Abstract
Bacterial pneumonia is a common and dangerous illness. Mononuclear phagocytes, which comprise monocyte, resident and recruited macrophage, and dendritic cell subsets, are critical to antimicrobial defenses, but the dynamics of their recruitment to the lungs in pneumonia is not established. We hypothesized that chemokine-mediated traffic of mononuclear phagocytes is important in defense against bacterial pneumonia. In a mouse model of Klebsiella pneumonia, circulating Ly6Chi and, to a lesser extent, Ly6Clo monocytes expanded in parallel with accumulation of inflammatory macrophages and CD11bhi dendritic cells and plasmacytoid dendritic cells in the lungs, whereas numbers of alveolar macrophages remained constant. CCR2 was expressed by Ly6Chi monocytes, recruited macrophages, and airway dendritic cells; CCR6 was prominently expressed by airway dendritic cells; and CX3CR1 was ubiquitously expressed by blood monocytes and lung CD11bhi dendritic cells during infection. CCR2-deficient, but not CCL2-, CX3CR1-, or CCR6-deficient animals exhibited worse outcomes of infection. The absence of CCR2 had no detectable effect on neutrophils but resulted in reduction of all subsets of lung mononuclear phagocytes in the lungs, including alveolar macrophages and airway and plasmacytoid dendritic cells. In addition, absence of CCR2 skewed the phenotype of lung mononuclear phagocytes, abrogating the appearance of M1 macrophages and TNF-producing dendritic cells in the lungs. Taken together, these data define the dynamics of mononuclear phagocytes during pneumonia.
Keywords: chemokine, host defense, pneumonia
aerobic gram-negative bacilli are the most common causes of health care-associated pneumonia (45), an illness that afflicts 250,000 hospitalized patients each year in the United States (24) and carries an attributable mortality of 14% (39). Progressive emergence of antibiotic resistance among hospital-associated Gram-negative bacteria has greatly complicated the treatment of these infections (40) and provides an important justification for investigating mechanisms of host defense against these infections because better understanding of these mechanisms can lead to development of new therapeutic interventions.
Mononuclear phagocytes have been recognized for their role in antimicrobial immunity since their initial description (7). Categorized as monocytes, macrophages, and dendritic cells (DC), the mononuclear phagocyte system is characterized by marked heterogeneity and incompletely defined cellular lineages (13). In the bone marrow compartment, the monocyte-DC progenitors give rise to monocytes expressing high levels of the glycosylphosphatidylinositol-anchored receptor Ly6C and the common DC progenitor. Under homeostatic conditions, Ly6Chi monocytes transit bidirectionally between the bone marrow and blood compartments and differentiate into Ly6Clo monocytes (17). In the absence of inflammation, mouse lungs contain at least five populations of mononuclear phagocytes, namely CD11bneg/lo CD11chi alveolar macrophages, CD11b+ CD11c− nonalveolar macrophages, CD11bhi conventional DC, CD103+ airway DC, and plasmacytoid DC (14).
A number of chemokine receptors and their ligands have been associated with migration of cells of the monocytic phagocyte system. Among these, CCR2 is expressed by Ly6Chi monocytes and is critical to their release from the bone marrow and homing to peripheral tissues in the context of inflammation (53). CCR2 has multiple ligands, including CCL2, CCL7, and CCL12 in mice and CCL2, CCL7, CCL8, CCL13, and CCL18 in humans. The relative contribution of these ligands to CCR2-mediated effects is incompletely defined, with evidence for both redundant and additive effects in different models (21, 38, 49). CX3CR1 is ubiquitously expressed on both populations of blood monocytes and in the resting state on lung CD11bhi DC; in some models, this receptor can contribute to migration of mononuclear phagocytes into tissue (29, 50). The sole ligand for CX3CR1 is CX3CL1. In the absence of inflammation, CCR6 is expressed by some lung DC populations and has been implicated in their traffic in several models of lung inflammation (19). The only chemokine ligand for CCR6 is CCL20; in addition, members of the β-defensin family can act as CCR6 ligands (64).
Although the monocytic phagocyte system is considered critical to defenses against many infections, the contribution of specific cells and specific chemokine receptors to host defense against bacterial pneumonia has not been defined. We sought to characterize the dynamics of the cells of the mononuclear phagocyte system in the bone marrow, blood, and lung during infection and to test the hypothesis that chemokine-mediated traffic of these cells is important to host defense against Gram-negative bacterial pneumonia.
MATERIALS AND METHODS
Animals and in vivo procedures.
Mice with enhanced green fluorescent protein (eGFP) knockin at the CX3CR1 locus (22) and mice deficient in CCR2 (25, 41), CCL2 (31), and CCR6 (46) were bred in our colony, and respective wild-types were purchased (Jackson Laboratories, Bar Harbor, ME). Heterozygous CX3CR1-eGFP animals were generated by crossing homozygous and wild-type animals and were used to identify CX3CR1-expressing cells, as described (22). Animals were maintained under pathogen-free conditions and in compliance with institutional animal care regulations. Age- and sex-matched 6- to 10-wk-old mice were used in all experiments. Experimental pneumonia was induced by intratracheal inoculation of Klebsiella pneumoniae (K. pneumoniae) (strain 43816; American Type Culture Collection, Manassas, VA), using inocula between 100 and 400 cfu in various experiments, as previously described (15, 37). In some experiments, circulating monocytes were depleted by twice daily intravenous injection of 100 μl of liposomes containing clodronate or PBS (Encapsula NanoSciences, Nashville, TN) starting 8 h before inoculation. In other experiments, neutrophil depletion was achieved with a single i.p. injection of 200 μg of a monoclonal Ab (clone 1A8) or isotype control (clone 2A3) 1 day before an intratracheal challenge with K. pneumoniae, resulting in peripheral blood neutropenia for ∼3 days (42).
Tissue harvest, histopathology, ELISA, and myeloperoxidase assays.
At designated time points, animals were euthanized by intraperitoneal administration of an overdose of heparinized ketamine and xylazine, blood was collected from the right ventricle, and the pulmonary vasculature was perfused with PBS containing 0.5 mM EDTA. In some experiments, bronchoalveolar lavage was performed; in other experiments, whole lungs and a femur were removed or tissue was processed for histology, as previously described (3, 44). Lung samples were homogenized and processed for myeloperoxidase activity and ELISA as described (37). ELISA for CCL2, CCL7, CCL12, CCL20, and CX3CL1 was performed according to manufacturer's instructions (CCL7 was from Peprotech, Rocky Hill, NJ; all others were from R&D Systems, Minneapolis, MN; minimum detectable concentrations were 50 pg/ml for CCL2, 100 pg/ml for CCL7 and CCL12, and 5 ng/ml for CCL20 and CX3CL1).
Flow cytometry.
Cell suspension of lungs, bone marrow, and peripheral blood were prepared as described (37, 42, 44, 46). The following reagents were used to label cells for flow cytometry (from BD Biosciences, San Jose, CA; eBiosciences, San Diego, CA; Miltenyi, Auburn, CA; or R&D Systems): 7-aminoactinomycin D (7-AAD), annexin V-FITC, anti-B220-FITC (clone RA3–6B2), anti-CCR2-PE (clone 475301), anti-CCR6-allophycocyanin (APC, clone 140706), anti-CD3-PE (clone 145–2C11), anti-CD4-FITC (clone GK1.5), anti-CD8-PE-Cy7 (clone 53–6.7), anti-CD11b-APC-Cy7 (clone M1/70), anti-CD11c-PE-Cy7 (clone HL3), anti-CD19-PE-Cy7 (clone 6D5), anti-CD45-PerCP (clone 30-F11), anti-CD69-PE (clone H1.2F3), anti-CD103-biotin (clone M209), anti-CD115-PE (clone AFS98), anti-CD124-biotin (clone mIL4R1-M1), anti-CD206-PE (clone MR5D3), anti-F4/80-Pacific blue (clone BM8), anti-I-A/I-E-Pacific blue (clone M5/114.15.2), anti-inducible nitric oxide synthetase (iNOS)-APC (polyclonal goat IgG), anti-Ly-6C-APC-Cy7 (clone AL-21), anti-Ly-6G-FITC (clone 1A8), anti-Mac3-PE (clone M3/84), anti-PDCA1-PE (clone JF05-IC2.4.1), and anti-TNF-PE (clone MP6-XT22). In some experiments, cells were stained for intracellular antigens; after being labeled for cell surface markers, samples were fixed and permeabilized using a commercial kit (Cytofix/Cytoperm, BD Biosciences) before intracellular staining. Data was acquired on a FACS Canto II instrument using Diva software (version 5.0.3, BD Biosciences) and analyzed using FlowJo software (version 8.8.6; Tree Star, Ashland, OR), using a previously published gating strategy (4). Apoptotic cells were defined as bronchoalveolar lavage cells that were positive for Annexin-V and negative for 7-AAD; dead cells were defined as cells staining with 7-AAD. The absolute number of each leukocyte subset was determined as the product of the percentage of the cell type and the total number of cells in the sample, as determined on an automated cell counter (Countess; Invitrogen, Carlsbad, CA).
Statistical analysis.
Data were analyzed on a Macintosh iMac computer using Prism statistical package (version 5; GraphPad Software, San Diego, CA). Survival data were compared using Fischer's exact test. Values between two groups over multiple times were compared with two-way ANOVA; comparisons between two groups at a single time were performed with unpaired two-tailed Mann-Whitney (nonparametric) test; and comparisons between multiple groups at a single time were compared using the Kruskal-Wallis test with Bonferroni posttest. Probability values were considered statistically significant if they were <0.05.
RESULTS
Expression of chemokine ligands and receptors.
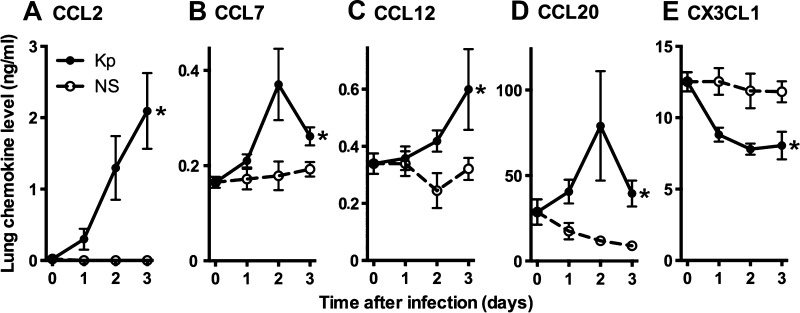
We began by measuring the concentration of ligands for the chemokine receptors under study, namely the CCR2 ligands CCL2, CCL7, and CCL12, the CCR6 ligand CCL20, and the CX3CR1 ligand CX3CL1, during the course of bacterial pneumonia (Fig. 1). Consistent with prior reports (38, 46–48), we found notable levels of CCL20 and CX3CL1, but little or no detectable CCR2 ligands, in the lungs of uninfected animals (Fig. 1). During the early course of infection, there was marked induction of CCR2 and CCR6 ligands but a decrease in total CX3CL1 protein (consisting of both cell-bound and soluble forms) as the infection progressed.
Fig. 1.
Protein levels of chemokine ligands in lung homogenates during experimental Gram-negative pneumonia. A–E: cytokine levels were measured at various times following intrapulmonary challenge with Klebsiella pneumoniae or sterile saline vehicle. Day 0 represents unchallenged animals. Data represent means ± SE of pooled data from 2 experiments; n = 6–8 mice per group per time point. *P < 0.05 comparing trend between the 2 groups over time (2-way ANOVA).
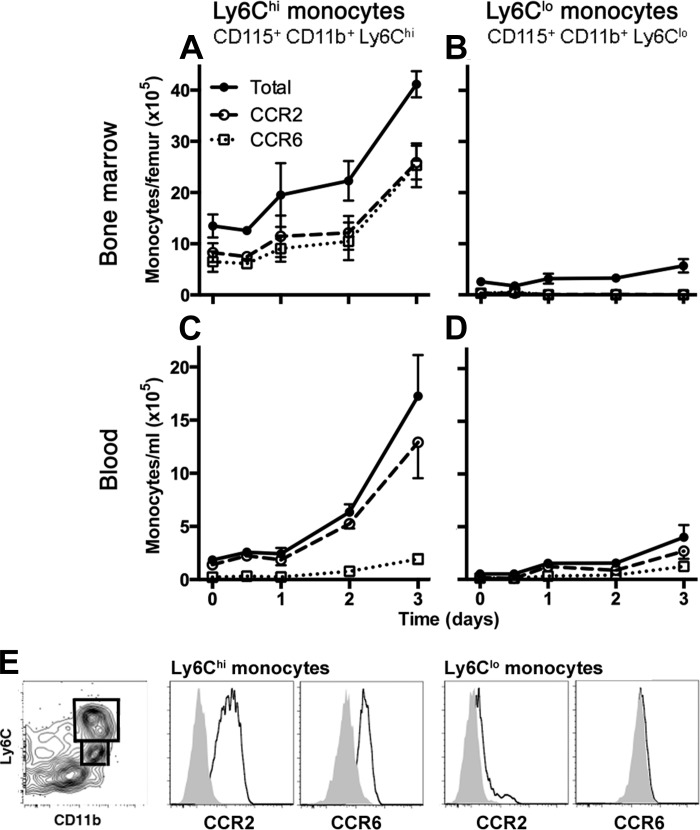
We next sought to assess the expression of the receptors CCR2, CCR6, and CX3CR1 on cells of the monocytic phagocyte system during the infection. We used standard surface staining for CCR2 and CCR6 using commercial monoclonal antibodies. To identify CX3CR1-expressing cells, we used mice heterozygote for eGFP transgene in the CX3CR1 locus, as previously described (22). As expected, both monocyte subsets in the bone marrow and blood (defined as Ly6Chi and Ly6Clo CD115-expressing cells) expressed CX3CR1 at all the examined time points (data not shown). We found a marked expansion of Ly6Chi monocytes in the bone marrow and an even larger expansion of these cells in the blood beginning on day 1 and continuing until day 3 after the onset of infection (Fig. 2, A and C). Approximately half of the Ly6Chi monocytes expressed CCR2 and CCR6 in the bone marrow; in contrast, in the blood compartment essentially all of the blood cells expressed CCR2, whereas very few expressed CCR6. The Ly6Clo monocytes were a small and stable population in the bone marrow throughout the infection that did not express detectable CCR2 or CCR6 (Fig. 2B). Although there was an expansion of the pool of blood Ly6Clo monocytes during the infection, these cells constituted a small fraction of total blood monocytes given the more marked expansion of Ly6Chi monocytes. In contrast to uninfected animals, we found detectable expression of CCR2 on the blood Ly6Clo monocytes in infected animals (Fig. 2D).
Fig. 2.
Kinetics and chemokine receptor expression of blood and bone marrow monocyte populations during experimental Gram-negative pneumonia. Number of bone marrow (A and B) and concentration of blood (C and D) monocytes, defined as CD45+ cells expressing the markers noted on each panel, are shown at various times after intrapulmonary challenge with Klebsiella. Data shown represent means ± SE; n = 4–5 mice per group per time point, representative data from 4 independent experiments. Time 0 represents unchallenged animals. Data represented in A, C, and D showed statistically significant change in cell numbers over time (P < 0.05, 2-way ANOVA). E: representative flow plots showing gating strategy and receptor expression. Left: CD45+ bone marrow cells on day 1 of infection, showing gating strategy to identify monocyte subsets. Gray histograms represent isotype controls.
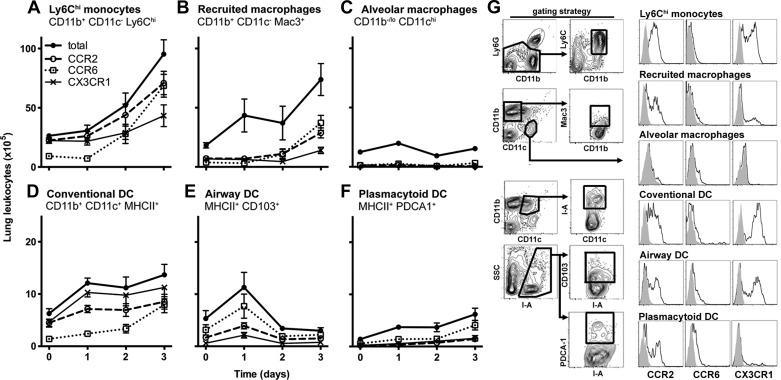
We next examined the expression of chemokine receptors on major populations of monocytic phagocytes in lungs. Because the lung vascular and interstitial compartments contain substantial populations of monocytic phagocytes (4), these measurements were made in whole lung single-cell suspensions. In samples in which the vasculature had been perfused to remove circulating leukocytes, we found no detectable CD115-expressing cells. We found a population of cells with similar cell surface markers to blood and bone marrow Ly6Chi monocytes (with the exception of the expression of CD115); we identified these lung monocytes as CD45+ CD11b+ Ly6Chi cells that did not express Ly6G, Mac3, CD11c, or lymphocyte markers. We noted a marked increase in the number of both the lung Ly6Chi monocytes and recruited macrophages over the course of the infection; in contrast, the number of alveolar macrophages remained unchanged (Fig. 3, A–C). CCR2 was expressed by most Ly6Chi monocytes and approximately half of the recruited macrophages, with a smaller proportion expressing CCR6 and CX3CR1. On the other hand, the alveolar macrophages did not express any of the receptors.
Fig. 3.
Kinetics and chemokine receptor expression of lung monocytic phagocytes during experimental Gram-negative pneumonia. Cells were enumerated in whole lung single-cell suspensions and populations identified as CD45+ cells expressing markers noted on each panel. Data shown represent means ± SE; n = 4–5 mice per group per time point, representative data from 4 independent experiments. Time 0 represents unchallenged animals. Data represented in A–F all showed statistically significant change in cell numbers over time (P < 0.05, 2-way ANOVA). DC: dendritic cells. G: representative flow plots showing gating strategy and histograms of receptor expression. Left: CD45+ lung cells on day 2 of infection, showing gating strategy to identify mononuclear phagocyte subsets. Gray histograms represent isotype controls (for CCR2 and CCR6) and wild-type mice (for CX3CR1).
Regarding the lung DC populations, there was a modest increase in the numbers of lung CD11bhi DC and plasmacytoid DC, but not airway DC, during the infection (Fig. 3, D–F). Similar to prior reports, we found most CD11bhi DC to express CX3CR1 (28), with half expressing CCR2 and smaller proportions expressing CCR6. Most airway DC, distinguished by the expression of CD103 (58), expressed CCR6 throughout the infection, whereas a minority expressed CCR2. CCR2 and CCR6 were expressed by plasmacytoid DC. These data provide descriptive evidence of the heterogeneous expression of CCR2, CCR6, and CX3CR1 on lung monocytic phagocytes during the early phase of bacterial pneumonia and, together with the expression of their ligands in the lungs, justified assessing their contribution to host defense in the infection.
Role of chemokine receptors in outcome of infection.
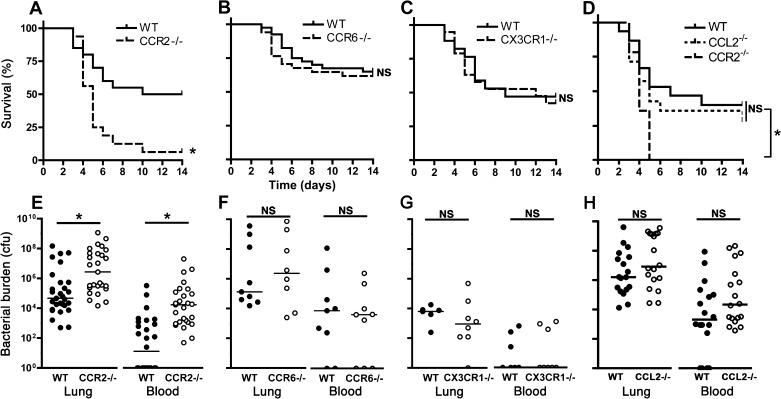
We next examined the outcome of infection in animals deficient in CCR2, CCR6, and CX3CR1. Mice deficient in CCR2, but not CCR6 or CX3CR1, had notably increased mortality compared with respective wild-types (Fig. 4, A–C). Consistent with this, CCR2-deficient mice had >100-fold higher lung bacterial burden, higher incidence of bacteremia, and ∼1,000-fold greater blood concentration of viable bacteria than wild-type animals on the third day of infection (Fig. 4E); in contrast, we found no significant difference in bacterial burden in CCR6 or CX3CR1-deficient mice compared with respective controls (Fig. 4, F–G). Interestingly, the host defense defect of CCR2-deficient mice was not replicated in CCL2-deficient animals, which exhibited similar mortality and organ bacterial burden as wild-type mice (Fig. 4, D and H), indicating redundancy between CCR2 ligands in the context of bacterial pneumonia. We therefore focused subsequent experiments on the mechanism of CCR2-mediated traffic of mononuclear phagocytes during pneumonia.
Fig. 4.
Outcome of experimental Gram-negative pneumonia in mice deficient in chemokine receptors. A–D: survival studies; n = 26–29 for each group, pooled from 2 experiments. WT, wild-type. E–H: lung and blood viable bacterial content on day 3 of infection. Each data point represents 1 animal, and horizontal lines indicate medians; samples with no recoverable bacteria are depicted as containing 1 colony-forming unit (cfu) on the logarithmic scale; pooled data from 3 experiments; *P < 0.05 compared with corresponding wild-type group; NS, no significant difference (Log-rank test for A–D; Mann-Whitney for E–H).
Role of CCR2 in traffic of mononuclear phagocytes.
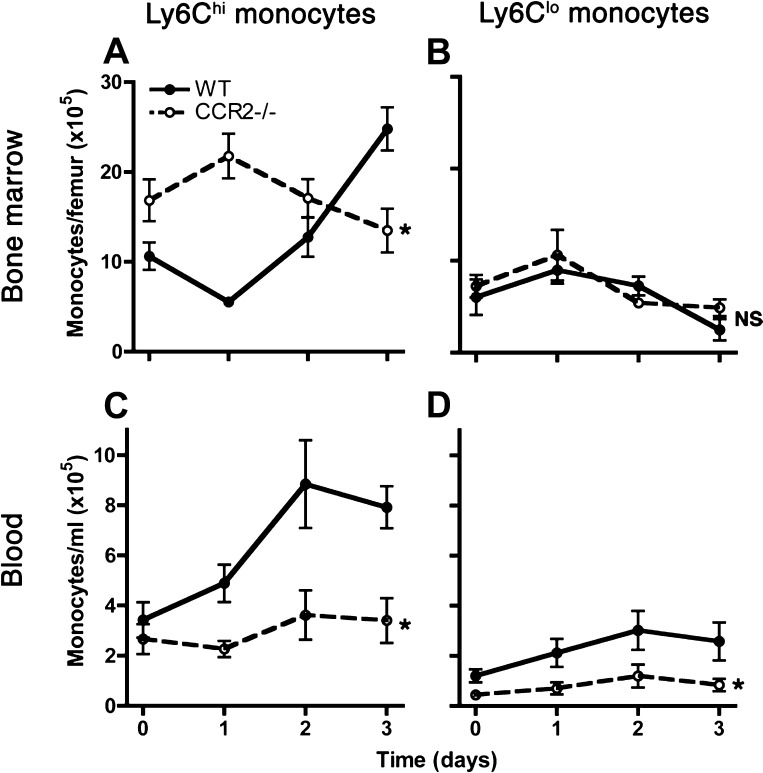
To assess the mechanism of CCR2-mediated host defense in Gram-negative pneumonia, we compared the kinetics of mononuclear phagocytes in infected CCR2-deficient mice to respective wild-types. Similar to reports in mice infected with L. monocytogenes (51), we found a paradoxical expansion of bone marrow Ly6Chi monocytes in CCR2-deficient mice, which, together with the lack of expansion in the blood pool of these cells, is consistent with failure to mobilize Ly6Chi cells from the bone marrow to the blood during infection (Fig. 5, A and C). We found no significant difference in Ly6Clo monocytes between wild-type and CCR2-deficient hosts in the bone marrow, but the concentration of blood Ly6Clo monocytes was lower in CCR2-deficient hosts compared with wild-type animals (Fig. 5, B and D).
Fig. 5.
Comparison of blood and bone marrow monocyte kinetics between wild-type and CCR2-deficient mice with experimental Gram-negative pneumonia. Number of bone marrow (A and B) and concentration of blood (C and D) monocytes, defined as noted in Fig. 2, are shown at various times after intrapulmonary challenge with Klebsiella. Day 0 represents uninfected mice. Data shown represent means ± SE of representative data from 3 experiments; n = 4–5 for each group at each time point. *P < 0.05 comparing trend between the 2 groups over time; NS, no significant difference (2-way ANOVA).
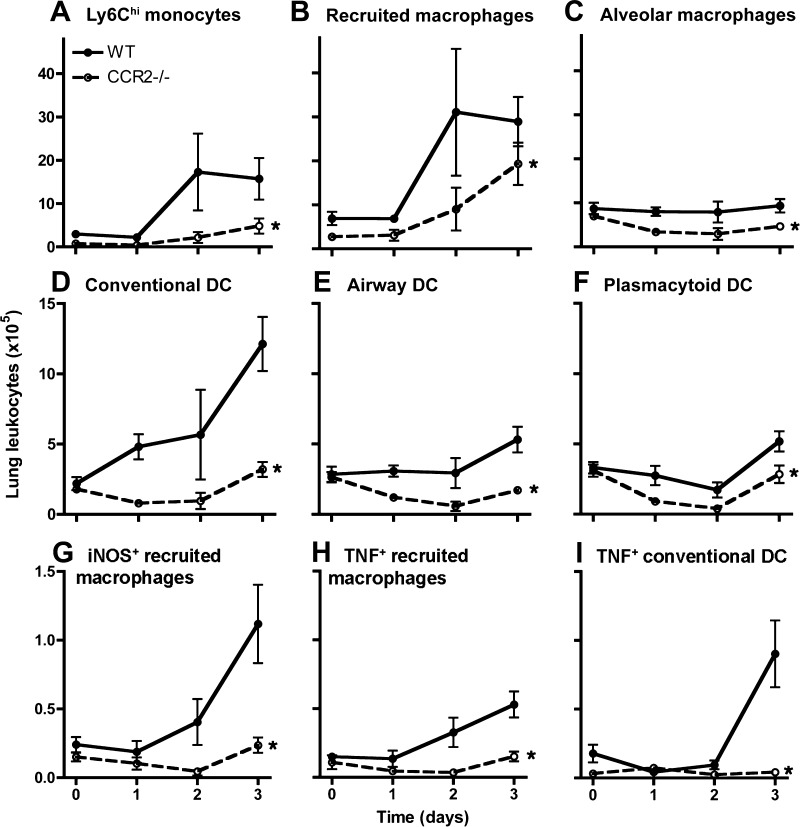
In the lungs, we found a profound defect in accumulation of all monocyte, macrophage, and DC subsets examined, which was most notable in lung Ly6Chi monocytes and CD11bhi DC (Fig. 6, A–F). The reduced numbers of alveolar macrophages were not attributable to increased rate of cell death in CCR2-deficient mice because the proportion of early apoptotic and dead alveolar macrophages in the bronchoalveolar lavage did not differ between infected wild-type and CCR2-deficient hosts (mean ± SE, 14.2 ± 1.0% vs. 17.6 ± 3.9% early apoptotic cells and 0.60 ± 0.1% vs. 0.55 ± 0.11% dead cells on day 1 of infection; 5–6 mice per group, P = 0.77 and 0.78, respectively). We also compared the activation phenotype of cells by assessing their expression of TNF and iNOS. Although there was an increase in lung-recruited macrophages in the CCR2-deficient mice (Fig. 6B), we found very few iNOS- or TNF-producing macrophages, consistent with the M1 phenotype, in the lungs of CCR2-deficient mice (Fig. 6, G and H). Similarly, we found essentially no TNF-producing CD11bhi DC in the lungs of CCR2-deficient mice (Fig. 6I). These data suggest that, in the context of pneumonia, the normal traffic of all examined mononuclear phagocytes is partially dependent on CCR2. Furthermore, they indicate that the absence of CCR2 results, not only in reduced number of lung mononuclear phagocytes, but also in markedly altered activation phenotype of recruited cells.
Fig. 6.
A–I: comparison of kinetics of lung monocytic phagocytes between wild-type and CCR2-deficient mice with experimental Gram-negative pneumonia. Cells enumerated in whole lung suspensions, identified as noted in Fig. 3, are shown at various times after intrapulmonary challenge with Klebsiella. Day 0 represents uninfected mice. *P < 0.05 comparing trend between the 2 groups over time (2-way ANOVA). Data shown represent means ± SE of representative data from 3 experiments; n = 4–5 for each group at each time point. iNOS, inducible nitric oxide synthase.
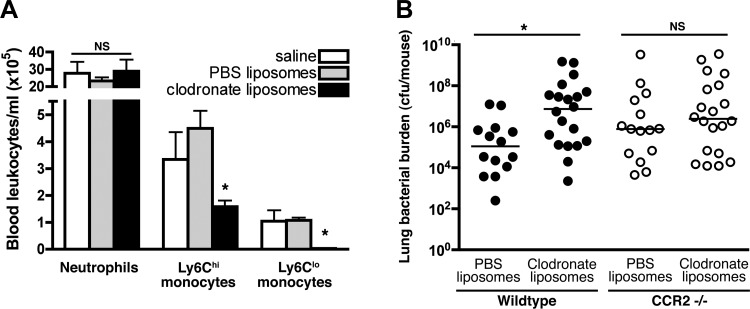
In addition to mononuclear phagocytes, CCR2 is also expressed by a subset of T cells and hematopoietic progenitor populations (33, 54), but we found no difference in the influx of activated T or B cells into the lungs of CCR2-deficient and wild-type animals with pneumonia (data not shown). To specifically test the hypothesis that the beneficial effect of CCR2 in Gram-negative pneumonia is mediated via mononuclear phagocytes, we sought to assess the effect of depletion of circulating monocytes on pathogen clearance in wild-type and CCR2-deficient animals with bacterial pneumonia. We first confirmed that, in the context of bacterial pneumonia, intravenous administration of clodronate liposomes resulted in partial depletion of circulating Ly6Chi monocytes and more complete depletion of LyClo monocytes but had no detectable effect on the number of circulating neutrophils (Fig. 7A). Administration of clodronate resulted in increased lung bacterial burden in wild-type, but not CCR2-deficient mice (Fig. 7B), indicating that monocytes are necessary for the beneficial effect of CCR2 on bacterial clearance.
Fig. 7.
Effect of depletion of circulating monocytes on CCR2-dependent host defense in mice with experimental Gram-negative pneumonia. A: comparison of blood neutrophil and monocyte concentration in mice treated with clodronate- or PBS-containing liposomes in mice on day 1 after intrapulmonary challenge with Klebsiella. Data represent means ± SE of representative data from 2 experiments; n = 3–5 for each group. B: lung viable bacterial content on day 3 after intrapulmonary challenge with Klebsiella in mice treated with clodronate- or PBS-containing liposomes. Each data point represents 1 animal, and horizontal lines indicate medians; pooled data from 3 experiments; *P < 0.05 (1-way ANOVA for A; Mann-Whitney for B).
Role of CCR2 in neutrophil-mediated host defense.
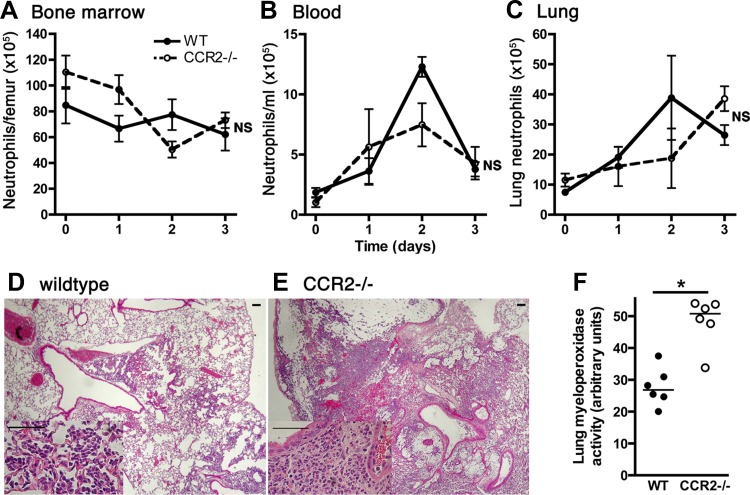
Several lines of evidence indicate a potential role for CCR2 in recruitment of neutrophils to inflamed tissues and, more broadly, that monocyte recruitment is necessary for optimal neutrophil recruitment (34, 57). Given the importance of neutrophils in Klebsiella pneumonia, we assessed the kinetics of neutrophil traffic in CCR2-deficient mice with bacterial pneumonia. We found no significant difference in the number of bone marrow, peripheral blood or lung neutrophils between wild-type and CCR2-deficient animals (Fig. 8, A–C). Histologically, lungs from both groups of animals showed multifocal alveolar filling with neutrophils and, to a lesser extent, mononuclear cells (Fig. 8, D–E). Lung myeloperoxidase activity, which is primarily attributable to neutrophils in this model, was higher in CCR2-deficient mice compared with wild-types on the third day of infection (Fig. 8F), consistent with somewhat higher neutrophil numbers in the lungs at this time point (Fig. 8C) and perhaps faster turnover of neutrophils in response to a higher burden of microorganisms (Fig. 4E). Taken together, these data suggest that CCR2 does not detectably impact the traffic of neutrophils in bacterial pneumonia.
Fig. 8.
Effect of CCR2 on neutrophil traffic in mice with experimental Gram-negative pneumonia. A–C: comparison of neutrophil numbers in the bone marrow, blood, and lung compartments at various times after intrapulmonary challenge with Klebsiella. Day 0 represents uninfected mice. NS, no significant difference between wild-type and CCR2-deficient hosts (2-way ANOVA). D and E: representative lung histological sections from wild-type and CCR2-deficient mice on day 3 of infection, stained with hematoxylin and eosin (original magnification ×40, scale bar = 100 μm) show alveolar consolidation. Insets: (original magnification ×400, scale bar = 50 μm) most of the intra-alveolar cells are neutrophils. F: lung myeloperoxidase activity on day 3 of infection. Each data point represents 1 animal and horizontal lines indicate medians; *P < 0.05 compared with wild-type group (Mann-Whitney).
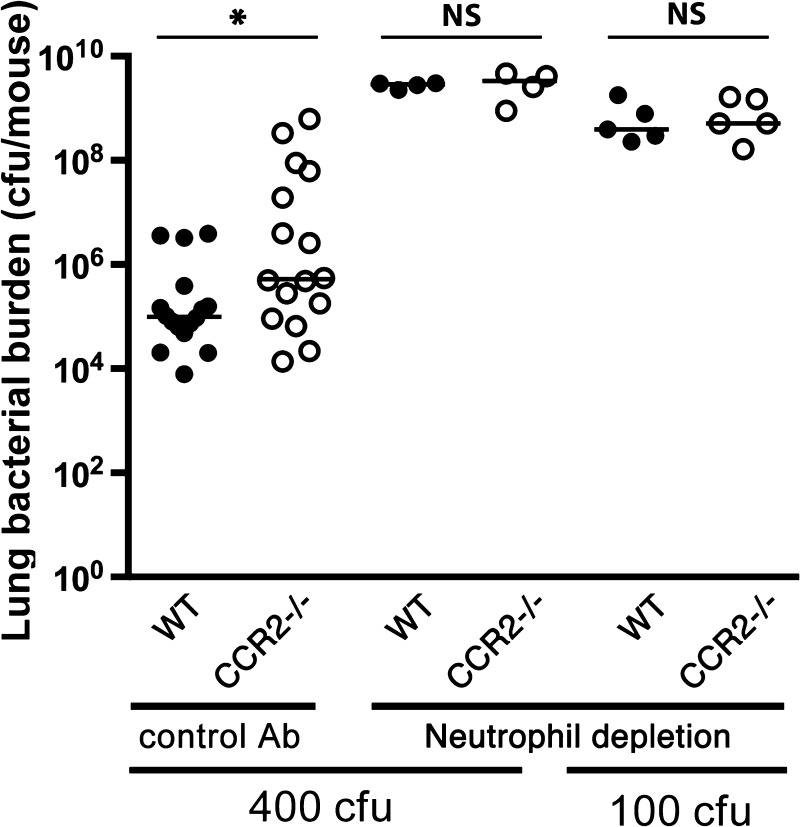
Having found no detectable effect of CCR2 to the traffic of neutrophils to the lungs, we lastly sought to assess the contribution of CCR2-mediated defense via the monocytic phagocytes relative to neutrophil-mediated defenses in hosts with Gram-negative pneumonia. As expected, neutrophil depletion rendered hosts highly susceptible to pneumonia, resulting in three to four orders of magnitude greater lung bacterial content in wild-type animals (Fig. 9). Depletion of neutrophils also resulted in increased bacterial burden in CCR2-deficient hosts, such that, in the context of neutrophil depletion, there was no difference in bacterial burden between wild-type and CCR2-deficient animals, even when challenged with a bacterial inoculum that is sublethal for wild-type nonneutropenic mice. These data suggest that the beneficial role of CCR2 in mediating monocytic phagocyte defenses is limited to hosts with intact neutrophil function.
Fig. 9.
Effect of neutrophil depletion on bacterial clearance in wild-type and CCR2-deficient animals with experimental Gram-negative pneumonia. Lung viable bacterial content on day 3 after intrapulmonary challenge with Klebsiella. Each data point represents 1 animal, and horizontal lines indicate medians; pooled data from 3 experiments; *P < 0.05; NS, no significant difference (Mann-Whitney).
DISCUSSION
The role of mononuclear phagocytes in antimicrobial host defense was defined in the intravenous inoculation model with the opportunistic Gram-positive intracellular bacterium, Listeria monocytogenes, where the primary sites of infection are the spleen and liver (51). Similar to the findings in other models, we report expansion of bone marrow and blood Ly6Chi monocytes in bacterial pneumonia and evidence for CCR2-dependent egress of these cells from the bone marrow to the blood. An unexpected finding in the present work is the broad-based reduction in the numbers of all examined cell types in the blood and lungs of CCR2-deficient hosts during infection. In this context, CCR2-deficient mice were previously reported to have fewer recruited lung macrophages in response to LPS and bacterial pneumonia (18, 35, 63); similarly, CCR2 was necessary for influx of Ly6Chi monocytes that gave rise to CD11bhi DCs in cryptococcosis (41), recruited macrophages, and CD11bhi DC in influenza (30). Interestingly, we found that CCR2 deficiency attenuated, but did not ablate, the traffic of mononuclear phagocytes to the lungs, suggesting that CCR2-independent mechanisms of mononuclear phagocyte recruitment to the lungs are operational but profoundly influenced the phenotype of the recruited macrophages and CD11bhi DCs. Specifically, iNOS- and TNF-producing recruited M1 macrophages and TNF-producing CD11bhi DC were essentially absent from the lungs of CCR2-deficient mice. Because nitric oxide and TNF are essential to host defense in this infection (26, 62), the absence of these cells is likely a key cause of worse outcomes in CCR2-deficient animals. Regarding the DC phenotype, the CD11bhi DC observed in wild-type infected lungs and absent from CCR2-deficient mice are similar to those in invasive pulmonary aspergillosis (42, 43) and differ from the TNF- and iNOS-producing DC (Tip-DC) first described in the spleens of animals with listeriosis (52) in that they do not express iNOS. At the early time points that we examined, we did not find any lung macrophages expressing M2 markers in either wild-type or CCR2-deficient mice, indicating that CCR2 is either necessary for recruitment of M1 macrophage precursors or for their development in the lung from uncommitted precursors.
Alveolar macrophages are critical to host defense in Gram-negative pneumonia (6), an effect that is likely independent of other lung mononuclear phagocyte populations. Several lines of evidence indicate that tissue-resident macrophages in general, and alveolar macrophages in particular, self-renew independent of bone marrow progenitors under steady state and Th2-inflammatory conditions (16, 20). However, our observation of high rate of alveolar macrophage apoptosis during pneumonia together with the reduced numbers of alveolar macrophages in CCR2-deficient animals with pneumonia is consistent with evidence for rapid turnover of alveolar macrophages described in pneumococcal pneumonia (60), and the replacement of alveolar macrophages from a blood precursor, via lung-recruited macrophage intermediates, after challenge with LPS (27). Because alveolar macrophages can be replenished by Ly6Clo blood monocytes (28), we speculate that the observed reduction in blood Ly6Clo monocytes in CCR2-deficient mice with pneumonia impairs the ability to replace dying alveolar macrophages during pneumonia, although our data do not preclude repopulation from nonmonocyte CCR2-expressing precursors (54). Interestingly, we noted a reduction in the number of Ly6Clo monocytes in the blood but not the bone marrow of CCR2-deficient mice with pneumonia, suggesting that, in the context of the infection, circulating Ly6Clo monocytes are derived from Ly6Chi counterparts in the blood rather than the bone marrow.
Our study demonstrates CCR6 and CX3CR1 to be dispensable for host defense in bacterial pneumonia. Although CX3CR1 has been implicated in some inflammatory settings (36, 59), it was not prominently expressed by lung mononuclear phagocytes during infection and was not essential to host defense in this setting. Prior data show that CCR6 is expressed by CD11bhi DC but also by most B cells, regulatory, and Th17 T cells and mediate their homing to epithelial surfaces expressing its high-affinity ligand, CCL20 (reviewed in Ref. 19). CCR6-mediated recruitment of CD11bhi DC to the lungs has been implicated in the pathogenesis of airway allergy (11, 32), cigarette-induced lung injury (5, 10), and viral and fungal infections (23, 46). On the other hand, similar to our findings in bacterial pneumonia, CCR6 was not expressed by lung CD11bhi DC and did not influence the response to helminth or mycobacterial antigens (9). In the context of bacterial pneumonia, we found CCR6 to be expressed by nearly all lung plasmacytoid DC; paradoxically, however, the homing of these cells to the lungs was dependent on CCR2. Several prior reports have documented the expression of CCR6 by blood plasmacytoid DC (8, 12) that home to mucosal associated lymphoid tissue (55); the role of these cells in bacterial pneumonia remains to be established.
Several lines of evidence support a cross-talk between neutrophils and mononuclear phagocytes at sites of inflammation; these interactions include mononuclear phagocyte-mediated neutrophil recruitment, neutrophil-mediated recruitment of inflammatory mononuclear cells, and functional modifications of mononuclear phagocytes after arriving in tissue, as recently reviewed (56). In contrast to prior work that implicated CCL2 in recruitment of neutrophils to the lungs in response to LPS or Gram-negative bacteria (1, 2, 34), we found no impairment in neutrophil recruitment to the lungs of CCR2-deficient animals with K. pneumoniae pneumonia. In fact, the much higher levels of lung myeloperoxidase in CCR2-deficient mice with pneumonia (Fig. 8F) support the contention that, in response to the higher bacterial burden, the process of recruitment, degranulation, and death of neutrophils is enhanced, and certainly not diminished, in this setting. This discrepancy may be explained by the marked differences between the biology of CCL2- and CCR2-deficient animals, as noted in other models (38, 49, 61), and by the observation that CCL2-deficient animals display a greater protection against Klebsiella pneumonia compared with CCR2-deficient mice (Fig. 4). Although we found no evidence of monocyte- or CCR2-mediated neutrophil recruitment, we report that neutrophils were necessary for the beneficial role of CCR2 to be observed. This observation supports a functional cooperation between neutrophils and mononuclear phagocytes in antibacterial host defenses in the lung.
The present work has a number of implications for future research. First, although we provide evidence for the importance of the mononuclear phagocyte system in bacterial pneumonia, the contribution of individual cell types, such as Ly6Clo monocytes, CD103 DC, and plasmacytoid DC, requires further study. Second, the relative contribution of CCR2 ligands to host defense in bacterial pneumonia awaits characterization. Specifically, CCR2 binds CCL2, CCL7, and CCL12 in mice and CCL2, CCL7, CCL8, CCL13, and CCL18 in humans. Although the relative contribution of these ligands in protection against pneumonia is not known, contrary to recent reports (1), our data indicate that CCL2 is dispensable to host defense in this infection. Finally, studies aimed at therapeutic agonism of CCR2 or increasing the pool of mononuclear phagocytes available for recruitment, as a potential therapeutic target may lead to novel treatments for bacterial pneumonia.
GRANTS
This work was supported by NIH grants HL073848 and HL098329.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: L.C., Z.Z., K.E.B., M.D.B., and B.M. performed experiments; L.C. and B.M. analyzed data; L.C., M.D.B., and B.M. interpreted results of experiments; L.C. and B.M. prepared figures; L.C., Z.Z., K.E.B., M.D.B., and B.M. approved final version of manuscript; M.D.B. and B.M. edited and revised manuscript; B.M. conception and design of research; B.M. drafted manuscript.
REFERENCES
- 1.Balamayooran G, Batra S, Balamayooran T, Cai S, Jeyaseelan S. Monocyte chemoattractant protein 1 regulates pulmonary host defense via neutrophil recruitment during Escherichia coli infection. Infect Immun 79: 2567–2577, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balamayooran G, Batra S, Theivanthiran B, Cai S, Pacher P, Jeyaseelan S. Intrapulmonary G-CSF rescues neutrophil recruitment to the lung and neutrophil release to blood in Gram-negative bacterial infection in MCP-1−/− mice. J Immunol 189: 5849–5859, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barletta KE, Cagnina RE, Burdick MD, Linden J, Mehrad B. Adenosine A(2B) receptor deficiency promotes host defenses against gram-negative bacterial pneumonia. Am J Respir Crit Care Med 186: 1044–1050, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barletta KE, Cagnina RE, Wallace KL, Ramos SI, Mehrad B, Linden J. Leukocyte compartments in the mouse lung: distinguishing between marginated, interstitial, and alveolar cells in response to injury. J Immunol Methods 375: 100–110, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bracke KR, D'Hulst AI, Maes T, Moerloose KB, Demedts IK, Lebecque S, Joos GF, Brusselle GG. Cigarette smoke-induced pulmonary inflammation and emphysema are attenuated in CCR6-deficient mice. J Immunol 177: 4350–4359, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Broug-Holub E, Toews GB, van Iwaarden JF, Strieter RM, Kunkel SL, Paine R, 3rd, Standiford TJ. Alveolar macrophages are required for protective pulmonary defenses in murine Klebsiella pneumonia: elimination of alveolar macrophages increases neutrophil recruitment but decreases bacterial clearance and survival. Infect Immun 65: 1139–1146, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cavaillon JM. The historical milestones in the understanding of leukocyte biology initiated by Elie Metchnikoff. J Leukoc Biol 90: 413–424, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Charles J, Di Domizio J, Salameire D, Bendriss-Vermare N, Aspord C, Muhammad R, Lefebvre C, Plumas J, Leccia MT, Chaperot L. Characterization of circulating dendritic cells in melanoma: role of CCR6 in plasmacytoid dendritic cell recruitment to the tumor. J Invest Dermatol 130: 1646–1656, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Chiu BC, Freeman CM, Stolberg VR, Hu JS, Zeibecoglou K, Lu B, Gerard C, Charo IF, Lira SA, Chensue SW. Impaired lung dendritic cell activation in CCR2 knockout mice. Am J Pathol 165: 1199–1209, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demedts IK, Bracke KR, Van Pottelberge G, Testelmans D, Verleden GM, Vermassen FE, Joos GF, Brusselle GG. Accumulation of dendritic cells and increased CCL20 levels in the airways of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 175: 998–1005, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Dua B, Watson RM, Gauvreau GM, O'Byrne PM. Myeloid and plasmacytoid dendritic cells in induced sputum after allergen inhalation in subjects with asthma. J Allergy Clin Immunol 126: 133–139, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Farrell E, O'Connor TM, Duong M, Watson RM, Strinich T, Gauvreau GM, O'Byrne PM. Circulating myeloid and plasmacytoid dendritic cells after allergen inhalation in asthmatic subjects. Allergy 62: 1139–1145, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Geissmann F, Gordon S, Hume DA, Mowat AM, Randolph GJ. Unravelling mononuclear phagocyte heterogeneity. Nat Rev Immunol 10: 453–460, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.GeurtsvanKessel CH, Lambrecht BN. Division of labor between dendritic cell subsets of the lung. Mucosal Immunol 1: 442–450, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Greenberger MJ, Strieter RM, Kunkel SL, Danforth JM, Goodman RE, Standiford TJ. Neutralization of IL-10 increases survival in a murine model of Klebsiella pneumonia. J Immunol 155: 722–729, 1995 [PubMed] [Google Scholar]
- 16.Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, Leboeuf M, Becker CD, See P, Price J, Lucas D, Greter M, Mortha A, Boyer SW, Forsberg EC, Tanaka M, van Rooijen N, Garcia-Sastre A, Stanley ER, Ginhoux F, Frenette PS, Merad M. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity 38: 792–804, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hashimoto D, Miller J, Merad M. Dendritic cell and macrophage heterogeneity in vivo. Immunity 35: 323–335, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herold S, Tabar TS, Janssen H, Hoegner K, Cabanski M, Lewe-Schlosser P, Albrecht J, Driever F, Vadasz I, Seeger W, Steinmueller M, Lohmeyer J. Exudate macrophages attenuate lung injury by the release of IL-1 receptor antagonist in gram-negative pneumonia. Am J Respir Crit Care Med 183: 1380–1390, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Ito T, Carson WFt Cavassani KA, Connett JM, Kunkel SL. CCR6 as a mediator of immunity in the lung and gut. Exp Cell Res 317: 613–619, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, van Rooijen N, MacDonald AS, Allen JE. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science 332: 1284–1288, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jia T, Serbina NV, Brandl K, Zhong MX, Leiner IM, Charo IF, Pamer EG. Additive roles for MCP-1 and MCP-3 in CCR2-mediated recruitment of inflammatory monocytes during Listeria monocytogenes infection. J Immunol 180: 6846–6853, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol 20: 4106–4114, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kallal LE, Schaller MA, Lindell DM, Lira SA, Lukacs NW. CCL20/CCR6 blockade enhances immunity to RSV by impairing recruitment of DC. Eur J Immunol 40: 1042–1052, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klevens RM, Edwards JR, Richards CL, Jr, Horan TC, Gaynes RP, Pollock DA, Cardo DM. Estimating health care-associated infections and deaths in US hospitals, 2002. Public Health Rep 122: 160–166, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuziel WA, Morgan SJ, Dawson TC, Griffin S, Smithies O, Ley K, Maeda N. Severe reduction in leukocyte adhesion and monocyte extravasation in mice deficient in CC chemokine receptor 2. Proc Natl Acad Sci USA 94: 12053–12058, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laichalk LL, Kunkel SL, Strieter RM, Danforth JM, Bailie MB, Standiford TJ. Tumor necrosis factor mediates lung antibacterial host defense in murine Klebsiella pneumonia. Infect Immun 64: 5211–5218, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Landsman L, Jung S. Lung macrophages serve as obligatory intermediate between blood monocytes and alveolar macrophages. J Immunol 179: 3488–3494, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Landsman L, Varol C, Jung S. Distinct differentiation potential of blood monocyte subsets in the lung. J Immunol 178: 2000–2007, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Lesnik P, Haskell CA, Charo IF. Decreased atherosclerosis in CX3CR1−/− mice reveals a role for fractalkine in atherogenesis. J Clin Invest 111: 333–340, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin KL, Suzuki Y, Nakano H, Ramsburg E, Gunn MD. CCR2+ monocyte-derived dendritic cells and exudate macrophages produce influenza-induced pulmonary immune pathology and mortality. J Immunol 180: 2562–2572, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Lu B, Rutledge BJ, Gu L, Fiorillo J, Lukacs NW, Kunkel SL, North R, Gerard C, Rollins BJ. Abnormalities in monocyte recruitment and cytokine expression in monocyte chemoattractant protein 1-deficient mice. J Exp Med 187: 601–608, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lundy SK, Lira SA, Smit JJ, Cook DN, Berlin AA, Lukacs NW. Attenuation of allergen-induced responses in CCR6−/− mice is dependent upon altered pulmonary T lymphocyte activation. J Immunol 174: 2054–2060, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Mack M, Cihak J, Simonis C, Luckow B, Proudfoot AE, Plachy J, Bruhl H, Frink M, Anders HJ, Vielhauer V, Pfirstinger J, Stangassinger M, Schlondorff D. Expression and characterization of the chemokine receptors CCR2 and CCR5 in mice. J Immunol 166: 4697–4704, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Maus UA, Waelsch K, Kuziel WA, Delbeck T, Mack M, Blackwell TS, Christman JW, Schlondorff D, Seeger W, Lohmeyer J. Monocytes are potent facilitators of alveolar neutrophil emigration during lung inflammation: role of the CCL2-CCR2 axis. J Immunol 170: 3273–3278, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Maus UA, Wellmann S, Hampl C, Kuziel WA, Srivastava M, Mack M, Everhart MB, Blackwell TS, Christman JW, Schlondorff D, Bohle RM, Seeger W, Lohmeyer J. CCR2-positive monocytes recruited to inflamed lungs downregulate local CCL2 chemokine levels. Am J Physiol Lung Cell Mol Physiol 288: L350–L358, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Medina-Contreras O, Geem D, Laur O, Williams IR, Lira SA, Nusrat A, Parkos CA, Denning TL. CX3CR1 regulates intestinal macrophage homeostasis, bacterial translocation, and colitogenic Th17 responses in mice. J Clin Invest 121: 4787–4795, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mehrad B, Park SJ, Akangire G, Standiford TJ, Wu T, Zhu J, Mohan C. The lupus-susceptibility locus, Sle3, mediates enhanced resistance to bacterial infections. J Immunol 176: 3233–3239, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Moore BB, Murray L, Das A, Wilke CA, Herrygers AB, Toews GB. The role of CCL12 in the recruitment of fibrocytes and lung fibrosis. Am J Respir Cell Mol Biol 35: 175–181, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muscedere JG, Day A, Heyland DK. Mortality, attributable mortality, and clinical events as end points for clinical trials of ventilator-associated pneumonia and hospital-acquired pneumonia. Clin Infect Dis 51, Suppl 1: S120–S125, 2010 [DOI] [PubMed] [Google Scholar]
- 40.Nordmann P, Naas T, Poirel L. Global spread of Carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis 17: 1791–1798, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Osterholzer JJ, Chen GH, Olszewski MA, Curtis JL, Huffnagle GB, Toews GB. Accumulation of CD11b+ lung dendritic cells in response to fungal infection results from the CCR2-mediated recruitment and differentiation of Ly-6Chigh monocytes. J Immunol 183: 8044–8053, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park SJ, Burdick MD, Brix WK, Stoler MH, Askew DS, Strieter RM, Mehrad B. Neutropenia enhances lung dendritic cell recruitment in response to Aspergillus via a cytokine-to-chemokine amplification loop. J Immunol 185: 6190–6197, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park SJ, Burdick MD, Mehrad B. Neutrophils mediate maturation and efflux of lung dendritic cells in response to Aspergillus fumigatus germ tubes. Infect Immun 80: 1759–1765, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park SJ, Wiekowski MT, Lira SA, Mehrad B. Neutrophils regulate airway responses in a model of fungal allergic airways disease. J Immunol 176: 2538–2545, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Peleg AY, Hooper DC. Hospital-acquired infections due to gram-negative bacteria. N Engl J Med 362: 1804–1813, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Phadke AP, Akangire G, Park SJ, Lira SA, Mehrad B. The role of CC chemokine receptor 6 in host defense in a model of invasive pulmonary aspergillosis. Am J Respir Crit Care Med 175: 1165–1172, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qiu B, Frait KA, Reich F, Komuniecki E, Chensue SW. Chemokine expression dynamics in mycobacterial (type-1) and schistosomal (type-2) antigen-elicited pulmonary granuloma formation. Am J Pathol 158: 1503–1515, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qiu Y, Zeltzer S, Zhang Y, Wang F, Chen GH, Dayrit J, Murdock BJ, Bhan U, Toews GB, Osterholzer JJ, Standiford TJ, Olszewski MA. Early induction of CCL7 downstream of TLR9 signaling promotes the development of robust immunity to cryptococcal infection. J Immunol 188: 3940–3948, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Quinones MP, Estrada CA, Jimenez F, Martinez H, Willmon O, Kuziel WA, Ahuja SK, Ahuja SS. CCL2-independent role of CCR2 in immune responses against Leishmania major. Parasite Immunol 29: 211–217, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Saederup N, Chan L, Lira SA, Charo IF. Fractalkine deficiency markedly reduces macrophage accumulation and atherosclerotic lesion formation in CCR2−/− mice: evidence for independent chemokine functions in atherogenesis. Circulation 117: 1642–1648, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol 7: 311–317, 2006 [DOI] [PubMed] [Google Scholar]
- 52.Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity 19: 59–70, 2003 [DOI] [PubMed] [Google Scholar]
- 53.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol 11: 762–774, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Si Y, Tsou CL, Croft K, Charo IF. CCR2 mediates hematopoietic stem and progenitor cell trafficking to sites of inflammation in mice. J Clin Invest 120: 1192–1203, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sisirak V, Vey N, Vanbervliet B, Duhen T, Puisieux I, Homey B, Bowman EP, Trinchieri G, Dubois B, Kaiserlian D, Lira SA, Puisieux A, Blay JY, Caux C, Bendriss-Vermare N. CCR6/CCR10-mediated plasmacytoid dendritic cell recruitment to inflamed epithelia after instruction in lymphoid tissues. Blood 118: 5130–5140, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Soehnlein O, Lindbom L. Phagocyte partnership during the onset and resolution of inflammation. Nat Rev Immunol 10: 427–439, 2010 [DOI] [PubMed] [Google Scholar]
- 57.Souto FO, Alves-Filho JC, Turato WM, Auxiliadora-Martins M, Basile-Filho A, Cunha FQ. Essential role of CCR2 in neutrophil tissue infiltration and multiple organ dysfunction in sepsis. Am J Respir Crit Care Med 183: 234–242, 2010 [DOI] [PubMed] [Google Scholar]
- 58.Sung SS, Fu SM, Rose CE, Jr, Gaskin F, Ju ST, Beaty SR. A major lung CD103 (alphaE)-beta7 integrin-positive epithelial dendritic cell population expressing Langerin and tight junction proteins. J Immunol 176: 2161–2172, 2006 [DOI] [PubMed] [Google Scholar]
- 59.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, Lira SA, Habenicht AJ, Randolph GJ. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest 117: 185–194, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Taut K, Winter C, Briles DE, Paton JC, Christman JW, Maus R, Baumann R, Welte T, Maus UA. Macrophage turnover kinetics in the lungs of mice infected with Streptococcus pneumoniae. Am J Respir Cell Mol Biol 38: 105–113, 2008 [DOI] [PubMed] [Google Scholar]
- 61.Traynor TR, Herring AC, Dorf ME, Kuziel WA, Toews GB, Huffnagle GB. Differential roles of CC chemokine ligand 2/monocyte chemotactic protein-1 and CCR2 in the development of T1 immunity. J Immunol 168: 4659–4666, 2002 [DOI] [PubMed] [Google Scholar]
- 62.Tsai WC, Strieter RM, Zisman DA, Wilkowski JM, Bucknell KA, Chen GH, Standiford TJ. Nitric oxide is required for effective innate immunity against Klebsiella pneumoniae. Infect Immun 65: 1870–1875, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Winter C, Herbold W, Maus R, Langer F, Briles DE, Paton JC, Welte T, Maus UA. Important role for CC chemokine ligand 2-dependent lung mononuclear phagocyte recruitment to inhibit sepsis in mice infected with Streptococcus pneumoniae. J Immunol 182: 4931–4937, 2009 [DOI] [PubMed] [Google Scholar]
- 64.Yang D, Chertov O, Bykovskaia SN, Chen Q, Buffo MJ, Shogan J, Anderson M, Schroder JM, Wang JM, Howard OM, Oppenheim JJ. Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science 286: 525–528, 1999 [DOI] [PubMed] [Google Scholar]