Abstract
The human ether-a-go-go-related gene (hERG) encodes a voltage-activated K+ channel that contributes to the repolarization of the cardiac action potential. Long QT syndrome type 2 (LQT2) is an autosomal dominant disorder caused by mutations in hERG, and patients with LQT2 are susceptible to severe ventricular arrhythmias. We have previously shown that nonsense and frameshift LQT2 mutations caused a decrease in mutant mRNA by the nonsense-mediated mRNA decay (NMD) pathway. The Q81X nonsense mutation was recently found to be resistant to NMD. Translation of Q81X is reinitiated at Met124, resulting in the generation of NH2-terminally truncated hERG channels with altered gating properties. In the present study, we identified two additional NMD-resistant LQT2 nonsense mutations, C39X and C44X, in which translation is reinitiated at Met60. Deletion of the first 59 residues of the channel truncated nearly one-third of the highly structured Per-Arnt-Sim domain and resulted in the generation of trafficking-defective proteins and a complete loss of hERG current. Partial deletion of the Per-Arnt-Sim domain also resulted in the accelerated degradation of the mutant channel proteins. The coexpression of mutant and wild-type channels did not significantly disrupt the function and trafficking properties of wild-type hERG. Our present findings indicate that translation reinitiation may generate trafficking-defective as well as dysfunctional channels in patients with LQT2 premature termination codon mutations that occur early in the coding sequence.
Keywords: human ether-a-go-go-related gene, long QT syndrome, Per-Arnt-Sim domain, protein trafficking, potassium channels
the rapidly activating delayed rectifier K+ current (IKr) in the heart functions in the repolarization of the cardiac action potential (22). IKr is generated by the human ether-a-go-go-related gene (hERG) K+ channel encoded by hERG (21, 31). Each pore-forming subunit of the tetrameric channel is composed of six membrane-spanning subdomains, which include the voltage-sensing domain and channel pore, that are flanked by highly structured, functionally important domains in the cytosolic NH2- and COOH-termini. The NH2-terminus of the channel is responsible for maintaining the slow deactivation of the channel (24, 32). The crystal structure of highly conserved residues 26–135 revealed a eukaryotic Per-Arnt-Sim (PAS) domain that directs protein-protein interactions between the hERG NH2-terminus and other regions of the channel (9, 15). The COOH-terminus of the channel contains a cyclic nucleotide-binding domain tethered to the transmembrane domain by a helical COOH-linker region.
Mutations in hERG cause long QT syndrome type 2 (LQT2), an autosomal dominant disorder characterized by prolonged action potential durations that may trigger severe arrhythmias and lead to syncope or sudden death (13, 23). Over 500 mutations have been identified to date in patients with LQT2. Missense mutations may give rise to nonfunctional channels, channels with trafficking defects, and channels with abnormal gating properties (3, 20, 35). hERG transcripts containing premature termination codons (PTCs) introduced by LQT2 nonsense and frameshift mutations have been shown be eliminated by the nonsense-mediated mRNA decay (NMD) pathway (8, 33). NMD is protective against severe forms of LQT2 by preventing the translation of truncated proteins that may dominantly suppress hERG current. Approximately one-third of the known LQT2 mutations are predicted to be targets of NMD. We (28) have recently reported on an NMD-resistant LQT2 nonsense mutation, Q81X, that generated NH2-terminally truncated channels with abnormal functional properties. Analysis of the hERG sequence reveals one in-frame ATG codon upstream of Met124. We hypothesized that LQT2 PTC mutations occurring upstream of Met60 will be resistant to NMD by translation reinitiation and that the deletion of 59 residues from the NH2-terminus of the channel will have significant functional consequences.
In this study, we characterized two LQT2 nonsense mutations, C39X and C44X, previously reported by Splawski et al. (27) and Fodstad et al. (5). We found that both mutations were resistant to NMD and were expressed as NH2-terminally truncated channel proteins. After premature termination, translation of the mutant channels was reinitiated at Met60, resulting in the deletion of nearly one-third of the PAS domain. The truncated channels were trafficking defective and did not express hERG current. The mutant channels were rapidly degraded compared with wild-type hERG, and the coexpression of mutant and wild-type channels did not prevent the trafficking or disrupt the functional properties of the wild-type channels. The present results provide evidence showing that translation reinitiation of hERG transcripts containing LQT2 PTC mutations generates trafficking-defective as well as dysfunctional channel proteins. This study further defines the positional requirements of NMD susceptibility in PTC-containing hERG transcripts.
MATERIALS AND METHODS
hERG minigene and cDNA constructs.
The hERG minigene is composed of cDNA from exon 1 to exon 10 and genomic DNA from intron 10 to the polyadenylation signal and was subcloned into the pcDNA5 vector as previously described (7). LQT2 nonsense mutations C39X and C44X and valine substitutions at Met60, Met124, Met133, and Met137 were generated using the pAlter mutagenesis system (Promega, Madison, WI). Wild-type and mutant minigenes were stably transfected into Flp-In human embryonic kidney (HEK)-293 cells (Life Technologies, Grand Island, NY) using the Effectene method (Qiagen, Valencia, CA) as previously described (30). Flp-In HEK-293 cells contain the Flp recombination target (FRT) site at a single genomic locus, allowing the stable integration of the gene of interest at a single, specific location by Flp recombinase-mediated recombination. C39X and C44X mutations were introduced into hERG cDNA constructs with an in-frame hemagglutinin (HA) epitope (YPYDVPDYA) inserted at the COOH-terminus. Wild-type hERG cDNA constructs contained an in-frame Flag epitope (DYKDDDDK) at the COOH-terminus. The design of the HA- and Flag-tagged hERG cDNA constructs has been previously described (6, 29). Wild-type and mutant cDNA constructs were stably cotransfected into Flp-Cre cells. Flp-Cre cells contain the FRT site and a single copy of the loxP2272/loxP target site. The design of the Flp-Cre cell line and the generation of double-stable Flp-Cre cell lines have been previously described (30). Briefly, Flag-tagged wild-type constructs were introduced into the loxP2272/loxP target site using Cre recombinase-mediated cassette exchange. HA-tagged mutant constructs were then introduced into the FRT target site by Flp recombinase-mediated recombination. Flp-In HEK-293 and Flp-Cre cells were cultured in DMEM supplemented with 10% FBS at 37°C in 5% CO2. Stable cell lines were cultured in the presence of 10 μM E-4031 for 24 h to test the drug-induced rescue of hERG trafficking.
RNase protection assay.
The isolation of RNA expressed from hERG minigenes and the RNase protection assay have been previously described (8). Briefly, a 409-nt probe was used to detect hERG RNA. The probe contained 277 nt specific to exons 12 and 13 and was flanked by sequences specific to the pCRII vector. A second 228-nt probe was used to detect RNA from the hygromycin B resistance gene and contained sequences from the pGEM vector at both ends. The expression of RNA from the hygromycin B resistance gene was used as a loading control. Probes were completely digested in the presence of yeast RNA. The relative quantitation of hERG RNA was determined by densitometry using ImageJ (1). Cells were cultured in the presence or absence of 100 μg/ml cycloheximide (CHX) for 3 h to inhibit protein synthesis and thus abrogate NMD.
Electrophysiology.
The recording of membrane currents in the whole cell configuration has been previously described (35). Cells were superfused with 10 mM HEPES-buffered solution (pH 7.4) containing 1.8 mM CaCl2, 4 mM KCl, 1 mM MgCl2, 137 mM NaCl, and 10 mM glucose. A 10 mM HEPES-buffered solution (pH 7.2) containing 130 mM KCl, 1 mM MgCl2, 5 mM MgATP, and 5 mM EGTA was used as the pipette solution. Experiments were performed at room temperature. Data were recorded using an Axopatch-200B patch-clamp amplifier and analyzed with pCLAMP10 software (Molecular Devices, Sunnyvale, CA).
Immunoblot analysis.
hERG channel proteins from whole cell lysates were detected using the anti-hERG, anti-HA, and anti-Flag antibody and visualized with Plus-ECL (Perkin-Elmer, Waltham, MA) as previously described (29, 35). Expression levels of β-tubulin and hygromycin B phosphotransferase (HPH) were used as loading controls as previously described (7).
CHX chase assay.
Wild-type and mutant cell lines were treated with 100 μg/ml CHX and harvested after 0, 2, 4, and 8 h. hERG channel proteins from whole cell lysates were detected by immunoblot analysis using anti-hERG antibody. The relative intensity of the total hERG protein was quantified by densitometry using ImageJ and normalized to the amount present at 0 h. The time at which a 50% decrease in the total amount of hERG protein occurred was reported as the channel half-life. The expression of β-tubulin, which has a long half-life compared with hERG, was used as a control.
Data analysis.
Data were analyzed and are presented as means ± SE using SigmaPlot (San Jose, CA). Student's t-test was used for statistical comparison involving two experimental groups. ANOVA with the Bonferroni correction was used for statistical comparison in tests containing more than two experimental groups. P values of <0.05 were considered statistically significant.
RESULTS
LQT2 nonsense mutations C39X and C44X are resistant to NMD.
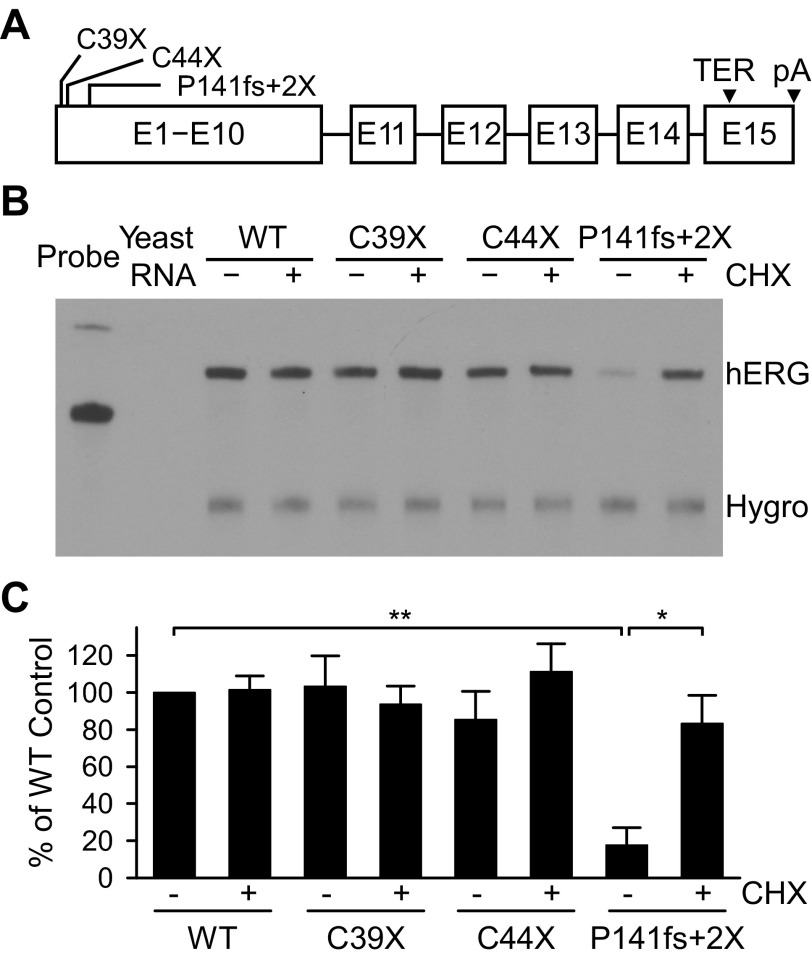
To test the susceptibility of LQT2 PTC mutations upstream of Met60 to NMD, we introduced the C39X and C44X nonsense mutations into full-length hERG minigenes (Fig. 1A). Wild-type and mutant minigenes were stably transfected into Flp-In HEK-293 cells. A stable cell line expressing the NMD-sensitive LQT2 mutation P141fs+2X was included as a control. Cells were cultured in the absence or presence of the protein synthesis inhibitor CHX, which has been shown to reverse NMD (11). The RNase protection assay was used to quantify the expression of hERG mRNA from wild-type and mutant minigenes. In the absence of CHX, wild-type, C39X, and C44X RNA were expressed at similar levels (Fig. 1B). In contrast, the amount of RNA expressed from minigenes containing the P141fs+2X mutation was significantly reduced compared with wild-type RNA (P < 0.01). Wild-type and mutant RNA expression levels were normalized and quantified and revealed no significant differences between wild-type, C39X, and C44X minigenes (P > 0.05; Fig. 1C). As previously described, treatment with CHX resulted in increased expression of P141fs+2X RNA (P < 0.05). These results indicated that hERG transcripts containing the C39X and C44X mutations are not degraded by NMD.
Fig. 1.
Analysis of hERG mRNA by the RNase protection assay. A: schematic of the full-length hERG minigene. The position of the C39X, C44X, and P141fs+2X mutations, the wild-type (WT) termination codon (TER), and the polyadenylation signal (pA) are indicated. B: RNase protection assay. hERG mRNA was harvested from cells stably transfected with WT and mutant minigenes. A probe specific to a 277-nt sequences spanning exons 12 and 13 was used to hybridize to hERG mRNA. The stable cell lines were treated with cycloheximide (CHX) for 3 h before RNA isolation (+) or left untreated (−). RNA expressed from the hygromycin B resistance gene (Hygro) served as a loading control. C: RNase protection assay signals were normalized to the hygromycin B resistance gene and are plotted as a percentage of the untreated WT. Values are means ± SE; n = 3. **P < 0.01; *P < 0.05.
C39X and C44X channels do not express hERG current.
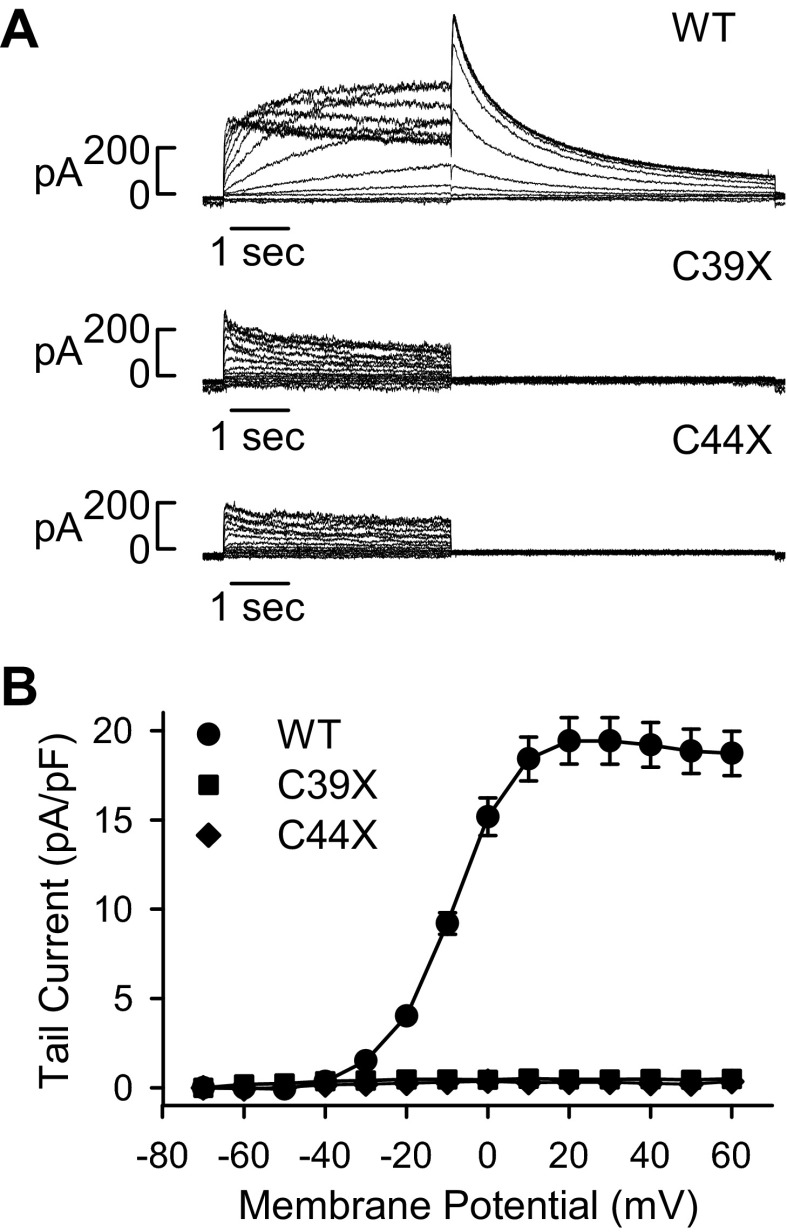
To test the functional properties of the mutant hERG channels, we performed whole cell patch-clamp analysis. hERG current was recorded by activating the channels with test potentials ranging between −70 and +60 mV from a −80-mV resting potential followed with a −50-mV repolarizing pulse. Representative current traces are shown in Fig. 2A, and the current-voltage relationship of tail current recorded at the onset of repolarization is shown in Fig. 2B. Wild-type channels exhibited an averaged current density of 19.4 ± 1.3 pA/pF after depolarization to +30 mV (n = 12). In contrast, hERG current was not detected from cells stably transfected with C39X (n = 6) and C44X (n = 8) minigenes.
Fig. 2.
Functional properties of WT and mutant hERG channels. A: representative currents from cells stably transfected with WT and mutant minigenes. hERG current was activated from a −80-mV holding potential with 4-s depolarizing test potentials between −70 and +60 mV and deactivated with a repolarizing pulse to −50 mV. B: current-voltage plot of tail currents of WT (n = 12), C39X (n = 6), and C44X (n = 8) currents. Tail currents were recorded at −50 mV after each depolarizing pulse. Data are plotted as means ± SE.
C39X and C44X are translated as truncated, trafficking-deficient channels.
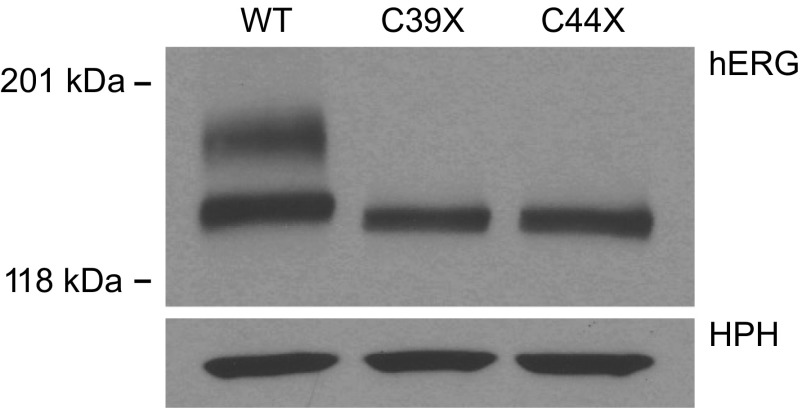
To identify the biochemical basis for the functional defects associated with the C39X and C44X mutations, we analyzed the proteins expressed from wild-type and mutant minigenes by immunoblot analysis (Fig. 3). hERG protein was detected using an antibody directed to the COOH-terminus of the channel. As previously shown, wild-type hERG was detected as two bands representing the immature, core-glycosylated channel located in the endoplasmic reticulum (135 kDa) and the fully glycosylated mature form (155 kDa) expressed at the cell surface (35). In contrast, C39X and C44X channel proteins were detected as a single band at a slightly lower molecular weight than the immature wild-type channel protein (∼130 kDa). This result suggested that the nonsense mutations resulted in the expression of truncated, trafficking-defective channel proteins. Immunoblot analysis also revealed that the mutant minigenes expressed significantly less total protein compared with the wild-type minigene. Quantitative analysis revealed that total proteins expressed from C39X and C44X minigenes were 28.5 ± 6.0% (n = 3) and 35.0 ± 9.0% (n = 3) of the total protein expressed from wild-type hERG (n = 3, P < 0.05).
Fig. 3.
Immunoblots of WT, C39X, and C44X channels. Proteins from whole cell lysates of cells expressing WT and mutant minigenes were detected using anti-hERG and anti-hygromycin B phosphotransferase (HPH) antibody. HPH levels served as a loading control. The mature, fully glycosylated WT channel was detected at 155 kDa, and the immature, core-glycosylated channel was detected at 135 kDa. Results shown are representative of 3 independent experiments.
Translation of C44X channels is reinitiated at Met60.
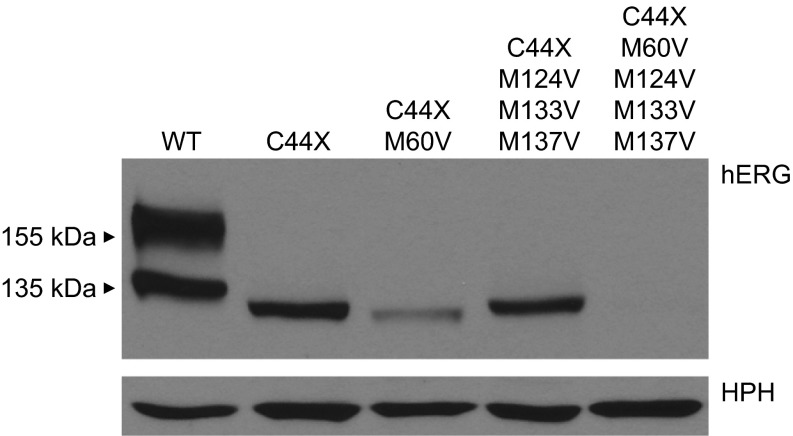
Translation reinitiation has been implicated in the NMD resistance of PTC mutations that occur early in the coding sequence of several genes, including hERG (4, 18, 28). Analysis of the hERG sequence downstream of the C39X and C44X mutations revealed several in-frame start codons, Met60, Met124, Met133, and Met137, that could potentially serve as the site of reinitiation. As shown in Fig. 4, mutating all four downstream methionine codons to valine resulted in the complete loss of C44X protein expression. This confirmed that the reinitiation of translation is associated with the NMD resistance of the PTC-containing transcripts. When Met124, Met133, and Met137 were mutated to valine, we observed a single band at the same molecular weight as found in C44X minigenes. This result strongly suggested that reinitiation of translation occurs at Met60 in C44X transcripts. Interestingly, we observed a faint band with a lower molecular weight expressed from minigenes containing the C44X and M60V mutations. This result indicated that in the absence of Met60, the downstream methionine codons Met124, Met133, and Met137 are able to reinitiate translation but with a significantly reduced efficiency, likely due to the increased distance between the C44X mutation and the downstream reinitiation site.
Fig. 4.
Translation of C44X channels is reinitiated at Met60. hERG channel proteins from whole cell lysates from cells stably transfected with WT, C44X, and C44X plus methionine to valine mutations are shown. Proteins were detected with anti-hERG and anti-HPH antibody. HPH expression levels served as a loading control. Results shown are representative of 3 independent experiments.
C39X and C44X mutations do not significantly disrupt the function or trafficking of wild-type hERG.
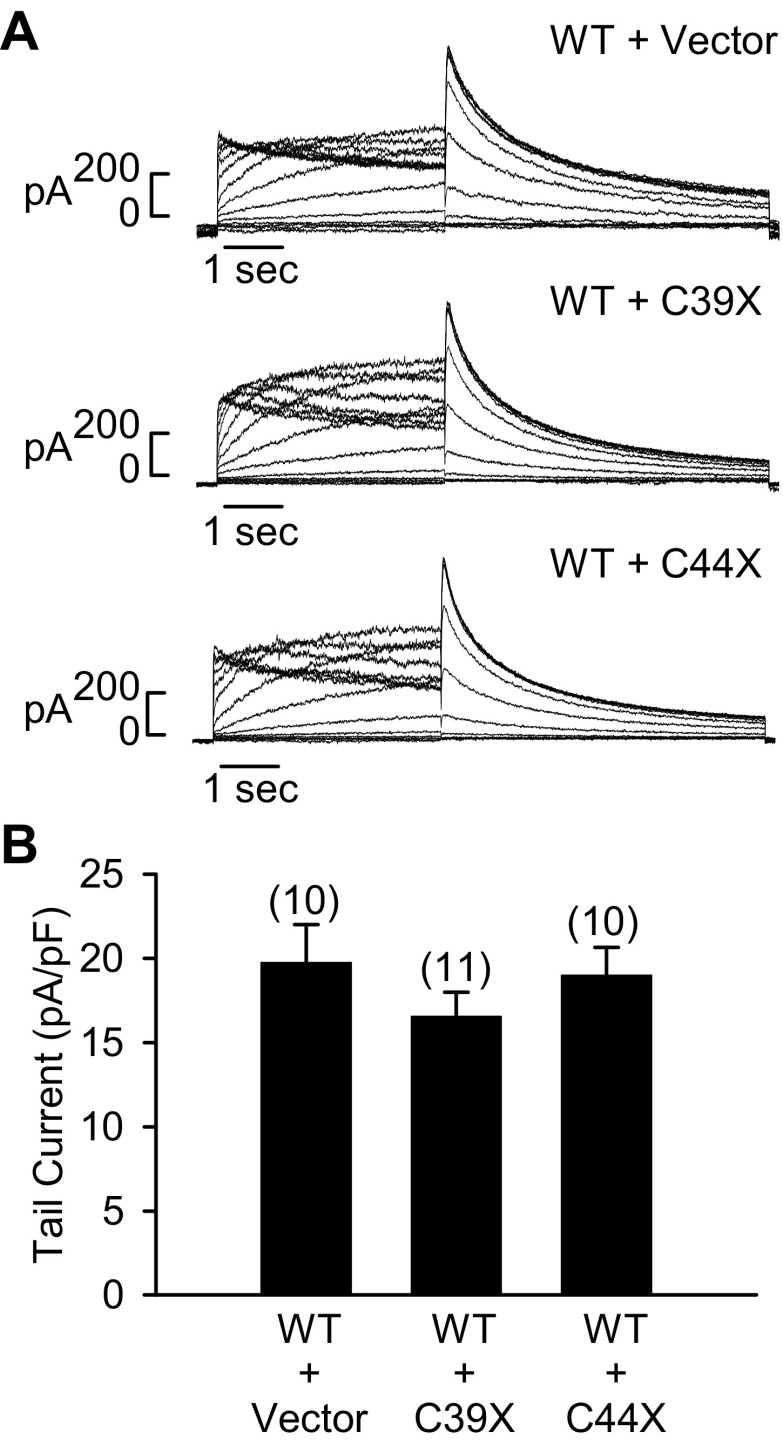
To determine whether C39X and C44X channels were able to dominantly disrupt the function and trafficking of wild-type channels, Flp-Cre cells were stably cotransfected with either wild-type + empty vector, wild-type + C39X, or wild-type + C44X cDNA constructs. hERG current was recorded using the voltage-clamp protocol shown in Fig. 2. Representative current traces are shown in Fig. 5A, and a plot of the averaged peak tail current densities is shown in Fig. 5B. Coexpression of the mutant and wild-type channels did not significantly decrease hERG current levels. The averaged peak tail current densities recorded after depolarizing potentials to +30 mV from cells coexpressing wild-type + empty vector, wild-type + C39X, and wild-type + C44X were 19.8 ± 2.3 pA/pF (n = 10), 16.6 ± 1.4 pA/pF (n = 11), and 19.0 ± 1.6 pA/pF (n = 10), respectively (P > 0.05).
Fig. 5.
Analysis of hERG current after coexpression of WT and mutant channels. A: representative currents from Flp-Cre cells stably cotransfected with WT + empty vector, WT + C39X, or WT + C44X. Current was recorded using the protocol described in Fig. 2. B: plot of averaged tail current amplitudes measured at −50 mV after depolarizing voltages to +30 mV. Data are plotted as means ± SE; numbers of cells are shown in parentheses.
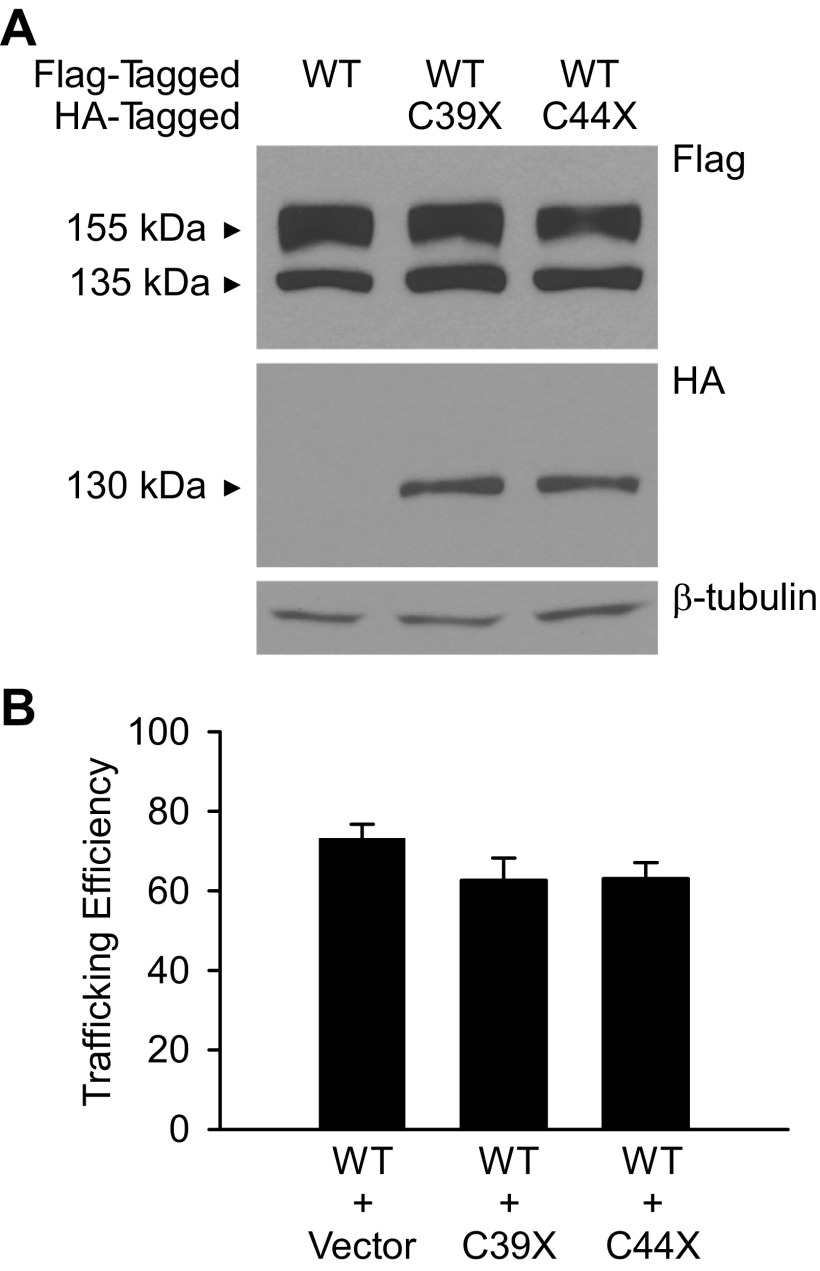
To determine the effect of the mutant channels on the trafficking of wild-type hERG, we performed immunoblot analysis of proteins expressed from Flp-Cre cell lines cotransfected with Flag-tagged wild-type hERG and either empty vector, HA-tagged C39X, or HA-tagged C44X. The expression of wild-type and mutant channels is shown in Fig. 6A. The trafficking efficiency of hERG was determined by the ratio of the upper, mature form of the band to the total hERG protein as detected by the anti-Flag antibody. As shown in Fig. 6B, the mutant channels did not significantly decrease the trafficking efficiency of wild-type hERG. The trafficking efficiencies from cells coexpressing wild-type + empty vector, wild-type + C39X, and wild-type + C44X were 73.1 ± 3.7% (n = 4), 62.7 ± 5.6% (n = 4), and 63.1 ± 4.0% (n = 4), respectively (P > 0.05).
Fig. 6.
Coexpression with mutant channels does not alter the trafficking properties of WT hERG. A: Flp-Cre cells were stably cotransfected with WT-Flag + empty vector, WT-Flag + C39X-hemagglutinin (HA), or WT-Flag + C44X-HA. hERG channels were detected using anti-Flag and anti-HA antibody. β-Tubulin served as a loading control. B: the trafficking efficiency of Flag-tagged WT channels was plotted as the ratio of upper, mature band to total hERG protein. Data are plotted as means ± SE; n = 3.
C44X channels are rapidly degraded and trafficking is not rescued by E-4031.
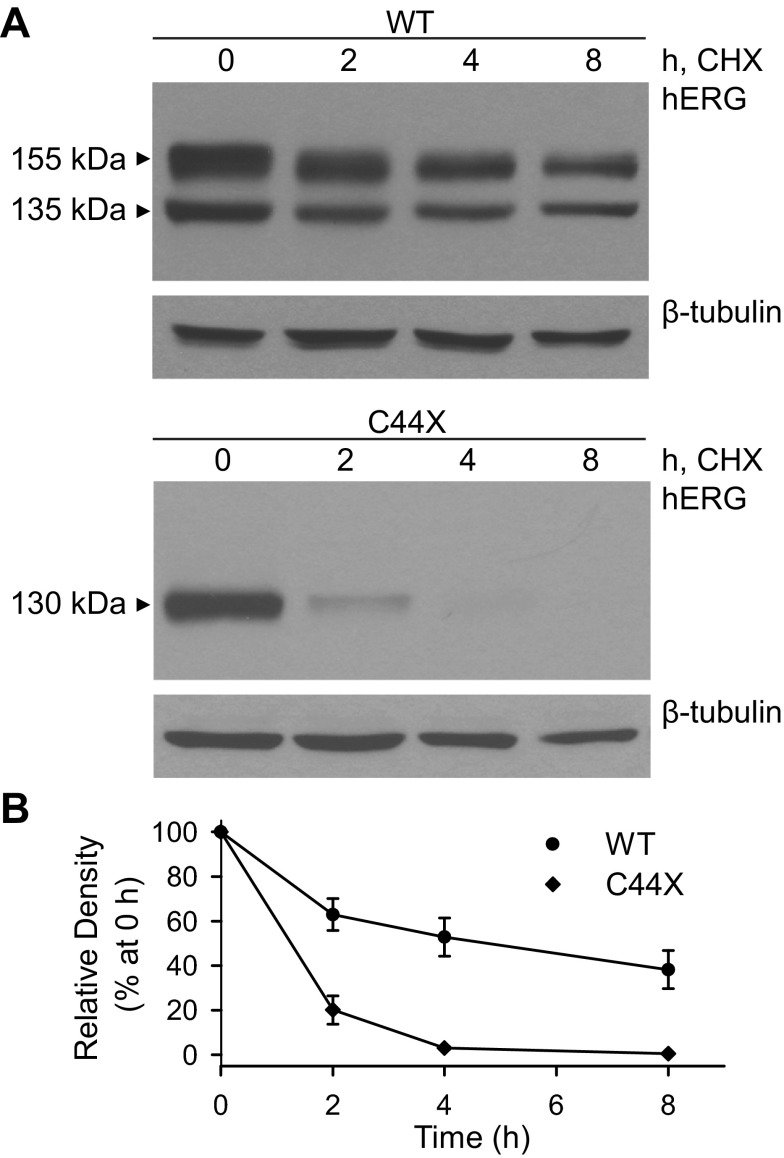
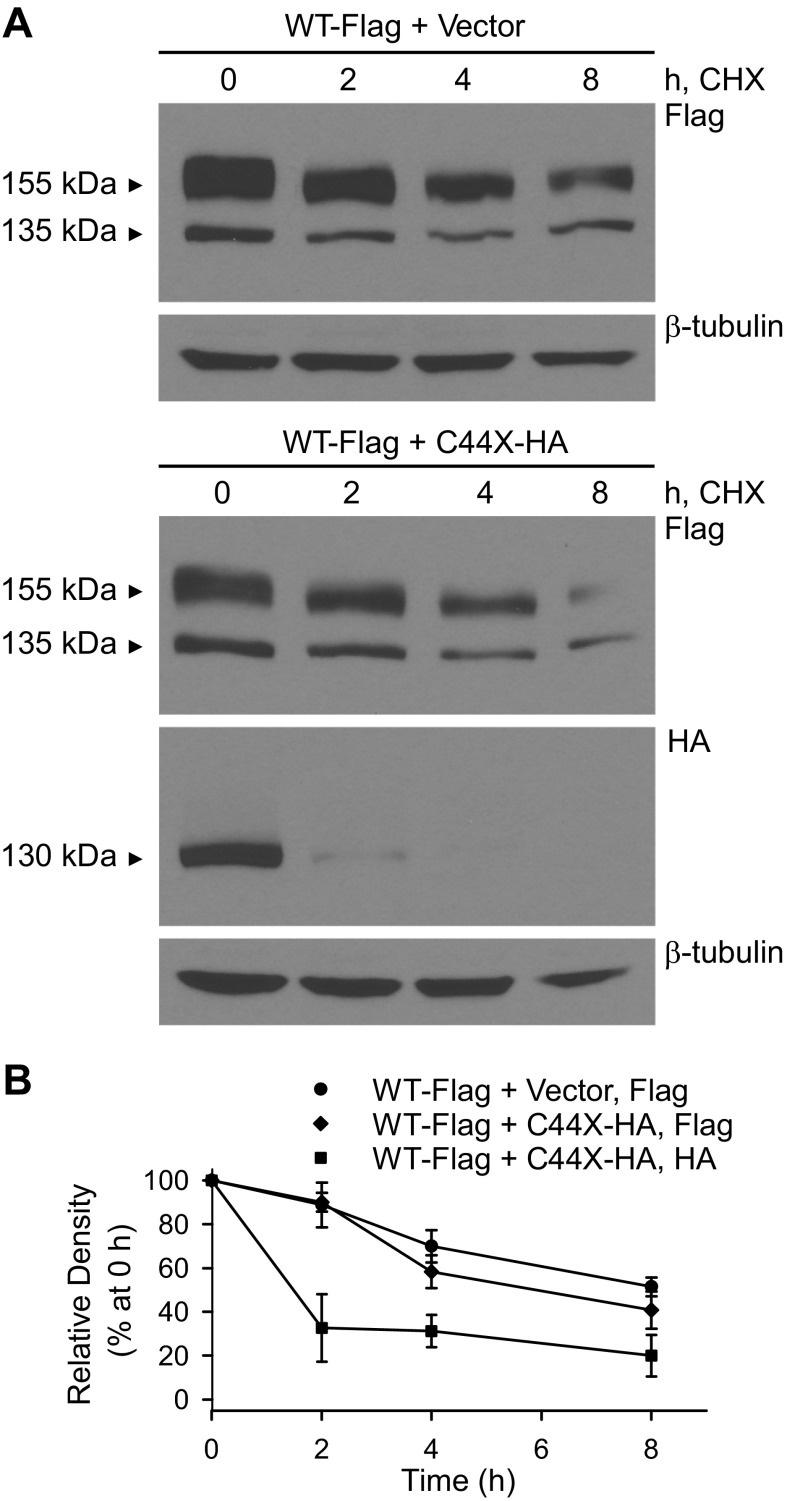
The immunoblot analyses revealed that the mutant channels were expressed at significantly lower levels than wild-type hERG. To determine whether the mutant channels exhibited increased rates of degradation, we analyzed the expression of wild-type and mutant proteins by immunoblot analysis after treatment with the protein synthesis inhibitor CHX for 0, 2, 4, and 8 h (Fig. 7A). Immunoblots were quantified by densitometry, and the relative intensity of wild-type and C44X was plotted as a function of the total protein at 0 h (Fig. 7B). The housekeeping gene β-tubulin served as a relatively stable control protein. As shown in Fig. 7, the mutant channels exhibited accelerated rates of degradation compared with wild-type hERG. The half-lives of the wild-type and C44X channels were 5.3 ± 1.7 h (n = 4) and 1.3 ± 0.1 h (n = 4, P < 0.05). We also compared the degradation rates of the differentially tagged wild-type and mutant channels coexpressed from the Flp-Cre cell lines (Fig. 8). Coexpression with the HA-tagged mutant channel did not significantly reduce the half-life of Flag-tagged wild-type hERG (7.2 ± 2.4 h, n = 3) compared with coexpression of wild-type channels with empty vector (8.0 ± 1.0 h, n = 3, P > 0.05). The half-life of the HA-tagged C44X channels coexpressed with Flag-tagged wild-type hERG (1.7 ± 0.7 h, n = 3) was similar to that found from the cell lines stably transfected with the C44X minigene shown in Fig. 7.
Fig. 7.
Characterization of the stability of WT and mutant channels. A: immunoblots of proteins harvested from cells stably transfected with WT and C44X minigenes. Protein synthesis was inhibited by treatment of the cells with 100 μg/ml CHX. Cells were harvested at 0, 2, 4, and 8 h after CHX treatment. Proteins were detected with hERG and β-tubulin antibody. B: the amount of total hERG protein was quantified using ImageJ and plotted as the percentage of the total protein at 0 h. Data are plotted as means ± SE; n = 4 for WT channels and 4 for C44X channels.
Fig. 8.
Effect of coexpression of mutant and WT channels on the stability of hERG channel proteins. A: immunoblots of proteins harvested from Flp-Cre cells stably cotransfected with Flag-tagged WT + empty vector or Flag-tagged WT + HA-tagged C44X. Cells were treated with 100 μg/ml CHX and harvested after 0, 2, 4, and 8 h. hERG channels were detected using anti-Flag and anti-HA antibody. β-Tubulin served as a loading control. B: the amount of total Flag-tagged or HA-tagged protein detected was quantified using ImageJ and plotted as the percentage of the total protein at 0 h. Data are plotted as means ± SE; n = 3 for WT-Flag + vector (detected by anti-Flag), 3 for WT-Flag + C44X-HA (detected by anti-Flag), and 3 for WT-Flag + C44X-HA (detected by anti-HA).
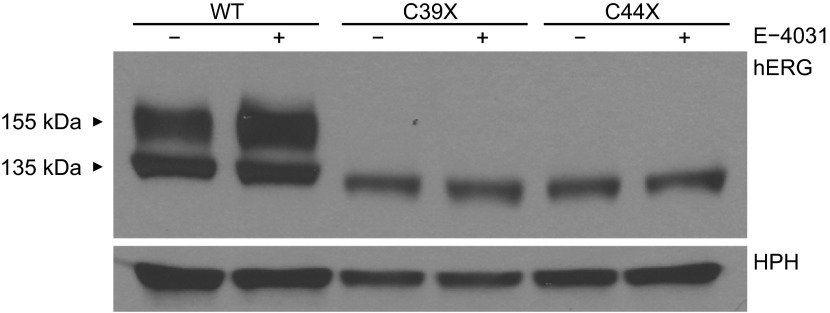
To further characterize the rapidly degraded mutant channels, we determined whether or not the trafficking defects associated with the partial deletion of the PAS domain could be reversed. Studies (2, 36) have shown that the trafficking defects of mutant hERG channels can be restored by the pore-blocking drug E-4031. We cultured wild-type and mutant stable cell lines in media containing E-4031 for 24 h and found that the drug was unable to rescue the trafficking-defective phenotype of the mutant channels (Fig. 9). The rapid degradation of the mutant channel and the failure of E-4031 to rescue trafficking strongly suggested that the NH2-terminus of the C39X and C44X channels is significantly destabilized.
Fig. 9.
Trafficking of C39X and C44X channels is not rescued by the hERG channel-blocking drug E-4031. Representative immunoblots of proteins harvested from cells stably transfected with WT, C39X, and C44X minigenes and detected using anti-hERG COOH-terminal and anti-HPH antibody are shown. Cells were incubated in the absence or presence of 10 μM E-4031 for 24 h before being harvested. Results shown are representative of 3 independent experiments.
DISCUSSION
In the present study, we identified two new NMD-resistant LQT2 nonsense mutations that were translated by reinitiation and generated nonfunctional NH2-terminally truncated channels. hERG channel dysfunction associated with the C39X and C44X nonsense mutations was characterized at the RNA, protein, and functional levels. We identified Met60 as the site of translation reinitiation. The deletion of the first 59 residues of the channel resulted in the truncation of the PAS domain and in the expression of trafficking-defective channels. This study establishes translation reinitiation as a pathogenic mechanism of LQT2 in patients with PTC mutations that occur near the translation start site and that early nonsense and frameshift mutations in hERG may give rise trafficking as well as functional defects.
NMD is an evolutionary conserved surveillance mechanism that eliminates PTC-containing transcripts and prevents the translation of potentially harmful COOH-terminally truncated proteins. According to the proposed rule of NMD in mammalian cells, PTCs occurring 50–55 nt upstream of the last exon-exon junction target the transcript for degradation (17). The activation of NMD is dependent on the interaction between the translation termination complex, formed at the site of the PTC, and NMD associated components of the exon-junction complex (EJC) (25). The EJC is a protein complex deposited by the spliceosome 20–24 nt upstream of the exon-exon junction after splicing and is normally displaced by the ribosome during the pioneer round of translation (12, 14). The EJC is displaced by the translating ribosome after reinitiation, resulting in the NMD resistance of early PTC mutations. The elimination of putative reinitiation codons by mutagenesis is an established method to confirm NMD resistance by translation reinitiation (34). The dependence of NMD on protein translation and splicing necessitates the use of hERG minigenes in the biochemical and functional characterization of LQT2 nonsense and frameshift mutations. Our findings that translation of C39X and C44X is reinitiated at Met60 is in agreement with an in silico analysis of translation initiation sites using the NetStart 1.0 artificial neural network server (19). NetStart 1.0 predicts the likelihood of translation in the context of local and global sequence information, and scores between 0.5 and 1.0 are strong predictors for translation initiation. Met60 received a score of 0.78, which indicated that it is a strong candidate site for translation reinitiation.
The structures of the hERG PAS domain obtained by X-ray crystallography and NMR are well described by a helix-loop-helix architecture (15, 16). The PAS domain encompasses residues 26–135 of the channel and is capped by a short amphiphatic helix that links the domain to 12 disordered residues at the channel NH2-terminus (16). A well-conserved hydrophobic patch on the surface of the PAS domain is proposed to mediate protein-protein interactions with other regions of the channel (15). A fluorescence resonance energy transfer study (9) has revealed that the functional effects of the PAS domain are mediated by physical interactions between the domain and the core of the channel. Functional studies (15, 32) of NH2-terminally truncated channels have shown that deletions up to residue 26 generate functional channels with accelerated deactivation rates. Increased deactivation rates were also observed in channels in which the PAS domain (residues 1–135) is deleted and in channels lacking the entire NH2-terminus (residues 1–354) (15, 26, 32). This is the first description of a naturally occurring mutation that partially deletes the PAS domain and generates NH2-terminally truncated, trafficking-defective hERG channels. A recently study by Harley et al. (10) reported that hERG trafficking defects were correlated with the misfolding and decreased stability of isolated PAS domains containing LQT2 missense mutations. We have previously reported trafficking defects associated with large deletions from highly structured cytosolic channel domains. The LQT2 splice site mutation G2592+1G>A mutation activates a cryptic splice site deleting 24 residues from the COOH-terminal cyclic nucleotide-binding domain, resulting in trafficking-defective channel proteins (29). It is likely that the partial deletion of the hERG NH2-terminus in C39X and C44X channels disrupted the proper folding of the PAS domain, resulting in trafficking-defective channels. The accelerated degradation rates and the failure of the pore-binding drug E-4031 to rescue trafficking provide further evidence of the significant misfolding the mutant channel proteins. The significantly decreased expression of the C39X and C44X mutants compared with the wild-type channels and the rapid degradation of the mutant channel proteins are also likely to be responsible for the minimal dominant negative effects observed in the coexpression experiments.
We recently identified a NMD-resistant LQT2 mutation, Q81X, which was translated by reinitiation at Met124 (28). In contrast to reinitiation at Met60, the deletion of the first 123 residues of the channel did not result in a trafficking-defective phenotype. Reinitiation at Met124 deleted nearly all of the PAS domain residues and resulted in the expression of channels that exhibited accelerated deactivation rates and decreased resurgent outward current during the late stages of ventricular action potential repolarization. The previous study also found that Met133 and Met137 were competent to reinitiate translation after premature termination at the Q81X PTC. Based on our previous study and our present findings, we propose that the reinitiation of LQT2 PTC mutations upstream of residue 60 will result in the translation of trafficking defective channels and that the reinitiation of PTC mutations occurring between residue 60 the methionine codons at residues 124, 133, and 137 will generate abnormal channels with altered gating properties that are expressed at the plasma membrane. Because the P141fs+2X mutation is sensitive to NMD, it appears that Met137 represents a boundary in the 5′-region of the hERG coding sequence that separates NMD-resistant and NMD-sensitive LQT2 PTC mutations.
In summary, the identification of two new LQT2 mutations, C39X and C44X, that are resistant to NMD establishes translation reinitiation as an important mechanism of disease in patients with early PTC mutations in hERG. This study highlights the importance of the folding and stability of the PAS domain in channel trafficking and contributes to the collective understanding of mechanisms by which hERG mutations cause LQT2.
GRANTS
This work was supported in part by National Institutes of Health (NIH) Grant HL-68854 (to Z. Zhou) and by NIH Training Grant T32-NL-094294 (to M. R. Stump). Z. Zhou is an Established Investigator of the American Heart Association.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.R.S., Q.G., and Z.Z. conception and design of research; M.R.S. and Q.G. performed experiments; M.R.S. and Z.Z. analyzed data; M.R.S. and Z.Z. interpreted results of experiments; M.R.S. prepared figures; M.R.S. drafted manuscript; M.R.S. and Z.Z. edited and revised manuscript; M.R.S., Q.G., and Z.Z. approved final version of manuscript.
REFERENCES
- 1.Abràmoff MD, Magalhães PJ, Ram SJ. Image processing with ImageJ. Biophotonics Int 11: 36–42, 2004 [Google Scholar]
- 2.Anderson CL, Delisle BP, Anson BD, Kilby JA, Will ML, Tester DJ, Gong Q, Zhou Z, Ackerman MJ, January CT. Most LQT2 mutations reduce Kv11.1 (hERG) current by a class 2 (trafficking-deficient) mechanism. Circulation 113: 365–373, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Berecki G, Zegers JG, Verkerk AO, Bhuiyan ZA, de Jonge B, Veldkamp MW, Wilders R, van Ginneken ACG. HERG channel (dys)function revealed by dynamic action potential clamp technique. Biophys J 88: 566–578, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buisson M, Anczuków O, Zetoune AB, Ware MD, Mazoyer S. The 185delAG mutation (c.68_69delAG) in the BRCA1 gene triggers translation reinitiation at a downstream AUG codon. Hum Mutat 27: 1024–1029, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Fodstad H, Swan H, Laitinen P, Piippo K, Paavonen K, Viitasalo M, Toivonen L, Kontula K. Four potassium channel mutations account for 73% of the genetic spectrum underlying long-QT syndrome (LQTS) and provide evidence for a strong founder effect in Finland. Ann Med 36, Suppl 1: 53–63, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Gong Q, Keeney DR, Robinson JC, Zhou Z. Defective assembly and trafficking of mutant HERG channels with C-terminal truncations in long QT syndrome. J Mol Cell Cardiol 37: 1225–1233, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Gong Q, Stump MR, Zhou Z. Inhibition of nonsense-mediated mRNA decay by antisense morpholino oligonucleotides restores functional expression of hERG nonsense and frameshift mutations in long-QT syndrome. J Mol Cell Cardiol 50: 223–229, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gong Q, Zhang L, Vincent GM, Horne BD, Zhou Z. Nonsense mutations in hERG cause a decrease in mutant mRNA transcripts by nonsense-mediated mRNA decay in human long-QT syndrome. Circulation 116: 17–24, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gustina AS, Trudeau MC. A recombinant N-terminal domain fully restores deactivation gating in N-truncated and long QT syndrome mutant hERG potassium channels. Proc Natl Acad Sci USA 106: 13082–13087, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harley CA, Jesus CSH, Carvalho R, Brito RMM, Morais-Cabral JH. Changes in channel trafficking and protein stability caused by LQT2 mutations in the PAS domain of the HERG channel. PLos One 7: e32654, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inoue K, Khajavi M, Ohyama T, Hirabayashi SI, Wilson J, Reggin JD, Mancias P, Butler IJ, Wilkinson MF, Wegner M, Lupski JR. Molecular mechanism for distinct neurological phenotypes conveyed by allelic truncating mutations. Nat Genet 36: 361–369, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Ishigaki Y, Li X, Serin G, Maquat LE. Evidence for a pioneer round of mRNA translation: mRNAs subject to nonsense-mediated decay in mammalian cells are bound by CBP80 and CBP20. Cell 106: 607–617, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Keating MT, Sanguinetti MC. Molecular and cellular mechanisms of cardiac arrhythmias. Cell 104: 569–580, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Le Hir H, Izaurralde E, Maquat LE, Moore MJ. The spliceosome deposits multiple proteins 20–24 nucleotides upstream of mRNA exon-exon junctions. EMBO J 19: 6860–6869, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morais-Cabral JH, Lee A, Cohen SL, Chait BT, Li M, Mackinnon R. Crystal structure and functional analysis of the HERG potassium channel N terminus: a eukaryotic PAS domain. Cell 95: 649–655, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Muskett FW, Thouta S, Thomson SJ, Bowen A, Stansfeld PJ, Mitcheson JS. Mechanistic insight into human ether-à-go-go-related gene (hERG) K+ channel deactivation gating from the solution structure of the EAG domain. J Biol Chem 286: 6184–6191, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagy E, Maquat LE. A rule for termination-codon position within intron-containing genes: when nonsense affects RNA abundance. Trends Biochem Sci 23: 198–199, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Paulsen M, Lund C, Akram Z, Winther JR, Horn N, Møller LB. Evidence that translation reinitiation leads to a partially functional Menkes protein containing two copper-binding sites. Am J Hum Genet 79: 214–229, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pedersen AG, Nielsen H. Neural network prediction of translation initiation sites in eukaryotes: perspectives for EST and genome analysis. Proc Int Conf Intell Syst Mol Biol 5: 226–233, 1997 [PubMed] [Google Scholar]
- 20.Sanguinetti MC, Curran ME, Spector PS, Keating MT. Spectrum of HERG K+-channel dysfunction in an inherited cardiac arrhythmia. Proc Natl Acad Sci USA 93: 2208–2212, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanguinetti MC, Jiang C, Curran ME, Keating MT. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell 81: 299–307, 1995 [DOI] [PubMed] [Google Scholar]
- 22.Sanguinetti MC, Jurkiewicz NK. Two components of cardiac delayed rectifier K+ current. Differential sensitivity to block by class III antiarrhythmic agents. J Gen Physiol 96: 195–215, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanguinetti MC. HERG1 channelopathies. Pflügers Arch 460: 265–276, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schönherr R, Heinemann SH. Molecular determinants for activation and inactivation of HERG, a human inward rectifier potassium channel. J Physiol 493: 635–642, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh G, Lykke-Andersen J. New insights into the formation of active nonsense-mediated decay complexes. Trends Biochem Sci 28: 464–466, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Spector PS, Curran ME, Zou A, Keating MT, Sanguinetti MC. Fast inactivation causes rectification of the IKr channel. J Gen Physiol 107: 611–619, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Splawski I, Shen J, Timothy KW, Lehmann MH, Priori S, Robinson JL, Moss AJ, Schwartz PJ, Towbin JA, Vincent GM, Keating MT. Spectrum of mutations in long-QT syndrome genes. KVLQT1, HERG, SCN5A, KCNE1, and KCNE2. Circulation 102: 1178–1185, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Stump MR, Gong Q, Packer JD, Zhou Z. Early LQT2 nonsense mutation generates N-terminally truncated hERG channels with altered gating properties by the reinitiation of translation. J Mol Cell Cardiol 53: 725–733, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stump MR, Gong Q, Zhou Z. Multiple splicing defects caused by hERG splice site mutation 2592+1G>A associated with long QT syndrome. Am J Physiol Heart Circ Physiol 300: H312–H318, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stump MR, Gong Q, Zhou Z. Isoform-specific dominant-negative effects associated with hERG1 G628S mutation in long QT syndrome. PLos One 7: e42552, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trudeau MC, Warmke JW, Ganetzky B, Robertson GA. HERG, a human inward rectifier in the voltage-gated potassium channel family. Science 269: 92–95, 1995 [DOI] [PubMed] [Google Scholar]
- 32.Wang J, Trudeau MC, Zappia AM, Robertson GA. Regulation of deactivation by an amino terminal domain in human ether-à-go-go-related gene potassium channels. J Gen Physiol 112: 637–647, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zarraga IG, Zhang L, Stump MR, Gong Q, Vincent GM, Zhou Z. Nonsense-mediated mRNA decay caused by a frameshift mutation in a large kindred of type 2 long QT syndrome. Heart Rhythm 8: 1200–1206, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang J, Maquat LE. Evidence that translation reinitiation abrogates nonsense-mediated mRNA decay in mammalian cells. EMBO J 16: 826–833, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou Z, Gong Q, Epstein ML, January CT. HERG channel dysfunction in human long QT syndrome. Intracellular transport and functional defects. J Biol Chem 273: 21061–21066, 1998 [DOI] [PubMed] [Google Scholar]
- 36.Zhou Z, Gong Q, January CT. Correction of defective protein trafficking of a mutant HERG potassium channel in human long QT syndrome. Pharmacological and temperature effects. J Biol Chem 274: 31123–31126, 1999 [DOI] [PubMed] [Google Scholar]