Abstract
Understanding the role of fibroblasts in pathologic conditions is hampered by the absence of specific markers. Fibroblast-specific protein (FSP)1 has been suggested as a fibroblast-specific marker in normal and fibrotic tissues; FSP1 reporter mice and FSP1-Cre-driven gene deletion are considered reliable strategies to investigate fibroblast biology. Because fibroblasts are abundant in normal and injured mammalian hearts, we studied the identity of FSP1+ cells in the infarcted and remodeling myocardium using mice with green fluorescent protein (GFP) expression driven by the FSP1 promoter. Neonatal and adult mouse hearts had low numbers of FSP1+ cells. Myocardial infarction induced marked infiltration with FSP1-expressing cells that peaked after 72 h of reperfusion. Using flow cytometry, we identified 50% of FSP1+ cells as hematopoietic cells; many endothelial cells were also FSP1+. Increased infiltration with FSP1+ cells was also noted in the pressure-overloaded myocardium. Although some FSP1+ cells had fibroblast morphology, >30% were identified as hematopoietic cells, endothelial cells, or vascular smooth muscle cells. In contrast, periostin did not stain leukocytes or vascular cells but labeled spindle-shaped interstitial cells and, as a typical matricellular protein, was deposited in the matrix. CD11b+ myeloid cells sorted from the infarcted heart had higher FSP1 expression than corresponding CD11b-negative cells, highlighting the predominant expression by hematopoietic cells. FSP1 is not a specific marker for fibroblasts in cardiac remodeling and fibrosis.
Keywords: cardiac fibrosis, myocardial infarction, fibroblast, cardiac remodeling, periostin
fibroblasts are the most abundant noncardiomyocytes in the adult mammalian myocardium and play an important role in cardiac homeostasis and disease (39). In the normal heart, fibroblasts not only secrete extracellular matrix proteins and provide structural support by maintaining the interstitial matrix network but may also interact with cardiomyocytes and vascular cells transducing signals essential for preservation of cardiac function (21, 42). Following myocardial injury, cardiac fibroblasts undergo dynamic changes and actively participate in reparative, fibrotic, and hypertrophic responses (9) by secreting structural and matricellular matrix proteins and by releasing cytokines and growth factors, thus modulating phenotype and function of cardiomyocytes and noncardiomyocytes (23, 41). Because of their abundance in normal and injured hearts, their phenotypic plasticity, and their broad range of secreted mediators, fibroblasts have attracted significant interest as potentially critical cellular effectors of cardiac remodeling. However, understanding of their role in vivo has been hampered by the lack of a reliable and specific fibroblast marker that could be used for cell type-specific gene targeting approaches.
Fibroblast specific protein (FSP)1 (also known as S100A-4) is a member of the S100 superfamily of cytoplasmic calcium binding proteins that is expressed by fibroblasts in fibrotic tissues and has been suggested as a fibroblast-specific marker. Differential hybridization studies identified FSP1 as a filament-associated protein that is expressed in fibroblasts but not in epithelial cells, mesangial cells, or embryonic endoderm (40). Antisera against FSP1 identified fibroblasts in vitro and in vivo; moreover, in vitro studies suggested a role for FSP1 in mediating epithelial to mesenchymal transition (35). In experimental models of pulmonary (25) and renal fibrosis (40) and in biopsied samples from patients with fibrotic disorders (25), FSP1 identified interstitial fibroblasts. On the basis of these observations, transgenic mice with green fluorescent protein (GFP) expression driven by the FSP1 promoter (FSP1.GFP mice) have been used to map cell fate in fibroblast populations (47), whereas FSP1-Cre animals have served as tools to dissect the effects of fibroblast-specific gene deletion in fibrotic and neoplastic conditions (4, 47). However, in recent years a growing body of evidence challenged the specificity of FSP1 as a fibroblast marker, suggesting that other cell types infiltrating injured tissues, such as inflammatory macrophages (36), dendritic cells (5), lymphocytes (26), and vascular smooth muscle cells (8) may also express FSP1. Although FSP1 upregulation has been demonstrated in the infarcted myocardium (37), the role of FSP1 as a fibroblast-specific marker in the injured and remodeling myocardium and its potential usefulness for fibroblast-specific gene targeting have not been studied.
Our study systematically investigates the phenotype of FSP1+ cells in two models of cardiac remodeling. To identify FSP1+ cells in the infarcted and pressure-overloaded myocardium, we used transgenic mice with GFP expression driven by the FSP1 promoter. Using flow cytometry and immunohistochemical staining, we found marked infiltration of the infarcted and remodeling myocardium with FSP1+ cells. In the infarct, almost half of the FSP1+ cells were leukocytes. Moreover, in the pressure-overloaded myocardium a large number of FSP1+ cells were identified as endothelial cells, inflammatory leukocytes, and arteriolar smooth muscle cells. Our studies suggest that FSP1 is not a specific marker for cardiac fibroblasts in the remodeling myocardium. In contrast, the matricellular protein periostin was predominantly localized in spindle-shaped interstitial cells with myofibroblast morphology and was not expressed by inflammatory or endothelial cells. Our findings discourage the use of FSP1 as a marker for cardiac fibroblasts and raise major concerns regarding the usefulness of FSP1-Cre animals as a tool for fibroblast-specific gene targeting in vivo.
MATERIALS AND METHODS
Animal protocols.
All animal studies were approved by the Institutional Animal Care and Use Committee at Albert Einstein College of Medicine. Transgenic reporter FSP1.GFP mice were purchased from Jackson Laboratories (strain number: 012893, background: C57BL/6N). Two- to four-month-old mice (18.0–24.0 g body wt) were anesthetized with inhaled isoflurane (2%) and underwent reperfused myocardial infarction protocols using a closed-chest model of coronary occlusion and reperfusion as previously described (12). Use of the closed chest model allows us to study the postinfarction inflammatory response while avoiding the confounding effects of surgical trauma and inflammation. The left anterior descending coronary artery was occluded for 1 h and then reperfused for 3 days (n = 9) or 28 days (n = 4). Control hearts were harvested from uninjured FSP1.GFP mice (n = 13). To examine whether FSP1+ cells infiltrate the myocardium during neonatal remodeling, uninjured hearts from 13-day-old mice were also harvested (n = 4). At the end of the experiment, hearts were used for flow cytometry (control, n = 5; and 3 days reperfusion, n = 5) or for histological studies (control, n = 8; 3 days, n = 4; and 28 days, n = 4).
To identify FSP1+ cells in the pressure-overloaded myocardium, 2- to 4-mo-old FSP1.GFP reporter mice were anesthetized with inhaled isoflurane and then underwent transverse aortic constriction (TAC) protocols for 7 or 28 days as previously described (45, 46). Briefly, a 6–0 suture was tied twice around a blunt 3-mm segment of a 27-gauge needle, which was positioned adjacent to the aorta and was removed after placement of the ligature. The severity of pressure overload was assessed by measuring right-to-left carotid artery flow velocity ratio after constricting the transverse aorta. Only mice with a flow ratio from 5:1 to 10:1 were used for analysis. At the end of the experiment, hearts were used for flow cytometry (7 days of TAC, n = 5) or for histological studies (7 days TAC, n = 6; and 28 days TAC, n = 5). Uninjured adult hearts (4 mo of age) from FSP1.GFP mice were used as controls (flow cytometry, n = 5; and histology, n = 8).
Immunohistochemistry, histology, and quantitative histologic analysis.
At the end of the experiment hearts were removed, fixed with zinc formalin for 48 h, and embedded in paraffin. Serial sections from paraffin embedded hearts cut at 5-μm intervals were stained with rabbit anti-GFP antibody (Cell Signaling), rabbit anti-periostin antibody (Abcam), and mouse anti-α-smooth muscle actin antibody (α-SMA; Sigma, St. Louis MO). Staining was performed with a peroxidase based technique using the Vectastain Elite rabbit kit for GFP, the Mouse to Mouse (MOM) kit for α-SMA, and the Ultravision LP kit (Thermo Scientific) for periostin. Quantitative assessment of FSP1 cell density was performed by assessing the density of FSP1+ cells in the infarct, the neighboring viable subepicardial and subendocardial myocardium (peri-infarct area), and the noninfarcted remote posterior septum (remote area). Three fields from each one of the three different areas for the infarcted sections and nine fields from the control and TAC animals were used for quantitation. Density was expressed as cells per millimeter squared.
Isolation of noncardiomyocytes from control, pressure-overloaded, and infarcted hearts and flow cytometry.
Single cell suspensions were obtained from control (n = 5), infarcted (3 days of reperfusion, n = 5), and pressure overloaded hearts (7 days TAC, n = 4). Briefly, heart tissue was minced and placed into a cocktail of 0.25 mg/ml Liberase Blendzyme 3 (Roche Applied Science), 20 U/ml DNase I (Sigma-Aldrich), 10 mmol/l HEPES (Invitrogen), and 0.1% sodium azide in HBSS with Ca2+ and Mg2+ (Invitrogen), and shaken at 37°C for 20 min. Subsequently cells were passed through 40-μm nylon mesh and centrifuged (10 min, 200 g, 4°C). Finally, cells were reconstituted with staining buffer (d-PBS without Ca2+ and Mg2+, 2% FBS, and 0.1% sodium azide) and total cell numbers were determined with trypan blue (Mediatech). The resulting single-cell suspensions were washed with HBSS supplemented with 0.2% (wt/vol) BSA and 1% (wt/vol) FCS and centrifuged. Cells were stained and analyzed using flow cytometry as previously described by our laboratory (10). The following dyes and antibodies were used: LIVE/DEAD Fixable Violet Dead Cell Stain single-color dyes (Invitrogen), APC-Cy7-conjugated anti-CD45, Alexa Fluor 700-conjugated anti-CD3 (both from BD Phamingen), PerCP/Cy5.5-labeled anti-CD31, PE/Cy7-labeled anti-F4/80 (both from Biolegend), and Cy3-conjugated anti-α-SMA (Sigma). Stained single cells were resuspended in staining buffer and immediately analyzed with a Becton Dickinson LSRII flow cytometer (BD Biosciences).
Harvesting of CD11b+ myeloid cells and CD11b-negative nonmyeloid cells from control and infarcted hearts.
Macrophages and fibroblasts were isolated from control and infarcted hearts (CD11b-negative control, n = 5; macrophages control, n = 7; CD11b-negative infarct 24 h, n = 8; CD11b-negative infarct 7 days, n = 8; macrophages infarct 24 h, n = 8; and macrophages infarct 7 days, n = 8) for RNA extraction as previously described (10). Briefly, single cell suspensions were obtained from infarcted hearts (1 h ischemia followed by 24 h or 7 days of reperfusion) or healthy hearts as described in Isolation of noncardiomyocytes from control, pressure-overloaded, and infarcted hearts and flow cytometry. Cells were reconstituted with staining buffer (d-PBS without Ca2+ and Mg2+, 2% FBS, and 0.1% sodium azide) and total cell numbers were determined with trypan blue (Mediatech). The resulting single-cell suspensions were washed with HBSS supplemented with 0.2% (wt/vol) BSA and 1% (wt/vol) FCS and centrifuged. Single cells were resuspended in a special MACS buffer containing PBS pH 7.2, 0.5% BSA, and 2 mM EDTA; incubated with CD11b+ microbeads (Miltenyi Biotec) 10 μl/107 cells at 4°C for 15 min; and then washed once and centrifuged. Resuspended cells went through a MACS Column (Miltenyi Biotec) set in a MACS Seperator (Miltenyi Biotec). Unlabeled cells that passed through were collected and washed once with PBS as the CD11b- fraction. These CD11b− cells were harvested for further experiments. The magnetically labeled CD11b+ cells were retained on the column. Approximately 2 ml of MACS buffer were applied onto the column. Cells were flushed out by firmly pushing the plunger and collected into a tube.
RNA extraction and quantitative PCR.
Total RNA was extracted from CD11b+ and CD11b-negative cells harvested from control and infarcted hearts, mRNA was reverse transcribed and amplified using the EvaGreen Supermix reagent and the C1000 thermal cycler apparatus from BIO-RAD following the manufacturer's instructions. The housekeeping gene GAPDH was used as internal control. Primers used were as follows: periostin (forward: 5′-CCTGGATTCTGACATTCGCA-3′ and reverse: 5′-CCATGCCGTGTTTCAGGTCC-3′), FSP1 (forward: 5′-TGTAATTGTGTCCACCTTCC-3′ and reverse: 5′-GCTCATCACCTTCTGGAATG-3′), and 18S (forward: 5′-TCAGATACCGTCGTAGTT-3′ and reverse: 5′-CTTTAAGTTTCAGCTTTGCA-3′). The quantitative PCR procedure was repeated for three times in independent runs; expression levels were calculated using the ΔΔCt method.
Statistical analysis.
Statistical analysis was performed using ANOVA followed by t-test corrected for multiple comparisons (Student-Newman-Keuls). Data are expressed as means ± SE. Statistical significance was set at 0.05.
RESULTS
Periostin, but not FSP1, is expressed in interstitial cells during neonatal cardiac remodeling.
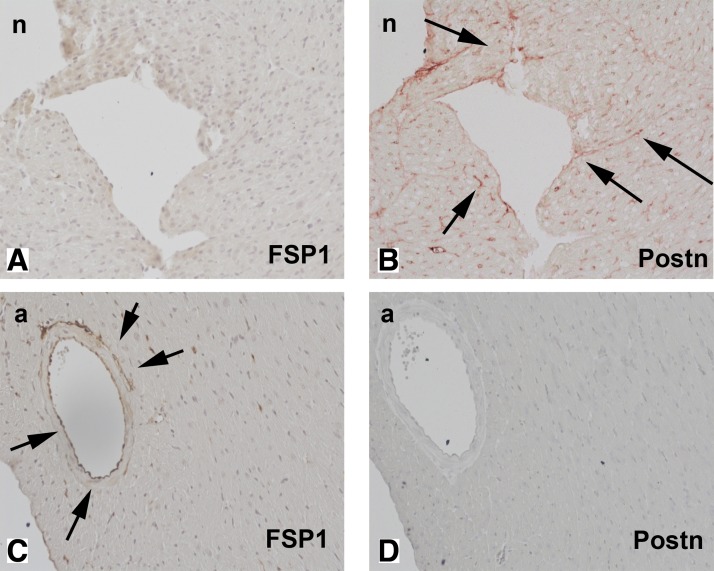
We used FSP1.GFP reporter mice to identify cells expressing FSP1 in uninjured mouse hearts. Because during the neonatal period, there is a significant increase in the number of myocardial fibroblasts (corresponding to the stage of neonatal cardiac remodeling), we studied both neonatal hearts (2 wk of age) and adult mouse hearts (3–4 mo of age). In the neonatal heart, no FSP1+ cells were present in the myocardium (Fig. 1A); in contrast, immunohistochemical staining identified a large number of interstitial cells expressing periostin (Fig. 1B). The adult mouse heart contained relatively few FSP1+ cells (predominantly endothelial and perivascular cells) and had practically no periostin-positive cells (Fig. 1, C and D).
Fig. 1.
Fibroblast-specific protein (FSP)1- and periostin (Postn)-expressing cells in neonatal (n: 2 wk of age) and adult (a: 3–4 mo of age) mouse myocardium. Neonatal hearts remodel after the transition from the fetal to adult circulation; neonatal remodeling is associated with an expansion of the cardiac fibroblast population. FSP1 expression in neonatal and adult mouse hearts in the absence of injury was studied in FSP1. Green fluorescent protein (GFP) reporter mice (A and C); serial sections were stained immunohistochemically for periostin using the Ultravision LP kit (red, B and D). Neonatal hearts exhibit no FSP1 expression (A); however, abundant interstitial cells are stained for periostin (B, arrows). In adult mouse hearts, rare FSP1+ cells are identified; these cells are either perivascular or endothelial (C, arrows). In contrast, negligible periostin immunoreactivity is found in the adult mouse myocardium.
FSP-1 + cells infiltrate the infarcted myocardium.
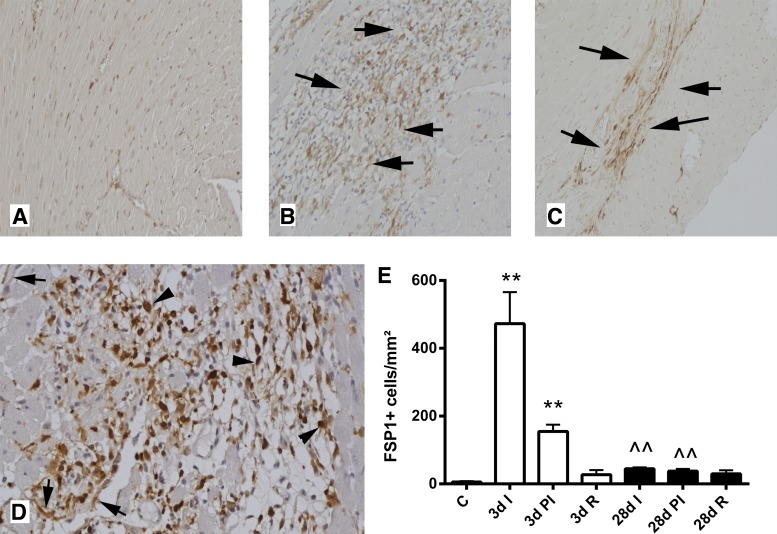
The reperfused infarcted myocardium exhibited intense infiltration with FSP1+ cells after 72 h of reperfusion (Fig. 2, A, B, D, and E). During scar maturation (28 days of reperfusion), FSP1+ cells became less abundant (Fig. 2, C–E). Most FSP1+ cells in the infarct were round, resembling leukocytes; occasional FSP1+ cells had spindle-shaped, fibroblast-like morphology (Fig. 2D). In contrast, border zone cardiomyocytes did not express FSP1. Quantitative analysis showed that FSP-1+ cells also accumulate in the peri-infarct area but not in the remote remodeling myocardium (Fig. 2E).
Fig. 2.
FSP1.GFP reporter mice were used to identify FSP1+ cells in the infarcted myocardium. Anti-GFP immunohistochemistry was performed in control heart (A) and infarcted myocardium after 3 days of reperfusion (B and D) and after 28 days of reperfusion (C). After 72 h of reperfusion, abundant FSP1+ cells are found in the infarcted heart (B, arrows). After 28 days of reperfusion, the mature scar contracts significantly and contains a lower number of FSP1+ cells (C, arrows). D: high magnification images show that although some FSP1+ cells are spindle-shaped (arrows), resembling fibroblast-like cells, most are round and have morphological characteristics of hematopoietic cells (arrowheads). E: quantitative analysis shows that, in the infarcted area (I), the number of FSP1+ cells peak after 3 days of reperfusion (D; **P < 0.01 vs. control). Increased numbers of FSP1+ cells are also found in the peri-infarct area (PI). The number of FSP1+ cells in the infarct and in the peri-infarct zone is significantly reduced after 28 days of reperfusion (∧∧P < 0.01 vs. corresponding 3-day cell densities). The number of FSP1+ cells is not increased in the remote remodeling myocardium (R).
The majority of FSP1+ cells in the infarcted myocardium are not myofibroblasts but are identified as hematopoietic cells or endothelial cells.
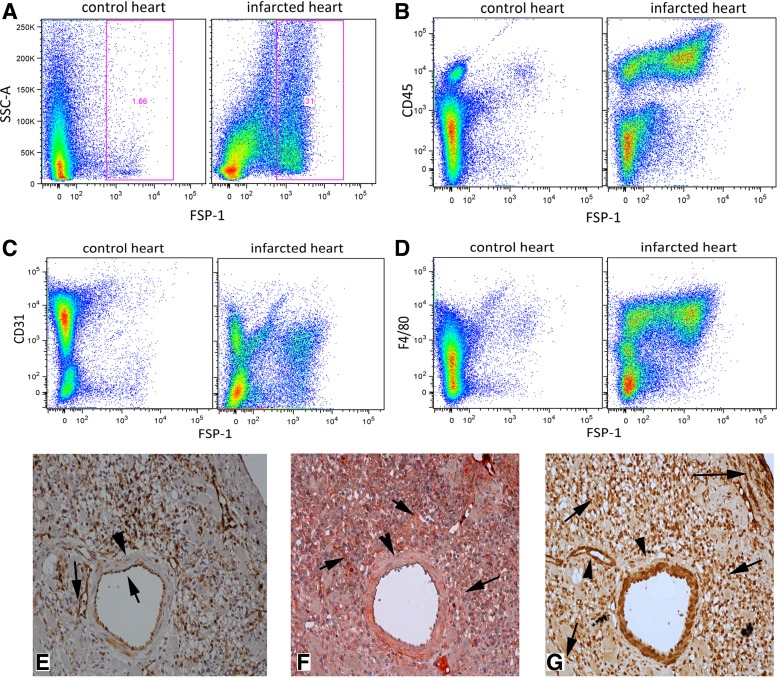
Flow cytometry and serial section immunohistochemistry were used to determine the cellular identity of FSP1+ cells infiltrating the infarcted myocardium. Flow cytometric analysis showed that in the control heart the majority of FSP1+ cells were CD31+ endothelial cells or α-SMA-expressing smooth muscle cells (Table 1). After 72 h of reperfusion, the number of FSP1+ cells showed a 34-fold increase when compared with control myocardium (Table 1 and Fig. 3A). The marked increase in FSP1+ cell numbers was due to influx of FSP1+/CD45+ hematopoietic cells that accounted for almost 50% of the FSP1+ population (Table 1 and Fig. 3B). Although CD3+ lymphocytes were only 1.3% of FSP1+ cells in the infarct, they exhibited a marked 66-fold increase compared with control hearts. Marked increases in the number of FSP1+/CD31+ endothelial cells (Table 1 and Fig. 3C) and FSP1+/F4/80 macrophages were also noted (Table 1 and Fig. 3D). Only 4% of FSP1+ cells were identified as α-SMA-expressing cells (myofibroblasts or smooth muscle cells). Immunohistochemical identification of FSP1+ cells supported the flow cytometric findings, suggesting lack of specificity of FSP1 for fibroblasts, as vascular endothelial cells in infarct vessels were also strongly FSP1+ (Fig. 3E). In contrast, periostin immunohistochemistry of infarcted hearts stained spindle-shaped cells with morphological characteristics of myofibroblasts but did not label endothelial cells (Fig. 3F). α-SMA+ smooth muscle cells in intramyocardial coronary vessels (Fig. 3G) did not express FSP1 (Fig. 3E) and periostin (Fig. 3F).
Table 1.
Cellular identity of the FSP1+ cells in the infarcted and pressure-overloaded myocardium
| Control |
Myocardial Infarction |
Pressure Overload |
||||
|---|---|---|---|---|---|---|
| % | n | % | n | % | n | |
| FSP+ | 100 | 17,163 ± 1,883 | 100 | 572,044 ± 136,559† | 100 | 95,010 ± 18,315 |
| CD3+/FSP+ | 0.8 ± 0.1 | 124 ± 13 | 1.3 ± 0.4 | 8,169 ± 3,869 | 0.2 ± 0.2 | 243 ± 186 |
| CD45+/FSP+ | 7.2 ± 0.8 | 1,237 ± 179 | 47.1 ± 3.3† | 278,930 ± 81,140† | 12.7 ± 4.1 | 12,359 ± 5,489 |
| F4/80+/FSP+ | 8.1 ± 0.7 | 1,368 ± 174 | 4.1 ± 0.8† | 24,191 ± 9,066* | 2.3 ± 0.7† | 2,226 ± 883 |
| α-SMA+/FSP+ | 27.8 ± 1.4 | 4,876 ± 740 | 1.6 ± 0.4† | 7,018 ± 947 | 8.4 ± 1.7† | 7,809 ± 2,266 |
| CD31+/FSP+ | 45.2 ± 2.3 | 7,624 ± 655 | 2.2 ± 0.6† | 9,132 ± 657 | 16.8 ± 3.0† | 14,928 ± 3,272* |
Values are means ± SE. FSP, fibroblast-specific protein; α-SMA, α-smooth muscle actin.
P < 0.05,
P < 0.01 vs. corresponding control.
Fig. 3.
Flow cytometry and serial section immunohistochemical staining demonstrates that the majority of FSP1+ cells infiltrating the infarcted myocardium are not fibroblasts. A: representative flow cytometric experiment studying noncardiomyocytes harvested from a control and an infarcted heart illustrates the marked increase in the number of FSP1+ cells in the infarct after 72 h of reperfusion. B: representative experiments show that the majority of FSP1+ cells in the infarct are CD45+ hematopoietic cells. C and D: significant percentage of infarct FSP1+ cells is identified as CD31+ endothelial cells (C) or as F4/80+ macrophages (D). Quantitative analysis of the findings is shown in Table 1. E–G: serial section immunohistochemical staining of reperfused infarcts for GFP to identify FSP1+ cells (stained with a peroxidase-based technique and developed with DAB, brown; E), with an anti-periostin antibody (stained with the Ultravision LP kit, red; F) and with an antibody against α-smooth muscle actin (α-SMA; G, brown). Note that vascular endothelial cells are positive for FSP1 (E, arrows). In contrast, periostin immunohistochemistry does not stain the endothelium but labels a large number of spinde-shaped interstitial cells (F, arrows) and, as a typical matricellular protein, is deposited in the matrix. G: α-SMA immunostaining identifies smooth muscle cells (arrowheads) and myofibroblasts (arrows) in the infarct. Vascular smooth muscle cells in the media of intramyocardial coronary vessels did not stain for FSP1 and periostin (arrowheads).
FSP1+ cells infiltrate the pressure-overloaded myocardium.
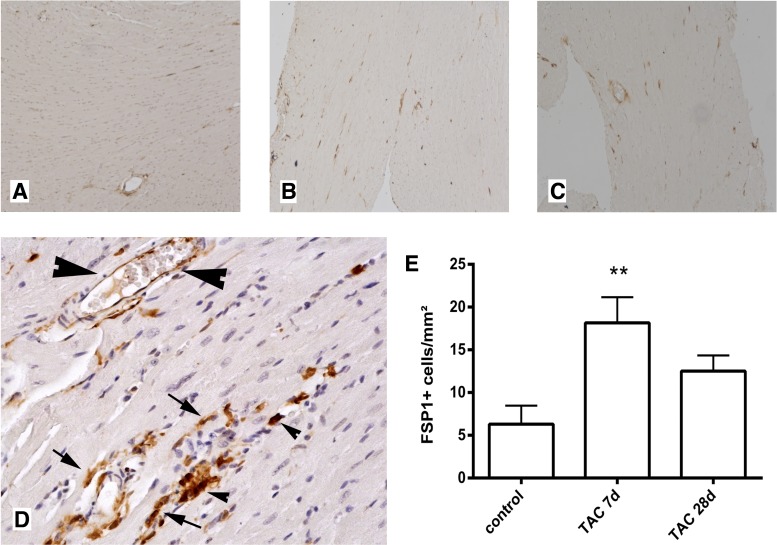
In pressure-overloaded hearts, FSP1+ cells were identified in the cardiac interstitium after 7 and 28 days of TAC (Fig. 4). The time points were selected to study the early hypertrophic phase (7days) and late dilative phase (28 days) of cardiac remodeling following pressure overload (46). FSP1+ cells had morphological characteristics of fibroblasts (spindle-shaped morphology), leukocytes (round morphology), or endothelial cells (Fig. 4D). In contrast, cardiomyocytes did not express FSP1. Quantitative analysis demonstrated a significant increase in FSP1+ cell density after 7 days of TAC (Fig. 4E); however, the number of FSP-1-expressing cells in pressure-overloaded myocardium was significantly lower than in healing infarcts.
Fig. 4.
FSP1+ cells infiltrate the pressure-overloaded myocardium. Anti-GFP staining is used to identify FSP1+ cells in control hearts (A) and in the pressure overloaded myocardium after 7 (B) and 28 days (C) of transverse aortic constriction (TAC). FSP1+ cells are identified in the cardiac interstitium (arrows). D: high magnification image shows that FSP1 is localized in spindle-shaped cells with morphological characteristics of fibroblasts (arrows), in endothelial cells (large arrowheads), or in round cells with leukocyte morphology (small arrowheads). E: quantitative analysis shows a significant increase in the density of myocardial FSP1+ cells after 7 days of TAC (**P < 0.01 vs. control myocardium).
Identification of FSP1+ cells in the pressure-overloaded myocardium.
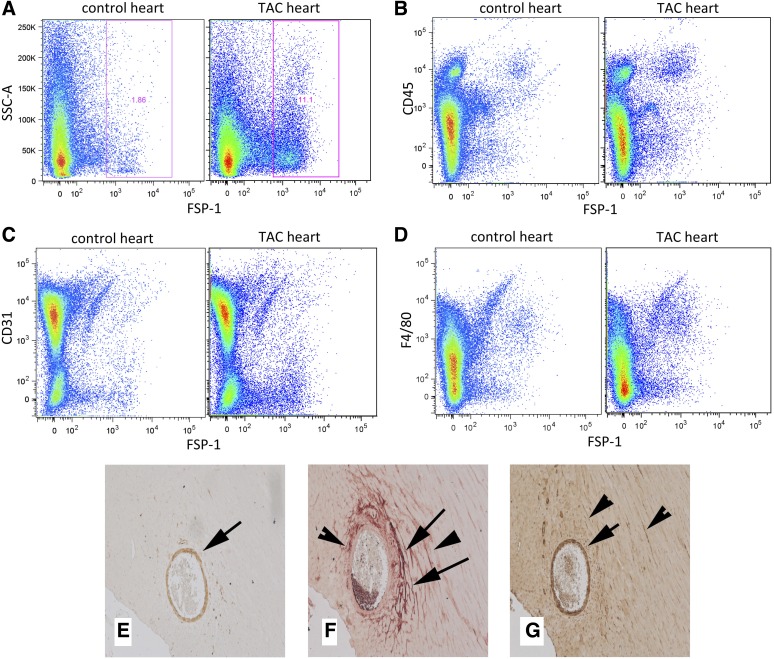
Flow cytometry was used to study the cellular identity of FSP1+ cells in the pressure-overloaded myocardium after 7 days of TAC. When compared with control heart, the pressure overloaded myocardium showed a 5.6-fold increase in the number of FSP1+ cells; about 17% of FSP1+ cells were identified as CD31+ endothelial cells, whereas 13% were hematopoietic cells (Fig. 5 and Table 1); 8.4% of FSP1+ cells were identified as α-SMA-expressing cells (myofibroblasts or smooth muscle cells) (Table 1). A significant percentage of FSP1+ cells infiltrating the pressure-overloaded myocardium (almost 60%) could not be identified with any of the cell-specific markers. Immunohistochemical staining of sections from pressure-overloaded hearts demonstrated that vascular smooth muscle cells in large myocardial arterioles and intramyocardial coronaries (identified through serial section staining for α-SMA) had intense upregulation of FSP-1 expression. In contrast, periostin did not stain vascular smooth muscle cells but identified spindle-shaped interstitial cells with morphological characteristics of fibroblasts and, as a typical matricellular protein, was incorporated into the extracellular matrix (Fig. 5).
Fig. 5.
Identification of FSP1+ cells in the pressure-overloaded myocardium using flow cytometry and serial section immunohistochemistry. A representative flow cytometry experiment illustrates a marked increase in the number of FSP1+ cells in the pressure-overloaded myocardium after 7 days of TAC (A). Representative experiments show that FSP1+ cells are identified as CD45+ hematopoietic cells (B), CD31+ endothelial cells (C), or F4/80+ macrophages (D). Quantitative analysis of the findings is shown in Table 1. Serial section immunohistochemistry in pressure-overloaded hearts after 28 days of TAC with an anti-EGFP antibody to identify FSP1+ cells (E, developed with DAB showing positive cells in brown color), with an anti-periostin antibody (F, red) and with an antibody to α-SMA that labels smooth muscle cells and myofibroblasts (G, brown). After 28 days of TAC, FSP1 expression is noted in α-SMA+ myocardial arterioles (E–G, arrows). In contrast, periostin staining does not label vascular smooth muscle cells but is localized in α-SMA+ myofibroblasts (F and G, arrowheads) and, in a typical matricellular manner, in the interstitial matrix (F, arrows).
FSP-1 is highly expressed in CD11b+ myeloid cells but not in CD11b-negative cells, harvested from the infarct, whereas periostin is markedly upregulated in the CD11b-negative fraction.
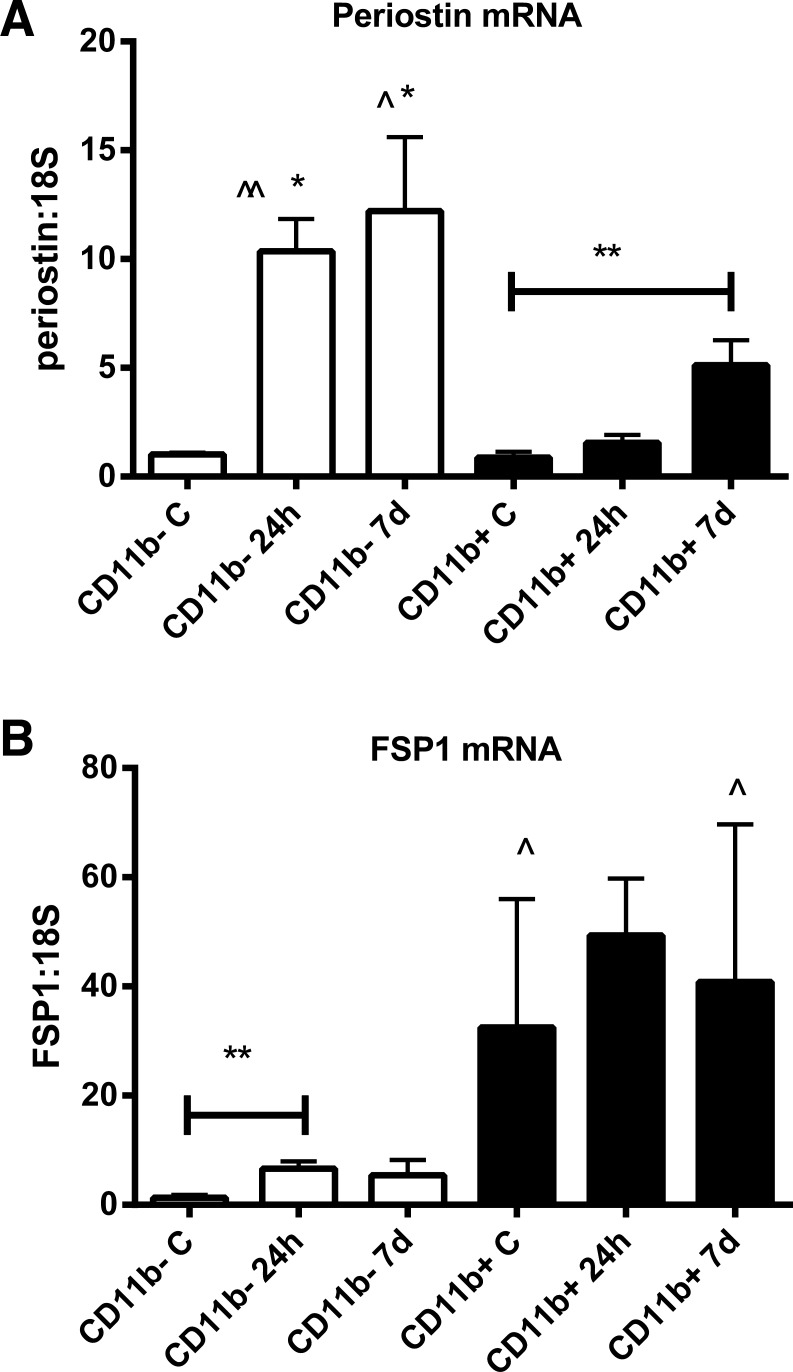
CD11b+ myeloid cells and CD11b-negative (nonmyeloid) cells were harvested from control hearts and from infarcted myocardium after 24 h and 7 days of reperfusion. Control CD11b-negative cells had very low expression of periostin and FSP1 mRNA. CD11b-negative cells harvested from the infarct exhibited marked increase in periostin mRNA expression but only a modest increase in FSP1 mRNA levels after 24 h of reperfusion (Fig. 6, A and B). Periostin mRNA expression remained low in CD11b+ myeloid cells harvested after 24 h of reperfusion but significantly increased in cells isolated after 7 days of reperfusion (Fig. 6A). CD11b+ cells isolated from control hearts and infarcts had high levels of FSP1 mRNA expression (Fig. 6B).
Fig. 6.
Expression of periostin and FSP1 mRNA by CD11b+ myeloid cells and by CD11b-negative (nonmyeloid cells) isolated from control and infarcted wild-type mouse hearts. A: CD11b+ and CD11b-negative cells isolated from control hearts show low levels of periostin mRNA expression. CD11b-negative cells harvested after 24 h and 7 days of reperfusion exhibit markedly increased periostin mRNA synthesis (∧∧P < 0.01, ∧P < 0.05 vs. control). CD11b+ myeloid cells have no increase in periostin mRNA levels after 24 h, but exhibit significantly increased expression after 7 days of reperfusion (**P < 0.01 vs. control CD11b+ cells). Periostin expression levels in cardiac CD11b-negative cells are significantly higher than in myeloid cells at the same time point (*P < 0.05 vs. corresponding CD11b+ cells). B: FSP1 expression is very low in nonmyeloid cells harvested from control hearts, but is increased significantly in nonmyeloid cells isolated after 24 h of reperfusion (**P < 0.01 vs. control). CD11b+ myeloid cells from control hearts exhibit high FSP1 mRNA expression, at levels significantly higher than corresponding nonmyeloid cells (∧P < 0.05). Myeloid cells isolated from infarcts after 7 days of reperfusion also have significantly higher levels of FSP1 mRNA expression than corresponding nonmyeloid cells.
DISCUSSION
Almost 20 yr ago, identification and characterization of FSP1 as a novel intracellular protein specifically expressed in fibroblasts generated considerable enthusiasm regarding its potential usefulness in mapping cell fate among fibroblasts and in studying the consequences of fibroblast-specific gene targeting in tissue homeostasis and in disease. Several high-profile studies have used FSP1 as a fibroblast-specific marker to investigate the role of fibroblasts in pathophysiology of disease through the development of fibroblast-specific knockout mice (4) or to study the origin of fibroblasts in fibrotic conditions (47). Recently, concerns were raised regarding the specificity of FSP1 as a fibroblast marker in hepatic and renal fibrosis (26, 36). Osterreicher et al. (36) demonstrated that in fibrotic liver FSP1 does not identify fibroblasts but labels a proinflammatory subpopulation of macrophages. Our study reports for the first time that FSP1 is not a specific marker for fibroblasts in the remodeling myocardium. In two distinct mouse models of cardiac fibrosis, FSP1 was primarily expressed by hematopoietic and vascular cells and was not specific for myofibroblast populations.
Periostin, and not FSP-1, labels interstitial cells in neonatal cardiac remodeling.
During the neonatal period, transition from the embryonic to the adult circulation increases intracardiac pressures and activates an adaptive myocardial response, resulting in increased thickness and stiffness of the left ventricular wall (6, 7). Neonatal cardiac remodeling is associated with a twofold increase in the number of interstitial fibroblasts (6); these cells transiently express periostin (33) and may be responsible for expansion of the interstitial collagenous network. Using immunohistochemical techniques, we identified abundant periostin-positive cells in the neonatal mouse heart; however, these cells did not express FSP1 (Fig. 1).
FSP1+ cells in the infarcted myocardium.
Myocardial infarction triggers an inflammatory cascade that ultimately results in formation of a scar (17). Repair of the infarcted myocardium can be divided into three distinct, but overlapping phases: the inflammatory phase, the proliferative phase, and the maturation phase (15). During the inflammatory phase, dying cardiomyocytes release danger signals, inducing cytokine and chemokine upregulation in the infarct through activation of innate immune mechanisms (43). Induction of chemokines, such as monocyte chemoattractant protein-1/CCL2, mediates recruitment of proinflammatory mononuclear cell into the infarcted myocardium that has prominent phagocytotic properties and clears the infarct from dead cells and matrix debris (32), (13). Removal of apoptotic leukocytes from the infarct marks the end of the inflammatory phase as proinflammatory signaling is suppressed and the infarct is infiltrated by myofibroblasts and vascular cells. During the proliferative phase activated myofibroblasts secrete matrix proteins (11, 14, 44), while vascular cells form a rich microvascular network that provides oxygen and nutrients to the metabolically active wound (18). As the scar matures, myofibroblast numbers in the infarct decrease and most of the infarct neovessels regress. Our experiments demonstrated that infiltration of the infarct with FSP1+ cells peaks during the proliferative phase of healing (Fig. 2). Although occasional FSP1 expressing cells were interstitial spindle-shaped cells with fibroblast-like morphological characteristics (Fig. 2D), and fibroblasts isolated from the infarct expressed FSP-1 (Fig. 6), the majority of FSP1+ cells in the infarcted heart were identified as hematopoietic cells or endothelial cells and only a small percentage (1.3%) expressed the myofibroblast marker α-SMA. Flow cytometric analysis demonstrated that almost 50% of FSP1+ cells in the infarcted heart were CD45+ hematopoietic cells; a small proportion of these cells were identified as F4/80-expressing macrophages (Table 1 and Fig. 3). Because progenitor cells of hematopoietic origin may differentiate into fibroblasts contributing to the development of cardiac fibrosis (19, 31), a small fraction of the CD45+/FSP1 cells may represent blood-derived fibroblast progenitors. FSP1 expression was also observed in endothelial cells (Table 1 and Fig. 3) and in CD3+ lymphocytes (Table 1) infiltrating the infarct. Cardiomyocytes in the infarct border zone and in the noninfarcted remodeling myocardium did not express FSP1. In contrast to our observations, Schneider et al. (37) have previously demonstrated FSP1 immunoreactivity in border zone cardiomyocytes in a rat model of myocardial infarction. The absence of FSP1 staining in infarct cardiomyocytes in FSP1.GFP reporter mice suggests that the previously reported immunohistochemical findings may reflect binding of FSP1 protein to injured cardiomyocytes. After 28 days of reperfusion, the number of FSP1+ cells significantly decreased, reflecting resolution of the inflammatory infiltrate and apoptosis of mesenchymal cells in the mature scar.
FSP1+ cells in the fibrotic pressure-overloaded myocardium.
Fibroblast activation and expansion of the cardiac interstitium through deposition of matrix proteins are hallmarks of the myocardial response to pressure overload (20, 27, 45). FSP1+ cells infiltrated the pressure-overloaded myocardium after 7 days of TAC (Fig. 4 and Table 1); however, their absolute number and cell density was markedly lower than in infarcted hearts (Fig. 4 and Table 1). Although some FSP1+ cells in the fibrotic heart had morphological characteristics of myofibroblasts (Table 1 and Fig. 4D), flow cytometric analysis demonstrated that almost 13% of FSP1-expressing cells were CD45+ hematopoietic cells (Fig. 5 and Table 1) and only 8% expressed the myofibroblast marker α-SMA. Vascular cells in the pressure-overloaded myocardium also expressed FSP1. The absolute number of myocardial endothelial cells expressing FSP1 almost doubled after 7 days of TAC, as almost 17% of the FSP1+ cells in pressure overloaded heart were identified as CD31+ endothelial cells (Figs. 4D and 5 and Table 1). After 28 days of TAC, intense FSP1 staining was noted in arteriolar smooth muscle cells (Fig. 5E), suggesting that FSP1 protein expression may be induced in coronary arterioles in response to chronic exposure to a pressure load. Similarly, previous investigations in porcine and human coronary arteries demonstrated negligible expression of FSP1 in normal vessels but showed marked upregulation in smooth muscle cells following vascular injury (8). Interestingly, almost 60% of the FSP1+ cells infiltrating the pressure-overloaded myocardium could not be identified with any of the markers used. These nonhematopoietic, nonendothelial cells may be quiescent fibroblasts or pericytes. In comparison, in the infarcted myocardium only 40% of FSP1+ cells were not labeled with any of the antibodies, reflecting the higher number of FSP1-expressing inflammatory cells recruited into the infarct.
Periostin as a fibroblast-specific marker.
Periostin is a matricellular protein that binds to the structural matrix and modulates cellular phenotype through integrin-mediated actions (16, 33). Periostin is highly expressed in the embryonic myocardium, and is absent from cells of cardiomyocyte lineage, but is localized in cardiac mesenchymal cells (22, 24). In adult hearts, periostin expression is very low; however, cardiac remodeling is consistently associated with induction of periostin in fibroblasts. Other mesenchymal cell types have been reported to express periostin upon stimulation. Periostin is not expressed by unstimulated smooth muscle cells but is markedly upregulated following vascular injury (28–30). Our experiments in both normal (Fig. 1) myocardium and in remodeling hearts (Figs. 3–4) suggest that intramyocardial coronaries in the infarcted and pressure-overloaded myocardium (Figs. 3–4) do not exhibit periostin immunoreactivity. Studies using periostin null mice have documented the critical role of periostin in activation of fibroblasts (34, 38) in myocardial infarction and in fibrosis induced by pressure overload. Because of the relatively specific localization of periostin in injury site fibroblasts, periostin-Cre-mediated gene deletion has been used to study the effects of fibroblast-specific gene targeting (41). Our study provides further support for the use of periostin as a marker of activated fibroblasts in the remodeling myocardium. In neonatal cardiac remodeling, periostin staining labeled abundant interstitial cells with morphological characteristics of fibroblasts; in contrast, periostin expression was negligible in normal adult mouse hearts (Fig. 1). In infarcted and pressure-overloaded myocardium, periostin staining identified spindle-shaped fibroblast-like cells, without labeling endothelial or vascular smooth muscle cells (Figs. 4 and 5). As a typical matricellular protein, periostin was also deposited in the interstitial matrix (Fig. 5). Because of its absence in normal cardiac matrix, the presence of periostin in the cardiac interstitium may be a specific indicator of active cardiac remodeling.
The specificity of periostin for fibroblasts was supported by experiments in sorted CD11b+ myeloid cells and in CD11b-negative nonmyeloid cells harvested from control and infarcted hearts. After 24 h of reperfusion, periostin mRNA was markedly induced in the CD11b-negative fraction but not in hematopoietic cells; in contrast, FSP1 mRNA was highly expressed by myeloid cells at all time points (Fig. 6). Interestingly, myeloid cells harvested at 7 days had periostin expression. Because periostin is not normally expressed by myeloid cell populations, the finding may reflect recruitment and differentiation of fibrocytes, a circulating CD11b+ subpopulation of monocytic cells, capable of differentiating into fibroblasts (1). The contribution of circulating progenitors in cardiac fibrosis has been suggested by experimental studies in a mouse model of nonreperfused myocardial infarction (31) and in ischemic fibrotic cardiomyopathy (19).
Implications of the findings for fibroblast identification and fibroblast-specific targeting in cardiac pathologic conditions.
Our findings have important implications regarding the choice of markers to identify fibroblasts and to study the role of fibroblast-specific signaling in cardiac remodeling and fibrosis. In experimental models of infarction and pressure overload-induced fibrosis, FSP1 is not specific for fibroblasts but identifies subpopulations of inflammatory leukocytes and vascular cells. Thus the study of FSP1 reporter mice to investigate the fate of cardiac fibroblasts and use of FSP1-Cre driven gene deletion to dissect the consequences of fibroblast-specific gene targeting are of limited value in understanding the pathobiology of cardiac disease. Periostin-Cre mice, on the other hand, may be a useful tool for genetic manipulation of activated injury-site fibroblasts. However, considering the negligible expression of periostin in normal hearts, these animals are of limited value for targeting gene expression in normal cardiac fibroblasts. Mice with inducible Cre under the control of a regulatory sequence from the proα2(I)collagen gene (48) have been used to investigate fibroblast-specific effects in models of wound healing and fibrosis; however, there is no information on their use to study the pathogenesis of cardiac fibrotic remodeling. Recently, Acharya et al. (3) have identified the transcription factor Tcf21, as a marker of cells committed to the cardiac fibroblast lineage and as an essential mediator in development of cardiac fibroblasts. Mice expressing an inducible Cre from the Tcf21 locus (2) may prove a useful tool for studies examining the role of fibroblast-specific signaling in cardiac homeostasis and disease. However, systematic investigation of Tcf21 expression in cardiac pathologic conditions has not been performed. Identification of new and specific fibroblast markers is urgently needed to advance understanding of the pathogenesis of cardiac fibrosis.
GRANTS
Support for this study was provided by National Heart, Lung, and Blood Institute Grants R01-HL-85440 and R01-HL-76246 and the Wilf Family Cardiovascular Research Institute (to the laboratory of N. G. Frangogiannis).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: P.K., P.C., A.S., and Y.S. performed experiments; P.K., P.C., A.S., and Y.S. analyzed data; P.K., P.C., and N.G.F. interpreted results of experiments; P.K., P.C., and N.G.F. prepared figures; P.K. and N.G.F. drafted manuscript; P.K., P.C., A.S., Y.S., and N.G.F. approved final version of manuscript; N.G.F. conception and design of research; N.G.F. edited and revised manuscript.
REFERENCES
- 1.Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol 166: 7556–7562, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Acharya A, Baek ST, Banfi S, Eskiocak B, Tallquist MD. Efficient inducible Cre-mediated recombination in Tcf21 cell lineages in the heart and kidney. Genesis 49: 870–877, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Acharya A, Baek ST, Huang G, Eskiocak B, Goetsch S, Sung CY, Banfi S, Sauer MF, Olsen GS, Duffield JS, Olson EN, Tallquist MD. The bHLH transcription factor Tcf21 is required for lineage-specific EMT of cardiac fibroblast progenitors. Development 139: 2139–2149, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S, Washington MK, Neilson EG, Moses HL. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science 303: 848–851, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Boomershine CS, Chamberlain A, Kendall P, Afshar-Sharif AR, Huang H, Washington MK, Lawson WE, Thomas JW, Blackwell TS, Bhowmick NA. Autoimmune pancreatitis results from loss of TGFbeta signalling in S100A4-positive dendritic cells. Gut 58: 1267–1274, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borg TK, Ranson WF, Moslehy FA, Caulfield JB. Structural basis of ventricular stiffness. Lab Invest 44: 49–54, 1981 [PubMed] [Google Scholar]
- 7.Borg TK, Rubin K, Lundgren E, Borg K, Obrink B. Recognition of extracellular matrix components by neonatal and adult cardiac myocytes. Dev Biol 104: 86–96, 1984 [DOI] [PubMed] [Google Scholar]
- 8.Brisset AC, Hao H, Camenzind E, Bacchetta M, Geinoz A, Sanchez JC, Chaponnier C, Gabbiani G, Bochaton-Piallat ML. Intimal smooth muscle cells of porcine and human coronary artery express S100A4, a marker of the rhomboid phenotype in vitro. Circ Res 100: 1055–1062, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Chen W, Frangogiannis NG. Fibroblasts in post-infarction inflammation and cardiac repair. Biochim Biophys Acta 1833: 945–953, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen W, Saxena A, Li N, Sun J, Gupta A, Lee DW, Tian Q, Dobaczewski M, Frangogiannis NG. Endogenous IRAK-M attenuates postinfarction remodeling through effects on macrophages and fibroblasts. Arterioscler Thromb Vasc Biol 32: 2598–2608, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cleutjens JP, Verluyten MJ, Smiths JF, Daemen MJ. Collagen remodeling after myocardial infarction in the rat heart. Am J Pathol 147: 325–338, 1995 [PMC free article] [PubMed] [Google Scholar]
- 12.Dewald O, Ren G, Duerr GD, Zoerlein M, Klemm C, Gersch C, Tincey S, Michael LH, Entman ML, Frangogiannis NG. Of mice and dogs: species-specific differences in the inflammatory response following myocardial infarction. Am J Pathol 164: 665–677, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dewald O, Zymek P, Winkelmann K, Koerting A, Ren G, Abou-Khamis T, Michael LH, Rollins BJ, Entman ML, Frangogiannis NG. CCL2/monocyte chemoattractant protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ Res 96: 881–889, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Dobaczewski M, Bujak M, Li N, Gonzalez-Quesada C, Mendoza LH, Wang XF, Frangogiannis NG. Smad3 signaling critically regulates fibroblast phenotype and function in healing myocardial infarction. Circ Res 107: 418–428, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frangogiannis NG. The immune system and cardiac repair. Pharmacol Res 58: 88–111, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frangogiannis NG. Matricellular proteins in cardiac adaptation and disease. Physiol Rev 92: 635–688, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frangogiannis NG. Regulation of the inflammatory response in cardiac repair. Circ Res 110: 159–173, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frangogiannis NG, Michael LH, Entman ML. Myofibroblasts in reperfused myocardial infarcts express the embryonic form of smooth muscle myosin heavy chain (SMemb). Cardiovasc Res 48: 89–100, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Haudek SB, Xia Y, Huebener P, Lee JM, Carlson S, Crawford JR, Pilling D, Gomer RH, Trial J, Frangogiannis NG, Entman ML. Bone marrow-derived fibroblast precursors mediate ischemic cardiomyopathy in mice. Proc Natl Acad Sci USA 103: 18284–18289, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herum KM, Lunde IG, Skrbic B, Florholmen G, Behmen D, Sjaastad I, Carlson CR, Gomez MF, Christensen G. Syndecan-4 signaling via NFAT regulates extracellular matrix production and cardiac myofibroblast differentiation in response to mechanical stress. J Mol Cell Cardiol 54: 73–81, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Kakkar R, Lee RT. Intramyocardial fibroblast myocyte communication. Circ Res 106: 47–57, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kern CB, Hoffman S, Moreno R, Damon BJ, Norris RA, Krug EL, Markwald RR, Mjaatvedt CH. Immunolocalization of chick periostin protein in the developing heart. Anat Rec A Discov Mol Cell Evol Biol 284: 415–423, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Kong P, Christia P, Frangogiannis NG. The pathogenesis of cardiac fibrosis. Cell Mol Life Sci 2013. May 7 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kruzynska-Frejtag A, Machnicki M, Rogers R, Markwald RR, Conway SJ. Periostin (an osteoblast-specific factor) is expressed within the embryonic mouse heart during valve formation. Mech Dev 103: 183–188, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Lawson WE, Polosukhin VV, Zoia O, Stathopoulos GT, Han W, Plieth D, Loyd JE, Neilson EG, Blackwell TS. Characterization of fibroblast-specific protein 1 in pulmonary fibrosis. Am J Respir Crit Care Med 171: 899–907, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Le Hir M, Hegyi I, Cueni-Loffing D, Loffing J, Kaissling B. Characterization of renal interstitial fibroblast-specific protein 1/S100A4-positive cells in healthy and inflamed rodent kidneys. Histochem Cell Biol 123: 335–346, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Leslie KO, Taatjes DJ, Schwarz J, vonTurkovich M, Low RB. Cardiac myofibroblasts express alpha smooth muscle actin during right ventricular pressure overload in the rabbit. Am J Pathol 139: 207–216, 1991 [PMC free article] [PubMed] [Google Scholar]
- 28.Li G, Jin R, Norris RA, Zhang L, Yu S, Wu F, Markwald RR, Nanda A, Conway SJ, Smyth SS, Granger DN. Periostin mediates vascular smooth muscle cell migration through the integrins alphavbeta3 and alphavbeta5 and focal adhesion kinase (FAK) pathway. Atherosclerosis 208: 358–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li G, Oparil S, Sanders JM, Zhang L, Dai M, Chen LB, Conway SJ, McNamara CA, Sarembock IJ. Phosphatidylinositol-3-kinase signaling mediates vascular smooth muscle cell expression of periostin in vivo and in vitro. Atherosclerosis 188: 292–300, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindner V, Wang Q, Conley BA, Friesel RE, Vary CP. Vascular injury induces expression of periostin: implications for vascular cell differentiation and migration. Arterioscler Thromb Vasc Biol 25: 77–83, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Mollmann H, Nef HM, Kostin S, von Kalle C, Pilz I, Weber M, Schaper J, Hamm CW, Elsasser A. Bone marrow-derived cells contribute to infarct remodelling. Cardiovasc Res 71: 661–671, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med 204: 3037–3047, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Norris RA, Borg TK, Butcher JT, Baudino TA, Banerjee I, Markwald RR. Neonatal and adult cardiovascular pathophysiological remodeling and repair: developmental role of periostin. Ann NY Acad Sci 1123: 30–40, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Oka T, Xu J, Kaiser RA, Melendez J, Hambleton M, Sargent MA, Lorts A, Brunskill EW, Dorn GW, 2nd, Conway SJ, Aronow BJ, Robbins J, Molkentin JD. Genetic manipulation of periostin expression reveals a role in cardiac hypertrophy and ventricular remodeling. Circ Res 101: 313–321, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okada H, Danoff TM, Kalluri R, Neilson EG. Early role of Fsp1 in epithelial-mesenchymal transformation. Am J Physiol Renal Physiol 273: F563–F574, 1997 [DOI] [PubMed] [Google Scholar]
- 36.Osterreicher CH, Penz-Osterreicher M, Grivennikov SI, Guma M, Koltsova EK, Datz C, Sasik R, Hardiman G, Karin M, Brenner DA. Fibroblast-specific protein 1 identifies an inflammatory subpopulation of macrophages in the liver. Proc Natl Acad Sci USA 108: 308–313, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schneider M, Kostin S, Strom CC, Aplin M, Lyngbaek S, Theilade J, Grigorian M, Andersen CB, Lukanidin E, Lerche Hansen J, Sheikh SP. S100A4 is upregulated in injured myocardium and promotes growth and survival of cardiac myocytes. Cardiovasc Res 75: 40–50, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Shimazaki M, Nakamura K, Kii I, Kashima T, Amizuka N, Li M, Saito M, Fukuda K, Nishiyama T, Kitajima S, Saga Y, Fukayama M, Sata M, Kudo A. Periostin is essential for cardiac healing after acute myocardial infarction. J Exp Med 205: 295–303, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Souders CA, Bowers SL, Baudino TA. Cardiac fibroblast: the renaissance cell. Circ Res 105: 1164–1176, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strutz F, Okada H, Lo CW, Danoff T, Carone RL, Tomaszewski JE, Neilson EG. Identification and characterization of a fibroblast marker: FSP1. J Cell Biol 130: 393–405, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takeda N, Manabe I, Uchino Y, Eguchi K, Matsumoto S, Nishimura S, Shindo T, Sano M, Otsu K, Snider P, Conway SJ, Nagai R. Cardiac fibroblasts are essential for the adaptive response of the murine heart to pressure overload. J Clin Invest 120: 254–265, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tian Y, Morrisey EE. Importance of myocyte-nonmyocyte interactions in cardiac development and disease. Circ Res 110: 1023–1034, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Timmers L, Pasterkamp G, de Hoog VC, Arslan F, Appelman Y, de Kleijn DP. The innate immune response in reperfused myocardium. Cardiovasc Res 94: 276–283, 2012 [DOI] [PubMed] [Google Scholar]
- 44.Willems IE, Havenith MG, De Mey JG, Daemen MJ. The alpha-smooth muscle actin-positive cells in healing human myocardial scars. Am J Pathol 145: 868–875, 1994 [PMC free article] [PubMed] [Google Scholar]
- 45.Xia Y, Dobaczewski M, Gonzalez-Quesada C, Chen W, Biernacka A, Li N, Lee DW, Frangogiannis NG. Endogenous thrombospondin 1 protects the pressure-overloaded myocardium by modulating fibroblast phenotype and matrix metabolism. Hypertension 58: 902–911, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xia Y, Lee K, Li N, Corbett D, Mendoza L, Frangogiannis NG. Characterization of the inflammatory and fibrotic response in a mouse model of cardiac pressure overload. Histochem Cell Biol 131: 471–481, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, Neilson EG, Sayegh MH, Izumo S, Kalluri R. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med 13: 952–961, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Zheng B, Zhang Z, Black CM, de Crombrugghe B, Denton CP. Ligand-dependent genetic recombination in fibroblasts: a potentially powerful technique for investigating gene function in fibrosis. Am J Pathol 160: 1609–1617, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]