Abstract
Airway smooth muscle hyperresponsiveness is a key component in the pathophysiology of asthma. Although calcium-activated chloride channel (CaCC) flux has been described in many cell types, including human airway smooth muscle (HASM), the true molecular identity of the channels responsible for this chloride conductance remains controversial. Recently, a new family of proteins thought to represent the true CaCCs was identified as the TMEM16 family. This led us to question whether members of this family are functionally expressed in native and cultured HASM. We further questioned whether expression of these channels contributes to the contractile function of HASM. We identified the mRNA expression of eight members of the TMEM16 family in HASM cells and show immunohistochemical evidence of TMEM16A in both cultured and native HASM. Functionally, we demonstrate that the classic chloride channel inhibitor, 5-nitro-2-(3-phenylpropylamino)benzoic acid (NPPB), inhibited halide flux in cultured HASM cells. Moreover, HASM cells displayed classical electrophysiological properties of CaCCs during whole cell electrophysiological recordings, which were blocked by using an antibody selective for TMEM16A. Furthermore, two distinct TMEM16A antagonists (tannic acid and benzbromarone) impaired a substance P-induced contraction in isolated guinea pig tracheal rings. These findings demonstrate that multiple members of this recently described family of CaCCs are expressed in HASM cells, they display classic electrophysiological properties of CaCCs, and they modulate contractile tone in airway smooth muscle. The TMEM16 family may provide a novel therapeutic target for limiting airway constriction in asthma.
Keywords: RT-PCR, MQAE, immunohistochemistry, electrophysiology, airway smooth muscle
asthma is a chronic disease with acute exacerbations of reversible airway narrowing, airway hyperresponsiveness, and inflammation affecting over 6 million Americans and costing over 56 billion dollars in 2009 (22). Since the 1980s, the prevalence and number of persons with asthma has steadily increased, suggesting that novel therapeutic approaches are needed to curtail this debilitating disease. One therapeutic approach involves the pharmacological manipulation of airway smooth muscle tone. Airway smooth muscle hyperresponsiveness is recognized as a major component of asthma.
The efflux of chloride from the airway smooth muscle cell following an exposure to a contractile mediator and an increase in intracellular calcium contributes to airway smooth muscle membrane depolarization (14, 16, 17, 19, 21). The relevance of chloride flux-induced membrane depolarization to functional smooth muscle contraction has been questioned (12, 13), but evidence exists for a link between calcium sparks, calcium-activated chloride channel (CaCC) activity resulting in spontaneous transient inward currents (STICs), and airway smooth muscle single cell contraction (15, 32). However, evidence for the functional effects of CaCC blockade in intact airway smooth muscle is limited (11).
Previously, chloride flux including calcium-induced chloride flux in airway smooth muscle was presumed to be occur via proteins of the ClCa, CLC-3, bestrophin, and Tweety families (5). However, in reconstituted cell systems these proteins did not demonstrate defining characteristics of a voltage-gated, calcium-sensing chloride channel. More specifically, these proteins failed to demonstrate requisite activation under induced membrane potential changes and/or an outward rectifying current at submaximal Ca2+ concentrations. These studies created considerable uncertainty as to the molecular identity of the channel responsible for calcium-mediated enhancement of chloride efflux in many cell types. Electrophysiological properties expected of a CaCC include an increase of intracellular calcium inducing an increase in a delayed outward rectifying Cl− current. Additionally, characteristics of CaCCs include sensitivity to Cl− channel blockers, small conductances, a voltage-dependent Ca2+ sensitivity (31), and a halide flux sensitivity of I− > Br− > Cl− > F− (7). These criteria were satisfied in 2008 when three separate laboratories discovered that one member of the TMEM16 or anoctamin family of proteins demonstrated electrophysiological and pharmacological characteristics typical of CaCCs (2, 26, 31). These seminal discoveries have been followed by rapid progress in the understanding of the molecular and biophysical characteristics of these ten family members. It appears that TMEM16A and TMEM16B are true CaCCs whereas the remaining eight members of the family may not be associated with the plasma membrane or have chloride-conducting properties (4, 30). In the present study we questioned 1) whether members of the TMEM16 family of CaCCs are expressed in human airway smooth muscle (HASM) cells, 2) whether HASM cells demonstrate classic electrophysiological evidence of CaCC activity, 3) whether members of this family are capable of generating a chloride flux in HASM, and 4) whether pharmacological targeting of these CaCCs may serve to relax tachykinin-mediated airway smooth muscle contractions.
METHODS
Materials.
N-(ethoxycarbonylmethyl)-6-methoxyquinolinium bromide (MQAE) and all cell culture reagents were purchased from Invitrogen (Carlsbad, CA). TRIzol reagent was obtained from Ambion-Applied Biosystems (Austin, TX). Conventional PCR and RT-PCR reagents were purchased from Clontech Laboratories (Mountain View, CA). Tannic acid and all other reagents were purchased from Sigma Aldrich (St. Louis, MO) unless otherwise indicated.
Cell culture.
All human airway tissue protocols were reviewed by the Columbia University Institutional Review Board (IRB) and were deemed not human subjects research under 45 CFR 46. Primary human bronchial smooth muscle cells were enzymatically dissociated by using a papain-collagenase kit according to the manufacturer's recommendations (Worthington). Additionally, human bronchial smooth muscle cells modified to stably express human telomerase reverse transcriptase were a kind gift from Dr. William Gerthoffer (University of South Alabama, Mobile, AL) (6). Smooth muscle cells were grown in SmBMII medium with manufacturer's recommended additives (Lonza, Walkersville, MD) or M199 medium containing 10% fetal bovine serum, 0.25 ng/ml epidermal growth factor, 1 ng/ml fibroblast growth factor, ITS supplement (1 mg/ml insulin, 0.55 mg/ml transferrin, 0.67 μg/ml sodium selenium), and antibiotics (100 units/ml penicillin G sodium, 100 μg/ml streptomycin sulfate, 0.25 μg/ml amphotericin B). Immortalized human airway epithelial cells (BEAS2B) cells were purchased from Lonza and maintained in BEBM medium (Lonza) with all additives recommended by the manufacturer. Cells were grown to confluence at 37°C in 95% air-5% CO2 in T75 cm2 flasks for total RNA or protein extraction, or in 96-well black-walled clear-bottom plates for MQAE halide flux. Cells for electrophysiological studies were plated on Thermanox 13-mm plastic coverslips (NUNC, Rochester, NY), coated with 0.5 mg/ml collagen type I.
Isolation of smooth muscle from human trachea.
All human airway tissue protocols were reviewed by the Columbia University IRB and were deemed not human subjects research under 45 CFR 46. Human tracheal tissue was obtained from discarded airway from healthy lung donors during transplantation surgery at Columbia University. The tracheal smooth muscle layer was removed by fine dissection of the airway and processed for immediate RNA extraction. Human whole brain, retina, stomach, skeletal muscle, total lung, trachea, and spinal cord total RNA was purchased from Clontech Laboratories to be used as RT-PCR positive controls.
RNA extraction and reverse transcription of cDNA.
Total RNA was extracted from native and cultured HASM cells using TRIzol (Ambion-Applied Biosystems) according to the manufacturer's recommendations. RNA purity and quantity was measured by 260/280 nm absorbance (Beckman Du 640). Total RNA from cultured or native human tracheal airway smooth muscle was diluted in RNAse-free water to a final concentration 500 μg/ml, aliquoted, and stored at −80°C. Complementary DNA synthesis was performed (2 μg total RNA in a final volume of 20 μl) with Superscript Vilo cDNA synthesis kit (Invitrogen) following the manufacturer's recommendations.
Conventional PCR.
Newly synthesized cDNA (5 μl of original 20-μl reaction) was used in PCR with the Advantage 2 PCR Kit (Clontech) on a MJ Research PTC-200 Peltier thermal cycler (Bio-Rad, Hercules, CA) following the manufacturer's protocol. Sense and antisense primers specific for 10 members of the TMEM family (TMEM 16A-K) were constructed by primer blast selecting default parameters and spanning an exon-exon junction (Table 1) and were purchased from Invitrogen. All cDNA samples were initially denatured at 94°C for 30 s. Optimal annealing temperatures for each primer set were initially established by using human cDNA from brain, retina, stomach, skeletal muscle, lung, and whole trachea purchased from Clontech and using 1-min annealing duration and 30-s extension duration for 40 cycles of PCR. The annealing temperatures for TMEM16A, B, C, D, E, F, G/H, I, J, and K were 64, 72, 72, 72, 69, 68, 69, 69, 68, and 68°C, respectively. For PCR, there was a completion step of 30 s. All PCR products were analyzed on 10% polyacrylamide gels stained with ethidium bromide (Molecular Probes, Eugene, OR) and visualized by use of a gel imager and Visioworks software (Biospectra UVP, Upland, CA). Samples were analyzed by loading all (5–10 μl) samples for a single TMEM16 subtype on a single gel.
Table 1.
PCR primers for TMEM16 family members
| Target | Human Accession Numbers | Sequence: 5′-to-3′ | Expected Base Pair Size | |
|---|---|---|---|---|
| TMEM16A | NM_018043 | s | GAGCCGCCGTGGTCGGAAAA | 416 |
| as | GGGAGAGGGTGCCCATCGGT | |||
| TMEM16B | NM_020373 | s | GAGCTGAGACGGCCGGATGC | 556 |
| as | CCCCACCTCCTGGGCTCCTC | |||
| TMEM16C | NM_031418 | s | TGCCAGCCCGAGCAACTGAC | 290 |
| as | TTTGGAACTCCAGGGCGGGC | |||
| TMEM16D | NM_178826 | s | TGGGGAGGGGGAGGACCAGT | 390 |
| as | CTGGGCGGCTTGCCACACTT | |||
| TMEM16E | NM_213599 | s | AGTGAGCAGAGCCTGAGCAGC | 413 |
| as | GGAGTGTGCTTAGGGCGGGG | |||
| TMEM16F | NM_001025356 | s | CCCCTGAGCCCAAGCGACAC | 755 |
| as | ATGCGGCTTCTGGTGGCTGG | |||
| TMEM16G/H | NM_001001891 | s | AGTGGTGGGCACACTGGTGTT | 141 |
| as | GCGCTGGAGAGCAGCCAGAAA | |||
| TMEM16I | NM_020959 | s | GCCAAGCAGGGAGAAGCACTCCACAA | 143 |
| as | ACCCATGACTTCATGAGGCGGTTCAGAATA | |||
| TMEM16J | NM_001012302 | s | AAGAAGACGTGGGCGCGGTG | 597 |
| as | GGAGGCCAGGACGCGGTAGA | |||
| TMEM16K | NM_018075 | s | GTGGAGCACGCACTCCTGGC | 373 |
| as | CCGAGCCAAGCCACTGCGAA |
s, Sense; as, antisense.
Western blot analysis.
All tissues and cells utilized for Western blot analysis (immortalized and primary human tracheal airway smooth muscle cells, BEAS2B, native human and guinea pig tracheal airway smooth muscle) were homogenized in ice-cold lysis buffer [50 mM Tris·HCl, pH 8.0, 1% Nonidet P-40, 0.1% SDS, 150 mM NaCl, 1 mM EDTA, 1:200 dilution of protease inhibitor cocktail III (Calbiochem)]. Following centrifugation (2,900 g, 20 min, 4°C) of the whole cell or tissue lysate, the supernatants were saved and protein concentrations were determined. Aliquots of the supernatants were solubilized by heating at 95°C for 5 min in sample buffer (final concentrations: 50 mM Tris·HCl, pH 6.8, 2.5% SDS, 6% glycerol, 2.5% 2-mercaptoethanol, and bromphenol blue). The supernatants of solubilized whole cell or tissue lysates were electrophoresed through a 7.5% Mini-PROTEAN TGX gel (Bio-Rad) and transferred to PVDF and probed with TMEM16A antibody (prediluted rabbit polyclonal was further diluted to 1:5, no. ab53213; Abcam, Cambridge, MA). Equivalent loading was confirmed by commensurate probing with β-actin (rabbit monoclonal diluted to 1:20,000, no. 4970; Cell Signaling, Cell Signaling Technology, Danvers, MA). The primary antibodies were detected by horseradish peroxidase-conjugated donkey anti-rabbit antibodies (1:5,000 for TMEM16A and 1:30,000 for β-actin). The signal from the immunoreactive bands was detected by enhanced chemiluminescence (SuperSignal West Femto; Thermo Scientific, no. 34095, Rockford, IL) according to the manufacturer's recommendations, developed on film (HyBlot CL Autoradiography Film; Denville Scientific, Metuchen, NJ), and analyzed using Vision Works LS software (UVP, Upland, CA).
Immunohistochemistry of HASM for TMEM16A protein expression.
Freshly obtained surgical discards of human trachea and first generation bronchi deemed as waste were utilized in accordance with IRB permission. Airway smooth muscle strips were immediately fixed in 4% paraformaldehyde (4°C overnight), then incubated in 30% sucrose in PBS for an additional 24 h prior to processing for cryostat sectioning (6 μm). The sections were washed in PBS, incubated with 0.1% Triton X-100 for 10 min, blocked with 15% goat serum and then incubated overnight at 4°C in primary antisera. In parallel experiments, a human telomerized airway smooth muscle cell line was cultured on coverslips in SmBMII smooth muscle medium (Lonza) for 2–3 days then fixed with 3% paraformaldehyde in PBS for 5 min. The primary antibodies used were 1) anti-TMEM16A (rabbit, monoclonal; Abcam no. ab64085, 1:100 dilution in PBS) for cultured cell immunocytochemistry, 2) anti-TMEM16A (rabbit polyclonal; Abcam no. ab53213, 1:100 dilution in PBS) for intact HASM tissue immunohistochemistry, and 3) anti-α smooth muscle actin (mouse, monoclonal; Sigma-Aldrich, no. A2547, 1:10,000 dilution in PBS). The secondary antibodies consisted of FITC-conjugated goat anti-rabbit IgG (1:400 dilution) and Alexa Fluor 594 goat anti-mouse IgG (1:400 dilution; Invitrogen) incubated for 1 h. Nuclear staining was achieved by employing mounting medium premixed with DAPI stain (Vector Laboratories, no. H-1500). Negative controls were performed on serial sections by omitting primary antibody. All the immunofluorescence experiments were repeated at least three times. Samples were viewed under confocal microscopy (Nikon Eclipse, Japan) and images were acquired with NIS software version 4.10.
Patch-clamp electrophysiological studies in HASM cells.
HASM cells were cultured in SmBMII smooth muscle medium (Lonza). For patch-clamp analysis, cells were placed on poly-l-lysine 12-mm coverslips (BD), coated with 0.5 mg/ml collagen type I (Sigma). The coverslips were transferred to a 0.5 ml chamber on the stage of an inverted microscope (Nikon). Membrane currents were recorded using whole cell configuration. The extracellular solution contained (in mM) 130 NaCl, 5.5 tetraethylammonium (TEA)-Cl, 2.2 CaCl2, 1 MgCl2, 10 HEPES, 10 glucose, pH adjusted to 7.35 with NaOH. The pipette solution contained (in mM): 75 CsCl, 64 Cs-gluconate, 1 MgC12, 10 HEPES, 3 Na2ATP, pH adjusted to pH 7.3 with CsOH (1). Cesium and TEA were used in intracellular and extracellular solutions, respectively, to block K+ channels to allow for the study of chloride channel activity. Whole-cell currents were recorded by using Axopatch 200B and digitized by use of 1322A. Patch pipette had a resistance of 3–6 MΩ. All recordings were performed at room temperature. Events were counted as STICs if their amplitude was two- to fourfold of the baseline as detected with pCLAMP10 and analysis by Origin 8 software; data are expressed as a percent of control. Niflumic acid (NFA) and 5-nitro-2-(3-phenylpropylamino) benzoic acid (NPPB) were dissolved in DMSO. The final concentration of DMSO was less than 0.05%. To demonstrate the effect of TMEM16A-specific blockade, we employed a functional antibody-mediated live cell blockade as described elsewhere (28). Cells were incubated for 2 h (in 37°C, 5% CO2) with either TMEM16A antibody (Abcam ab53213, 1:5 dilution) or with the vehicle diluent used in the antibody as a control (0.1% sodium azide, 1% BSA, 50 mM Tris, pH 7.6), after which STIC activity was assessed.
Functional measurements of chloride channels in HASM.
For measurement of halide flux through chloride channels, HASM cells were grown to confluence in 96-well black-walled, clear-bottom plates. Cells were loaded for 10 h with 10 mM MQAE (Invitrogen) in 50 μl/well of basal medium (M199). Cells were washed 3× with physiological buffer solution (composition in mM: 10 HEPES with pH 7.4, 10 glucose, 2.4 K2PO4, 0.6 K2HPO4, 1 MgSO4, 1 CaSO4, and 110 Na isethionate) and left in a final volume of 90 μl of buffer. Cells were pretreated for 30 min at 37°C with the chloride channel blocker NPPB (100 μM) or vehicle (0.1% DMSO). Baseline fluorescence was continuously measured at 2-s intervals by using an excitation wavelength of 360 nm and an emission wavelength of 460 nm with a cutoff filter of 455 nm (3). After baseline fluorescent readings for 2 min, halide flux was induced by injecting 50 mM NaI (final concentration) by using the automated injector function of the Flexstation 3 plate reader (Molecular Devices, Sunnyvale, CA). Continuous recording of fluorescence continued for an additional 3 min before maximal quenching of MQAE fluorescent was determined by a final injection of 150 mM (final concentration) potassium thiocyanate. Halide (iodide) flux was compared between control and antagonist-pretreated cells by comparing the slopes of the quenching of fluorescence with the introduction of NaI (3, 25). Since cells have no endogenous intracellular iodide, NaI was utilized instead of NaCl since CaCCs are known to allow for an enhanced conductance of iodide ions compared with chloride ions and significant extracellular iodide creates a maximum extracellular-intracellular halide gradient to facilitate halide influx (19).
Impaired contraction of airway smooth muscle by TMEM16 channel inhibitors.
All animal protocols were approved by the Institutional Animal Care and Use Committee of Columbia University. Hartley male guinea pigs (400 g) were anesthetized by intraperitoneal pentobarbital to effect. Tracheas were promptly removed and dissected into two closed cartilaginous ring units with mucosa, connective tissue, and epithelium removed and attached to a fixed tissue hook in a 4-ml bath (Radnoti Glass Technology, Monrovia, CA) and a Grass FT03 force transducer (Grass Telefactor, West Warwick, RI), by using silk thread as previously described (18). BioPac hardware and Acknowledge 3.7.3 software (Biopac Systems, Goleta, CA) continuously recorded muscle force throughout all experiments. Rings were equilibrated at 1 g of isotonic force for 1 h with Krebs-Henseleit buffer (18) (with 10 μM indomethacin, pH 7.4, 37°C) replaced every 15 min in buffer continuously bubbled with 95% O2 and 5% CO2. Following equilibration, epithelium-denuded tracheal rings were contracted with 10 μM of the capsaicin analog N-vanillylnonanamide to activate and deplete nonadrenergic, noncholinergic nerves present in the tracheal ring preparation. Following buffer exchanges and resetting of resting tension to 1.0 g, rings were contracted with increasing concentrations of acetylcholine (100 nM to 100 μM) for two cycles with buffer exchanges and resetting of resting tension to 1.0 g between and after acetylcholine dose-response curves. Rings were then contracted with a maximal contractile concentration of KCl (80 mM) followed by buffer exchanges and resetting of resting tension. Inhibition of endogenous cholinergic nerves and mast cell degranulation was achieved by pretreating tracheal rings with 1 μM tetrodotoxin and 10 μM pyrilamine, respectively. Following results from preliminary dose-response experiments used to determine the lowest concentration of tannic acid capable of relaxing a substance P contraction, rings were then randomly treated with vehicle (buffer) or with the TMEM16 inhibitor tannic acid (100 μM) or benzbromarone (100 μM) for 10 min before contractions were induced with the tachykinin receptor agonist substance P (1 μM). The maximum force induced by substance P was expressed as a percentage of maximum force induced by the acetylcholine pretreatment in each individual ring. The pH was measured and maintained at 7.4 throughout the experiment in both the control and treatment groups with no significant change noted after addition of tannic acid.
Statistical analysis.
Muscle force comparisons in organ bath experiments and electrophysiological measurements of current and frequency were analyzed by unpaired, two-tailed t-tests. Data are expressed as means ± SE and a P value <0.05 was considered significant.
RESULTS
Qualitative expression of mRNA encoding TMEM16 family members in native and cultured HASM.
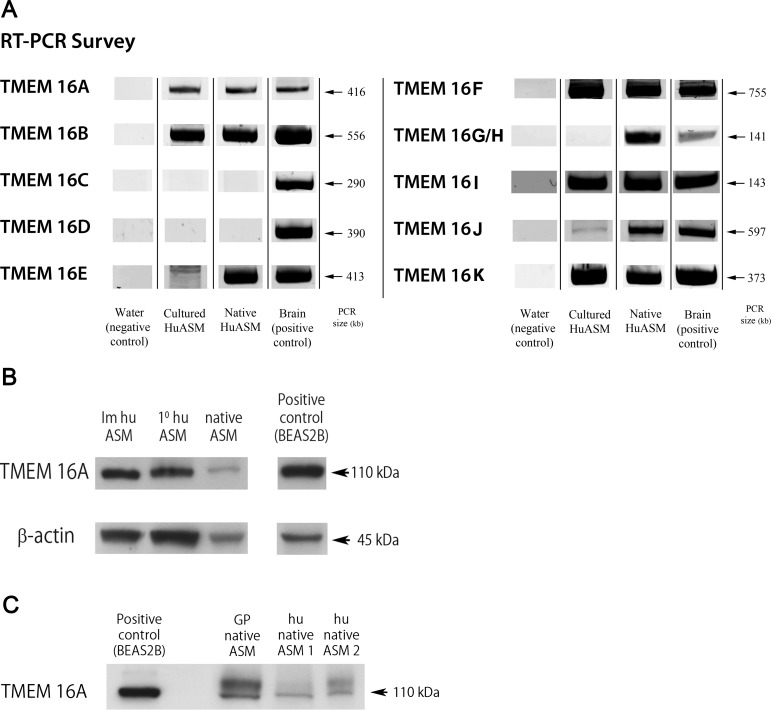
Messenger RNA encoding TMEM16A, B, E, F, G/H, I, J, and K (ANO 1, 2, 5, 6, 7, 8, 9, and 10) was identified in native HASM isolated from trachea from human lung transplant donors. In immortalized cultures of HASM, mRNA encoding six of these TMEM16 members (A, B, F, I, J, and K) were identified. In contrast, TMEM16 E and G/H demonstrated expression in native HASM but not in cultured cells. TMEM16 C and D were not identified in airway smooth muscle from either native or cultured cells despite the detection of the mRNA in appropriate positive control samples (Fig. 1A).
Fig. 1.
TMEM16 mRNA and protein expression in human airway smooth muscle. A: representative gel images of RT-PCR products of the 10 members of the TMEM16 (anoctamin) family of calcium-activated chloride channels (CaCCs). Cultured and freshly isolated (native) human airway smooth muscle (HuASM) expressed 6 isoforms (A, B, F, I, J, and K). All isoforms were detected in their respective positive control tissues. Total RNA positive controls included human total brain (A, B, C, D, E, F, G/H, I, K) and human stomach (J). Results are representative of at least 3 samples. B: representative immunoblot demonstrating expression of TMEM16A in both grossly dissected native human airway smooth muscle (native ASM) and from immortalized and primary cultures of human cultured airway smooth muscle cells (Im huASM and 1° huASM, respectively). Human airway epithelial cells (BEAS2B) were used as a positive control (31). C: representative immunoblot demonstrating expression of TMEM16A in both grossly dissected human (hu native airway smooth muscle) and guinea pig airway smooth muscle (GP native airway smooth muscle). Human airway epithelial cells (BEAS2B) were used as a positive control (31).
Immunoblot analysis of Ano-1/TMEM16A in airway smooth muscle.
Lysates from freshly dissected HASM (airway smooth muscle) tissue and cultures of primary and telomerized HASM cells were subjected to immunoblot analysis using a specific ANO-1 receptor antibody (Fig. 1B). To illustrate interspecies conservation (between human and guinea pig) of TMEM16A expression in fresh airway smooth muscle we also performed separate immunoblot analyses (Fig. 1C). Immunoreactive bands of the expected molecular mass (∼110 kDa) were detected from all four sources and substantiate that the guinea pig airway smooth muscle is a reasonable surrogate for HASM for TMEM16A functional studies. In all cases, cultured human airway epithelial cells (BEAS-2B) were used as a positive control for TMEM16A immunoblotting.
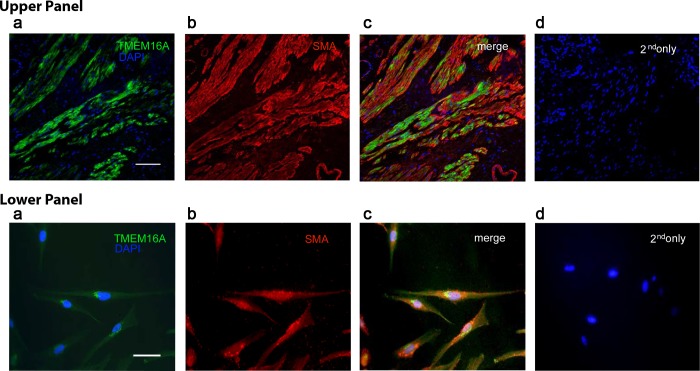
Immunohistochemistry reveals protein expression of TMEM16A in HASM cells.
To confirm the localization of TMEM16A (ANO-1) protein to HASM, immunohistochemistry was also performed using two distinct commercially obtained antibodies (Fig. 2). Specific immunoreactivity was detected in airway smooth muscle cells of fresh human tissues (upper panel) and a human immortalized cultured cell line (lower panel) with no staining observed in the negative controls (omission of primary antibodies with only secondary antibodies used). In addition, double and triple staining was performed with fluorescent-linked actin and DAPI to illustrate that staining was specific to airway smooth muscle cells.
Fig. 2.
Detection of TMEM16A on intact human airway smooth muscle and immortalized cultured human airway smooth muscle cells. Confocal microscopy images employing single, double, and triple staining of immunofluorescence labeling with antibodies directed against TMEM16A (green), α-smooth muscle actin (SMA) (red), and/or the nuclear counterstain DAPI (blue). Representative images from native human tracheal airway smooth muscle (upper panel) and human cultured airway smooth muscle cells (lower panel) are depicted. a, DAPI and TMEM16A costaining; b, α-SMA alone; c, triple staining and merging of TMEM16A and α-SMA; d, DAPI counterstain with primary antibodies omitted as negative control. Calibration bars represent 60 μm in upper panel and 35 μm in lower panel.
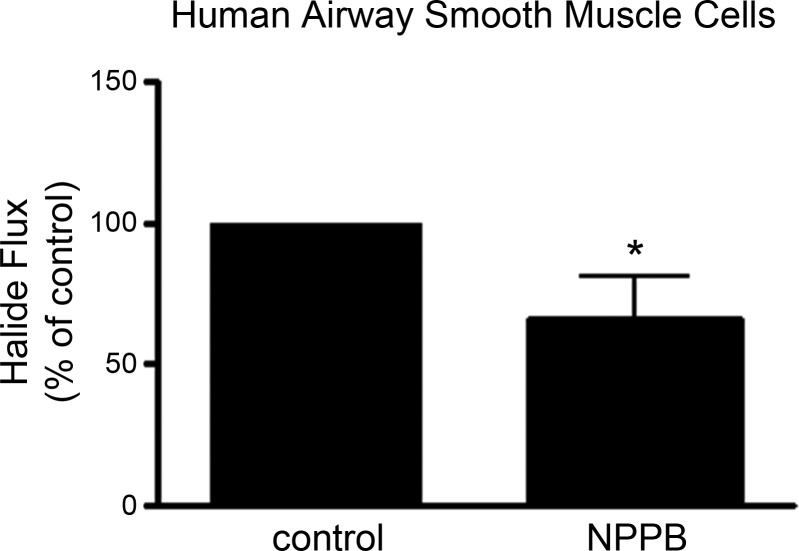
Chloride channel halide flux in cultured HASM.
Iodide flux through chloride channels was measured by the quenching of MQAE fluorescence in the absence or presence of pretreatment with a chloride channel antagonist. Pretreatment with the chloride channel blocker NPPB blocked halide flux in cultured immortalized HASM cells (n = 9) (P = 0.04) (Fig. 3).
Fig. 3.
Halide flux is inhibited by chloride channel blockers in cultured human airway smooth muscle cells. Halide flux was determined by quenching of MQAE fluorescence in the absence or presence of the chloride channel inhibitor 5-nitro-2-(3-phenylpropylamino)benzoic acid (NPPB) in response to 50 mM NaI in immortalized cultured human airway smooth muscle cells (n = 9) (*P ≤ 0.05).
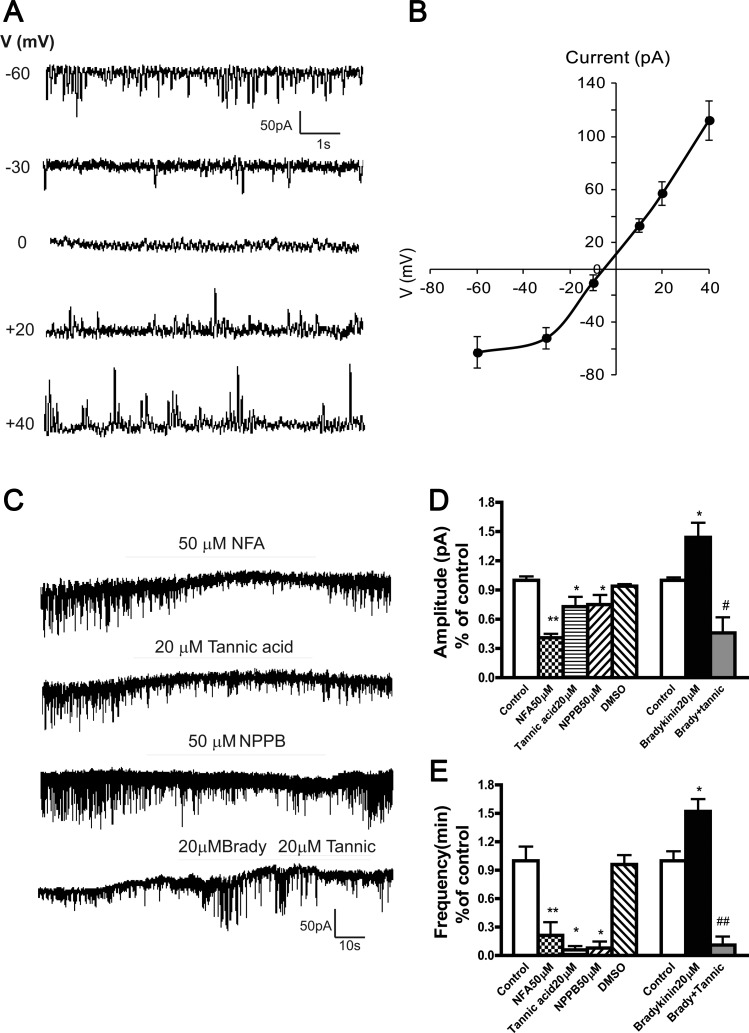
Patch-clamp electrophysiological studies on HASM.
STICs were recorded from immortalized HASM cells under buffer conditions selective for chloride currents. Under whole cell voltage-clamp configuration, spontaneous rhythmic inward currents were identified from HASM cells with use of a holding potential of −60 mV (Fig. 4A, upper panel tracing). The average peak amplitudes and frequencies of the current were 93.2 ± 21.2 pA and 116.1 ± 11.7 min−1, respectively (calculated 947 events from 16 cells). The average of this current reached a peak within 21.6 ± 4.0 ms (rise time, τ) with a single exponential function and decayed with a τ of 93.4 ± 20.7 ms (calculated 947 events from 16 cells). Figure 4A displays an example, representative of three cells, in which STICs were recorded at different holding voltages. In these three cells the average current was −61 ± 8 pA at −60 mV, which correlated with earlier work by Yang et al. (31). STICs reversed potential at −5.3 ± 0.8 mV (n = 3) (Fig. 4B), close to the predicted chloride reversal potential (ECl) = −16.2 mV. At potentials more negative than approximately −10 mV, STICs were inward, and at holding potentials above the predicted ECl, they changed direction from inward to outward (Fig. 4A), consistent with a chloride current. This correlated with the study of TMEM16A channels by Yang et al. in which reversal potentials shifted from −9.0 ± 0.6 mV to +12.5 ± 2.9 mV. At a holding potential +40 mV, the current was 112 ± 8 pA (n = 3) (Fig. 4B). STICs were reduced in amplitude and frequency when extracellular calcium was reduced (data not shown). These features indicate that STICs result from the opening of calcium-sensitive chloride channels. Next we sought to demonstrate a calcium-dependent increase in this chloride channel activity.
Fig. 4.
Current-voltage relationship of spontaneous transient inward current (STIC) and effects of Ca2+-activated chloride channel blockers on baseline and G protein-coupled receptor (bradykinin)-stimulated spontaneous transient inward currents in immortalized human airway smooth muscle cells. A: representative traces of spontaneous transient inward currents recorded at different holding potentials (V). B: relationship between mean peak amplitude of STICs (n = 3) and voltage holding potential. The reverse potential for STICs is −5.3 ± 0.8 mV (n = 3). C: representative whole cell recordings of STICs in response to niflumic acid (NFA), tannic acid (Tannic), NPPB, and bradykinin (Brady) in human immortalized airway smooth muscle cells. D and E: summary of effects of chloride channel inhibitors on the amplitude and frequency of STICs (n = 4 ∼ 12, *P < 0.05 **P < 0.01 compared with control; #P < 0.05; ##P < 0.01 compared with bradykinin).
Effects of bradykinin-induced increases in intracellular calcium and the chloride channel blockers NFA, tannic acid, NPPB, and TMEM16A antibody on STICs in immortalized HASM cells.
We found that 50 μM NFA (n = 4), 20 μM tannic acid (n = 4), or 50 μM NPPB (n = 4) rapidly inhibited STICs, expressed as reduction of both amplitude and frequency (Fig. 4, C–E); 20 μM bradykinin (used to increase intracellular calcium) increased the amplitude and frequency of STICs in HASM cells, and this stimulation was inhibited by tannic acid, an antagonist of the TMEM16A family member of CaCCs (23) (Fig. 4C, bottom tracing, D, and E).
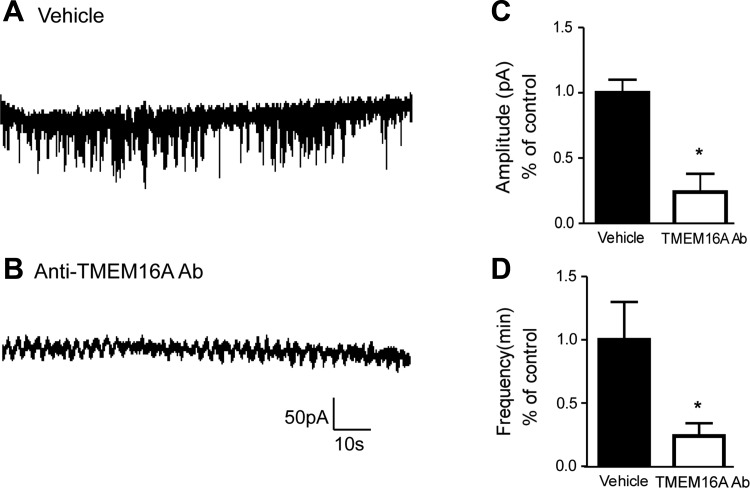
In a separate group of studies (Fig. 5), antibody-mediated blockade of TMEM16A led to a functional loss of STIC activity compared with vehicle-treated cells. We found complete abrogation of STIC activity in 21 of the 26 cells following incubation in TMEM16A antibody (compared to a lack of STIC activity in 3 of 17 patched cells following incubation with vehicle). Moreover, even in the 5 cells exposed to antibody that did display STIC activity, there was significant attenuation of amplitude (Fig. 5C) and frequency (Fig. 5D) compared with vehicle controls. These results again suggest that TMEM16A-mediated Ca2+-activated chloride currents in HASM cells constitute the major contribution to both baseline and G protein-coupled receptor-stimulated increases in STICs an electrophysiological phenomenon of CaCC activity.
Fig. 5.
TMEM16A-specific blockade via functional antibody treatment corroborates pharmacological TMEM16A antagonist effects on STICs. A and B: representative whole cell recordings of STICs in immortalized human airway smooth muscle cells following incubation with vehicle (A) or TMEM16A antibody (B). C and D: antibody-mediated blockade of TMEM16A led to a functional loss of STIC amplitude and frequency compared with vehicle-treated cells (*P < 0.05, and *P < 0.05, respectively).
Functional effects of TMEM16A chloride channel blockade on acetylcholine- and tachykinin-induced airway smooth muscle contraction.
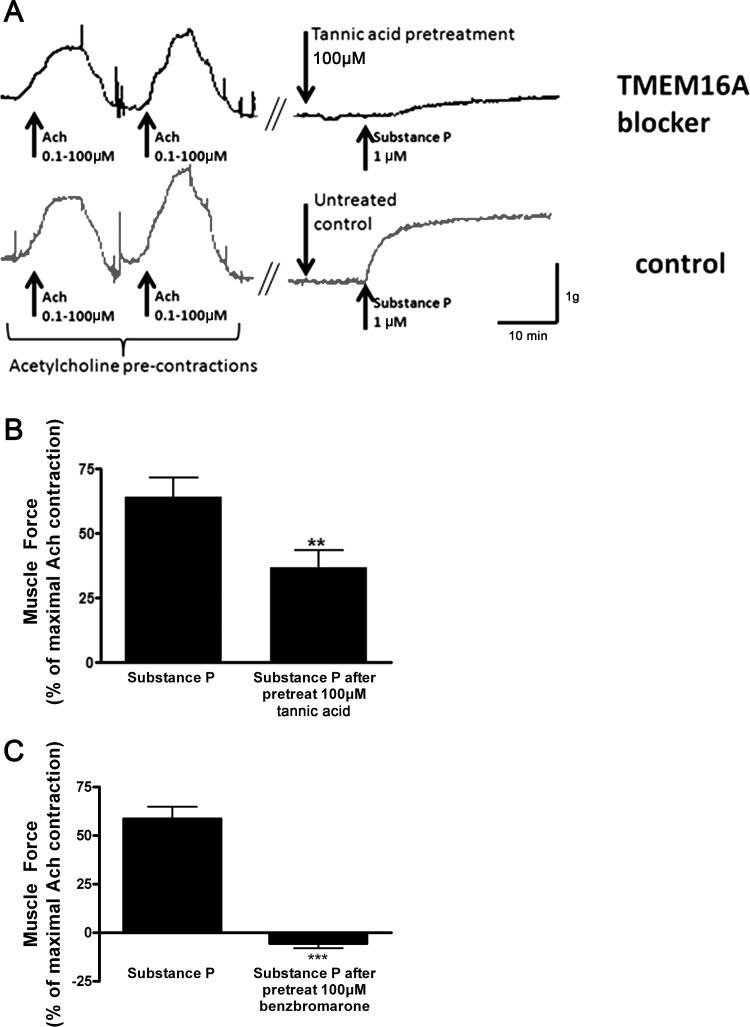
Dose-response studies examining tannic acid-mediated relaxation from a 1 μM substance P contraction demonstrated the lowest concentration (100 μM) that was functionally effective (data not shown). In an effort to maintain selectivity, this concentration was used to determine the functional relevance of TMEM16A blockade on contractile tone in intact airway smooth muscle by a pretreatment approach. Following pretreatment of ex vivo guinea pig airway smooth muscle with vehicle or the TMEM16A antagonists tannic acid or benzbromarone (100 μM), we found significant attenuation of a subsequent substance P contraction consistent with a role for TMEM16 CaCCs in airway smooth muscle contraction (P < 0.01, n = 8 and P < 0.001 respectively; Fig. 6). The contraction achieved with substance P in each airway ring was compared with a previous maximal contraction achieved with 100 μM acetylcholine in each individual ring.
Fig. 6.
Effect of TMEM16A chloride channel blockers (tannic acid and benzbromarone) on muscle force measurements in guinea pig tracheal rings. A: representative muscle force tracing of guinea pig tracheal rings precontracted with 1 μM substance P with or without pretreatment with the TMEM16A inhibitor tannic acid (100 μM). B: pretreatment with tannic acid significantly attenuates a subsequent substance P contraction consistent with a role for TMEM16A calcium-activated chloride channels in airway smooth muscle contraction. **P < 0.01, n = 8. C: benzbromarone pretreatment prior to a substance P contractile challenge also results in significant attenuation in airway smooth muscle force generation compared with vehicle control. ***P < 0.001, n = 8.
DISCUSSION
The primary findings of the present study are that multiple members of the TMEM16 family of CaCCs are expressed at the mRNA level in cultured and native HASM cells and that TMEM16A is immunohistochemically detected in native and cultured HASM. Consistent with previous studies (1, 13–15, 19, 32), we have demonstrated that airway smooth muscle cells express channels with classic electrophysiological properties of chloride channels that are modulated by a classic stimulus of increased intracellular calcium (bradykinin mediated). In the present study we demonstrate that STICs 1) occur under conditions of K+ channel blockade, 2) demonstrate voltage dependence, 3) reverse direction near the chloride equilibrium potential, 4) increase in magnitude and frequency by increasing calcium, 5) are attenuated by a number of chloride channel antagonists known to block TMEM16A chloride channels, and 6) are selectively blocked by incubating human smooth muscle airway cells with a specific antibody directed against the human TMEM16A channel. These findings are consistent with TMEM16A representing a CaCC in airway smooth muscle responsible for STICs. We have demonstrated that halide quenching of the fluorescent indicator MQAE is attenuated by the chloride channel inhibitor NPPB consistent with antagonism of functional chloride channels. Finally, in organ bath studies in intact tracheal rings we demonstrate that blockade of these channels with TMEM16A inhibitors (tannic acid and benzbromarone) attenuates a tachykinin-induced contraction of airway smooth muscle.
Our findings agree with those recently published in mouse airway myocytes (1) in which the kinetics of STICs under identical extracellular and pipette buffer conditions were very similar to those in the present study. Moreover, STICs in these mouse airway myocytes were demonstrated to be temporally and spatially related to calcium sparks linking calcium release via sarcoplasmic reticulum ryanodine receptors to activation of CaCCs accounting for STIC activity. Subsequently these authors demonstrated that biphasic membrane potential transients mediated by calcium transients opening large-conductance KCa channels causing spontaneous transient outward currents coupled with ClCa-mediated STICs serve to stabilize plasma membrane potential (32).
A fundamental feature of a CaCC is a voltage dependence that is modulated by intracellular Ca2+ and is activated by positive membrane potentials at submaximal Ca2+ concentrations with an inherent outwardly rectifying current-voltage relationship (5, 9). At maximal concentrations this relationship should become linear. Prior to 2008, the CLC, ClCa, bestrophin, and Tweety families were all thought to have some characteristics of CaCCs; however, the ionic currents conducted by these proteins did not exhibit the expected Ca2+ sensitivity, voltage dependence, or kinetics expected at submaximal Ca2+ concentrations (8).
In 2008, three separate laboratories using different approaches demonstrated that the TMEM16 family of proteins exhibits the expected electrophysiological characteristics of a voltage- and calcium-regulated chloride channel (2, 26, 31). Yang et al. (31) showed that TMEM16A coexpressed with endothelin receptor A into HEK293 cells can produce robust endothelin-induced chloride currents that were outwardly rectified at low activation levels but nonrectifying at maximal activation. These outward currents could be blocked by NFA (31). This Gq-coupled receptor increase in intracellular calcium is functionally analogous to the use of bradykinin in the present study and calcium-induced increases in chloride currents by acetylcholine, histamine, substance P, and caffeine in previous studies in airway smooth muscle cells (13, 14, 16, 17, 20, 21, 29). Schroeder et al. (26), using an expression system of Xenopus laevis for CaCC mRNA, generated CaCC currents that became less dependent upon voltage and showed little rectification at high intracellular calcium levels. These currents were activated by carbachol and blocked by NFA and 4,4-diisothiocyanatostilibene 2,2-disulfonic acid (DIDS) (26). Moreover, this family of ion channels was shown to be involved in a number of cellular functions including cell volume regulation, neuron excitability, smooth muscle contraction, and epithelial electrolyte and fluid transport (2).
In our study, we have demonstrated in airway smooth muscle that six of the ten known members of the TMEM16 family of CaCCs are expressed at the mRNA level in both native and cultured HASM cells. Two members, TMEM16C and TMEM16D, were not detected in either native or cultured HASM. TMEM16E and G/H were detected in native but not cultured airway smooth muscle. These discrepancies in detection between native and cultured cells may reflect impurities of cell type in the natively dissected airway smooth muscle or induced changes that occur in cell culture. Prior to the present study, only TMEM16A mRNA expression had been demonstrated in mouse airway smooth muscle, where it is believed to be critical for the normal epithelial/smooth muscle development of the airway (24) and attenuates acetylcholine-induced contraction (11).
Numerous previous studies have demonstrated spontaneous transient inward currents in airway smooth muscle cells and calcium-activated increases in chloride currents, but none of these previous studies identified the specific protein responsible for this conductance. In fact, most of these previous studies occurred before the molecular characterization of the TMEM16 (anoctamin) family of CaCCs, which are now recognized as the protein responsible for these chloride currents in many other cell types (30). The molecular function of this ten-member family has been further refined as TMEM16A and TMEM16B appear to account for plasma membrane calcium-regulated chloride conductance while the other eight family members are either expressed in other cellular compartments (4) or serve other functional roles (27). Thus TMEM16A (and perhaps TMEM16B) identified in airway smooth muscle in the present study likely account for calcium-induced chloride currents that account for STIC activity (1, 13–15, 19, 32) modulated by spatially close ryanodine receptors demonstrated in airway smooth muscle cells (1). Given this fact, the abundant expression of TMEM16A, and the commercially available antibodies that have been used successfully for immunohistochemistry and functional blockade of TMEM16A, we decided to focus our initial efforts on this family member. However, we realize that ANO-2/TMEM16B may also play a role in modulating airway smooth muscle calcium-activated chloride flux.
Until recently, specific antagonism of the TMEM16A channel has been elusive, with the pharmacological armamentarium being limited to nonspecific chloride channel blockade with NFA, DIDS, or NPPB. Although selective pharmacological antagonists that unequivocally target only TMEM16A are lacking, in 2010 Namkung et al. (23) discovered that gallotannins, including tannic acid, in red wine and green tea selectively inhibited TMEM16A and not CFTR Cl− channels. In the present study, we demonstrated that the calcium-mediated increase in chloride channel activity (measured as STICs) was decreased by tannic acid and that pretreatment of isolated guinea pig tracheal rings with tannic acid attenuated a subsequent substance P-induced smooth muscle contraction. These results are in close agreement to those in human bronchial smooth muscle in which a TMEM16A small molecule inhibitor (benzbromarone) inhibited methacholine- but not KCl-induced contractions in isolated human bronchi (11). These authors suggested that TMEM16A blockade may specifically attenuate muscarinic receptor-mediated contractions. However, the present study demonstrates that a contraction induced by another Gq-coupled receptor (neurokinin receptor-activated by substance P) is also attenuated by TMEM16A blockade, a novel finding that expands this target's usefulness in suppressing other contractile agonists. We also observed recovery of contractility to substance P following washout and rechallenge with substance P (data not shown), suggesting a reversible drug effect. In addition, to corroborate our pharmacological data, we also employed functional TMEM16A channel blockade via a TMEM16A specific antibody to assess whether acute blockade of the TMEM16A receptor by an antibody shown to functionally antagonize this receptor on live cells by other investigators (28), could replicate our electrophysiological findings obtained by using tannic acid. Utilizing a similar methodology, we demonstrate that antibody-mediated TMEM16A specific blockade leads to functional obliteration of STIC activity in HASM cells.
Although the role of ANO1/TMEM16A in airway smooth muscle has been reported in the literature (10, 11, 24), there has been limited functional characterization of its role especially in human tissues. What is known about ANO1 and the airway is predominantly from studies focusing on murine models. For example, ANO1 is critical to the development of the trachea since global ANO1 knockout mice die within 1 mo of birth and exhibit severe tracheomalacia including a defect in airway smooth muscle development on the posterior wall of the trachea and a failure of airway epithelial stratification (24). ANO1 has been immunochemically localized to mouse airway smooth muscle and was shown to colocalize with α-smooth muscle actin (10). Human bronchi were shown to contract less effectively in the presence of benzbromarone an antagonist of ANO1 channels (11). However, this pharmacological ligand of ANO1 has not been shown to be devoid of antagonism at other chloride or other ion channels.
In summary, we demonstrate the mRNA expression of multiple members of the ANO/TMEM16 family of CaCCs in HASM as well as protein expression of TMEM16A (now thought to account for calcium-activated chloride currents in many cells). We demonstrate that calcium-induced increases in chloride channel activity are attenuated by tannic acid, an inhibitor of TMEM16A, and that tannic acid and benzbromarone attenuate a substance P contraction in intact airway smooth muscle. These studies suggest that TMEM16A is the elusive chloride channel responsible for calcium-induced chloride currents in airway smooth muscle cells.
GRANTS
This work was supported by National Institutes of Health grants [G. Gallos (GM093137); C. W. Emala (GM065281); and P. D. Yim (GM008464)]; The Foundation for Anesthesia Education and Research (H. S. Chang); and the National Center for Research Resources ULI RR 024156.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
G.G., K.E.R., P.D.Y., and C.W.E. conception and design of research; G.G., K.E.R., J.D., H.F., X.W.F., H.-Y.S.C., and D.X. performed experiments; G.G., J.D., H.F., X.W.F., H.-Y.S.C., D.X., and C.W.E. analyzed data; G.G., K.E.R., J.D., P.D.Y., and C.W.E. interpreted results of experiments; G.G., J.D., H.F., X.W.F., H.-Y.S.C., and C.W.E. prepared figures; G.G. and K.E.R. drafted manuscript; G.G., K.E.R., J.D., X.W.F., and C.W.E. edited and revised manuscript; G.G., K.E.R., J.D., H.F., X.W.F., H.-Y.S.C., and C.W.E. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. William Gerthoffer, Department of Biochemistry and Molecular Biology, University of South Alabama, for providing the immortalized HASM cells used in this study. We also thank Dr. Theresa Swayne for assistance with confocal imaging.
REFERENCES
- 1.Bao R, Lifshitz LM, Tuft RA, Bellve K, Fogarty KE, ZhuGe R. A close association of RyRs with highly dense clusters of Ca2+-activated Cl− channels underlies the activation of STICs by Ca2+ sparks in mouse airway smooth muscle. J Gen Physiol 132: 145–160, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, Pfeffer U, Ravazzolo R, Zegarra-Moran O, Galietta LJ. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science 322: 590–594, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Carlos MA, Nwagwu C, Ao M, Venkatasubramanian J, Boonkaewwan C, Prasad R, Chowdhury SA, Vidyasagar D, Rao MC. Epidermal growth factor stimulates chloride transport in primary cultures of weanling and adult rabbit colonocytes. J Pediatr Gastroenterol Nutr 44: 300–311, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Duran C, Qu Z, Osunkoya AO, Cui Y, Hartzell HC. ANOs 3–7 in the anoctamin/Tmem16 Cl− channel family are intracellular proteins. Am J Physiol Cell Physiol 302: C482–C493, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galietta LJ. The TMEM16 protein family: a new class of chloride channels? Biophys J 97: 3047–3053, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gosens R, Stelmack GL, Dueck G, McNeill KD, Yamasaki A, Gerthoffer WT, Unruh H, Gounni AS, Zaagsma J, Halayko AJ. Role of caveolin-1 in p42/p44 MAP kinase activation and proliferation of human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 291: L523–L534, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Hartzell C, Putzier I, Arreola J. Calcium-activated chloride channels. Annu Rev Physiol 67: 719–758, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Hartzell HC. Physiology. CaCl-ing channels get the last laugh. Science 322: 534–535, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Hartzell HC, Yu K, Xiao Q, Chien LT, Qu Z. Anoctamin/TMEM16 family members are Ca2+-activated Cl− channels. J Physiol 587: 2127–2139, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang F, Rock JR, Harfe BD, Cheng T, Huang X, Jan YN, Jan LY. Studies on expression and function of the TMEM16A calcium-activated chloride channel. Proc Natl Acad Sci USA 106: 21413–21418, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang F, Zhang H, Wu M, Yang H, Kudo M, Peters CJ, Woodruff PG, Solberg OD, Donne ML, Huang X, Sheppard D, Fahy JV, Wolters PJ, Hogan BL, Finkbeiner WE, Li M, Jan YN, Jan LY, Rock JR. Calcium-activated chloride channel TMEM16A modulates mucin secretion and airway smooth muscle contraction. Proc Natl Acad Sci USA 109: 16354–16359, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janssen LJ. Ionic mechanisms and Ca2+ regulation in airway smooth muscle contraction: do the data contradict dogma? Am J Physiol Lung Cell Mol Physiol 282: L1161–L1178, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Janssen LJ, Sims SM. Acetylcholine activates non-selective cation and chloride conductances in canine and guinea-pig tracheal myocytes. J Physiol 453: 197–218, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janssen LJ, Sims SM. Histamine activates Cl− and K+ currents in guinea-pig tracheal myocytes: convergence with muscarinic signalling pathway. J Physiol 465: 661–677, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janssen LJ, Sims SM. Spontaneous transient inward currents and rhythmicity in canine and guinea-pig tracheal smooth muscle cells. Pflügers Arch 427: 473–480, 1994 [DOI] [PubMed] [Google Scholar]
- 16.Janssen LJ, Sims SM. Substance P activates Cl− and K+ conductances in guinea-pig tracheal smooth muscle cells. Can J Physiol Pharmacol 72: 705–710, 1994 [DOI] [PubMed] [Google Scholar]
- 17.Janssen LJ, Sims SM. Ca2+-dependent Cl− current in canine tracheal smooth muscle cells. Am J Physiol Cell Physiol 269: C163–C169, 1995 [DOI] [PubMed] [Google Scholar]
- 18.Jooste EH, Sharma A, Zhang Y, Emala CW. Rapacuronium augments acetylcholine-induced bronchoconstriction via positive allosteric interactions at the M3 muscarinic receptor. Anesthesiology 103: 1195–1203, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Kotlikoff MI, Wang YX. Calcium release and calcium-activated chloride channels in airway smooth muscle cells. Am J Respir Crit Care Med 158: S109–S114, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Liu C, Tazzeo T, Lippton H, Janssen LJ. Role of tyrosine phosphorylation in U46619-induced vasoconstriction of pulmonary vasculature and its modulation by genistein, daidzein, and equol. J Cardiovasc Pharmacol 50: 441–448, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Liu X, Farley JM. Acetylcholine-induced Ca++-dependent chloride current oscillations are mediated by inositol 1,4,5-trisphosphate in tracheal myocytes. J Pharmacol Exp Ther 277: 796–804, 1996 [PubMed] [Google Scholar]
- 22.Moorman J, Zahran H, Truman B, Molla M. Current asthma prevalence—United States, 2006–2008. MMWR Surveill Summ 60 Suppl: 84–86, 2011 [PubMed] [Google Scholar]
- 23.Namkung W, Thiagarajah JR, Phuan PW, Verkman AS. Inhibition of Ca2+-activated Cl− channels by gallotannins as a possible molecular basis for health benefits of red wine and green tea. FASEB J 24: 4178–4186, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rock JR, Futtner CR, Harfe BD. The transmembrane protein TMEM16A is required for normal development of the murine trachea. Dev Biol 321: 141–149, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Sahi J, Goldstein JL, Layden TJ, Rao MC. Cyclic AMP- and phorbol ester-regulated Cl− permeabilities in primary cultures of human and rabbit colonocytes. Am J Physiol Gastrointest Liver Physiol 266: G846–G855, 1994 [DOI] [PubMed] [Google Scholar]
- 26.Schroeder BC, Cheng T, Jan YN, Jan LY. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell 134: 1019–1029, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scudieri P, Sondo E, Ferrera L, Galietta LJ. The anoctamin family: TMEM16A and TMEM16B as calcium-activated chloride channels. Exp Physiol 97: 177–183, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Thomas-Gatewood C, Neeb ZP, Bulley S, Adebiyi A, Bannister JP, Leo MD, Jaggar JH. TMEM16A channels generate Ca2+-activated Cl− currents in cerebral artery smooth muscle cells. Am J Physiol Heart Circ Physiol 301: H1819–H1827, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang YX, Kotlikoff MI. Muscarinic signaling pathway for calcium release and calcium-activated chloride current in smooth muscle. Am J Physiol Cell Physiol 273: C509–C519, 1997 [DOI] [PubMed] [Google Scholar]
- 30.Winpenny JP, Gray MA. The anoctamin (TMEM16) gene family: calcium-activated chloride channels come of age. Exp Physiol 97: 175–176, 2012 [DOI] [PubMed] [Google Scholar]
- 31.Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, Park SP, Lee J, Lee B, Kim BM, Raouf R, Shin YK, Oh U. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature 455: 1210–1215, 2008 [DOI] [PubMed] [Google Scholar]
- 32.ZhuGe R, Bao R, Fogarty KE, Lifshitz LM. Ca2+ sparks act as potent regulators of excitation-contraction coupling in airway smooth muscle. J Biol Chem 285: 2203–2210, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]