Abstract
Based on mosaic theory, hypertension is a multifactorial disorder that develops because of genetic, environmental, anatomical, adaptive neural, endocrine, humoral, and hemodynamic factors. It has been recently proposed that oxidative stress may contribute to all of these factors and production of reactive oxygen species (ROS) play an important role in the development of hypertension. Previous studies focusing on the role of vascular NADPH oxidases provided strong support of this concept. Although mitochondria represent one of the most significant sources of cellular ROS generation, the regulation of mitochondrial ROS generation in the cardiovascular system and its pathophysiological role in hypertension are much less understood. In this review, the role of mitochondrial oxidative stress in the pathophysiology of hypertension and cross talk between angiotensin II signaling, pathways involved in mechanotransduction, NADPH oxidases, and mitochondria-derived ROS are considered. The possible benefits of therapeutic strategies that have the potential to attenuate mitochondrial oxidative stress for the prevention/treatment of hypertension are also discussed.
Keywords: hypertension, mitochondria, oxidative stress, superoxide, antioxidant
this article is part of a collection on Mitochondria in Cardiovascular Physiology and Disease. Other articles appearing in this collection, as well as a full archive of all collections, can be found online at http://ajpheart.physiology.org/.
Introduction
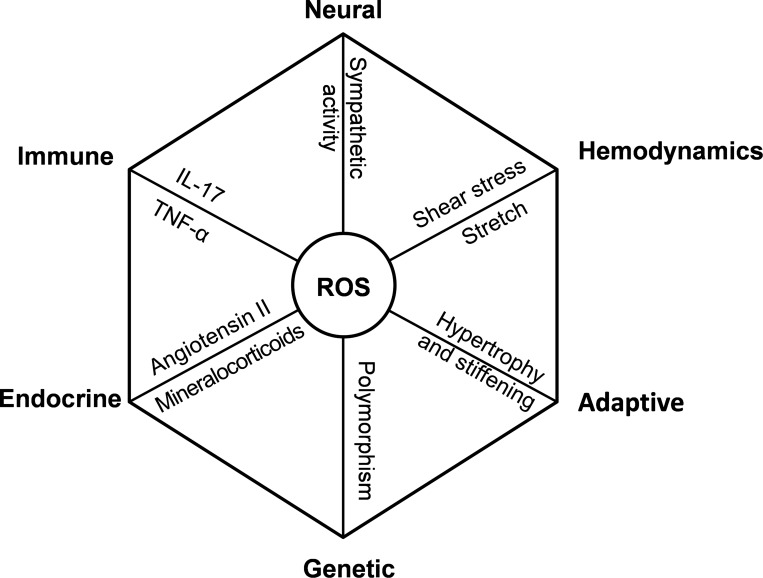
In 1967, Dr. Irvine H. Page proposed the mosaic theory of multiple causes of hypertension (103). The theory states that hypertension is a result of perturbation of interdigitating regulatory mechanisms acting on the variety of tissues, all of which control blood pressure. This includes alterations in neural, endocrine, and immune systems and increased vascular shear stress and stretch due to increased hemodynamics, genetic factors, and maladaptive changes (Fig. 1). Recently, Dr. David Harrison pointed out that a common molecular and cellular event manifested in various organs underlie many features of the mosaic theory (59), namely, enhanced production of reactive oxygen species (ROS; Fig. 1).
Fig. 1.
The central role of reactive oxygen species (ROS) in the mosaic theory of hypertension (59). The octagon indicates a closed system in equilibrium with the interdigitating regulatory mechanisms on each focal point. Note that ROS production contributes to each component of the mosaic theory.
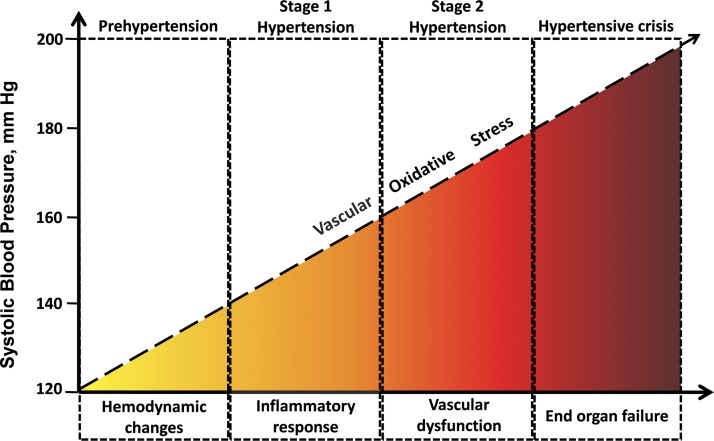
In the past two decades, a number of preclinical studies have been published implicating enhanced production of reactive oxygen and nitrogen species (ROS/RNS) in the development and progression of hypertension (Fig. 2) (25, 61, 145). Increased vascular oxidative stress is also thought to play a key role in the pathophysiological consequences of hypertension (including vascular remodeling, inflammation, endothelial dysfunction, atherosclerosis, vascular cognitive impairment, stroke, and aorta aneurysm formation). Accordingly, animal studies show that attenuation of cellular oxidative stress by overexpression of superoxide dismutase (SOD) or treatment with antioxidants scavenging superoxide attenuate hypertension, whereas depletion of SOD expression exacerbates hypertension (46). Similarly, attenuation of cellular oxidative stress by molecular or pharmacological methods was shown to confer multifaceted cardiac and vascular protective effects, preventing/delaying the development of complications of hypertension in animal models (140). Duffy et al. (42) demonstrated a beneficial effect of vitamin C on blood pressure. Yet, no large studies have confirmed this effect. Recent clinical studies on the role of antioxidant supplements in hypertension failed to show any effect of low-dose antioxidants or yielded mixed results (27). A number of clinical trials showed that the routinely used antioxidants per se are ineffective in preventing or treating cardiovascular diseases and hypertension (50). Yet, many antihypertensive drugs were shown to inhibit ROS production and reduce vascular oxidative stress (60).
Fig. 2.
Proposed conceptual model showing that development of hypertension and hypertension-related vascular alterations are associated with increases in vascular oxidative stress. The model predicts that the link between vascular oxidative stress and hypertension is bidirectional: oxidative stress both promotes the development of hypertension and contributes to hypertension-induced pathologies (including atherosclerosis, kidney failure, stroke, aorta aneurysms, vascular cognitive impairment). This emerging new concept is supported by both animal studies and clinical observations (24, 37, 66, 76, 78, 92, 95, 108, 113, 117).
According to the model using a system biology approach, recently proposed by Dr. Harrison based on the mosaic theory of hypertension (59), ROS production represents an important common pathway (a “node”, as depicted in Fig. 1) in vascular remodeling, vasoconstriction, endocrine dysregulation, anatomical maladaptation, neural, and emotional factors that lead to development of hypertension. To identify molecular mechanisms of oxidative stress that can be targeted pharmacologically for treatment of hypertension, it is, therefore, important to investigate separately the different sources of vascular ROS production. In general, oxidative stress is a result of an imbalance between ROS production and activity of cellular antioxidant system. Major sources of vascular ROS production include NADPH oxidases, xanthine oxidase, uncoupled nitric oxide synthase, and mitochondria (33). In recent years, a number of studies have been published on the role of NADPH oxidases, xanthine oxidase, and uncoupled nitric oxide synthase in hypertension-related cardiovascular pathologies [reviewed recently (138)]. Although mitochondria represent one of the most significant sources of cellular ROS generation (mitochondrial ROS production can reach up to 2% of the electron flow), the regulation of mitochondrial ROS generation and its pathophysiological role in hypertension are much less understood. In this review, the role of mitochondrial oxidative stress in the pathophysiology of hypertension and cross talk between angiotensin II signaling, pathways involved in mechanotransduction, NADPH oxidases, and mitochondria-derived ROS are considered. The possible benefits of therapeutic strategies that have the potential to attenuate mitochondrial oxidative stress for the prevention/treatment of hypertension are also discussed.
Dysregulation of Mitochondrial ROS Production in the Cardiovascular System in Hypertension
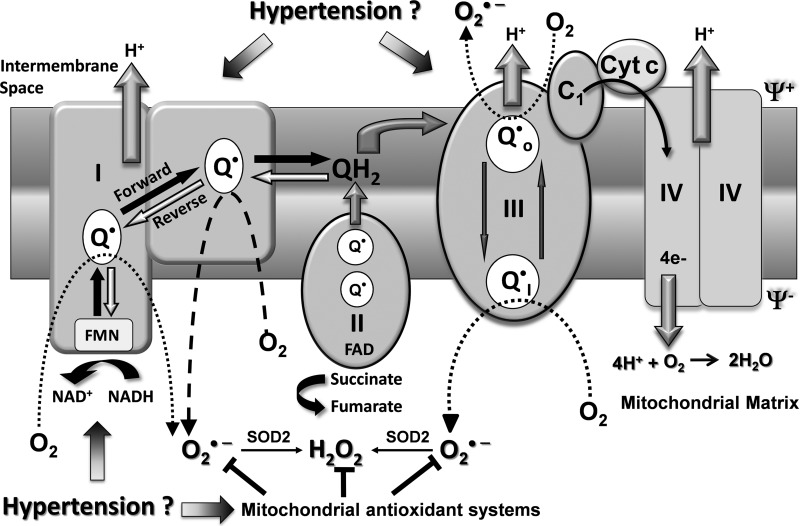
Mitochondria, in addition to generating most of the cell's supply of ATP by oxidative phosphorylation, are also involved in regulation of apoptosis, maintenance of cellular redox status, and ROS production (33). ATP synthesis is based on electron transfer through the mitochondrial respiratory chain (Fig. 3). Electrons can be supplied by either NADH at complex I or by succinate at complex II. Ubiquinone (QH2) mediates electron transfer to complex III, which in turn reduces complex IV. Complex IV couples oxygen reduction to water and the proton pump, transporting protons (H+) from the matrix to the intermembrane space (19). The resultant proton motive force across the inner mitochondrial membrane is used by complex V for ATP synthesis. Since mitochondria is a major source of cellular superoxide (O2·−), it has been previously suggested that electrons “leak” from electron transport chain to O2 producing O2·− (11, 55). It was called a nonenzymatic reaction where O2 interacts with the ubisemiquinone radical of the respiratory chain leading to a one electron reduction of O2. In the past decade, it has been shown that free ubiqunone does not represent a significant source of mitochobdrial O2·− but complex I (33) and complex III (123, 126) are the main sources of mitochondrial O2·− (Fig. 3). Other sources of mitochondrial O2·− may include α-ketoglutarate dehydrogenase, pyruvate dehydrogenase (124), glycerol 3-phosphate dehydrogenase, and fatty acid β-oxidation (12). A new concept therefore is emerging that production of mitochondrial ROS is a highly regulated enzymatic process, and it is a part of mitochondrial physiological function (54). Complex I can produce O2·− both in the forward and reverse electron transfer (Fig. 3). It has been shown that succinate-dependent reverse electron transfer is much more efficient in O2·− production compared with NADH-dependent forward electron transfer (104). Reverse electron transport is physiologically regulated by several mechanisms including: 1) control of succinate dehydrogenase activity in the citric acid cycle (106), 2) fatty acids (107), 3) and mitochondrial membrane potential (99). Complex I mediated production of mitochondrial O2·− has been implicated in various pathophysiological processes, including ischemic preconditioning (15) and in aging. Recent findings demonstrate increased production of O2·− in the mitochondrial matrix by complex I by reverse electron flow from complex II in the setting of hypertension (97) (Fig. 3). O2·− produced by complex I mainly on the matrix side is rapidly dismutated to H2O2 by mitochondrial SOD2 (16, 56). H2O2 is a neutral molecule and will easily leave mitochondria regardless of mitochondrial energization. Complex III produces O2·− both in mitochondrial matrix and intermembrane space (Fig. 3) under condition of hypoxia and complex IV inhibition (94). It is thought to be important in oxygen sensing and redox-dependent cell signaling (54).
Fig. 3.
Possible sites of ROS production in mitochondria, which can contribute to cellular oxidative stress in the cardiovascular system in hypertension. O2·−, superoxide; Cyt c, cytochrome c; FAD, flavin adenine dinucleotide; FMN, flavin mononucleotide; Q·, ubiquinone; QH2, ubiquinone; Ψ+, membrane potential.
There is increasing evidence that hypertension is associated with an increased mitochondria-derived production of ROS in various animal models, including angiotensin II-infused mice (36, 37, 40). Mitochondria also contribute to increased vascular ROS production in mesenteric resistance arteries and the aorta from DOCA-salt rats (136). There are other mechanisms by which mitochondria may affect vascular function in hypertension. For example, deficiency of mitochondrial aldehyde dehydrogenase, an enzyme that detoxifies aldehydes to carboxylic acids, is known to increase oxidative stress. Interestingly, recent studies suggest that mitochondrial aldehyde dehydrogenase attenuates vascular contractions in angiotensin-II treated hypertensive mice (22).
In addition to the role of mitochondria in the peripheral vasculature in hypertension, there is increasing evidence that mitochondria-derived ROS play an important role in the central regulation of systemic cardiovascular function as well (17, 96). Recent data suggest that in the brain activation of the renin-angiotensin system elicits intraneuronal signaling, which involves an increased production of mitochondrial O2·− (17), modulating ion channel activity and increasing neuronal firing (144). The aforementioned mitochondrial redox signaling pathways are thought to play a key role in central regulation of blood pressure. Accordingly, overexpression of MnSOD in the brain effectively abolishes the central angiotensin II-induced pressor response and decreases blood pressure in rodent models of hypertension (18, 149).
The critical role of mitochondrial ROS in hypertension-induced cardiomyopathy in angiotensin II-infused rodents is suggested by the findings that mice overexpressing catalase targeted to mitochondria (mCat), but not mice overexpressing peroxisomal catalase, are resistant to hypertension-induced cardiac hypertrophy, fibrosis, and diastolic dysfunction (29). Future studies should explore whether mCat mice are also resistant to hypertension-induced pathological alterations in the vasculature as well.
Mitochondria are not only the major source of O2·− and H2O2 in vascular cells (7, 84) but also represent a targets of cellular ROS (7). Mitochondrial membranes, proteins, and mitochondrial DNA seem particularly sensitive to oxidative damage (8, 143). Oxidative macromolecular damage in the mitochondria in vascular cells is known to adversely affect mitochondrial function (8). ROS posttranslationally modify mitochondrial proteins leading to their inactivation, as in the case of SOD2 and aconitase, or alter their function as occurs with cytochrome c (14, 21, 87). Superoxide reacts with 4Fe-4S clusters of complex I, complex II, and aconitase, resulting in release of Fe3+ and altered protein function (45). It has been shown that oxidative damage to complex I and complex II, presumably at the level of 4Fe-4S clusters, increases mitochondrial O2·− production. Interestingly, a decrease in complex II activity due to oxidative modification increases its O2·− production by three- to fourfold (105). Oxidative mitochondrial DNA damage may affect the synthesis of components of the respiratory chain, which in turn can further increase ROS production, initiating a vicious cycle. Interestingly, mutations in mitochondrial DNA also associate with increased risk for hypertension (43, 114, 128).
Potential Role of Mitochondrial Antioxidant Systems in Attenuating Hypertension-Induced Oxidative Stress in the Cardiovascular System
Mitochondrial antioxidant systems play an important role in protecting mitochondria and attenuating vascular oxidative stress. SOD2 and glutathione peroxidase are major scavengers of mitochondrial O2·− and H2O2 (45, 139). SOD2 plays an important role in regulation of redox-sensitive signaling pathways and control mitochondrial O2·− (98). By inhibiting the reaction of O2·− with 4Fe-4S clusters, this enzyme prevents inactivation of aconitase, complex I and complex II (110). SOD2 is inactivated by peroxynitrite (112), and its activity is reduced with age (142). Expression of SOD2 is regulated in a redox-dependent manner (121). SOD2 overexpression attenuates H2O2-induced apoptosis (115), decreases lipid peroxidation, and reduces the age-related decline in mitochondrial ATP (72). Multiple lines of evidence suggest that impaired function of mitochondrial antioxidant systems is causally linked to the pathogenesis of hypertension. Depletion of mitochondrial SOD2 predisposes mice to both age-related and salt-induced hypertension (116). Earlier reports have found that hypertension and cardiac hypertrophy were associated with reduced expression of SOD1 and SOD2 in spontaneously hypertensive rats compared with Wistar-Kyoto rats (70). Furthermore, increased SOD2 expression in intracerebroventricular region using adenoviral vector AdSOD2 abolished angiotensin II-induced changes in blood pressure and heart rate (149). In that regard it is interesting that in angiotensin II-stimulated neurons, mitochondrial-localized NADPH oxidase 4 was shown to contribute to increased mitochondrial O2·− production (17). In humans, SOD2 coding is in a region of chromosome 6 linked to susceptibility to hypertension (120). Interestingly, failure to induce SOD2 in response to oxygen treatment may contribute to the development of persistent pulmonary hypertension as well (6). Treatment with l-buthionine sulfoximine, which elicits mitochondrial oxidative stress by depleting GSH, elicits hypertension in rats (9). Recent studies also indicate that genetic Gpx1 deficiency exacerbates cardiac hypertrophy and dysfunction in angiotensin II-dependent hypertension (4). Another major antioxidant defense system against mitochondrial ROS (in particular, H2O2) is thiol-reducing systems, including the thioredoxin (thioredoxin 2, thioredoxin reductase 2, and peroxiredoxin 3), glutaredoxin, and the glutathione system. Recent studies using transgenic mice overexpressing thioredoxin 2 showed that this mitochondrial antioxidant system plays a key role in attenuation of mitochondrial ROS production in the aorta, endothelial protection, and regulation of blood pressure in mice with angiotensin II-induced hypertension (139). Overexpression of thioredoxin 2 was also shown to inhibit cardiac hypertrophy and cardiac oxidative stress in mice with chronic angiotensin II infusion (139). The aforementioned studies suggest that imbalance between ROS production and mitochondrial antioxidants contribute to the pathogenesis of hypertension and the development of various vascular pathologies associated with hypertension. There are studies extant showing that mice overexpressing peroxiredoxin 3, the mitochondria-specific peroxidase linked to thioredoxin 2, exhibit improved survival under conditions of increased mitochondrial oxidative stress (88), but the role of peroxiredoxin 3 in hypertension remains elusive. It has been recently suggested that oxidative stress is a result of ROS-induced ROS release due to feed-forward regulation of ROS sources (33, 150). Indeed, production of mitochondrial ROS is redox dependent, and overexpression of mitochondrial SOD2 and thioredoxin 2 simultaneously reduces both production of mitochondrial and cytoplasmic ROS (45, 139). Mitochondrial ATP-sensitive K+ channel (mitoKATP) and PKCε are potential target of mitochondrial and cellular ROS, because they are ROS sensitive (23, 111, 147). Importantly, stimulation of mitoKATP and PKCε has been shown to induce production of mitochondrial ROS (3, 23), representing a potential mechanism of ROS-induced mitochondrial ROS release.
Increased O2·− production activates series of feedback protective mechanisms including increased antioxidant expression by NF-E2-related factor 2 (NRF2) activation (74) or stimulation of mitochondrial uncoupling protein 2 (UCP2) (85). UCP2 dissipates the mitochondrial proton gradient that contributes to O2·− formation by reverse electron flow from complex II to complex I. It has been shown that UCP2 has a compensatory role to offset the hypertensive effects of the high-salt diet and partially offset salt-induced vascular dysfunction (86). Interestingly, angiotensin receptor blockade in Otsuka Long-Evans Tokushima fatty mice reduces blood pressure and recovers hepatic UCP2 expression (91). This may suggest that protective role of UCP2 is impaired in hypertension. Indeed, high-salt diet downregulates the UCP2 gene and protein expression in kidneys of stroke-prone spontaneously hypertensive rats, but not of stroke-resistant rats (32). These data suggest that UCP2 is an important protein to prevent oxidative stress in the settings of hypertension.
Cross Talk Between Mitochondrial ROS and NADPH Oxidases: Role in the Pathophysiology of Hypertension
In the past decade an important role of NADPH oxidases in hormone and cytokine responses have been found (79). ROS production by NADPH oxidases has been implicated both in physiological and pathological conditions. For example, NADPH oxidases regulates endothelial nitric oxide synthase activity (81) but overexpression of NADPH oxidases contributes to development of diabetes (118) and hypertension (38). We have found that activation of vascular NADPH oxidases increases production of mitochondrial O2·− (37, 40). For example, stimulation of endothelial cells with angiotensin II increases mitochondrial O2·−, and depletion of p22phox, a docking subunit of NADPH oxidase complex, abolishes this effect (40). This can be mediated by Nox1 or Nox2 isoforms that are required for angiotensin II-stimulated O2·− (34).
Nox1 is expressed in smooth muscle and other vascular cells, whereas Nox2 is present in endothelial and phagocytic cells (79). Nox4 is constitutively expressed and active in vascular smooth muscle and endothelial cells (2, 63) and is responsible for the basal production of H2O2, whereas increased Nox5 Ca2+-dependent H2O2 production in human endothelial cells has been implicated in cardiovascular diseases (53). Angiotensin II increases activity of Nox1 and/or Nox2 (80) via angiotensin II type 1 receptor-dependent activation of PKC and c-Src pathways (82). It is important to note that activation of c-Src is redox sensitive and stimulated by H2O2 (135), which appears to represent a feed-forward mechanism whereby H2O2-mediated activation of c-Src amplifies NADPH oxidase activity. In addition to Nox1 and Nox2, there is increasing evidence that Nox4 also plays an important role in the pathophysiology of hypertension. Importantly, Nox4 is localized to the mitochondrial membrane and its activation increases mitochondrial oxidative stress (28). The expression of Nox4 is reported to be increased in spontaneously hypertensive rats (13). Moreover, angiotensin II induces expression of Nox4 in smooth muscle cells (13) and in cardiac myocytes (28). A recent study also reported that mitochondrial-located Nox4 is a major source of pressure overload-induced oxidative stress in the heart (77). In addition of direct production of O2·− by Nox4 it is also possible that ROS produced by Nox4 alter mitochondrial electron transports chain exacerbating ROS production from this source as well. The fact that mitochondria-targeted catalase overexpression but not cytoplasmic catalase attenuates hypertension-induced cardiac hypertrophy in angiotensin II-infused mice emphasize the key role of ROS amplification within mitochondria (29).
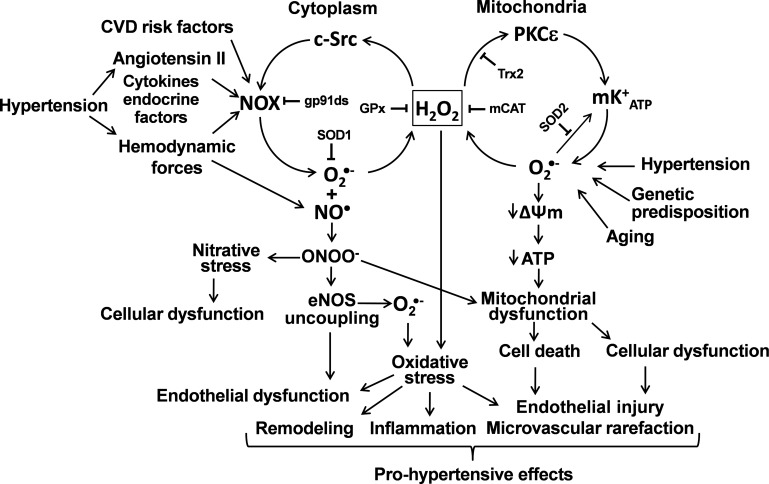
The cross talk between mitochondria and NADPH oxidases is bidirectional. Mitochondria apparently regulate both expression (45) and activity of NADPH oxidases (35). Partial depolarization reduces Ca2+ uptake by mitochondrial uniporter and increases Ca2+-dependent activation of phagocytic NADPH oxidase (35), whereas depletion of SOD2 results in increase of basal and stimulated vascular NADPH oxidase activity (37). These data show that mitochondrial ROS provide feed-forward regulation of NADPH oxidases which is likely mediated by activation of redox sensitive c-Src with mitochondrial H2O2 (Fig. 4). Recently, it has been reported that functional angiotensin II type 2 receptors are present on mitochondrial inner membranes and that binding of angiotensin II to these receptors activate mitochondrial nitric oxide production (1). Mitochondria-derived nitric oxide. has been shown to regulate oxygen consumption by inhibiting the electron transport chain (44, 109). It is tempting to speculate that in hypertension, when mitochondria-derived O2·− production is also increased, this mechanism would contribute to the increased generation of peroxynitrite in mitochondria, promoting nitrosative damage to mitochondrial proteins. Manganese SOD (SOD2) is a key mitochondrial antioxidant enzyme that is inactivated by reacting with peroxynitrite. Previous studies demonstrated that angiotensin II-induced hypertension is indeed associated with increased tyrosine nitration of SOD2 (52), suggesting that the aforementioned pathway may further exacerbate mitochondrial oxidant stress in the vasculature. Mitochondria may also scavenge cellular ROS and thereby represent an important check-point down regulating production of cellular ROS. ROS production by NADPH oxidases was reduced by SOD2 overexpression (37), by overexpression of mitochondrial thioredoxin 2 (139), by treatment with mitochondria targeted SOD mimetic mitoTEMPO (37) or supplementation with mitoKATP blocker 5-hydroxydecanoic acid (40). Taken together, these data suggest that mitochondria can attenuate or amplify production of cellular ROS. Since mitochondrial ROS have been proposed as second messengers for regulations provided by many hormones and cytokines such as angiotensin II, VEGF, TNF-α, and IL-1 (20, 37, 119), we suggest that mitochondrial dysfunction (7) may result in dysregulation of cellular redox status and overproduction of ROS leading to the development vicious cycle of oxidative stress (Fig. 4).
Fig. 4.
Proposed role of redox-dependent cross talk between mitochondria and NADPH oxidases (NOXs) in vascular dysfunction in hypertension. CVD, cardiovascular disease; NO, nitirc oxide; ONOO−, peroxynitrite; eNOS, endothelial NO synthase; GPx, glutathione peroxidase; Trx2, thioredoxin 2; mCat, catalase targeted to mitochondria; mKATP, ATP-sensitive K+ channel; ΔΨm, mitochondrial transmembrane potential.
Potential Role of Hemodynamic Forces in Regulation of Mitochondrial ROS Production
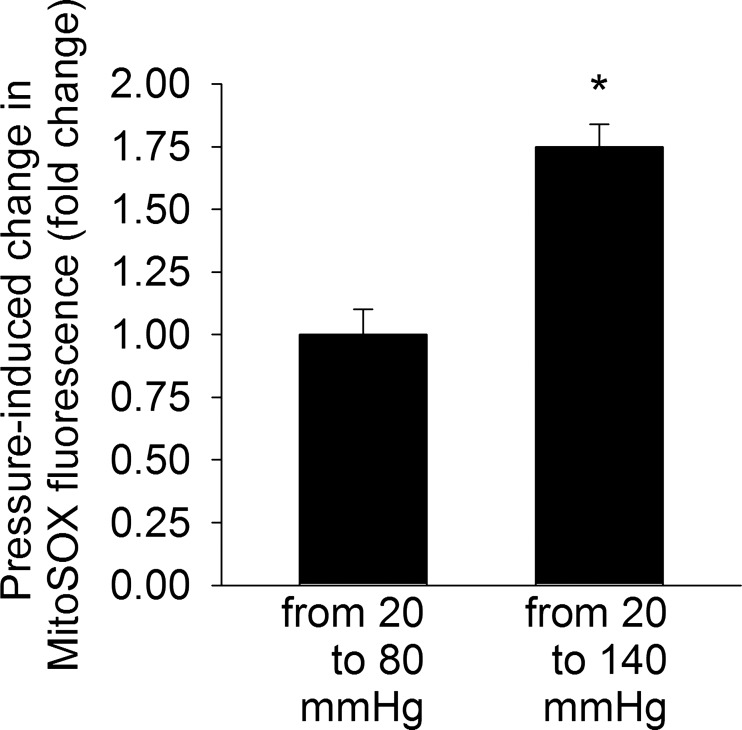
Several line of evidence suggests that changes in the hemodynamic environment associated with hypertension, either directly or indirectly, can activate vascular ROS production (131). A primary role for high intraluminal pressure in the upregulation of vascular ROS generation is supported by the findings that in aortic banded rats (in which only blood vessels proximal to the coarctation are exposed to high pressure), regional increases in blood pressure result in selective increases in vascular O2·− production (132). Furthermore, exposure of isolated arteries to high pressure in vitro was shown to increase vascular ROS production (131), eliciting endothelial dysfunction (67). Short-term increases in blood pressure in vivo also impair endothelial function and promote oxidative stress (30, 47, 75, 137). It is likely that in the aforementioned experiments wall tension-dependent cellular stretch is the primary mechanical stimulus for increased vascular ROS production, because exposure of isolated arterial rings to in vitro stretching was also reported to activate NADPH oxidase-mediated O2·− generation (101), mimicking the effects of high pressure. Previous studies also demonstrate that in cultured endothelial and smooth muscle cells subjected to in vitro stretching production of ROS also significantly increases (51, 64, 65). The available evidence suggests that the prooxidative effects of high pressure itself and the downstream effects angiotensin II signaling are synergistic. In addition to the established role of NADPH oxidases in mechanosensitive ROS production (131), there is also evidence suggesting that high pressure (similar to angiotensin II) can also increase ROS production by the mitochondria. An elegant study by Ichimura et al. (69) demonstrated that elevation of pulmonary capillary pressure increases the amplitude of cytosolic Ca2+ oscillations in endothelial cells, which trigger increases in the amplitude of mitochondrial Ca2+ oscillations. The aforementioned study provided evidence that pressure-induced mitochondrial Ca2+ signals promote mitochondrial ROS production in endothelial cells, which likely play a role in proinflammatory signaling pathways (69). Recently we found that exposure of isolated mouse middle cerebral arteries to high pressure in vitro also promote mitochondria-derived ROS generation (Fig. 5), although further studies are needed to elucidate the underlying signaling pathways. Taken together, the aforementioned studies raise the possibility that mechanosensitive mitochondrial ROS production exacerbates vascular oxidative stress in hypertension (37). Importantly, aging was reported to exacerbate high pressure-induced vascular ROS production (71). There is also evidence suggesting that aging renders cardiac mitochondria more susceptible to hypertension-induced dysfunction (5), although a detailed characterization of aging and hypertension on mitochondrial ROS generation in cardiac myocytes has yet to be performed. Because vascular aging is associated with increased propensity for mitochondrial ROS generation (134), abnormal mitochondrial ROS detoxification, and impaired resistance to mitochondrial oxidative stressors (130), future studies should investigate the interaction among aging, hypertension, and mitochondrial ROS production and its role in increased vulnerability of the elderly to complications of hypertension.
Fig. 5.
Pressure-induced increases in mitochondrial ROS production in isolated, cannulated branches of the mouse middle cerebral artery. Data are relative changes in the buildup of MitoSox fluorescence intensities induced by stepwise increase in intraluminal pressure. Note that high pressure upregulates vascular mitochondrial ROS generation. MitoSox loading was performed as previously reported (26). Data are means ± SE (n = 6 in each group). *P < 0.01.
In addition to pressure, other modalities of blood flow, such as shear stress, also regulate vascular ROS production. During hypertension, the hemodynamic energy dissipation increases due to changes in wall shear stress along the arterial tree. Hypertension is also associated with increases in retrograde and oscillatory shear in large arteries (102). Recent clinical studies in patients with uncomplicated hypertension demonstrate flow reversal in the descending aorta (62). Moreover, hypertension is often associated with aortic regurgitation, which also leads to flow reversal in the thoracic aorta. It is likely that the aforementioned changes in the hemodynamic microenvironment in hypertension affect ROS production in vascular cells. Shear stress was demonstrated to promote the release of H2O2 in human coronary arterioles (isolated from hearts of cardiac patients) (90). In cultured endothelial cells, oscillatory shear stress is also a potent stimulus of ROS production (31, 58, 68, 89). The available data suggest that mitochondria is an important source of shear stress-mediated endothelial ROS production both in intact vessels (84) and cultured endothelial cells (127). In addition, there are studies extant showing that shear stress can also increase endothelial ROS production via stimulation of NADPH oxidases (68). Recent studies suggest that shear stress promotes mitochondrial ROS generation via a pathway that involves both NADPH oxidase and JNK activation (127), suggesting that a cross talk exist between the two pathways. Laminar shear stress-induced nitric oxide synthesis and simultaneous increase in mitochondrial O2·− generation were reported to lead to mitochondrial peroxynitrite formation and suppression of respiration in endothelial cells (73). Interestingly, oscillatory shear stress was shown to upregulate Nox2 and Nox1 while decreasing Nox4 expression in human endothelial cells (122). Although it has been proposed that acute administration of H2O2 elicits vasodilation (141), it is likely that chronic mechanosensitive increases in vascular H2O2 production predominantly have signaling roles (e.g., activation of NF-κB, MAPKs), regulating cell proliferation and structural remodeling, and/or contribute to oxidative vascular injury. Importantly, recent data suggest that shear stress-induced mitochondria-derived release of H2O2 contributes to shear stress-mediated Nrf2 activation in endothelial cells as well (57). Taken together, some of the effects exerted by changes in hemodynamic forces on vascular endothelial and smooth muscle cells are similar to those induced by humoral factors associated with hypertension (including angiotensin II), e.g., upregulation of mitochondria-derived ROS production and the signaling pathways leading to NADPH oxidase activation. Future studies on the effects of mechanical forces on signal transduction regulating mitochondrial function, mitochondrial biogenesis, and mitochondrial gene expression will provide insights into the molecular mechanisms by which hemodynamic factors and humoral factors interact in hypertension to regulate vascular physiology and pathophysiology.
Mitochondria-Targeted Antioxidants: Role in Pharmacological Treatment and Prevention of Hypertension
Recent preclinical studies by the Dikalov laboratory (37) show that SOD2 overexpression attenuate hypertension. These studies provided important proof of concept for the development of pharmacological strategies to attenuate mitochondrial oxidative stress for the prevention/treatment of hypertension. Importantly, supplementation with a mitochondria-targeted SOD mimetic mitoTEMP also significantly attenuates angiotensin II-induced hypertension in mice (37). Infusion of mitoTEMPO in mice after onset of both angiotensin II- and DOCA-salt induced hypertension resulted in a significant decrease (by ∼25 mmHg) of blood pressure (37). MitoTempo treatment also inhibited vascular oxidative stress and completely restored endothelial-dependent relaxation (37). In 2009, it has been reported that 8-wk treatment of spontaneously hypertensive rats with mitochondria-targeted antioxidant MitoQ significantly reduced systolic blood pressure by 25 mmHg (49). MitoQ supplementation, however, may have unwanted side effects, including promotion of inflammation (39, 93, 100). A recent study reported that treatment with rotenone (as an effort to inhibit production of mitochondrial ROS by inhibiting complex I) attenuates DOCA-salt induced hypertension (146). Rotenone, however, causes neurodegeneration (105), and it is not appropriate for treatment of humans. Treatment of angiotensin II-infused mice with the mitochondrial-targeted antioxidant Szeto-Schiller (SS) compound SS-31 was reported to reduce oxidative stress, improve cardiac function, and attenuate cardiac hypertrophy, despite the absence of blood pressure-lowering effect (28). In that regard it should be noted that the mechanism of action of various mitochondria-targeted antioxidants is different, for example, SS-31 is able to scavenge H2O2 and peroxynitrite (125, 148), which may explain their differential effect on blood pressure.
Recent findings demonstrate that in the cardiovascular system of healthy animals in response to increased production of ROS, adaptive mechanisms are invoked that involve induction of Nrf2-driven antioxidant defense mechanisms (129, 130, 133). Nrf2 is an evolutionarily highly conserved redox sensitive transcription factor that upregulates the expression of numerous antioxidant systems (e.g., enzymes involved in glutathione synthesis). This homeostatic response serves to attenuate mitochondrial and cytoplasmic oxidative stress and limit the damage caused by the increased production of ROS. In recent years, Nrf2 has emerged as a potential target for pharmacological attenuation of mitochondrial oxidative stress in the cardiovascular system (129). Recent studies report that treatment of hypertensive rodents with naturally occurring Nrf2-activating compounds (10, 48), lower blood pressure, restore endothelial function, and decrease vascular oxidative stress. Collectively, the aforementioned studies demonstrate that mitochondrial ROS production is important for the development of hypertension and that antioxidant strategies targeting mitochondria ROS confer multifaceted therapeutic benefits in hypertension.
Perspectives
A large body of evidence supports an important role for mitochondrial oxidative stress in the development of hypertension, vascular pathologies, and cardiac dysfunction, which characterize the hypertensive disease. We are at the beginning of an exciting phase of research on understanding the genetic and epigenetic mechanisms underlying increased individual susceptibility for mitochondrial alterations and development of hypertension. Within the vascular wall mitochondria-derived ROS are generated, both in endothelial and vascular smooth muscle cells, and both function as signaling molecules, regulating many aspects of cellular function and contribute to oxidative macromolecular damage. Importantly, a consensus should be reached whether contribution of mitochondria-derived ROS to cardiovascular pathologies occur primarily via increased macromolecular damage or other mechanisms by which mitochondria-derived ROS and signaling affect the cellular function play an equally important role. The role of neurohormonal factors, including the interplay between angiotensin II signaling and mechanosensitive signaling pathways, in hypertension-related mitochondrial alterations needs to be further elucidated. In hypertension, excess generation of ROS cannot be counterbalanced by endogenous mitochondrial protective antioxidant mechanisms, leading to a state of increased mitochondrial oxidative stress. Despite the fact that contribution of mitochondrial ROS in pathophysiology of hypertension is well documented, we still do not know the precise mechanism of upregulation and the specific sources of mitochondrial ROS. This information will be critical for the development of treatment of mitochondrial oxidative stress. Further studies are warranted to determine whether upregulation of endogenous antioxidant defense mechanisms (e.g., by induction of Nrf2-regulated pathways) is a viable option to attenuate mitochondrial oxidative stress for the prevention/treatment of hypertension. The available evidence, suggesting that pharmacological treatment with mitochondria-targeted antioxidants confers cardiovascular protective effects in animal models of hypertension, looks particularly promising. Further experimental and clinical studies examining the interaction between mitochondria-targeted antioxidants and inhibitors of the renin-angiotensin system in hypertension are necessary. We think that these future studies will discover new pharmacological targets and will advance the development of new therapies of hypertension and possibly other oxidative stress-related conditions.
GRANTS
This work was supported by funding from National Institutes of Health Grants HL-094469 and AT-006526 (to S. I. Dikalov and Z. Ungvari, respectively) and a grant from the American Heart Association (to Z. Ungvari).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Z.I.U. and S.I.D. prepared figures; Z.I.U. and S.I.D. drafted manuscript; Z.I.U. and S.I.D. edited and revised manuscript; Z.I.U. and S.I.D. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. David G. Harrison for fruitful discussion and advice and Dr. Peter Toth (Reynolds Oklahoma Center on Aging, University of Oklahoma Health Sciences Center, Oklahoma City, OK) for help with the MitoSox measurements.
REFERENCES
- 1.Abadir PM, Foster DB, Crow M, Cooke CA, Rucker JJ, Jain A, Smith BJ, Burks TN, Cohn RD, Fedarko NS, Carey RM, O'Rourke B, Walston JD. Identification and characterization of a functional mitochondrial angiotensin system. Proc Natl Acad Sci USA 108: 14849–14854, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ago T, Kitazono T, Ooboshi H, Iyama T, Han YH, Takada J, Wakisaka M, Ibayashi S, Utsumi H, Iida M. Nox4 as the major catalytic component of an endothelial NAD(P)H oxidase. Circulation 109: 227–233, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Andrukhiv A, Costa AD, West IC, Garlid KD. Opening mitoKATP increases superoxide generation from complex I of the electron transport chain. Am J Physiol Heart Circ Physiol 291: H2067–H2074, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Ardanaz N, Yang XP, Cifuentes ME, Haurani MJ, Jackson KW, Liao TD, Carretero OA, Pagano PJ. Lack of glutathione peroxidase 1 accelerates cardiac-specific hypertrophy and dysfunction in angiotensin II hypertension. Hypertension 55: 116–123, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asemu G, O'Connell KA, Cox JW, Dabkowski ER, Xu W, Ribeiro RF, Jr, Shekar KC, Hecker PA, Rastogi S, Sabbah HN, Hoppel CL, Stanley WC. Enhanced resistance to permeability transition in interfibrillar cardiac mitochondria in dogs: effects of aging and long-term aldosterone infusion. Am J Physiol Heart Circ Physiol 304: H514–H528, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asikainen TM, Heikkila P, Kaarteenaho-Wiik R, Kinnula VL, Raivio KO. Cell-specific expression of manganese superoxide dismutase protein in the lungs of patients with respiratory distress syndrome, chronic lung disease, or persistent pulmonary hypertension. Pediatr Pulmonol 32: 193–200, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Ballinger SW. Mitochondrial dysfunction in cardiovascular disease. Free Radic Biol Med 38: 1278–1295, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Ballinger SW, Patterson C, Yan CN, Doan R, Burow DL, Young CG, Yakes FM, Van Houten B, Ballinger CA, Freeman BA, Runge MS. Hydrogen peroxide- and peroxynitrite-induced mitochondrial DNA damage and dysfunction in vascular endothelial and smooth muscle cells. Circ Res 86: 960–966, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Banday AA, Lau YS, Lokhandwala MF. Oxidative stress causes renal dopamine D1 receptor dysfunction and salt-sensitive hypertension in Sprague-Dawley rats. Hypertension 51: 367–375, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Bhatt SR, Lokhandwala MF, Banday AA. Resveratrol prevents endothelial nitric oxide synthase uncoupling and attenuates development of hypertension in spontaneously hypertensive rats. Eur J Pharmacol 667: 258–264, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Boveris A. Determination of the production of superoxide radicals and hydrogen peroxide in mitochondria. Methods Enzymol 105: 429–435, 1984 [DOI] [PubMed] [Google Scholar]
- 12.Brand MD. The sites and topology of mitochondrial superoxide production. Exp Gerontol 45: 466–472, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Briones AM, Tabet F, Callera GE, Montezano AC, Yogi A, He Y, Quinn MT, Salaices M, Touyz RM. Differential regulation of Nox1, Nox2 and Nox4 in vascular smooth muscle cells from WKY and SHR. J Am Soc Hypertens 5: 137–153, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Brookes PS, Zhang J, Dai L, Zhou F, Parks DA, Darley-Usmar VM, Anderson PG. Increased sensitivity of mitochondrial respiration to inhibition by nitric oxide in cardiac hypertrophy. J Mol Cell Cardiol 33: 69–82, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Burwell LS, Nadtochiy SM, Tompkins AJ, Young S, Brookes PS. Direct evidence for S-nitrosation of mitochondrial complex I. Biochem J 394: 627–634, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cadenas E, Boveris A, Ragan CI, Stoppani AO. Production of superoxide radicals and hydrogen peroxide by NADH-ubiquinone reductase and ubiquinol-cytochrome c reductase from beef-heart mitochondria. Arch Biochem Biophys 180: 248–257, 1977 [DOI] [PubMed] [Google Scholar]
- 17.Case AJ, Li S, Basu U, Tian J, Zimmerman MC. Mitochondrial-localized NADPH oxidase 4 is a source of superoxide in angiotensin II-stimulated neurons. Am J Physiol Heart Circ Physiol 305: H19–H28, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan SH, Wu KL, Chang AY, Tai MH, Chan JY. Oxidative impairment of mitochondrial electron transport chain complexes in rostral ventrolateral medulla contributes to neurogenic hypertension. Hypertension 53: 217–227, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Chance B, Williams GR. Respiratory enzymes in oxidative phosphorylation. III. The steady state. J Biol Chem 217: 409–427, 1955 [PubMed] [Google Scholar]
- 20.Chen K, Thomas SR, Albano A, Murphy MP, Keaney JF., Jr Mitochondrial function is required for hydrogen peroxide-induced growth factor receptor transactivation and downstream signaling. J Biol Chem 279: 35079–35086, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Chen YR, Deterding LJ, Sturgeon BE, Tomer KB, Mason RP. Protein oxidation of cytochrome C by reactive halogen species enhances its peroxidase activity. J Biol Chem 277: 29781–29791, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Choi H, Tostes RC, Webb RC. Mitochondrial aldehyde dehydrogenase prevents ROS-induced vascular contraction in angiotensin-II hypertensive mice. J Am Soc Hypertens 5: 154–160, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Costa AD, Garlid KD. Intramitochondrial signaling: interactions among mitoKATP, PKCε, ROS, and MPT. Am J Physiol Heart Circ Physiol 295: H874–H882, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cottone S, Mule G, Guarneri M, Palermo A, Lorito MC, Riccobene R, Arsena R, Vaccaro F, Vadala A, Nardi E, Cusimano P, Cerasola G. Endothelin-1 and F2-isoprostane relate to and predict renal dysfunction in hypertensive patients. Nephrol Dial Transplant 24: 497–503, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Cruzado MC, Risler NR, Miatello RM, Yao G, Schiffrin EL, Touyz RM. Vascular smooth muscle cell NAD(P)H oxidase activity during the development of hypertension: effect of angiotensin II and role of insulinlike growth factor-1 receptor transactivation. Am J Hypertens 18: 81–87, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Csiszar A, Labinskyy N, Perez V, Recchia FA, Podlutsky A, Mukhopadhyay P, Losonczy G, Pacher P, Austad SN, Bartke A, Ungvari Z. Endothelial function and vascular oxidative stress in long-lived GH/IGF-deficient Ames dwarf mice. Am J Physiol Heart Circ Physiol 295: H1882–H1894, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Czernichow S, Bertrais S, Blacher J, Galan P, Briancon S, Favier A, Safar M, Hercberg S. Effect of supplementation with antioxidants upon long-term risk of hypertension in the SU.VI.MAX study: association with plasma antioxidant levels. J Hypertens 23: 2013–2018, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Dai DF, Chen T, Szeto H, Nieves-Cintron M, Kutyavin V, Santana LF, Rabinovitch PS. Mitochondrial targeted antioxidant peptide ameliorates hypertensive cardiomyopathy. J Am Coll Cardiol 58: 73–82, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dai DF, Johnson SC, Villarin JJ, Chin MT, Nieves-Cintron M, Chen T, Marcinek DJ, Dorn GW, 2nd, Kang YJ, Prolla TA, Santana LF, Rabinovitch PS. Mitochondrial oxidative stress mediates angiotensin ii-induced cardiac hypertrophy and Gαq overexpression-induced heart failure. Circ Res 108: 837–846, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Bruyn VH, Nuno DW, Cappelli-Bigazzi M, Dole WP, Lamping KG. Effect of acute hypertension in the coronary circulation: role of mechanical factors and oxygen radicals. J Hypertens 12: 163–172, 1994 [PubMed] [Google Scholar]
- 31.De Keulenaer GW, Chappell DC, Ishizaka N, Nerem RM, Alexander RW, Griendling KK. Oscillatory and steady laminar shear stress differentially affect human endothelial redox state: role of a superoxide-producing NADH oxidase. Circ Res 82: 1094–1101, 1998 [DOI] [PubMed] [Google Scholar]
- 32.Di Castro S, Scarpino S, Marchitti S, Bianchi F, Stanzione R, Cotugno M, Sironi L, Gelosa P, Duranti E, Ruco L, Volpe M, Rubattu S. Differential modulation of uncoupling protein 2 in kidneys of stroke-prone spontaneously hypertensive rats under high-salt/low-potassium diet. Hypertension 61: 534–541, 2013 [DOI] [PubMed] [Google Scholar]
- 33.Dikalov S. Cross talk between mitochondria and NADPH oxidases. Free Radic Biol Med 51: 1289–1301, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dikalov SI, Dikalova AE, Bikineyeva AT, Schmidt HH, Harrison DG, Griendling KK. Distinct roles of Nox1 and Nox4 in basal and angiotensin II-stimulated superoxide and hydrogen peroxide production. Free Radic Biol Med 45: 1340–1351, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dikalov SI, Li W, Doughan AK, Blanco RR, Zafari AM. Mitochondrial reactive oxygen species and calcium uptake regulate activation of phagocytic NADPH oxidase. Am J Physiol Regul Integr Comp Physiol 302: R1134–R1142, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dikalov SI, Nazarewicz RR. Angiotensin II-induced production of mitochondrial reactive oxygen species: potential mechanisms and relevance for cardiovascular disease. Antioxid Redox Signal. 2012. May 21 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dikalova AE, Bikineyeva AT, Budzyn K, Nazarewicz RR, McCann L, Lewis W, Harrison DG, Dikalov SI. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ Res 107: 106–116, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dikalova AE, Gongora MC, Harrison DG, Lambeth JD, Dikalov S, Griendling KK. Upregulation of Nox1 in vascular smooth muscle leads to impaired endothelium-dependent relaxation via eNOS uncoupling. Am J Physiol Heart Circ Physiol 299: H673–H679, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Doughan AK, Dikalov SI. Mitochondrial redox cycling of mitoquinone leads to superoxide production and cellular apoptosis. Antioxid Redox Signal 9: 1825–1836, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Doughan AK, Harrison DG, Dikalov SI. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res 102: 488–496, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Duffy SJ, Gokce N, Holbrook M, Hunter LM, Biegelsen ES, Huang A, Keaney JF, Jr, Vita JA. Effect of ascorbic acid treatment on conduit vessel endothelial dysfunction in patients with hypertension. Am J Physiol Heart Circ Physiol 280: H528–H534, 2001 [DOI] [PubMed] [Google Scholar]
- 43.Elango S, Govindaraj P, Vishwanadha VP, Reddy AG, Tamang R, Muthusami U, Kunnoth S, Koyilil VK, Lakshman M, Shanmugasundharam N, Singh L, Thangaraj K. Analysis of mitochondrial genome revealed a rare 50 bp deletion and substitutions in a family with hypertension. Mitochondrion 11: 878–885, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Finocchietto PV, Franco MC, Holod S, Gonzalez AS, Converso DP, Arciuch VG, Serra MP, Poderoso JJ, Carreras MC. Mitochondrial nitric oxide synthase: a masterpiece of metabolic adaptation, cell growth, transformation, and death. Exp Biol Med (Maywood) 234: 1020–1028, 2009 [DOI] [PubMed] [Google Scholar]
- 45.Fridovich I. Mitochondria: are they the seat of senescence? Aging Cell 3: 13–16, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Fukai T, Ushio-Fukai M. Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid Redox Signal 15: 1583–1606, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ghaleh B, Hittinger L, Kim SJ, Kudej RK, Iwase M, Uechi M, Berdeaux A, Bishop SP, Vatner SF. Selective large coronary endothelial dysfunction in conscious dogs with chronic coronary pressure overload. Am J Physiol Heart Circ Physiol 274: H539–H551, 1998 [DOI] [PubMed] [Google Scholar]
- 48.Gomez-Guzman M, Jimenez R, Sanchez M, Zarzuelo MJ, Galindo P, Quintela AM, Lopez-Sepulveda R, Romero M, Tamargo J, Vargas F, Perez-Vizcaino F, Duarte J. Epicatechin lowers blood pressure, restores endothelial function, and decreases oxidative stress and endothelin-1 and NADPH oxidase activity in DOCA-salt hypertension. Free Radic Biol Med 52: 70–79, 2012 [DOI] [PubMed] [Google Scholar]
- 49.Graham D, Huynh NN, Hamilton CA, Beattie E, Smith RA, Cocheme HM, Murphy MP, Dominiczak AF. Mitochondria-targeted antioxidant MitoQ10 improves endothelial function and attenuates cardiac hypertrophy. Hypertension 54: 322–328, 2009 [DOI] [PubMed] [Google Scholar]
- 50.Griendling KK, Harrison DG. Out, damned dot: studies of the NADPH oxidase in atherosclerosis. J Clin Invest 108: 1423–1424, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grote K, Flach I, Luchtefeld M, Akin E, Holland SM, Drexler H, Schieffer B. Mechanical stretch enhances mRNA expression and proenzyme release of matrix metalloproteinase-2 (MMP-2) via NAD(P)H oxidase-derived reactive oxygen species. Circ Res 92: e80–e86, 2003 [DOI] [PubMed] [Google Scholar]
- 52.Guo W, Adachi T, Matsui R, Xu S, Jiang B, Zou MH, Kirber M, Lieberthal W, Cohen RA. Quantitative assessment of tyrosine nitration of manganese superoxide dismutase in angiotensin II-infused rat kidney. Am J Physiol Heart Circ Physiol 285: H1396–H1403, 2003 [DOI] [PubMed] [Google Scholar]
- 53.Guzik TJ, Chen W, Gongora MC, Guzik B, Lob HE, Mangalat D, Hoch N, Dikalov S, Rudzinski P, Kapelak B, Sadowski J, Harrison DG. Calcium-dependent NOX5 NADPH oxidase contributes to vascular oxidative stress in human coronar artery disease. J Am Coll Cardiol 52: 1803–1809, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hamanaka RB, Chandel NS. Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. Trends Biochem Sci 35: 505–513, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Han D, Antunes F, Canali R, Rettori D, Cadenas E. Voltage-dependent anion channels control the release of the superoxide anion from mitochondria to cytosol. J Biol Chem 278: 5557–5563, 2003 [DOI] [PubMed] [Google Scholar]
- 56.Han D, Williams E, Cadenas E. Mitochondrial respiratory chain-dependent generation of superoxide anion and its release into the intermembrane space. Biochem J 353: 411–416, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Han Z, Varadharaj S, Giedt RJ, Zweier JL, Szeto HH, Alevriadou BR. Mitochondria-derived reactive oxygen species mediate heme oxygenase-1 expression in sheared endothelial cells. J Pharmacol Exp Ther 329: 94–101, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harrison D, Griendling KK, Landmesser U, Hornig B, Drexler H. Role of oxidative stress in atherosclerosis. Am J Cardiol 91: 7A–11A, 2003 [DOI] [PubMed] [Google Scholar]
- 59.Harrison DG. The Mosaic Theory revisited: common molecular mechanisms coordinating diverse organ and cellular events in hypertension. J Am Soc Hypertens 7: 68–74, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harrison DG, Gongora MC. Oxidative stress and hypertension. Med Clin North Am 93: 621–635, 2009 [DOI] [PubMed] [Google Scholar]
- 61.Harrison DG, Gongora MC, Guzik TJ, Widder J. Oxidative stress and hypertension. J Am Soc Hypertens 1: 30–44, 2007 [DOI] [PubMed] [Google Scholar]
- 62.Hashimoto J, Ito S. Aortic stiffness determines diastolic blood flow reversal in the descending thoracic aorta: potential implication for retrograde embolic stroke in hypertension. Hypertension 62: 542–549, 2013 [DOI] [PubMed] [Google Scholar]
- 63.Hilenski LL, Clempus RE, Quinn MT, Lambeth JD, Griendling KK. Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 24: 677–683, 2004 [DOI] [PubMed] [Google Scholar]
- 64.Hishikawa K, Luscher TF. Pulsatile stretch stimulates superoxide production in human aortic endothelial cells. Circulation 96: 3610–3616, 1997 [DOI] [PubMed] [Google Scholar]
- 65.Hishikawa K, Oemar BS, Yang Z, Luscher TF. Pulsatile stretch stimulates superoxide production and activates nuclear factor-kappa B in human coronary smooth muscle. Circ Res 81: 797–803, 1997 [DOI] [PubMed] [Google Scholar]
- 66.Hozawa A, Ebihara S, Ohmori K, Kuriyama S, Ugajin T, Koizumi Y, Suzuki Y, Matsui T, Arai H, Tsubono Y, Sasaki H, Tsuji I. Increased plasma 8-isoprostane levels in hypertensive subjects: the Tsurugaya Project. Hypertens Res 27: 557–561, 2004 [DOI] [PubMed] [Google Scholar]
- 67.Huang A, Sun D, Kaley G, Koller A. Superoxide released to high intra-arteriolar pressure reduces nitric oxide-mediated shear stress- and agonist-induced dilations. Circ Res 83: 960–965, 1998 [DOI] [PubMed] [Google Scholar]
- 68.Hwang J, Saha A, Boo YC, Sorescu GP, McNally JS, Holland SM, Dikalov S, Giddens DP, Griendling KK, Harrison DG, Jo H. Oscillatory shear stress stimulates endothelial production of O2 from p47phox-based NAD(P)H oxidase leading to monocyte adhesion. J Biol Chem 278: 47291–47298, 2003 [DOI] [PubMed] [Google Scholar]
- 69.Ichimura H, Parthasarathi K, Quadri S, Issekutz AC, Bhattacharya J. Mechano-oxidative coupling by mitochondria induces proinflammatory responses in lung venular capillaries. J Clin Invest 111: 691–699, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ito H, Torii M, Suzuki T. Decreased superoxide dismutase activity and increased superoxide anion production in cardiac hypertrophy of spontaneously hypertensive rats. Clin Exp Hypertens 17: 803–816, 1995 [DOI] [PubMed] [Google Scholar]
- 71.Jacobson A, Yan C, Gao Q, Rincon-Skinner T, Rivera A, Edwards J, Huang A, Kaley G, Sun D. Aging enhances pressure-induced arterial superoxide formation. Am J Physiol Heart Circ Physiol 293: H1344–H1350, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jang YC, Perez VI, Song W, Lustgarten MS, Salmon AB, Mele J, Qi W, Liu Y, Liang H, Chaudhuri A, Ikeno Y, Epstein CJ, Van Remmen H, Richardson A. Overexpression of Mn superoxide dismutase does not increase life span in mice. J Gerontol A Biol Sci Med Sci 64: 1114–1125, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jones CI, 3rd, Han Z, Presley T, Varadharaj S, Zweier JL, Ilangovan G, Alevriadou BR. Endothelial cell respiration is affected by the oxygen tension during shear exposure: role of mitochondrial peroxynitrite. Am J Physiol Cell Physiol 295: C180–C191, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kaspar JW, Niture SK, Jaiswal AK. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic Biol Med 47: 1304–1309, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kinugawa S, Post H, Kaminski PM, Zhang X, Xu X, Huang H, Recchia FA, Ochoa M, Wolin MS, Kaley G, Hintze TH. Coronary microvascular endothelial stunning after acute pressure overload in the conscious dog is caused by oxidant processes: the role of angiotensin II type 1 receptor and NAD(P)H oxidase. Circulation 108: 2934–2940, 2003 [DOI] [PubMed] [Google Scholar]
- 76.Kumar KV, Das UN. Are free radicals involved in the pathobiology of human essential hypertension? Free Radic Res Commun 19: 59–66, 1993 [DOI] [PubMed] [Google Scholar]
- 77.Kuroda J, Ago T, Matsushima S, Zhai P, Schneider MD, Sadoshima J. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc Natl Acad Sci USA 107: 15565–15570, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lacy F, Kailasam MT, O'Connor DT, Schmid-Schonbein GW, Parmer RJ. Plasma hydrogen peroxide production in human essential hypertension: role of heredity, gender, and ethnicity. Hypertension 36: 878–884, 2000 [DOI] [PubMed] [Google Scholar]
- 79.Lassegue B, Griendling KK. NADPH oxidases: functions and pathologies in the vasculature. Arterioscler Thromb Vasc Biol 30: 653–661, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lassegue B, Sorescu D, Szocs K, Yin Q, Akers M, Zhang Y, Grant SL, Lambeth JD, Griendling KK. Novel gp91(phox) homologues in vascular smooth muscle cells: nox1 mediates angiotensin II-induced superoxide formation and redox-sensitive signaling pathways. Circ Res 88: 888–894, 2001 [DOI] [PubMed] [Google Scholar]
- 81.Laude K, Cai H, Fink B, Hoch N, Weber DS, McCann L, Kojda G, Fukai T, Schmidt HH, Dikalov S, Ramasamy S, Gamez G, Griendling KK, Harrison DG. Hemodynamic and biochemical adaptations to vascular smooth muscle overexpression of p22phox in mice. Am J Physiol Heart Circ Physiol 288: H7–H12, 2005 [DOI] [PubMed] [Google Scholar]
- 82.Lavoie JL, Sigmund CD. Minireview: overview of the renin-angiotensin system—an endocrine and paracrine system. Endocrinology 144: 2179–2183, 2003 [DOI] [PubMed] [Google Scholar]
- 84.Liu Y, Zhao H, Li H, Kalyanaraman B, Nicolosi AC, Gutterman DD. Mitochondrial sources of H2O2 generation play a key role in flow-mediated dilation in human coronary resistance arteries. Circ Res 93: 573–580, 2003 [DOI] [PubMed] [Google Scholar]
- 85.Lombard JH. Uncoupling protein 2 (UCP2): another player in the complex drama of vascular salt sensitivity. Am J Hypertens 23: 816, 2010 [DOI] [PubMed] [Google Scholar]
- 86.Ma S, Ma L, Yang D, Luo Z, Hao X, Liu D, Zhu Z. Uncoupling protein 2 ablation exacerbates high-salt intake-induced vascular dysfunction. Am J Hypertens 23: 822–828, 2010 [DOI] [PubMed] [Google Scholar]
- 87.MacMillan-Crow LA, Cruthirds DL, Ahki KM, Sanders PW, Thompson JA. Mitochondrial tyrosine nitration precedes chronic allograft nephropathy. Free Radic Biol Med 31: 1603–1608, 2001 [DOI] [PubMed] [Google Scholar]
- 88.Matsushima S, Ide T, Yamato M, Matsusaka H, Hattori F, Ikeuchi M, Kubota T, Sunagawa K, Hasegawa Y, Kurihara T, Oikawa S, Kinugawa S, Tsutsui H. Overexpression of mitochondrial peroxiredoxin-3 prevents left ventricular remodeling and failure after myocardial infarction in mice. Circulation 113: 1779–1786, 2006 [DOI] [PubMed] [Google Scholar]
- 89.McNally JS, Davis ME, Giddens DP, Saha A, Hwang J, Dikalov S, Jo H, Harrison DG. Role of xanthine oxidoreductase and the NAD(P)H oxidase in endothelial superoxide production in response to oscillatory shear stress. Am J Physiol Heart Circ Physiol 285: H2290–H2297, 2003 [DOI] [PubMed] [Google Scholar]
- 90.Miura H, Bosnjak JJ, Ning G, Saito T, Miura M, Gutterman DD. Role for hydrogen peroxide in flow-induced dilation of human coronary arterioles. Circ Res 92: e31–e40, 2003 [DOI] [PubMed] [Google Scholar]
- 91.Montez P, Vazquez-Medina JP, Rodriguez R, Thorwald MA, Viscarra JA, Lam L, Peti-Peterdi J, Nakano D, Nishiyama A, Ortiz RM. Angiotensin receptor blockade recovers hepatic UCP2 expression and aconitase and SDH activities and ameliorates hepatic oxidative damage in insulin resistant rats. Endocrinology 153: 5746–5759, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Montezano AC, Touyz RM. Oxidative stress, Noxs, and hypertension: experimental evidence and clinical controversies. Ann Med 44, Suppl 1: S2–S16, 2012 [DOI] [PubMed] [Google Scholar]
- 93.Mukherjee TK, Mishra AK, Mukhopadhyay S, Hoidal JR. High concentration of antioxidants N-acetylcysteine and mitoquinone-Q induces intercellular adhesion molecule 1 and oxidative stress by increasing intracellular glutathione. J Immunol 178: 1835–1844, 2007 [DOI] [PubMed] [Google Scholar]
- 94.Muller FL, Liu Y, Van Remmen H. Complex III releases superoxide to both sides of the inner mitochondrial membrane. J Biol Chem 279: 49064–49073, 2004 [DOI] [PubMed] [Google Scholar]
- 95.Nakazono K, Watanabe N, Matsuno K, Sasaki J, Sato T, Inoue M. Does superoxide underlie the pathogenesis of hypertension? Proc Natl Acad Sci USA 88: 10045–10048, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nautiyal M, Arnold AC, Chappell MC, Diz DI. The brain renin-angiotensin system and mitochondrial function: influence on blood pressure and baroreflex in transgenic rat strains. Int J Hypertens 2013: 136028, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nazarewicz RR, Dikalova AE, Bikineyeva A, Dikalov SI. Nox2 as a potential target of mitochondrial superoxide and its role in endothelial oxidative stress. Am J Physiol Heart Circ Physiol. 10.1152/ajpheart.00063.2013 Epub ahead of print 2013. August 16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nishikawa T, Araki E. Impact of mitochondrial ROS production in the pathogenesis of diabetes mellitus and its complications. Antioxid Redox Signal 9: 343–353, 2007 [DOI] [PubMed] [Google Scholar]
- 99.Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 404: 787–790, 2000 [DOI] [PubMed] [Google Scholar]
- 100.O'Malley Y, Fink BD, Ross NC, Prisinzano TE, Sivitz WI. Reactive oxygen and targeted antioxidant administration in endothelial cell mitochondria. J Biol Chem 281: 39766–39775, 2006 [DOI] [PubMed] [Google Scholar]
- 101.Oeckler RA, Kaminski PM, Wolin MS. Stretch enhances contraction of bovine coronary arteries via an NAD(P)H oxidase-mediated activation of the extracellular signal-regulated kinase mitogen-activated protein kinase cascade. Circ Res 92: 23–31, 2003 [DOI] [PubMed] [Google Scholar]
- 102.Padilla J, Simmons GH, Fadel PJ, Laughlin MH, Joyner MJ, Casey DP. Impact of aging on conduit artery retrograde and oscillatory shear at rest and during exercise: role of nitric oxide. Hypertension 57: 484–489, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Page IH. The mosaic theory of arterial hypertension—its interpretation. Perspect Biol Med 10: 325–333, 1967 [DOI] [PubMed] [Google Scholar]
- 104.Panov A, Dikalov S, Shalbuyeva N, Hemendinger R, Greenamyre JT, Rosenfeld J. Species- and tissue-specific relationships between mitochondrial permeability transition and generation of ROS in brain and liver mitochondria of rats and mice. Am J Physiol Cell Physiol 292: C708–C718, 2007 [DOI] [PubMed] [Google Scholar]
- 105.Panov A, Dikalov S, Shalbuyeva N, Taylor G, Sherer T, Greenamyre JT. Rotenone model of Parkinson disease: multiple brain mitochondria dysfunctions after short term systemic rotenone intoxication. J Biol Chem 280: 42026–42035, 2005 [DOI] [PubMed] [Google Scholar]
- 106.Panov A, Schonfeld P, Dikalov S, Hemendinger R, Bonkovsky HL, Brooks BR. The neuromediator glutamate, through specific substrate interactions, enhances mitochondrial ATP production and reactive oxygen species generation in nonsynaptic brain mitochondria. J Biol Chem 284: 14448–14456, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Panov AV, Vavilin VA, Lyakhovich VV, Brooks BR, Bonkovsky HL. Effect of bovine serum albumin on mitochondrial respiration in the brain and liver of mice and rats. Bull Exp Biol Med 149: 187–190, 2010 [DOI] [PubMed] [Google Scholar]
- 108.Park JB, Touyz RM, Chen X, Schiffrin EL. Chronic treatment with a superoxide dismutase mimetic prevents vascular remodeling and progression of hypertension in salt-loaded stroke-prone spontaneously hypertensive rats. Am J Hypertens 15: 78–84, 2002 [DOI] [PubMed] [Google Scholar]
- 109.Poderoso JJ, Carreras MC, Lisdero C, Riobo N, Schopfer F, Boveris A. Nitric oxide inhibits electron transfer and increases superoxide radical production in rat heart mitochondria and submitochondrial particles. Arch Biochem Biophys 328: 85–92, 1996 [DOI] [PubMed] [Google Scholar]
- 110.Powell CS, Jackson RM. Mitochondrial complex I, aconitase, and succinate dehydrogenase during hypoxia-reoxygenation: modulation of enzyme activities by MnSOD. Am J Physiol Lung Cell Mol Physiol 285: L189–L198, 2003 [DOI] [PubMed] [Google Scholar]
- 111.Queliconi BB, Wojtovich AP, Nadtochiy SM, Kowaltowski AJ, Brookes PS. Redox regulation of the mitochondrial K(ATP) channel in cardioprotection. Biochim Biophys Acta 1813: 1309–1315, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Quijano C, Hernandez-Saavedra D, Castro L, McCord JM, Freeman BA, Radi R. Reaction of peroxynitrite with Mn-superoxide dismutase. Role of the metal center in decomposition kinetics and nitration. J Biol Chem 276: 11631–11638, 2001 [DOI] [PubMed] [Google Scholar]
- 113.Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest 97: 1916–1923, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rea IM, McNerlan SE, Archbold GP, Middleton D, Curran MD, Young IS, Ross OA. Mitochondrial J haplogroup is associated with lower blood pressure and anti-oxidant status: findings in octo/nonagenarians from the BELFAST Study. Age (Dordr) 35: 1445–1456, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Reddy VN, Kasahara E, Hiraoka M, Lin LR, Ho YS. Effects of variation in superoxide dismutases (SOD) on oxidative stress and apoptosis in lens epithelium. Exp Eye Res 79: 859–868, 2004 [DOI] [PubMed] [Google Scholar]
- 116.Rodriguez-Iturbe B, Sepassi L, Quiroz Y, Ni Z, Wallace DC, Vaziri ND. Association of mitochondrial SOD deficiency with salt-sensitive hypertension and accelerated renal senescence. J Appl Physiol 102: 255–260, 2007 [DOI] [PubMed] [Google Scholar]
- 117.Russo C, Olivieri O, Girelli D, Faccini G, Zenari ML, Lombardi S, Corrocher R. Anti-oxidant status and lipid peroxidation in patients with essential hypertension. J Hypertens 16: 1267–1271, 1998 [DOI] [PubMed] [Google Scholar]
- 118.San Martin A, Du P, Dikalova A, Lassegue B, Aleman M, Gongora MC, Brown K, Joseph G, Harrison DG, Taylor WR, Jo H, Griendling KK. Reactive oxygen species-selective regulation of aortic inflammatory gene expression in Type 2 diabetes. Am J Physiol Heart Circ Physiol 292: H2073–H2082, 2007 [DOI] [PubMed] [Google Scholar]
- 119.Schulze-Osthoff K, Los M, Baeuerle PA. Redox signalling by transcription factors NF-kappa B and AP-1 in lymphocytes. Biochem Pharmacol 50: 735–741, 1995 [DOI] [PubMed] [Google Scholar]
- 120.Shao J, Chen L, Marrs B, Lee L, Huang H, Manton KG, Martin GM, Oshima J. SOD2 polymorphisms: unmasking the effect of polymorphism on splicing. BMC Med Genet 8: 7, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shatrov VA, Brune B. Induced expression of manganese superoxide dismutase by non-toxic concentrations of oxidized low-density lipoprotein (oxLDL) protects against oxLDL-mediated cytotoxicity. Biochem J 374: 505–511, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sorescu GP, Song H, Tressel SL, Hwang J, Dikalov S, Smith DA, Boyd NL, Platt MO, Lassegue B, Griendling KK, Jo H. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress induces monocyte adhesion by stimulating reactive oxygen species production from a nox1-based NADPH oxidase. Circ Res 95: 773–779, 2004 [DOI] [PubMed] [Google Scholar]
- 123.St-Pierre J, Buckingham JA, Roebuck SJ, Brand MD. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J Biol Chem 277: 44784–44790, 2002 [DOI] [PubMed] [Google Scholar]
- 124.Starkov AA, Fiskum G, Chinopoulos C, Lorenzo BJ, Browne SE, Patel MS, Beal MF. Mitochondrial alpha-ketoglutarate dehydrogenase complex generates reactive oxygen species. J Neurosci 24: 7779–7788, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Szeto HH. Mitochondria-targeted cytoprotective peptides for ischemia-reperfusion injury. Antioxid Redox Signal 10: 601–619, 2008 [DOI] [PubMed] [Google Scholar]
- 126.Tahara EB, Navarete FD, Kowaltowski AJ. Tissue-, substrate-, and site-specific characteristics of mitochondrial reactive oxygen species generation. Free Radic Biol Med 46: 1283–1297, 2009 [DOI] [PubMed] [Google Scholar]
- 127.Takabe W, Jen N, Ai L, Hamilton R, Wang S, Holmes K, Dharbandi F, Khalsa B, Bressler S, Barr ML, Li R, Hsiai TK. Oscillatory shear stress induces mitochondrial superoxide production: implication of NADPH oxidase and c-Jun NH2-terminal kinase signaling. Antioxid Redox Signal 15: 1379–1388, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Teng L, Zheng J, Leng J, Ding Y. Clinical and molecular characterization of a Han Chinese family with high penetrance of essential hypertension. Mitochondrial DNA 23: 461–465, 2012 [DOI] [PubMed] [Google Scholar]
- 129.Ungvari Z, Bagi Z, Feher A, Recchia FA, Sonntag WE, Pearson K, de Cabo R, Csiszar A. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am J Physiol Heart Circ Physiol 299: H18–H24, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ungvari Z, Bailey-Downs L, Sosnowska D, Gautam T, Koncz P, Losonczy G, Ballabh P, de Cabo R, Sonntag WE, Csiszar A. Vascular oxidative stress in aging: a homeostatic failure due to dysregulation of Nrf2-mediated antioxidant response. Am J Physiol Heart Circ Physiol 301: H363–H372, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ungvari Z, Csiszar A, Huang A, Kaminski PM, Wolin MS, Koller A. High pressure induces superoxide production in isolated arteries via protein kinase C-dependent activation of NAD(P)H oxidase. Circulation 108: 1253–1258, 2003 [DOI] [PubMed] [Google Scholar]
- 132.Ungvari Z, Csiszar A, Kaminski PM, Wolin MS, Koller A. Chronic high pressure-induced arterial oxidative stress: Involvement of protein kinase C-dependent NAD(P)H oxidase and local renin-angiotensin system. Am J Pathol 165: 219–226, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ungvari ZI, Bailey-Downs L, Gautam T, Jimenez R, Losonczy G, Zhang C, Ballabh P, Recchia FA, Wilkerson DC, Sonntag WE, Pearson KJ, de Cabo R, Csiszar A. Adaptive induction of NF-E2-related factor-2-driven antioxidant genes in endothelial cells in response to hyperglycemia. Am J Physiol Heart Circ Physiol 300: H1133–H1140, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ungvari ZI, Orosz Z, Labinskyy N, Rivera A, Xiangmin Z, Smith KE, Csiszar A. Increased mitochondrial H2O2 production promotes endothelial NF-kB activation in aged rat arteries. Am J Physiol Heart Circ Physiol 293: H37–H47, 2007 [DOI] [PubMed] [Google Scholar]
- 135.Ushio-Fukai M, Griendling KK, Becker PL, Hilenski L, Halleran S, Alexander RW. Epidermal growth factor receptor transactivation by angiotensin II requires reactive oxygen species in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 21: 489–495, 2001 [DOI] [PubMed] [Google Scholar]
- 136.Viel EC, Benkirane K, Javeshghani D, Touyz RM, Schiffrin EL. Xanthine oxidase and mitochondria contribute to vascular superoxide anion generation in DOCA-salt hypertensive rats. Am J Physiol Heart Circ Physiol 295: H281–H288, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wei EP, Kontos HA, Christman CW, DeWitt DS, Povlishock JT. Superoxide generation and reversal of acetylcholine-induced cerebral arteriolar dilation after acute hypertension. Circ Res 57: 781–787, 1985 [DOI] [PubMed] [Google Scholar]
- 138.Weseler AR, Bast A. Oxidative stress and vascular function: implications for pharmacologic treatments. Curr Hypertens Rep 12: 154–161, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Widder JD, Fraccarollo D, Galuppo P, Hansen JM, Jones DP, Ertl G, Bauersachs J. Attenuation of angiotensin II-induced vascular dysfunction and hypertension by overexpression of Thioredoxin 2. Hypertension 54: 338–344, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wilcox CS. Effects of tempol and redox-cycling nitroxides in models of oxidative stress. Pharmacol Ther 126: 119–145, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wolin MS, Burke TM. Hydrogen peroxide elicits activation of bovine pulmonary arterial soluble guanylate cyclase by a mechanism associated with its metabolism by catalase. Biochem Biophys Res Commun 143: 20–25, 1987 [DOI] [PubMed] [Google Scholar]
- 142.Wu JL, Wu QP, Yang XF, Wei MK, Zhang JM, Huang Q, Zhou XY. l-Malate reverses oxidative stress and antioxidative defenses in liver and heart of aged rats. Physiol Res 57: 261–268, 2008 [DOI] [PubMed] [Google Scholar]
- 143.Yakes FM, Van Houten B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc Natl Acad Sci USA 94: 514–519, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yin JX, Yang RF, Li S, Renshaw AO, Li YL, Schultz HD, Zimmerman MC. Mitochondria-produced superoxide mediates angiotensin II-induced inhibition of neuronal potassium current. Am J Physiol Cell Physiol 298: C857–C865, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zalba G, Beaumont FJ, San Jose G, Fortuno A, Fortuno MA, Etayo JC, Diez J. Vascular NADH/NADPH oxidase is involved in enhanced superoxide production in spontaneously hypertensive rats. Hypertension 35: 1055–1061, 2000 [DOI] [PubMed] [Google Scholar]
- 146.Zhang A, Jia Z, Wang N, Tidwell TJ, Yang T. Relative contributions of mitochondria and NADPH oxidase to deoxycorticosterone acetate-salt hypertension in mice. Kidney Int 80: 51–60, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhang DX, Chen YF, Campbell WB, Zou AP, Gross GJ, Li PL. Characteristics and superoxide-induced activation of reconstituted myocardial mitochondrial ATP-sensitive potassium channels. Circ Res 89: 1177–1183, 2001 [DOI] [PubMed] [Google Scholar]
- 148.Zhao K, Zhao GM, Wu D, Soong Y, Birk AV, Schiller PW, Szeto HH. Cell-permeable peptide antioxidants targeted to inner mitochondrial membrane inhibit mitochondrial swelling, oxidative cell death, and reperfusion injury. J Biol Chem 279: 34682–34690, 2004 [DOI] [PubMed] [Google Scholar]
- 149.Zimmerman MC, Lazartigues E, Lang JA, Sinnayah P, Ahmad IM, Spitz DR, Davisson RL. Superoxide mediates the actions of angiotensin II in the central nervous system. Circ Res 91: 1038–1045, 2002 [DOI] [PubMed] [Google Scholar]
- 150.Zinkevich NS, Gutterman DD. ROS-induced ROS release in vascular biology: redox-redox signaling. Am J Physiol Heart Circ Physiol 301: H647–H653, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]