Abstract
The sympathetic nervous system plays a pivotal role in homeostasis through its direct innervation and functional impact on a variety of end organs. In rats, a number of methods are available to assess sympathetic nervous system function. Traditionally, direct recording of sympathetic nerve activity (SNA) has been restricted to acute, anesthetized preparations or conscious animals within a few days after electrode implantation. However, these approaches provide short-term data in studies designed to investigate changes in SNA during chronic disease states. Over the last several years, chronic SNA recording has been pioneered in rabbits and more recently in rats. The purpose of this article is to provide insights and a “how to” guide for chronic SNA recordings in rats based on experiences from two independent laboratories. We will present common methodologies used to chronically record SNA, characteristics and methods to distinguish sympathetic bursts versus electrical artifacts (and provide corresponding audio clips when available), and provide suggestions for analysis and presentation of data. In many instances, these same guidelines are applicable to acute SNA recordings. Using the surgical approaches described herein, both laboratories have been able to chronically record SNA in >50% of rats for a duration >3 wk. The ability to record SNA over the time course of several weeks will, undoubtedly, greatly impact the field of autonomic and cardiovascular physiology.
Keywords: renal, lumbar, splanchnic, telemetry, blood pressure
this article is part of a collection on Standards and Guidelines. Other articles appearing in this collection, as well as a full archive of all collections, can be found online at http://ajpheart.physiology.org/.
Introduction
The sympathetic nervous system plays a pivotal role in homeostasis through its direct innervation and functional influence on a variety of organs including the vasculature, kidney, adrenal gland, heart, and gastrointestinal tract. Importantly, altered sympathetic nervous system function is a well-established contributor to the development, maintenance, and pathophysiology of numerous cardiovascular diseases (9, 12, 18, 22, 33). A number of methods are available to assess sympathetic nervous system function including measurement of plasma, urinary, and tissue catecholamines; acute or chronic blockade of ganglionic transmission or peripheral adrenergic receptors; nerve transection/organ denervation; tissue or regional norepinephrine spillover; spectral analysis; and direct recording of sympathetic nerve activity (SNA). Each of these approaches has unique advantages and disadvantages. For example, spectral analysis or measurement of norepinephrine can be readily performed in chronic disease states; however, these approaches may have limited sensitivity or lack the ability to detect changes in sympathetic outflow to a specific end organ. Indeed, the importance of specificity was recently shown in studies with humans and experimental models, which demonstrated that the central nervous system can differentially control sympathetic outflow by increasing SNA to one organ while decreasing SNA to another (9, 21, 28, 29, 35, 37).
Traditionally, direct recording of SNA has been performed in anesthetized preparations or conscious animals within 0–48 h after implantation of the electrodes (the duration of the recordings have been limited because of nerve viability). While such recordings can provide insight into differential control of SNA and sympathetic nervous system function, this approach has been limited by effects of anesthesia, stress due to surgery, difficulties associated with between animal comparisons of SNA, and the lack of temporal resolution during chronic disease states due to short-term nerve viability. Truly chronic recordings of SNA, lasting greater than several days, have been pioneered in rabbits for more than a decade (1–4, 6, 7, 10, 11, 22, 24, 34, 35). More recently, similar long-term recordings have been performed in rats (30, 39, 40), and the demonstration of renal sympathetic nerve recording for 5 days in mice by Hamza and Hall (13) indicates that chronic studies will soon be possible in this species. There is no doubt that the ability to record SNA over the time course of several weeks could greatly impact the field of autonomic and cardiovascular physiology. The purpose of this article is to provide practical insights into this approach from two independent laboratories that have successfully performed chronic SNA recordings in rats. We discuss common methodologies used within both laboratories to record chronic lumbar, renal, and splanchnic SNA, characteristics and methodologies to distinguish sympathetic bursts versus electrical artifacts or noise including corresponding audio clips (when available), and suggestions for analysis and presentation of data.
Instrumentation and Surgery
The methods and findings described herein reflect a collection of methodologies from the authors' laboratories and published research papers. To this end, the experimental procedures from the authors' laboratories conform to the National Institutes of Health's Guide for the Health and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committees at the Pennsylvania State University College of Medicine and Lehman College. The Stocker laboratory has used the Data Sciences International F50-W-F2 transmitter for SNA and PA-C40 transmitter for blood pressure. The transmitters can be placed in the peritoneal cavity and subcutaneously on the flank/back. Both laboratories have used the rechargeable Telemetry Research TR46SP transmitter that can record both blood pressure and SNA. Data from either system can be digitized, recorded, and analyzed using any analog-to-digital converter and software platform. It is noteworthy that the charging apparatus for the Telemetry Research System will produce electrical interference with the Data Sciences International System. Thus the end user will need to decide on the appropriate system and experimental design.
Animals and anesthesia.
Initially, investigators should use younger rats (200–300 g) that have less connective tissue, adipose tissue, and vascularization, providing an easier and less traumatic dissection. The surgical procedure should be conducted using sterile, aseptic technique with antibiotic therapy to avoid infection and accompanying inflammation. Postsurgical inflammation should be minimized by using analgesic agents such as Carprogen and Ketoprofen. Pain can also be alleviated with buprenex. Although all anesthetics can substantially alter arterial blood pressure (ABP) and SNA, our laboratories recommend isoflurane (1.5–2.5 in 100% O2). This is because characteristic renal, lumbar, and splanchnic sympathetic bursts having pulse or cardiac cycle-related activity can easily be visualized along with reasonable mean ABPs (70–100 mmHg) while using isoflurane anesthesia (1.5 in 100% O2).
Electrodes.
Whether the electrode wires are homemade (13, 25, 26, 40) or come prefabricated as a wireless telemetry unit (30, 39), they must be precut and prebent to exact dimensions for their designated implantation site [for an excellent example, see Hamza and Hall (13)]. This is because cutting and bending the wires in the restricted surgery space is very difficult and frequently results in damage to the selected nerve section. Once formed, the implantable electrode can be sterilized in ozone (TSO3), gluteraldehyde, or Cidex. In our hands, successful nerve recordings have been performed using single- or multi-stranded wire.
Surgical procedure and electrode implantation.
The nerve dissections can be visualized with a high-power dissecting microscope, and the addition of a foot-controlled powered focus drive decreases surgery time and improves sterility. For dissection of the nerve, there are several techniques that can improve long-term viability: 1) ensure a constant bathing of the dissection site in warmed, sterile saline (37.5°C); 2) choose a 2- to 3-mm section of nerve that is free of incoming blood supply; 3) avoid damage to nearby lymph vessels or blood vessels that will produce leakage of fluid into the area; 4) gently isolate the selected nerve section from the surrounding tissue using fine, straight forceps (No. 5 micro-dissecting tweezers, Roboz Surgical Instrument, Rockville, MA) or fine, round-ended, straight glass probes (made by pulling 100-μl glass pipets over a flame and melting the broken ends into a rounded shape); and 5) take great care not to stretch, pick up, pull, or touch the nerve section during the dissection (10, 13, 22, 25).
The lumbar sympathetic nerve can be isolated through a ventral midline abdominal incision. After the intestines are gently retracted, the abdominal aorta or vena cava caudal to the renal vessels should be pulled aside to expose the lumbar nerve. After nerve isolation, the electrode wires can be anchored to the aorta using several 6-0 sutures placed through the aortic adventitia (30) (see Fig. 1A). Alternatively, the lumbar nerve can be approached by gentle retraction of the vena cava, and the electrode leads can be sutured to adjacent muscle. When telemetry devices are used, the transmitter can be placed in the peritoneal cavity and sutured to the abdominal wall or placed subcutaneously on the flank.
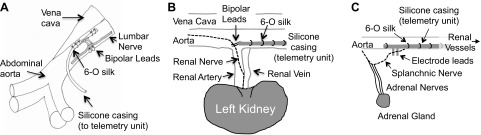
Fig. 1.
Schematic representation of the chronic electrode implantation around the lumbar (A), renal (B), and splanchnic sympathetic nerve (C). For lumbar, note that a window (darkened area) was cut out of the teflon surrounding the bipolar leads to provide nerve contact with the underlying lead wires. The 6-0 silk was anchored through the adventitia of the abdominal aorta. For the renal and splanchnic nerve, the electrode was anchored to the aortic adventitia with 6-0 silk.
For the renal and splanchnic sympathetic nerves, several laboratories have visualized and isolated these nerves through a retroperitoneal incision. The leads exit the peritoneal cavity through the same incision (25, 26, 40). The electrode can be anchored to surrounding tissue either by tissue adhesive or by suturing onto the renal arterial wall. In our laboratory, we have used an alternative approach similar to that described for the lumbar nerve. As illustrated in Fig. 1, B and C, the nerves are visualized and isolated through a retroperitoneal incision, but the electrode leads are led from the back of the animal or peritoneal cavity, placed parallel to the aorta, and anchored to the aortic wall via sutures or tissue adhesive for ∼2 to 3 cm distal to the renal vessels. This anchoring is achieved such that the leads are placed above the renal sympathetic nerve near the junction of the aorta and renal artery. Since the anatomical location of the renal nerve differs from animal to animal (i.e., alongside the renal artery vs. between the renal artery and vein), the nerve should be dissected first. The electrodes can then be placed to fit the course of the renal nerve. Essentially, the electrode and leads are placed on the dorsolateral side of the aorta. This location along with the additional sutures placed on the aorta wall helps to limit electrode movement and help longevity of the nerve recording. A similar placement is used for the splanchnic sympathetic nerve, but the leads are placed more rostrally on the aorta (Fig. 1C). Alternatively, the leads can be anchored into the overlying muscle using sutures. In our hands, stabilization of the electrode leads to an adjacent tissue or major artery (vs. free floating or unanchored) greatly enhances the duration of the nerve viability because this prevents a shifting contact between the nerve bundle and electrode wires. The ground wire is sutured into adjacent tissue.
Although the above description and placement of the electrode leads are important for chronic SNA recordings, work with acute, anesthetized preparations do not require suturing of electrode leads or tissue adhesive. Instead, the electrode leads can be placed perpendicular to the nerves through the incision site where they can be exteriorized to the amplifier.
Once the electrode wires are anchored, the nerve bundle (lumbar, renal, or splanchnic) can be gently lifted with angled forceps (No. 5, 45° micro-dissecting tweezers, Roboz Surgical Instrument) or fine, hook-shaped glass probes, and the electrode tips can be carefully slipped underneath the nerve. For correct nerve-to-wire contact, several factors are important: 1) the nerve must touch both of the electrode wires (the uninsulated electrode wires cannot contact one another); 2) although the electrode wires usually cause a slight lifting of the nerve, this should be minimized to avoid stretching the nerve, which compromises blood flow; 3) the nerve should be free from any connective tissue; and 4) contact between the “underside” of the electrode wires and surrounding tissues should be avoided by placing a small piece of Parafilm between the nerve/wire bundle and the tissues (25, 26, 40) or by cutting a window through the top side of the Teflon insulation (Fig. 1A). After correct placement is verified, all of the fluid around the electrode wires and nerve should be removed using absorbent spears, and the quality of the SNA signal should be verified by visualizing raw nerve bursts on an oscilloscope and/or by assessing nerve sound using an audio monitor. A two-component silicone elastomer (Kwik-Sil, WPI, Sarasota, FL) should immediately be applied, such that the silicone pools under and around the nerve/electrode bundle for full electronic isolation. However, care should be taken to use the smallest amount of silicone to cover the assembly, because larger silicone “blobs” can be displaced by organ or muscle movement to cause a stretching of the enclosed nerve segment or a shifting contact between the nerve and wires (22).
Distinguishing Between Sympathetic Nerve Bursts, Background Noise, and Electrical Artifacts
A major challenge associated with acute or chronic SNA recordings is the ability to distinguish sympathetic nerve bursts versus background or artifact-related noise. While an audio monitor can assist with identification of sympathetic nerve bursts, investigators need to visually distinguish these electrical signals for two reasons: 1) unless laboratory personnel can listen to audio signals continuously throughout the 24-h period to identify artifacts, data analysis will typically be performed after data collection has been completed and require visual inspection of raw traces for artifact-free segments, and 2) the volume on many audio monitors is set at a level to hear the sympathetic nerve bursts rather than low-amplitude noise, electrocardiogram (ECG), or other small artifacts. In this section, we attempt to illustrate these different types of signals (visually and through audio examples) and provide suggestions for identifying a viable sympathetic nerve recording.
Sympathetic bursts display pulse-synchronous and cardiac cycle-related activity.
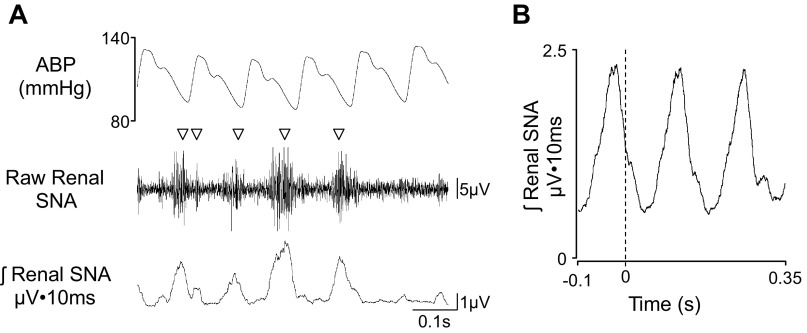
A characteristic of renal, splanchnic, and lumbar SNA is the presence of pulse-synchronous or cardiac cycle-related nerve bursts (10, 22). Figure 2 illustrates a chronic telemetry-based recording of renal SNA from a rat using Data Sciences International F50-W-F2 and PA-C40 transmitters (see online supplement for corresponding audio clip). The majority of nerve bursts can be identified as a cluster of vertical lines or compound action potentials that are synchronized to the raw blood pressure signal at a normal ABP (90–120 mmHg). These bursts are typically observed after the peak in systolic blood pressure. It is noteworthy that recordings from the renal, splanchnic, and lumbar nerves in conscious rats display visible sympathetic bursts within the majority of cardiac cycles (∼60–80%), and more than one burst can appear within a cardiac cycle. This is consistent with the high level of sympathetic tone observed in rats. Investigators should also be aware that each nerve has a characteristic sound and appearance: renal SNA is entirely rhythmic with a sound that corresponds to the cardiac cycle. Lumbar and splanchnic SNA is semirhythmic with a majority of activity corresponding to the cardiac cycle. Within this report, we provide examples of lumbar, renal, and splanchnic SNA recordings. A second proof-of-concept method to confirm the presence of sympathetic bursts is to determine whether the activity is cardiac cycle related (10, 22). This analysis can be performed two ways: 1) subjectively by visual inspection of the location of the sympathetic burst relative to the cardiac cycle or pulsatile blood pressure or 2) spike-triggered averaging of the rectified nerve signal from the start of each cardiac cycle (Fig. 2B). The advantage of this latter analysis is that it does not require external intravenous (IV) lines for drug administration or any experimental manipulation to inhibit/activate SNA. The above visualization and analysis can be performed with the ABP signal or the QRS complex of an ECG recorded from two leads placed in the fore/hind paws or subcutaneously on the chest and back. Renal, lumbar, and splanchnic sympathetic nerve bursts (in acute or chronic preparations) will display this pulse-synchronous activity and cardiac cycle-related rhythm.
Fig. 2.
A: 0.7-s segment of arterial blood pressure (ABP), raw renal sympathetic nerve activity (SNA), and rectified/integrated (10-s time constant) renal SNA in a conscious rat (315 g body wt) at 15 days after surgical implantation (raw renal SNA was filtered 100–1,000 Hz and digitized at 2 kHz). Note the presence of sympathetic nerve bursts in the majority of cardiac cycles and located between systolic and diastolic blood pressure (bursts are denoted by ▽). Listen to the corresponding audio clip embedded in the PowerPoint slides of the online supplement. B: cardiac cycle-related average of renal SNA triggered by diastolic blood pressure from the corresponding 0.7-s trace. The vertical dashed line denotes diastolic blood pressure.
Sympathetic bursts are abolished by ganglionic blockade or baroreflex loading.
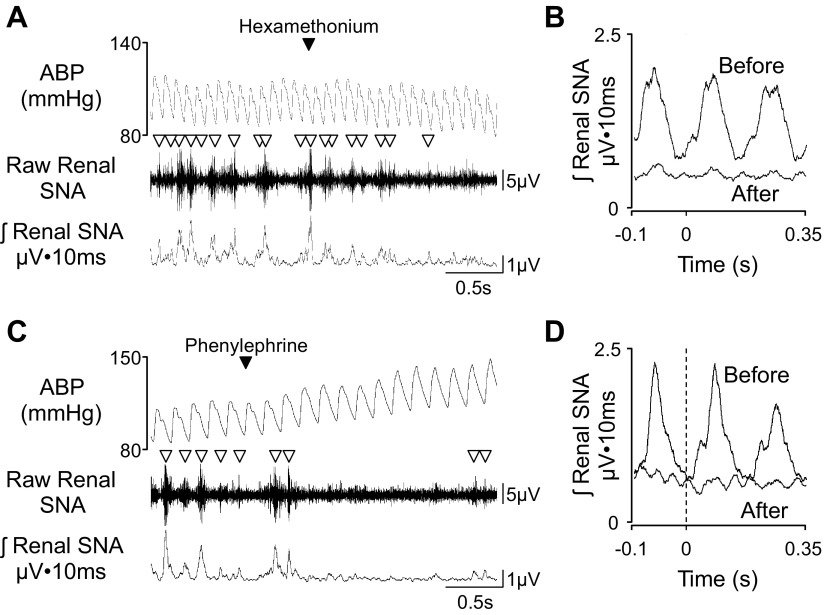
A common experimental manipulation to test the presence of sympathetic bursts is ganglionic blockade or baroreflex loading (7, 10, 22). For example, IV administration of ganglionic blockers (i.e., chlorisondamine or hexamethonium) will block neurotransmission between pre- and postganglionic neurons and result in an abrupt cessation of the sympathetic burst activity, pulse-synchronous activity, and cardiac cycle-related activity (Fig. 3, A and B; and listen to corresponding audio clip embedded in figures within the online supplemental material related to this article). Baroreflex loading produced by pharmacological agents (i.e., phenylephrine) increases blood pressure and reflexively decreases SNA to cause disruption of pulse-synchronous and cardiac cycle-related activity (Fig. 3, C and D, and listen to corresponding audio clip embedded in figures within the online supplement). Both experimental manipulations will help to identify the presence of a viable nerve and will also provide a value for background noise (see below). While baroreflex unloading with pharmacological agents such as sodium nitroprusside will decrease blood pressure and increase SNA, this manipulation will not necessarily facilitate discrimination between a viable sympathetic nerve and noise.
Fig. 3.
Example of 3-s segments of ABP, raw renal SNA, and rectified/integrated renal SNA in a conscious rat (278 g body wt) and injected with ganglionic blocker hexamethonium (30 mg/kg iv; A) or α-adrenergic agonist phenylephrine (C). Note the cessation of sympathetic bursts (denoted by ▽) after either treatment. Corresponding spike-triggered averaging of integrated renal SNA from the diastolic blood pressure (dashed vertical lines) before and after hexamethonium (B) or phenylephrine (D). Note that both treatments eliminated the cycle nature of renal SNA. This rat was implanted with the F50-W-F2 transmitter for SNA and PA-C40 transmitters from Data Sciences International at 13 days before either manipulation. The renal SNA was filtered at 100–1,000 Hz and digitized at 2 kHz. Listen to the corresponding audio clips embedded within the figures of the online supplement.
Background noise.
The background noise is characterized by its constancy: the raw nerve signal shows a nearly uniform band, the rectified/integrated signal is almost flat, and the sound is that of a consistent and quiet “hiss” (see Fig. 3 for examples). As indicated above, the background can be clearly observed after ganglionic blockade, after baroreceptor loading with phenylephrine, or in a preparation that has lost all of its sympathetic activity (7, 10, 22). However, “true” background noise must be distinguished from electrical noise artifacts, which can be identified through audio monitor output, oscilloscope, or visual inspection of the raw digitized traces. The purpose of the following section is to discuss and present examples of common electrical noise artifacts that mask “true noise.”
Electrical noise artifacts.
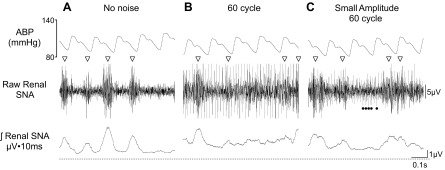
The most common form of artifact is 60-cycle (or 60 Hz), high-voltage electrical noise, which causes extremely high voltages on the raw and integrated channels. Voltage levels can be so high that it is nearly impossible to detect background noise or sympathetic bursts through an audio monitor, oscilloscope, or digitized traces. This is usually attributed to improper electrical shielding of the experimental room, presence of a nearby major power supply, or the absence of an electrical ground. Nevertheless, both of our laboratories have observed occasional and smaller amplitude 60-cycle noise in chronic SNA recordings regardless of the system used. Figure 4 illustrates a high-quality, chronic renal SNA recording and two examples of smaller amplitude 60-cycle noise (listen to corresponding audio clips embedded within figures of online supplement). In Fig. 4B, sympathetic nerve bursts can be observed or heard through an audio monitor, but there is a constant “hum” and appearance of continuous voltage events that have a shorter duration than typical sympathetic bursts (<5 ms); these voltage events are not eliminated by phenylephrine or ganglionic blockade (data not shown). In Fig. 4C, the sympathetic nerve bursts are more obvious and prominent (visually and audibly), but a smaller amplitude electrical noise is still present. This noise is clearly smaller when compared with that in Fig. 4B (visually and audibly). Typically, these noise artifacts have a constant amplitude, and the duration of each voltage artifact is much shorter than that of a sympathetic burst. In each case, the noise can be heard through an audio monitor at a louder volume setting and is visible on an oscilloscope or a very short digitized trace (0.5–1 s). The presence of electrical noise will influence the rectified voltage values and likely result in greater variability and/or type I or type II errors in experimental results.
Fig. 4.
Example of 0.7-s segments of ABP, raw renal SNA, and rectified/integrated renal SNA of a conscious rat (305 g body wt) with very little electrical noise (A), standard 60-cycle noise (B), and small-amplitude 60-cycle noise (denoted by ●; C). Sympathetic nerve bursts are denoted by ▽. In C, the smaller amplitude noise is present throughout the trace, but the closed circles denote the electrical noise for part of the raw trace. Note the higher level of integrated voltage and a less clear delineation of the sympathetic nerve bursts. Listen to corresponding audio clips embedded in figures of online supplement. This rat was implanted with the F50-W-F2 transmitter for SNA and PA-C40 transmitters from Data Sciences International at 10 days before either manipulation. The renal SNA was filtered at 100–1,000 Hz and digitized at 2 kHz.
ECG and respiratory artifacts.
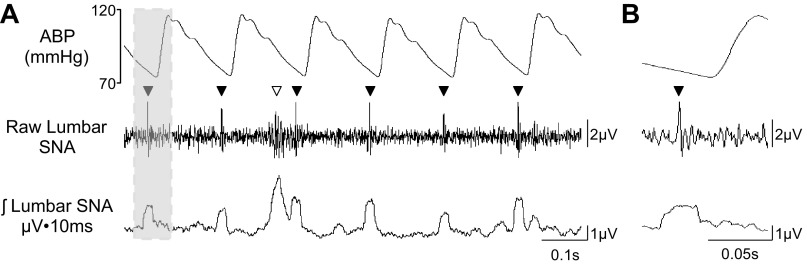
ECG signals create additional artifacts that are easily identified on the raw nerve signal as thin vertical lines or single action potentials instead of the thicker clustered shape of SNA bursts (Fig. 5). In addition, ECGs are entirely rhythmic and exhibit a heart rate frequency that is characteristic of the species studied. Figure 5 illustrates a chronic lumbar SNA recording from an adult rat with a viable nerve and ECG artifact. The ECG has a much shorter duration than a sympathetic burst and is time locked to the pulsatile blood pressure signal (or cardiac cycle). On the rectified and integrated waveform, it resembles a sympathetic burst but is not eliminated by ganglionic blockade or baroreflex loading. In our experience, ECG signals were the artifacts most easily confounded with true sympathetic burst activity. In several cases, the ECG signal can be attenuated or eliminated by electronic filters that increase the high-pass filter to 300 Hz. In addition to the ECG, respiration can also cause electrical artifacts because of the electromyogram of the diaphragm and intercostal muscles or movement of the electrode. The appearance of the electrical event is similar to an ECG but has a smaller frequency that is synchronized to the respiratory cycle. In our experience, respiratory-related artifacts are not common but more frequently observed with splanchnic SNA recordings. Again, these can be minimized by a higher high-pass filter (i.e., 300 Hz).
Fig. 5.
A: example of 1-s segment of ABP, raw lumbar SNA, and rectified/integrated lumbar SNA of a conscious rat with an ECG artifact. Sympathetic nerve bursts are denoted by ▽; ECG artifacts are denoted by ▼. The ECG artifact appears as thin vertical lines in the raw lumbar SNA and appears as a reasonable SNA on the rectified and integrated traces. B: highlighted example of a signal ECG artifact (see gray box). Note two characteristics: 1) the waveform appears as a single action potential, and 2) the ECG is time locked to the cardiac cycle.
Electromyogram or animal movement.
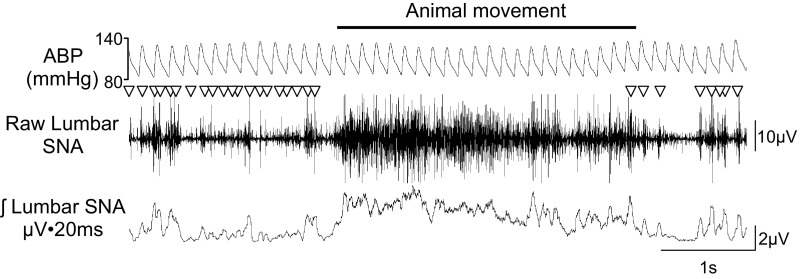
A final electrical artifact present in chronic SNA recordings is related to movement of the animal or the electromyogram. These electrical events are caused by movement of the electrode leads/wires or the massive electrical depolarization associated with contraction of nearby skeletal muscle. Figure 6 illustrates an animal movement artifact on the raw and integrated lumbar SNA channels. Note that the sympathetic bursts in the raw and rectified/integrated channels are associated with the cardiac cycle before animal movement. During movement, these synchronous bursts are no longer evident but are replaced by continuous activity or voltage increases that extend over several cardiac cycles. This continuous activity results in raw and rectified nerve traces that lack the “interburst trough” periods normally seen in SNA recordings. Although movement artifacts can be seen in chronic SNA recordings using any telemetry or hard-wired system, animal movements do not always result in electrical artifacts. In our experience, we have not identified a solution to eliminate these artifacts. Instead, we have identified and removed these segments from the analysis through visual inspection of the raw traces.
Fig. 6.
ABP, raw lumbar SNA, and rectified/integrated lumbar SNA of a conscious rat with an animal movement or electromyogram artifact. Sympathetic nerve bursts are denoted by ▽. The artifact associated with animal movement persists over multiple cardiac cycles and raises the integrated voltage value, and clear sympathetic bursts cannot be identified in the raw or integrated trace. The F50-W-F2 transmitter for SNA and PA-C40 transmitters from Data Sciences International were implanted at 22 days before this recording. The lumbar SNA was filtered at 100–1,000 Hz and digitized at 2 kHz.
As an alternative to visually identifying artifacts on traces, some investigators have restricted SNA recordings to a few hours a day when the animals can be monitored for animal movement (1, 19, 20). This has clear advantages and can provide useful data regarding the disease state and responses to acute experimental manipulations in conscious animals. However, major advantages of chronic SNA recordings include the ability to record SNA over time (days to weeks), to relate changes in SNA to changes in ABP, and to observe effects of experimental manipulations on the circadian cycles (day vs. night) of heart rate, ABP, and SNA. Since it is difficult to monitor animal movement throughout the 24-h cycle, visual inspection of the raw traces will be needed.
Time Course and Reasonable Expectations of Chronic SNA Recordings in Rats
Postimplant recovery period.
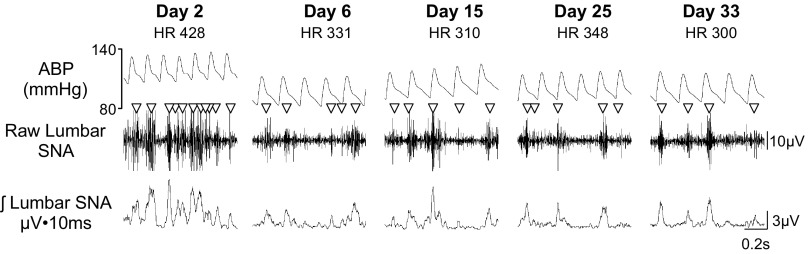
Postsurgery stress activates the hypothalamic-pituitary axis, disrupts normal fluid and food intake, and elevates ABP, heart rate, and sympathetic outflow (as reflected by plasma catecholamines levels). These alterations persist for several days before returning to new levels. SNA appears to follow a similar time course with an initial large increase in SNA that stabilizes at 4 to 6 days after the procedure (3, 30, 39). Figure 7 illustrates this time course after implantation of a Data Sciences International telemetry transmitter in a normal male Sprague-Dawley rat. On day 2, we observe much larger amplitude bursts in raw SNA and a higher burst incidence equating to >90% of the cardiac cycles. In some instances, multiple bursts are associated with a single cardiac cycle. As the animal recovers over the initial 5 days, ABP and heart rate return to normal values and food and fluid intake return to presurgical levels (data not shown). SNA (lumbar, renal, or splanchnic) slowly and steadily declines to stabilize at a lower level. In both of our laboratories, this stable level is evident after a 4-day minimum recovery period regardless of the system used to record SNA. This time frame also corresponds to the “reestablishment” of the circadian rhythm in blood pressure, heart rate, and SNA (10). While there is no “true baseline” SNA due to potential damage to the nerve, we suggest that conscious SNA recordings should wait several days until many of these cardiovascular variables stabilize.
Fig. 7.
ABP, raw lumbar SNA, and rectified/integrated lumbar SNA of a conscious rat (292 g initial body wt; and 371 g final body wt) at various times after surgical implantation of the telemetry units. Sympathetic nerve bursts are denoted by ▽. Traces are 1-s examples. Heart rate (HR) values are indicated below the day. Note that much higher level of sympathetic activity as reflected by the larger amplitude bursts and burst frequency at day 2. This rodent was implanted with the F50-W-F2 transmitter for SNA and PA-C40 transmitters from Data Sciences International. The lumbar SNA was filtered at 100–1,000 Hz and digitized at 2 kHz.
Success rates with chronic recordings.
The 4–6-day recovery period also represents a “danger zone,” because we and others have found that sympathetic signals degrade to zero activity in ∼40% of the animals during this early stage of the recording (1, 25, 30). This loss of SNA is thought to be caused by 1) loss of blood supply to the nerve segment (by destruction of blood vessels during surgery or by inflammation and expansion of the nerve bundle within the nonexpandable Sil-Gel covering); 2) growth of inflammatory tissue between the nerve and electrode wires; 3) infiltration of blood, lymph fluid, or both into the spaces between the gel, nerve, and electrode wires; 4) muscle contractions or organ movements, which move the electrode wires away from the nerve (6, 10, 22, 25); and 5) excessive handling of the animals, which also moves the electrodes off the nerve (if possible, rats with viable recordings should not be handled at all). In many instances, this loss of SNA can be replaced by the appearance of different types of nonsympathetic noise that can be mistaken for burst activity. Therefore, it is critically important to distinguish between the background noise, SNA, and nonsympathetic noise (discussed above).
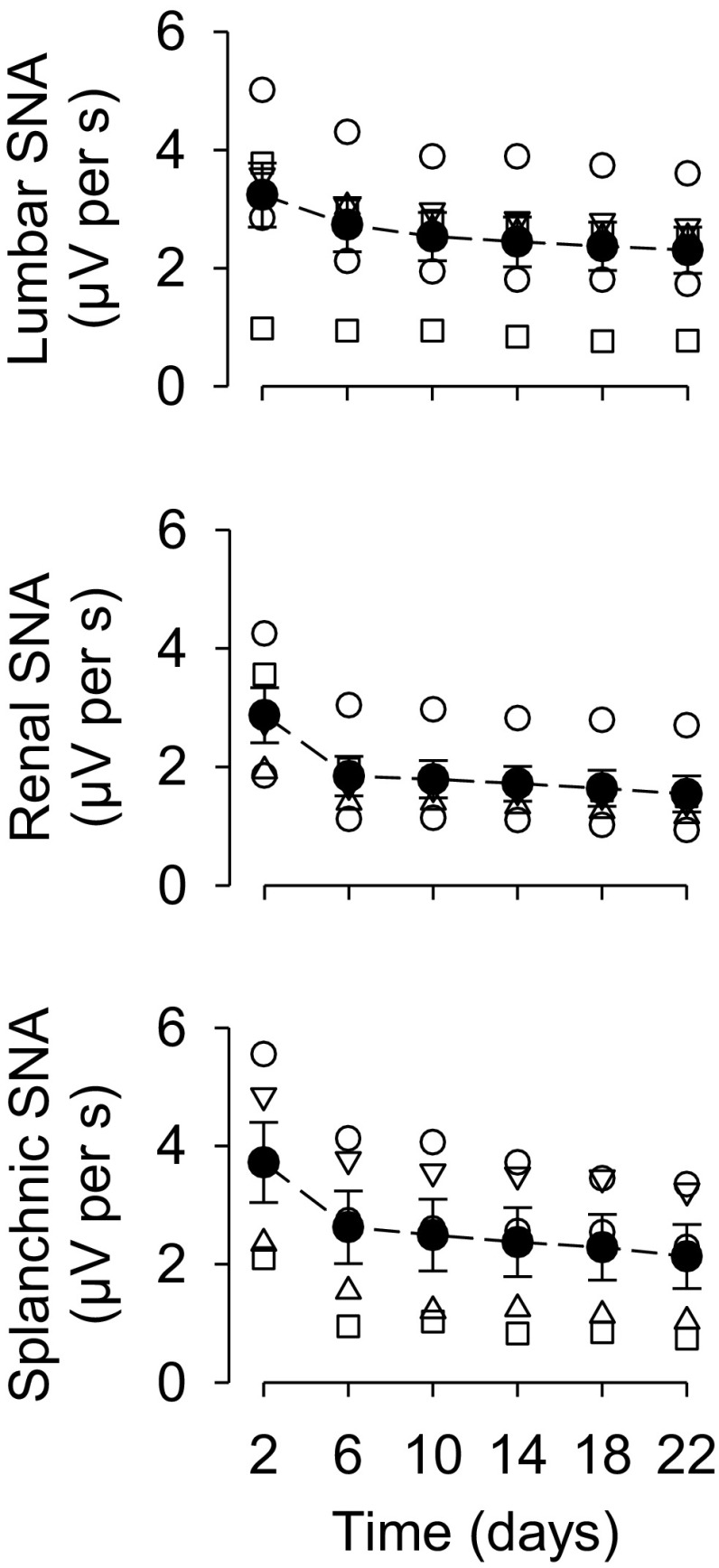
If a rat exhibits a stable, viable nerve at 5 or 6 days after implant, as assessed by cardiac cycle-related bursts, a majority of the remaining animals (>80%) maintain a stable nerve for several weeks. Figure 8 illustrates results from SNA implants on the lumbar, renal, and splanchnic nerves using the methods described above. For this example, 10 implants were performed for each nerve, and the success rates at day 6 were 60, 50, and 50% for the lumbar, renal, and splanchnic nerves, respectively. Figure 8 also illustrates two additional points: 1) initial time-related declines in SNA after implantation and 2) the variability in voltage values across the different nerves. These success rates were similar within both our laboratories. However, it is noteworthy that both laboratories already had extensive experience with acute SNA recordings and that our success rates for chronic SNA recordings were poor when our laboratories began using the approach. As surgical skill and trial and error improved/refined the technique; the success rates and duration of the recordings improved. These success rates also have implications for relevant power analyses during experimental design.
Fig. 8.
Means ± SE of voltage values (in μV) for lumbar, renal, and splanchnic SNA at various times after implantation of the chronic nerve electrode. The results are based on 10 animals per group, but only a subset of these animals had a viable nerve at day 6. Animals without a viable nerve are not illustrated. Note the time-related decline in voltage and variability across animals.
Long-term assessment of nerve viability.
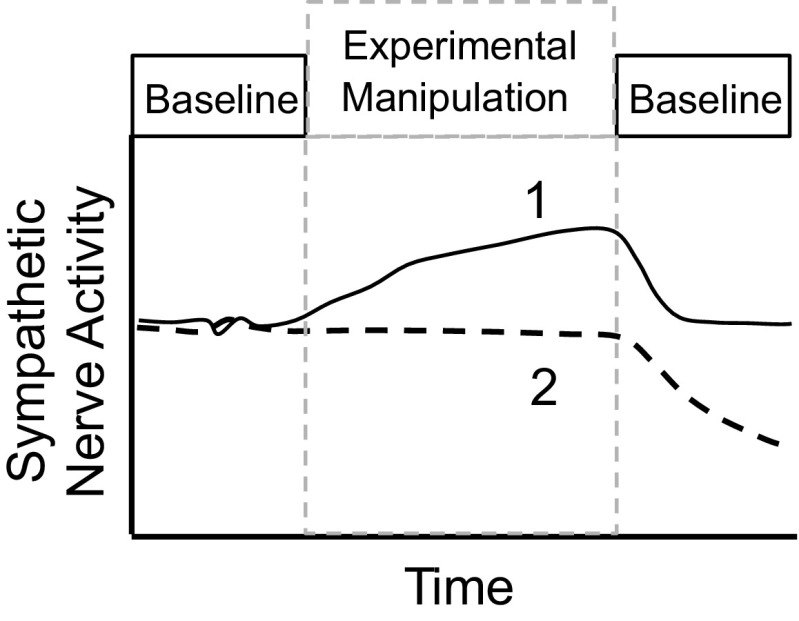
As discussed above, SNA signals in many animals will degrade over time or during the experimental manipulation. Part of the difficulty associated with chronic SNA recordings is to identify whether any decline in SNA represents a physiological response versus a nonviable nerve recording. One method to distinguish between viable and nonviable recordings is the use of experimental designs that consist of three time frames: 1) baseline, 2) experimental manipulation, and 3) baseline postexperimental manipulation. Figure 9 illustrates a common experimental pitfall associated with chronic SNA recordings. In example 1, the animal exhibits an increase in SNA during the experimental manipulation and SNA returns to baseline values during the postexperimental baseline period. In example 2, the animal displays no change in SNA, which may represent a real physiological response to the experimental manipulation. Alternatively, the gradual loss of nerve signal or viability could offset an excitatory physiological response and result in no net change in SNA. This would be difficult to detect without an “off” period. This “off-on-off” method has been successfully applied during chronic recordings in rabbits and rats (1, 2, 4, 35, 40) and could equally be applied to acute experimental manipulations. A disadvantage of this approach for chronic changes in SNA is that it requires longer recording durations, which still represent a challenge for this relatively new methodology. Another technique to assess long-term viability uses nasopharyngeal stimuli (small puff of cigarette smoke to the face of the animal) to cause maximal increases in renal SNA and the resulting level has been classified as 100% (6, 7, 10, 22). This technique is easy to administer, is highly reproducible within rabbits, and can help determine whether decay has occurred in long-term SNA recordings (6, 7, 10, 22, 36). However, this method assumes that responses to nasopharyngeal stimuli are not altered by the experimental manipulation or time (10). Although rats appear to show similar nasopharyngeal reflex-induced elevations in renal and lumbar SNA (27, 31), the reproducibility of the response has not been assessed in this species.
Fig. 9.
Schematic illustration of two possible results for a chronic SNA experiment. Example 1 represents a clear increase in SNA over time during the experimental manipulation that returns near baseline values during recovery. Example 2 represents a common pitfall in chronic SNA recording experiments in which SNA does not change because of a loss of nerve viability that masks any increase in SNA.
Analysis of SNA Signals
Sympathetic nerve signals can be analyzed using a number of techniques, and much of this methodology has been previously reviewed (6, 7, 10, 22). Initially, the SNA recording is filtered (low pass, 1–5 kHz; and high pass, 50–100 Hz with or without a 60-Hz notch filter). Ultimately, the filter settings will be determined by the end user to optimize signal-to-noise ratio and may be limited by the telemetry system. Although the nerve signal can be processed through a window discriminator to count the number of action potentials, this method does not account for different sizes of the voltage events (amplitude and duration) that may reflect summated action potentials in several axons within a nerve bundle or the distance between the depolarized axons and the nerve electrode. A more common approach is to rectify and integrate the filtered nerve signal to quantify entire voltage changes in the nerve bundle. The integration can be performed using a resetting voltage integrator, but this type of integration loses a number of potentially insightful measurements including burst size, shape, and frequency. Alternatively, the majority of software platforms can rectify and integrate the filtered nerve signal and permit the investigator to quantify the level of SNA in a number of different ways.
Assessment of the background noise.
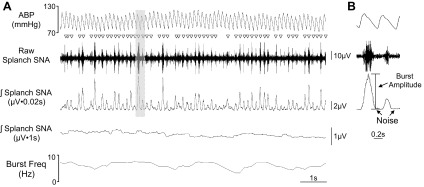
Every electrical recording contains two components: 1) electrical background noise and 2) the physiological signal. To isolate the physiological signal and quantify how this changes over time, investigators need to quantify the background noise and, more importantly, assess whether the background noise changes over time in chronic experiments. As noted above, two common manipulations to decrease SNA are ganglionic blockade and baroreflex loading (see Fig. 3). When used appropriately to completely suppress SNA, the resulting voltage represents the background noise level. However, such manipulations cannot be routinely performed throughout the experimental paradigm or may not be possible due to the unavailability of an external IV line. Furthermore, ganglionic blockade removes only about 90% of lumbar SNA when recorded at the level of L3–L5 because some of the fibers are preganglionic (30, 38). Alternatively, the electrical signal between SNA bursts can be used to estimate background noise. Several studies have concluded that this voltage level is comparable to those after euthanasia, ganglionic blockade, or baroreflex loading with phenylephrine (10, 30, 39, 40). Importantly, investigators using voltage values obtained between bursts as estimates of background noise must employ small time constants for integration (10–50 ms). The advantage of this method is that it does not require an external manipulation and can be routinely assessed every second through the duration of the experiment. In our experience, the background noise can be easily calculated during chronic SNA recordings by using small data bins (1 s). For example, many software programs can detect average integrated voltage, burst frequency, burst amplitude, as well as minimum voltage. The latter is reflective of background noise and changes in minimum voltage may reflect changes in background noise. Figure 10 illustrates a conscious recording of splanchnic SNA and shows various analyses, including rectified and integrated SNA with two different time constants, burst frequency, burst amplitude, and estimation of noise using the interburst voltage level.
Fig. 10.
A: ABP, raw splanchnic SNA, and rectified/integrated splanchnic SNA with two different time constants (0.02 and 1 s), and burst frequency (Freq) of a conscious rat. Sympathetic nerve bursts are denoted by ▽. B: example of analyses for burst amplitude and interburst voltage for an estimation of noise. The F50-W-F2 transmitter for SNA and PA-C40 transmitters from Data Sciences International were implanted at 8 days before this recording. The splanchnic SNA was filtered at 100–1,000 Hz and digitized at 2 kHz.
Normalizing SNA data.
Any voltage event above the background noise level (aside from electrical artifacts) can be defined as SNA. The challenge to the investigator is how to quantify these changes. A common presentation of SNA is to subtract background noise from the integrated SNA values and normalize data by setting baseline values to 100% for each animal. While this provides a powerful and sensitive tool for within animal analysis, both the data and changes in activity can be distorted if there are baseline SNA differences between control and experimental groups (7). Other laboratories have normalized SNA values to a maximum response produced by baroreceptor unloading or the nasopharyngeal reflex (1, 6, 10, 24, 30, 39, 40). While this method removes some variability with chronic SNA recordings, it assumes that the response to these stimuli do not change over time or by treatment (10). Indeed, many chronic disease states are associated with changes in the responsiveness of central circuits regulating sympathetic outflow (5, 8, 14–17). Although nasopharyngeal stimulation to rats also produces very large increases in renal (31) and lumbar SNA (27), this method has not yet been used to scale SNA in this species. While using any of these analyses, baseline values should be presented in microvolts. The SNA recording can be scaled by factoring the amplification; however, a more precise method is to apply a known stimulus through the recording electrodes to calibrate the transmitter and amplifier. Based on this method, the SNA signal can be scaled, calibrated, and reported in microvolts.
Amplitude and frequency of SNA bursts.
Further analyses can focus on the patterning of sympathetic discharges. Changes in SNA can be reflected by increases in the number of sympathetic bursts or the amplitude of individual bursts (10, 22). This type of analysis depends on the investigator's ability to rigorously identify each sympathetic burst. Typically, a sympathetic burst can be detected using a threshold voltage on the integrated signal above the background noise level. Unfortunately, the criteria used to determine this threshold voltage have differed from study to study. In chronically instrumented rats, Muntzel and colleagues (30) found that rejection of any integrated spike with an amplitude <3 μV above the background noise resulted in inclusion of 96% of all visible spikes. Guild and colleagues (10) suggested that the threshold voltage can be a percentage of the maximal burst amplitude (i.e., 25% of maximum burst amplitude). A key point with this analysis is that the integration time constant can greatly influence these criteria. For example, a longer integration time constant will result in a smaller burst amplitude on the integrated signal (and vice versa). Figure 10 illustrates the impact of different time constants on SNA burst amplitude.
Once a threshold voltage for SNA bursts on the integrated signal is established, the amplitude and frequency of the bursts can be easily calculated. As recently reviewed (22), there is good evidence in different species for a differential control of amplitude and frequency for different physiological stimuli. In theory, greater amplitude of a sympathetic burst may reflect a population recruitment or activation of new fibers in the nerve bundle. On the other hand, a change in frequency may reflect differences in the activity of active neurons. The functional importance of a change in amplitude or frequency of sympathetic bursts is still unclear; however, such an analysis can easily be performed, normalized for heart rate, and eventually may provide insight into differences across physiological conditions or pathophysiological disease states. This may be particularly important when changes in SNA of experimental models are compared with those reported in human studies.
Analyses for amplitude and frequency have revealed important differences in baseline SNA across species. In healthy humans (20–30 yr of age), the majority of studies report a baseline burst frequency of ∼0.3–0.5 Hz with a heart rate of ∼60 beats/min and burst incidence of ∼40–60 bursts/100 heart beats (23, 32). In chronically instrumented rats, the baseline burst frequency is much higher in the lumbar, renal, and splanchnic sympathetic nerves. For example, Muntzel and colleagues (30) reported a lumbar SNA burst frequency of ∼6–8 Hz with a heart rate of ∼350 beats/min and estimated burst incidence of 100–130 bursts/100 heart beats. Within the Stocker laboratory, we have observed similar numbers (unpublished observations). As reflected in numerous traces within this manuscript, a sympathetic burst within the lumbar, renal, or splanchnic nerves are associated with the majority of cardiac cycles, and, at times, more than one burst is present in one cardiac cycle. In conscious rabbits (34), renal SNA burst frequency was ∼6 Hz with a heart rate of ∼190 beats/min and burst incidence of ∼200 bursts/100 heart beats. The functional significance of this difference across species is not clear, but this could indicate that increased SNA in experimental models could be reflected by a preferential increase in burst amplitude. Clearly, future studies together with the development of chronic SNA recordings in rats will address this issue.
Summary
The ability to implement chronic SNA recordings from rats into experimental models will substantially impact the field of autonomic-cardiovascular physiology. However, the success of this approach will depend on a number of factors: 1) surgical stability of the electrode leads and nerve, 2) a differentiation between electrical noise artifacts and nerve bursts, and 3) surgical skill. In regard to the latter, sympathetic nerve recordings will require significantly more practice and skill versus other common surgical techniques (i.e., vascular catheterization, blood pressure telemetry, and brain cannulas). Furthermore, we strongly recommend that investigators visually inspect raw data traces and provide raw data examples that reflect the quality of the nerve recording. While the experimental manipulation may last minutes to weeks, a compressed raw data figure will provide little insight into the quality of the SNA recording. Instead, we recommend that studies employing SNA recordings (acute or chronic) provide raw data traces that reflect the duration of the experiment while also providing much shorter examples (0.5–2.0 s) that reflect the quality of the SNA signal or signal-to-noise ratio. With the techniques and approaches described within this article, chronic SNA recordings are achievable with a >50% success rate and a duration of >20 days. As this technique and approach continues to evolve, the field will benefit from a continued discussion of approaches used to maximize stability of nerve recordings as well as analysis procedures that may be used.
GRANTS
This work was supported by National Institutes of Health Grants R01-HL-090826 (to S. D. Stocker), R01-HL-113270 (to S. D. Stocker), and 1SC1-DK-083062 (to M. S. Muntzel); an American Heart Association Established Investigator Grant (to S. D. Stocker); and the Pennsylvania Department of Health using Tobacco CURE Funds (to S. D. Stocker). The Department specifically disclaims responsibility for any analyses, interpretations, or conclusions.
DISCLOSURES
Sean D. Stocker is a consultant for Data Sciences International.
AUTHOR CONTRIBUTIONS
S.D.S. and M.S.M. conception and design of research; S.D.S. and M.S.M. performed experiments; S.D.S. and M.S.M. analyzed data; S.D.S. and M.S.M. interpreted results of experiments; S.D.S. and M.S.M. prepared figures; S.D.S. and M.S.M. drafted manuscript; S.D.S. and M.S.M. edited and revised manuscript; S.D.S. and M.S.M. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We thank numerous students and staff within our laboratories including both previous mentors and colleagues that have contributed to these discussions over the past several years.
REFERENCES
- 1.Armitage JA, Burke SL, Prior LJ, Barzel B, Eikelis N, Lim K, Head GA. Rapid onset of renal sympathetic nerve activation in rabbits fed a high-fat diet. Hypertension 60: 163–171, 2012. [DOI] [PubMed] [Google Scholar]
- 2.Barrett CJ, Guild SJ, Ramchandra R, Malpas SC. Baroreceptor denervation prevents sympathoinhibition during angiotensin II-induced hypertension. Hypertension 46: 168–172, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Barrett CJ, Navakatikyan MA, Malpas SC. Long-term control of renal blood flow: what is the role of the renal nerves? Am J Physiol Regul Integr Comp Physiol 280: R1534–R1545, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Barrett CJ, Ramchandra R, Guild SJ, Lala A, Budgett DM, Malpas SC. What sets the long-term level of renal sympathetic nerve activity: a role for angiotensin II and baroreflexes? Circ Res 92: 1330–1336, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Bergamaschi C, Campos RR, Schor N, Lopes OU. Role of the rostral ventrolateral medulla in maintenance of blood pressure in rats with Goldblatt hypertension. Hypertension 26: 1117–1120, 1995. [DOI] [PubMed] [Google Scholar]
- 6.Burke SL, Head GA. Method for in vivo calibration of renal sympathetic nerve activity in rabbits. J Neurosci Methods 127: 63–74, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Burke SL, Lambert E, Head GA. New approaches to quantifying sympathetic nerve activity. Curr Hypertens Rep 13: 249–257, 2011. [DOI] [PubMed] [Google Scholar]
- 8.D'Angelo G, Mintz JD, Tidwell JE, Schreihofer AM, Pollock DM, Stepp DW. Exaggerated cardiovascular stress responses and impaired beta-adrenergic-mediated pressor recovery in obese Zucker rats. Hypertension 48: 1109–1115, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Esler M, Straznicky N, Eikelis N, Masuo K, Lambert G, Lambert E. Mechanisms of sympathetic activation in obesity-related hypertension. Hypertension 48: 787–796, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Guild SJ, Barrett CJ, McBryde FD, Van Vliet BN, Head GA, Burke SL, Malpas SC. Quantifying sympathetic nerve activity: problems, pitfalls and the need for standardization. Exp Physiol 95: 41–50, 2010. [DOI] [PubMed] [Google Scholar]
- 11.Guild SJ, McBryde FD, Malpas SC, Barrett CJ. High dietary salt and angiotensin II chronically increase renal sympathetic nerve activity: a direct telemetric study. Hypertension 59: 614–620, 2012. [DOI] [PubMed] [Google Scholar]
- 12.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci 7: 335–346, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Hamza SM, Hall JE. Direct recording of renal sympathetic nerve activity in unrestrained, conscious mice. Hypertension 60: 856–864, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang BS, Ahmad M, Deng AY, Leenen FH. Neuronal responsiveness to central Na+ in 2 congenic strains of Dahl salt-sensitive rats. Hypertension 49: 1315–1320, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Huang BS, Wang H, Leenen FH. Enhanced sympathoexcitatory and pressor responses to central Na+ in Dahl salt-sensitive vs. -resistant rats. Am J Physiol Heart Circ Physiol 281: H1881–H1889, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Huber DA, Schreihofer AM. Attenuated baroreflex control of sympathetic nerve activity in obese Zucker rats by central mechanisms. J Physiol 588: 1515–1525, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ito S, Komatsu K, Tsukamoto K, Sved AF. Tonic excitatory input to the rostral ventrolateral medulla in Dahl salt-sensitive rats. Hypertension 37: 687–691, 2001. [PubMed] [Google Scholar]
- 18.Leenen FH, Ruzicka M, Huang BS. The brain and salt-sensitive hypertension. Curr Hypertens Rep 4: 129–135, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Lim K, Burke SL, Armitage JA, Head GA. Comparison of blood pressure and sympathetic activity of rabbits in their home cage and the laboratory environment. Exp Physiol 97: 1263–1271, 2012. [DOI] [PubMed] [Google Scholar]
- 20.Lim K, Burke SL, Head GA. Obesity-related hypertension and the role of insulin and leptin in high-fat-fed rabbits. Hypertension 61: 628–634, 2013. [DOI] [PubMed] [Google Scholar]
- 21.Madden CJ, Morrison SF. Hypoxic activation of arterial chemoreceptors inhibits sympathetic outflow to brown adipose tissue in rats. J Physiol 566: 559–573, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malpas SC. Sympathetic nervous system overactivity and its role in the development of cardiovascular disease. Physiol Rev 90: 513–557, 2010. [DOI] [PubMed] [Google Scholar]
- 23.Matsukawa T, Sugiyama Y, Watanabe T, Kobayashi F, Mano T. Gender difference in age-related changes in muscle sympathetic nerve activity in healthy subjects. Am J Physiol Regul Integr Comp Physiol 275: R1600–R1604, 1998. [DOI] [PubMed] [Google Scholar]
- 24.McBryde FD, Malpas SC, Guild SJ, Barrett CJ. A high-salt diet does not influence renal sympathetic nerve activity: a direct telemetric investigation. Am J Physiol Regul Integr Comp Physiol 297: R396–R402, 2009. [DOI] [PubMed] [Google Scholar]
- 25.Miki K, Kosho A, Hayashida Y. Method for continuous measurements of renal sympathetic nerve activity and cardiovascular function during exercise in rats. Exp Physiol 87: 33–39, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Miki K, Oda M, Kamijyo N, Kawahara K, Yoshimoto M. Lumbar sympathetic nerve activity and hindquarter blood flow during REM sleep in rats. J Physiol 557: 261–271, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moffitt JA, Grippo AJ, Holmes PV, Johnson AK. Olfactory bulbectomy attenuates cardiovascular sympathoexcitatory reflexes in rats. Am J Physiol Heart Circ Physiol 283: H2575–H2583, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Morgan DA, Balon TW, Ginsberg BH, Mark AL. Nonuniform regional sympathetic nerve responses to hyperinsulinemia in rats. Am J Physiol Regul Integr Comp Physiol 264: R423–R427, 1993. [DOI] [PubMed] [Google Scholar]
- 29.Morrison SF. Differential control of sympathetic outflow. Am J Physiol Regul Integr Comp Physiol 281: R683–R698, 2001. [DOI] [PubMed] [Google Scholar]
- 30.Muntzel MS, Al-Naimi OA, Barclay A, Ajasin D. Cafeteria diet increases fat mass and chronically elevates lumbar sympathetic nerve activity in rats. Hypertension 60: 1498–1502, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakamura T, Hayashida Y. Autonomic cardiovascular responses to smoke exposure in conscious rats. Am J Physiol Regul Integr Comp Physiol 262: R738–R745, 1992. [DOI] [PubMed] [Google Scholar]
- 32.Narkiewicz K, Phillips BG, Kato M, Hering D, Bieniaszewski L, Somers VK. Gender-selective interaction between aging, blood pressure, and sympathetic nerve activity. Hypertension 45: 522–525, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Osborn JW, Fink GD, Kuroki MT. Neural mechanisms of angiotensin II-salt hypertension: implications for therapies targeting neural control of the splanchnic circulation. Curr Hypertens Rep 13: 221–228, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prior LJ, Eikelis N, Armitage JA, Davern PJ, Burke SL, Montani JP, Barzel B, Head GA. Exposure to a high-fat diet alters leptin sensitivity and elevates renal sympathetic nerve activity and arterial pressure in rabbits. Hypertension 55: 862–868, 2010. [DOI] [PubMed] [Google Scholar]
- 35.Ramchandra R, Barrett CJ, Guild SJ, Malpas SC. Evidence of differential control of renal and lumbar sympathetic nerve activity in conscious rabbits. Am J Physiol Regul Integr Comp Physiol 290: R701–R708, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Ramchandra R, Barrett CJ, Guild SJ, McBryde F, Malpas SC. Role of renal sympathetic nerve activity in hypertension induced by chronic nitric oxide inhibition. Am J Physiol Regul Integr Comp Physiol 292: R1479–R1485, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Ramchandra R, Hood S, Frithiof R, McKinley MJ, May CN. The role of the paraventricular nucleus of the hypothalamus in the regulation of cardiac and renal sympathetic nerve activity in conscious normal and heart failure sheep. J Physiol 591: 93–107, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scislo TJ, Augustyniak RA, O'Leary DS. Differential arterial baroreflex regulation of renal, lumbar, and adrenal sympathetic nerve activity in the rat. Am J Physiol Regul Integr Comp Physiol 275: R995–R1002, 1998. [DOI] [PubMed] [Google Scholar]
- 39.Stocker SD. Assessment of chronic sympathetic nerve recording using Data Sciences International telemetry (Abstract). FASEB J 26: 1091.1047, 2012. [Google Scholar]
- 40.Yoshimoto M, Miki K, Fink GD, King A, Osborn JW. Chronic angiotensin II infusion causes differential responses in regional sympathetic nerve activity in rats. Hypertension 55: 644–651, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.