Abstract
Quantitative real-time PCR was used to test whether cavernous nerve injury leads to a decrease in major pelvic ganglia (MPG) neuronal nicotinic ACh receptor (nAChR) subunit and postsynaptic density (PSD)-93 transcript levels. Subunits α3, β4, and α7, commonly expressed in the MPG, were selected for analysis. After 72 h in explant culture, MPG transcript levels for α3, β4, α7, and PSD-93 were significantly depressed. Three days after cavernous nerve axotomy or crush in vivo, transcript levels for α3, β4, and PSD-93, but not for α7, were significantly depressed. Three days after dissection of the cavernous nerve free of underlying tissue and application of a 5-mm lateral stretch (manipulation), transcript levels for α3 and PSD-93 were also significantly decreased. Seven days after all three surgical procedures, α3 transcript levels remained depressed, but PSD-93 transcript levels were still decreased only after axotomy or nerve crush. At 30 days postsurgery, transcript levels for the nAChR subunits and PSD-93 had recovered. ACh-induced currents were significantly smaller in MPG neurons dissociated from 3-day explant cultured ganglia than from those recorded in neurons dissociated from acutely isolated ganglia; this observation provides direct evidence showing that a decrease in nAChR function was coincident with a decrease in nAChR subunit transcript levels. We conclude that a downregulation of nAChR subunit and PSD-93 expression after cavernous nerve injury, or even manipulation, could interrupt synaptic transmission within the MPG and thus contribute to the loss of neural control of urogenital organs after pelvic surgeries.
Keywords: major pelvic ganglia, neuronal nicotinic receptor subunits, axotomy of postganglionic neurons, neuronal response to injury
it is well documented for the sympathetic superior cervical ganglia (SCG) that after axotomy or nerve crush, synaptic transmission is depressed for prolonged periods of time (8, 16). Matthews and Nelson (8) quantified the time course of loss of connectivity between pre- and postganglionic neurons in the rat SCG after axotomy, and Purves (16) showed that synaptic depression also followed nerve crush in the guinea pig SCG. A retraction of preganglionic fiber terminals from postganglionic neurons was first proposed to underlie the prolonged synaptic depression. Connectivity was reestablished upon reinnervation of the target tissues (16). In subsequent experiments, Zhou et al. (25) showed that a second mechanism likely contributing to the synaptic failure is a decreased expression of neuronal nicotinic ACh receptors (nAChRs) at synapses on the postganglionic neurons. This decrease in receptor subunit transcript expression occurred within a few days after axotomy. More recently, McCann et al. (9) showed that after nerve crush, synaptic depression also occurs within parasympathetic ganglia innervating the submandibular gland. The depressed ganglionic transmission resulted from a loss of postganglionic cell sensitivity to ACh followed by a loss of pre- to postganglionic neuron contact. Recovery of synaptic transmission progressed slowly over a 3-wk period (9).
The major pelvic ganglia (MPG) are the primary source of autonomic input to urogenital organs and pelvic viscera (7). The MPG are mixed ganglia containing both sympathetic and parasympathetic postganglionic neurons, which are selectively innervated by the hypogastric and pelvic nerves, respectively (7). A number of postganglionic nerve bundles emanate from the MPG to innervate different visceral targets (7). Both sympathetic and parasympathetic postganglionic neurons within the mouse MPG have no dendrites so that the synaptic connections are made on the soma (19, 21). In humans, MPG neurons and their axons are susceptible to injury during pelvic surgeries such as hysterectomy, prostatectomy, and tumor removal from the lower bowel. In the course of these procedures, extensive neuronal or axonal injury often results, which leads to neurogenic surgical complications such as urinary and fecal incontinence as well as erectile dysfunction. Urinary incontinence and erectile dysfunction are the two major causes of postsurgical morbidity in individuals undergoing surgical intervention for bladder, prostate, and colorectal cancers (2). Prior animal studies conducted to address the loss of function after pelvic surgical procedures have focused on the identification of pharmacological interventions that may enhance the regeneration of crushed or severed cavernous nerve axons in an effort to reduce the time course for recovery (2). No animal studies have assessed directly whether disruption of synaptic transmission occurs within the MPG after cavernous nerve axotomy or crush, a change that very likely contributes to the loss of neuronal control after axonal injury. However, Palma and Keast (14) presented histological data from rat MPG suggesting there was a marked decrease in synaptophysin- or synapsin-stained varicosities surrounding the cell somas of putative penile projecting neurons 7–8 days after nerve injury. These observations are consistent with a separation of preganglionic terminals from penile projecting postganglionic neurons. However, these authors did not assess whether there was a change in AChR subunit expression.
In previous studies, we (3, 4) identified changes in the chemical phenotype of neurons within the male mouse MPG that change in response to axotomy. In mice, the MPG are readily accessible, and axons from a subset of MPG neurons project via the well-defined cavernous nerve to penile tissues (7). In the present study, we quantified the extent and time course of a decrease in transcript level expression of α3, β4, and α7 neuronal nAChR subunits and the receptor scaffolding protein postsynaptic density (PSD)-93 after cavernous nerve cut or crush. A previous study (15) has shown that α3 and β4 neuronal nAChR subunits are the subunits primarily responsible for fast synaptic transmission in the rat MPG. As McCann et al. (9) have shown that α7 expression is decreased in the mouse mandibular ganglion after axotomy, we also tested whether α7 transcript levels are decreased in the mouse MPG after injury to the cavernous nerve. PSD-93 is the scaffolding protein that maintains the stability of nAChRs in the postsynaptic membrane (9). We showed that after axotomy or crush of the cavernous nerve, transcript levels for α3 and β4, but not α7, as well as transcript levels for PSD-93 decreased and recovered slowly. As a control, we tested whether simply dissecting the cavernous nerve away from the surrounding tissue and stretching it (manipulation) without any visible injury (indicated by an absence of color change of the nerve bundle) might also produce changes in transcript expression. Quite unexpectedly, our results indicate that a relatively simple manipulation of the cavernous nerve can produce a significant decrease in MPG neuron transcript levels for the α3 nAChR subunit and PSD-93. Thirty days postsurgery, the decrease in nAChR subunit and PSD-93 transcript levels had returned to control levels. A decrease in transcript level expression of α3 and β4 neuronal nAChR subunits and the receptor scaffolding protein PSD-93 was also noted in the MPG maintained in explant culture for 72 h. In separate electrophysiological experiments, ACh-induced currents were significantly smaller in MPG neurons dissociated from 3-day explant cultured ganglia than from those recorded in neurons dissociated from acutely isolated ganglia, an observation providing direct evidence showing that a decrease in nAChR function was coincident with a decrease in nAChR subunit transcript levels.
MATERIALS AND METHODS
All experiments were performed in male C57BL6 mice (3–5 mo) following protocols approved by the Institutional Animal Care and Use Committee of University of Vermont and conformed with NIH guidelines. As in previous studies (3, 4), mice were euthanized by an overdose of isoflurane followed by thoracotomy.
Explant cultures.
Intact whole mount MPG preparations, which contained both neural and non-neural cells, were removed under sterile conditions after euthanasia as previously described (3, 4). Whole mounts were maintained at 37°C in culture media consisting of DMEM-F-12 (1:1) containing 10% horse serum, gentamicin (10 g/ml), amphotericin B (3.75 g/ml), penicillin (100 U/ml), and streptomycin (100 g/ml, Sigma, St. Louis, MO). Preparations were pinned on a Sylgard-coated petri dish, which was placed on a wave platform shaker in a 5% CO2-95% air incubator (37°C) and kept for 72 h, with the culture media replaced either after 24 or 48 h depending on the experimental protocol. No difference in results was evident between the two conditions.
Surgical procedures.
For all surgical procedures, mice were anesthetized with isofluorane (2%), and an abdominal incision ∼1 cm long was made into the lower abdominal cavity to expose the MPG and cavernous nerve. Once one cavernous nerve was exposed (unilateral), one of three procedures was performed. In some animals, the cavernous nerve was sectioned on one side where the nerve travels over the surface of the prostate (axotomy). In a second group of animals, the cavernous nerve on one side was manually crushed at two locations, 2–3 mm from the ganglion, using fine forceps (Dumont Medical no. 3) held closed for 5 s (crush). Crush lesions were readily identifiable as the color of the nerve changed from white to gray in the area of crush. In the last group of animals, the cavernous nerve on one side was dissected carefully away from the underlying prostatic tissue using fine forceps, and, once separated, the nerve was displaced by a lateral stretch of ∼5 mm (manipulation).
Quantitative real-time PCR.
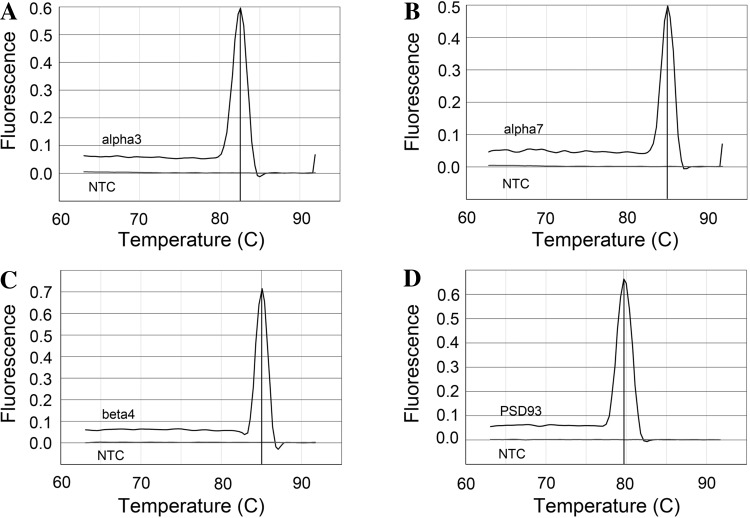
The quantitative real-time PCR procedures followed those used in our previous studies (3, 4). In brief, total RNA was extracted under RNAse-free conditions. The RNA sample was used to synthesize cDNA using Moloney murine leukemia virus reverse transcriptase and a mix of random hexamer and oligo dT primers from Promega. Real-time quantitative PCR was performed on an Applied Biosystems 7500 Fast real-time PCR system (Applied Biosystems, Foster City, CA). The amplified product from these amplification parameters was subjected to SYBR green I melting analysis. A single DNA melting profile was observed under these dissociation assay conditions demonstrating amplification of a single unique product free of primer dimers or other anomalous products (Fig. 1). Data were analyzed at the termination of each assay using Sequence Detection software (version 1.3.1, Applied Biosystems). All quantitative real-time PCR data are expressed as the relative quantity of the gene of interest normalized to the relative quantity of the reference gene L32 (a ribosomal marker) and the relative quantity of the gene of interest obtained from the ipsilateral (operated) MPG given as a fraction of that determined for the contralateral (unoperated) MPG, which served as a surgical control. The following primers were used: galanin, forward 5′-CACAGATCATTTAGCGACAAGCAT-3′ and reverse 5′-GACGATATTGCTCTCAGGCAG-3′; PSD-93, forward 5′-CTGTGTGCCTCATACTACGA-3′ and reverse 5′-TGGTTAAGTATAAATTGTCATTG-3′; β4, forward 5′-TGTACAACAATGCCGATGGG-3′ and reverse 5′-CCTGTGGGTTCACTGTCCTT-3′; α3, forward 5′-TGTCCCTGTCTGCTCTGTCACCA-3′ and reverse 5′-GCGAGCCTTGGCGAACAGGT-3′; α7, forward 5′-CGTGGGCCTCTCAGTGGTCG-3′ and reverse 5′-CGCCGAGGCTTGTGCTGACA-3′; and L32, forward 5′-CCTGGCGTTGGGATTGGTGA-3′ and reverse 5′-GAAAAGCCATCGTAGAAAGA-3′.
Fig. 1.
Melting point profiles for α3 (A), α7 (B), and β4 (C) neuronal nicotinic ACh receptor (nAChR) subunits as well as postsynaptic density (PSD)-93 (D). The amplified product for each gene was subjected to SYBR green I melting analysis by ramping the temperature of the reaction samples from 60 to 95°C. A single DNA melting profile was observed under these dissociation assay conditions, demonstrating amplification of a single unique product, free of primer dimers or other anomalous products. Melting temperatures of the quantitative real-time PCR products were 82.6, 85.1, 85, and 79.7°C for α3, α7, β4, and PSD-93, respectively. NTC equals no template control.
Electrophysiological recordings.
ACh-induced currents were recorded from either acutely dissociated MPG neurons or neurons dissociated from the MPG maintained in explant culture for 72 h. After euthanasia, MPG were removed and either prepared immediately for enzymatic dissociation or put into explant culture. Freshly dissected or cultured MPG were dissociated by an incubation at 37°C in 10 mg/ml collagenase D (Roche Molecular Biochemicals, Indianapolis, IN), 6 mg/ml trypsin (Sigma Chemical, St. Louis, MO), and 0.1 mg/ml DNase (Worthington Biochemical, Lakewood, NJ) for 80 min. After dissociation, neurons were placed in Eagle MEM (HEPES modification) containing 2 mM CaCl2 and supplemented with 10% FBS, 0.1% BSA, 0.1 mg/ml DNase I, 100 U/ml penicillin, 100 μg/ml streptomycin, and 100 μg/ml gentamicin (all from Sigma Chemical except for DNase I, which was from Worthington Biochemical). Cells were stored overnight in a 37°C incubator before being recorded.
For recording, isolated neurons were continuously superfused with HEPES-buffered physiological solution, which contained (in mM) 121 NaCl, 5.9 KCl, 2.5 CaCl2, 1.2 MgCl2, 26 Na-HEPES, and 8 glucose (pH 7.3). The temperature of the bathing solution was warmed to 34–35°C using an in-line solution heater controlled by a single channel heater controller (Warner Instruments, Hamden, CT). ACh-induced currents were recorded using the perforated-patch configuration of the whole cell patch-clamp recording technique (10, 18) with cells maintained at −50 mV, a value close to the membrane potential determined in current-clamp mode. Voltage-clamp experiments were controlled, and ACh-induced currents were recorded using an Axopatch-C amplifier in combination with a Digidata 1322A acquisition system and pCLAMP 9 software (Molecular Devices, Sunnyvale, CA). ACh-induced currents were digitized at 2 kHz and expressed in pA/pF to control for cell size. Resting membrane potentials and action potentials were monitored using the current-clamp recording mode. The pipette solution contained (in mM) 110 potassium aspartate, 30 KCl, 10 NaCl, 5 MgCl2, 10 mM HEPES-KOH (pH 7.2), and patch pipettes were backfilled with 0.2 mg/ml amphotericin B (Sigma Chemical).
ACh-induced currents were elicited by a puffer application of ACh (100 μM, 6 psi for 1 s) to neurons voltage clamped to −50 mV and with the puffer pipette positioned ∼80 μm from the cell (18).
Statistical analysis.
Statistical analysis was done using Graphpad Prism (Graphpad Software, La Jolla, CA). Averaged data are presented as means ± SE and were analyzed using either one-way ANOVA or an unpaired t-test. Differences were considered statistically significant if P ≤ 0.05.
RESULTS
Neuronal nAChR subunit transcript levels are decreased in the 3-day explant cultured MPG.
Zhou et al. (25) showed that nAChR subunit expression is decreased when rodent SCG are maintained in explant culture. Furthermore, these authors determined that the decreased expression of nAChR subunit transcripts noted in cultured ganglia in vitro closely mimicked the decrease in expression of the same receptor subunits after axotomy in vivo (25). In previous studies, we (3, 4) used an explant cultured male mouse MPG whole mount as an in vitro model to study the neuronal response to injury. Our previous studies established that after 2–3 days in culture, MPG neurons in whole mount ganglia explant preparations increase expression, both transcript and protein levels, of three molecules (activating transcription factor 3, pituitary adenylate cyclase-activating polypeptide, and galanin) that are known to be upregulated after axotomy in vivo in other autonomic ganglia (1, 6, 11, 12, 17, 20, 24). Consequently, we initially determined, as part of this study, whether transcript levels of the nAChR subunits α3, β4, and α7 were decreased when the MPG were maintained in culture for 3 days. All three subunits are expressed in freshly isolated ganglia, although α3- and β4-subunits are thought to form the nAChR primarily mediating synaptic transmission (15). We determined α7-subunit expression because this subunit is decreased in the mouse mandibular ganglion after axotomy (9). We also tested whether expression of the nAChR subunit scaffolding protein PSD-93 was downregulated after explant culture (9).
As shown in Fig. 2, transcript levels of all three nAChR subunits were significantly decreased after 3 days in culture. Similarly, expression of PSD-93 transcript was also significantly reduced. To quantify results, all transcript levels are normalized to transcript levels for L32 and expressed as fold decreases in levels determined in extracts from freshly isolated ganglia. These results suggested that nAChR subunit transcript levels were depressed in the explant cultured MPG, as previously noted for the explant cultured rat SCG (25).
Fig. 2.
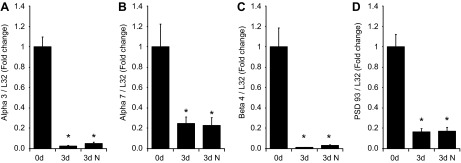
Comparison of mRNA levels for nAChR subunits (α3, α7, and β4) and the receptor scaffolding protein PSD-93 in extracts of acutely isolated (0 d) and 3-day explant cultured (3 d) whole mount preparations of the major pelvic ganglia (MPG). Transcript levels were normalized to the transcript level for L32. A–D: α3 (A), α7 (B), β4 (C), and PSD-93 (D) transcript levels were significantly decreased (P = 0.0001 in A, P = 0.002 in B, P = 0.0001 in C, and P = 0.0001 in D) in 3-day cultured MPG. The presence of 10 ng/ml neurturin in the culture media (3d N) did not reverse the decrease in transcript levels after explant culture. Data are means ± SE from six acutely isolated ganglia and six 3-day cultured ganglia.
It has been suggested that a loss of target-derived nerve growth factor might be one component of the trophic signal leading to downregulation of nAChR subunit transcript levels after axotomy of rat SCG neurons (13, 25). The development and differentiation of many parasympathetic postganglionic neurons and enteric ganglia are supported by members of the glial-derived neurotrophic factor family, such as neurturin (22, 23). Consequently, we postulated that one signal contributing to the decrease in nAChR subunit and PSD-93 transcript expression might be a loss of target-derived neurturin. However, when 10 ng/ml neurturin was added to the culture media, there was no reversal of the injury-induced downregulation of MPG nAChR or PSD-93 transcript levels (Fig. 2).
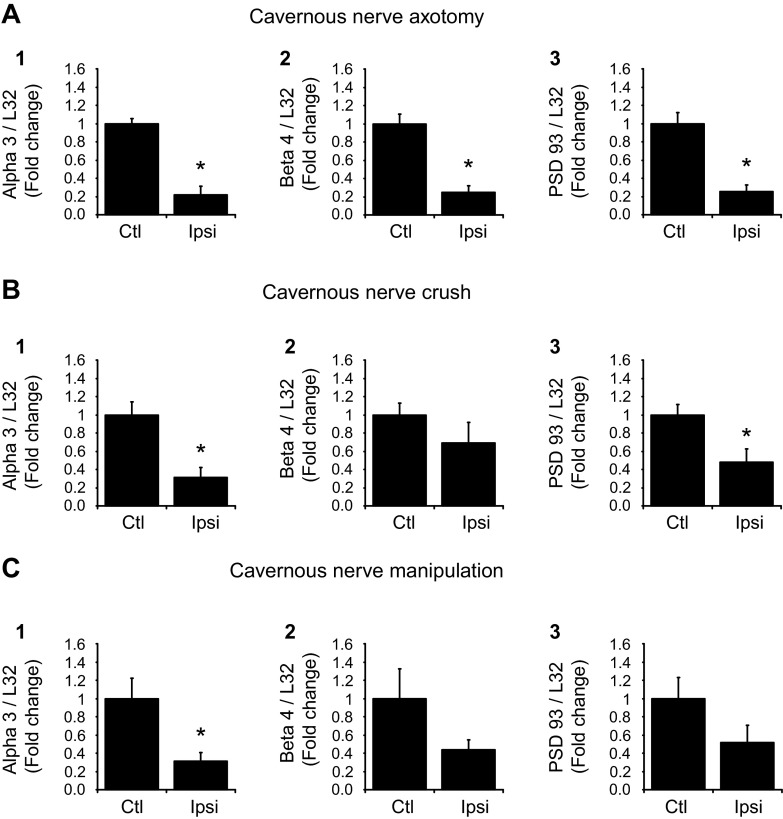
MPG nAChR subunit and PSD-93 transcript levels are decreased 3 days after axotomy or crush of the cavernous nerve.
The results with the explant cultured MPG indicated that there was an injury-associated decrease in the levels of nAChR subunit and PSD-93 transcript levels in the mouse MPG. Thus, we tested whether a similar decrease in the ipsilateral MPG occurred after unilateral transection (axotomy) or crush of the cavernous nerve. To control for the effect of the surgical procedure, we compared nAChR subunit and PSD-93 transcript levels in extracts from the ipsilateral operated MPG with those determined in extracts from the contralateral unoperated MPG. To quantify results, all nAChR subunit and PSD-93 transcript levels were normalized to L32 transcript levels, and the change was denoted as the fold decrease in the ipsilateral operated MPG relative to that in the contralateral unoperated MPG. Three days after either axotomy (Fig. 3A) or nerve crush (Fig. 3B), transcript levels for α3, β4, and PSD-93 were significantly (P ≤ 0.05) less in extracts from the ipsilateral operated MPG compared with the contralateral unoperated MPG. In contrast, the transcript level for α7 was not significantly reduced after axotomy or crush (Fig. 3, A and B).
Fig. 3.
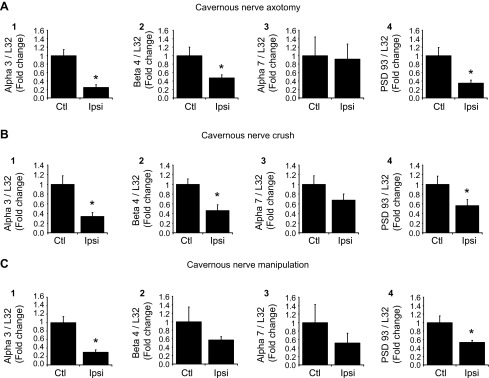
nAChR subunit and PSD-93 transcript levels are decreased 3 days after cavernous nerve axotomy (A), crush (B), or manipulation (C). All transcript levels were normalized to the transcript level for L32, and the normalized transcript levels from the ipsilateral (Ipsi) operated side are expressed as a fraction of normalized transcript levels in the contralateral unoperated MPG, which served as a control (Ctl). A and B: after cavernous nerve axotomy or crush, transcript levels for α3, β4, and PSD-93 were significantly decreased (P = 0.002 in A,1, P = 0.01 in B,1, P = 0.04 in A,2, P = 0.018 in B,2, P = 0.01 in A,4, and P = 0.05 in B,4), whereas α7 transcript levels were not changed (P = 0.89 in A,3 and P = 0.18 in B,3). C: after dissection/stretch (manipulation), transcript levels for α3 and PSD-93 were significantly decreased (P = 0.003 in C,1 and P = 0.02 in C,4), whereas β4 and α7 transcript levels were not significantly changed (P = 0.26 in C,2 and P = 0.69 in C,3). Data are means ± SE of transcript levels determined in MPG extracts from 4–6 animals/group.
Transcript levels for the α3-nAChR subunit and PSD-93 are decreased 3 days after dissection/stretch (manipulation) of the cavernous nerve.
We also determined whether only unilateral dissection of the cavernous nerve to free a short segment of the nerve from surrounding tissues followed by a lateral stretch of ∼5 mm would change ipsilateral MPG nAChR subunit or PSD-93 transcript levels. We determined that transcript levels of α3 and PSD-93 were significantly (P ≤ 0.02) decreased by this procedure (Fig. 3C). In contrast, transcript levels for β4 and α7 were not significantly decreased (Fig. 3C).
Transcript levels for nAChR subunits and PSD-93 recover over time.
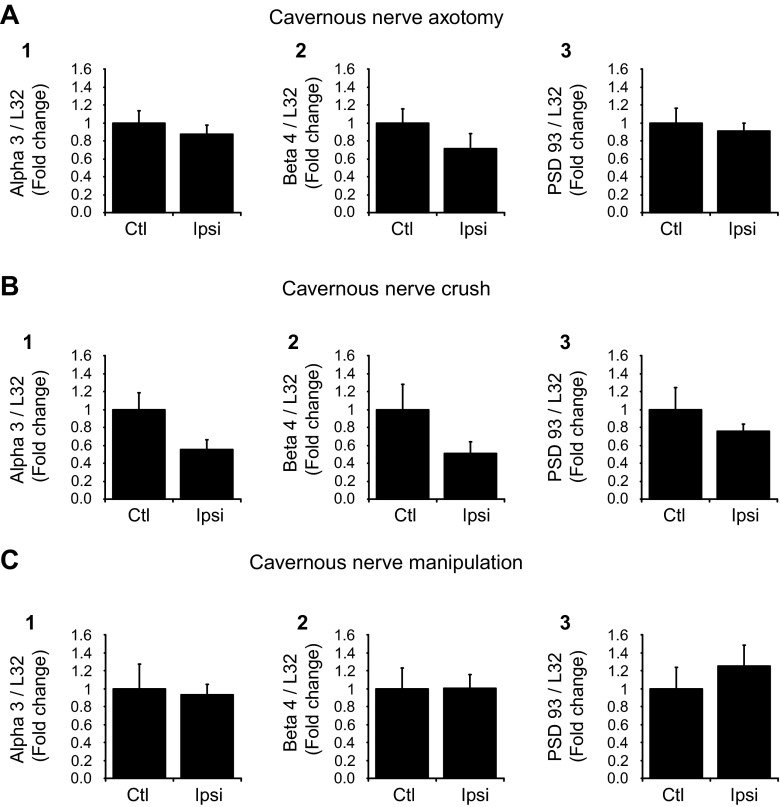
Next, we determined whether the decrease in nAChR subunit and PSD-93 transcript levels reversed over time. For these experiments, we compared transcript levels from the MPG on the unoperated side with those obtained from the operated MPG taken from mice at 7 or 30 days postsurgery. Because there were no significant changes in MPG α7 transcript levels for any of the surgical procedures at 3 days postsurgery, we focused on changes in α3, β4, and PSD-93 transcript levels at subsequent time points [7 days (Fig. 4) and 30 days (Fig. 5)]. After a 7-day recovery period, transcript levels for α3 remained significantly (P ≤ 0.03) less in extracts from the MPG on the operated side from that in extracts from the unoperated MPG with axotomy, crush, and dissection/stretch of the cavernous nerve. In contrast, β4 transcript levels remained significantly (P ≤ 0.002) reduced after axotomy but not with nerve crush or dissection/stretch. Also, after a 7-day recovery, PSD-93 transcript levels were significantly (P ≤ 0.02) reduced after both axotomy and nerve crush but not with nerve dissection/manipulation.
Fig. 4.
nAChR subunit and PSD-93 transcript levels determined in MPG extracts 7 days after cavernous nerve axotomy (A), cavernous nerve crush (B), or cavernous nerve manipulation (C). All transcript levels were normalized to the transcript level for L32, and transcript levels from the ipsilateral operated side are expressed as a fraction of transcript levels in the contralateral unoperated MPG. A: after cavernous nerve axotomy, transcript levels for α3, β4, and PSD-93 were significantly decreased (P = 0.0005 in A,1, P = 0.002 in A,2, and P = 0.005 in A,3). B: after cavernous nerve crush, transcript levels for α3 and PSD-93 were significantly decreased (P = 0.01 in B,1 and P = 0.02 in B,3), whereas β4 transcript levels were not changed (P = 0.17 in B,2). C: after nerve manipulation, transcript levels for α3 were significantly decreased (P = 0.03 in C,1), whereas β4 and PSD-93 transcript levels were not changed (P = 0.06 in C,2 and P = 0.32 in C,3). Data are means ± SE of MPG extracts from 4 animals/group.
Fig. 5.
nAChR subunit and PSD-93 transcript levels determined in MPG extracts 30 days after cavernous nerve axotomy (A), cavernous nerve crush (B), or cavernous nerve manipulation (C). All transcript levels were normalized to the transcript level for L32, and transcript levels from the ipsilateral operated side are expressed as a fraction of transcript levels in the contralateral unoperated MPG. A–C: 30 days after cavernous nerve axotomy, crush, or manipulation, transcript levels for α3, β4, and PSD-93 were not significantly different in the operated MPG compared with the unoperated MPG. Data are means ± SE of MPG extracts from 4–6 animals/group.
When animals were allowed to recover for 30 days after all surgical procedures, axotomy, nerve crush, or dissection/stretch, there were no significant differences in nAChR subunit or PSD-93 transcript levels in extracts from the operated MPG compared with those in extracts from the unoperated MPG. Thus, the decrease in transcript levels had reversed by 30 days (Fig. 5).
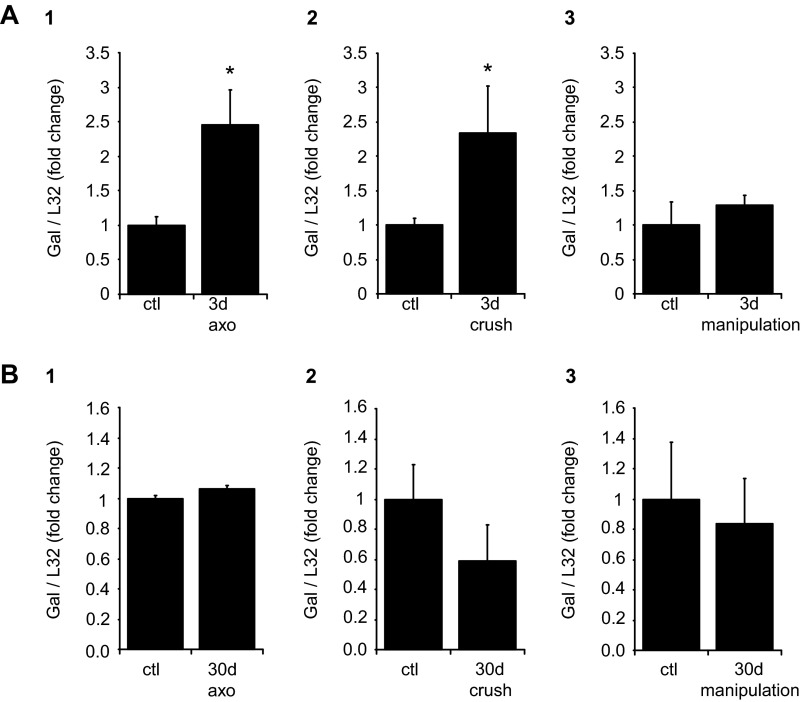
MPG galanin transcript levels are increased after cavernous nerve transection and crush but not after dissection/stretch.
Expression of the neuropeptide galanin is known to increase in the SCG after axonal transection or crush (11, 12, 17). We (3) have previously shown that galanin transcript levels increase in explant cultured MPG and in the ipsilateral MPG after unilateral cavernous nerve axotomy. We extended these observations in the present study to test whether ipsilateral MPG galanin transcript levels were also increased after cavernous nerve crush or the dissection/stretch procedure. As shown in Fig. 6, galanin transcript levels were significantly increased at 3 days after nerve crush or axotomy but not after the dissection/stretch procedure. Given that galanin expression is a good indicator of neuronal injury (11, 12, 17), the lack of change in galanin expression after nerve dissection/stretch suggested that this procedure did not produce marked axonal injury. After recovery for 30 days, galanin transcript levels returned to control levels after axotomy or nerve crush (Fig. 6).
Fig. 6.
MPG galanin (Gal) transcript levels determined after cavernous nerve axotomy (axo), crush, or manipulation. All transcript levels were normalized to the transcript level for L32, and transcript levels from the ipsilateral operated side are expressed as a fraction of transcript levels in the contralateral unoperated side. A: 3 days after cavernous nerve axotomy or crush, galanin transcript levels increased significantly in the operated MPG (P = 0.02 in A,1 and P = 0.01 in A,2), whereas there was no difference in galanin transcript levels with nerve manipulation (P = 0.46 in A,3). B: 30 days after cavernous nerve axotomy, crush, or manipulation, there were no differences in galanin transcript levels in the operated or unoperated MPG. Data are means ± SE of MPG extracts from 4–6 animals/group.
ACh-induced currents are decreased after explant culture of the MPG.
In the mouse MPG, only a subpopulation of the neurons project via the cavernous nerve to innervate their targets, and, under the conditions of the present study, we cannot identify penile projecting neurons. Consequently, no attempt was made as part of this study to correlate changes in the expression of the AChR in the plasma membrane with the demonstrated decrease in transcript level after the cavernous nerve injury or manipulation. However, to provide evidence for mouse MPG neurons showing that a decrease in plasma membrane AChR expression does accompany a decrease in AChR subunit transcript levels, we compared ACh-induced current amplitudes in neurons dissociated from the acutely dissected MPG with those recorded in neurons dissociated from MPG after explant culture for 72 h. As all neurons are axotomized in the explant cultured MPG, we predicted that the density of AChRs in neurons dissociated from cultured MPG should be less than that in neurons dissociated from the acutely isolated MPG.
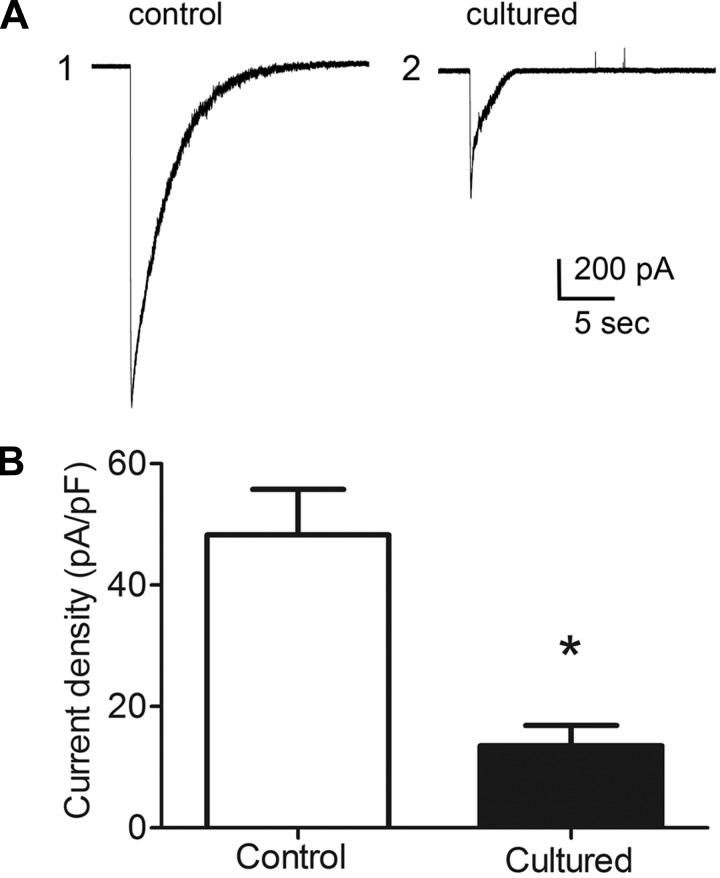
Neurons dissociated from the explant cultured and acutely isolated MPG had comparable resting membrane potentials (−50 ± 2.6 mV in 10 neurons from 4 acutely isolated ganglia and −51.0 ± 4.9 mV in 11 neurons from 4 cultured MPG), and all neurons in both groups exhibited robust action potential generation elicited by depolarizing pulses. Recordings were obtained from 10 cells dissociated from 4 freshly isolated MPG (control) and from 11 cells from 4 MPG that were maintained in explant culture for 72 h (cultured). ACh-induced currents, elicited by a puffer application of ACh to neurons voltage clamped to −50 mV, were significantly (P ≤ 0.0003) smaller in neurons dissociated from the cultured MPG than from neurons dissociated from the acutely isolated MPG (Fig. 7).
Fig. 7.
ACh-induced currents from neurons dissociated from the 3-day cultured MPG are significantly smaller than those recorded from neurons dissociated from the acutely isolated MPG. A: examples of current traces illustrating ACh-induced currents recorded from a neuron dissociated from an acutely isolated MPG (1) and from a neuron dissociated from a MPG cultured for 3 days (2). The capacitance of the two cells was similar (41 pF in 1 and 42 pF in 2). B: averaged value of ACh-induced currents from 10 neurons dissociated from the acutely isolated MPG and 11 neurons dissociated from the 3-day cultured MPG. ACh-induced currents are expressed as current density (in pA/pF) to take into account cell size. *P = 0.0003.
DISCUSSION
A key observation of the present study is that AChR subunit and PSD-93 transcript levels are significantly decreased in the MPG after cut or crush of the cavernous nerve and that the depression of transcript levels persists for more than a week. Similarly, AChR subunit and PSD-93 transcript levels were decreased after explant culture of the MPG. Furthermore, separate whole cell voltage-clamp recordings demonstrated that neurons dissociated from the cultured MPG had significantly smaller ACh-induced currents than those recorded from neurons dissociated from the acutely isolated MPG. These latter observations supported our hypothesis that a decrease in protein expression accompanied the decrease in transcript levels, a view consistent with all other ganglia studied to date. These observations provide new results for the MPG demonstrating that decreases in AChR subunit expression after cavernous nerve injury could contribute to the loss of neural control of visceral organs after pelvic surgeries. Our results also show, for the first time, that even a simple nerve bundle manipulation without noticeable injury can lead to diminished α3-subunit and PSD-93 transcript expression. A decrease in the expression of the α3-subunit is particularly relevant to fast synaptic transmission because this subunit contains the ACh-binding site and PSD-93 expression is critical for the stability of nAChRs in the postsynaptic membrane.
In a recent study (5), castration was shown to decrease nicotinic receptor expression in rat MPG neurons. The decreased receptor expression was evident from reduced transcript levels and ACh-evoked currents. Also, the diminished receptor responsiveness was more evident in the parasympathetic neurons within the MPG, and the authors suggested that downregulation of AChR in these neurons, due to a loss of testosterone after castration, likely contributed to the loss of erectile function in these animals.
A similar correlation between a decrease in AChR subunit transcript levels and diminished ACh-induced currents was noted in the present study after explant culture of the MPG for 72 h. We tested ACh responsiveness in neurons from the culture model because the axons of all postganglionic neurons are severed and thus would elicit an axotomy response. We recognize that in the culture injury model, the preganglionic inputs are also severed. However, it has been previously shown that the increase in galanin, activating transcription factor 3, or AChR subunit expression noted after axotomy is not recapitulated by cutting the preganglionic innervation to the SCG, an indication that increased expression of these molecules is due to axotomy rather than elimination of preganglionic inputs (12, 17, 25, 26). We suggest that the decreased expression of the AChR seen after explant culture of the MPG also is primarily due to axotomy rather than a loss of preganglionic innervation. We further suggest that the decrease in ACh-induced currents noted in neurons after explant culture provides evidence supporting our working hypothesis that a similar decrease in AChR expression and diminished ACh-induced response occurs in vivo after cavernous nerve injury and possibly after simple nerve manipulation with minimal axonal damage. This hypothesis will be tested further in future studies.
For the whole cell recordings made as part of the present study, no attempt was made to identify whether the MPG neurons tested were parasympathetic or sympathetic postganglionic MPG neurons. Rather, cells were randomly selected based on their suitability for patch formation. In the rat MPG, parasympathetic and sympathetic postganglionic neurons have identifying characteristics (5). To date, we have not determined the electrical or chemical characteristics that would allow identification of mouse MPG neurons as being either parasympathetic or sympathetic postganglionic. The purpose of the present experiments was to test whether a decrease in ACh response was correlated with decreased expression of AChR transcripts. We understand that the explant cultured whole mount can only be considered as a first approximation for the in vivo system. Furthermore, we recognize that our present results, obtained using the in vitro injury model, will need to be confirmed in future studies of the changes that occur after axotomy in vivo.
The surgical procedures in the present study focused on the response of MPG neurons to injury or manipulation of the cavernous nerve. However, only a subset of MPG neurons contributes axons that traverse through the cavernous nerve to their targets (7), and, as we have not identified the affected neurons, we compared changes in overall transcript levels. We also suggest that most of the affected neurons were parasympathetic MPG neurons as sympathetic fibers traveling in the cavernous nerve are suggested to arise from neurons residing outside of the MPG (7). Significant changes in transcript levels were evident in extracts of the entire MPG even though not all MPG neurons were affected, suggesting that the decreases in individual affected neurons were more extensive than those shown for the whole neuronal population. This would explain why the decrease in transcript levels was greater after explant culture than nerve injury. All MPG neurons were axotomized with explant culture. An unexpected result was that after nerve manipulation only, α3 and PSD-93 transcript levels were also significantly depressed for prolonged periods. As the α3-subunit contains the ACh-binding site, a decrease in α3-subunit protein expression could lead to synaptic depression as well after nerve manipulation.
After explant culture, transcript levels for all AChR subunits were significantly decreased. In contrast, α7 transcript levels were not significantly decreased after any of the surgical procedures on the cavernous nerve. At present, we can only speculate about what was responsible for this difference. One possibility is that for neurons in the MPG, expression of the α7-subunit is not affected by nerve injury to the same extent as occurs in the submandibular ganglia (9). Alternatively, expression of the α7-subunit could be greater on neurons that do not project through the cavernous nerve. Thus, with cavernous nerve injury, little change in the expression of α7 transcript levels would be noted, whereas when all neurons are axotomized, as occurs with explant culture, the change would be significant. In the freshly dissected, unfixed male MPG, α-bungarotoxin, which specifically labels α7-subunits, binds to both tyrosine hydroxylase-immunoreactive neurons and those not tyrosine hydroxylase immunoreactive, an indication that both neuron types express α7-subunits in the cell membrane (Dr. Rae Nishi, personal communication). However, α-bungarotoxin binding has not been quantified for the subpopulations of neurons projecting to different urogenital targets.
In previous in vivo axotomy or explant culture studies, application of exogenous nerve growth factor partially blunted the decrease in SCG AChR subunit expression (25). Consequently, we tested whether loss of neurturin, suggested to be a key trophic molecule regulating parasympathetic neurons (22, 23), might be a mechanism signaling the downregulation of nicotinic receptors and their scaffolding protein. This was tested by the addition of neurturin to the media bathing the MPG whole mounts. As shown in Fig. 2, the presence of neurturin had no effect on AChR subunit or PSD-93 transcript levels.
Galanin transcript levels increased in the cultured MPG and in the MPG after cavernous transection or crush, as would be expected after nerve injury or axotomy. In contrast, no increase in galanin transcript level was found after cavernous nerve manipulation. Also, there was no obvious visual evidence of nerve injury with the dissection/stretch procedure. The lack of change in galanin transcript levels provides independent support for the view that the nerve was not injured by the cavernous nerve manipulation procedure.
In conclusion, the present results indicate that a decrease in AChR subunit expression in parasympathetic postganglionic MPG neurons results after cavernous nerve injury and, to some extent, even after manipulation without significant nerve fiber damage. Consistent with results reported for all other ganglia studied to date, this could lead to a marked depression of synaptic transmission to penile projecting postganglionic neurons, whose axons traverse within the cavernous nerve. Thus, we suggest that a loss of synaptic transmission very likely is a factor contributing to the loss of neural control of urogenital function after surgical procedures that cut or crush cavernous nerve fibers.
GRANTS
This work was supported in part by National Institutes of Health Grants P20-RR-16435 and P30-RR-032135/P30-GM-103498 (to R. L. Parsons), DK-051369, DK-060481, and DK-065989 (to M. A. Vizzard), and DK-081444 (to J. D. Tompkins).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: B.M.G. and L.A.M. performed experiments; B.M.G. and L.A.M. analyzed data; B.M.G. and L.A.M. prepared figures; B.M.G., L.A.M., J.D.T., M.A.V., and R.L.P. edited and revised manuscript; B.M.G., L.A.M., J.D.T., M.A.V., and R.L.P. approved final version of manuscript; J.D.T., M.A.V., and R.L.P. conception and design of research; M.A.V. and R.L.P. interpreted results of experiments; R.L.P. drafted manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Rae Nishi for providing primers for the α3- and α7-subunits.
REFERENCES
- 1.Boeshore KL, Schreiber RC, Vaccariello SA, Hyatt-Sachs H, Salazar R, Lee J, Ratan RR, Leahy P, Zigmond RE. Novel changes in gene expression following axotomy of a sympathetic ganglion: a microarray analysis. J Neurobiol 59: 216–235, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Burnett AL, Lue TF. Neuromodulatory therapy to improve erectile function recovery outcomes after pelvic surgery. J Urol 176: 882–887, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Girard BM, Galli JR, Vizzard MA, Parsons RL. Galanin expression in the mouse major pelvic ganglia during explant culture and following cavernous nerve transection. J Mol Neurosci 48: 713–720, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Girard BM, Galli JR, Young BA, Vizzard MA, Parsons RL. PACAP expression in explant cultured mouse major pelvic ganglia. J Mol Neurosci 42: 370–377, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang XZ, Park JT, Kim HG, Lee CK, Won YJ, Park BG, Jeong SW. Phenotype-specific down-regulation of nicotinic acetylcholine receptors in the pelvic ganglia of castrated rats: implications for neurogenic erectile dysfunction. Neurosci Lett 501: 55–59, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Hyatt-Sachs H, Schreiber Shoemaker SE, Sabe A, Reed E, Zigmond RE. Activating transcription factor 3 induction in sympathetic neurons after axotomy: response to decreased neurotrophin availability. Neuroscience 15: 887–897, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Keast JR. Plasticity of pelvic autonomic ganglia and urogenital innervation. Int Rev Cytol 248: 141–208, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Matthews MR, Nelson VH. Detachment of structurally intact nerve endings from chromatolytic neurones of rat superior cervical ganglion during the depression of synaptic transmission induced by post-ganglionic axotomy. J Physiol 245: 91–135, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCann CM, Tapia JC, Kim H, Coggan JS, Lichtman JW. Rapid and modifiable neurotransmitter receptor dynamics at a neuronal synapse in vivo. Nat Neurosci 11: 807–815, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Merriam LA, Barstow KL, Parsons RL. Pituitary adenylate cyclase-activating polypeptide (PACAP) enhances the hyperpolarization activated nonselective cationic conductance, Ih, in dissociated guinea pig intracardiac neurons. Regul Pept 13: 123–133, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Mohney RP, Siegel RE, Zigmond RE. Galanin and vasoactive intestinal peptide messenger RNAs increase following axotomy of adult sympathetic neurons. J Neurobiol 25: 108–118, 1994 [DOI] [PubMed] [Google Scholar]
- 12.Moller K, Reimer M, Ekblad E, Hannibal J, Fahrenkrug J, Kanje M, Sundler F. The effects of axotomy and preganglionic denervation on the expression of pituitary adenylate cyclase activating peptide (PACAP), galanin and PACAP type 1 receptors in the rat superior cervical ganglion. Brain Res 775: 166–182, 1997 [DOI] [PubMed] [Google Scholar]
- 13.Nja A, Purves D. The effects of nerve growth factor and its antiserum on synapses in the superior cervical ganglion of the guinea-pig. J Physiol 277: 53–75, 1978 [PMC free article] [PubMed] [Google Scholar]
- 14.Palma CA, Keast JR. Structural effects and potential changes in growth factor signalling in penis-projecting autonomic neurons after axotomy. BMC Neurosci 7: 41, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park KS, Cha SK, Kim MJ, Kim DR, Jeong SW, Lee JW, Kong ID. An α3β4 subunit combination acts as a major functional nicotinic acetylcholine receptor in male rat pelvic ganglion neurons. Pflügers Arch 452: 775–783, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Purves D. Functional and structural changes in mammalian sympathetic neurones following interruption of their axons. J Physiol 252: 429–463, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schreiber RC, Hyatt-Sachs H, Bennett TA, Zigmond RE. Galanin expression increases in adult rat sympathetic neurons after axotomy. Neuroscience 60: 17–27, 1994 [DOI] [PubMed] [Google Scholar]
- 18.Tompkins JD, Parsons RL. Exocytotic release of ATP and activation of P2X receptors in dissociated guinea pig stelate neurons. Am J Physiol Cell Physiol 291: C1062–C1071, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Tompkins JD, Vizzard MA, Parsons RL. Synaptic transmission at parasympathetic neurons of the major pelvic ganglia from normal and diabetic male mice. J Neurophysiol 109: 988–995, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsujino H, Kondo E, Fukuoka T, Dai Y, Tokunaga A, Miki K, Yonenobu K, Ochi T, Noguchi K. Activating transcription factor 3 (ATF3) induction by axotomy in sensory and motoneurons: a novel neuronal marker of nerve injury. Mol Cell Neurosci 15: 170–182, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Wanigasekara Y, Kepper ME, Keast JR. Immunohistochemical characteristics of pelvic autonomic ganglia in mice. Cell Tissue Res 311: 175–185, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Wanigasekara Y, Keast JR. Neurturin has multiple neurotrophic effects on adult rat sacral parasympathetic ganglion neurons. Eur J Neurosci 22: 595–604, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Yan H, Keast JR. Neurturin regulates postnatal differentiation of parasympathetic pelvic ganglion neurons, initial axonal projections, and maintenance of terminal fields in male urogenital organs. J Comp Neurol 507: 1169–1183, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Young BA, Girard BM, Parsons RL. Neurturin suppresses injury-induced neuronal activating transcription factor 3 (ATF-3) expression in cultured guinea pig cardiac ganglia. J Comp Neurol 508: 795–805, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Zhou Y, Deneris E, Zigmond RE. Differential regulation of levels of nicotinic receptor subunit transcripts in adult sympathetic neurons after axotomy. J Neurobiol 34: 164–178, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Zigmond RE, Vaccariello SA. Activating transcription factor 3 immunoreactivity identifies small populations of axotomized neurons in rat cervical sympathetic ganglia after transection of the preganglionic cervical sympathetic trunk. Brain Res 1159: 119–123, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]