Abstract
Idiopathic uric acid nephrolithiasis is characterized by elevated urinary net acid excretion and insufficient buffering by ammonium, resulting in excessively acidic urine and titration of the relatively soluble urate anion to insoluble uric acid. Patients with type 2 diabetes have similar changes in urinary pH, net acid excretion, and ammonium in 24-h urine collections at baseline, even after controlling for dietary factors, and are at increased risk for uric acid nephrolithiasis. However, not all patients with type 2 diabetes develop kidney stones, suggesting that uric acid stone formers may have additional urinary defects, perhaps not apparent at baseline. We performed a metabolic study of 14 patients with idiopathic uric acid nephrolithiasis, 13 patients with type 2 diabetes, and 8 healthy control subjects of similar body mass index. After equilibration on a fixed diet for 5 days, subjects were given a single oral acid load (50 meq ammonium chloride), and urine was collected hourly for 4 h. Uric acid stone formers had a lower ammonium excretory response to acute acid loading compared with diabetic and nondiabetic nonstone formers, suggesting that an ammonium excretory defect unique to uric acid stone formers was unmasked by the acid challenge. The Zucker diabetic fatty rat also did not show impaired urinary ammonium excretion in response to acute acid challenge. A blunted renal ammonium excretory response to dietary acid loads may contribute to the pathogenesis of idiopathic uric acid nephrolithiasis.
Keywords: kidney stones, diabetes, obesity
the burden of kidney stone disease in the United States has steadily increased over the past decades, with the most recent prevalence estimates surpassing 8% of the adult population (8, 18) and with up to 1 in 10 analyzed stones composed of uric acid (7, 11). The term “idiopathic uric acid nephrolithiasis” (IUAN) is applicable to the vast majority of uric acid stone cases, which are not secondary to an identifiable primary disorder such as gout, chronic diarrhea, neoplasms, or known genetic mutations (13). The pathophysiologic underpinnings of IUAN are incompletely understood, but one key intermediate determinant of uric acid stone risk is low urinary pH, leading to titration of the relatively water-soluble urate anion to poorly soluble uric acid (13). Unduly acidic urine in patients with IUAN has been attributed to a combination of dietary acid excess, diet-independent increased net acid excretion (NAE), and lower proportion of NAE excreted as ammonium, the principal urinary buffer (3, 16).
Both type 2 diabetes (T2DM) and obesity are associated with low urinary pH and increased risk for IUAN (5, 6, 9). Based on 24-h urine data obtained under controlled dietary conditions, patients with T2DM but no kidney stones have NAE and ammonium excretion defects of similar magnitude as uric acid stone formers (9, 16). Since clearly not all patients with T2DM and/or obesity develop stones, we hypothesized that uric acid stone formers have more profound urinary acidification defects that are not discernible at baseline, additional factors not present in diabetic nonstone formers, or both.
To test this hypothesis, we used an experimental acute acid load to challenge the renal acid excretion machinery in body mass index (BMI)-matched patients with IUAN, nonstone formers with T2DM, and healthy volunteers after equilibration on a fixed metabolic diet. We had previously piloted this technique to amplify preexisting differences in ammonium excretion between patients with IUAN and nonstone-forming control subjects without controlling for body size or diabetes status (17).
METHODS
Human subjects and definitions.
We studied a total of 35 subjects in 3 groups. Patients with IUAN without T2DM (n = 14) were recruited from the Kidney Stone Clinic at the University of Texas Southwestern Medical Center. BMI-matched nonstone formers with diagnosed T2DM (n = 13) and healthy control subjects (nondiabetic nonstone formers, n = 8) were recruited through local advertisements. IUAN was defined by stone analysis, in the absence of any identifiable underlying etiology. Only patients with stones composed of 100% uric acid (no mixed stones) were included. Nonstone formers were individuals with no confirmed or suspected history of kidney stones of any type. Included in the study were subjects over 30 yr of age of any race/ethnicity and sex. Eligibility for the study was determined during a screening visit including medical history, examination, fasting serum biochemistry, urinalysis, and pregnancy testing for women. Exclusion criteria for all groups were pregnancy, weight loss of >4.5 kg during the preceding 6 mo, recurrent urinary tract infections, chronic diarrhea, myeloproliferative disorders, genetic kidney diseases, creatinine clearance of <70 ml/min, proteinuria, treatment with alkali, ANG II receptor antagonists, uricosuric medications, insulin or thiazolidinediones, and any acute illness at the time of the study. Subjects taking diuretics were instructed to stop the medication 10 days before beginning the study. Subjects with diagnosed diabetes mellitus or fasting plasma glucose of ≥126 mg/dl were excluded from the uric acid stone former group and from the control group of nondiabetic nonstone former group.
Human study protocol.
This study was approved by the Institutional Review Board of the University of Texas Southwestern Medical Center, and all participants provided informed consent. The duration of the study was 6 days for each subject, with 3 days as an outpatient and 3 days as an inpatient in the University of Texas Southwestern Medical Center Clinical and Translational Research Center (CTRC). During the outpatient phase, subjects were equilibrated on a frozen metabolic diet containing 30% calories from fat, 55% from carbohydrate, and 15% from protein (9). Subjects were then admitted to the CTRC and provided the same metabolic diet during the inpatient phase. While in the CTRC, each subject provided two 24-h urine collections under mineral oil. Urine samples were stored at 4°C and processed within 24 h for measurements of total volume, pH, sodium, potassium, calcium, magnesium, phosphorus, chloride, sulfate, oxalate, citrate, bicarbonate, uric acid, ammonium, titratable acidity (TA), and creatinine. Two fasting blood samples were obtained for the measurements of sodium, potassium, chloride, bicarbonate, uric acid, creatinine, glucose, triglycerides, and cholesterol. All reported baseline urine and serum values represent the average of two independent measurements from samples obtained on consecutive days from the same individual. An ammonium chloride loading test (17) was performed in the morning of the last day of the inpatient study after a 12-h overnight fast. Subjects voided at 7:00 AM, and hourly urine samples were collected from 8:00 AM to 12:00 PM under mineral oil, stored at 4°C, and processed within 24 h for measurement of total volume, pH, creatinine, ammonium, TA, and bicarbonate. Immediately after the first hourly collection, 50 meq ammonium chloride gelatin capsules were administered orally with 250 ml water in the presence of CTRC staff.
Animal study protocol.
The use of animals in this study was in strict accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (8th ed., National Academy Press, 2011), and the study protocol was approved by the Institutional Animal Care and Use Committee of the University of Texas Southwestern Medical Center. Sixteen male Zucker diabetic fatty (ZDF; fa/fa) rats and lean control littermates (Charles River Laboratories, Wilmington, MA) were studied at 16 wk of age, when ZDF rats have established T2DM and baseline urinary biochemical abnormalities similar to those of human uric acid stone formers (1, 2). For baseline urine biochemistry, 24-h urine collections were obtained from pair-fed (LabDiet 5008 rodent chow, Purina Mills, St. Louis, MO) rats individually housed in metabolic cages with free access to water. For the acute acid loading experiment, a single dose of ammonium chloride (10 mmol/kg) was given by orogastric gavage, with urine collected in metabolic cages at baseline and at 4 and 8 h after the acid load in fasting animals. To minimize experimental variability associated with putative circadian differences in ammoniagenesis or ammonium transport, ammonium chloride loading experiments were performed on 4 consecutive days with two ZDF and two lean animals studied on each day at the same time of day.
Analytic procedures and calculations.
Serum measurements were performed by Quest Diagnostics (Madison, NJ). The methodology for urine biochemistry has been previously described in detail (17). Briefly, pH and Pco2 were measured with electrodes, creatinine with the Jaffe alkaline picrate assay, ammonium using the glutamate dehydrogenase method, citrate with the citrate lyase assay, and uric acid by the urate oxidase method from an alkalinized urine aliquot to prevent uric acid precipitation. TA was measured using an automated burette end-point titration system (Radiometer, Copenhagen, Denmark) in a urine aliquot, as the amount of OH− (in meq) required to bring the original pH of the aliquot to 7.4, with the resulting value used to calculate TA for the entire volume of the 24-h collection. NAE was calculated as the sum of 24-h urine citrate + bicarbonate subtracted from the sum of TA + ammonium (all expressed in meq).
Statistical analyses.
The Kruskal-Wallis test was used to compare continuous variables between the three study groups, and Student's t-test was used for pairwise comparisons. The response to ammonium chloride loading was analyzed with mixed-model repeated-measures analysis. Analysis of covariance was used to adjust for demographic characteristics (age and race/ethnicity). Student's t-test was used to evaluate statistical significance in the animal study. Results are reported as means ± SD unless otherwise noted. Two-sided P values of <0.05 were considered statistically significant. Statistical analyses were performed using SAS 9.3 (SAS Institute, Cary, NC).
RESULTS
Characteristics of subjects.
Demographic characteristics of the three study groups are shown in Table 1. Subjects were matched for BMI, with the majority classifiable as obese (BMI ≥ 30), and had similar height and body mass across groups. Patients with IUAN were of similar age as those with T2DM and no kidney stones but were overall older than control subjects. Uric acid stone formers were numerically more often male and white, but these differences were not statistically significant. Age and race/ethnicity were not significant covariates for renal ammonium excretory response to acid loading, and separate analyses conducted with adjustment for these factors yielded similar results as the unadjusted analyses (not shown). Similarly, separate analyses conducted only in men across the three groups yielded similar results as analyses including both sexes.
Table 1.
Demographic data
| Control Group | UASF Group | T2DM Group | P Value (by Kruskal-Wallis Test) | |
|---|---|---|---|---|
| Number of subjects | 8 | 14 | 13 | |
| Height, cm | 170 ± 12 | 173 ± 6 | 175 ± 11 | 0.71 |
| Body mass, kg | 94 ± 14 | 102 ± 18 | 102 ± 25 | 0.67 |
| Body mass index, kg/m2 | 32 ± 7 | 34 ± 6 | 33 ± 6 | 0.81 |
| Age, yr | 47 ± 5 | 57 ± 10* | 53 ± 9 | 0.04 |
| Sex, men/women | 4/4 | 13/1 | 8/5 | 0.07 |
| Race, nonhispanic white/hispanic/black | 5/0/3 | 11/1/2 | 6/3/4 | 0.21 |
Data are means ± SD. T2DM, type 2 diabetes mellitus.
Statistical significance by pairwise comparison for uric acid stone former (UASF) versus control groups (P < 0.05).
Baseline plasma/serum chemistry.
There were no significant differences in plasma glucose and serum triglycerides, cholesterol, and uric acid between groups (Table 2). There were no significant intraindividual or within-group differences in the biochemistry of the consecutive study samples, and no significant differences between study visit and screening visit serum biochemistry. The unadjusted differences in serum creatinine between groups were no longer statistically significant after adjustment for age and sex.
Table 2.
Baseline plasma/serum biochemistry
| Control Group | UASF Group | T2DM Group | P Value (by Kruskal-Wallis Test) | |
|---|---|---|---|---|
| Sodium, meq/l | 137 ± 4 | 139 ± 2 | 139 ± 3 | 0.61 |
| Potassium, meq/l | 4.3 ± 0.3 | 4.1 ± 0.4* | 4.8 ± 0.7 | 0.03 |
| Chloride, meq/l | 105 ± 2 | 107 ±2*† | 104 ± 3 | 0.05 |
| Bicarbonate, meq/l | 29 ± 3 | 28 ± 3 | 27 ± 4 | 0.31 |
| Urate, mg/dl | 6.4 ± 1.3 | 7.6 ± 1.2 | 7.4 ± 1.5 | 0.15 |
| Creatinine, mg/dl | 1.0 ± 0.2 | 1.2 ± 0.2*† | 1.0 ± 0.2 | 0.01 |
| Glucose, mg/dl | 94 ± 12 | 94 ± 6 | 108 ± 26 | 0.36 |
| Triglycerides, mg/dl | 131 (25, 274) | 170 (95, 326) | 187 (83, 735) | 0.21 |
| Total cholesterol, mg/dl* | 209 ± 29 | 211 ± 58 | 199 ± 53 | 0.64 |
| LDL-cholesterol, mg/dl | 139 ± 31 | 136 ± 54 | 116 ± 38 | 0.29 |
| HDL-cholesterol, mg/dl | 44 ± 15 | 37 ± 9 | 39 ± 7 | 0.60 |
Data are means ± SD or medians (interquartile ranges). For total cholesterol, cholesterol-lowering medications were used by 1 of 8 subjects in the control group, 1 of 14 patients in the UASF group, and 4 of 13 patients in the T2DM group.
Statistical significance by pairwise comparison for UASF vs. T2DM groups (P < 0.05);
statistical significance by pairwise comparison for UASF vs. control groups (P < 0.05).
Baseline 24-h urine chemistry.
After equilibration on a fixed metabolic diet, patients with IUAN and nonstone formers with T2DM had lower 24-h urinary pH, higher daily NAE, lower citrate and bicarbonate, and a lower proportion of net acid excreted as ammonium (ammonium/NAE) compared with control subjects (Table 3). There were no significant differences in creatinine clearance and in the daily excretion of uric acid and other measured anions and cations across groups, with the exception of sulfate, which was lower in patients with T2DM.
Table 3.
Baseline 24-hour urine biochemistry
| Control Group | UASF Group | T2DM Group | P Value (by Kruskal-Wallis Test) | |
|---|---|---|---|---|
| Total volume, liters | 2.7 ± 0.2 | 2.6 ± 0.5 | 2.5 ± 0.4 | 0.42 |
| pH | 5.92 ± 0.40 | 5.49 ± 0.31† | 5.41 ± 0.25‡ | 0.01 |
| NH4+, meq/day | 36 ± 8 | 33 ± 13 | 38 ± 6 | 0.34 |
| NAE, meq/day | 44 ± 15 | 57 ± 16 | 62 ± 16‡ | 0.06 |
| NH4+/NAE | 0.9 ± 0.2 | 0.6 ± 0.1† | 0.6 ± 0.1‡ | 0.002 |
| Citrate, meq/day | 10 ± 3 | 6 ± 4† | 7 ± 4 | 0.02 |
| Bicarbonate, meq/day | 3.9 ± 3.0 | 0.8 ± 1.5† | 0.8 ± 1.7‡ | 0.008 |
| Sulfate, meq/day | 42 ± 5 | 42 ± 5* | 36 ± 6‡ | 0.02 |
| Total uric acid, mg/day | 538 ± 136 | 494 ± 177 | 547 ± 138 | 0.47 |
| Oxalate, mg/day | 23 ± 3 | 26 ± 4 | 28 ± 6‡ | 0.10 |
| Phosphorus, mg/day | 594 ± 163 | 706 ± 166 | 589 ± 254 | 0.17 |
| Chloride, meq/day | 94 ± 19 | 92 ± 23 | 78 ± 16 | 0.10 |
| Sodium, meq/day | 101 ± 31 | 97 ± 31 | 73 ± 30 | 0.10 |
| Potassium, meq/day | 36 ± 4 | 35 ± 9 | 37 ± 13 | 0.91 |
| Calcium, mg/day | 185 ± 76 | 123 ± 54 | 139 ± 86 | 0.14 |
| Magnesium, mg/day | 97 ± 24 | 90 ± 26 | 72 ± 21 | 0.10 |
| Creatinine clearance, ml/min | 109 ± 25 | 111 ± 31 | 116 ± 24 | 0.31 |
Data are means ± SD.
NAE, net acid excretion.
Statistical significance by pairwise comparison for UASF vs. T2DM groups (P < 0.05);
statistical significance by pairwise comparison for UASF vs. control groups (P < 0.05);
statistical significance by pairwise comparison for T2DM vs. control groups (P < 0.05).
Renal ammonium excretory response to an acute acid load.
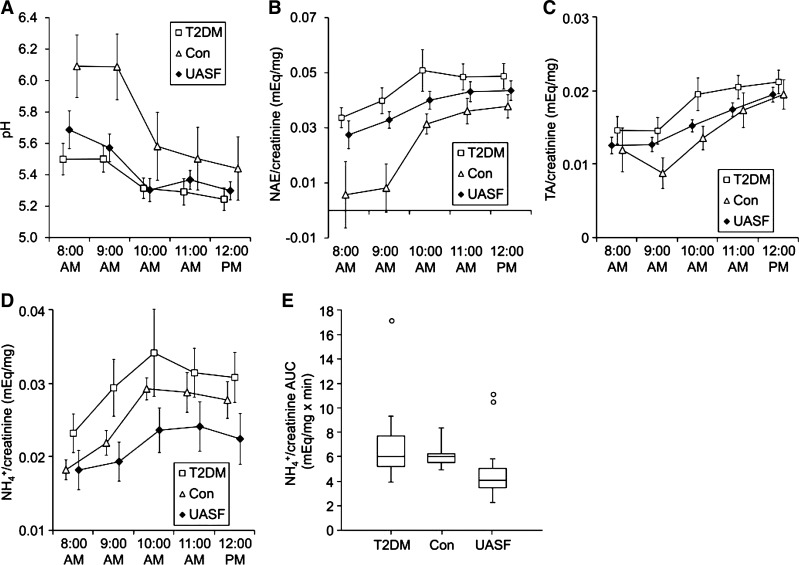
Because there were no detectable differences in urinary pH or ammonium excretion at baseline between uric acid stone formers and nonstone formers with T2DM, we used a standard acute acid loading protocol to challenge the renal ammonium excretory capacity and unmask potential differences that were not evident at baseline. The acid load test was performed after 5 days of equilibration on a fixed metabolic diet, including 2 days as inpatients. As shown in Fig. 1A, urinary pH was consistently lower in uric acid stone formers and in patients with T2DM compared with control subjects (P < 0.05 for each of the first three hourly collections), although the difference was no longer statistically significant at the end of the experiment. Urinary NAE was similar in uric acid stone formers and in patients with T2DM (although numerically higher in the latter) for all hourly collections, was lower at baseline in control subjects (P < 0.05 vs. patients with T2DM), and reached comparable levels in all three groups by the end of the experiment (Fig. 1B). Throughout the study, TA (Fig. 1C) was numerically lower in control subjects compared with the other two groups, and citrate (not shown) was numerically higher, but these differences did not reach statistical significance. Uric acid stone formers had the lowest ammonium excretory response to the acute acid load (Fig. 1D), and the area under the ammonium excretory response curve was significantly lower in uric acid stone formers compared with each group of nonstone formers (Fig. 1E).
Fig. 1.
Urinary pH, net acid excretion (NAE), titratable acidity (TA), and ammonium after an acute acid load. Uric acid stone formers (UASFs), nonstone-forming patients with type 2 diabetes (T2DM), and nondiabetic nonstone-forming control (Con) subjects were equilibrated on a fixed metabolic diet for 5 days, including 2 days as inpatients. Five hourly urine samples were collected on day 6 after a 12-h overnight fast. Immediately after the first collection (8:00 AM), subjects were given a single oral dose of NH4Cl (50 meq). A: hourly urine pH. B: hourly urine net acid excretion (NAE)/creatinine. C: hourly urine titratable acidity (TA)/creatinine. D: hourly urine NH4+/creatinine. Data in A–D are presented as means ± SE. E: area under the curve (AUC) for urine NH4+/creatinine [box represents the median and interquartile range (IQR); whiskers represent the entire data range except for data points of >3 × IQR away from the box, which are plotted individually]. P < 0.05 for NH4+/creatinine AUC comparisons of UASF vs. T2DM groups and UASF vs. Con groups.
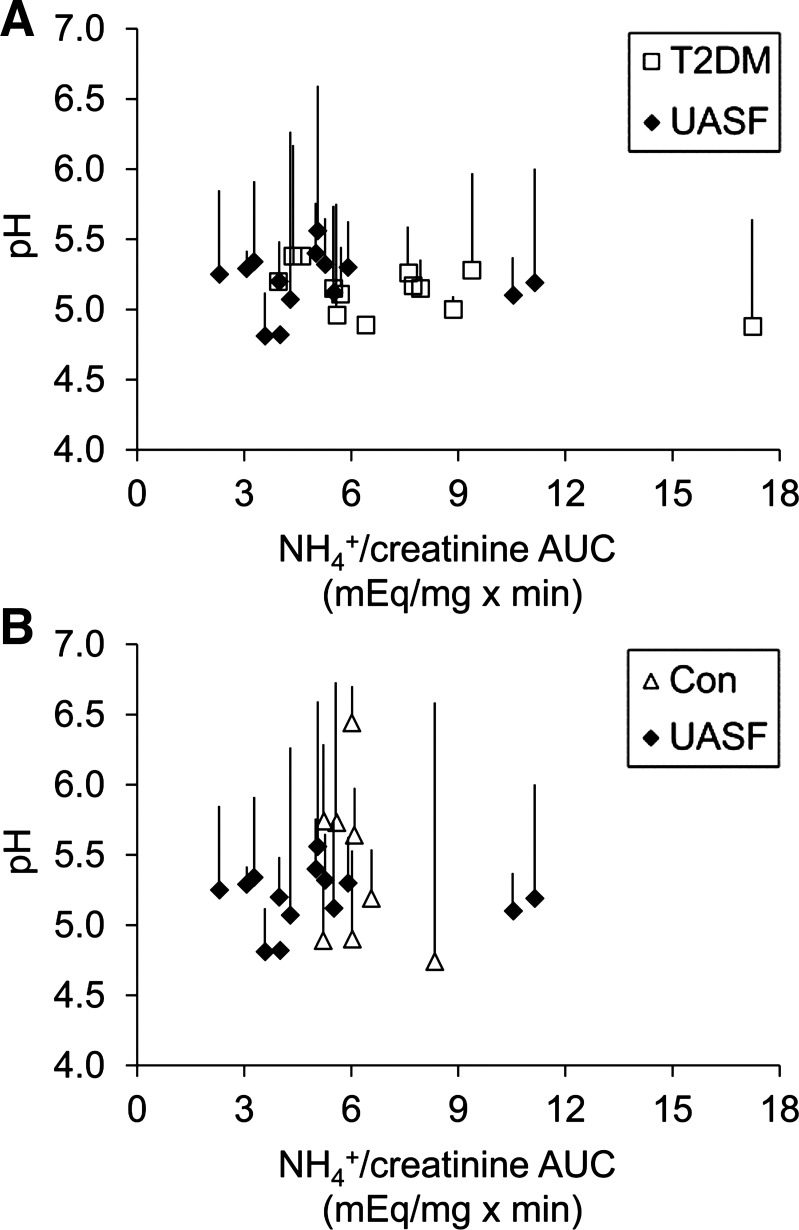
Figure 2, A and B, shows the ammonium excretory response and corresponding urine pH change for each individual subject, with the two groups of nonstone formers plotted separately for clarity. All but two uric acid stone formers are clustered to the left on the x-axis, representing low urinary ammonium excretion responses. Minimum pH reached after the acid load (symbols) and pH drop from baseline (lines) were not statistically different between the groups, but there was a numerical trend for greater pH drop in uric acid stone formers compared with patients with T2DM (Fig. 2A).
Fig. 2.
Urinary pH and NH4+ after an acute acid load in each individual subject. The AUC for urine NH4+/creatinine was plotted against urinary pH. Each symbol represents the lowest urinary pH measured after acute acid load in one subject. Lines indicate the change from starting (baseline) urinary pH. The two groups of nonstone formers were plotted separately for clarity. A: UASFs versus nonstone-forming patients with T2DM. B: UASF versus nondiabetic nonstone forming Con subjects.
As diminished ammonium excretion has been described in aging, we examined the relationship between the area under the ammonium excretory response curve and age across the three groups but did not observe any age dependence (data not shown).
Acute ammonium excretory response in ZDF rats.
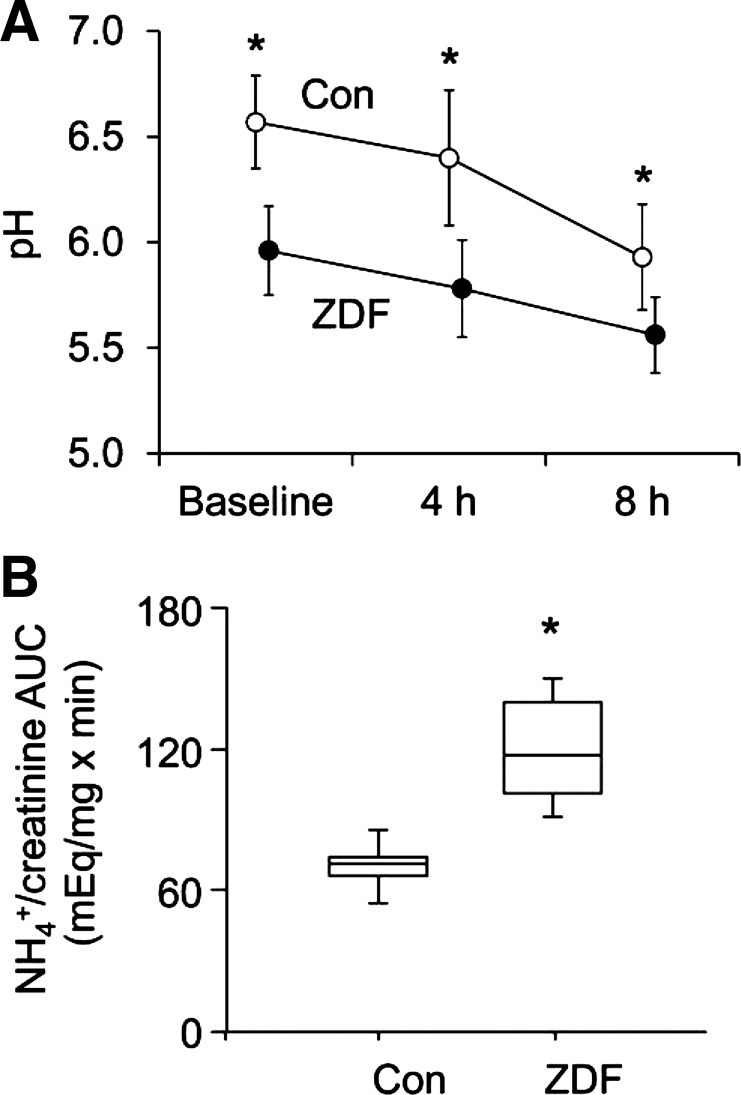
Since further investigation into the cellular and molecular mechanisms of an impaired ammonium excretory response in humans is limited by inaccessibility of kidney tissue, we next sought to reproduce the effects of acute acid loading in an established rodent model. Similar to humans with uric acid nephrolithiasis, urinary pH was consistently lower in ZDF versus control animals, both at baseline and after a standard acute acid load (10 mmol/kg ammonium chloride by orogastric gavage; Fig. 3A). However, in contrast with human uric acid stone formers, ZDF rats mounted a robust renal ammonium excretory response to the acid load, exceeding that of control animals (Fig. 3B).
Fig. 3.
Acute acid load in rats. Zucker diabetic fatty (ZDF) rats and Con littermates were housed in metabolic cages. After a baseline urine collection, rats were given a single dose of NH4Cl (10 mmol/kg) by orogastric gavage, and urine was collected every 4 h. A: urinary pH (means ± SD). *P < 0.01 for Con vs. ZDF rats. B: AUC for urine NH4+/creatinine (median, IQR, and data range). *P < 0.001 for Con vs. ZDF rats.
DISCUSSION
We conducted a detailed metabolic study comparing patients with IUAN, nonstone formers with T2DM, and control subjects, with the primary aim of uncovering novel factors that may contribute to uric acid stone risk. The three groups were matched for BMI, because body mass and BMI have been inversely associated with baseline urine pH and stone risk in previous studies (10, 19), and were examined under carefully controlled dietary conditions, to exclude any confounding effects related to variable nutrient intake.
Key findings and interpretations.
Although uric acid stone formers and nonstone formers with T2DM had similar urinary acidification defects compared with control subjects at baseline when averaged over 24 h, an ammonium excretory defect unique to uric acid stone formers was unmasked using an acute acid challenge. Renal ammonium excretion is the net result of ammonium production in proximal tubule mitochondria followed by several steps of transepithelial, largely protein-mediated transport along the nephron (4). It is possible (although not proven) that one or more components of this complex machinery may function close to capacity in uric acid stone formers, with little physiological reserve for upregulation.
Strengths and limitations.
A total of 35 subjects were equilibrated for 5 days on a fixed metabolic diet, including 2 days as inpatients, before the acid challenge. The number of subjects included in each of the three groups was relatively small but was sufficient to provide power to detect significant differences in urinary biochemical parameters both at baseline and after the acid load. Matching for age, sex, and race/ethnicity between groups was not ideal, but separate analyses with adjustment for these factors yielded similar results as the unadjusted analyses. Importantly, our data showed an association between IUAN and a blunted ammonium excretory response to acute acid loading, but whether this defect has a direct causal role in uric acid stone formation requires further study. Additional research should also test whether our findings in nondiabetic uric acid stone formers can be extrapolated to patients with concomitant IUAN and T2DM, not included in this study.
Comparison with previous clinical studies.
The lower baseline (24-h urine) pH, higher NAE, and lower ammonium/NAE in uric acid stone formers and in patients with T2DM compared with control subjects in this study are consistent with previous reports (9, 17). Our ammonium chloride loading data build on a previous study (17) comparing obese uric acid stone formers with leaner controls, by demonstrating defective ammonium response to acute acid loading in uric acid stone formers even after a match for BMI. The present results are also consistent with a previous circadian study (3) of patients with IUAN versus nonstone formers fed a fixed metabolic diet, showing lower urinary pH and ammonium excretion in IUAN throughout the entire day. However, the purpose of that study was not to discern the effect of meals per se on urine acidification, and the metabolic meals provided to study participants were not designed to provide a high acid load.
Comparison with previous bench studies.
The ZDF rat, an established model of obesity and T2DM, is the best-studied rodent model with a urinary biochemical profile similar with that of patients with IUAN, including low urine pH and inadequate ammonium excretion compared with lean control rats (1). In previous studies (1, 2) using ZDF rats and a cell culture model of the proximal tubule, we have shown that renal lipid accumulation and lipotoxicity may play a causal role in impaired ammonium excretion. In the present study, ZDF rats responded with a robust increase in ammonium excretion after an experimental acute acid load, unlike uric acid stone formers. We infer that the ZDF rat is appropriate for further studies examining the mechanisms of disturbed urinary acidification in obesity and T2DM but may not be adequate for the study of defects that are unique to uric acid stone formers, such as an impaired ammonium excretory response to acute acid loading.
Pathophysiological significance.
While most people will not ingest ammonium chloride capsules in their day-to-day life, occasional intake of large meals providing high acid content (such as meats and hard cheeses) may present physiologically equivalent or even greater acute acid loads to the kidney (14, 15). We used ammonium chloride rather than a large acid-providing meal in this study to minimize potential experimental variability from interindividual differences in digestion, absorption, metabolism of acid precursors, and endocrine responses (e.g., insulin) to a caloric load. Our finding of a blunted ammonium excretory response to ammonium chloride loading in uric acid stone formers suggests that similar changes in urinary milieu may also occur in these patients after dietary acid ingestion, creating transient conditions that are conducive to uric acid precipitation.
Of note, it is evident from the results shown in Figs. 1E and 2 that two patients with IUAN had distinctly better ammonium excretion after the acute acid load compared with all other IUAN subjects. Although any interpretation of this finding in the context of our study is inherently speculative, it is possible that IUAN is more heterogeneous than one normally believes. A subset of patients with IUAN may respond well to an acid challenge, with uric acid stone risk in these patients attributable to other, yet-unidentified mechanisms.
These findings underscore the need for further research on the molecular mechanisms of impaired ammonium excretion in both IUAN and T2DM, from metabolic and genetic studies in humans to detailed animal studies of proximal tubule ammoniagenesis and transepithelial ammonium transport in various nephron segments.
Conclusions.
Most therapeutic approaches currently available for nephrolithiasis focus on kidney stones post factum, at ever-increasing financial costs. Understanding the pathophysiologic underpinnings of stone formation will allow for a more rational and economical approach, with a focus on prevention and causal rather than symptomatic treatment (12). The present study contributes to this overall objective by describing one important mechanism that may contribute to stone risk specifically in IUAN. Uric acid stone formers, but not patients with T2DM or normal volunteers of similar body size, have a blunted renal ammonium excretory response to an acute acid load. This defect is postulated to contribute to the pathogenesis of IUAN.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants R01-DK-081423 (to K. Sakhaee), K01-DK-090282 (to I. A. Bobulescu), R21-DK-097476 (to N. M. Maalouf), and R01-DK-041612 and R01-DK-091392 (O. W. Moe), the O'Brien Kidney Research Center (through NIH Grant P30-DK-07938), the Simmons Family Foundation, and the Charles and Jane Pak Foundation. The University of Texas Southwestern Clinical and Translational Research Center is supported in part by NIH Grant UL1-TR-000451.
DISCLOSURES
I. A. Bobulescu and N. M. Maalouf received research funding from Takeda Pharmaceutical Company Limited via the Investigator-Initiated Sponsored Research mechanism. O. W. Moe received an investigator-initiated GRIP grant from Genzyme. There was no funding and no involvement from any commercial entity in any aspect of this article.
AUTHOR CONTRIBUTIONS
Author contributions: I.A.B., N.M.M., and T.R.R. performed experiments; I.A.B., N.M.M., G.C., B.A.-H., O.W.M., and K.S. analyzed data; I.A.B., N.M.M., O.W.M., and K.S. interpreted results of experiments; I.A.B. prepared figures; I.A.B. drafted manuscript; I.A.B., N.M.M., O.W.M., and K.S. edited and revised manuscript; I.A.B., N.M.M., G.C., B.A.-H., T.R.R., O.W.M., and K.S. approved final version of manuscript; O.W.M. and K.S. conception and design of research.
ACKNOWLEDGMENTS
The authors are grateful for the skilled assistance of the nursing and technical staff at the University of Texas Southwestern Clinical and Translational Research Center, the Charles and Jane Pak Center for Mineral Metabolism and Clinical Research, and the Mineral Metabolism Laboratory, the expertise of John Poindexter for database management, and the technical assistance of Anthony Nguyen for animal experiments.
REFERENCES
- 1.Bobulescu IA, Dubree M, Zhang J, McLeroy P, Moe OW. Effect of renal lipid accumulation on proximal tubule Na+/H+ exchange and ammonium secretion. Am J Physiol Renal Physiol 294: F1315–F1322, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bobulescu IA, Dubree M, Zhang J, McLeroy P, Moe OW. Reduction of renal triglyceride accumulation: effects on proximal tubule Na+/H+ exchange and urinary acidification. Am J Physiol Renal Physiol 297: F1419–F1426, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cameron M, Maalouf NM, Poindexter J, Adams-Huet B, Sakhaee K, Moe OW. The diurnal variation in urine acidification differs between normal individuals and uric acid stone formers. Kidney Int 81: 1123–1130, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Curthoys NP. Renal ammonium ion production and excretion. In: Seldin and Giebisch's The Kidney Physiology and Pathophysiology (5th ed.), edited by Alpern RJ, Caplan M, Moe OW. San Diego, CA: Academic Press, 2013, p. 1995–2019. [Google Scholar]
- 5.Daudon M, Traxer O, Conort P, Lacour B, Jungers P. Type 2 diabetes increases the risk for uric acid stones. J Am Soc Nephrol 17: 2026–2033, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Daudon M, Lacour B, Jungers P. Influence of body size on urinary stone composition in men and women. Urol Res 34: 193–199, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Gault MH, Chafe L. Relationship of frequency, age, sex, stone weight and composition in 15,624 stones: comparison of resutls for 1980 to 1983 and 1995 to 1998. J Urol 164: 302–307, 2000 [PubMed] [Google Scholar]
- 8.Goldfarb DS. Increasing prevalence of kidney stones in the United States. Kidney Int 63: 1951–1952, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Maalouf NM, Cameron MA, Moe OW, Sakhaee K. Metabolic basis for low urine pH in type 2 diabetes. Clin J Am Soc Nephrol 5: 1277–1281, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maalouf NM, Sakhaee K, Parks JH, Coe FL, Adams-Huet B, Pak CY. Association of urinary pH with body weight in nephrolithiasis. Kidney Int 65: 1422–1425, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Mandel NS, Mandel GS. Urinary tract stone disease in the United States veteran population. II. Geographical analysis of variations in composition. J Urol 142: 1516–1521, 1989 [DOI] [PubMed] [Google Scholar]
- 12.Matlaga BR. Toward a better understanding of kidney stone disease: platinum priorities. Eur Urol 62: 166–167, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Pak CY, Sakhaee K, Peterson RD, Poindexter JR, Frawley WH. Biochemical profile of idiopathic uric acid nephrolithiasis. Kidney Int 60: 757–761, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Remer T. Influence of nutrition on acid-base balance–metabolic aspects. Eur J Nutr 40: 214–220, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Remer T, Manz F. Potential renal acid load of foods and its influence on urine pH. J Am Diet Assoc 95: 791–797, 1995 [DOI] [PubMed] [Google Scholar]
- 16.Sakhaee K. Recent advances in the pathophysiology of nephrolithiasis. Kidney Int 75: 585–595, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakhaee K, Adams-Huet B, Moe OW, Pak CY. Pathophysiologic basis for normouricosuric uric acid nephrolithiasis. Kidney Int 62: 971–979, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Scales CD, Jr, Smith AC, Hanley JM, Saigal CS. Urologic Diseases in America Project. Prevalence of kidney stones in the United States. Eur Urol 62: 160–165, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor EN, Curhan GC. Body size and 24-hour urine composition. Am J Kidney Dis 48: 905–915, 2006 [DOI] [PubMed] [Google Scholar]