Abstract
Sympathoactivation may be excessive during exercise in subjects with hypertension, leading to increased susceptibility to adverse cardiovascular events, including arrhythmias, infarction, stroke, and sudden cardiac death. The muscle metaboreflex is a powerful cardiovascular reflex capable of eliciting marked increases in sympathetic activity during exercise. We used conscious, chronically instrumented dogs trained to run on a motor-driven treadmill to investigate the effects of hypertension on the mechanisms of the muscle metaboreflex. Experiments were performed before and 30.9 ± 4.2 days after induction of hypertension, which was induced via partial, unilateral renal artery occlusion. After induction of hypertension, resting mean arterial pressure was significantly elevated from 98.2 ± 2.6 to 141.9 ± 7.4 mmHg. The hypertension was caused by elevated total peripheral resistance. Although cardiac output was not significantly different at rest or during exercise after induction of hypertension, the rise in cardiac output with muscle metaboreflex activation was significantly reduced in hypertension. Metaboreflex-induced increases in left ventricular function were also depressed. These attenuated cardiac responses caused a smaller metaboreflex-induced rise in mean arterial pressure. We conclude that the ability of the muscle metaboreflex to elicit increases in cardiac function is impaired in hypertension, which may contribute to exercise intolerance.
Keywords: exercise, pressor response, arterial pressure, vasoconstriction, ergoreceptors
dynamic exercise elicits workload-dependent increases in sympathetic activity, heart rate (HR), cardiac output (CO), and arterial blood pressure. The increases in arterial blood pressure during exercise may be exaggerated in hypertensive subjects, thereby increasing risk factors for cardiac arrhythmia, infarction, stroke, and sudden cardiac death (5, 10, 32, 49, 57). A potential source for the enhanced sympathoactivation in response to exercise in hypertension is the muscle metaboreflex, a powerful pressor response triggered by afferent nerve endings within the active skeletal muscle that respond to the accumulation of metabolites (1, 30, 39, 71). In humans, recent studies support (17, 56) and refute (50) enhanced metaboreflex responsiveness in hypertension. Using a decerebrate rat model, Smith and colleagues (38, 43, 44, 61) concluded that the increases in arterial pressure and renal sympathetic nerve activity in response to stimulation of muscle metaboreceptors are enhanced in spontaneously hypertensive rats (SHR) vs. normotensive (Wistar-Kyoto) controls.
Muscle metaboreflex-induced increases in arterial pressure during exercise can occur via increases in CO and/or peripheral vasoconstriction. The relative roles of each of these may be intimately dependent on how the reflex is activated and the contractile abilities of the heart. For example, in heart failure, the ability of the reflex to increase CO is markedly attenuated, yet the peripheral vasoconstrictor responses are enhanced (13, 22, 33). Furthermore, mechanisms mediating metaboreflex pressor responses may also depend on the type and intensity of the exercise, as well as whether the reflex is activated during exercise or during postexercise recovery (63). Also, comparison of responses across different groups of individuals or animals of different genetic strains can introduce further secondary complexities, which may complicate the conclusions.
In the present study we investigated the effects of hypertension on the strength and mechanisms of the muscle metaboreflex in a longitudinally designed experiment. We studied the same animals before and after induction of hypertension. The muscle metaboreflex was activated during mild dynamic exercise, a setting in which cardiac reserve is substantial (23). We found that the CO component of the reflex was markedly reduced after induction of hypertension. This restrained ability of the reflex to raise CO may contribute to exercise intolerance in hypertensive subjects.
METHODS
Seven adult female mongrel dogs (∼20–25 kg) were selected on the basis of their willingness to run on a motor-driven treadmill. There was no intent for selection based on sex. The protocols used in the present study were reviewed and approved by the Institutional Animal Care and Use Committee of Wayne State University and complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Each animal was instrumented via two sterile surgical procedures: left thoracotomy and left abdominal retroperitoneal surgery, in that order. The animals were initially sedated with acepromazine (0.4–0.5 mg/kg im) and anesthetized with a combination of ketamine and diazepam (5.0 and 0.22 mg/kg iv, respectively). Anesthesia was maintained with isoflurane gas (1–3%) following endotracheal intubation. Cefazolin (antibiotic, 30 mg/kg iv), carprofen (analgesic, 2.0 mg/kg iv), buprenorphine (analgesic, 0.01 mg/kg im), and fentanyl [analgesic, 125–175 μg/h (72 h) total daily dose] were administered preoperatively. Prior to the left thoracotomy, bupivacaine HCl (2.0 mg/kg) was administered to achieve selective intercostal nerve blockade. After each surgical procedure, animals received cefazolin (30 mg/kg iv) and prophylactic cephalexin [antibiotic, 30 mg/kg (twice per day) po] therapy for the term of the experimental protocol. During the 12-h postoperative period, animals were closely monitored and received buprenorphine and acepromazine (0.05 and 0.5 mg/kg iv, respectively) as needed to control any potential discomfort. For the following 10 days, animals received carprofen [4 mg/kg (once per day) po].
In the first surgical procedure, the thoracic cavity was opened via a left thoracotomy (4th intercostal space) approach. An ultrasonic perivascular flow probe (model 20PAU, Transonic Systems) was positioned around the ascending aorta to measure CO. Approximately 10 cm caudal to the thoracotomy incision, an implantable telemetry blood pressure transmitter (model TA11 PA-D70, Data Sciences International) was tethered subcutaneously. The catheter of the transmitter was tunneled into the thoracic cavity through the seventh intercostal space, and the tip was inserted and secured inside the left ventricle for measurement of left ventricular pressure (LVP). For studies unrelated to the present investigation, a blood flow transducer was also placed on the left circumflex artery. The pericardium was loosely reapproximated, the cables were tunneled subcutaneously and exteriorized between the scapulae, and the chest was closed in layers.
After ≥10 days of recovery, via a left retroperitoneal approach, the abdominal aorta was exposed and an ultrasonic perivascular flow probe (model 10PAA, Transonic Systems) was positioned around the terminal aorta for measurement of hindlimb blood flow (HLBF). All arterial side branches between the common iliac arteries and the flow probe were ligated and severed. Two perivascular hydraulic occluders (8–10 mm; DocXS Biomedical Products) were positioned around the terminal aorta distal to the flow probe to reduce HLBF for metaboreflex activation. A catheter was advanced through a ligated lumbar artery and secured into the terminal aorta cranial to the probe and occluders to measure arterial pressure. A blood flow transducer and vascular occluder were placed on the left renal artery. The cables and vascular occluder tubing were tunneled subcutaneously and exteriorized between the scapulae, and the abdomen was closed in layers.
Data acquisition.
After ≥7 days of recovery, each animal was brought to the laboratory and allowed to roam freely and acclimate for ∼15–20 min. The animal was then directed onto the treadmill, where the instrumentation was connected to the data acquisition system (model TS420, Transonic Systems; blood flow meter, Gould; amplifiers, Data Science International; telemetry system, LabScribe, iWorx). The animal stood quietly on the treadmill for ∼10 min. The treadmill was then started and adjusted to 3.2 km/h, and the animal walked until steady state was achieved (∼3–5 min). HLBF was reduced to activate the muscle metaboreflex. After ≥1 min at steady state, the occluder was deflated and the treadmill was stopped.
Induction of hypertension.
After completion of control experiments, hypertension was induced via the classical approach developed by Goldblatt and colleagues (19, 20). Blood flow to the left kidney was reduced to a target level of ∼30% of control via partial inflation of the vascular occluder. The level of renal blood flow was checked at least twice per day, and the vascular occluder was adjusted until renal blood flow was stable. Hypertension gradually developed over the next several weeks. We defined hypertension as systolic pressure ≥140 mmHg and diastolic pressure ≥90 mmHg. The experiments were repeated after 30.9 ± 4.2 days of sustained hypertension. Thus the experiments were longitudinal in nature, and each animal served as its own control.
Data analysis.
CO, HLBF, LVP, HR, and mean arterial pressure (MAP) data were continuously recorded during each experiment. Other parameters, including stroke volume (SV), nonischemic vascular conductance [NIVC, which is calculated as (CO − HLBF)/MAP and reflects vascular conductance to all areas except the hindlimbs], maximal and minimal rates of change in LVP (dP/dtmax and dP/dtmin), and total peripheral resistance (TPR, calculated as MAP/CO), were calculated offline. One-minute averages of steady-state data were calculated at rest, during exercise, and during metaboreflex activation. Average responses for each animal were analyzed via a two-way repeated-measures ANOVA to compare hemodynamic data for the effects of settings (rest, exercise, and metaboreflex activation) and conditions (normal and hypertension). In the event of a significant settings-condition interaction, a C-matrix test for simple effects was performed. Values are means ± SE; n = 7 for all data with the exception of LVP, for which n = 5.
RESULTS
Table 1 shows the average levels of HLBF at rest, during mild exercise, and during muscle metaboreflex activation in the same animals before and after induction of hypertension. As expected, HLBF rose with mild exercise and was significantly lower with imposed hindlimb occlusion. There were no significant differences between control and hypertensive states. After induction of hypertension, HLBF was reduced to the same levels for metaboreflex activation as in the control experiments.
Table 1.
Hindlimb blood flow at rest, during exercise, and during muscle metaboreflex activation before and after induction of hypertension
| Normal | Hypertension | |
|---|---|---|
| Rest | 0.68 ± 0.07 | 0.83 ± 0.08 |
| Ex | 1.29 ± 0.10 | 1.30 ± 0.10 |
| Ex + MMA | 0.45 ± 0.03 | 0.41 ± 0.05 |
Values are means ± SE in l/min. Ex, exercise; MMA, muscle metaboreflex activation.
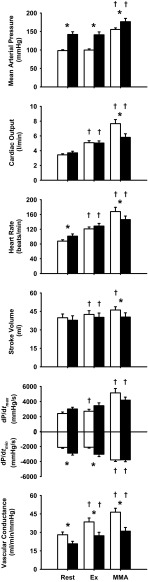
Figure 1 shows the average values of MAP, CO, HR, SV, dP/dtmax, dP/dtmin, and NIVC at rest, during mild exercise, and with muscle metaboreflex activation in control experiments and in the same animals after induction of hypertension. Figure 2 shows the changes in these variables in response to exercise and in response to metaboreflex activation before and after induction of hypertension. At rest, MAP was substantially elevated after induction of hypertension, which was due to peripheral vasoconstriction. TPR increased at rest from 29.0 ± 1.8 to 39.3 ± 3.4 mmHg·l−1·min after induction of hypertension (P < 0.01). There was no significant change in CO. HR was slightly, but significantly, higher, whereas SV was slightly lower; however, this difference was not statistically significant. On average, dP/dtmax was slightly elevated after induction of hypertension, and this difference approached statistical significance (P = 0.06), whereas dP/dtmin was significantly different after induction of hypertension.
Fig. 1.
Hemodynamic values during rest, mild exercise (Ex), and muscle metaboreflex activation (MMA) before (open bars) and after (filled bars) induction of hypertension. dP/dtmax and dP/dtmin, maximal and minimal rates of change in left ventricular pressure, respectively. *P < 0.05 between conditions. †P < 0.05 vs. previous setting.
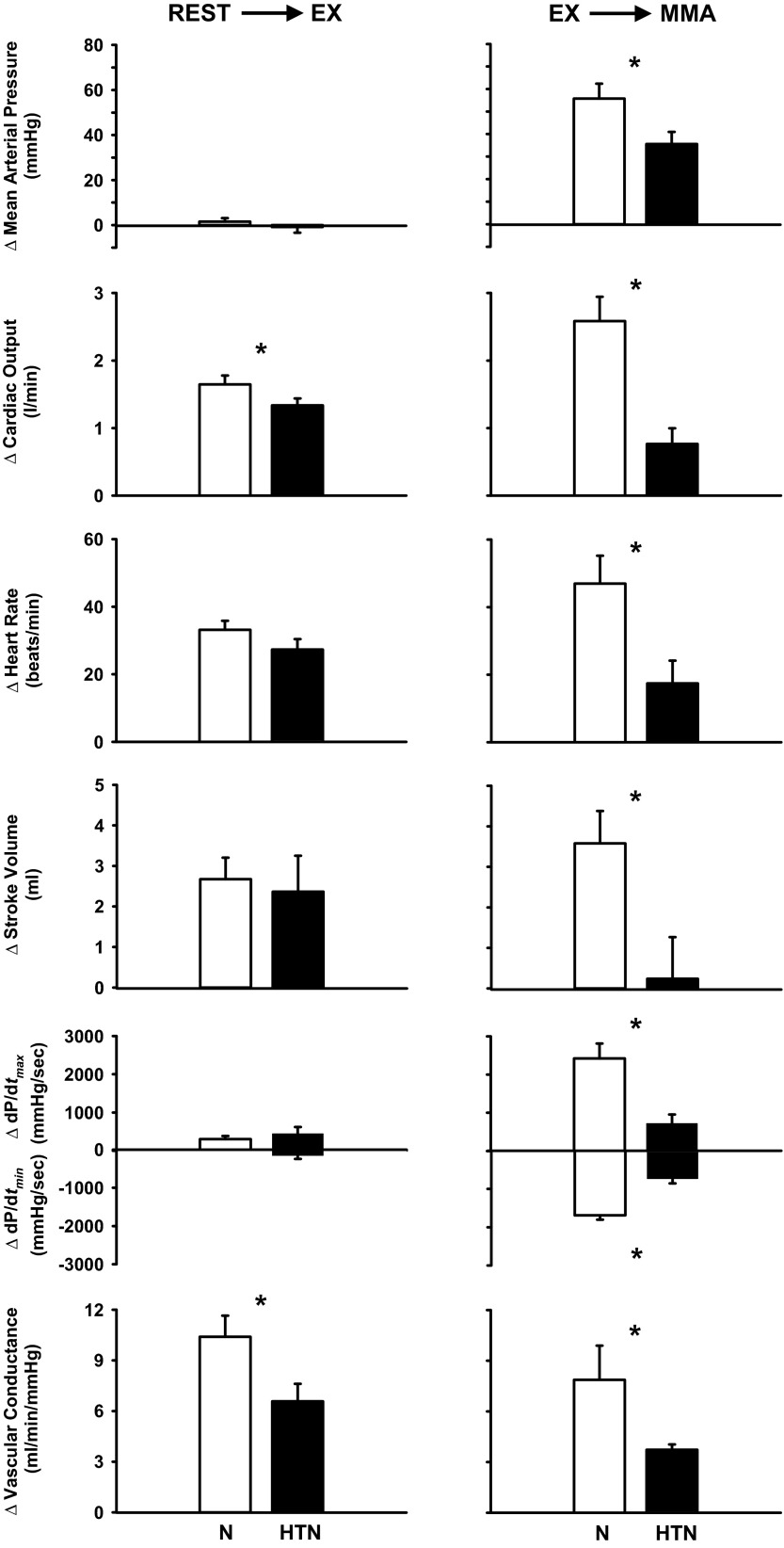
Fig. 2.
Average changes in hemodynamic parameters from rest to steady-state mild exercise (left) and from steady-state exercise to muscle metaboreflex activation (right) before [normal (N)] and after induction of hypertension (HTN). *P < 0.05 between conditions.
The cardiovascular responses from rest to steady-state mild exercise were similar before and after induction of hypertension with two exceptions. Whereas CO at rest and during mild exercise was not statistically affected by hypertension, the change in CO from rest to exercise was slightly lower after induction of hypertension. Furthermore, vasodilation in the active skeletal muscle was also smaller after induction of hypertension, as evidenced by the smaller rise in NIVC as well as hindlimb vascular conductance (+6.0 ± 0.5 vs. +3.5 ± 0.4 ml·min−1·mmHg−1). From rest to mild exercise, similar increases in HR and CO occurred in each condition, and no significant changes from rest occurred in MAP or dP/dtmax. In contrast, hypertension markedly affected the responses to metaboreflex activation. In control experiments, metaboreflex activation caused substantial increases in MAP, HR, CO, dP/dtmax, and dP/dtmin. After induction of hypertension, the metaboreflex pressor responses were attenuated due to much smaller cardiac responses. The reflex increases in HR, CO, and ventricular function were significantly smaller in hypertension. In control experiments, NIVC rose significantly with metaboreflex activation, and this rise in NIVC was significantly smaller after induction of hypertension.
DISCUSSION
This is the first study to investigate the effects of hypertension on the mechanisms of the muscle metaboreflex during dynamic exercise in conscious subjects. Our major new finding is that although the cardiovascular responses to mild exercise are little changed from normal, the increases in ventricular function with muscle metaboreflex activation are markedly impaired in hypertension. This results in a smaller rise in CO and arterial pressure and, therefore, likely limits the ability of the muscle metaboreflex to restore blood flow to the ischemic active skeletal muscle.
Few studies have examined the effects of hypertension on the muscle metaboreflex, and the conclusions have been somewhat mixed on whether the reflex is accentuated or attenuated, with most studies showing an enhanced response. For all the studies in humans (17, 50, 56), the technique of postexercise muscle ischemia (PEMI) was used to isolate the effects of the muscle metaboreflex during the recovery from exercise. Rondon et al. (50) observed that muscle sympathetic nerve activity (MSNA) was higher at rest and during handgrip exercise in middle-aged never-treated hypertensive subjects. However, during PEMI, MSNA returned to baseline levels, whereas it remained elevated in normotensive controls. In both groups, the rise in MAP in response to exercise was similar and was maintained during PEMI. How arterial pressure could remain elevated, yet MSNA could decline, in the hypertensive subjects was not addressed and may indicate that MSNA does not reflect sympathetic activity in all vascular beds. Using similar methodology, others observed that the pressor (17, 56) and MSNA (17) responses to handgrip and PEMI were enhanced in older hypertensive subjects. An enhanced pressor response during PEMI was also observed in prehypertensive young adults (10).
The only previous animal studies investigating the effect of hypertension on the muscle metaboreflex were performed by Smith and colleagues using a decerebrate rat model. They found that the pressor (38, 43) and renal sympathetic nerve activity (43) responses to intra-arterial infusion of capsaicin into the hindlimb were accentuated in SHR vs. Wistar-Kyoto (control normotensive) rats. Capsaicin activates transient receptor potential vanilloid (TRPv1) receptors, which exist on group IV afferents (24). Many metaboreceptors are group IV afferents, as are other afferents, including nociceptors (30). Furthermore, Mizuno et al. (43) recently demonstrated that the pressor response to ischemic electrically induced static muscle contraction of the hindlimb was accentuated in the SHR and that this could be attenuated by an antagonist of the TRPv1 receptor. These investigators concluded that the muscle metaboreflex is accentuated in hypertension and that sensitization of the metaboreceptor may play a key role.
In the present study, the rise in MAP with muscle metaboreflex activation was attenuated after induction of hypertension. A key difference between the present and previous studies is that we activated the muscle metaboreflex during dynamic exercise, rather than during recovery from exercise (e.g., during PEMI) or during electrically induced static muscle contraction or intra-arterial infusion of drugs. These differences in settings may likely affect the mechanisms mediating the metaboreflex responses and possibly the afferent activity eliciting the reflex. Ischemia during exercise may sensitize afferents that are also mechanosensitive, as many group III and IV afferents are polymodal (29–31, 52, 53). Whether mechanoreceptors continue activity during PEMI or whether intra-arterial infusion of drugs elicits activity from receptors that are mechanosensitive is unclear. When the reflex is activated during dynamic exercise, the primary mechanism mediating the pressor response is an increase in CO (6, 18, 34, 54, 72). In contrast, during PEMI, the role of CO in mediating the pressor response varies as HR declines with the recovery from exercise, yet ventricular contractility remains elevated, which may increase SV (7, 9, 13–15, 48, 60, 63). The extent to which the metaboreflex pressor response in the studies in rats (38, 43) occurs via an increase in CO vs. peripheral vasoconstriction is unknown. However, given the small amount of tachycardia and lack of skeletal muscle pump aiding ventricular preload, we speculate that most, if not all, of the rise in MAP in these studies is due to peripheral vasoconstriction. Other differences between the current and previous studies are species and sex used as the model. Findings from our canine studies on the mechanisms mediating baroreflex and metaboreflex function have been substantiated in humans (13, 47). In the present study, because of animal availability, all animals were female. No experiments were performed when the animals were in estrus. Previously, we showed that, in normal dogs, sex difference has no significant effect on the strength or mechanisms of the muscle metaboreflex (37).
In the present study, we saw a variable, but significant, systemic vasodilation during metaboreflex activation, as we have seen in some earlier studies (6, 11, 12, 34). This vasodilation may be due to metaboreflex-mediated epinephrine release from the adrenal glands activating vascular β2-receptors (35). It is also possible that, with reduced HLBF, work done by nonischemic muscles may increase. NIVC reflects the sum of conductance in all vascular beds except the ischemic hindlimbs, and a large fraction of this is conductance to the other active skeletal muscles (34). This increase in NIVC with metaboreflex activation was significantly smaller after induction of hypertension, which may reflect exaggerated peripheral vasoconstriction. In other settings when increases in ventricular function are limited (e.g., heart failure, pharmacological or mechanical restrictions on cardiac function, or maximal exercise), the metaboreflex elicits more pronounced peripheral vasoconstriction (13, 22, 26, 58). Whether this increased peripheral sympathetic drive vasoconstricts the active muscle is unknown.
Several factors, including alterations in receptor function, central processing of the afferent signals, and depressed cardiac responses, may explain the attenuated ability to increase cardiac function after induction of hypertension. Inasmuch as Mizuno et al. (43) concluded that metaboreceptors are sensitized in hypertension, impaired afferent function is unlikely. The pressor response induced by metaboreceptor stimulation is normally buffered by the arterial baroreflex (34, 59). This buffering is mediated primarily by attenuating or preventing metaboreflex-induced peripheral vasoconstriction (34). Arterial baroreflex gain (or strength) in the control of HR at rest is diminished in hypertensive subjects (27, 62). Whether baroreflex control of sympathetic activity is depressed in hypertension is controversial (16, 28, 40, 41, 51, 55, 65). To our knowledge, no study has shown enhanced baroreflex sensitivity in hypertension. Therefore, improved baroreflex buffering of metaboreflex-induced sympathoactivation is unlikely to explain the attenuated cardiac responses we observed after induction of hypertension. Thus we would not expect that the CO responses in hypertension would return to normal levels after baroreceptor denervation, but this has yet to be investigated. By definition, afterload is markedly elevated after induction of hypertension, and this may contribute importantly to the impaired ability to further raise CO during metaboreflex activation. Previous studies have shown reduced cardiac contractile responses to β-receptor stimulation in hypertension. This may or may not be due to reduced cardiac β-receptor density (42). Impaired β-receptor-mediated stimulation of adenylyl cyclase has also been observed in hypertension (73), which may be related to increased inhibitory G protein function (8). We recently showed that the increase in cardiac sympathetic activity during metaboreflex activation functionally vasoconstricts the coronary vasculature, and this restraint of coronary hyperemia limits increases in ventricular function (11, 12). This metaboreflex-mediated coronary vasoconstriction is exacerbated after induction of heart failure (12). The extent to which coronary vasoconstriction contributes to the impaired ventricular function during metaboreflex activation in hypertension is unknown.
Perspectives and significance.
The Goldblatt model is a widely used and well-characterized model of human renovascular hypertension. The incidence of renal stenosis associated with hypertension in humans is growing and is especially prevalent in patients with concurrent coronary artery disease (36, 67–70). A distinct advantage of this model is that we could induce hypertension in our animals after completion of the control studies, which allowed for a longitudinally designed paired study where each animal served as its own control. This form of hypertension is characterized by an initial marked activation of the renin-angiotensin system; however, Verburg et al. (66) showed that renin levels were no longer above control levels after 2 wk of renovascular hypertension in dogs, which would indicate that the sustained peripheral vasoconstriction is mediated via elevated sympathetic activity. At rest, our observation of significant tachycardia and faster dP/dtmin after >1 mo of sustained hypertension, along with the increased TPR, is consistent with higher sympathetic activity.
Exercise in hypertensive subjects may elicit large increases in sympathetic activity and arterial pressure, which may increase risk factors for adverse cardiovascular events (5, 10, 32, 49, 57). The mechanisms mediating these responses remain elusive. Inasmuch as ventricular dysfunction can result from chronic hypertension, it is possible that skeletal muscle perfusion may be compromised, especially during whole body, dynamic exercise, which could elicit exaggerated activation of the muscle metaboreflex. In the present study, the vasodilation accompanying exercise was slightly attenuated during exercise. This would not activate the muscle metaboreflex in our model, since HLBF must be reduced substantially before the metaboreflex is triggered during mild exercise (72), even in animals with marked heart failure (22). Similar results were observed in our preliminary studies: before and after hypertension, small decreases in HLBF caused no metaboreflex responses. However, during moderate exercise, this reflex appears to be tonically active, as any reduction in skeletal muscle blood flow engages further pressor responses (6, 72). Recent findings in humans also indicate a role for skeletal muscle afferents in mediating the cardiovascular responses to relatively moderate exercise (2). Thus, during moderate to heavy exercise in hypertensive subjects, underperfusion of active skeletal muscle, via inherent cardiac dysfunction or impaired peripheral vasodilation, may elicit exaggerated metaboreflex activation. Metaboreflex-induced peripheral vasoconstriction is buffered by the arterial baroreflex (34), and baroreflex function may be impaired in hypertension (3, 27, 41, 55). Thus, enhanced metaboreflex stimulation combined with attenuated baroreflex buffering may allow for exaggerated sympathoactivation during exercise. This could engage a “vicious cycle” positive-feedback scenario, further restraining skeletal muscle (45) and even coronary (4, 11, 12, 25, 46) vasodilation, which could further compromise cardiac function and further limit muscle perfusion, ultimately until exercise intolerance develops (21).
In summary, in a longitudinally designed study, we found that hypertension impairs muscle metaboreflex-mediated left ventricular chronotropic, inotropic, and lusitropic responses. This limits the ability of the reflex to raise CO, which is the primary mechanism by which the reflex acts to improve blood flow to ischemic active skeletal muscle. This impaired ability to raise ventricular function may contribute to exercise intolerance in hypertensive subjects (64).
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL-095819 and HL-055473 and by the Multidisciplinary Research Group Incubator Program at Wayne State University.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.A.S.-M. and D.S.O. are responsible for conception and design of the research; J.A.S.-M., M.D.S., R.A.-H., J.K., M.C., D.S., and D.S.O. performed the experiments; J.A.S.-M., M.D.S., R.A.-H., J.K., R.A.A., and D.S.O. analyzed the data; J.A.S.-M., M.D.S., J.K., R.A.A., and D.S.O. interpreted the results of the experiments; J.A.S.-M. and D.S.O. drafted the manuscript; J.A.S.-M., M.D.S., and D.S.O. edited and revised the manuscript; J.A.S.-M., M.D.S., R.A.-H., J.K., M.C., D.S., R.A.A., and D.S.O. approved the final version of the manuscript; M.D.S., J.K., and D.S.O. prepared the figures.
ACKNOWLEDGMENTS
We thank Jody Helme-Day and Alberto Alvarez for expert technical assistance.
REFERENCES
- 1.Adreani CM, Kaufman MP. Effect of arterial occlusion on responses of group III and IV afferents to dynamic exercise. J Appl Physiol 84: 1827–1833, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Group III and IV muscle afferents contribute to ventilatory and cardiovascular response to rhythmic exercise in humans. J Appl Physiol 109: 966–976, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angell-James JE, George MJ, Peters CJ. Baroreflex sensitivity in rabbits during the development of experimental renal hypertension and medial sclerosis. Clin Exp Hypertens 2: 321–340, 1980 [DOI] [PubMed] [Google Scholar]
- 4.Ansorge EJ, Shah SH, Augustyniak R, Rossi NF, Collins HL, O'Leary DS. Muscle metaboreflex control of coronary blood flow. Am J Physiol Heart Circ Physiol 283: H526–H532, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Aoki K, Sato K, Kondo S, Pyon CB, Yamamoto M. Increased response of blood pressure to rest and handgrip in subjects with essential hypertension. Jpn Circ J 47: 802–809, 1983 [DOI] [PubMed] [Google Scholar]
- 6.Augustyniak RA, Collins HL, Ansorge EJ, Rossi NF, O'Leary DS. Severe exercise alters the strength and mechanisms of the muscle metaboreflex. Am J Physiol Heart Circ Physiol 280: H1645–H1652, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Bastos BG, Williamson JW, Harrelson T, Nobrega AC. Left ventricular volumes and hemodynamic responses to postexercise ischemia in healthy humans. Med Sci Sports Exerc 32: 1114–1118, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Bohm M, Gierschik P, Knorr A, Larisch K, Weismann K, Erdmann E. Desensitization of adenylate cyclase and increase of Giα in cardiac hypertrophy due to acquired hypertension. Hypertension 20: 103–112, 1992 [DOI] [PubMed] [Google Scholar]
- 9.Bonde-Petersen F, Rowell LB, Murray RG, Blomqvist GG, White R, Karlsson E, Campbell W, Mitchell JH. Role of cardiac output in the pressor responses to graded muscle ischemia in man. J Appl Physiol 45: 574–580, 1978 [DOI] [PubMed] [Google Scholar]
- 10.Choi HM, Stebbins CL, Lee OT, Nho H, Lee JH, Chun JM, Kim KA, Kim JK. Augmentation of the exercise pressor reflex in prehypertension: roles of the muscle metaboreflex and mechanoreflex. Appl Physiol Nutr Metab 38: 209–215, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Coutsos M, Sala-Mercado JA, Ichinose M, Li Z, Dawe EJ, O'Leary DS. Muscle metaboreflex-induced coronary vasoconstriction functionally limits increases in ventricular contractility. J Appl Physiol 109: 271–278, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coutsos M, Sala-Mercado JA, Ichinose M, Li Z, Dawe EJ, O'Leary DS. Muscle metaboreflex-induced coronary vasoconstriction limits ventricular contractility during dynamic exercise in heart failure. Am J Physiol Heart Circ Physiol 304: H1029–H1037, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crisafulli A, Salis E, Tocco F, Melis F, Milia R, Pittau G, Caria MA, Solinas R, Meloni L, Pagliaro P, Concu A. Impaired central hemodynamic response and exaggerated vasoconstriction during muscle metaboreflex activation in heart failure patients. Am J Physiol Heart Circ Physiol 292: H2988–H2996, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Crisafulli A, Piras F, Filippi M, Piredda C, Chiappori P, Melis F, Milia R, Tocco F, Concu A. Role of heart rate and stroke volume during muscle metaboreflex-induced cardiac output increase: differences between activation during and after exercise. J Physiol Sci 61: 385–394, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crisafulli A, Salis E, Pittau G, Lorrai L, Tocco F, Melis F, Pagliaro P, Concu AN. Modulation of cardiac contractility by muscle metaboreflex following efforts of different intensities in humans. Am J Physiol Heart Circ Physiol 291: H3035–H3042, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Dampney RA, Horiuchi J, Killinger S, Sheriff MJ, Tan PS, McDowall LM. Long-term regulation of arterial blood pressure by hypothalamic nuclei: some critical questions. Clin Exp Pharmacol Physiol 32: 419–425, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Delaney EP, Greaney JL, Edwards DG, Rose WC, Fadel PJ, Farquhar WB. Exaggerated sympathetic and pressor responses to handgrip exercise in older hypertensive humans: role of the muscle metaboreflex. Am J Physiol Heart Circ Physiol 299: H1318–H1327, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eiken O, Bjurstedt H. Dynamic exercise in man as influenced by experimental restriction of blood flow in the working muscles. Acta Physiol Scand 131: 339–345, 1987 [DOI] [PubMed] [Google Scholar]
- 19.Goldblatt H, Haas E, Klick RL, Lewis LV. The effect of main artery occlusion of one kidney on blood pressure of dogs. Proc Natl Acad Sci USA 73: 1722–1724, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldblatt H, Lynch J, Hanzal RF, Summerville WW. Studies on experimental hypertension. J Exp Med 59: 347–379, 1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guazzi M, Brenner DA, Apstein CS, Saupe KW. Exercise intolerance in rats with hypertensive heart disease is associated with impaired diastolic relaxation. Hypertension 37: 204–208, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Hammond RL, Augustyniak RA, Rossi NF, Churchill PC, Lapanowski K, O'Leary DS. Heart failure alters the strength and mechanisms of the muscle metaboreflex. Am J Physiol Heart Circ Physiol 278: H818–H828, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Hammond RL, Augustyniak RA, Rossi NF, Lapanowski K, Dunbar JC, O'Leary DS. Alteration of humoral and peripheral vascular responses during graded exercise in heart failure. J Appl Physiol 90: 55–61, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Hoheisel U, Reinohl J, Unger T, Mense S. Acidic pH and capsaicin activate mechanosensitive group IV muscle receptors in the rat. Pain 110: 149–157, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Huang AH, Feigl EO. Adrenergic coronary vasoconstriction helps maintain uniform transmural blood flow distribution during exercise. Circ Res 62: 286–298, 1988 [DOI] [PubMed] [Google Scholar]
- 26.Ichinose MJ, Sala-Mercado JA, Coutsos M, Li Z, Ichinose TK, Dawe E, O'Leary DS. Modulation of cardiac output alters the mechanisms of the muscle metaboreflex pressor response. Am J Physiol Heart Circ Physiol 298: H245–H250, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones JV, Floras JS. Baroreflex sensitivity changes during the development of Goldblatt two-kidney one-clip hypertension in rats. Clin Sci (Lond) 59: 347–352, 1980 [DOI] [PubMed] [Google Scholar]
- 28.Judy WV, Farrell SK. Arterial baroreceptor reflex control of sympathetic nerve activity in the spontaneously hypertensive rat. Hypertension 1: 605–614, 1979 [DOI] [PubMed] [Google Scholar]
- 29.Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol 55: 105–112, 1983 [DOI] [PubMed] [Google Scholar]
- 30.Kaufman MP, Rybicki KJ. Discharge properties of group III and IV muscle afferents: their responses to mechanical and metabolic stimuli. Circ Res 61: I60–I65, 1987 [PubMed] [Google Scholar]
- 31.Kaufman MP, Rybicki KJ, Waldrop TG, Ordway GA. Effect of ischemia on responses of group III and IV afferents to contraction. J Appl Physiol 57: 644–650, 1984 [DOI] [PubMed] [Google Scholar]
- 32.Kazatani Y, Hamada M, Shigematsu Y, Hiwada K, Kokubu T. Beneficial effect of a long-term antihypertensive therapy on blood pressure response to isometric handgrip exercise in patients with essential hypertension. Am J Ther 2: 165–169, 1995 [DOI] [PubMed] [Google Scholar]
- 33.Kim JK, Sala-Mercado JA, Hammond RL, Rodriguez J, Scislo TJ, O'Leary DS. Attenuated arterial baroreflex buffering of muscle metaboreflex in heart failure. Am J Physiol Heart Circ Physiol 289: H2416–H2423, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Kim JK, Sala-Mercado JA, Rodriguez J, Scislo TJ, O'Leary DS. Arterial baroreflex alters strength and mechanisms of muscle metaboreflex during dynamic exercise. Am J Physiol Heart Circ Physiol 288: H1374–H1380, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Kitchen AM, Scislo TJ, O'Leary DS. NTS purinoceptor activation elicits hindlimb vasodilation primarily via a β-adrenergic mechanism. Am J Physiol Heart Circ Physiol 278: H1775–H1782, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Landwehr DM, Vetrovec GW, Cowley MJ, Parker VE. Association of renal-artery stenosis with coronary-artery disease in patients with hypertension and/or chronic renal-insufficiency. Kidney Int 25: 170, 1984 [Google Scholar]
- 37.LaPrad SL, Augustyniak RA, Hammond RL, O'Leary DS. Does gender influence the strength and mechanisms of the muscle metaboreflex during dynamic exercise in dogs? Am J Physiol Regul Integr Comp Physiol 276: R1203–R1208, 1999 [DOI] [PubMed] [Google Scholar]
- 38.Leal AK, Williams MA, Garry MG, Mitchell JH, Smith SA. Evidence for functional alterations in the skeletal muscle mechanoreflex and metaboreflex in hypertensive rats. Am J Physiol Heart Circ Physiol 295: H1429–H1438, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.MacLean DA, Imadojemu VA, Sinoway LI. Interstitial pH, K+, lactate, and phosphate determined with MSNA during exercise in humans. Am J Physiol Regul Integr Comp Physiol 278: R563–R571, 2000 [DOI] [PubMed] [Google Scholar]
- 40.Matsukawa T, Gotoh E, Hasegawa O, Miyajima E, Shionoiri H, Tochikubo O, Ishii M. Reduced arterial baroreflex control of muscle sympathetic nerve activity in young borderline hypertensives. Funct Neurol 6: 113–120, 1991 [PubMed] [Google Scholar]
- 41.Matsukawa T, Gotoh E, Hasegawa O, Shionoiri H, Tochikubo O, Ishii M. Reduced baroreflex changes in muscle sympathetic nerve activity during blood pressure elevation in essential hypertension. J Hypertens 9: 537–542, 1991 [DOI] [PubMed] [Google Scholar]
- 42.Michel MC, Brodde OE, Insel PA. Peripheral adrenergic receptors in hypertension. Hypertension 16: 107–120, 1990 [DOI] [PubMed] [Google Scholar]
- 43.Mizuno M, Murphy MN, Mitchell JH, Smith SA. Antagonism of the TRPv1 receptor partially corrects muscle metaboreflex overactivity in spontaneously hypertensive rats. J Physiol 589: 6191–6204, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mizuno M, Murphy MN, Mitchell JH, Smith SA. Skeletal muscle reflex-mediated changes in sympathetic nerve activity are abnormal in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 300: H968–H977, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O'Leary DS, Robinson ED, Butler JL. Is active skeletal muscle functionally vasoconstricted during dynamic exercise in conscious dogs? Am J Physiol Regul Integr Comp Physiol 272: R386–R391, 1997 [DOI] [PubMed] [Google Scholar]
- 46.O'Leary DS, Sala-Mercado JA, Hammond RL, Ansorge EJ, Kim JK, Rodriguez J, Fano D, Ichinose M. Muscle metaboreflex-induced increases in cardiac sympathetic activity vasoconstrict the coronary vasculature. J Appl Physiol 103: 190–194, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Ogoh S, Fadel PJ, Nissen P, Jans O, Selmer C, Secher NH, Raven PB. Baroreflex-mediated changes in cardiac output and vascular conductance in response to alterations in carotid sinus pressure during exercise in humans. J Physiol 550: 317–324, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pawelczyk JA, Pawelczyk RA, Warberg J, Mitchell JH, Secher NH. Cardiovascular and catecholamine responses to static exercise in partially curarized humans. Acta Physiol Scand 160: 23–28, 1997 [DOI] [PubMed] [Google Scholar]
- 49.Pickering TG. Pathophysiology of exercise hypertension. Herz 12: 119–124, 1987 [PubMed] [Google Scholar]
- 50.Rondon MU, Laterza MC, de Matos LD, Trombetta IC, Braga AM, Roveda F, Alves MJ, Krieger EM, Negrao CE. Abnormal muscle metaboreflex control of sympathetic activity in never-treated hypertensive subjects. Am J Hypertens 19: 951–957, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Rossi NF, Maliszewska-Scislo M, Chen H, Black SM, Sharma S, Ravikov R, Augustyniak RA. Neuronal nitric oxide synthase within paraventricular nucleus: blood pressure and baroreflex in two-kidney, one-clip hypertensive rats. Exp Physiol 95: 845–857, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rotto DM, Kaufman MP. Effect of metabolic products of muscular contraction on discharge of group III and IV afferents. J Appl Physiol 64: 2306–2313, 1988 [DOI] [PubMed] [Google Scholar]
- 53.Rotto DM, Shultz HD, Kaufman MP. Sensitization of group III muscle afferents to static contraction by arachidonic acid. J Appl Physiol 68: 861–867, 1990 [DOI] [PubMed] [Google Scholar]
- 54.Rowell LB, Savage MV, Chambers J, Blackmon JR. Cardiovascular responses to graded reductions in leg perfusion in exercising humans. Am J Physiol Heart Circ Physiol 261: H1545–H1553, 1991 [DOI] [PubMed] [Google Scholar]
- 55.Sapru HN, Wang SC. Modification of aortic baroreceptor resetting in the spontaneously hypertensive rat. Am J Physiol 230: 664–674, 1976 [DOI] [PubMed] [Google Scholar]
- 56.Sausen MT, Delaney EP, Stillabower ME, Farquhar WB. Enhanced metaboreflex sensitivity in hypertensive humans. Eur J Appl Physiol 105: 351–356, 2009 [DOI] [PubMed] [Google Scholar]
- 57.Seguro C, Sau F, Zedda N, Scano G, Cherchi A. Arterial blood pressure behavior during progressive muscular exercise in subjects with stable arterial hypertension. Cardiologia 36: 867–877, 1991 [PubMed] [Google Scholar]
- 58.Sheriff DD, Augustyniak RA, O'Leary DS. Muscle chemoreflex-induced increases in right atrial pressure. Am J Physiol Heart Circ Physiol 275: H767–H775, 1998 [DOI] [PubMed] [Google Scholar]
- 59.Sheriff DD, O'Leary DS, Scher AM, Rowell LB. Baroreflex attenuates pressor response to graded muscle ischemia in exercising dogs. Am J Physiol Heart Circ Physiol 258: H305–H310, 1990 [DOI] [PubMed] [Google Scholar]
- 60.Shoemaker JK, Mattar L, Kerbeci P, Trotter S, Arbeille P, Hughson RL. WISE 2005: stroke volume changes contribute to the pressor response during ischemic handgrip exercise in women. J Appl Physiol 103: 228–233, 2007 [DOI] [PubMed] [Google Scholar]
- 61.Smith SA, Williams MA, Leal AK, Mitchell JH, Garry MG. Exercise pressor reflex function is altered in spontaneously hypertensive rats. J Physiol 577: 1009–1020, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Souza HC, Martins-Pinge MC, Dias da Silva VJ, Borghi-Silva A, Gastaldi AC, Blanco JH, Tezini GC. Heart rate and arterial pressure variability in the experimental renovascular hypertension model in rats. Auton Neurosci 139: 38–45, 2008 [DOI] [PubMed] [Google Scholar]
- 63.Spranger MD, Sala-Mercado JA, Coutsos M, Kaur J, Stayer D, Augustyniak RA, O'Leary DS. Role of cardiac output versus peripheral vasoconstriction in mediating muscle metaboreflex pressor responses: dynamic exercise versus postexercise muscle ischemia. Am J Physiol Regul Integr Comp Physiol 304: R657–R663, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Takamura T, Onishi K, Sugimoto T, Kurita T, Fujimoto N, Dohi K, Tanigawa T, Isaka N, Nobori T, Ito M. Patients with a hypertensive response to exercise have impaired left ventricular diastolic function. Hypertens Res 31: 257–263, 2008 [DOI] [PubMed] [Google Scholar]
- 65.Thames MD, Gupta BN, Ballon BJ. Central abnormality in baroreflex control of renal nerves in hypertension. Am J Physiol Heart Circ Physiol 246: H843–H850, 1984 [DOI] [PubMed] [Google Scholar]
- 66.Verburg KM, Freeman RH, Villarreal D, Brands MW. Atrial natriuretic factor in dogs with one-kidney, one-clip Goldblatt hypertension. Am J Physiol Heart Circ Physiol 253: H1623–H1627, 1987 [DOI] [PubMed] [Google Scholar]
- 67.Vetrovec GW, Cowley MJ, Landwehr DM, Parker VE. High prevalence of renal-artery stenosis in patients with hypertension undergoing cardiac-catheterization (Abstract). Clin Res 31: A845, 1983 [Google Scholar]
- 68.Vetrovec GW, Cowley MJ, Landwehr DM, Parker VE. High prevalence of renal-artery stenosis in hypertensive patients with coronary-artery disease (Abstract). J Am Coll Cardiol 3: 518, 1984 [Google Scholar]
- 69.Vetrovec GW, Woods AC, Lewis SE, Cowley MJ, Mukharji J, Hirsh PD, Parker VE. Frequency of renal-artery stenosis in hypertensive patients undergoing coronary angiography (Abstract). Clin Res 34: A205, 1986 [Google Scholar]
- 70.Vetrovec GW, Woods AC, Lewis SE, Cowley MJ, Mukharji J, Hirsh PD, Parker VE. High prevalence of renal-artery stenosis in patients undergoing coronary angiography—relationship to the presence of hypertension (Abstract). Clin Res 34: A351, 1986 [Google Scholar]
- 71.Victor RG, Bertocci LA, Pryor SL, Nunnally RL. Sympathetic nerve discharge is coupled to muscle cell pH during exercise in humans. J Clin Invest 82: 1301–1305, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wyss CR, Ardell JL, Scher AM, Rowell LB. Cardiovascular responses to graded reductions in hindlimb perfusion in exercising dogs. Am J Physiol Heart Circ Physiol 245: H481–H486, 1983 [DOI] [PubMed] [Google Scholar]
- 73.Yurenev AP, Parfyonova EV, Krasnikova TL, Aripova NA. Alteration of β-adrenoceptor function in hypertensive patients with different degrees of left ventricular hypertrophy. Am J Hypertens 5: 164S–168S, 1992 [DOI] [PubMed] [Google Scholar]