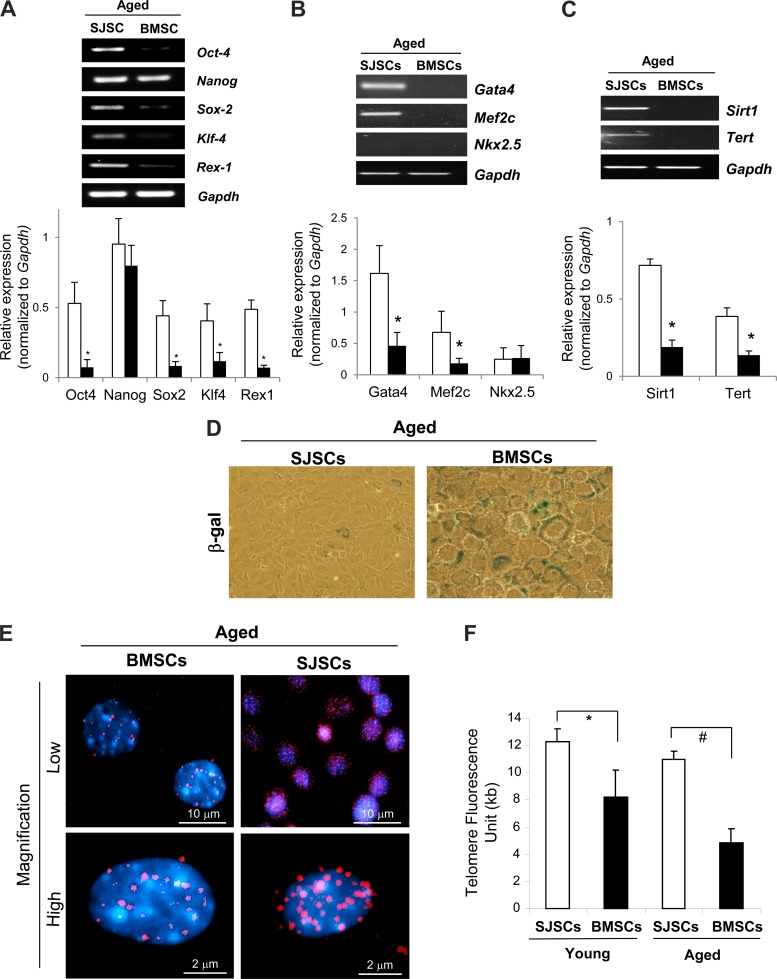
We confirmed that SJSCs expressed pluripotency markers, including Oct-4, Nanog, sex-determining region Y box 2 (Sox-2), Kruppel-like factor 4 (Klf-4), and Rex-1, as determined by RT-PCR (Fig. 2A). In addition, cardiogenic differentiation markers, such as Gata-4 and myocyte-specific enhancer factor 2C (Mef2c), were also observed in SJSCs (Fig. 2B), whereas standard BMSCs did not show significant expression of these pluripotency and cardiac differentiation markers. Expression of key antiaging genes, including telomerase reverse transcriptase and sirtuin 1, were also significantly higher in aged SJSCs (Fig. 2C). In addition, we observed lower expression of senescence-associated β-gal expression in aged SJSCs compared with aged BMSCs (Fig. 2D). Moreover, we analyzed telomere density as well as length in both aged SJSCs and aged BMSCs using quantitative FISH and confocal microscopy. Intriguingly, significant higher telomere density and longer telomeres were observed in aged SJSCs compared with aged BMSCs (Fig. 2, E and F), suggesting that SJSCs have greater antiaging properties in aged bone marrow compared with standard BMSCs.
Fig. 2.
Higher expression of pluripotency, cardiac lineage, and antiaging markers with longer telomeres in SJSCs. Compared with aged BMSCs, aged SJSCs showed higher expression of pluripotency markers such as octamer-binding transcription factor 4 (Oct-4), Nanog, sex-determining region Y box 2 (Sox-2), Kruppel-like factor 4 (Klf-4), and Rex-1 (A), cardiogenic differentiation markers such as Gata-4 and myocyte-specific enhancer factor 2C (Mef2c) (B), and antiaging markers such as sirtuin 1 (Sirt1) and telomerase reverse transcriptase (Tert) (C), as examined by RT-PCR. *P < 0.01 vs. BMSCs. D: aged SJSCs showed less senescence-associated β-galactosidase (β-gal) expression compared with aged BMSCs. E: confocal microscopy showed that aged SJSCs had a higher density of telomeres compared with aged BMSCs. F: Quantitative fluorescence in situ hybridization (FISH) analysis demonstrated that SJSCs maintain longer telomeres compared with BMSCs. Telomere shortening was delayed in aged SJSCs compared with aged BMSCs. *P < 0.05; #P < 0.001.
Higher efficacy of SJSCs for the functional recovery of the ischemic heart in vivo.
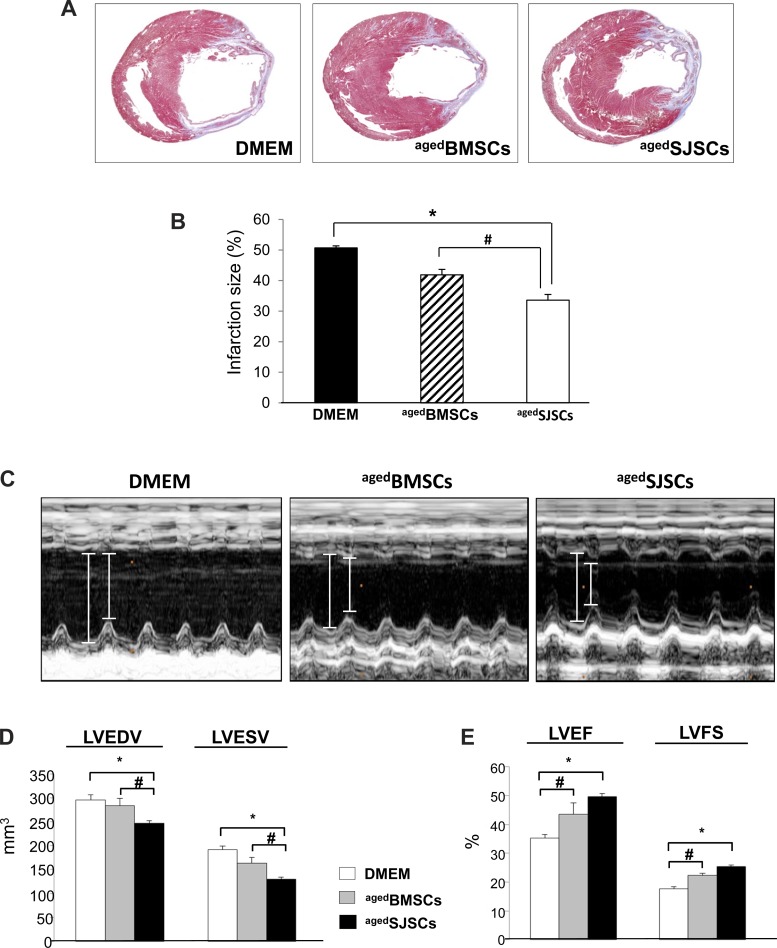
To evaluate the therapeutic potential of SJSCs for the recovery of the ischemic heart in vivo, we transplanted BMSCs or SJSCs in the infarcted rat heart. All animals survived the full length of the study, and there were no deaths related to cell transplantation. Animals (n = 10 animals/group) were euthanized at 4 wk after the respective treatments. Transplantation of both aged BMSCs and aged SJSCs in and around the infarcted myocardium attenuated infarct size expansion compared with the DMEM-treated group. Importantly, transplantation of aged SJSCs significantly reduced infarction size compared with that of aged BMSCs (DMEM: 50.7 ± 0.7%, aged BMSCs: 41.9 ± 1.8%, and aged SJSCs: 33.6 ± 1.9%; Fig. 3, A and B). We also performed echocardiography to evaluate LV function after cell transplantation. The indexes of LV contractile function were well preserved in the aged SJSCs-injected group, whereas the aged BMSC-injected group showed minor effects (Fig. 3, C–E). There were preserved indexes of global heart function in the aged SJSC-transplanted group, including LV diastolic volume (DMEM: 295.6 ± 10.6 mm3, aged BMSCs: 283.4 ± 14.8 mm3, and aged SJSCs: 246.6 ± 5.2 mm3, P < 0.05), LV ejection fraction (DMEM: 35.3 ± 1.2%, aged BMSCs: 43.4 ± 3.9%, and aged SJSCs: 49.7 ± 1.0%, P < 0.01), and LV fractional shortening (DMEM: 17.6 ± 0.7%, aged BMSCs: 22.4 ± 0.6%, and aged SJSCs: 25.3 ± 0.6%, P < 0.01).
Fig. 3.
Effect of SJSCs on the functional recovery of the ischemic heart in vivo. A and B: histological assessment of left ventricular (LV) cross-sections after Masson's trichrome staining, which stains the visible scar region as blue. Infarction size was significantly reduced in aged BMSC- and aged SJSC-injected groups, whereas the DMEM-injected group showed extensive scar formation. The aged SJSC-injected group showed a higher efficacy for infarction size reduction. *P < 0.01; #P < 0.05. C: representative M-mode echocardiography was performed 4 wk after myocardial infarction. D: functional recovery of the LV was significantly improved by aged SJSC transplantation, whereas DMEM- and aged BMSC-transplanted groups showed fewer effects, as analyzed by LV end-diastolic and end-systolic volume (LVEDV and LVESV, respectively; D) and LV ejection fraction and fractional shortening (LVEF and LVFS, respectively; E) using echocardiography. *P < 0.01; #P < 0.05.
SJSCs promote neovascularization and cardiac differentiation in the infarcted heart in vivo.
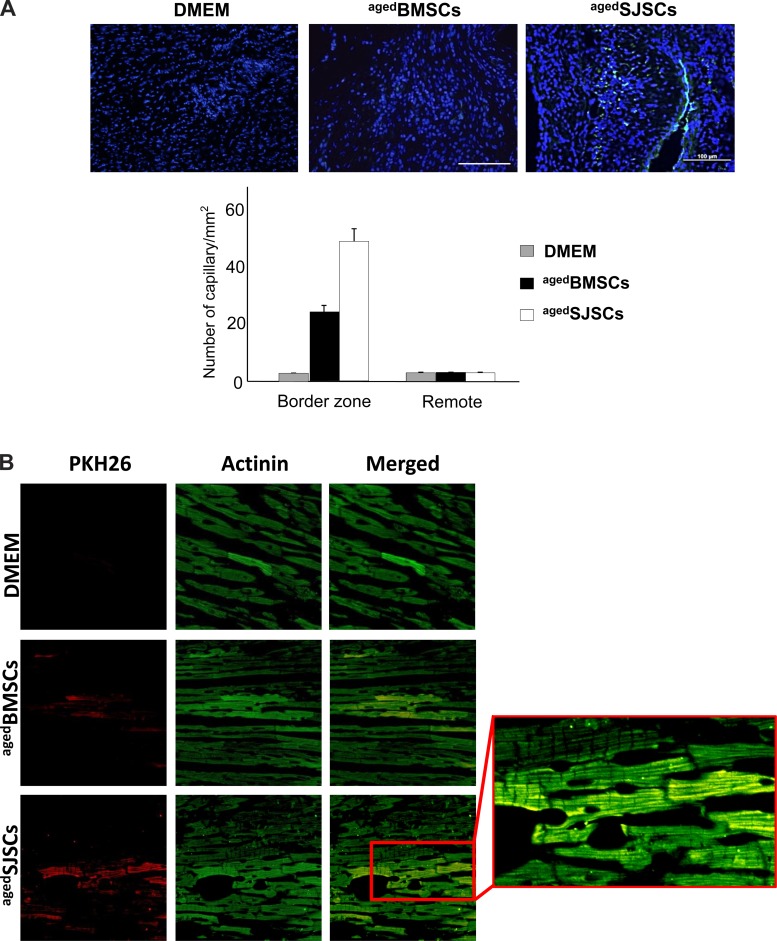
Fluorescent immunocytochemical analysis of histological tissue sections on cell-transplanted groups of animal hearts revealed that neovascularization was elevated in the border zone in the aged SJSC-transplanted group (aged BMSCs: 22.4 ± 1.7 capillaries/plaque-forming unit and aged SJSCs: 44.9 ± 4.7 capillaries/plaque-forming unit, P < 0.01), as evaluated by measurements of capillary density (vWF staining; Fig. 4A). Moreover, actinin staining also showed remarkable myogenic differentiation of transplanted PKH26-labeled aged SJSCs compared with DMEM or aged BMSCs (Fig. 4B).
Fig. 4.
Angiomyogenesis by SJSCs in the infarcted heart in vivo. A: capillary density analysis of the border zone in heart tissue sections at 4 wk after myocardial infarction showed that transplantation of aged SJSCs had a marked effect on blood vessel formation compared with that of aged BMSCs. The graph shows the number of capillaries per 1 mm3 of heart tissue. B: representative images of fluorescence immunostaining of histological sections for actinin expression at 4 wk after different treatments. Cells were labeled with PKH26 before transplantation. Extensive myogensis was observed in the peri-infarct regions of aged SJSC-injected groups, whereas aged BMSC injection was less effective. Inset: higher-magnification image of the boxed region.
DISCUSSION
A number of studies have indicated that the function of stem cells involved in neural, cardiac, and vascular regeneration is compromised with age. The loss of stem cell functions is, at least in part, due to genetic changes by aging. Thus, adult stem cells appear to lack some of the protective mechanisms that may be essential to long-term survival and function, limiting the regenerative capacity with age. BMSCs are a heterogeneous population including small cells and large cells, and, generally, the BMSC population isolated from aged animals contains more senescent large cells than that from young animals (23). In the present study, we discovered that aged BMSCs still contained a subpopulation of small, young, and partially pluripotent cells, termed SJSCs. The major findings of this study are as follows: 1) SJSCs possessed high proliferation and differentiation potential to adopt osteogenic, chondrogenic, and adipogenic phenotypes; 2) SJSCs expressed markers for pluripotency, cardiac differentiation, and antiaging; 3) SJSCs showed higher density and longer length of telomeres compared with BMSCs; and 4) transplantation of these small cells into the infarcted rat heart significantly reduced the infarction size and improved the recovery of the ischemic heart, suggesting that SJSCs have a great therapeutic potential for the treatment of ischemic heart diseases. Although this study showed that SJSCs promoted neovascularization and cardiogenic differentiation for the recovery of the ischemic heart, further indepth investigation may be required to determine the molecular mechanisms on how SJSCs imparted these beneficial effects in the infarcted heart.
Transplantation of pluripotent stem cells in the infarcted heart produced malignant tumors. Due to their pluripotent nature, we expected that SJSCs might increase the chance of tumorigenicity as well. However, injection of SJSCs (106 cells) into nude mice did not form teratomas, whereas induced pluripotent stem cells produced tumors in 4 wk (see Supplemental Fig. S2 in the Supplemental Material).1 It is well known that the accumulation of dysfunctional senescent cells augments the risk of cancer development, and rejuvenation of aged cells is also associated with cancer prevention. Further studies are required to elucidate the antitumorigenic potential of SJSCs.
Recently, it has been reported that adult murine bone marrow harbors a population of nonhematopoietic Sca-1+/lin−/CD45−/CXCR4+ cells (VSELs) enriched for GATA-4 and Nkx2.5 (11). These VSELs express cardiac and endothelial markers as well as some developmental markers, such as Oct-4, Nanog, and SSEA-1. VSELs can differentiate into cell lineages from all three germ layers, including mesoderm-derived cardiomyocytes. Moreover, transplantation of a relatively small number of VSELs was sufficient to improve LV function and alleviate myocyte hypertrophy after MI, supporting the potential therapeutic utility of these cells for cardiac repair. However, the number of these VSELs in bone marrow gradually decreases with aging, and their ability to form spheres containing primitive stem cells declines with time (11, 21, 26). VSELs and SJSCs are similar to each other in many aspects, such as 1) cell size (VSELs: 2–10 μm vs. SJSCs: 3–6 μm); 2) pluripotency marker expression (VSELs: Oct-4+, Nanog+, Rex-1+, developmental pluripotency-associated protein 3+, and Rif-1+ vs. SJSCs: Oct-4+, Nanog+, Sox-2+, Rex-1+, and Klf-2+); 3) lack of major histocompatibility complex expression (data not shown); and 4) excellent efficiency for repair of the ischemic heart. However, there are also several discrepancies between VSELs and SJSCs even though these two small cell populations are similar in cell size and pluripotency. First, SJSCs isolated from aged bone marrow continue their proliferation and differentiation, whereas the function of VSELs deteriorates with aging. Second, SJSCs originate from a mesenchymal stem cell population, whereas VSELs have been isolated from a mononuclear cell population in bone marrow. Finally, SJSCs are positive for CD90, whereas VSELs do not express CD90. It is still not clear whether or not SJSCs and VSELs originated from the same parent cells, and further indepth investigation is needed to determine the precise function as well as direct comparison of these two populations using molecular and cellular biological approaches, including global gene expression and epigenetics profiles. In fact, the existence of pluripotent VSELs in adult tissue is still controversial. During the review of this manuscript, Miyanishi et al. (14) recently reported that they were unable to find pluripotent VSELs in mouse bone marrow as VSELs failed to form embryoid body-like spheres, Oct-4 expression, and hematopoietic differentiation, thereby indicating that it is very important for the reproducibility of results by multiple laboratories to confirm the developmental potential of these pluripotent stem cells before considering their application in a clinical setting. In the present study, although these cells showed partial expression of pluripotency markers such as Oct-4 and Sox-2, it still remained undetermined whether SJSCs are fully pluripotent stem cells or just a juvenile subpopulation to support the regenerative functions of mesenchymal stem cells in bone marrow.
It has been reported that relatively large cells in the BMSC population propagate very slowly, whereas small cells proliferate rapidly. The large cells are referred as mature marrow stromal cells, and the smaller ones are referred to as recycling stem or rapidly self-renewing (RS) cells (3, 7, 22). RS cells have multipotent differentiation potential compared with mature marrow stromal cells. It is not clear whether SJSCs differ from RS cells only because of cell size (RS cells: 7 μm) (20), and it is also unknown whether RS cells express Oct-4. However, we do not rule out the possibility that growing SJSCs may contain RS cells because RS cells showed similar characteristics for proliferation and differentiation. Further work is required to elucidate differences between SJSCs and RS cells.
The number of SJSCs isolated from aged mice was not different from that of young mice (Supplemental Fig. S3). It has been thought that adult tissues contain only tissue-committed stem cells, such as epidermal stem cells and skeletal myoblasts, which have a limited potential for differentiation. However, we and other researchers consider that some pluripotent stem cells, including SJSCs, reside in adult tissues, such as bone marrow, as a supporting resource for pluripotent stem cells that renews the pool of tissue-committed stem cells. Adult pluripotent stem cells may play a role in the rejuvenation of aged cells or repair of damaged tissues. Further indepth studies are required in the future to understand the role of pluripotent stem cells in adult tissues.
One of the most important mechanisms for replicative senescence is the downregulation of telomerase and resulting loss of telomerase activity. Telomere shortening induces disruption of chromosome integrity because uncapped telomeres activate signaling pathways that correlate with DNA damage and apoptosis. Telomerase activity tends to decline with age, and telomeres are progressively lost with each round of cell division. It is noteworthy that patients with chronic heart failure also have 25% shorter cardiac telomeres compared with healthy age control subjects (16), suggesting that telomere length is associated with the severity of heart failure. Telomerase knockout mice have severely reduced telomere length and suffer from severe LV failure (12). In the present study, SJSCs showed high expression of telomerase as well as long telomere length, indicating the youthfulness of SJSCs. Compared with aged BMSCs, transplantation of aged SJSCs into the infarcted heart significantly reduced the infarction size and improved the LV function. Our data demonstrated that transplanted SJSCs directly differentiated into cardiomyocytes in the ischemic heart, as examined by immunohistochemical analysis. These beneficial effects may be mediated possibly through the transfer of antiaging factors including telomerase mRNA from SJSCs to damaged cardiomyocytes. Further studies are under investigation in our laboratory to determine the molecular mechanisms for a role of telomerase in SJSCs and repair of aged hearts.
In conclusion, the present study demonstrates that SJSCs present in aged bone marrow have a higher potential for proliferation and differentiation and are novel candidates for antiaging strategies and repair of the aged ischemic heart.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants R37-HL-074272, HL-095375, and HL-087246 (to M. Ashraf).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K.I., H.W.K., and M.A. conception and design of research; K.I., M.O., and H.W.K. performed experiments; K.I., M.O., and H.W.K. analyzed data; K.I., M.O., H.W.K., and M.A. interpreted results of experiments; K.I., M.O., and H.W.K. prepared figures; K.I., M.O., H.W.K., and M.A. approved final version of manuscript; M.O., H.W.K., and M.A. drafted manuscript; M.O., H.W.K., and M.A. edited and revised manuscript.
ACKNOWLEDGMENTS
The authors thank Kaoru Mats-ura for technical assistance.
Footnotes
Supplemental Material for this article is available at the American Journal of Physiology-Heart and Circulatory Physiology website.
REFERENCES
- 1.Centurione L, Antonucci A, Miscia S, Grilli A, Rapino M, Grifone G, Di Giacomo V, Di Giulio C, Falconi M, Cataldi A. Age-related death-survival balance in myocardium: an immunohistochemical and biochemical study. Mech Ageing Dev 123: 341–350, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Chimenti C, Kajstura J, Torella D, Urbanek K, Heleniak H, Colussi C, Di Meglio F, Nadal-Ginard B, Frustaci A, Leri A, Maseri A, Anversa P. Senescence and death of primitive cells and myocytes lead to premature cardiac aging and heart failure. Circ Res 93: 604–613, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Colter DC, Class R, DiGirolamo CM, Prockop DJ. Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proc Natl Acad Sci USA 97: 3213–3218, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colter DC, Sekiya I, Prockop DJ. Identification of a subpopulation of rapidly self-renewing and multipotent adult stem cells in colonies of human marrow stromal cells. Proc Natl Acad Sci USA 98: 7841–7845, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cristofalo VJ, Allen RG, Pignolo RJ, Martin BG, Beck JC. Relationship between donor age and the replicative lifespan of human cells in culture: a reevaluation. Proc Natl Acad Sci USA 95: 10614–10619, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet 3: 393–403, 1970 [DOI] [PubMed] [Google Scholar]
- 7.Javazon EH, Colter DC, Schwarz EJ, Prockop DJ. Rat marrow stromal cells are more sensitive to plating density and expand more rapidly from single-cell-derived colonies than human marrow stromal cells. Stem Cells 19: 219–225, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Jiang S, Haider KH, Idris NM, Salim A, Ashraf M. Supportive interaction between cell survival signaling and angiocompetent factors enhances donor cell survival and promotes angiomyogenesis for cardiac repair. Circ Res 99: 776–784, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Jones DL, Rando TA. Emerging models and paradigms for stem cell ageing. Nat Cell Biol 13: 506–512, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim HW, Jiang S, Ashraf M, Haider KH. Stem cell-based delivery of Hypoxamir-210 to the infarcted heart: implications on stem cell survival and preservation of infarcted heart function. J Mol Med 90: 997–1010, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kucia M, Reca R, Campbell FR, Zuba-Surma E, Majka M, Ratajczak J, Ratajczak MZ. A population of very small embryonic-like (VSEL) CXCR4(+)SSEA-1(+)Oct-4+ stem cells identified in adult bone marrow. Leukemia 20: 857–869, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Leri A, Franco S, Zacheo A, Barlucchi L, Chimenti S, Limana F, Nadal-Ginard B, Kajstura J, Anversa P, Blasco MA. Ablation of telomerase and telomere loss leads to cardiac dilatation and heart failure associated with p53 upregulation. EMBO J 22: 131–139, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maggioni AA, Maseri A, Fresco C, Franzosi MG, Mauri F, Santoro E, Tognoni G. Age-related increase in mortality among patients with first myocardial infarctions treated with thrombolysis. N Engl J Med 329: 1442–1448, 1993 [DOI] [PubMed] [Google Scholar]
- 14.Miyanishi M, Mori Y, Seita J, Chen JY, Karten S, Chan CKF, Nakauchi H, Weissman IL. Do pluripotent stem cells exist in adult mice as very small embryonic stem cells? Stem Cell Rep 1: 198–208, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neuhuber B, Swanger SA, Howard L, Mackay A, Fisher I. Effect of plating density and culture time on bone marrow stromal cell characteristics. Exp Hematol 36: 1176–1185, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oh H, Wang SC, Prahash A, Sano M, Moravec CS, Taffet GE, Michael LH, Youker KA, Entman ML, Schneider MD. Telomere attrition and Chk2 activation in human heart failure. Proc Natl Acad Sci USA 100: 5378–5383, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Owen M. Marrow stromal stem cells. J Cell Sci Suppl 10: 63–76, 1988 [DOI] [PubMed] [Google Scholar]
- 18.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science 284: 143–147, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Poon SS, Martens UM, Ward RK, Lansdorp PM. Telomere length measurements using digital fluorescence microscopy. Cytometry 36: 267–278, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Prockop DJ, Sekiya I, Colter DC. Isolation and characterization of rapidly self-renewing stem cells from cultures of human marrow stromal cells. Cytotherapy 3: 393–396, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Ratajczak MZ, Zuba-Surma EK, Shin DM, Ratajczak J, Kucia M. Very small embryonic-like (VSEL) stem cells in adult organs and their potential role in rejuvenation of tissues and longevity. Exp Gerontol 43: 1009–1017, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith JR, Pochampally R, Perry A, Hsu SC, Prockop DJ. Isoalation of a highly clonogenic and multipotential subfraction of adult stem cells from bone marrow stroma. Stem Cells 22: 823–831, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Stolzing A, Jones E, McGonagle D, Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequence for cell therapies. Mech Ageing Dev 129: 163–173, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Wagner W, Horn P, Castoldi M, Diehlmann A, Bork S, Saffrich R, Benes V, Blake J, Pfister S, Eckstein V, Ho AD. Replicative senescence of mesenchymal stem cells: a continuous and organized process. PLoS One 4: e5846, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou S, Greenberg JS, Epperly MW, Goff JP, Adler C, LeBoff MS, Glowacki J. Age-related intrinsic changes in human bone marrow-derived mesenchymal stem cells and their differentiation to osteoblasts. Aging Cell 7: 335–343, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zuba-Surma EK, Wu W, Ratajczak J, Kucia M, Ratajczak MZ. Very small embryonic-like stem cells in adult tissues-potential implications for aging. Mech Ageing Dev 130: 58–66, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]