Abstract
Cyclooxygenase (COX)-derived prostanoids contribute to angiotensin II (ANG II) hypertension (HTN). However, the specific mechanisms by which prostanoids act are unclear. ANG II-induced HTN is caused partly by increased sympathetic nervous system activity, especially in a setting of high dietary salt intake. This study tested the hypothesis that COX-derived prostanoids cause ANG II-salt sympathoexcitation and HTN. Experiments were conducted in conscious rats. Infusion of ANG II (150 ng·kg−1·min−1 sc) caused a marked HTN in rats on 2% salt diet, but a much smaller increase in blood pressure in rats on 0.4% salt diet. The nonselective COX inhibitor ketoprofen (2 mg/kg sc) given throughout the ANG-II infusion period attenuated HTN development in rats on 2% NaCl diet, but not in rats on 0.4% NaCl diet. The acute depressor response to ganglion blockade was used to assess neurogenic pressor activity in rats on 2% NaCl diet. Ketoprofen-treated rats showed a smaller fall in arterial pressure in response to ganglion blockade during ANG-II infusion than did nontreated controls. In additional experiments, ketoprofen-treated rats exhibited smaller increases in plasma norepinephrine levels and whole body norepinephrine spillover than we previously reported in ANG II-salt HTN. Finally, the effects of the selective COX-1 inhibitor SC560 (10 mg·kg−1·day−1 ip) and the selective COX-2 inhibitor nimesulide (10 mg·kg−1·day−1 ip) were investigated. Treatment with SC560 but not nimesulide significantly reduced blood pressure and the depressor response to ganglion blockade in ANG II-salt HTN rats. The results suggest that COX-1 products are critical for sympathoexcitation and the full development of ANG II-salt HTN in rats.
Keywords: cyclooxygenase, angiotensin II, salt, sympathetic, hypertension
angiotensin ii is involved in the etiology of systemic hypertension (HTN) through a variety of mechanisms. These include direct contraction of blood vessels and subsequent remodeling of their structure (12), reductions in renal sodium excretion (27), release of aldosterone (41), and activation of the sympathetic nervous system (SNS) (4, 9). In 1970, McGiff et al. (24) proposed the idea that the renin-angiotensin system influences eicosanoid formation and reported the release of prostaglandin (PG)-like substances during infusion of angiotensin II (ANG II). Eicosanoids are a class of lipids that act as local hormones and are synthesized “on demand.” The enzyme phospholipase A2 acts on cell membrane phospholipids to produce arachidonic acid. Arachidonic acid is then converted into the PGs, thromboxanes and leukotrienes (LT)—collectively called the eicosanoids—by the enzymes cyclooxygenase, lipoxygenase, and various other synthases (35). The cyclooxygenase-derived prostanoids (e.g., PGE2, PGF2α, PGD2, PGI2, thromboxane A2) are generated by either the largely constitutive isoform COX-1 or by the inducible isoform COX-2 (34, 36, 37). In either case, prostanoids cause both prohypertensive and antihypertensive effects by acting on the kidneys, blood vessels, endocrine organs, and brain.
But are prostanoids involved in ANG-II HTN? This question has been addressed in numerous previous studies through the use of COX-inhibiting drugs (3, 8, 38) and COX knockout mice (3, 31, 43). However, these efforts produced contradictory conclusions, possibly because the studies employed quite varied treatment regimens, treatment durations, and methods of measuring blood pressure. Furthermore, the relative importance of COX-1 versus COX-2 in ANG-II HTN is disputed (3, 31, 38).
Sympathoexcitation is an important cause of HTN during ANG-II infusion, particularly in the setting of high dietary salt intake (18, 33). For example, we previously found significant elevations in plasma norepinephrine (NE) levels and whole body NE spillover (indexes of sympathetic activity) during infusion of ANG II in rats on a high-salt intake (2.0% NaCl diet; ANG II-salt HTN), but not in rats on a normal salt intake (0.4% NaCl diet) (18). The mechanisms by which ANG II causes sympathoexcitation remain to be fully elucidated. Both older (15, 22) and recent (3) experiments indicate that the SNS is activated by prostanoids in experimental ANG-II HTN and/or that prostanoids are involved in mediating neurogenic HTN. Therefore, in this study we used radiotelemetric methods and standardized ANG-II HTN and ANG II-salt HTN protocols to identify the importance of prostanoids in these two models of HTN. A special emphasis was on determining if prostanoids affect sympathetic control of blood pressure. We also then used pharmacological tools to determine the relative contribution of COX-1 and COX-2 to sympathetic control of blood pressure in ANG II-salt HTN.
METHODS
Animals
Male Sprague-Dawley rats weighing 225 to 250 g were obtained from Charles River Laboratories (Wilmington, MA) and allowed to acclimate for a week before experimentation. They were maintained in light- and temperature-controlled animal rooms and allowed free access to a 0.4% or 2% NaCl diet (Research Diets, New Brunswick, NJ) and distilled water starting 7 days before telemeter or catheter implantation. Animals were maintained on normal (0.4%) or high-salt (2% NaCl) diet until the end of the experiment. All experiments were done in compliance with the National Institutes of Health's laboratory animal care and use guidelines and with Institutional Animal Care and Use Committee approval at Michigan State University.
Surgery
Telemeter implantation and catheterization.
Telemeter implantation and postoperative procedures were similar to our previous studies (19). Briefly, the tip of a radiotelemeter (Data Science International, St. Paul, MN) catheter was introduced into the abdominal aorta just cranial to the aortic bifurcation through the left femoral artery under general anesthesia. The body of the transmitter was placed in a subcutaneous pocket along the caudoventral abdomen. Antimicrobial prophylaxis and postoperative analgesia were as previously described (19). The freely moving rats were then individually housed in cages placed over a radiotelemetry receiver.
Catheterization and postoperative procedures were performed as previously described (21). Briefly, rats were chronically instrumented with femoral arterial and venous catheters under isoflurane anesthesia for the measurement of arterial pressure and for blood sampling, respectively. Antimicrobial prophylaxis and postoperative analgesia were as previously described (21). The rats were then loosely tethered in their home cages to allow undisturbed blood sampling during the experiment.
Hemodynamic measurements.
Arterial pressure measurements in telemetered rats were obtained for 10 s every 10 min for the entire experimental period as previously described (19). In radiotelemeter-implanted rats, continuous 24-h measurements of mean arterial pressure (MAP) and heart rate (HR) were obtained using a commercially available radiotelemetry data acquisition program (Dataquest ART 4.1, Data Sciences International). In catheterized rats, MAP and HR data were obtained by connecting the exteriorized arterial catheter to a pressure transducer, which was attached to a digital pressure monitor (Digit-Med BPA-400; Micro-Med, Louisville, KY), which was then linked to a computerized data acquisition program (DMSI-400; Micro-Med). Blood pressure was recorded for 15–30 min daily in the morning.
NE spillover.
The radioisotope dilution technique was used to determine NE clearance and spillover (17). Briefly, 3H-NE was infused intravenously at a constant rate for 90 min, at the end of which 1 ml of blood was drawn from the arterial catheter. The blood was centrifuged and plasma was used to determine the levels of catecholamines using reverse-phase high-performance liquid chromatography separation with coulometric detection. After high-performance liquid chromatography, 3H-NE was quantified using a liquid scintillation analyzer. Total NE clearance and spillover were calculated using established methods (6, 17).
Protein isolation and Western blot analysis of COX-1 and COX-2 in hypertensive rat tissues.
Aorta, cerebral blood vessels, mesenteric artery, and brains were collected from rats (fed a 2% salt diet) infused with ANG II (150 ng·kg−1·min−1 for 14 days) or saline (vehicle) after anesthesia with pentobarbital sodium (50 mg/kg ip) and were snap frozen on dry ice. Cryostat sections of brain (500 μm) were made and paraventricular nucleus, rostral ventrolateral medulla, and subfornical organ were punched using Palkovits microdissection technique. Lysis buffer, containing 0.5 mmol/l Tris·HCl (pH 6.8), 10% SDS, and 10% glycerol, with protease inhibitors, consisting of 0.5 mmol/l PMSF, 10 μg/μl aprotinin, and 10 μg/μl leupeptin, was added to the tissue before homogenization. Protein concentrations were measured using bicinchoninic acid protein assay (Sigma, St. Louis, Mo). Tissue samples were run on SDS-PAGE 10% gel with positive and negative controls and transferred to immobilon-P membrane. The membrane was later blocked for 3 h with blocking buffer, consisting of Tris-buffered saline (TBS) with Tween 20, 4% chick egg ovalbumin, and 2.5% sodium azide, and incubated overnight (4°C) with rabbit polyclonal primary antibody anti-COX-1 (70 kDa; lot no. DAM 1412889, Cayman Chemicals, Ann Arbor, MI) or rabbit polyclonal anti-COX-2 (72 kDa; lot no. 0409461-1, Cayman Chemicals) with mouse monoclonal anti-tubulin (55 kDa; lot no. LV1581100, Millipore; Temecula, CA). After being rinsed in TBS-Tween 20 three times and final rinse in TBS, the blots were incubated using secondary antibody for 1 h at 4°C. Blots were visualized after incubating them with enhanced chemiluminescence reagent.
Experimental Protocols
Nonselective COX inhibition in chronic ANG II-salt hypertensive rats.
Rats were fed a normal (0.4% NaCl) or high-salt (2% NaCl) diet and had radiotelemeters installed. They were allowed a 5- to 7-day recovery period. After 3 days of baseline blood pressure recordings, saline (vehicle) or the nonselective COX inhibitor ketoprofen (2 mg/kg) was injected subcutaneously once daily for 18 days (from day 4–21). After 4 days of COX inhibition or vehicle injection, ANG II (Sigma) or saline was infused at a constant rate of 150 ng·kg−1·min−1 sc for 14 days (from days 7–21) using a miniosmotic pump (2ML2, Alzet, Cupertino, CA). The dose of ketoprofen was chosen based on previous reports of using the drug in rats (1, 29). Arterial pressure and HR were measured daily for the duration of the experiment.
Nonselective COX inhibition and ANG II-salt mediated SNS activation.
To investigate the effect of cyclooxygenase inhibition on SNS activation in ANG II-salt HTN, an additional group of animals was catheterized to allow measurements of whole body NE spillover. Rats acclimatized to 2% NaCl diet for 7 days were instrumented with an arterial and venous catheter. After 5–7 days of recovery and 3 days of recording baseline blood pressure, ANG II (150 ng·kg−1·min−1 sc) was delivered by miniosmotic pump for 14 days. Ketoprofen (2 mg/kg sc) or vehicle (saline) was administered daily for the entire duration of the experiment. Endogenous plasma NE levels and total body NE spillover and clearance were determined before ANG-II infusion (day 2) and on days 7 and 14 of ANG-II infusion. Ganglionic blockade was achieved with hexamethonium (30 mg/kg ip; Sigma) on day 14 of the experiment (39). The fall in MAP 15 min later was recorded, and the magnitude was used as an estimate of neurogenic pressor activity. The 15-min time point was chosen based on our experience that after intraperitoneal injection of hexamethonium, the peak fall in MAP generally occurs around 15 min later. In addition, using this time point should minimize the impact on our measurement of the short-lived direct vasodilator effects of hexamethonium and the slower hormonal compensatory responses to the initial fall in MAP.
Selective COX-1 or COX-2 inhibition in chronic ANG II-salt hypertensive rats.
Rats implanted with radiotelemeters and fed a high-salt diet were used in this experiment. After a 5–7-day recovery period and 3 days of baseline blood pressure recordings, DMSO (vehicle) or a selective COX-1 inhibitor SC560 (10 mg/kg ip) or a selective COX-2 inhibitor nimesulide (10 mg/kg ip) was injected once daily for the remainder of the study. The doses for COX-1 and COX-2 inhibitor were chosen based on previous reports (13, 40) of the use of this particular dose of 10 mg/kg ip in mimicking the effects of the widely used COX inhibitor aspirin as well as the successful reduction of prostanoid levels in various tissues. After 4 days of COX inhibition or DMSO injection, ANG II or physiological saline infusion was initiated using a miniosmotic pump (2ML2, Alzet). ANG II was infused at the rate of 150 ng·kg−1·min−1 sc for 14 days. MAP and HR were measured for the entire duration of the experiment. Animals were subjected to ganglionic blockade with hexamethonium 10 days after starting ANG-II administration to assess neurogenic pressor activity as described above.
Statistical Analysis
Changes in MAP and other variables were assessed by one-way repeated-measures ANOVA, followed by post hoc multiple comparisons using Dunnett's procedure (GraphPad Instat 3, La Jolla, CA). Between-group differences were assessed by a two-way mixed-design ANOVA, and post hoc testing at each time point was performed using Bonferroni's procedure to correct for multiple comparisons (GraphPad Prism 4). A P value of <0.05 was considered statistically significant. All results are presented as means ± SE.
RESULTS
Effect of Nonselective COX Inhibition on Chronic ANG-II HTN in Rats on Normal and High-Salt Diets
The effect of nonselective cyclooxygenase inhibition on chronic ANG-II HTN is displayed in Fig. 1. In rats fed 0.4% NaCl (Fig. 1A), ketoprofen treatment had no effect on MAP before or during the ANG-II infusion period. The MAP on days 14, 17, and 21 of the protocol (7, 10 and 14 days post-ANG-II infusion) was compared with control period (day 3) using repeated-measures ANOVA and Dunnett's posttest. The MAP was significantly higher on days 10 and 14 of ANG-II treatment in vehicle-treated 0.4% NaCl-fed (119 ± 6 and 120 ± 12 mmHg, respectively) rats compared with the control baseline period (101 ± 2 mmHg). Similarly, the MAP was significantly higher only on day 14 of ANG-II treatment in ketoprofen-treated 0.4% NaCl-fed (118 ± 6 mmHg) rats compared with the control period (104 ± 1 mmHg). By day 14 of ANG-II infusion in rats fed 0.4% NaCl, MAP increased to a similar extent in control (17 ± 5 mmHg) and ketoprofen-treated (14 ± 5 mmHg) rats. In rats fed a 2% salt diet, MAPs in vehicle and ketoprofen groups were not different during the ANG-II preinfusion period, and a comparable increase in MAP was observed in control (22 ± 5 mmHg) and ketoprofen-treated (20 ± 5 mmHg) rats during the first few days of ANG-II infusion. However, as seen in Fig. 1B, by day 14 of ANG-II infusion, MAP had increased significantly greater in control rats (36 ± 12 mmHg) compared with ketoprofen-treated rats (2 ± 1 mmHg).
Fig. 1.
The effect of chronic cyclooxygenase inhibition on mean arterial pressure (MAP) response to ANG II. Rats were fed 0.4% (A) or 2% (B) NaCl from the beginning of the study. *P < 0.05 on days 10 and 14 of ANG-II treatment in vehicle-treated group (n = 5) compared with baseline control period (day 3). $P < 0.05 on day 14 of ANG-II treatment in ketoprofen-treated group (n = 5) compared with baseline control period (day 3). #P < 0.05 compared with vehicle-treated group (n = 5, each group).
Effect of Nonselective COX Inhibition on Chronic ANG II-Salt HTN Measured by Exteriorized Catheter
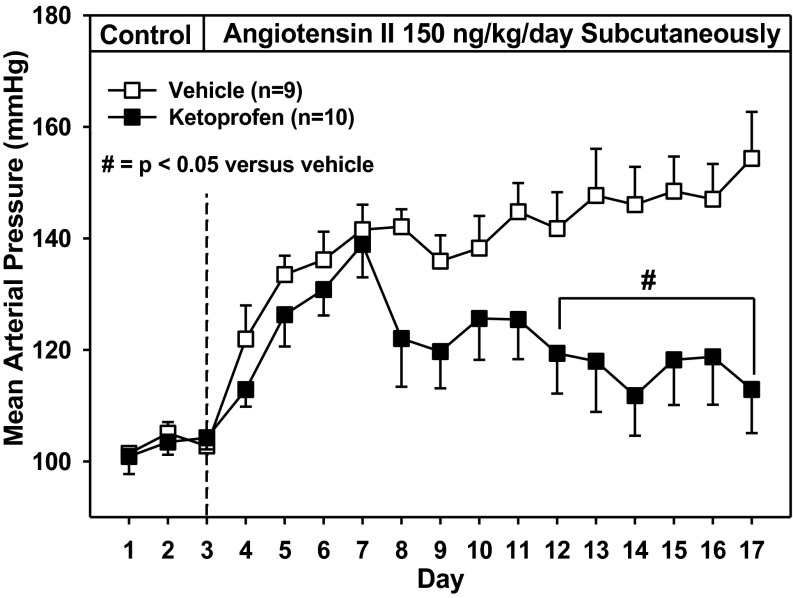
Figure 2 depicts changes in MAP after ANG-II infusion in vehicle- and ketoprofen-treated rats (ingesting 2.0% NaCl diet) that had chronically implanted arterial catheters. There were no differences in MAP between vehicle- and ketoprofen-treated rats before ANG-II infusion. In the vehicle-treated group, ANG-II infusion increased MAP from 103 ± 2 mmHg during the control period to 142 ± 4 mmHg on day 4 of ANG-II infusion, and thereafter MAP gradually increased to 154 ± 8 mmHg by day 14. Although in ketoprofen-treated rats a similar increase in MAP was observed during the first 4 days of ANG-II infusion, a reduction in MAP was seen from day 5 (122 ± 9 mmHg) through day 14 (113 ± 8 mmHg) of infusion. MAP was significantly lower in the ketoprofen-treated group (compared with the vehicle-treated group) from days 9–14 of ANG-II infusion.
Fig. 2.
The effect of chronic cyclooxygenase inhibition on MAP response to ANG II in catheterized rats fed a high-salt diet (2% NaCl). Rats were treated daily with the nonselective cyclooxygenase inhibitor ketoprofen (n = 10) or vehicle (n = 9). #P < 0.05 compared with vehicle-treated group.
Effect of Ganglionic Blockade on Pressor Response after Nonselective COX Inhibition in ANG II-Salt HTN
Peak falls in MAP in response to ganglion blockade with hexamethonium in ANG II-salt HTN rats are shown in Fig. 3. Ganglionic blockade with hexamethonium was performed on day 10 of ANG-II infusion. Hexamethonium treatment produced a marked drop in MAP in ANG II salt-treated rats (−91 ± 14 mmHg) that was substantially greater than the fall observed in ketoprofen-treated ANG II salt-treated rats (−63 ± 8 mmHg), but the difference was not significantly different (P = 0.11).
Fig. 3.
Depressor response to ganglionic blockade in control and ketoprofen-treated ANG II-salt hypertensive rats. Peak fall in MAP in response to hexamethonium administration (30 mg/kg iv) on day 10 of ANG-II infusion in rats fed 2% NaCl treated daily with the nonselective cyclooxygenase inhibitor ketoprofen (n = 10) or vehicle (n = 9).
Effect of Nonselective COX Inhibition on NE Clearance, Plasma NE, and Whole Body NE Spillover in ANG II-Salt HTN
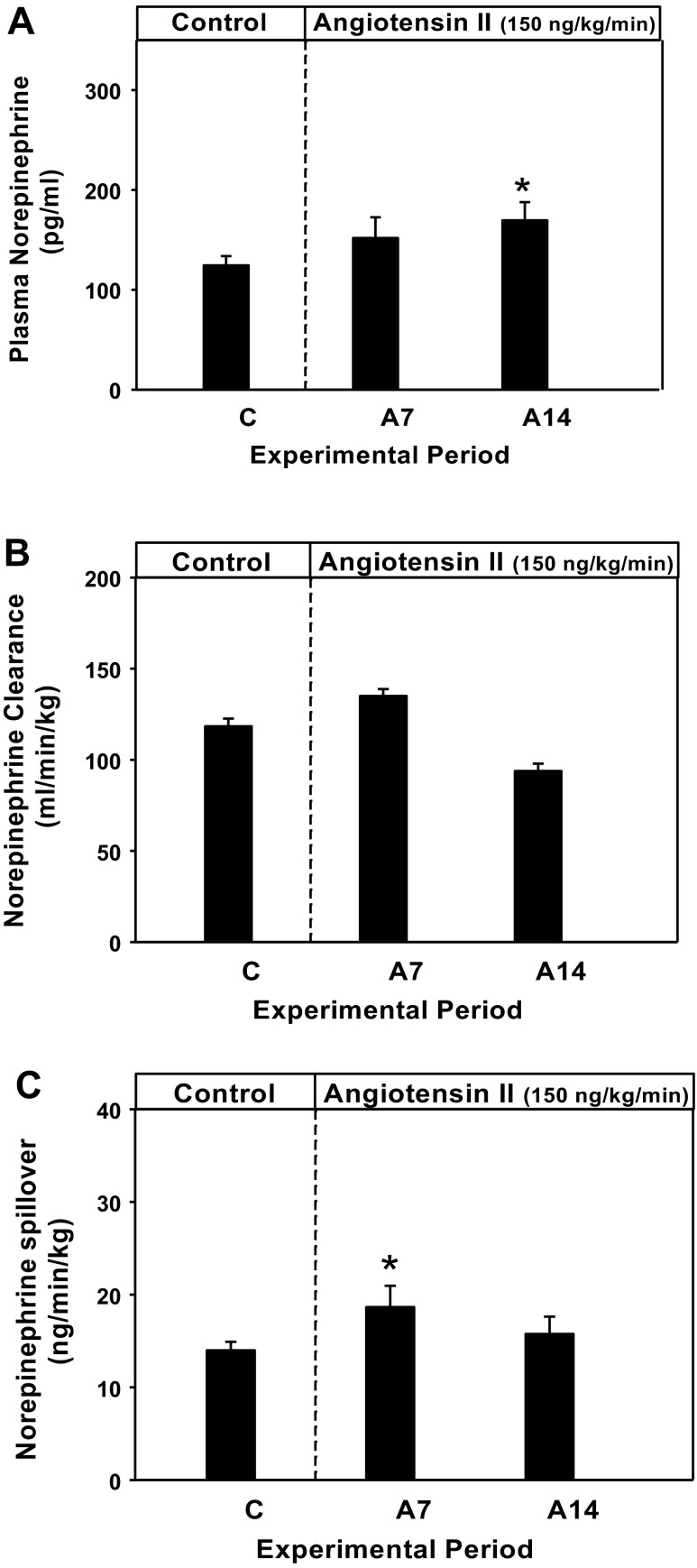
Figure 4, A–C, shows the effect of ketoprofen treatment on plasma NE, NE clearance, and NE spillover, respectively, on day 2 of the pretreatment control period and days 7 and 14 of 2% NaCl-fed and ANG II-infused rats. Plasma NE level during the pretreatment control period was 124 ± 9 pg/ml. On day 7 of ANG-II infusion, it was 152 ± 21 pg/ml and on day 14 was 169 ± 18 pg/ml. The day 14 plasma NE levels were significantly higher than the pretreatment control period (P < 0.05 vs. control). The NE clearance was 118 ± 4 on pretreatment day 2 and 135 ± 4 and 94 ± 4 ml·min−1·kg−1 on days 7 and 14 of ANG-II infusion, respectively. Calculated NE spillover was 14 ± 1 on pretreatment control day 2 and 16 ± 2 ng·min−1·kg−1 on days 7 and 14 of ANG-II infusion, respectively. The day 7, but not the day 14, the value was significantly higher (P < 0.05) than during the pretreatment control period.
Fig. 4.
The effect of chronic cyclooxygenase inhibition on plasma norepinephrine (NE; A), NE clearance (B), and NE spillover (C) in rats (n = 10) infused with ANG II and fed a high-salt diet (2% NaCl) compared with vehicle-treated rats (n = 9). *P < 0.05 vs. control period (C).
COX-1 and COX-2 Expression in ANG II-Salt Hypertensive Rats
Western blot analyses were performed to determine if COX protein expression was increased in tissues from 2% NaCl fed rats on day 14 of ANG-II administration. The data are shown in Fig. 5. Both COX-1 and COX-2 expression in brain subfornical organ (Fig. 5A), paraventricular nucleus (Fig. 5B), rostral ventrolateral medulla (Fig. 5C), and blood vessels viz., aorta (Fig. 5D), mesenteric artery (Fig. 5E), and cerebral artery (Fig. 5F) of hypertensive rats (n = 6) were not significantly different from that found in normotensive control rats (n = 6) (high-salt diet alone).
Fig. 5.
Western blot analysis of cyclooxygenase (COX)-1 (70 kDa) and COX-2 (72 kDa) protein expression in 2% NaCl-fed rats treated with ANG II (n = 6) or saline as vehicle (n = 6) for 14 days. COX-1 and COX-2 protein expression in 14 day ANG II-treated rats in subfornical organ (SFO; A), paraventricular nucleus (PVN; B), rostral ventrolateral medulla (RVLM; C), aorta (D), mesenteric artery (E) and cerebral artery (F).
Effect of Selective COX-1 and COX-2 Inhibition on Chronic ANG II-Salt HTN
COX-1 inhibition with SC560.
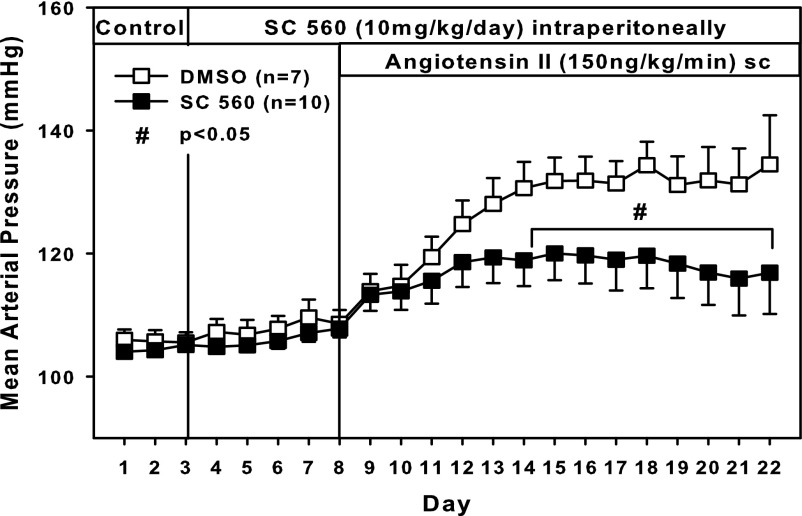
In vehicle (DMSO)-treated rats fed a high-salt diet, MAP was 105 ± 2 mmHg on pretreatment day 3 and gradually increased to 135 ± 8 mmHg on day 14 of ANG-II infusion. In the SC-560-treated group, MAP was not different from that of the vehicle-treated group during the pretreatment period or during treatment with SC560 alone. During ANG-II administration, however, MAP rose to only 117 ± 1 mmHg, and was significantly lower than MAP in the vehicle group on days 6–14 of ANG-II infusion (Fig. 6).
Fig. 6.
Effect of selective COX-1 inhibition on MAP. Response to ANG II in rats fed a high-salt diet (2% NaCl). Rats were treated daily with the selective COX-1 inhibitor SC560 or vehicle. #P < 0.05 SC560 (n = 10) vs. DMSO (n = 7).
COX-2 inhibition with nimesulide.
Treatment with nimesulide alone did not affect MAP relative to the vehicle (DMSO)-treated control group. Furthermore, ANG-II infusion caused the same magnitude of HTN in vehicle- and nimesulide-treated rats (Fig. 7).
Fig. 7.
Effect of selective COX-2 inhibition on MAP. Rats fed a high-salt diet (2% NaCl), were treated with the selective COX-2 inhibitor nimesulide (n = 5) or vehicle (n = 5) and were infused with subcutaneous ANG II.
Neurogenic pressor activity.
The maximum falls in MAP after ganglionic blockade on day 10 of ANG-II infusion in control rats and in rats subjected to selective COX inhibition are shown in Fig. 8. In experiments with SC560, ganglion blockade decreased MAP significantly more in vehicle- (39 ± 4 mmHg) versus drug-treated (27 ± 4 mmHg) rats. In experiments using nimesulide, the fall in MAP with ganglion blockade was similar in vehicle- (58 ± 9 mmHg) and drug-treated (62 ± 12 mmHg) animals.
Fig. 8.
Depressor response to ganglionic blockade 10 days after high-salt (2% NaCl) feeding and ANG-II infusion in vehicle- and specific COX inhibitor-treated rats. Peak fall in MAP in response to hexamethonium administration (30 mg/kg ip) in SC560 (A) (n = 10) compared with vehicle DMSO (n = 7) and nimesulide (NM, n = 5) (B) compared with vehicle DMSO-treated (n = 5) rats. #P < 0.05 SC560 vs. DMSO.
DISCUSSION
In this article our goal was to explore the involvement of COX products in the development of HTN. In the past, result from clinical trials and meta-analysis of randomized trials involving use of nonsteroidal anti-inflammatory drugs (NSAIDs)—targeting the cyclooxygenase enzyme—have led to a general disagreement regarding the effects of COX-inhibiting drugs on blood pressure in human patients. Meta-analyses show a 3- to 5-mmHg blood pressure rise in hypertensive patients who take NSAIDs (16, 30). Furthermore, NSAID use among patients for inflammatory disease increases the risk of HTN development in men and women (10, 11). Nevertheless, COX products can affect blood pressure in a wide variety of ways and, based on animal experimentation, very likely have both pro- and antihypertensive actions under some circumstances. The focus of our work was specifically on angiotensin-high salt-induced HTN.
Previous investigators have used both pharmacological and gene knockout approaches to determine if prostanoids play a critical role in ANG II-mediated HTN, but their results have led to conflicting conclusions (3, 31, 43). Prostanoid products can exert both pro- and antihypertensive effects: thus their net effect on blood pressure will depend on where increased formation occurs and on which specific products are released. Earlier studies showed that dietary salt intake is one factor that influences the contribution of prostanoids to ANG II-dependent HTN (15, 25). Our initial experiment here confirmed such an influence: we found that COX inhibition with ketoprofen did not prevent ANG-II HTN in rats on a 0.4% NaCl diet, but virtually eliminated ANG-II HTN in rats on a 2.0% NaCl diet.
There are multiple potential explanations for why prostanoids could make a higher contribution to ANG-II HTN under conditions of high-salt diet. However, others and we have shown that high-salt intake amplifies the role of the SNS in ANG-II HTN (18, 28, 33). Furthermore, we showed that selective removal of the splanchnic sympathetic innervation significantly attenuated chronic ANG II-salt HTN (19), indicating that splanchnic SNS activity is important for HTN development in this model. Since direct recordings in conscious rats with chronic ANG-II HTN showed an increase in splanchnic SNS activity that was associated with a significant rise in urinary excretion of cyclooxygenase products (22), we hypothesized that COX-derived prostanoids could increase blood pressure in the ANG II-salt model by stimulating SNS activity.
This idea was further supported by our findings here that ketoprofen treatment had a more prominent effect on ANG II-salt HTN development during the later days of ANG-II infusion. We have previously reported that neurogenic mechanisms appear to play a greater role in this HTN model after 4 to 5 days of ANG-II infusion (18–20). The increase in MAP on days 1–4 appears to be caused by either the direct pressor actions of ANG II or perhaps renal sodium and water retention. We hypothesize that these nonneurogenic mechanisms also account for the mild HTN seen in our rats fed a 0.4% NaCl diet. The relative contribution of different mechanisms causing ANG-II HTN likely depends on both the rate of ANG-II infusion and dietary salt intake (among other factors).
We tested the role of COX products in the neurogenic actions of ANG II in two ways. First, we assessed SNS activity using measurement of whole body NE spillover in ketoprofen-treated rats. In an earlier study, we observed a statistically significant 71% increase in whole body NE spillover on days 7 and 14 of ANG-II infusion in rats on a high-salt diet (18). In the present study, in rats treated with ketoprofen, we observed a much smaller (though statistically significant) increase in whole body NE spillover on day 7 of ANG-II infusion (36% vs. control) and a statistically nonsignificant increase (14% vs. control) on day 14 of ANG-II infusion in high salt-fed rats. We conclude from these results that the global SNS activation caused by ANG-II infusion, as indicated by significant elevation in plasma NE and whole body NE spillover as shown by us (18), was largely but not completely prevented by nonselective COX inhibition.
We assessed neurogenic pressor activity (the acute contribution of the autonomic nervous system to arterial pressure) to test further whether prostanoid-related SNS activity contributed to ANG II-salt HTN. Vehicle-treated ANG II-salt HTN rats exhibited a marked drop in MAP during ganglionic blockade; ANG II-salt rats treated with ketoprofen showed a substantially smaller drop in pressure, although the difference was not statistically significant. Together, these data indicate that COX products may not be the sole cause of, but at least contribute to, the neurogenically mediated rise in MAP in ANG II-salt HTN rats.
The next issue we addressed was whether prostanoids involved in ANG II-salt HTN were derived from the COX-1 or COX-2 isoform of the enzyme. ANG II exerts physiologically significant proinflammatory effects that contribute to numerous cardiovascular diseases (5, 7). ANG II appears to produce inflammatory responses predominantly in a localized manner in target tissues such as blood vessels (5), kidney (42), and brain (44). COX-2 is the isoform most strongly implicated in generating inflammatory prostanoids, so it is logical to assume that it is the key player in ANG II-salt HTN. Some published studies support this conclusion. For example, pretreatment with COX-2 inhibitors like refecoxib and nimesulide attenuated ANG II-HTN in Sprague-Dawley rats (23, 32, 43). However, other studies indicate that COX-1 is the source of prostanoids involved in ANG-II HTN (2). In a recent report, Cao and colleagues (3) showed that ANG-II HTN was attenuated in COX-1−/− mice but not COX-2−/− mice. The contradictory reports from various laboratories could be attributed to the differences in animal models used, blood pressure measurement methods like tail-cuff plethysmography versus radiotelemetry, or the mode of drug administration.
Although COX-1 and COX-2 expression have been reported to be increased in other models of ANG-II HTN, in our ANG II-salt HTN model COX protein expression in blood vessels and brain of hypertensive rats was similar to that observed in control rats. This could be because our measurement was made only after HTN was established or because we did not evaluate the key blood vessels or brain regions. Alternatively, our model may involve a change in COX activity, rather than a change in new protein synthesis. This possibility requires further investigation. Nevertheless, the key prostanoids appear to be derived from the COX-1 and not the COX-2 isoform: HTN development was markedly and significantly attenuated by treatment with the COX-1 inhibitor SC560, but not by treatment with the COX-2 inhibitor nimesulide. However, with selective COX-1 inhibition, we did not see as complete an attenuation of HTN as we observed with the nonselective COX inhibitor ketoprofen. This may be due to 1) additional pharmacological actions of ketoprofen or 2) involvement of COX-2 in the development of HTN, although our results with the selective COX-2 inhibition do not support the latter idea. It is further notable that COX-1 inhibition with SC560 significantly reduced neurogenic pressor activity in ANG II-salt HTN rats. This result supports the hypothesis that prostanoids increase arterial pressure in ANG II-salt HTN in part by increasing SNS activity. It should be noted that we observed a smaller fall in MAP after hexamethonium treatment in control rats treated with DMSO as vehicle (as seen in Fig. 8) compared with control rats treated with saline as vehicle (as seen in Fig. 3). This could be due to differences in the effect of DMSO and saline on sympathetic nerve activity but more likely reflects the distinct experimental conditions at the time of the hexamethonium treatment in the two studies, i.e., tethered rats (with higher MAP and possibly sympathetic activity) in Fig. 3 compared with telemetered rats in Fig. 8.
In conclusion, this study suggests that in rats fed a high-salt diet, chronic ANG-II infusion stimulates the formation of prostanoids from COX-1 that could activate the SNS and increase arterial pressure. Our data do not allow us to establish where these prostanoids are produced, or where they act, to possibly increase SNS activity or arterial pressure. However, since central injections of prostanoids can increase SNS activity (26) and arterial pressure (14) and a recent study in mice strongly implicated brain COX-1 activity in ANG-II HTN (3), future studies are needed to investigate the role of brain prostanoids derived from COX-1 in ANG II-salt HTN.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants RO1-HL-076312 and RCO-HL-060363.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
N.A.-J., A.J.K., C.A.N., and G.D.F. conception and design of research; N.A.-J., A.J.K., C.A.N., and S.M. performed experiments; N.A.-J., A.J.K., C.A.N., S.M., and G.D.F. analyzed data; N.A.-J., A.J.K., C.A.N., and G.D.F. interpreted results of experiments; N.A.-J., A.J.K., C.A.N., and S.M. prepared figures; N.A.-J. drafted manuscript; N.A.-J., A.J.K., C.A.N., and G.D.F. edited and revised manuscript; N.A.-J., A.J.K., C.A.N., S.M., and G.D.F. approved final version of manuscript.
ACKNOWLEDGMENTS
This work was performed as part of the Neurogenic Cardiovascular Diseases Consortium project.
REFERENCES
- 1.Anneken JH, Cunningham JI, Collins SA, Yamamoto BK, Gudelsky GA. MDMA increases glutamate release and reduces parvalbumin-positive GABAergic cells in the dorsal hippocampus of the rat: role of cyclooxygenase. J Neuroimmune Pharmacol 8: 58–65, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Araujo M, Welch WJ. Tubuloglomerular feedback is decreased in COX-1 knockout mice after chronic angiotensin II infusion. Am J Physiol Renal Physiol 298: F1059–F1063, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao X, Peterson JR, Wang G, Anrather J, Young CN, Guruju MR, Burmeister MA, Iadecola C, Davisson RL. Angiotensin II-dependent hypertension requires cyclooxygenase 1-derived prostaglandin E2 and EP1 receptor signaling in the subfornical organ of the brain. Hypertension 59: 869–876, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cox BF, Bishop VS. Neural and humoral mechanisms of angiotensin-dependent hypertension. Am J Physiol Heart Circ Physiol 261: H1284–H1291, 1991 [DOI] [PubMed] [Google Scholar]
- 5.Davis PA, Mussap M, Pagnin E, Bertipaglia L, Savica V, Semplicini A, Calo LA. Early markers of inflammation in a high angiotensin II state—results of studies in Bartter's/Gitelman's syndromes. Nephrol Dial Transplant 21: 1697–1701, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Eisenhofer G. Sympathetic nerve function—assessment by radioisotope dilution analysis. Clin Auton Res 15: 264–283, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Ferrario CM, Strawn WB. Role of the renin-angiotensin-aldosterone system and proinflammatory mediators in cardiovascular disease. Am J Cardiol 98: 121–128, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Fierro-Carrion GA, Ram CV. Nonsteroidal anti-inflammatory drugs (NSAIDs) and blood pressure. Am J Cardiol 80: 775–776, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Fink GD. Long-term sympatho-excitatory effect of angiotensin II: a mechanism of spontaneous and renovascular hypertension. Clin Exp Pharmacol Physiol 24: 91–95, 1997 [DOI] [PubMed] [Google Scholar]
- 10.Forman JP, Rimm EB, Curhan GC. Frequency of analgesic use and risk of hypertension among men. Arch Intern Med 167: 394–399, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Forman JP, Stampfer MJ, Curhan GC. Non-narcotic analgesic dose and risk of incident hypertension in US women. Hypertension 46: 500–507, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Griffin SA, Brown WC, MacPherson F, McGrath JC, Wilson VG, Korsgaard N, Mulvany MJ, Lever AF. Angiotensin II causes vascular hypertrophy in part by a non-pressor mechanism. Hypertension 17: 626–635, 1991 [DOI] [PubMed] [Google Scholar]
- 13.Gu B, Desjardins P, Butterworth RF. Selective increase of neuronal cyclooxygenase-2 (COX-2) expression in vulnerable brain regions of rats with experimental Wernicke's encephalopathy: effect of nimesulide. Metab Brain Dis 23: 175–187, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Inoue M, Crofton JT, Share L. Interactions between the brain renin-angiotensin system and brain prostanoids in the control of vasopressin secretion. Exp Brain Res 83: 131–136, 1990 [DOI] [PubMed] [Google Scholar]
- 15.Jia Z, Zhang A, Zhang H, Dong Z, Yang T. Deletion of microsomal prostaglandin E synthase-1 increases sensitivity to salt loading and angiotensin II infusion. Circ Res 99: 1243–1251, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Johnson AG, Nguyen TV, Day RO. Do nonsteroidal anti-inflammatory drugs affect blood pressure? A meta-analysis. Ann Intern Med 121: 289–300, 1994 [DOI] [PubMed] [Google Scholar]
- 17.Keeton TK, Biediger AM. The measurement of norepinephrine clearance and spillover rate into plasma in conscious spontaneously hypertensive rats. Naunyn Schmiedebergs Arch Pharmacol 338: 350–360, 1988 [DOI] [PubMed] [Google Scholar]
- 18.King AJ, Novotny M, Swain GM, Fink GD. Whole body norepinephrine kinetics in ANG II-salt hypertension in the rat. Am J Physiol Regul Integr Comp Physiol 294: R1262–R1267, 2008 [DOI] [PubMed] [Google Scholar]
- 19.King AJ, Osborn JW, Fink GD. Splanchnic circulation is a critical neural target in angiotensin II salt hypertension in rats. Hypertension 50: 547–556, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Kuroki MT, Guzman PA, Fink GD, Osborn JW. Time-dependent changes in autonomic control of splanchnic vascular resistance and heart rate in ANG II-salt hypertension. Am J Physiol Heart Circ Physiol 302: H763–H769, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lau YE, Galligan JJ, Kreulen DL, Fink GD. Activation of ETB receptors increases superoxide levels in sympathetic ganglia in vivo. Am J Physiol Regul Integr Comp Physiol 290: R90–R95, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Luft FC, Wilcox CS, Unger T, Kuhn R, Demmert G, Rohmeiss P, Ganten D, Sterzel RB. Angiotensin-induced hypertension in the rat. Sympathetic nerve activity and prostaglandins. Hypertension 14: 396–403, 1989 [DOI] [PubMed] [Google Scholar]
- 23.Martinez-Revelles S, Avendano MS, Garcia-Redondo AB, Alvarez Y, Aguado A, Perez-Giron JV, Garcia-Redondo L, Esteban V, Redondo JM, Alonso MJ, Briones AM, Salaices M. Reciprocal relationship between reactive oxygen species and cyclooxygenase-2 and vascular dysfunction in hypertension. Antioxid Redox Signal 18: 51–65, 2013 [DOI] [PubMed] [Google Scholar]
- 24.McGiff JC, Crowshaw K, Terragno NA, Lonigro AJ. Release of a prostaglandin-like substance into renal venous blood in response to angiotensin II. Circ Res 27: 121–130, 1970 [PubMed] [Google Scholar]
- 25.Mistry M, Nasjletti A. Role of pressor prostanoids in rats with angiotensin II-salt-induced hypertension. Hypertension 11: 758–762, 1988 [DOI] [PubMed] [Google Scholar]
- 26.Okuno T, Lindheimer MD, Oparil S. Central effects of prostaglandin E2 on blood pressure and plasma renin activity in rats. Role of the sympathoadrenal system and vasopressin. Hypertension 4: 809–816, 1982 [DOI] [PubMed] [Google Scholar]
- 27.Osborn JL. Relation between sodium intake, renal function, and the regulation of arterial pressure. Hypertension 17: I91–I96, 1991 [DOI] [PubMed] [Google Scholar]
- 28.Osborn JW, Fink GD, Sved AF, Toney GM, Raizada MK. Circulating angiotensin II and dietary salt: converging signals for neurogenic hypertension. Curr Hypertens Rep 9: 228–235, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Parsadaniantz SM, Lebeau A, Duval P, Grimaldi B, Terlain B, Kerdelhue B. Effects of the inhibition of cyclo-oxygenase 1 or 2 or 5-lipoxygenase on the activation of the hypothalamic-pituitary-adrenal axis induced by interleukin-1beta in the male Rat. J Neuroendocrinol 12: 766–773, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Pope JE, Anderson JJ, Felson DT. A meta-analysis of the effects of nonsteroidal anti-inflammatory drugs on blood pressure. Arch Intern Med 153: 477–484, 1993 [PubMed] [Google Scholar]
- 31.Qi Z, Hao CM, Langenbach RI, Breyer RM, Redha R, Morrow JD, Breyer MD. Opposite effects of cyclooxygenase-1 and -2 activity on the pressor response to angiotensin II. J Clin Invest 110: 61–69, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quilley J. COX-2 and angiotensin II-induced hypertension and oxidative stress. Am J Hypertens 24: 1188, 2011 [DOI] [PubMed] [Google Scholar]
- 33.Sato Y, Ogata E, Fujita T. Role of chloride in angiotensin II-induced salt-sensitive hypertension. Hypertension 18: 622–629, 1991 [DOI] [PubMed] [Google Scholar]
- 34.Simmons DL, Botting RM, Hla T. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol Rev 56: 387–437, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Smith WL, Garavito RM, DeWitt DL. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2. J Biol Chem 271: 33157–33160, 1996 [DOI] [PubMed] [Google Scholar]
- 36.Smith WL, Marnett LJ, DeWitt DL. Prostaglandin and thromboxane biosynthesis. Pharmacol Ther 49: 153–179, 1991 [DOI] [PubMed] [Google Scholar]
- 37.Smith WL, Urade Y, Jakobsson PJ. Enzymes of the cyclooxygenase pathways of prostanoid biosynthesis. Chem Rev 111: 5821–5865, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smyth EM, Grosser T, Wang M, Yu Y, FitzGerald GA. Prostanoids in health and disease. J Lipid Res 50 Suppl: S423–S428, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Touw KB, Haywood JR, Shaffer RA, Brody MJ. Contribution of the sympathetic nervous system to vascular resistance in conscious young and adult spontaneously hypertensive rats. Hypertension 2: 408–418, 1980 [DOI] [PubMed] [Google Scholar]
- 40.Von der Weid PY, Hollenberg MD, Fiorucci S, Wallace JL. Aspirin-triggered, cyclooxygenase-2-dependent lipoxin synthesis modulates vascular tone. Circulation 110: 1320–1325, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Weir MR, Dzau VJ. The renin-angiotensin-aldosterone system: a specific target for hypertension management. Am J Hypertens 12: 205S–213S, 1999 [DOI] [PubMed] [Google Scholar]
- 42.Wilcox CS. Reactive oxygen species: roles in blood pressure and kidney function. Curr Hypertens Rep 4: 160–166, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Wu R, Duchemin S, Laplante MA, De Champlain J, Girouard H. Cyclo-oxygenase-2 knockout genotype in mice is associated with blunted angiotensin II-induced oxidative stress and hypertension. Am J Hypertens 24: 1239–1244 [DOI] [PubMed] [Google Scholar]
- 44.Zimmerman MC, Lazartigues E, Sharma RV, Davisson RL. Hypertension caused by angiotensin II infusion involves increased superoxide production in the central nervous system. Circ Res 95: 210–216, 2004 [DOI] [PubMed] [Google Scholar]