Abstract
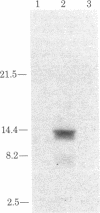
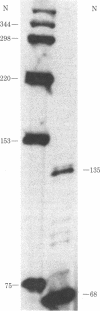
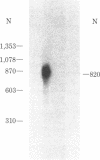
Molecular cloning has established the primary structures of two precursors of the human pancreas growth hormone-releasing factor (hpGRF-44), somatocrinin. Both polypeptides contain the sequence of hpGRF-44 flanked by basic processing sites. Furthermore, the precursors include a putative signal sequence and a carboxyl-terminal amidation signal for hpGRF-44. The two forms of mRNA code for pre-pro-GRF-107 and pre-pro-GRF-108. Pre-pro-GRF-108 differs from pre-pro-GRF-107 by the insertion of a serine in the carboxyl-terminal portion of the precursor. In vitro translation of tumor poly(A)+ RNA followed by immunoprecipitation with GRF-specific antiserum and gel electrophoresis showed the molecular weight of preprosomatocrinin to be approximately 13,000, which is in good agreement with the molecular weight deduced from the sequences of the cDNA clones.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal K. L., Brunstedt J., Noyes B. E. A general method for detection and characterization of an mRNA using an oligonucleotide probe. J Biol Chem. 1981 Jan 25;256(2):1023–1028. [PubMed] [Google Scholar]
- Benoist C., Chambon P. In vivo sequence requirements of the SV40 early promotor region. Nature. 1981 Mar 26;290(5804):304–310. doi: 10.1038/290304a0. [DOI] [PubMed] [Google Scholar]
- Benoit R., Böhlen P., Ling N., Briskin A., Esch F., Brazeau P., Ying S. Y., Guillemin R. Presence of somatostatin-28-(1-12) in hypothalamus and pancreas. Proc Natl Acad Sci U S A. 1982 Feb;79(3):917–921. doi: 10.1073/pnas.79.3.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch B., Brazeau P., Ling N., Bohlen P., Esch F., Wehrenberg W. B., Benoit R., Bloom F., Guillemin R. Immunohistochemical detection of growth hormone-releasing factor in brain. Nature. 1983 Feb 17;301(5901):607–608. doi: 10.1038/301607a0. [DOI] [PubMed] [Google Scholar]
- Bradbury A. F., Finnie M. D., Smyth D. G. Mechanism of C-terminal amide formation by pituitary enzymes. Nature. 1982 Aug 12;298(5875):686–688. doi: 10.1038/298686a0. [DOI] [PubMed] [Google Scholar]
- Brazeau P., Ling N., Böhlen P., Esch F., Ying S. Y., Guillemin R. Growth hormone releasing factor, somatocrinin, releases pituitary growth hormone in vitro. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7909–7913. doi: 10.1073/pnas.79.24.7909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Esch F. S., Böhlen P., Ling N. C., Brazeau P. E., Wehrenberg W. B., Guillemin R. Primary structures of three human pancreas peptides with growth hormone-releasing activity. J Biol Chem. 1983 Feb 10;258(3):1806–1812. [PubMed] [Google Scholar]
- Guillemin R., Brazeau P., Böhlen P., Esch F., Ling N., Wehrenberg W. B. Growth hormone-releasing factor from a human pancreatic tumor that caused acromegaly. Science. 1982 Nov 5;218(4572):585–587. doi: 10.1126/science.6812220. [DOI] [PubMed] [Google Scholar]
- Hanahan D., Meselson M. Plasmid screening at high colony density. Gene. 1980 Jun;10(1):63–67. doi: 10.1016/0378-1119(80)90144-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Land H., Schütz G., Schmale H., Richter D. Nucleotide sequence of cloned cDNA encoding bovine arginine vasopressin-neurophysin II precursor. Nature. 1982 Jan 28;295(5847):299–303. doi: 10.1038/295299a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi K., Arentzen R., Huang T., Itakura K. Solid-phase synthesis of polynucleotides. IV. Usage of polystyrene resins for the synthesis of polydeoxyribonucleotides by the phosphostriester method. Nucleic Acids Res. 1980 Nov 25;8(22):5507–5517. doi: 10.1093/nar/8.22.5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgard M. V., Tocci M. J., Monahan J. J. On the cloning of eukaryotic total poly(A)-RNA populations in Escherichia coli. J Biol Chem. 1980 Aug 25;255(16):7665–7672. [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payvar F., Schimke R. T. Methylmercury hydroxide enhancement of translation and transcription of ovalbumin and conalbumin mRNA's. J Biol Chem. 1979 Aug 25;254(16):7636–7642. [PubMed] [Google Scholar]
- Peacock S. L., McIver C. M., Monahan J. J. Transformation of E. coli using homopolymer-linked plasmid chimeras. Biochim Biophys Acta. 1981 Sep 28;655(2):243–250. doi: 10.1016/0005-2787(81)90014-9. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Shen L. P., Pictet R. L., Rutter W. J. Human somatostatin I: sequence of the cDNA. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4575–4579. doi: 10.1073/pnas.79.15.4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. J. DNA sequence analysis by primed synthesis. Methods Enzymol. 1980;65(1):560–580. doi: 10.1016/s0076-6879(80)65060-5. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Quinn P. S., Chan S. J., Marsh J., Tager H. S. Processing mechanisms in the biosynthesis of proteins. Ann N Y Acad Sci. 1980;343:1–16. doi: 10.1111/j.1749-6632.1980.tb47238.x. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]