Abstract
Obesity increases the risk of arrhythmias and sudden cardiac death, but the mechanisms are unknown. This study tested the hypothesis that obesity-induced cardiac sympathetic outgrowth and hyperinnervation promotes the development of arrhythmic events. Male Sprague-Dawley rats (250–275 g), fed a high-fat diet (33% kcal/fat), diverged into obesity-resistant (OR) and obesity-prone (OP) groups and were compared with rats fed normal chow (13% kcal/fat; CON). In vitro experiments showed that both OR and OP rats exhibited hyperinnervation of the heart and high sympathetic outgrowth compared with CON rats, even though OR rats are not obese. Despite the hyperinnervation and outgrowth, we showed that, in vivo, OR rats were less susceptible to arrhythmic events after an intravenous epinephrine challenge compared with OP rats. On examining total and stimulus-evoked neurotransmitter levels in an ex vivo system, we demonstrate that atrial acetylcholine content and release were attenuated in OP compared with OR and CON groups. OP rats also expressed elevated atrial norepinephrine content, while norepinephrine release was suppressed. These findings suggest that the consumption of a high-fat diet, even in the absence of overt obesity, stimulates sympathetic outgrowth and hyperinnervation of the heart. However, normalized cardiac parasympathetic nervous system control may protect the heart from arrhythmic events.
Keywords: arrhythmia, autonomic imbalance, neurite outgrowth, obesity
obesity is associated with numerous comorbidities, including the development of type 2 diabetes and arterial hypertension. Moreover, clinical studies have identified obesity as a high risk factor for the development of cardiac arrhythmias (30, 32, 37, 40). Some arrhythmias are brief and benign, while prolonged arrhythmias can have severe consequences, such as sudden cardiac death (11). The increased prevalence of arrhythmias in humans over the past 30 yr is of great concern, especially since the trend is not directly associated with age (34). Clinical investigations have identified a positive correlation between the body mass index and an increased risk of atrial (33, 37, 40) and ventricular arrhythmias (30, 32), while weight loss can reverse the prevalence of arrhythmic events (33). However, the specific mechanisms that link obesity to the development of cardiac arrhythmias are currently unknown.
Cardiac arrhythmias are attributed to a disorganization of electrical signals within the heart, resulting in the inability of the atria and ventricles to contract in a regular rhythm. To date, myocardial infarction (MI) is the only pathology that has established a mechanistic explanation for development of arrhythmias and electrical remodeling (7). Previous findings in rats (16, 18), dogs (42), and humans (26) suggest that this electrical remodeling is due to the release of nerve growth factor (NGF) in the peri-infarct region, which stimulates regenerative axonal outgrowth through tyrosine kinase A (TrkA) receptor signaling.
The increased risk of arrhythmias during obesity may also be associated with neuronal outgrowth and hyperinnervation of the heart. Aubin et al. (2) demonstrated that female Sprague-Dawley rats fed a high-fat diet (HFD) exhibit sympathetic hyperinnervation of the heart and an enhanced risk of arrhythmias after MI or electrical stimulation of the left ventricle; however, this has not been investigated in a male model of diet-induced obesity (DIO). Furthermore, the mechanism by which obesity enhances sympathetic hyperinnervation has not been identified in either sex. Adipose tissue produces NGF (17, 29), which could support sympathetic sprouting during obesity in a similar fashion to MI.
Therefore, we developed the hypothesis that obesity-induced cardiac sympathetic outgrowth and hyperinnervation promotes the development of arrhythmic events. To test this hypothesis, we used a model of DIO in male rats, in which after 4–6 wk of a moderately HFD, the rats diverge into obesity-resistant (OR) and obesity-prone (OP) groups. We determined whether the OP rats exhibit 1) cardiac hyperinnervation; 2) accelerated sympathetic neurite outgrowth from sympathetic superior cervical ganglia (SCG) tissue explants; and 3) a higher prevalence of cardiac arrhythmias during an intravenous epinephrine challenge. In contrast to our hypothesis, we found that both OR and OP rats expressed hyperinnervation of the heart and high sympathetic growth from SCG ganglia compared with aged-matched control (CON) rats fed standard chow. However, the OR rats were resistant to arrhythmic events after the epinephrine challenge compared with OP rats. Since the parasympathetic nervous system protects the heart against arrhythmic events (4, 35), we, therefore, determined whether DIO reduces neuronal acetylcholine (ACh) stores and stimulus-evoked release in an ex vivo atrial preparation system.
METHODOLOGY
Animals
Male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) were individually housed in a temperature controlled room (22 ± 2°C) set to a 12:12-h dark-light cycle. Rats were provided chow and water ad libitum. All experimental protocols were conducted in agreement with the National Institutes of Health's Guide for the Health and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee at Oregon Health and Science University.
DIO Model
Rats (57–59 days, 250–275 g) were placed on a HFD (33% kcal fat, Purina 571R, Richmond, IN) for 4–6 wk, as previously described (25, 41). After 2 wk, the bottom tertile of weight gain was classified as the OR group, while the top tertile was classified as the OP group. The middle third was not used. In addition, an aged-matched CON group was fed normal chow (Purina 5001, 13% kcal from fat) for the same period of time. The nutritional profile of the CON diet is 23% protein, 4.5% fat, and 6% fiber. The HFD is a derivative of the 5001 diet with 10% lard added to the mix, composed of 22% protein, 15% fat, and 5% fiber. Experiments commenced when the animals were on the HFD for 4–6 wk.
Experimental Protocols
Protocol 1: Does DIO promote sympathetic hyperinnervation of the heart?
IMMUNOHISTOCHEMISTRY.
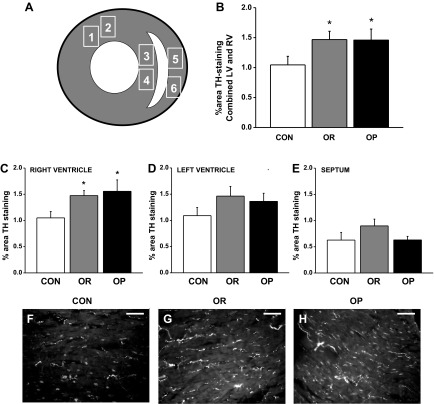
Hearts were rapidly excised from rats, measured for weight and length, and divided into four main transverse sections. The top and bottom quarters were discarded. The second quarter of the heart was immersed in 4% paraformaldehyde for 1–2 h, rinsed with 1× PBS, cryoprotected in 30% sucrose (15%–30%) overnight, and frozen in Tissue Tek OCT compound (Fisher Scientific, Pittsburg, PA). The third quarter was divided into the left and right ventricle, flash frozen on dry ice, and stored at −80°C for subsequent quantification of norepinephrine (NE) by HPLC (see protocol 4). Immunohistochemistry was performed on 10-μm cryostat sections on a set of five slides per animal with three serial sections per slide. Heart sections were incubated overnight with a primary rabbit anti-rat tyrosine hydroxylase (TH)-specific antibody (1:300; AB152, Millipore, Billerica, MA), followed by incubation for 1.5-h with an Alexa fluor 488-conjugated rabbit IgG-specific antibody (1:300; Life Technologies, Grand Island, NY). Slides were analyzed under a Zeiss Axiophot II fluorescent microscope (Thornwood, NY) with a ×40 objective. Photomicrographs of the heart were taken in two representative areas of the left ventricle, septum, and right ventricle (Fig. 1A). The fluorescent TH nerve fiber area was quantified with threshold discrimination by two blinded reviewers using ImageJ software and expressed relative to the total sectional cardiomyocyte area (%TH-immunoreactive area). Values for each region were averaged from five equidistant sections 50 μm apart. Data shown for each region are the average of two separate analyses.
Fig. 1.
Immunohistochemical quantification of cardiac tyrosine hydroxylase (TH+) nerve fiber density in control (CON), obesity-resistant (OR), and obesity-prone (OP) rats. A: diagram showing the regions of the heart that were analyzed for innervation density of TH+ fibers; 1 and 2, left ventricle (LV); 3 and 4, septum; 5 and 6, right ventricle (RV). B: compared with CON rats (n = 6), the combined sympathetic innervation of the LV and RV was higher in OR (n = 5) and OP (n = 5) rats (P < 0.05). When analyzed separately, hyperinnervation was prominent in the RV (C), but not in the LV (D) or in the septum (E). Representative images are shown at ×40 magnification of TH+ fibers in the RV from a CON (F), OR (G), and OP (H) rat. Scale bar = 50 μm. Values are means ± SE. *P < 0.05 vs. CON.
Protocol 2: Does DIO stimulate sympathetic nerve growth?
SYMPATHETIC NEURITE OUTGROWTH FROM SCG EXPLANTS.
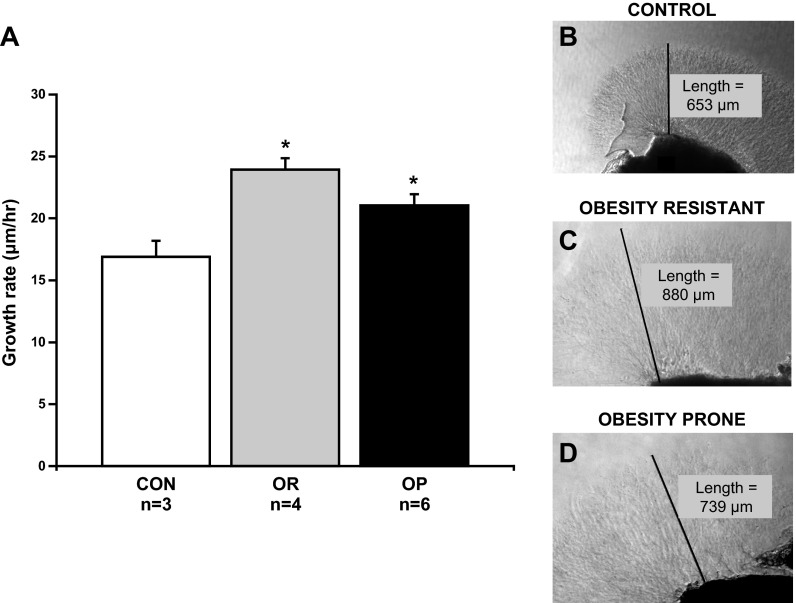
Rat SCGs were removed from the same group of animals used in protocol 1. Once removed, SCGs were halved and explanted into a 12-well tissue culture plate and covered with 35 μl Matrigel (BD Biosciences, San Jose, CA). Serum-free DMEM/F-12 media (Life Technologies, Grand Island, NY) + 1% penicillin/streptomycin was layered over the solidified Matrigel, and wells were treated with 2 ng/ml NGF. Culture plates were placed in a humidified incubator of 95% O2/5% CO2 at 37°C. Axon outgrowth was visualized with phase-contrast microscopy, and axon length was measured in the images using Nikon Elements AR 3.0 software (Melville, NY). Initial images for “time zero” were taken 24 h after plating. Images were taken at time zero and again 24-h later (time 24-h). Images were analyzed blindly, and the rate of neurite outgrowth (μm/h) was calculated from the difference in axon growth at time zero and time 24 h, and then dividing this value over the duration of the treatment. Since SCGs were halved, neurite outgrowth was measured in two wells for each animal. The values from each well were averaged to determine the mean rate of neurite outgrowth for each animal.
Protocol 3: Does DIO increase the risk of evoked arrhythmic events?
SURGERY.
In a separate group of rats, OR (n = 3) and OP (n = 5) rats were anesthetized (2.5% isoflurane in 100% O2) and aseptically instrumented with a femoral arterial catheter (PE-50) to measure pulse pressure and heart rate (HR) and two venous catheters (PE-10 attached to PE-50) for intravenous access. The catheters were tunneled subcutaneously and exteriorized between the scapulae. Catheter patency was maintained by flushing with sterile heparinized saline (100 U/ml) at least three times per week. At least 5 days were allowed for recovery before experiments were performed.
EXPERIMENTAL PROTOCOL.
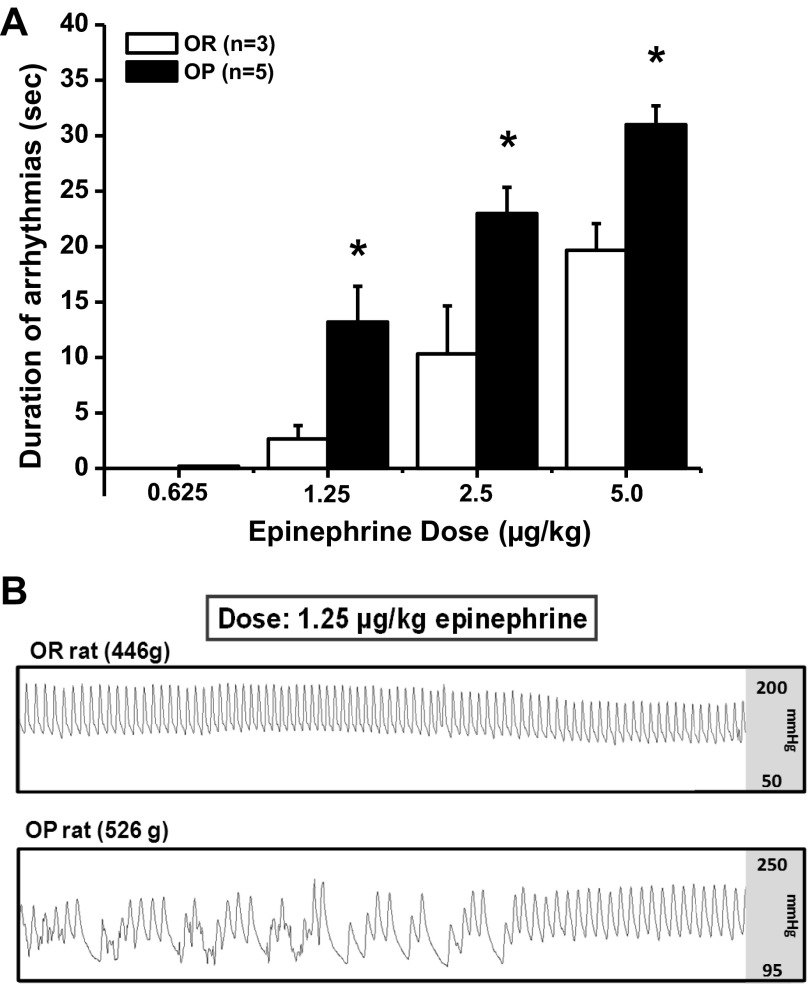
Baseline mean arterial pressure and HR was measured for ∼30 min in conscious animals. Rats were then challenged with increasing intravenous doses of epinephrine (0.625, 1.25, 2.5, and 5.0 μg/kg). After each bolus was administered, HR and pulse pressure were allowed to return to baseline before the next dose was applied.
Data were sampled at 2,000 Hz using the Biopac MP100 data acquisition system and analyzed offline (AcqKnowledge 3.0, Biopac, Goleta, CA). Arrhythmias were defined as a continuous irregular HR for more than five beats. Arrhythmia duration was calculated as the number of seconds over which arrhythmias persisted without interruption.
Protocol 4: Does DIO alter atrial neurotransmitter release?
FIELD STIMULATION OF ATRIAL EXPLANTS.
Using a separate group of rats, the right atrium was removed (n = 4–7 per group) from rats under isofluorane anesthesia and pinned to a thin layer of Sylgard (Dow Corning, Midland, MI) in a preheated (37°C), continuously oxygenated, water-jacketed organ bath containing 2 ml of Ringer solution (120 mM NaCl, 1.2 mM KH2PO4, 4.7 mM KCl, 1.2 mM MgSO4, 2 mM CaCl2, 25 mM NaHCO3, 11 mM glucose, pH 7.4). The atrial tissue was placed between platinum stimulating electrodes and stimulated as in our laboratory's previous study (17). Briefly, a baseline sample was taken (5-min incubation) followed by atrial stimulation using an S88X Stimulator (Grass Technologies, West Warwick, RI) in constant-voltage mode (15 V, 5 Hz, 0.1-ms pulse duration) for 1 min to trigger neurotransmitter release. Two 5-min samples were collected poststimulation. Each 1-ml sample was split into 0.5-ml samples for analysis of ACh and NE. Baseline levels of transmitter were subtracted from the amounts recovered during the stimulation and recovery periods. At the end of the experiment, atria were incubated in 1 ml of 0.1 M perchloric acid (PCA) for 24 h to extract total remaining ACh and NE, which were quantified by HPLC coupled with mass spectrometry (HPLC-MS) or electrochemical detection (HPLC-ED), respectively. Stimulation-evoked release was the amount of ACh or NE in the bath after stimulation, less the ACh or NE present before stimulation.
HPLC-MS FOR ACH QUANTIFICATION.
The tissue used for this set of experiments was the same as for the field stimulation experiments. As in our laboratory's previous study (17), 0.5-ml aliquots of the collected samples and a 0.5-ml aliquot of the PCA extract of the atrial tissue were spiked with deuterated ACh (d4 ACh; 0.1 pg/μl, CDN Isotopes, Quebec, Canada). Deuterated ACh was also added to standards of known concentrations of ACh (10 fg/μl to 100 pg/μl, Sigma-Aldrich). Samples were chromatographically separated on a hydrophilic interaction chromatography mode column (HILIC; PolyLC, Columbia, MD; 30-μl injection volume, mobile phase A: ammonium formate, B: acetonitrile) and detected and quantified by a linear ion trap mass spectrometer (Applied Biosystems MDS SCIEX 4000 QTrap mass spectrometer, Carlsbad, CA). Multiple reaction monitoring was used to quantify d4 ACh (mass-to-charge ratio 150→91.1) and ACh (mass-to-charge ratio 146→87). Injections of 2.05 fmol (300 fg in 30 μl) ACh consistently gave signal-to-noise ratios above 3, indicating the detection limit for ACh in our system (lower limit mean for 21 HPLC-MS runs = 2.40 ± 0.04 fmol). Data were acquired using Analyst software (AB SCIEX, Framingham, MA). For both atrial content and stimulus-evoked release measurements, the amount of ACh was calculated by comparing the ratio of ACh to d4 ACh in samples to those ratios from known standards for ACh and d4 ACh run in parallel.
HPLC-ED FOR NE QUANTIFICATION.
The atrial tissue used for this set of experiments was the same as for the field stimulation experiments. The ventricular tissue used was from the same set of heart samples as in protocol 1. With a similar protocol to that previously described (17), a 0.5-ml aliquot of each sample, and a 0.5-ml aliquot of the PCA extract from atria, left ventricle, and right ventricle were spiked with the internal standard dihydroxybenzylamine (DHBA, 9 nM; Sigma-Aldrich). A similar amount of DHBA was also added to standards of known amounts of NE (4–40 nM), and catecholamines were purified from samples and standards by alumina extraction (15 mg, 30 min). Samples were chromatographically separated by reverse-phase HPLC (50 μl injection volume with C18 column; 15 × 0.46 cm, 5-μm particle size; Varian, Lake Forest, CA) using a mobile phase containing 75 mM NaH2PO4 (pH 3.0), 1.7 mM sodium octane sulfonate, and 4% (vol/vol) acetonitrile. A coulometric detector (ESA, Bedford, MA) was used to detect and quantify NE (electrode potential 180 mV, 50 nA) with area under curve normalized to DHBA area. Detection limits were ∼50 fmol with recoveries >60%. Data were acquired with LCsolution software (Shimadzu, Columbia, MD). NE levels were determined by comparison of the ratios of NE to DHBA in the samples to those ratios from known standards for NE and DHBA run in parallel with the experimental samples.
Statistical analysis.
All between-group differences were compared with one-way ANOVA. Specific comparisons were assessed using a Neuman-Keuls post hoc test, and P < 0.05 indicated significance.
RESULTS
Baseline Measurements
As shown in Table 1, OP rats weighed more (P < 0.05) than OR and CON rats. Furthermore, the heart weight (P < 0.05) and heart length (P < 0.05) were greater in OP compared with OR and CON rats. Body weight, heart weight, and heart length were similar between OR and CON rats, even though the OR rats were fed the HFD.
Table 1.
Body weight, heart weight, and heart length were greater in OP rats compared with CON and OR
| CON |
OR |
OP |
|||||
|---|---|---|---|---|---|---|---|
| Parameters | Protocol | Means ± SE | n | Means ± SE | n | Means ± SE | n |
| Body weight, g | All | 459 ± 14 | 10 | 444 ± 5 | 12 | 564 ± 12*† | 20 |
| Heart weight, g | 2 | 1.28 ± 0.06 | 6 | 1.28 ± 0.02 | 5 | 1.49 ± 0.08*† | 5 |
| Heart weight/body weight | 2 | 2.77 ± 0.19 | 6 | 2.68 ± 0.10 | 5 | 2.62 ± 0.10 | 5 |
| Heart length, mm | 2 | 14.8 ± 0.3 | 6 | 14.3 ± 0.3 | 5 | 15.8 ± 0.5*† | 5 |
n, No. of animals. CON, control; OR, obesity resistant; OP, obesity prone. There was no difference in any of the baseline parameters between CON and OR rats. Heart dimensions were measured in a subset of the total experimental group.
P < 0.05 vs. CON.
P < 0.05 vs. OR rats.
High-fat Feeding Leads to Sympathetic Hyperinnervation of the Ventricles
To test the hypothesis that obesity causes sympathetic hyperinnervation of the heart, we utilized immunohistochemistry to determine whether OP rats expressed a higher percentage of TH-immunoreactive ventricular nerves compared with OR and CON rats. In agreement with our hypothesis, combined TH-positive fibers in the left and right ventricles was 40% higher in OP rats (n = 5) compared with CON rats (n = 6; Fig. 1B, P < 0.05). However, OR rats (n = 5) also expressed 40% higher TH expression compared with CON rats, with no difference between OR and OP groups. When analyzed for each chamber, the hyperinnervation observed in OR and OP rats was particularly prominent in the right ventricle (Fig. 1, C and D; P < 0.05 vs. CON), whereas TH expression in the left ventricle (Fig. 1D) and septum (Fig. 1E) did not differ significantly between groups.
High-fat Feeding Accelerates Basal Sympathetic Outgrowth
To test the hypothesis that obesity promotes sympathetic outgrowth, we determined whether the rate of SCG neurite outgrowth was higher in OP rats compared with OR and CON rats. In agreement with our hypothesis, SCG neurite outgrowth rate was 25% higher in OP rats (n = 5) compared with CON rats (n = 3; Fig. 2A, P < 0.05). However, the rate of growth was also higher (42%) in OR rats (n = 4) compared with CON (Fig. 2A, P < 0.05) and similar to OP rats. Interestingly, the elevated outgrowth parallels the hyperinnervation observed in OR and OP rats.
Fig. 2.
Basal neurite outgrowth in superior cervical ganglia (SCG) explants from CON (n = 3), OR (n = 4), and OP (n = 5) rats. A: average basal sympathetic neurite outgrowth from SCG explants was greater in OR and OP rats compared with CON rats. B–D: photos of SCG explants at time 24 h from a CON, OR, and OP rat, respectively. Values are means ± SE. *P < 0.05 vs. CON.
DIO Increases the Prevalence of Cardiac Arrhythmic Events
To determine whether DIO is associated with a higher occurrence of arrhythmias, we assessed the duration of arrhythmic events after an intravenous epinephrine challenge in conscious OR (n = 3) and OP (n = 5) rats. Baseline mean arterial pressure (OR: 105 ± 5 mmHg, OP: 101 ± 3 mmHg) and HR (OR: 354 ± 9 beats/min, OP: 333 ± 7 beats/min) did not differ between groups. The duration of arrhythmias was directly dose dependent in both groups (P < 0.05), with the minimal occurrence of arrhythmias at the lowest doses, and the longest events at the highest dose. The lowest dose of epinephrine (0.625 μg/kg) evoked arrhythmias in one OP rat. The higher doses of epinephrine (1.25, 2.5, and 5 μg/kg) evoked arrhythmias in both groups; however, the OP rats exhibited a sensitization to the epinephrine challenge, as reflected by a prolonged duration of arrhythmias compared with OR rats (Fig. 3A; P < 0.05 at each dose).
Fig. 3.
Assessment of arrhythmic events in OR (n = 3) and OP (n = 5) rats. A: the duration of arrhythmic events evoked by intravenous epinephrine were greater in OP compared with OR rats. B: representative arterial pulse pressure trace in an OR and OP rat during an epinephrine challenge. Values are means ± SE. *P < 0.05 vs. OR.
DIO Depresses Atrial ACh Content and Release
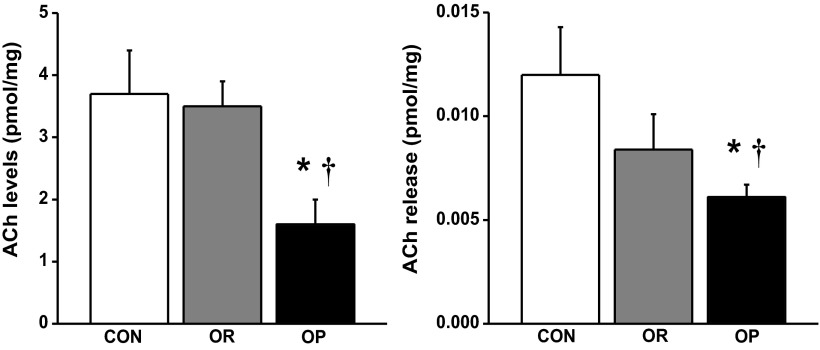
Utilizing an atrial ex vivo system, we simultaneously stimulated autonomic parasympathetic and sympathetic cardiac atrial nerves and measured neurotransmitter release. Atrial weights were not significantly different between groups, and these weights were used for normalizing both the stimulus-evoked release of neurotransmitter and the total pool of neurotransmitter remaining in atria after stimulation. The total pool of ACh in atria from OP rats (n = 7) was lower than that in CON (57%, P < 0.05) and OR (n = 4; 54%, P < 0.05) atria (Fig. 4, left). The fraction of ACh released after field stimulation constituted a small fraction (0.2–0.5%) of the total pool of ACh in all groups. Following field stimulation, all groups demonstrated a statistically significant increase in ACh release from baseline (P < 0.05, data not shown). However, ACh release levels from OP atria were significantly less (48%, P < 0.05) than those from CON rat atria (Fig. 4, right).
Fig. 4.
HPLC-mass spectrometry quantification of atrial acetylcholine (ACh) content and release. Total atrial ACh content (left) and release (right) were lower in OP rats (n = 7) compared with CON (n = 4) and OR (n = 4) rats. Values are means ± SE. *P < 0.05 vs. CON. †P < 0.05 vs. OR.
DIO Elevates Atrial NE Content, But Lowers Release
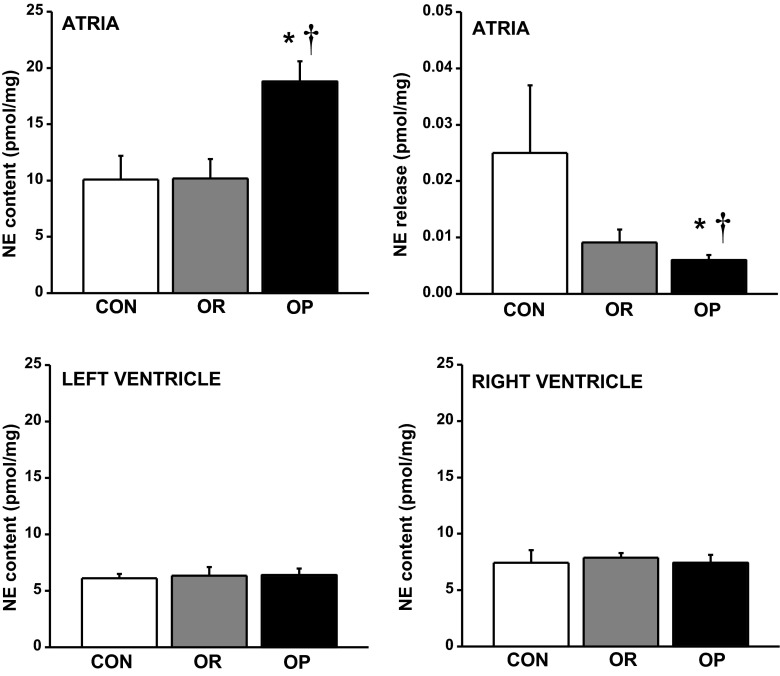
In contrast to ACh, the total pool of NE in atria from OP rats (n = 7) was significantly greater than that in CON (n = 4; 87%, P < 0.01) and OR (n = 4; 84%, P < 0.01) atria (Fig. 5, top left). The fraction of NE released after atrial field stimulation was limited, being only 0.1–0.2% of the total pool of atrial NE across all groups. Following stimulus-evoked release, NE release from OP rats was significantly lower (76%, P < 0.05) than that from CON atria (Fig. 5, top right). Even when normalized to atrial content, or to atrial weight as a fraction of body weight, NE release from OP atria was still significantly lower than CON tissue (data not shown). In contrast to the atria, NE content of the left (Fig. 5, bottom left) and right (Fig. 5, bottom right) ventricles was similar between groups.
Fig. 5.
HPLC-electrochemical detection quantification of atrial norepinephrine (NE) content and release. OP rats (n = 7) exhibited high atrial NE content (top left) but low NE release (top right) compared with CON (n = 4) and OR rats (n = 4). NE content in the LV (bottom left) and RV (bottom right) did not differ between groups. Values are means ± SE. *P < 0.05 vs. CON. †P < 0.05 vs. OR.
DISCUSSION
The purpose of this study was to test the hypothesis that obesity-induced sympathetic outgrowth and cardiac hyperinnervation increases the risk of arrhythmic events. In agreement with our hypothesis, we showed that OP rats exhibit accelerated SCG neurite outgrowth, express hyperinnervation of the ventricles compared with CON rats, and are more susceptible to arrhythmic events during an epinephrine challenge. In contrast to our hypothesis, OR rats also exhibit sympathetic outgrowth and hyperinnervation of the ventricles, but are not as susceptible to arrhythmic events, despite hyperinnervation of the heart. Interestingly, OP rats have attenuated atrial ACh content and release compared with OR and CON atria. OP rats also exhibit elevated atrial NE content, but NE release is dramatically suppressed. Together, these findings suggest that the consumption of the HFD enhances sympathetic growth and cardiac hyperinnervation expressed in both OR and OP rat populations. However, normal atrial ACh content and release may protect OR rats from the development of potentially lethal arrhythmias that would normally be triggered by hyperinnervation of the heart.
Obesity is associated with a higher occurrence of arrhythmic events (30, 32, 37, 40), but few mechanistic details are understood. Since human obesity is rarely attributed to a specific genetic mutation (8), we chose to investigate these mechanisms in rats with DIO. The DIO model utilizes outbred genetically diverse rats, which represents the polygenetic profile of clinical obesity (24). Excessive weight gain in OP rats is attributed to a high food intake and a dysregulation of energy balance, most likely due to central overexpression of neuropeptide Y (23). Furthermore, OP rats have insulin resistance (6, 9, 22, 41) and lower rates of fat oxidation (6), modeling the characteristics of human obesity.
In the present study, OP rats were fed a HFD for 4–6 wk, unlike other DIO models that utilize a longer high-fat feeding regimen (10–12 wk) (10). Our present and previous findings show that OP rats fed a short-term HFD do not develop arterial hypertension, but exhibited severely impaired cardiac baroreflex function and autonomic imbalance (25, 41), similar to human obesity (1, 15, 36). This suggests that changes in autonomic tone may precede, and perhaps be causal, in the development of hypertension in obesity (14, 20, 21). Indeed, OP rats fed a HFD for 10–12 wk can develop modest hypertension and arterial hypertrophy (10). Our findings demonstrate that OP rats can exhibit arrhythmic events without the confounding influence of arterial hypertension. This is likely linked to the autonomic imbalance and dysfunction, especially since the development of arrhythmias is associated with heterogeneous cardiac sympathetic hyperinnervation (27, 42) and electrical remodeling of the heart (7). Along these lines, our study also shows that OP rats have a higher prevalence of arrhythmias and exhibit a greater percentage of TH-positive ventricular sympathetic nerves, suggesting a link between autonomic imbalance and the development of cardiac arrhythmias during the obese state.
Further analysis showed that the hyperinnervation was specific to the right ventricle, which is in agreement with clinical findings that the development of right ventricular dysfunction is directly related to body mass index (39). Despite the absence of overt changes in systemic arterial pressure, elevated pulmonary artery pressure in our model cannot be ruled out, especially since obesity is a risk factor for pulmonary hypertension (5, 13). If pulmonary hypertension is present, increased load on the right ventricular cardiomyocytes may be causal to the right-sided hyperinnervation. However, this mechanism will require further investigation. Although our assessment of arrhythmias cannot distinguish between atrial and ventricular events, the pronounced hyperinnervation in the right ventricle may trigger preventricular contractions in OP rats, especially since preventricular contractions mainly originate from the right ventricle in humans (19).
In agreement with our hypothesis, the hyperinnervation of the heart in OP rats was paralleled by accelerated SCG neurite outgrowth in explant culture. In adult neurons, NGF stimulates nerve growth via TrkA signaling (12). Obesity is a chronic state of low-grade inflammation (38), but it is not clear if inflammatory cell-NGF release is prominent in the obese heart. However, in the present study, OR rats also exhibit greater sympathetic outgrowth and hyperinnervation of the ventricles, despite the absence of overt obesity. Since the common factor between OR and OP rats is the HFD, these data suggest that the neurite outgrowth and hyperinnervation are also stimulated by the consumption of the HFD. Our data are consistent with Aubin et al. (2), who found that female Sprague-Dawley rats fed a HFD that were not obese also exhibited sympathetic hyperinnervation of the heart. Higher circulating levels of free fatty acids from the HFD may directly stimulate growth and hyperinnervation, especially since fatty acids, by binding to residues on regulatory proteins, support growth cone function and neurite outgrowth (28). Alternatively, the consumption of the HFD may sensitize TrkA receptor signaling, but this mechanism would require further investigation.
If consumption of a HFD stimulates sympathetic outgrowth and hyperinnervation of the heart, then why were OR rats not sensitized to arrhythmias? Normal organization of ventricular gap junctions could lower the occurrence of arrhythmias, despite the hyperinnervation; however, this mechanism is unlikely since high-fat feeding in nonobese female rats stimulates abnormal connexin-43 expression and phosphorylation (2). Alternatively, the lack of arrhythmia generation in OR rats may be attributed to the difference in cardiac autonomic balance between OR and OP rats. Previously, our laboratory showed that the bradycardic response to aortic depressor nerve stimulation in OP rats is predominantly mediated by withdrawal of sympathetic nerve activity, rather than activation of central parasympathetic pathways (25). In contrast, OR rats exhibit robust decreases in HR for the same degree of stimulation, which are highly dependent on the parasympathetic system. In addition, our present study examined how DIO affects neurotransmitter release and content in the right atrium. In agreement with previous findings (25), we found that the OR rats have higher atrial ACh content and release compared with OP rats. Interestingly, parasympathetic stimulation of the heart suppresses the development of ventricular fibrillation (4, 35). Therefore, the parasympathetic nervous system may protect OR rats against arrhythmic events that would otherwise be triggered by the sympathetic hyperinnervation of the ventricles.
We also observed that OP rats had higher atrial NE content compared with OR and CON rats, while stimulus-evoked NE release from atrial terminals was diminished. Therefore, even without central input in our ex vivo field stimulation system, there appear to be basal differences in atrial sympathetic nerves from OP rats that promote attenuated release of NE from atrial terminals. Our field-stimulation data for NE parallel findings in obese humans (36) and our laboratory's previous observation that the withdrawal of cardiac sympathetic activity is suppressed in OP rats after aortic depressor nerve stimulation (25). Although stimulus-evoked NE release levels were only significantly decreased in OP rats, NE release in OR rats also trended in the same direction. Therefore, we suspect that both OR and OP groups may have deficiencies in atrial NE release. However, obesity also attenuates parasympathetic control of the heart (3, 25, 31) and may be the most physiologically relevant factor in protecting OR rats from developing potentially fatal arrhythmias.
In contrast to the atria, obesity did not change the NE content in the right ventricle, despite evidence of hyperinnervation. The lack of change in NE content of the OP rat right ventricle may reflect a disconnect between synthetic enzymes and NE processing. In particular, we have preliminary data suggesting that phosphorylation of TH, which occurs as TH is activated, may be decreased in OP rat sympathetic ganglia (data not shown); however, this suggestion warrants further study. It is evident that neurotransmitter synthesis, release, and cardiac distribution are all altered in obesity, resulting in autonomic imbalance at multiple sites within the heart.
Our novel findings, while largely ex vivo, suggest that sympathetic hyperinnervation of the heart and atrial autonomic imbalance may lower the threshold for evoked arrhythmic events in rats with DIO. However, the consumption of the diet itself enhances neurite outgrowth and sympathetic hyperinnervation of the heart. OR rats are less susceptible to arrhythmic events, despite hyperinnervation of the heart, perhaps due to a protective role of relatively elevated atrial ACh content and release in the OR heart. These complex findings open new avenues of research to investigate how the consumption of a HFD, with and without obesity, affects autonomic control and electrical remodeling of the heart, and how these factors contribute to the development of cardiac arrhythmias.
GRANTS
This work was supported by the American Heart Association (7500041 B. H. McCully; 2060630 V. L. Brooks), National Institutes of Health (HL-068231 and HL-093056 B. A. Habecker; HL-088552 V. L. Brooks, NS077063 W. Hasan), and used instrumentation in the Oregon Health and Science University Bioanalytical Shared Resource and Pharmacokinetics core.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: B.H.M., W.H., C.T.S., J.C.H., W.R.W., G.D.G., V.L.B., and B.A.H. conception and design of research; B.H.M., W.H., C.T.S., J.C.H., and W.R.W. performed experiments; B.H.M., W.H., C.T.S., J.C.H., and W.R.W. analyzed data; B.H.M., W.H., C.T.S., J.C.H., W.R.W., G.D.G., V.L.B., and B.A.H. interpreted results of experiments; B.H.M., W.H., and J.C.H. prepared figures; B.H.M. and W.H. drafted manuscript; B.H.M., W.H., C.T.S., J.C.H., W.R.W., G.D.G., V.L.B., and B.A.H. edited and revised manuscript; B.H.M., W.H., C.T.S., J.C.H., W.R.W., G.D.G., V.L.B., and B.A.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Core Director Dr. Dennis Koop for assistance setting up the ACh method, Jenny Luo and Amanda Macek for expert technical assistance, and Laura Gotthardt for assistance with data analysis.
REFERENCES
- 1.Arone LJ, Mackintosh R, Rosenbaum M, Leibel RL, Hirsch J. Autonomic nervous system activity in weight gain and weight loss. Am J Physiol Regul Integr Comp Physiol 269: R222–R225, 1995 [DOI] [PubMed] [Google Scholar]
- 2.Aubin MC, Cardin S, Comtois P, Clement R, Gosselin H, Gillis MA, Le Quang K, Nattel S, Perrault LP, Calderone A. A high-fat diet increases risk of ventricular arrhythmia in female rats: enhanced arrhythmic risk in the absence of obesity or hyperlipidemia. J Appl Physiol 108: 933–940, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Beske SD, Taylor JA. Obesity and autonomic function. Clin Auton Res 11: 61–62, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Brack KE, Patel VH, Coote JH, Ng GA. Nitric oxide mediates the vagal protective effect on ventricular fibrillation via effects on action potential duration restitution in the rabbit heart. J Physiol 583: 695–704, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burger CD, Foreman AJ, Miller DP, Safford RE, McGoon MD, Badesch DB. Comparison of body habitus in patients with pulmonary arterial hypertension enrolled in the Registry to Evaluate Early and Long-term PAH Disease Management with normative values from the National Health and Nutrition Examination Survey. Mayo Clin Proc 86: 105–112, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang S, Graham B, Yakubu F, Lin D, Peters JC, Hill JO. Metabolic differences between obesity-prone and obesity-resistant rats. Am J Physiol Regul Integr Comp Physiol 259: R1103–R1110, 1990 [DOI] [PubMed] [Google Scholar]
- 7.Chen PS, Chen LS, Cao JM, Sharifi B, Karagueuzian HS, Fishbein MC. Sympathetic nerve sprouting, electrical remodeling and the mechanisms of sudden cardiac death. Cardiovasc Res 50: 409–416, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Choquet H, Meyre D. Genetics of obesity: what have we learned? Curr Genomics 12: 169–179, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clegg DJ, Benoit SC, Reed JA, Woods SC, Dunn-Meynell A, Levin BE. Reduced anorexic effects of insulin in obesity-prone rats fed a moderate-fat diet. Am J Physiol Regul Integr Comp Physiol 288: R981–R986, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Dobrian AD, Davies MJ, Prewitt RL, Lauterio TJ. Development of hypertension in a rat model of diet-induced obesity. Hypertension 35: 1009–1015, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Duflou J, Virmani R, Rabin I, Burke A, Farb A, Smialek J. Sudden death as a result of heart disease in morbid obesity. Am Heart J 130: 306–313, 1995 [DOI] [PubMed] [Google Scholar]
- 12.Francis NJ, Landis SC. Cellular and molecular determinants of sympathetic neuron development. Annu Rev Neurosci 22: 541–566, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Friedman SE, Andrus BW. Obesity and pulmonary hypertension: a review of pathophysiologic mechanisms. J Obes 2012: 505274, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grassi G, Seravalle G, Cattaneo BM, Bolla GB, Lanfranchi A, Colombo M, Giannattasio C, Brunani A, Cavagnini F, Mancia G. Sympathetic activation in obese normotensive subjects. Hypertension 25: 560–563, 1995 [DOI] [PubMed] [Google Scholar]
- 15.Grassi G, Seravalle G, Colombo M, Bolla G, Cattaneo BM, Cavagnini F, Mancia G. Body weight reduction, sympathetic nerve traffic, and arterial baroreflex in obese normotensive humans. Circulation 97: 2037–2042, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Hasan W, Jama A, Donohue T, Wernli G, Onyszchuk G, Al-Hafez B, Bilgen M, Smith PG. Sympathetic hyperinnervation and inflammatory cell NGF synthesis following myocardial infarction in rats. Brain Res 1124: 142–154, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hasan W, Woodward WR, Habecker BA. Altered atrial neurotransmitter release in transgenic p75(−/−) and gp130 KO mice. Neurosci Lett 529: 55–59, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hiltunen JO, Laurikainen A, Vakeva A, Meri S, Saarma M. Nerve growth factor and brain-derived neurotrophic factor mRNAs are regulated in distinct cell populations of rat heart after ischaemia and reperfusion. J Pathol 194: 247–253, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Kamakura S, Shimizu W, Matsuo K, Taguchi A, Suyama K, Kurita T, Aihara N, Ohe T, Shimomura K. Localization of optimal ablation site of idiopathic ventricular tachycardia from right and left ventricular outflow tract by body surface ECG. Circulation 98: 1525–1533, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Lambert E, Sari CI, Dawood T, Nguyen J, McGrane M, Eikelis N, Chopra R, Wong C, Chatzivlastou K, Head G, Straznicky N, Esler M, Schlaich M, Lambert G. Sympathetic nervous system activity is associated with obesity-induced subclinical organ damage in young adults. Hypertension 56: 351–358, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Lambert GW, Straznicky NE, Lambert EA, Dixon JB, Schlaich MP. Sympathetic nervous activation in obesity and the metabolic syndrome–causes, consequences and therapeutic implications. Pharmacol Ther 126: 159–172, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Levin BE, Dunn-Meynell AA. Reduced central leptin sensitivity in rats with diet-induced obesity. Am J Physiol Regul Integr Comp Physiol 283: R941–R948, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Levin BE, Dunn-Meynell AA. Dysregulation of arcuate nucleus preproneuropeptide Y mRNA in diet-induced obese rats. Am J Physiol Regul Integr Comp Physiol 272: R1365–R1370, 1997 [DOI] [PubMed] [Google Scholar]
- 24.Madsen AN, Hansen G, Paulsen SJ, Lykkegaard K, Tang-Christensen M, Hansen HS, Levin BE, Larsen PJ, Knudsen LB, Fosgerau K, Vrang N. Long-term characterization of the diet-induced obese and diet-resistant rat model: a polygenetic rat model mimicking the human obesity syndrome. J Endocrinol 206: 287–296, 2010 [DOI] [PubMed] [Google Scholar]
- 25.McCully BH, Brooks VL, Andresen MC. Diet-induced obesity severely impairs myelinated aortic baroreceptor reflex responses. Am J Physiol Heart Circ Physiol 302: H2083–H2091, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meloni M, Caporali A, Graiani G, Lagrasta C, Katare R, Van Linthout S, Spillmann F, Campesi I, Madeddu P, Quaini F, Emanueli C. Nerve growth factor promotes cardiac repair following myocardial infarction. Circ Res 106: 1275–1284, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parrish DC, Alston EN, Rohrer H, Hermes SM, Aicher SA, Nkadi P, Woodward WR, Stubbusch J, Gardner RT, Habecker BA. Absence of gp130 in dopamine beta-hydroxylase-expressing neurons leads to autonomic imbalance and increased reperfusion arrhythmias. Am J Physiol Heart Circ Physiol 297: H960–H967, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patterson SI, Skene JH. Novel inhibitory action of tunicamycin homologues suggests a role for dynamic protein fatty acylation in growth cone-mediated neurite extension. J Cell Biol 124: 521–536, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peeraully MR, Jenkins JR, Trayhurn P. NGF gene expression and secretion in white adipose tissue: regulation in 3T3–L1 adipocytes by hormones and inflammatory cytokines. Am J Physiol Endocrinol Metab 287: E331–E339, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Pietrasik G, Goldenberg I, McNitt S, Moss AJ, Zareba W. Obesity as a risk factor for sustained ventricular tachyarrhythmias in MADIT II patients. J Cardiovasc Electrophysiol 18: 181–184, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Rossi M, Marti G, Ricordi L, Fornasari G, Finardi G, Fratino P, Bernardi L. Cardiac autonomic dysfunction in obese subjects. Clin Sci (Lond) 76: 567–572, 1989 [DOI] [PubMed] [Google Scholar]
- 32.Russo V, Ammendola E, De Crescenzo I, Ricciardi D, Capuano P, Topatino A, Docimo L, Santangelo L, Calabro R. Effect of weight loss following bariatric surgery on myocardial dispersion of repolarization in morbidly obese patients. Obes Surg 17: 857–865, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Tedrow UB, Conen D, Ridker PM, Cook NR, Koplan BA, Manson JE, Buring JE, Albert CM. The long- and short-term impact of elevated body mass index on the risk of new atrial fibrillation the WHS (women's health study). J Am Coll Cardiol 55: 2319–2327, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsang TS, Petty GW, Barnes ME, O'Fallon WM, Bailey KR, Wiebers DO, Sicks JD, Christianson TJ, Seward JB, Gersh BJ. The prevalence of atrial fibrillation in incident stroke cases and matched population controls in Rochester, Minnesota: changes over three decades. J Am Coll Cardiol 42: 93–100, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Vanoli E, De Ferrari GM, Stramba-Badiale M, Hull SS, Jr, Foreman RD, Schwartz PJ. Vagal stimulation and prevention of sudden death in conscious dogs with a healed myocardial infarction. Circ Res 68: 1471–1481, 1991 [DOI] [PubMed] [Google Scholar]
- 36.Vaz M, Jennings G, Turner A, Cox H, Lambert G, Esler M. Regional sympathetic nervous activity and oxygen consumption in obese normotensive human subjects. Circulation 96: 3423–3429, 1997 [DOI] [PubMed] [Google Scholar]
- 37.Wang TJ, Parise H, Levy D, D'Agostino RB, Sr, Wolf PA, Vasan RS, Benjamin EJ. Obesity and the risk of new-onset atrial fibrillation. JAMA 292: 2471–2477, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Wisse BE. The inflammatory syndrome: the role of adipose tissue cytokines in metabolic disorders linked to obesity. J Am Soc Nephrol 15: 2792–2800, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Wong CY, O'Moore-Sullivan T, Leano R, Hukins C, Jenkins C, Marwick TH. Association of subclinical right ventricular dysfunction with obesity. J Am Coll Cardiol 47: 611–616, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Zacharias A, Schwann TA, Riordan CJ, Durham SJ, Shah AS, Habib RH. Obesity and risk of new-onset atrial fibrillation after cardiac surgery. Circulation 112: 3247–3255, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Zhao D, McCully BH, Brooks VL. Rosiglitazone improves insulin sensitivity and baroreflex gain in rats with diet-induced obesity. J Pharmacol Exp Ther 343: 206–213, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou S, Chen LS, Miyauchi Y, Miyauchi M, Kar S, Kangavari S, Fishbein MC, Sharifi B, Chen PS. Mechanisms of cardiac nerve sprouting after myocardial infarction in dogs. Circ Res 95: 76–83, 2004 [DOI] [PubMed] [Google Scholar]