Abstract
Melatonin attenuates muscle sympathetic nerve responses to sympathoexcitatory stimuli, but it is unknown whether melatonin similarly attenuates reflex changes in skin sympathetic nerve activity (SSNA). In this double-blind, placebo-controlled, crossover study, we tested the hypothesis that melatonin (3 mg) would attenuate the SSNA response to mental stress (mental arithmetic). Twelve healthy subjects underwent experimental testing on two separate days. Three minutes of mental stress occurred before and 45 min after ingestion of melatonin (3 mg) or placebo. Skin temperature was maintained at 34°C. Reflex increases in SSNA (peroneal nerve), mean arterial pressure, and heart rate (HR) to mental stress before and after melatonin were determined. Melatonin lowered HR (pre, 66 ± 3 beats/min; and post, 62 ± 3 beats/min, P = 0.046) and SSNA (pre, 14,282 ± 3,706 arbitrary units; and post, 9,571 ± 2,609 arbitrary units, P = 0.034) at rest. In response to mental stress, SSNA increases were significantly attenuated following melatonin ingestion (second minute, 114 ± 30 vs. 74 ± 14%; and third minute, 111 ± 29 vs. 54 ± 12%, both P < 0.05). The mean arterial pressure increase to mental stress was blunted in the third minute (20 ± 2 vs. 17 ± 2 mmHg, P = 0.032), and the HR increase was blunted in the first minute (33 ± 3 vs. 29 ± 3 beats/min, P = 0.034) after melatonin. In summary, exogenous melatonin attenuates the SSNA response to mental stress.
Keywords: autonomic nervous system, blood pressure, heart rate
melatonin is an endogenous hormone secreted by the pineal gland in a circadian rhythm. This hormone is mechanistically linked to the sleep-wake cycle, and peak plasma levels are evident in the early morning (1:00 to 5:00 am) (19, 27). For this reason, melatonin is commonly ingested in high doses to improve sleep (i.e., 3 to 5 mg, which leads to >50 times above physiological levels). In addition to its effectiveness as a sleep aid, mounting evidence suggests that exogenous melatonin has widespread effects on immune response, aging, and cancer (c.f., 7, 37). Furthermore, melatonin supplementation has been reported to reduce blood pressure in patients with metabolic syndrome (20) and essential hypertension (30). One mechanism by which melatonin decreases blood pressure could be its effect on cardiovascular reflexes.
In previous studies, our laboratory demonstrated that melatonin attenuated increases in muscle sympathetic nerve activity (MSNA) to both lower body negative pressure (24) and head-down rotation (14). These findings suggest that melatonin attenuates reflex changes in sympathetic outflow during postural change. However, the effect of melatonin on reflex changes in skin sympathetic nerve activity (SSNA) is unknown. The interaction of melatonin on SSNA is important to understand because both participate in thermoregulation (13). For example, Aoki et al. (2) reported that ingestion of melatonin attenuated the reflex cutaneous vasoconstriction in response to whole body cooling. However, this study did not measure reflex changes in SSNA to ascertain whether the reduced vasoconstriction was neurally mediated or due to direct effects of melatonin on vascular smooth muscle. Therefore, we tested the hypothesis that reflex changes in SSNA would be attenuated by melatonin (3 mg oral dose). We used an established laboratory model of mental stress to elicit marked increases in SSNA (23). Our results further support the concept that melatonin has a significant effect on reflex changes in sympathetic nerve activity in humans.
METHODS
Subjects.
Twelve subjects (8 men, and 4 women) with a mean age of 26 ± 1 yr, height of 1.76 ± 0.04 m, and weight of 76.4 ± 4.2 kg participated in this study. Subjects were determined to be healthy via medical history and physical examination and were not taking medication. All subjects refrained from caffeine, alcohol, and exercise for 24 h before the study and arrived to the laboratory in a semifasted state (i.e., at least 4–6 h after their last meal). The study protocols were approved in advance by the Institutional Review Board of the Penn State Milton S. Hershey Medical Center and conformed to the Declaration of Helsinki. Each participant provided written, informed consent.
Design.
This study employed a randomized, double-blind, placebo-controlled, crossover design. On separate days, subjects underwent mental stress before and after ingestion of either 3 mg melatonin or placebo (cellulose). Two trials occurred each day. Neither the investigators nor the subject was aware of the treatment, and the code was broken when all data analysis was completed. Because SSNA recordings cannot be obtained from the exact same anatomical location between days, responses within the same day (pre-melatonin, post-melatonin) were considered to be the primary outcome measure in this study. Our laboratory recently reported that under control conditions (without drug), the reflex increases in SSNA, mean arterial pressure (MAP), and heart rate (HR) in response to mental stress are reproducible within and between days (23). Thus, in the current study, any alterations in the SSNA response to mental stress were attributed to melatonin and not time/order effects. It is also important to emphasize that 3 mg of melatonin is a common dose that people take to improve sleep. Twenty-five to 50 min after ingestion of 3 mg melatonin, plasma melatonin reaches peak levels (i.e., >50 times above baseline), and plasma levels remain in the supraphysiological range for several hours (14, 24).
Protocol.
All experiments were conducted in the supine posture during the morning hours (8:00 to 11:30 am) in a dimly lit, quiet, thermoneutral laboratory (22–25°C). To maintain neutral skin temperature, each subject wore a custom-designed tube-lined suit (Med-Eng Systems, Ottawa, ON, Canada). The suit covered the entire body except for the head, hands, feet, and the left lower leg (i.e., where SSNA was measured). Water was perfused at 34 to 35°C for the entire study.
Upon arrival at the laboratory, subjects were outfitted with several hemodynamic and thermal monitoring devices (see below). They were familiarized to the procedures and encouraged to respond as quickly and correctly as possible during the upcoming mental stress trial. Following successful nerve recording and instrumentation of the subject, resting blood pressure was obtained. Next, a 5-min baseline occurred to quantify resting SSNA. The total duration from when the subject was placed in the thermoneutral suit until the start of baseline was always >30 min. The baseline period was followed by 3 min of fast-paced verbal mental arithmetic, as previously described from our laboratory (10–12, 22, 23, 25). Specifically, subjects were provided a two- or three-digit number and were instructed to subtract the number seven as fast as possible. The eyes remained closed during testing, and subjects were encouraged to speak softly but quickly. Investigators provided a new number from which to subtract every 5–10 s. A 3-min recovery period occurred following mental stress. These procedures were repeated ∼45 min later after ingesting either melatonin or placebo. On a separate day (∼7–10 days later), subjects returned to the laboratory and underwent the same procedures ingesting the opposite treatment.
Instrumentation and measurements.
Mean skin temperature was measured via weighted average of three thermocouples (model TC-1000, Sable Systems) attached to the skin (8, 29). These thermocouples were underneath the suit but insulated from contacting the suit itself. Tympanic temperature, an index of core temperature (6), was measured before and after testing with an automated device (Genius 3, AccuSystem). HR was measured via three-lead EKG, and beat-by-beat MAP was determined by photoplethysmography (Finometer, FMS, The Netherlands). Blood pressures at rest were obtained by automated oscillometry (Dinamap XL, Critikon/GE).
Multifiber recordings of SSNA were made with a tungsten microelectrode inserted in the peroneal nerve at the fibular head. A reference electrode was placed 2 to 3 cm from the recording electrode. The recording electrode was adjusted until a site was found in which SSNA bursts were clearly identified using previously established criteria (15, 33). In brief, these included 1) light stroking of the skin within the innervated region resulted in afferent discharge and 2) deep inspiration and arousal stimuli resulted in activity not synchronous with the cardiac cycle. The nerve signal was amplified, passed through a band-pass filter with a bandwidth of 700–2,000 Hz, and integrated with a time constant of 0.1 s (Iowa Bioengineering, Iowa City, IA). Mean voltage neurograms were visually displayed and recorded on a data acquisition system (16SP PowerLab, ADInstruments, New Castle, Australia) and routed to a loudspeaker for monitoring throughout the study. SSNA responsiveness to auditory stimuli and deep inspiration was confirmed at the very end of experiments to ensure a consistent recording site.
Once the recording nerve site was established, two skin blood flow lasers (Moor Instruments, local heater set at 34°C) and one thermocouple were carefully attached to the dorsal foot (within the region of innervation on the foot) (28, 31, 36). Sweat rate was measured on the contralateral dorsal foot via capacitance hygrometry (Vaisala, Woburn, MA) by perfusing 100% nitrogen at a flow rate of 150 ml/min through a ventilated capsule (surface area, 2.0 cm2). Perception of stress was quantified after the bout of mental arithmetic (0 = not stressful, 1 = somewhat stressful, 2 = stressful, 3 = very stressful, and 4 = very very stressful) (9). Thermal sensation and thermal comfort were also determined before and after testing (16).
For all trials, beat-by-beat physiological measurements were recorded electronically and analyzed offline (16SP, PowerLab, ADInstruments). Perceptual variables were obtained by verbal report.
Data analysis and statistics.
Sympathetic nerve recordings that demonstrated possible electrode site shifts or electromyographic artifact were excluded from analysis (n = 1; leaving 11 full SSNA data sets for the melatonin trial). Consistent with previous experiments in our laboratory (23, 26, 35, 36) and others (18, 32, 34), SSNA was expressed as a percent change in total area under the mean voltage neurogram relative to the preceding baseline (5 min average). This analysis was achieved using computer software (Chart 5, ADInstruments), and the focus was on the first 10 s of mental stress (arousal response), as well as averages of minutes 1–3. Repeated-measures ANOVA (pre, post × minute 1, minute 2, minute 3) was used to determine if melatonin attenuated reflex responses. Cutaneous vascular conductance (CVC) was calculated as the quotient of skin blood flow flux and MAP. Changes in CVC due to mental stress were expressed as a percent change from the preceding 5-min baseline. Absolute changes in HR, MAP, sweat rate, and skin temperature were also determined. All values are reported as means ± SE, and P values < 0.05 were considered statistically significant.
RESULTS
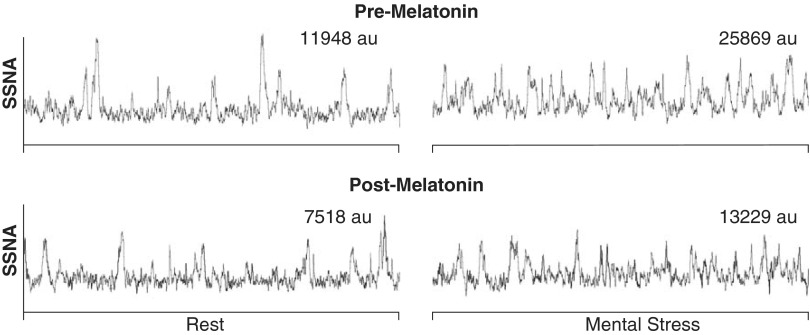
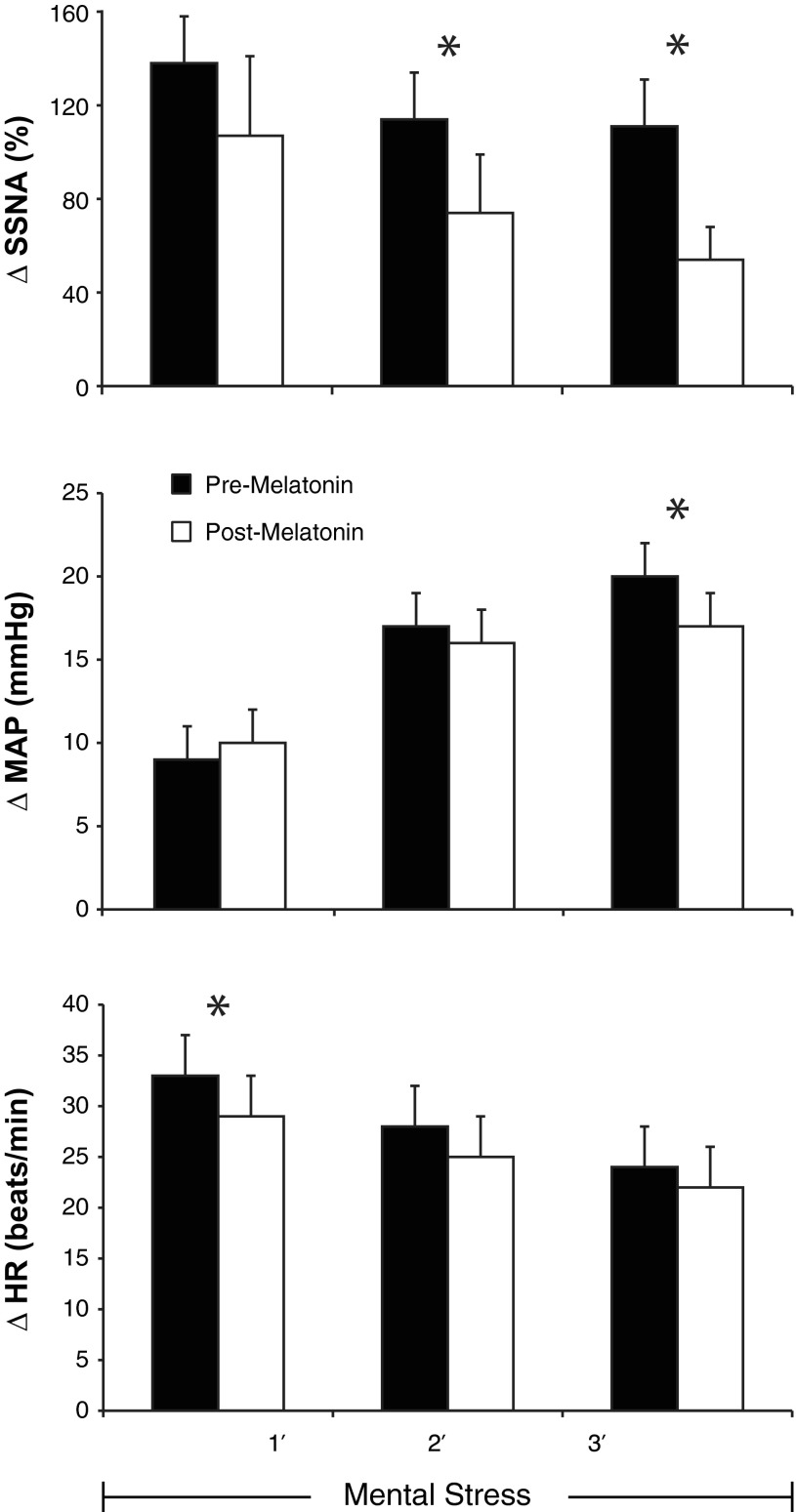
In response to mental stress, perceived stress level was comparable before (2.8 ± 0.2) and after (2.8 ± 0.2) melatonin. As displayed in Table 1, melatonin ingestion lowered HR at rest (P = 0.05) but did not have a significant effect on blood pressure or body temperature. In a subset of subjects, where we were confident no electrode shift occurred during the entire trial (n = 6), resting SSNA was also lower following melatonin ingestion [pre, 14,282 ± 3,706 arbitrary units (AU); and post, 9,571 ± 2,609 AU, P = 0.03]. Representative recordings of SSNA at baseline and during mental stress are displayed in Fig. 1. Depicted in Fig. 2 are the reflex changes in SSNA, MAP, and HR in response to mental stress. For SSNA, repeated-measures ANOVA revealed a treatment by time interaction (P = 0.02) such that melatonin attenuated the reflex increase in SSNA in response to mental stress. During the second (P = 0.03) and third minute (P = 0.04) of mental stress, SSNA responses were significantly lower after melatonin ingestion. Likewise, the MAP response was blunted in the third minute (P = 0.03) and HR was blunted in the first minute (P = 0.034). In the first 10 s of mental stress (arousal response), there was no observed effect of melatonin (pre, 173 ± 47 AU; and post, 160 ± 34 AU, P = 0.61). The end-organ responses to mental stress measured on the dorsal foot were also not significantly impacted by melatonin (Table 2). Before and after mental stress, individuals consistently reported that they felt “neutral” and “comfortable.” During the third minute of mental stress, the rate pressure product (an index of myocardial oxygen demand) was ∼5% lower with melatonin (12,450 ± 760 vs. 11,767 ± 805, P = 0.04).
Table 1.
Resting baseline parameters before and after ingestion of melatonin or placebo
| Pre-Melatonin | Post-Melatonin | |
|---|---|---|
| SBP, mmHg | 116 ± 2 | 116 ± 2 |
| DBP, mmHg | 66 ± 2 | 63 ± 2 |
| MAP, mmHg | 86 ± 1 | 85 ± 1 |
| HR, beats/min | 66 ± 3 | 62 ± 3* |
| Ttym, °C | 36.6 ± 0.2 | 36.8 ± 0.1 |
| Tfoot, °C | 27.1 ± 0.3 | 26.5 ± 0.3 |
| MST, °C | 34.1 ± 0.3 | 34.5 ± 0.3 |
| CVC, AU | 0.29 ± 0.03 | 0.32 ± 0.04 |
| SR, mg·cm−2·min−1 | 0.18 ± 0.02 | 0.16 ± 0.02 |
| Pre-Placebo | Post-Placebo | |
| SBP, mmHg | 115 ± 2 | 116 ± 2 |
| DBP, mmHg | 65 ± 2 | 64 ± 2 |
| MAP, mmHg | 84 ± 1 | 85 ± 1 |
| HR, beats/min | 64 ± 3 | 64 ± 3 |
| Ttym, °C | 36.5 ± 0.1 | 36.5 ± 0.2 |
| Tfoot, °C | 26.9 ± 0.3 | 26.7 ± 0.2 |
| MST, °C | 34.2 ± 0.2 | 34.4 ± 0.2 |
| CVC, AU | 0.32 ± 0.02 | 0.33 ± 0.03 |
| SR mg·cm−2·min−1 | 0.17 ± 0.03 | 0.14 ± 0.03 |
Values are means ± SE; for the melatonin trials, n = 11, and for placebo, n = 8.
SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; HR, heart rate; Ttym, tympanic membrane temperature; Tfoot, dorsal foot temperature; MST, mean skin temperature; CVC, cutaneous vascular conductance; AU, arbitrary units; SR, sweat rate.
P < 0.05, significant difference from the preingestion value.
Fig. 1.
Representative recordings of skin sympathetic nerve activity (SSNA) at rest (left) and during mental stress (right) in the same subject. All recordings are 60 s in duration. The mental stress recordings were both taken during the third minute. AU, arbitrary units.
Fig. 2.
Reflex changes (Δ) in SSNA (top), mean arterial blood pressure (MAP, middle), and heart rate (HR, bottom) in response to 3-min bouts of mental stress before (pre, black bars) and after (post, white bars) ingestion of 3 mg melatonin, n = 11; means ± SE; *P < 0.05 between trials.
Table 2.
Thermal response to mental arithmetic
| Minute |
||||||
|---|---|---|---|---|---|---|
| Δ1′ | Δ2′ | Δ3′ | Trial | Time | Trial × Time | |
| Tfoot, °C | ||||||
| Pre | 0.01 ± 0.01 | 0.04 ± 0.02 | 0.10 ± 0.04 | 0.082 | 0.029 | 0.150 |
| Post | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.03 ± 0.02 | |||
| CVC, % | ||||||
| Pre | −9 ± 8 | 9 ± 13 | 8 ± 13 | 0.350 | 0.147 | 0.695 |
| Post | −12 ± 12 | 1 ± 13 | 5 ± 14 | |||
| SR, mg·cm−2·min−1 | ||||||
| Pre | 0.00 ± 0.00 | 0.01 ± 0.01 | 0.03 ± 0.02 | 0.342 | 0.074 | 0.079 |
| Post | 0.01 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 | |||
Subjects underwent 3 min of fast-paced, verbal, mental arithmetic in the supine posture. Tfoot, CVC, and SR were measured, and changes (Δ) from baseline were calculated. Trials occurred before (pre) and after (post) blinded ingestion of melatonin (n = 11). Values are means ± SE.
Eight of the 12 subjects who completed the melatonin trial also completed the placebo trial (Table 1). SSNA at rest was not changed in response to placebo ingestion (pre, 8,945 ± 1,955 AU; and post, 11,414 ± 1,597 AU, P = 0.38). Responses of SSNA, MAP, and HR to mental stress were also not altered by placebo ingestion. Specifically, the change in SSNA in the first 10 s (198 ± 44 vs. 259 ± 61%), first minute (119 ± 25 vs. 154 ± 29%), second minute (63 ± 18 vs. 118 ± 28%), and third minute (93 ± 18 vs. 144 ± 44%) of mental stress were not different before and after placebo ingestion (drug × time interaction, P = 0.64). The changes in MAP during mental stress were not different before and after placebo ingestion (first minute, 9 ± 2 vs. 6 ± 2 mmHg; second minute, 17 ± 4 vs. 15 ± 3 mmHg; and third minute, 12 ± 4 vs. 8 ± 3 mmHg; drug × time interaction, P = 0.43). The changes in HR to mental stress were not different before and after placebo ingestion (first minute, 32 ± 5 vs. 32 ± 5 beats/min; second minute, 30 ± 7 vs. 27 ± 6 beats/min; and third minute, 29 ± 7 vs. 24 ± 6 beats/min; drug × time interaction, P = 0.30).
DISCUSSION
Our previous studies demonstrated that melatonin attenuates MSNA responses to postural changes (14, 24). These studies indicated that melatonin directly affects neural control of reflex changes in MSNA. In the current study, we tested the hypothesis that melatonin would alter reflex changes in SSNA in a similar manner to that observed with MSNA. Consistent with our hypothesis, melatonin attenuated the SSNA response to mental stress. This finding might provide an important mechanistic explanation of the interaction that melatonin has on skin blood flow and thermoregulation in humans.
In response to sympathoexcitatory stress, the human body activates several reflex pathways to maintain cardiovascular homeostasis. Studies from our laboratory have demonstrated that melatonin attenuates the reflex increases in MSNA to lower body negative pressure and head-down rotation (14, 24). Along the same lines, Arangino et al. (3) reported that plasma norepinephrine in response to standing was reduced following melatonin ingestion. In the current study, we observed that reflex SSNA responses to mental stress were attenuated following melatonin. We chose mental stress because it is a robust and reproducible activator of SSNA and it does not impact body temperature (15, 17, 23). The use of mental stress is an important strength in this study because both melatonin and SSNA contribute to thermoregulation. If we heated or cooled the body in this study, we would have been unable to discern whether the effects of melatonin were due to altered autonomic control or due to a shift in hypothalamic thermoregulatory set point (1, 5). Because we used a short-duration, nonthermal stimulus, it is not surprising that CVC, sweat rate, and skin temperature were not altered by melatonin (Table 2). Aoki et al. (2) observed that the cutaneous vasoconstrictor response to whole body cooling was blunted following melatonin. This study was not able to determine if the effect of melatonin on skin blood flow was because of its local vasodilator effect on smooth muscle or inhibition of skin vasoconstriction activity by reduced SSNA. The current study suggests that reduced SSNA by melatonin contributed to the decrease in cutaneous vascular resistance. Our past and current studies clearly indicate melatonin is capable of attenuating reflex responses to both physiological and psychological stress. Mental and social stress can trigger myocardial ischemia and adverse cardiovascular events (4, 21). Our current data indicate that HR and MAP responses to mental stress are blunted after melatonin ingestion, which would presumably reduce myocardial oxygen demand, thereby lessening episodes of angina. During the third minute of mental stress, the rate pressure product, an index of myocardial oxygen demand, was ∼5% lower with melatonin. Whether this translates into a clinically relevant cardioprotective effect on the heart requires further study.
Conclusions.
In the current study, exogenous melatonin attenuated the SSNA response to mental stress. These data in healthy humans, along with previously published work from our laboratory (14, 24), support the concept that acute ingestion of melatonin attenuates reflex changes in sympathetic outflow to skin and muscle. Therefore, melatonin can have a profound effect on autonomic regulation in humans.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant HL-109952.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.D.M. and C.A.R. conception and design of research; M.D.M., C.L.S., and C.A.R. performed experiments; M.D.M. analyzed data; M.D.M., C.L.S., and C.A.R. interpreted results of experiments; M.D.M. prepared figures; M.D.M. and C.A.R. drafted manuscript; M.D.M., C.L.S., and C.A.R. edited and revised manuscript; M.D.M., C.L.S., and C.A.R. approved final version of manuscript.
REFERENCES
- 1.Aoki K, Stephens DP, Zhao K, Kosiba WA, Johnson JM. Modification of cutaneous vasodilator response to heat stress by daytime exogenous melatonin administration. Am J Physiol Regul Integr Comp Physiol 291: R619–R624, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Aoki K, Zhao K, Yamazaki F, Sone R, Alvarez GE, Kosiba WA, Johnson JM. Exogenous melatonin administration modifies cutaneous vasoconstrictor response to whole body skin cooling in humans. J Pineal Res 44: 141–148, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Arangino S, Cagnacci A, Angiolucci M, Vacca AM, Longu G, Volpe A, Melis GB. Effects of melatonin on vascular reactivity, catecholamine levels, and blood pressure in healthy men. Am J Cardiol 83: 1417–1419, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Bhattacharyya MR, Steptoe A. Emotional triggers of acute coronary syndromes: strength of evidence, biological processes, and clinical implications. Prog Cardiovasc Dis 49: 353–365, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Bini G, Hagbarth KE, Hynninen P, Wallin BG. Thermoregulatory and rhythm-generating mechanisms governing the sudomotor and vasoconstrictor outflow in human cutaneous nerves. J Physiol 306: 537–552, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brinnel H, Cabanac M. Tympanic temperature is a core temperature in humans. J Therm Biol 14: 47–53, 1988 [Google Scholar]
- 7.Brzezinski A. Melatonin in humans. N Engl J Med 336: 186–195, 1997 [DOI] [PubMed] [Google Scholar]
- 8.Burton AC. Human calorimetry: the average temperature of the tissues of the body. J Nutr 9: 1935 [Google Scholar]
- 9.Callister R, Suwarno NO, Seals DR. Sympathetic activity is influenced by task difficulty and stress perception during mental challenge in humans. J Physiol 454: 373–387, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carter JR, Cooke WH, Ray CA. Forearm neurovascular responses during mental stress and vestibular activation. Am J Physiol Heart Circ Physiol 288: H904–H907, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Carter JR, Kupiers NT, Ray CA. Neurovascular responses to mental stress. J Physiol 564: 321–327, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carter JR, Ray CA, Cooke WH. Vestibulosympathetic reflex during mental stress. J Appl Physiol 93: 1260–1264, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Claustrat B, Brun J, Chazot G. The basic physiology and pathophysiology of melatonin. Sleep Med Rev 9: 11–24, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Cook JS, Ray CA. Melatonin attenuates the vestibulosympathetic but not vestibulocollic reflexes in humans: selective impairment of the utricles. J Appl Physiol 109: 1697–1701, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delius W, Hagbarth KE, Hongell A, Wallin BG. Manoeuvres affecting sympathetic outflow in human skin nerves. Acta Physiol Scand 84: 177–186, 1972 [DOI] [PubMed] [Google Scholar]
- 16.DuBois AB, Harb ZF, Fox SH. Thermal discomfort of respiratory protective devices. Am Ind Hyg Assoc J 51: 550–554, 1990 [DOI] [PubMed] [Google Scholar]
- 17.Hagbarth KE, Hallin RG, Hongell A, Torebjork HE, Wallin BG. General characteristics of sympathetic activity in human skin nerves. Acta Physiol Scand 84: 164–176, 1972 [DOI] [PubMed] [Google Scholar]
- 18.Iwase S, Ikeda T, Kitazawa H, Hakusui S, Sugenoya J, Mano T. Altered response in cutaneous sympathetic outflow to mental and thermal stimuli in primary palmoplantar hyperhidrosis. J Auton Nerv Syst 64: 65–73, 1997 [DOI] [PubMed] [Google Scholar]
- 19.Kennaway DJ, Voultsios A. Circadian rhythm of free melatonin in human plasma. J Clin Endocrinol Metab 83: 1013–1015, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Kozirog M, Poliwczak AR, Duchnowicz P, Koter-Michalak M, Sikora J, Broncel M. Melatonin treatment improves blood pressure, lipid profile, and parameters of oxidative stress in patients with metabolic syndrome. J Pineal Res 50: 261–266, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Krantz DS, Kop WJ, Santiago HT, Gottdiener JS. Mental stress as a trigger of myocardial ischemia and infarction. Cardiol Clin 14: 271–287, 1996 [PubMed] [Google Scholar]
- 22.Kuipers NT, Sauder CL, Carter JR, Ray CA. Neurovascular responses to mental stress in the supine and upright postures. J Appl Physiol 104: 1129–1136, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muller MD, Sauder CL, Ray CA. Mental stress elicits sustained and reproducible increases in skin sympathetic nerve activity. Physiol Rep 1: 1–8, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ray CA. Melatonin attenuates the sympathetic nerve responses to orthostatic stress in humans. J Physiol 551: 1043–1048, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ray CA, Carter JR. Effects of aerobic exercise training on sympathetic and renal responses to mental stress in humans. Am J Physiol Heart Circ Physiol 298: H229–H234, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ray CA, Wilson TE. Comparison of skin sympathetic nerve responses to isometric arm and leg exercise. J Appl Physiol 97: 160–164, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Reiter RJ, Tan DX, Paredes SD, Fuentes-Broto L, Fuentes-Broto L. Beneficial effects of melatonin in cardiovascular disease. Ann Med 42: 276–285, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Sawasaki N, Iwase S, Mano T. Effect of skin sympathetic response to local or systemic cold exposure on thermoregulatory functions in humans. Auton Neurosci 87: 274–281, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Sawka MN, Gonzalez RR, Young AJ, Muza SR, Pandolf KB, Latzka WA, Dennis RC, Valeri CR. Polycythemia and hydration: effects on thermoregulation and blood volume during exercise-heat stress. Am J Physiol Regul Integr Comp Physiol 255: R456–R463, 1988 [DOI] [PubMed] [Google Scholar]
- 30.Scheer FA, Van Montfrans GA, van Someren EJ, Mairuhu G, Buijs RM. Daily nighttime melatonin reduces blood pressure in male patients with essential hypertension. Hypertension 43: 192–197, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Sugenoya J, Iwase S, Mano T, Ogawa T. Identification of sudomotor activity in cutaneous sympathetic nerves using sweat expulsion as the effector response. Eur J Appl Physiol Occup Physiol 61: 302–308, 1990 [DOI] [PubMed] [Google Scholar]
- 32.Toma K, Walkowski S, Metzler-Wilson K, Wilson TE. Acupuncture attenuates exercise-induced increases in skin sympathetic nerve activity. Auton Neurosci 162: 84–88, 2011 [DOI] [PubMed] [Google Scholar]
- 33.Vallbo AB, Hagbarth KE, Torebjork HE, Wallin BG. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev 59: 919–957, 1979 [DOI] [PubMed] [Google Scholar]
- 34.Vissing SF, Scherrer U, Victor RG. Stimulation of skin sympathetic nerve discharge by central command. Differential control of sympathetic outflow to skin and skeletal muscle during static exercise. Circ Res 69: 228–238, 1991 [DOI] [PubMed] [Google Scholar]
- 35.Wilson TE, Dyckman DJ, Ray CA. Determinants of skin sympathetic nerve responses to isometric exercise. J Appl Physiol 100: 1043–1048, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Wilson TE, Kuipers NT, McHugh EA, Ray CA. Vestibular activation does not influence skin sympathetic nerve responses during whole body heating. J Appl Physiol 97: 540–544, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Wirtz PH, Bartschi C, Spillmann M, Ehlert U, von Kanel R. Effect of oral melatonin on the procoagulant response to acute psychosocial stress in healthy men: a randomized placebo-controlled study. J Pineal Res 44: 358–365, 2008 [DOI] [PubMed] [Google Scholar]