Abstract
The purpose of this study was to characterize changes in antioxidant and age-related gene expression in aorta and aortic valve with aging, and test the hypothesis that increased mitochondrial oxidative stress accelerates age-related endothelial and aortic valve dysfunction. Wild-type (MnSOD+/+) and manganese SOD heterozygous haploinsufficient (MnSOD+/−) mice were studied at 3 and 18 mo of age. In aorta from wild-type mice, antioxidant expression was preserved, although there were age-associated increases in Nox2 expression. Haploinsufficiency of MnSOD did not alter antioxidant expression in aorta, but increased expression of Nox2. When compared with that of aorta, age-associated reductions in antioxidant expression were larger in aortic valves from wild-type and MnSOD haploinsufficient mice, although Nox2 expression was unchanged. Similarly, sirtuin expression was relatively well-preserved in aorta from both genotypes, whereas expression of SIRT1, SIRT2, SIRT3, SIRT4, and SIRT6 were significantly reduced in the aortic valve. Expression of p16ink4a, a marker of cellular senescence, was profoundly increased in both aorta and aortic valve from MnSOD+/+ and MnSOD+/− mice. Functionally, we observed comparable age-associated reductions in endothelial function in aorta from both MnSOD+/+ and MnSOD+/− mice. Interestingly, inhibition of NAD(P)H oxidase with apocynin or gp91ds-tat improved endothelial function in MnSOD+/+ mice but significantly impaired endothelial function in MnSOD+/− mice at both ages. Aortic valve function was not impaired by aging or MnSOD haploinsufficiency. Changes in antioxidant and sirtuin gene expression with aging differ dramatically between aorta and aortic valve. Furthermore, although MnSOD does not result in overt cardiovascular dysfunction with aging, compensatory transcriptional responses to MnSOD deficiency appear to be tissue specific.
Keywords: aging, mitochondrial oxidative stress, endothelial function, aorta, aortic valve
increasing age is associated with increases in reactive oxygen species (ROS), which is thought to contribute to the development of age-related cardiovascular disease. Previous work in aged humans and animals has shown that NAD(P)H oxidase contributes to age-related endothelial dysfunction and vascular fibrosis and that reducing SOD1 (cytosolic), SOD2 (mitochondrial), or SOD3 (extracellular) worsens endothelial function with aging (3, 4, 12, 13, 19, 25). Furthermore, we have previously shown that aortic valve calcification is strongly associated with increases in oxidative stress and reductions in antioxidant defense mechanisms in humans (27), and several studies have shown that the balance between oxidative stress and nitric oxide bioavailability is an important determinant of vascular and valvular calcification in vitro (27, 39, 41). Interestingly, emerging data from in vitro experiments also suggest that, once initiated, ROS production may self-perpetuate (ROS-induced ROS-release), accelerate development of endothelial dysfunction, and accelerate development of age-related disease (15). Mechanisms downstream of oxidative stress that regulate these phenotypic and functional changes are unclear.
Sirtuin deacetylases protect against cellular senescence and play a major role in protecting against several age-related diseases. For example, SIRT6-deficient mice have profound reductions in bone mineral density, exaggerated kypholordosis, and a dramatically shortened lifespan compared with their wild-type littermates (32). Furthermore, the observations that SIRT1-dependent deacetylation of endothelial nitric oxide synthase (eNOS) is essential for eNOS activation (26) and that experimentally reducing SIRT1 increases pro-inflammatory gene expression in aorta (9) provide evidence for a more direct role of sirtuins in regulation of age-related cardiovascular disease. Although limited data from in vitro model systems suggest that increased oxidative stress may decrease sirtuin function, we are not, however, aware of studies examining whether reductions in antioxidant capacity and induction of ROS-induced ROS release alter sirtuin expression and cardiovascular function in vivo.
Thus the aims of the current study were to determine whether reducing mitochondrial antioxidant capacity results in induction of ROS-induced ROS-release in young and aged mice in vivo, to determine whether reducing mitochondrial antioxidant capacity exacerbates reductions in aging- and senescence-associated gene expression, and to examine the consequences of reducing mitochondrial antioxidant capacity on aortic and aortic valve function in young and aged mice. Our working hypothesis was that reducing mitochondrial antioxidant capacity would initiate ROS-induced ROS-release, reduce nitric oxide bioavailability, exaggerate reductions in longevity-related genes, and ultimately accelerate age-related aortic and aortic valve dysfunction.
METHODS
Animals.
Wild-type and manganese SOD heterozygous mice (MnSOD+/+ and MnSOD+/−, respectively; from Strain 002973, B6.129S7-Sod2tm1Leb/J, The Jackson Laboratory) crossed onto a C57Bl/6J background (Strain 000664; The Jackson Laboratory) >10 times and were aged to two different time points for these studies: young (3 mo) and old (18–22 mo). Mice were housed in climate-controlled facilities under a 12-h:12-h light/dark cycle with free access to water and normal chow (PicoLab Mouse Diet 5053). Mice were generated from MnSOD+/+ × MnSOD+/− breeder pairs, and offspring used in these studies were littermate-matched within each age group whenever possible. All experimental protocols were approved by the Institutional Animal Care and Use Committee at Mayo Clinic.
Gene expression.
The thoracic aorta was removed en bloc, and perivascular adipose tissue and adventitial tissue was removed. The aortic arch was minced and stored in TRIzol (Invitrogen). Aortic valve cusps were carefully dissected and stored in TRIzol. RNA was isolated using spin columns and chloroform extractions (Invitrogen), as described previously. After conversion to cDNA (VILO reverse transcriptase; Invitrogen), quantitative real-time PCR was performed on a StepOne Plus RT-PCR machine (Applied Biosystems). Genes analyzed can be broadly categorized into five groups: antioxidant genes (SOD1, SOD2, SOD3), antioxidant regulation genes (Nrf2, FOXO1, FOXO3, FOXO4, DJ-1, and Nqo1) and pro-oxidant genes (Nox1, Nox2, Nox4, p47phox), nitric oxide synthases (NOS) isoforms [eNOS, inducible NOS (iNOS), and neuronal NOS], pro-inflammatory genes (VCAM-1, ICAM-1, TNF-α, and IL-6), and aging/progeria/longevity-related genes (sirtuins 1–7 and WRN).
Measurement of ROS in aorta.
Levels of ROS in descending thoracic aorta were measured using lucigenin-enhanced chemiluminescence, as described previously (27). In brief, excess adventitial tissue and perivascular fat were removed, and four sections of ∼4 mm in length were placed in cuvettes containing Leibovitz's L15 medium (a CO2-independent medium) and 5 μM lucigenin. Chemiluminescence was measured using an FB12 luminometer (Zylux).
Hydrogen peroxide levels in aorta were examined using Amplex Red Assay (Invitrogen). Briefly, excess adventitial tissue and perivascular fat were removed, and four sections of ∼4 mm in length were placed in a 96-well plate with 50 μM Amplex Red Reagent and incubated at 37° for 30 min. Fluorescence (excitation, 570/emission, 585 nm) was measured using a plate reader (Flexstation 3; Molecular Devices).
Measurement of ROS in aortic valve.
Superoxide levels in aortic valve were measured in 10 μm thick cryosections using dihydroethidium (DHE; Lifetech) fluorescence. In brief, sections were incubated with 5 μM DHE and imaged using a confocal microscope (Zeiss LSM780; excitation, 488/emission, 585–615), as described previously (27).
Hydrogen peroxide levels in aortic valve were measured in 10 μm thick cryosections using fluorescence of carboxymethyl-dichlorofluorescein diacetate (CM-H2DCFDA; Lifetech). In brief, sections were incubated with 10 μM CM-H2DCFDA and imaged using a confocal microscope (Zeiss LSM780; excitation, 488/emission, 515–585), as described previously (27).
Measurement of changes in vascular and aortic valve calcification.
Calcium levels were measured in aortic valve using Alizarin Red staining followed by semi-quantitative analysis using Adobe Photoshop, as described in detail previously (31).
Vasomotor function.
Rings from descending thoracic aorta were used for vasomotor function studies, as described previously (28). In brief, excess adventitial tissue and perivascular fat were removed, and sections of ∼3 to 4 mm in length were mounted on stainless-steel hooks. The vessels were maintained in an organ bath chamber containing oxygenated (95% O2-5% CO2) Krebs solution at 37°C and allowed to equilibrate for 1 h before evaluation of vasomotor function. Vessels were rinsed for a minimum of 30 min between vasomotor function curves. Responses to serotonin, acetylcholine, nitroprusside, PGF-2α, and hydrogen peroxide were evaluated. Responses to acetylcholine were also examined in the presence of apocynin (100 μM; Sigma-Aldrich), gp91ds-tat (500 nM; Anaspec) (34), catalase (200 units/ml; Sigma-Aldrich), and Nω-nitro-l-arginine methyl ester (100 μM; Cayman Chemical).
Cardiac and aortic valve function.
Cardiac and aortic valve function were assessed using echocardiography (GE Vivid 7 ultrasound system, 13 MHz linear probe) under light isoflurane anesthesia to avoid anesthesia-induced cardiodepression (heart rates were maintained above >475 beats/min). Cardiac function was evaluated from parasternal short-axis images at the level of the papillary muscles. Aortic valve cusp separation distance was measured from M-mode images taken from the parasternal long-axis view, and peak transvalvular velocities were measured with Doppler velocimetry from the suprasternal notch view.
Blood pressure.
Blood pressure was measured non-invasively in conscious mice using a tail-cuff system (CODA; Kent Scientific). Blood pressure was measured for five consecutive days, and systolic and diastolic measurements were recorded.
Statistical analyses.
All data are expressed as means ± SE. Main effects of age, genotype, and age-by-genotype interactions were detected using two-way ANOVA testing. Subsequent comparisons across groups were made using Bonferroni-corrected t-tests.
RESULTS
Antioxidant gene expression in aorta.
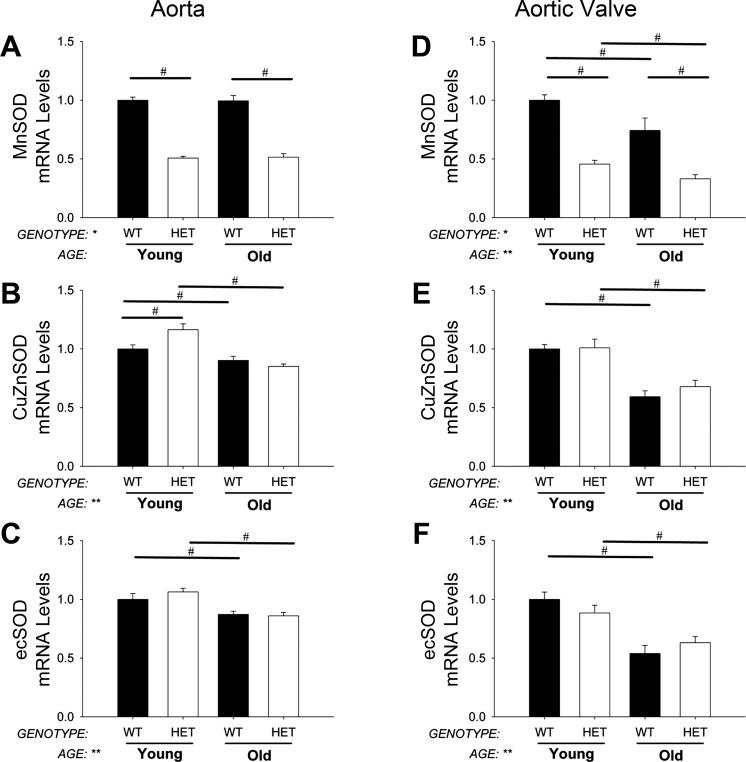
As expected, expression of MnSOD was reduced by ∼50% in MnSOD+/− mice compared with MnSOD+/+ mice in both young and old groups. MnSOD-deficiency did not affect expression of copper-zinc SOD (CuZnSOD) and extracellular SOD (ecSOD) in young mice, nor did it affect age-related reductions in these genes (Fig. 1, A–C).
Fig. 1.
Antioxidant gene expression levels. Manganese SOD (MnSOD) expression was reduced by ∼50% in MnSOD+/− mice (P < 0.01) in aorta and aortic valve (A and D). Note that there was no compensatory increase in other antioxidant mechanisms in aorta or aortic valve due to reductions of MnSOD (B and C and E and F). Copper-zinc SOD (CuZnSOD) and extracellular SOD (ecSOD) were, however, significantly decreased with aging in aorta (B and C) and aortic valve (E and F). Values are means ± SE; n = 4–14 mice/group. *Significant main effect of genotype with P < 0.01; **significant main effect of age with P value < 0.01; #individual group differences with P < 0.05. WT, wild-type; Het, heterozygous mice.
Antioxidant gene expression in aortic valve.
As expected, expression of MnSOD was reduced by ∼50% in aortic valves from MnSOD+/− mice compared with age-matched MnSOD+/+ mice. Expression of CuZnSOD and ecSOD was markedly reduced by aging, and these reductions were not affected by MnSOD deficiency (Fig. 1, D–F).
Genes regulating antioxidant responses in aorta.
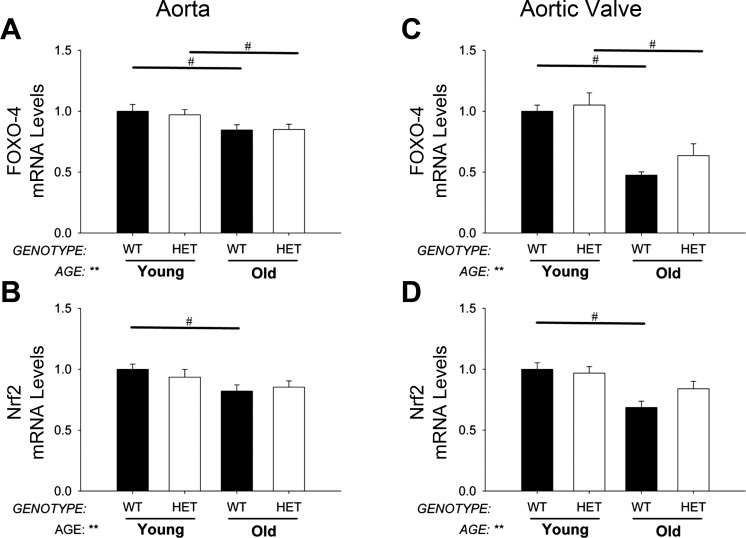
Expression of FOXO1, FOXO3, and FOXO4 were significantly reduced with aging, but these changes were not altered by MnSOD-deficiency (Fig. 2A). Nrf2 was slightly decreased with age but not affected by MnSOD-deficiency (Fig. 2B). Nqo1 and DJ-1 were relatively unchanged across both age and genotype groups (data not shown).
Fig. 2.
Expression of transcription factors regulating antioxidant gene expression. Increasing age significantly decreased FOXO-4 and Nrf2 gene expression in aorta (A and B) and aortic valve (C and D), but these changes were relatively unaffected by MnSOD haploinsufficiency. Values are means ± SE; n = 4–14 mice/group. **Significant main effect of age with P value < 0.01; #individual group differences with P < 0.05.
Genes regulating antioxidant responses in aortic valve.
Expression of FOXO1, FOXO3, and FOXO4 were reduced in aortic valve with aging, and, similar to aorta, these changes were not altered by MnSOD-deficiency (Fig. 2C). Reduction of MnSOD also did not affect age-related declines in Nrf2 (Fig. 2D) or DJ-1 (data not shown).
Pro-oxidant and pro-inflammatory gene expression in aorta.
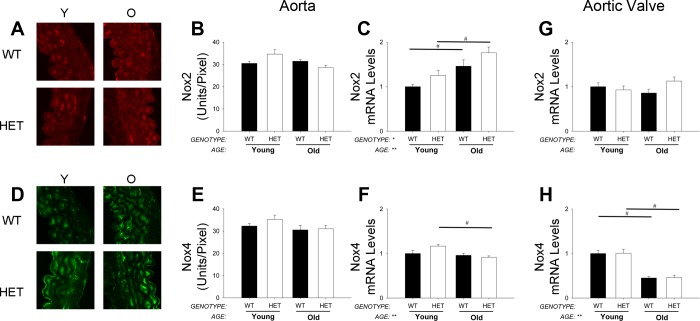
Similar to MnSOD+/+ mice, expression of Nox1 was not detectable in aorta from MnSOD+/− mice (data not shown), and expression of Nox4 was slightly but significantly reduced with aging only in MnSOD+/− mice (Fig. 3F). mRNA levels of Nox2 in aorta were elevated in both young and old MnSOD+/− mice compared with age-matched MnSOD+/+ mice (Fig. 3C) and were paralleled by changes in p47phox expression (data not shown). Although similar changes in Nox2 and Nox4 protein were observed in young cohorts of mice (Fig. 3, A and B and D and E), increases in Nox2 were not evident from immunohistochemical analysis of protein levels in aorta from aged mice (Fig. 3, A and B). Changes in expression of TNF-α were similar between MnSOD+/+ and MnSOD+/− mice within and across age groups (data not shown).
Fig. 3.
Expression of NAD(P)H oxidase subunits in aorta and aortic valve. Immunohistochemical analyses of mouse aorta suggested Nox2 protein levels were slightly increased from young (Y) mice (A: raw images; B: quantitation). mRNA levels of Nox2, however, were increased in aorta by aging and MnSOD deficiency (C). Conversely, Nox4 protein (D and E) and mRNA (F) levels were only modestly altered with aging in aorta. Interestingly, mRNA levels of Nox2 remained relatively unchanged in aortic valve from young and aged mice of either genotype (G). In aortic valve, Nox4 mRNA levels were significantly reduced with age but were not affected by MnSOD deficiency (H). Values are means ± SE; n = 4–14 mice/group. *Significant genotype effect; **significant age effect with P value < 0.01; #significance between young MnSOD+/− and old (O) MnSOD+/− groups. Nox, NAD(P)H oxidase. Protein levels are expressed as fluorescent units per area of tissue.
Pro-oxidant and pro-inflammatory gene expression in aortic valve.
In aortic valves from MnSOD+/− mice, Nox4 mRNA levels were decreased by aging to a similar extent to that observed in MnSOD+/+ mice (Fig. 3H). Unlike aorta, however, mRNA levels of Nox2 were not increased in MnSOD+/− mice compared with age-matched MnSOD+/+ mice (Fig. 3G). Expression of ICAM, VCAM, and TNF-α in young and aged MnSOD+/− mice were similar to levels observed in their age-matched MnSOD+/+ littermates (data not shown).
Superoxide and H2O2 analysis in aorta.
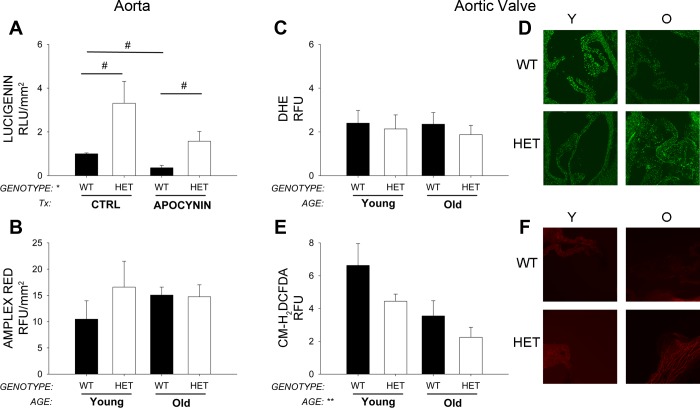
Lucigenin-enhanced chemiluminescence suggested that superoxide levels were significantly increased in young MnSOD+/− compared with young MnSOD+/+ mice (Fig. 4A). After incubation with apocynin, there were significant reductions in superoxide levels in MnSOD+/− mice (Fig. 4A). Similar changes were observed in a small subset of aged mice (data not shown).
Fig. 4.
Superoxide and H2O2 analysis in aorta and aortic valve. Superoxide levels measured by lucigenin are significantly increased in aorta from MnSOD+/− mice compared with aorta from MnSOD+/+ mice (A; Tx, in vitro drug treatment conditions). Hydrogen peroxide levels tended to be increased with age in aorta, but these changes were not affected by MnSOD-haploinsufficiency (B). In aortic valve, dihydroethidium (DHE) fluorescence was largely unchanged by aging or MnSOD-haploinsufficiency and age (C and D). Surprisingly, hydrogen peroxide levels were reduced in aortic valve with increasing age and MnSOD-deficiency (E and F). Values are means ± SE; n = 4–14 mice/group. *Significant genotype effect; **significant age effect with P value < 0.01; #individual group differences with P < 0.05. Ctrl, control; CM-H2DCFDA, carboxymethyl-dichlorofluorescein diacetate.
Amplex red fluorescence suggested that H2O2 levels were slightly increased in young MnSOD+/− mice compared with young MnSOD+/+ mice (Fig. 4B). Hydrogen peroxide levels also tended to be increased aged MnSOD+/+ mice but were not increased further in MnSOD+/− mice.
Superoxide and H2O2 analysis in aortic valve.
Surprisingly, superoxide levels were unchanged in aortic valve across all groups (Fig. 4, C and D). H2O2 levels in aortic valve were also decreased with MnSOD-deficiency and with age (Fig. 4, E and F).
Changes in NOS isoform expression in aorta.
In aorta, eNOS expression was not affected by aging in MnSOD+/+ or MnSOD+/− mice. Similarly, expression of iNOS was not significantly affected by aging in MnSOD+/+ or MnSOD+/− mice (Table 1).
Table 1.
Effects of aging and MnSOD-haploinsufficiency on expression of NOS isoforms
| Aorta |
Aortic Valve |
|||||||
|---|---|---|---|---|---|---|---|---|
| Young |
Old |
Young |
Old |
|||||
| WT | Het | WT | Het | WT | Het | WT | Het | |
| NOS isoforms | ||||||||
| Endothelial NOS | 1.0 ± 0.1 | 1.1 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 0.7 ± 0.2** | 0.7 ± 0.1** |
| Inducible NOS | 1.0 ± 0.3 | 0.9 ± 0.3 | 1.9 ± 0.7 | 1.4 ± 0.3 | 1.0 ± 0.3 | 0.95 ± 0.3 | 3.7 ± 0.8** | 5.0 ± 1.0** |
| Neuronal NOS | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
Values are means ± SE; n = 4–14 mice/group. WT, wild-type; HET, heterozygous; MnSOD, manganese SOD; NOS, nitric oxide synthase; n.d., not detectable.
Significance versus young mice of same genotype.
Changes in NOS isoform expression in aortic valve.
MnSOD-deficiency did not alter eNOS expression in aortic valves from young mice, and MnSOD+/− mice had similar age-related reductions in eNOS compared with MnSOD+/+ littermates (Table 1). Age-related increases in iNOS expression in aortic valve were similar between MnSOD+/+ and MnSOD+/− mice (Table 1). Expression of neuronal NOS was not detectable in young or aged MnSOD+/− mice (Table 1).
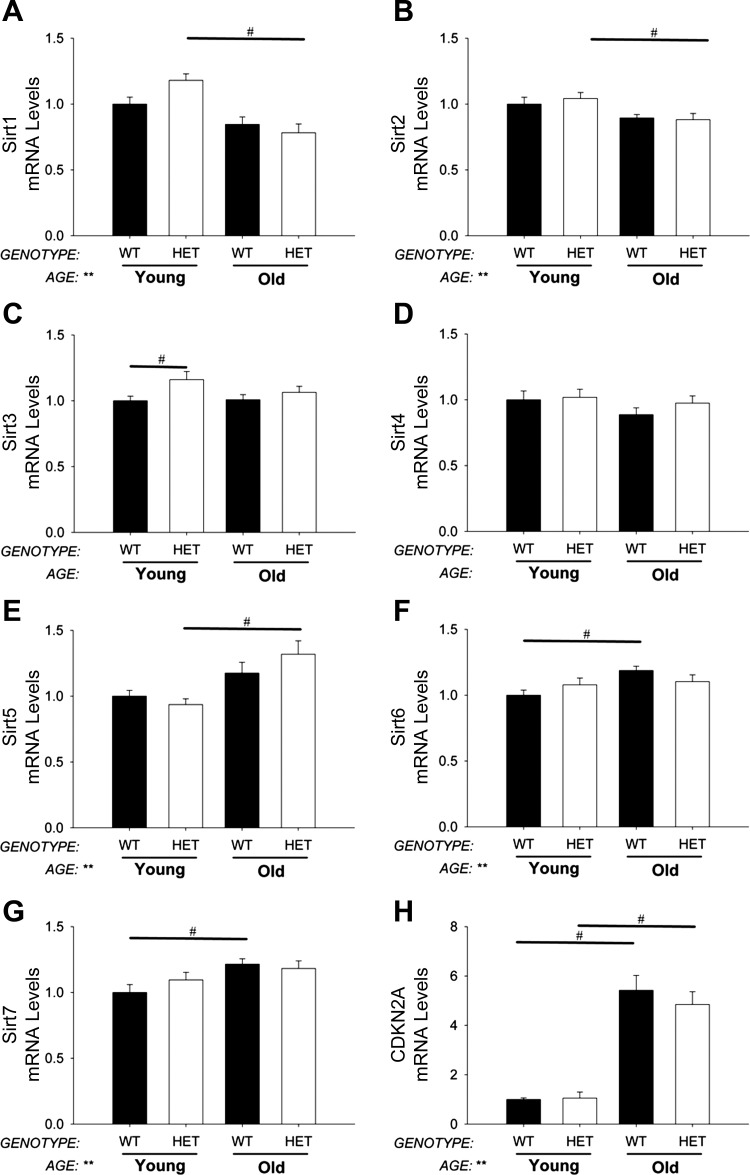
Aging- and senescence-related gene expression in aorta.
There were significant age-related decreases in expression of SIRT1, SIRT2 (Fig. 5, A and B), and WRN (data not shown) in aorta. SIRT5, SIRT6, and SIRT7 were increased with age but were not affected by reduction of MnSOD (Fig. 5, E–G). Expression of CDKN2A was significantly increased in aged mice but was not affected by reduction of MnSOD (Fig. 5H).
Fig. 5.
Expression of aging- and senescence-related genes in aorta. Sirtuin (Sirt) expression is largely unchanged with aging (A–G). Cyclin-dependent kinase inhibitor 2A (CDKN2A) expression, a marker of cellular senescence, was significantly increased by aging in aorta (H). MnSOD-haploinsufficiency did not significantly impact sirtuin or CDKN2A expression in the aorta from young or aged mice. Values are means ± SE; n = 4–14 mice/group. *Significant main effect of genotype with P < 0.01; **significant main effect of age with P value < 0.01; #individual group differences with P < 0.05.
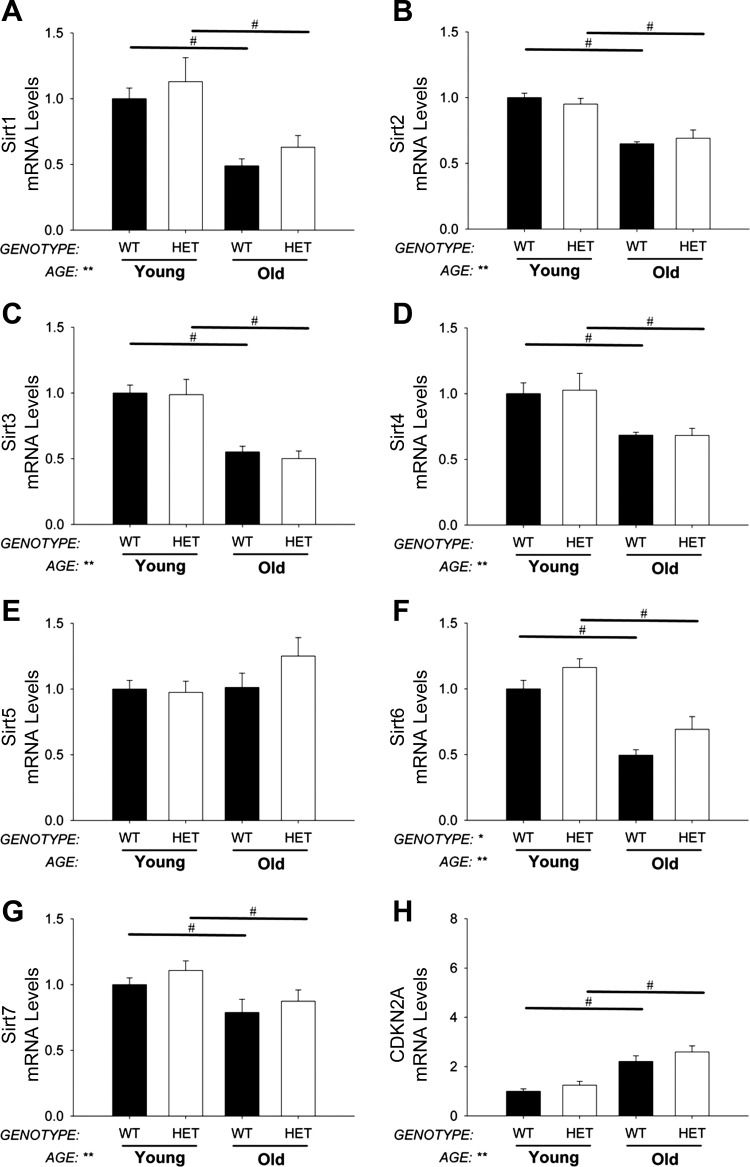
Aging- and senescence-related gene expression levels in aortic valve.
Expression of sirtuin isoforms was not affected by MnSOD deficiency in young mice, and age-related reductions in SIRT1, SIRT2, SIRT3, SIRT4, SIRT6, SIRT7 (Fig. 6, A–D and F and G), and Wrn (data not shown) were similar in aortic valves from MnSOD+/− and MnSOD+/+ mice. Expression of SIRT5, however, was increased in aged MnSOD+/− mice compared with young MnSOD+/− mice (Fig. 6E). Expression of CDKN2A was significantly increased in aged mice and was not affected by MnSOD deficiency (Fig. 6H).
Fig. 6.
Expression of aging- and senescence-related genes in aortic valve. Expression of most sirtuin isoforms is significantly reduced by aging in aorta (A–G). CDKN2A expression, a marker of cellular senescence, was significantly increased by aging in aortic valve (H). MnSOD-haploinsufficiency did not significantly impact sirtuin or CDKN2A expression in the aortic valve from young or aged mice. Values are means ± SE; n = 4–14 mice/group. *Significant main effect of genotype with P < 0.01; **significant main effect of age with P value < 0.01; #individual group differences with P < 0.05.
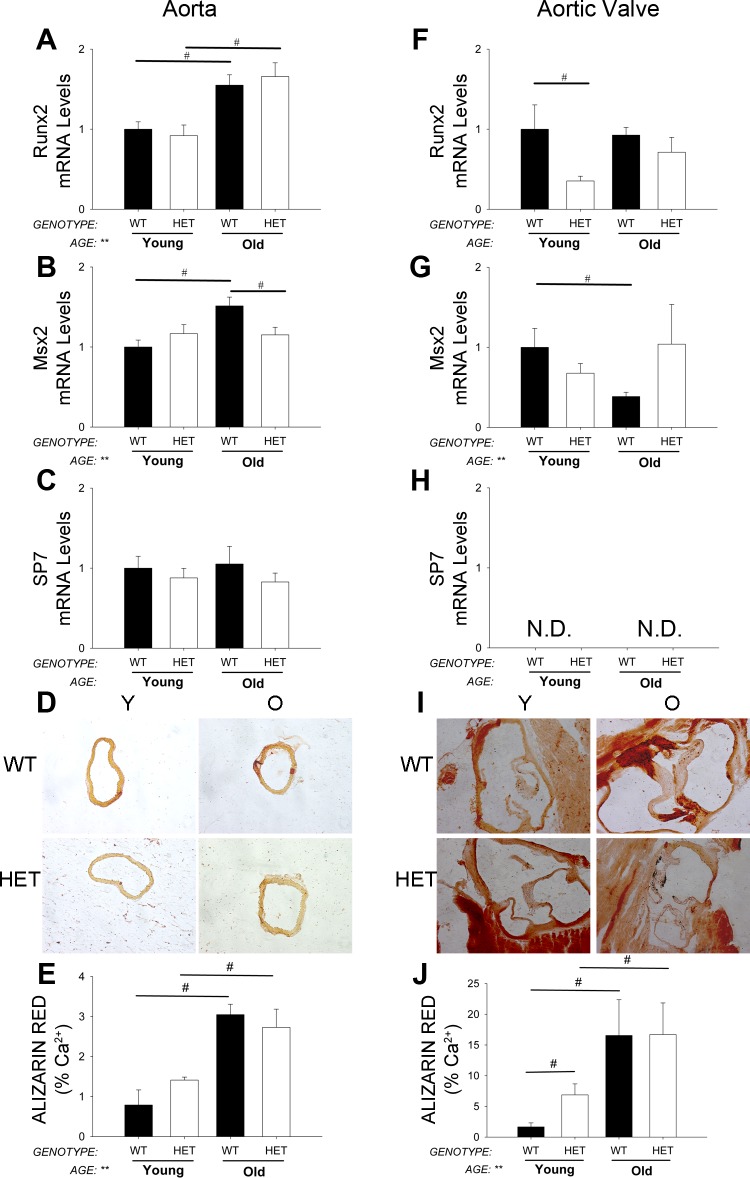
Pro-osteogenic gene expression levels and calcium deposition in aorta.
Runx2 and Msx2 were significantly increased with aging in aorta (Fig. 7, A and B), but expression of Sp7 was unchanged (Fig. 7C). Similarly, calcium burden was also significantly increased with aging in MnSOD+/+ mice (Fig. 7, D and E). MnSOD-deficiency, however, did not augment age-related increases in aortic calcium (Fig. 7, D and E).
Fig. 7.
Expression of osteogenic genes and quantification of calcium. Early markers of osteogenesis (A and B; quantitative real-time RT-PCR) and calcium burden (D and E; Alizarin red micrographs and quantitation) were significantly increased by aging and were not affected by MnSOD-haploinsufficiency in aorta. SP7 mRNA levels, however, were unchanged across all groups in aorta (C). In aortic valve, mRNA levels of osteogenic genes were relatively unchanged or even tended to decrease with aging, and these changes were not significantly altered by MnSOD-haploinsufficiency (F–H). Calcium burden, however, was significantly increased with age in mice from both genotypes (I and J). Values are means ± SE; n = 4–14 mice/group. *Significant main effect of genotype with P < 0.01; **significant main effect of age with P value < 0.01; #individual group differences with P < 0.05. ND, not detectable.
Pro-osteogenic gene expression levels and calcium burden in aortic valve.
Surprisingly, expression of pro-osteogenic markers was not increased with age (Fig. 7, F–H). Osteogenic gene expression was not significantly affected by MnSOD-deficiency in aortic valve (Fig. 7, F–H). Calcium burden, however, was substantially increased with age but was not affected by reductions in MnSOD levels (Fig. 7, I and J).
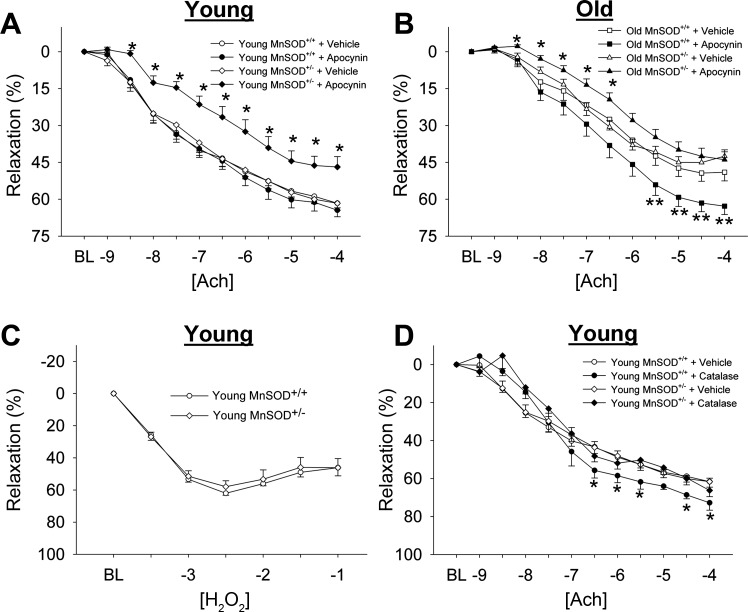
Vasomotor function.
Vascular relaxation to acetylcholine was significantly reduced in aged MnSOD+/+ mice compared with young MnSOD+/+ mice. Inhibition of NAD(P)H oxidase with apocynin did not affect responses to acetylcholine in young MnSOD+/+ mice, and significantly improved responses to acetylcholine in aged MnSOD+/+ mice (Fig. 8, A and B). Responses to sodium nitroprusside were unaffected by aging (Table 2). Maximum tension in response to PGF2α was augmented in aged MnSOD+/+ mice compared with young MnSOD+/+ mice (Table 2).
Fig. 8.
Vasomotor function in aorta. A and B: vasomotor responses to ACh in young and old mice. Vessels were treated with vehicle or with apocynin, an NAD(P)H oxidase inhibitor. Note the relaxation to ACh is significantly impaired in MnSOD+/− mice after incubation of apocynin. *Significance in HET treatment group vs. HET control group with P < 0.05; **significance in WT treatment group vs. WT control group with P < 0.05. C: vasomotor responses to hydrogen peroxide. D: vasomotor responses to ACh after incubation with catalase. BL, baseline level of preconstruction before initiation of the dose-response curve. *Significance in treatment group vs. control group with P < 0.05. Values are means ± SE; n = 8–23 mice/group.
Table 2.
Effects of aging and MnSOD-haploinsufficiency on vasomotor function in aorta
| Aorta |
||||
|---|---|---|---|---|
| Young |
Old |
|||
| WT | Het | WT | Het | |
| Relaxation | ||||
| Maximum relaxation to ACh, % | 62 ± 3 | 62 ± 1 | 49 ± 3** | 43 ± 3** |
| Concentration of ACh eliciting 50% of maximum relaxation to ACh | 7.5 ± 0.13 | 7.2 ± 0.1 | 7.0 ± 0.2 | 7.1 ± 0.1 |
| Maximum relaxation to sodium nitroprusside, % | 90 ± 2 | 91 ± 2 | 94 ± 2 | 96 ± 1** |
| Constriction | ||||
| Peak tension (PGF2α) | 1.2 ± 0.04 | 1.1 ± 0.06 | 1.78 ± 0.1** | 1.74 ± 0.1** |
| Peak tension (5HT) | 0.68 ± 0.07 | 0.68 ± 0.07 | 0.90 ± 0.04** | 0.86 ± 0.04** |
Values are means ± SE; n = 4–14 mice/group.
Significance versus young mice of same genotype.
Relaxation to acetylcholine was not impaired in aorta from young MnSOD+/− mice compared with MnSOD+/+ littermates, and age-related impairments in endothelial function were similar in aorta from the two genotypes of mice (Fig. 8, A and B). In contrast, NAD(P)H oxidase inhibition significantly impaired responses to acetylcholine in both young and old MnSOD+/− mice (Fig. 8, A and B). Similar responses were observed following incubation with gp91ds-TAT, an inhibitor of the Nox2 catalytic subunit (data not shown). Addition of exogenous hydrogen peroxide did not result in vasodilation at low concentrations (data not shown) but did elicit substantial vasodilation at relatively high concentrations (Fig. 8C). Interestingly, incubation of vessels with catalase improved relaxation to acetylcholine in MnSOD+/+ mice but did not alter relaxation to acetylcholine in aorta from MnSOD+/− mice (Fig. 8D). Responses to sodium nitroprusside were similar between MnSOD+/− and MnSOD+/+ mice in both age groups (Table 2). Responses to PGF2α in MnSOD+/− mice were also similar to those observed in MnSOD+/+ mice within both age groups (Table 2).
Aortic valve function.
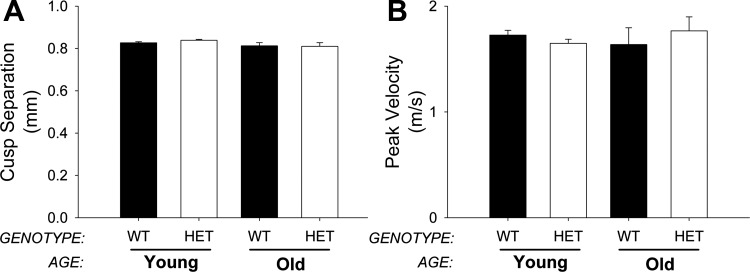
Aortic valve cusp separation was not significantly different between young and old MnSOD+/+ mice and was not altered by MnSOD haploinsufficiency (Fig. 9A). Similarly, peak transvalvular velocity was largely unchanged between groups (Fig. 9B). Changes in cardiac function did not contribute significantly to alterations in aortic valve function across genotypes or with aging (data not shown), and we did not detect differences in the prevalence of aortic valve regurgitation between the two groups (data not shown).
Fig. 9.
Aortic valve function. Cusp separation (A) and peak transvalvular velocity (B) were measured by echocardiography. MnSOD-haploinsufficiency did not affect aortic valve function and was not affected by age. Values are means ± SE; n = 8–23 mice/group.
Blood pressure.
Similar to previous reports, systolic blood pressure was not affected by MnSOD haploinsufficiency in young mice but was markedly increased in aged MnSOD+/− mice compared with their MnSOD+/+ littermates (data not shown).
DISCUSSION
The main findings of this study are 1) there are dramatically different transcriptional responses to aging in aorta and aortic valve, 2) the functional consequences of NAD(P)H oxidase-derived radicals are critically dependent on mitochondrial antioxidant levels in aorta in both young and aged mice, 3) despite apparent deleterious molecular changes, aortic valve function is remarkably well preserved with increasing age in mice, and 4) reductions in mitochondrial antioxidant capacity modulate, but do not independently initiate, osteogenic signaling in aorta.
Transcriptional responses to aging in aorta.
In aorta from wild-type mice, we found that aging was associated with changes in transcriptional patterns that favored a pro-oxidative environment. Specifically, levels of ecSOD and CuZnSOD were significantly reduced and expression of Nox2 was significantly increased in aorta from aged wild-type mice. This reduced antioxidant capacity is consistent with data from previous reports in aging mice and may be in part due to age-associated reductions the transcription factors DJ-1 and members of the FOXO family, ultimately resulting in reductions in Nrf2 (8, 38). NAD(P)H oxidase-derived ROS have also been shown to be increased with aging and to play a key role in limiting nitric oxide bioavailability (20, 33) and promoting fibrosis in aged conduit vessels (17). When combined with age-related reductions in eNOS and SIRT1 expression, these molecular changes would be expected to strongly favor perivascular fibrosis and vascular stiffening with aging (16).
In the present study we found that Nox2 expression in aorta was increased in young and old MnSOD-deficient mice compared with their wild-type littermates. This finding is consistent with previous work in cultured endothelial cells demonstrating a positive feedback loop between mitochondria and NAD(P)H oxidase-derived ROS (15), a phenomenon more commonly referred to as ROS-induced ROS release. This pro-oxidative environment did not, however, augment age-related reductions in expression of eNOS or sirtuin enzymes, nor did these changes augment age-associated increases in pro-calcific gene expression, vascular calcification, or vasomotor dysfunction. Thus, at least in the context of these processes, these data provide compelling evidence against the oxidative stress hypothesis of cardiovascular aging (2) and instead support a working model in which ROS-induced ROS-release initiated by losses in mitochondrial antioxidant capacity is not a primary driver of molecular changes promoting age-related vascular disease. Instead, alterations in mitochondrial oxidative stress may alter age-related vascular phenotypes in a highly context-dependent manner in aorta and may require additional stressors (e.g., hypercholesterolemia) to elicit a phenotypic/functional consequence.
Transcriptional responses to aging in aortic valve.
Similar to our findings in aorta, our data from aortic valves suggest that age-related reductions in antioxidant expression in wild-type mice also favor a pro-oxidative environment. Unlike aorta, however, expression of NAD(P)H oxidase subunits was not increased with aging, and expression of Nox4 was significantly decreased. To our knowledge, these are the first data characterizing changes in pro- and anti-oxidant enzymes in aged (but functionally normal) aortic valves and the first to identify a fundamental difference in expression of redox-related genes in aorta and aortic valve with aging. Although these age-related reductions in antioxidant gene expression are remarkably similar to those observed in stenotic aortic valves (27), we did not observe increases in procalcific (Fig. 7) or profibrotic (data not shown) gene expression in aortic valves from aged MnSOD+/+ mice. Thus our data suggest that aging-associated reductions in antioxidant levels are not a primary driver of profibrotic and procalcific signaling in aortic valve. Alternatively, activation of profibrotic signaling may be more closely linked to NAD(P)H oxidase activation. More specifically, expression of Nox4 was significantly reduced in aortic valves from aged mice, and Nox4 has been shown to be a critical component to the induction of profibrotic signaling cascades in myocardium (11) and is likely to augment profibrotic signaling in aged aorta (17, 18).
The lack of an increase in profibrotic and procalcific gene expression in aortic valves from aged MnSOD+/+ mice was particularly surprising given the observation that expression of multiple sirtuin isoforms was markedly reduced in aortic valves with aging. Sirtuin deacetylases are emerging as important regulators of gene expression and function in the cardiovascular system. In particular, SIRT1 has been shown to inhibit TGF-β-induced gene expression and fibrosis in aorta and myocardium with aging (23) and has been shown to be critical for maintenance of NOS function (6, 20). Interactions between TGF-β superfamily signaling, increases in NAD(P)H oxidase-derived ROS, and epigenetics in aorta are clearly in need of further investigation.
In aortic valves from MnSOD+/− mice, we did not observe significant changes in NAD(P)H oxidase expression. Importantly, these are the first data suggesting that mitochondria-initiated ROS-induced ROS release may occur in a highly tissue specific manner (and does not occur in aortic valve) and represent a fundamental difference in the molecular responses to aging and increased mitochondrial oxidative stress in aorta and aortic valve. Similar to our findings in aorta from MnSOD+/− mice, however, MnSOD deficiency did not elicit further reductions in sirtuin expression or increases in profibrotic signaling in aortic valves from young or aged mice. Collectively, these data suggest that reductions in MnSOD are not likely to be a primary driver of profibrotic or procalcific gene expression in aortic valves from young or aged mice and instead may play a modulatory or permissive role when profibrotic or procalcific stimuli are present.
Functional consequences of aging and MnSOD deficiency in aorta.
We observed significant reductions in endothelial function with aging in wild-type mice that were largely reversible with the NAD(P)H oxidase inhibitors apocynin or gp91ds-tat. This is consistent with previous work demonstrating that NAD(P)H oxidase (and Nox2-derived radicals in particular) are important contributors to age-related declines in endothelial function (10, 20, 33).
Consistent with previous reports, we also found that endothelial function was not impaired in young MnSOD+/− mice compared with their MnSOD+/+ littermates (1, 4). We were, however, somewhat surprised to find that endothelial function in aged mice was not further impaired by MnSOD haploinsufficiency, as other groups have reported greater impairment of endothelial function in aged MnSOD+/− mice (1, 4). One potential explanation for this difference in findings is that our aged mice were significantly younger than those used in previous studies (∼18 mo old vs. 24+ mo old). Differences in animal husbandry, cumulative environmental stress, and diet may also significantly impact metabolic state and endothelial function with aging (21).
A major novel finding of this study, however, is that inhibition of NAD(P)H oxidase significantly impaired endothelial function in both young and aged MnSOD+/− mice. This finding was contrary to our hypothesis that ROS-induced ROS-release would impair endothelial function in aorta. Numerous studies have shown that, under a variety of stressed conditions, Nox2 or mitochondria-derived radicals impair endothelial function via reductions in nitric oxide bioavailability (7, 14, 15, 40). Conversely, several studies have reported that mitochondria-derived H2O2 elicits vasodilation in coronary arteries (24, 35, 36, 42) and that Nox4-derived ROS may play a central role in maintaining nitric oxide bioavailability (37). Interestingly, incubation of vessels from MnSOD+/+ with catalase improved relaxation to acetylcholine, an effect that is likely due to increased NO bioavailability. In contrast, vascular responses to acetylcholine were not improved by catalase in vessels from MnSOD+/− mice. To explain these findings, we suggest that reductions in H2O2 result in two key changes that serve to counterbalance one another, namely, a reduction in H2O2-dependent endothelium-derived hyperpolarizing factor (EDHF) activity and an increase in nitric oxide bioavailability due to decreased H2O2-driven peroxynitrite formation. Our observation that addition of exogenous H2O2 elicits similar relaxation in aorta from MnSOD+/+ and MnSOD+/− mice supports the notion that H2O2 can act as an EDHF in mouse aorta regardless of mitochondrial antioxidant status and suggests that the net effect of H2O2 scavenging in aorta is dependent upon the relative contributions of nitric oxide and endothelium-derived EDHFs. Collectively, our data support a working model in which the vasomotor consequences of increased hydrogen peroxide levels are dependent not only on the level of physiological stress an organism is facing but also on mitochondrial antioxidant status.
Functional consequences of aging and MnSOD deficiency in aortic valve.
Aortic valve function in wild-type mice was unchanged with increasing age. Somewhat surprisingly, MnSOD deficiency did not impact aortic valve function compared with wild-type mice in both age groups. We anticipated that losses in mitochondrial antioxidant capacity would negatively impact aortic valve function with aging, due to previous studies suggesting that increases in oxidative stress are strongly associated with calcification in tissue from humans with calcific aortic valve disease (27), and experimental data suggesting that increases in oxidative stress and reductions in nitric oxide bioavailability accelerate cardiovascular cell calcification in vitro (5, 22). A possible explanation for the contrast in findings is that the mice used in this study were not exposed to significant exogenous stressors (e.g., high fat diet, hypertensive stress, etc.), and the combination of hypercholesterolemia and oxidative stress is needed to induce aortic valve disease in aging mice (29–31).
Limitations.
An important limitation to the current study relates to the quantitation of protein levels in aortic valve tissue. More specifically, the normal murine aortic valve yields exceedingly small amounts of protein, which make it not conducive for Western blotting or other quantitative measurements of protein levels. Furthermore, there is a limited amount of tissue that can be obtained for histology and/or immunohistochemical analyses of protein levels (especially when multiple sections are used during the analysis of each stain as was the case herein). Thus, following evaluation of ROS and calcium levels, we did not have sufficient samples remaining to examine changes in protein levels of NADPH oxidase isoforms in valves from aged animals. Close examination of our young cohort, however, suggests that key molecular changes that occur from protein in aorta can be approximated from changes in mRNA levels (e.g., Nox2/Nox4). This is particularly impressive given the fact that we would only expect gross correlations between the magnitude of change in mRNA from aortic homogenates and changes in aortic protein levels from fluorescent immunohistochemistry given the semi-quantitative nature of the latter.
Similar limitations exist for evaluation of ROS in murine tissues, where the mass/volume of tissue that can be obtained often requires substantial pooling of tissue for quantitative methods (which is logistically difficult with aging animals), and semi-quantitative measurement using fluorescent dyes, such as dihydroethidium, is a well-established alternative approach in such limited tissues.
Finally, our mechanistic studies of aortic endothelial function were done predominantly in young mice (e.g., multiple inhibitors and/or hydrogen peroxide-induced vascular relaxation). Although it is possible that different compensatory mechanisms may evolve throughout the lifetime of an aging animal, our fundamental observation that inhibition of NAD(P)H oxidase paradoxically impairs endothelium-dependent relaxation in MnSOD-deficient mice was robust in both young and aged animals. Thus we believe it is highly likely that similar mechanisms (i.e., generation of an endothelium-derived EDHF in MnSOD-deficient mice) are present and conserved in both young and aged mice.
Conclusions
Collectively, our data demonstrate that the transcriptional responses and phenotypes elicited by aging and alterations in mitochondrial antioxidant capacity differ dramatically between aorta and aortic valve. Furthermore, the functional consequences of NAD(P)H-derived ROS and ROS-induced ROS-release appear to be critically dependent upon mitochondrial antioxidant capacity in aorta from unstressed mice. These data support a working model in which age-related reductions in mitochondrial antioxidant capacity are an important modulator, but not primary driver, of age-related cardiovascular disease.
GRANTS
This study was supported by funding from National Heart, Lung, and Blood Institute Grants HL-092235 and HL-111121 (to J. D. Miller), the Mayo Clinic Center for Regenerative Medicine, and the Mayo Clinic Kogod Center on Aging.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.M.R. and J.D.M. conception and design of research; C.M.R., M.A.H., B.Z., E.A.O., and A.A. performed experiments; C.M.R., M.A.H., E.A.O., A.A., and J.D.M. analyzed data; C.M.R., M.A.H., E.A.O., and J.D.M. interpreted results of experiments; C.M.R. prepared figures; C.M.R. drafted manuscript; C.M.R., M.A.H., B.Z., E.A.O., A.A., and J.D.M. approved final version of manuscript; M.A.H., B.Z., A.A., and J.D.M. edited and revised manuscript.
REFERENCES
- 1.Andresen JJ, Faraci FM, Heistad DD. Vasomotor responses in MnSOD-deficient mice. Am J Physiol Heart Circ Physiol 287: H1141–H1148, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Bachschmid MM, Schildknecht S, Matsui R, Zee R, Haeussler D, C RA, Pimental D, Loo BV. Vascular aging: chronic oxidative stress and impairment of redox signaling-consequences for vascular homeostasis and disease. Ann Med 45: 17–36, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown KA, Chu Y, Lund DD, Heistad DD, Faraci FM. Gene transfer of extracellular superoxide dismutase protects against vascular dysfunction with aging. Am J Physiol Heart Circ Physiol 290: H2600–H2605, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Brown KA, Didion SP, Andresen JJ, Faraci FM. Effect of aging, MnSOD deficiency, and genetic background on endothelial function: evidence for MnSOD haploinsufficiency. Arterioscler Thromb Vasc Biol 27: 1941–1946, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Byon CH, Javed A, Dai Q, Kappes JC, Clemens TL, Darley-Usmar VM, McDonald JM, Chen Y. Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor Runx2 by AKT signaling. J Biol Chem 283: 15319–15327, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Z, Peng IC, Cui X, Li YS, Chien S, Shyy JY. Shear stress, SIRT1, and vascular homeostasis. Proc Natl Acad Sci USA 107: 10268–10273, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chrissobolis S, Banfi B, Sobey CG, Faraci FM. Role of Nox isoforms in angiotensin II-induced oxidative stress and endothelial dysfunction in brain. J Appl Physiol 113: 184–191, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins AR, Lyon CJ, Xia X, Liu JZ, Tangirala RK, Yin F, Boyadjian R, Bikineyeva A, Pratico D, Harrison DG, Hsueh WA. Age-accelerated atherosclerosis correlates with failure to upregulate antioxidant genes. Circ Res 104: e42–e54, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Csiszar A, Labinskyy N, Jimenez R, Pinto JT, Ballabh P, Losonczy G, Pearson KJ, de Cabo R, Ungvari Z. Anti-oxidative and anti-inflammatory vasoprotective effects of caloric restriction in aging: role of circulating factors and SIRT1. Mech Ageing Dev 130: 518–527, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Csiszar A, Ungvari Z, Edwards JG, Kaminski P, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res 90: 1159–1166, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Cucoranu I, Clempus R, Dikalova A, Phelan PJ, Ariyan S, Dikalov S, Sorescu D. NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ Res 97: 900–907, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Didion SP, Kinzenbaw DA, Schrader LI, Faraci FM. Heterozygous CuZn superoxide dismutase deficiency produces a vascular phenotype with aging. Hypertension 48: 1072–1079, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Didion SP, Ryan MJ, Didion LA, Fegan PE, Sigmund CD, Faraci FM. Increased superoxide and vascular dysfunction in CuZnSOD-deficient mice. Circ Res 91: 938–944, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Dikalov S. Cross talk between mitochondria and NADPH oxidases. Free Radic Biol Med 51: 1289–1301, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dikalova AE, Bikineyeva AT, Budzyn K, Nazarewicz RR, McCann L, Lewis W, Harrison DG, Dikalov SI. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ Res 107: 106–116, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donato AJ, Magerko KA, Lawson BR, Durrant JR, Lesniewski LA, Seals DR. SIRT-1 and vascular endothelial dysfunction with ageing in mice and humans. J Physiol 589: 4545–4554, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fleenor BS, Marshall KD, Durrant JR, Lesniewski LA, Seals DR. Arterial stiffening with ageing is associated with transforming growth factor-β1-related changes in adventitial collagen: reversal by aerobic exercise. J Physiol 588: 3971–3982, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fleenor BS, Seals DR, Zigler ML, Sindler AL. Superoxide-lowering therapy with TEMPOL reverses arterial dysfunction with aging in mice. Aging Cell 11: 269–276, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fukai T. Extracellular SOD and aged blood vessels. Am J Physiol Heart Circ Physiol 297: H10–H12, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamilton CA, Brosnan MJ, Al-Benna S, Berg G, Dominiczak AF. NAD(P)H oxidase inhibition improves endothelial function in rat and human blood vessels. Hypertension 40: 755–762, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Imumorin IG, Dong Y, Zhu H, Poole JC, Harshfield GA, Treiber FA, Snieder H. A gene-environment interaction model of stress-induced hypertension. Cardiovasc Toxicol 5: 109–132, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Kennedy JA, Hua X, Mishra K, Murphy GA, Rosenkranz AC, Horowitz JD. Inhibition of calcifying nodule formation in cultured porcine aortic valve cells by nitric oxide donors. Eur J Pharmacol 602: 28–35, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Kume S, Haneda M, Kanasaki K, Sugimoto T, Araki S, Isshiki K, Isono M, Uzu T, Guarente L, Kashiwagi A, Koya D. SIRT1 inhibits transforming growth factor beta-induced apoptosis in glomerular mesangial cells via Smad7 deacetylation. J Biol Chem 282: 151–158, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, Bubolz AH, Mendoza S, Zhang DX, Gutterman DD. H2O2 is the transferrable factor mediating flow-induced dilation in human coronary arterioles. Circ Res 108: 566–573, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lund DD, Chu Y, Miller JD, Heistad DD. Protective effect of extracellular superoxide dismutase on endothelial function during aging. Am J Physiol Heart Circ Physiol 296: H1920–H1925, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mattagajasingh I, Kim CS, Naqvi A, Yamamori T, Hoffman TA, Jung SB, DeRicco J, Kasuno K, Irani K. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci USA 104: 14855–14860, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller JD, Chu Y, Brooks RM, Richenbacher WE, Pena-Silva R, Heistad DD. Dysregulation of antioxidant mechanisms contributes to increased oxidative stress in calcific aortic valvular stenosis in humans. J Am Coll Cardiol 52: 843–850, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller JD, Peotta VA, Chu Y, Weiss RM, Zimmerman K, Brooks RM, Heistad DD. MnSOD protects against COX1-mediated endothelial dysfunction in chronic heart failure. Am J Physiol Heart Circ Physiol 298: H1600–H1607, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller JD, Weiss RM, Heistad DD. Calcific aortic valve stenosis: methods, models, and mechanisms. Circ Res 108: 1392–1412, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller JD, Weiss RM, Serrano KM, Brooks RM, 2nd, Berry CJ, Zimmerman K, Young SG, Heistad DD. Lowering plasma cholesterol levels halts progression of aortic valve disease in mice. Circulation 119: 2693–2701, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller JD, Weiss RM, Serrano KM, Castaneda LE, Brooks RM, Zimmerman K, Heistad DD. Evidence for active regulation of pro-osteogenic signaling in advanced aortic valve disease. Arterioscler Thromb Vasc Biol 30: 2482–2486, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mostoslavsky R, Chua KF, Lombard DB, Pang WW, Fischer MR, Gellon L, Liu P, Mostoslavsky G, Franco S, Murphy MM, Mills KD, Patel P, Hsu JT, Hong AL, Ford E, Cheng HL, Kennedy C, Nunez N, Bronson R, Frendewey D, Auerbach W, Valenzuela D, Karow M, Hottiger MO, Hursting S, Barrett JC, Guarente L, Mulligan R, Demple B, Yancopoulos GD, Alt FW. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell 124: 315–329, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Podlutsky A, Ballabh P, Csiszar A. Oxidative stress and endothelial dysfunction in pulmonary arteries of aged rats. Am J Physiol Heart Circ Physiol 298: H346–H351, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Rey FE, Cifuentes ME, Kiarash A, Quinn MT, Pagano PJ. Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular O2− and systolic blood pressure in mice. Circ Res 89: 408–414, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Saitoh S, Kiyooka T, Rocic P, Rogers PA, Zhang C, Swafford A, Dick GM, Viswanathan C, Park Y, Chilian WM. Redox-dependent coronary metabolic dilation. Am J Physiol Heart Circ Physiol 293: H3720–H3725, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Saitoh S, Zhang C, Tune JD, Potter B, Kiyooka T, Rogers PA, Knudson JD, Dick GM, Swafford A, Chilian WM. Hydrogen peroxide: a feed-forward dilator that couples myocardial metabolism to coronary blood flow. Arterioscler Thromb Vasc Biol 26: 2614–2621, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Schroder K, Zhang M, Benkhoff S, Mieth A, Pliquett R, Kosowski J, Kruse C, Luedike P, Michaelis UR, Weissmann N, Dimmeler S, Shah AM, Brandes RP. Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase. Circ Res 110: 1217–1225, 2012 [DOI] [PubMed] [Google Scholar]
- 38.Ungvari Z, Bailey-Downs L, Gautam T, Sosnowska D, Wang M, Monticone RE, Telljohann R, Pinto JT, de Cabo R, Sonntag WE, Lakatta EG, Csiszar A. Age-associated vascular oxidative stress, Nrf2 dysfunction, and NF-κB activation in the nonhuman primate Macaca mulatta. J Gerontol A Biol Sci Med Sci 66: 866–875, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weiss RM, Ohashi M, Miller JD, Young SG, Heistad DD. Calcific aortic valve stenosis in old hypercholesterolemic mice. Circulation 114: 2065–2069, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Wind S, Beuerlein K, Armitage ME, Taye A, Kumar AH, Janowitz D, Neff C, Shah AM, Wingler K, Schmidt HH. Oxidative stress and endothelial dysfunction in aortas of aged spontaneously hypertensive rats by NOX1/2 is reversed by NADPH oxidase inhibition. Hypertension 56: 490–497, 2010 [DOI] [PubMed] [Google Scholar]
- 41.Yoshii T, Iwai M, Li Z, Chen R, Ide A, Fukunaga S, Oshita A, Mogi M, Higaki J, Horiuchi M. Regression of atherosclerosis by amlodipine via anti-inflammatory and anti-oxidative stress actions. Hypertens Res 29: 457–466, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Zhang DX, Borbouse L, Gebremedhin D, Mendoza SA, Zinkevich NS, Li R, Gutterman DD. H2O2-induced dilation in human coronary arterioles: role of protein kinase G dimerization and large-conductance Ca2+-activated K+ channel activation. Circ Res 110: 471–480, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]