Abstract
Club cell secretory protein (CCSP) is an indirect phospholipase A2 inhibitor with some immunosuppressive and antiproliferative properties that is expressed in bronchiolar Club cells. In our murine bone marrow transplant (BMT) model of obliterative bronchiolitis (OB), CCSP is diminished; however, its role is unknown. To determine the role of CCSP, B6 wild-type (WT) or CCSP-deficient (CCSP−/−) mice were lethally conditioned and given allogeneic bone marrow with a sublethal dose of allogeneic splenic T cells to induce OB. We found that CCSP−/− mice demonstrated a higher mortality following BMT-induced OB compared with WT mice. Mice were analyzed 60 days post-BMT for protein expression, pulmonary function, and histology. CCSP levels were reduced in WT mice with BMT-induced OB, and lower levels correlated to decreased lung compliance. CCSP−/− had a higher degree of injury and fibrosis as measured by hydroxy proline, along with an increased lung resistance and the inflammatory markers, leukotriene B4 and CXCL1. Replacement with recombinant intravenous CCSP partially reversed the weight loss and improved survival in the CCSP−/− mice. In addition, CCSP replacement improved histology and decreased inflammatory cells and markers. These findings indicate that CCSP has a regulatory role in OB and may have potential as a preventive therapy.
Keywords: Club cell secretory protein, obliterative bronchiolitis, bone marrow transplant
obliterative bronchiolitis (OB) is one of the late complications of allogeneic bone marrow transplantation (BMT) and is a major cause of increased mortality following lung transplantation (7, 20, 25). We have previously reported a BMT model of OB that demonstrated a decrease in Club cell secretory protein (CCSP, formerly called Clara cell secretory protein) in affected airways (19). CCSP is a 16-kDa homodimeric protein expressed primarily in the bronchiolar Club cell, the observed site for the lesion of OB (5, 9, 23). The primary physiological function of CCSP remains unknown; however, it has demonstrated anti-inflammatory effects (22, 24). Specifically, CCSP has been shown to inhibit phospholipase A2 (PLA2) in vitro and through this mechanism downregulates inflammation (4, 6, 33). The ability of CCSP to modulate activity of inflammatory markers reflects its potential to act as a protective protein during lung injury. Moreover, the high concentration of CCSP in the epithelial lining fluid suggests a role in lung repair (5) because Club cells containing CCSP are airway epithelium progenitors (1).
CCSP-deficient (−/−) mice have been used to identify the immunological response to lung injury (30). These mice show a normal phenotype at birth with no marked physiological, reproductive, or pathological abnormalities including normal pulmonary function (27). However, CCSP−/− mice demonstrate an exaggerated response to oxidant injury (12). Although other Club cell-specific proteins such as surfactant proteins and CCSP 26 are not altered, the cellular organelles of Club cells are drastically altered in knockout mice (9). Proteomic studies using 2D gels reveal altered protein patterns between wild-type (WT) and CCSP−/− mice, supporting the notion that CCSP deficiency alters, not only CCSP, but also airway homeostasis (28). With these findings in mind, recombinant human CCSP (hrCCSP) has been used clinically to treat lung injury and respiratory distress syndrome in premature infants (5).
Our study focuses on the role CCSP plays post-BMT in our mouse model of OB (19). The lung damage present in OB is persistent, and the pathophysiology is different than acute trauma. It has been shown that patients receiving lung transplants who develop OB have lower levels of CCSP in bronchiolar lavage fluid and serum (16, 34). In this study, we compare the lung function and repair ability of mice deficient of CCSP vs. WT controls in our murine BMT model of OB. Once we established that CCSP−/− mice post-BMT performed worse in pulmonary function tests, collagen content, and lung injury scores, we investigated the potential therapeutic effects of administering rhCCSP in our OB model.
MATERIALS AND METHODS
Mice.
C57BL/6 (H2b) and B10.BR (H2k) were purchased from Jackson Laboratories (Bar Harbor, ME). CCSP−/− mice were obtained from Dr. Susan Reynolds at the University of Colorado Denver (29). Mice were housed in a specific pathogen-free facility at the University of Minnesota and cared for according to the Research Animal Resources guidelines of our institution. Experiments were approved by the Institutional Animal Care and Use Committee of the University of Minnesota. Sentinel mice were found to be negative for infectious microorganisms known to cause pulmonary pathology, such as pneumonia virus, K virus, and Sendai. For BMT, donors were 8–12 wk of age, and recipients were used at 8–10 wk of age.
BMT.
B6 WT or CCSP−/− mice received intraperitoneal PBS or cyclophosphamide (Cytoxan; Bristol Myers Squibb, Seattle, WA), 120 mg/kg per day as a conditioning regimen pre-BMT on days −3 and −2. All mice were lethally irradiated the day before BMT (7.5 Gy TBI) by X-ray at a dose rate of 0.41 Gy/min as described previously (2). Our BMT protocol has been previously described (18). Briefly, donor B10.BR bone marrow was T cell depleted (TCD) with anti-Thy 1.2 monoclonal antibody (mAb; clone 30-H-12, rat IgG2b; kindly provided by Dr. David Sachs, Charlestown, MA) plus complement (Nieffenegger, Woodland, CA). B6 WT or CCSP−/− recipient mice were transplanted via caudal vein with 20 × 106 TCD B10.BR (H2k) marrow with 1 × 106 splenocytes (BMS) or without splenocytes (BM) as a source of allogeneic T cells. The BMS model develops OB (19).
Pulmonary function tests.
Lung function was assessed by whole body plethysmography using the Flexivent system (Scireq, Montreal, PQ, Canada) and Flexivent software version 5. The Flexivent was calibrated for open- and closed-tube systems for each pulmonary test performed. Each mouse was anesthetized, weighed, and orally intubated. The weight was integrated to the parameters for the perturbations used. The mice were allowed a brief period to acclimate to the ventilator. The maximum pressure was set at 30 cm H2O for pressure/volume analysis. The positive end-expiratory pressure remained constant at ∼2.5 cm H2O.
Serum collection.
At the time of death, blood was collected by cardiac puncture and placed immediately at 4°C. The serum was separated and subsequently stored at −80°C.
Hydroxyproline.
Collagen content was determined by hydroxyproline (OH-proline) levels and was measured by oxidation of 4-OH-l-proline to pyrrole and reaction with p-dimethylaminobenzaldehyde (absorbance read at 560 nm).
Lung protein extracts and gene expression.
After exsanguination, the left lung was homogenized in 1 ml PBS containing protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN) and centrifuged at 3,000 revolution/min for 10 min. The supernatant was filtered through a 1.2-μm syringe filter and stored at −80°C until assayed. RNA was isolated using Trizol method (Invitrogen, Carlsbad, CA) for qRT-PCR analysis that was run on Applied Biosystems 7500 real-time PCR system. The probe set for CCSP qRT-PCR was from Applied Biosystems (Mn00442046_ml), and GAPDH1 was used as an endogenous control.
Proteomic analysis.
Equivalent amounts of protein (50 μg) from 1) healthy WT mice, 2) BM WT control, and 3) WT BMS mouse lungs were subjected to trypsin digestion and iTRAQ labeling as outlined in the manufacturer's protocol. Briefly, disulfides were reduced and alkylated, and samples were trypsin digested. Each sample was labeled with one of the four isobaric iTRAQ reagents with reporter masses of 114, 115, 116, and 117, respectively. The samples were pooled, and the resulting mixture was speed-vacuumed to dryness, dissolved in deionized water, and purified by adsorption and elution from a Sep-Pak C18 cartridge using standard methods (Waters, Milford, MA). The recovered peptide mixture was speed-vacuumed to dryness and dissolved in 350 μl of strong cation exchange (SCX) loading buffer (20% vol/vol acetonitrile, 5 mM KH2PO4, pH 3.2) for subsequent fractionation. The first dimension of separation used a SCX polysulfoethyl A column (150 × 1.0 mm, 5 μm, 300 Å; The Nest Group, Southborough, MA) mounted on a Magic 2002 HPLC system (Michrom Bioresources, Auburn, CA). Peptide fractions were dried by speed vacuum, and fractions 10 through 28 were dissolved in 30 μl of reverse-phase loading buffer (98:2, H2O:ACN, 0.1% triflouroacetic acid). Each fraction was then desalted by use of an LCP C18 nanoprecolumn (5 mm × 0.3 mm), eluted at 350 nl/min using an LCP Ultimate LC system (Dionex, Sunnyvale, CA), and further separated on a reverse-phase C18 column (13 cm × 5 μm, 200 Å pore size; Michrom BioResources), which was coupled online to a Quadrupole-TOF mass spectrometer (QSTAR Pulsar I; Applied Biosystems, Foster City, CA) equipped with Protana's nanoelectrospray source. Protein identification and relative quantification of peptides were performed using ProQuant software (Applied Biosystems, Version 1.0). Fragment ion spectra were searched against the murine database with the Interrogator Algorithm and a 0.35-Da mass tolerance for both parent (MS) and fragment ions (MS/MS). Protein Pilot 3.0 was used to generate compiled protein identification and quantification results from the ProQuant database for peptides with a ProScore of greater than 1.3 or 95% confidence of peptide identification.
Computational analysis.
We performed gene ontology enrichment analysis. Functional annotation clustering analysis was performed using online software Database for Annotation, Visualization, and Integrated Discovery (DAVID) (http://david.abcc.ncifcrf.gov) (8). DAVID generates an enrichment score by testing the relatedness of different combinations of genes according to common biological function, chromosomal location, or regulation. A high enrichment score for a group of genes indicates that annotation term members are playing important roles in a given study, and an enrichment score of 1.3 is equivalent to a non-log scale P value of 0.05. The P value is also termed the EASE score and represents a modified Fisher exact test; the smaller the P value, the more significant the gene association. The Benjamini test globally corrects enrichment P values to provide false discovery rates <0.05.
Frozen tissue preparation.
A mixture of 0.5 ml of optimal cutting temperature compound (OCT; Miles, Elkhart, IN) to PBS (3:1) was infused via the trachea into the lungs. Lung tissue was embedded in OCT, frozen in liquid nitrogen, and stored at −80°C.
Immunohistochemistry and tissue scoring.
After anesthesia with pentobarbital sodium, mice were killed by cervical dislocation, and the lung was removed, snap-frozen in liquid nitrogen, and stored at −80°C. Frozen sections (6 μm) were cut, and cryosections were fixed in acetone and stained with hematoxylin and eosin or Masson's Trichrome stain. To assess lung injury, tissues were scored on a scale of 0 to 4+ as previously described (3, 17, 26). Additional lung cryosections (6 mm) were fixed in acetone and immunoperoxidase-stained using biotinylated mAbs specific for CD4, CD11b, and Gr-1 (all from BDPharmingen, San Diego, CA) with avidin-biotin blocking reagents, ABC-peroxidase conjugate, and DAB chromogen purchased from Vector Laboratories (Burlingame, CA). Sections were counterstained with methyl green, and positive cells were enumerated as a percentage of total nucleated cells under ×200 magnification on an Olympus BX51 microscope.
Protein measurements.
CCSP levels in lung protein extracts were evaluated by sandwich ELISA. Wells were coated overnight at 4°C with goat anti-CC10 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) at 1 μg/ml in bicarbonate coating buffer. After being blocked with 10% BSA in PBS and washed (wash buffer; R&D Systems, Minneapolis, MN), lung protein extracts were added at a 1:10 dilution in PBS, incubated for 2 h at room temperature, and washed. Rabbit-anti-mouse CC10 antibody (clone FL-96, Santa Cruz) was then added at 1 μg/ml, incubated for 1 h at room temperature, and washed, and peroxidase-conjugated goat-anti-rabbit IgG (Bio-Rad, Hercules, CA) was added at 1:100 dilution for 1 h at room temperature. After being further washed, peroxidase substrate was added (R&D Systems color reagents A and B) and incubated for 30 min in the dark at room temperature, and the reaction was stopped with 2N H2SO4. Plates were read at 450 nm, with subtraction at 580 nm, using a Bio-Rad 550 Plate reader. Data for samples were expressed as O.D. reading after subtracting background levels. All samples were assayed in duplicate. Leukotriene B4 levels were measured by commercially available ELISA (Cayman Chemical, Ann Arbor, MI). Lung protein extract and serum levels of CXCL1 (KC) were determined using the Luminex system (Austin, TX) and a mouse-specific CXCL-1 bead set (R&D Systems; sensitivity 2 pg/ml).
CCSP injections.
rhCCSP (Clarassance, Rockville, MD) was intravenously administered at 50 μg/0.5 ml per mouse via caudal tail vein once every 7 days for 60 days post-BMT. Purified recombinant protein was diluted in sterile saline and stored at 4°C.
Statistical analysis.
Survival data were analyzed by Kaplan-Meier plots with nonparametric statistical tests. Other data were analyzed by analysis of variance or Student's t-test. P values ≥0.05 were considered statistically significant.
RESULTS
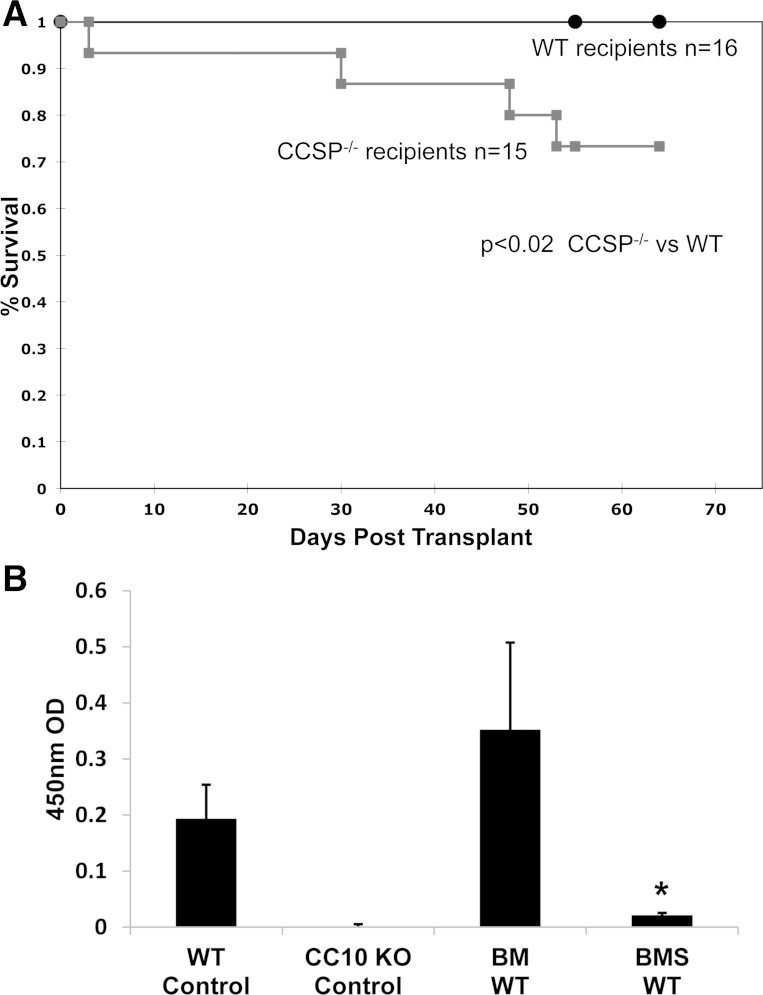
We previously reported that CCSP is diminished in airways from patients with OB and our murine BMT model of OB (19). To determine whether CCSP influences survival, we induced OB via a BMT supplemented with splenocytes (BMS) in WT and CCSP−/− mice and observed them for 60 days post-BMT. We found a significantly higher mortality in CCSP−/− mice relative to WT mice (Fig. 1A, P < 0.02). Although WT mice demonstrated histological OB following BMS, survival was not impaired at 60 days (P = NS). However, CCSP−/− mice demonstrated an increase in mortality compared with both WT BMS mice (P < 0.02) and CCSP−/− BM mice transplanted without splenocytes (P < 0.02). When WT and CCSP−/− mice were transplanted with a higher dose of splenocytes (5,000,000), an even higher mortality was observed in the CCSP−/− mice with little significant change in WT mortality during the same time period (P < 0.00002, data not shown). The CCSP−/− mice did not appear more sensitive to BMT conditioning because both WT and CCSP−/− mice had long-term survival and were fully engrafted when given allogenic BM alone, where OB was not induced (data not shown).
Fig. 1.
Survival and Club cell secretory protein (CCSP) levels in CCSP−/− and wild-type (WT) mice. A: WT and CCSP−/− mice underwent a bone marrow transplant (BMT) with 1 × 10−6 splenocytes (BMS) to induce obliterative bronchiolitis (OB), and survival was observed up to 60 days. n = 15 animals for CCSP−/− and n = 16 for WT. P < 0.02 comparing CCSP−/− with WT. B: CCSP levels were determined by ELISA in whole lung homogenates from WT or CCSP−/− (knockout, KO) mice at 60 days post-BMT. Controls consisted of healthy WT and CCSP KO. WT mice underwent BMT with splenocytes (BMS) to induce OB or without splenocytes (BM). Means ± SD are indicated for 6 mice per group. *P < 0.05 compared with WT controls and BM WT controls.
To characterize protein expression, we performed protein profiling with iTRAQ labeling combined with mass spectrometry on lung extracts of healthy WT mice and WT mice at day 60 after a BMT with splenocytes (BMS) or without splenocytes (BM) to identify proteins that are differentially expressed. Mass spectrometry with iTRAQ labeling allows the comparisons of labeled groups to give relative changes in protein. We identified 239 proteins, of which 26 were differentially expressed statistically when comparing the BMS model to the BM control (Supplemental Table S1; supplemental material for this article is available online at the American Journal of Physiology Lung Cellular and Molecular Physiology website). Table 1 depicts the top four proteins that were decreased or increased; among these, CCSP was decreased twofold. In comparing the BMS model with healthy controls, the decrease in CCSP was more pronounced with a sevenfold decrease (data not shown). This is consistent with our previously reported findings of a decrease in CCSP immunohistochemical staining post-BMT when splenocytes are given as a source of allogeneic T cells (19). To validate this finding, we measured CCSP in lung homogenates with an ELISA from WT and CCSP−/− control mice and those that underwent a BMT with and without splenocytes at day 60. As was anticipated, CCSP−/− did not have measureable CCSP. The WT mice that underwent a BMT with splenocytes (BMS) develop OB and had a marked decrease in CCSP protein expression compared with both untreated controls and BMT controls (BM) (Fig. 1B).
Table 1.
Changes in lung proteome in WT mice
| Protein | Fold Decrease | P Value |
|---|---|---|
| Proteins decreased in BMS model vs. WT BM control | ||
| Serum albumin precursor | 1.28 | 1.0 × 10−23 |
| Lung carbonyl reductase | 2.24 | 6.4 × 10−14 |
| Superoxide dismutase | 1.59 | 1.6 × 10−11 |
| CCSP | 2.16 | 2.3 × 10−9 |
| Proteins increased in BMS model vs. WT BM control | ||
| Eosinophilic chemotactic cytokine | 4.93 | 1.7 × 10−20 |
| Transferrin | 1.49 | 3.1 × 10−15 |
| Hemopexin precursor | 1.77 | 4.4 × 10−11 |
| Vimentin | 2.63 | 1.0 × 10−8 |
WT, wild-type; CCSP, Club cell secretory protein; BMS, bone marrow transplant with splenocytes; BM, bone marrow transplant without splenocytes.
To determine the biological relevance of these 26 differentially expressed proteins identified by iTRAQ in the BMS model, we performed gene ontology enrichment analysis using the annotation-clustering tool available in DAVID. As one protein can be annotated to multiple biological processes, we used the functional annotation tool to reduce the burden of associating similar terms and focusing on the biological interpretation. We identified proteins associated with the response to wounding (P < 2.4 × 10−4), cofactors in metabolic processes (P < 0.001), and homeostasis (P < 0.004).
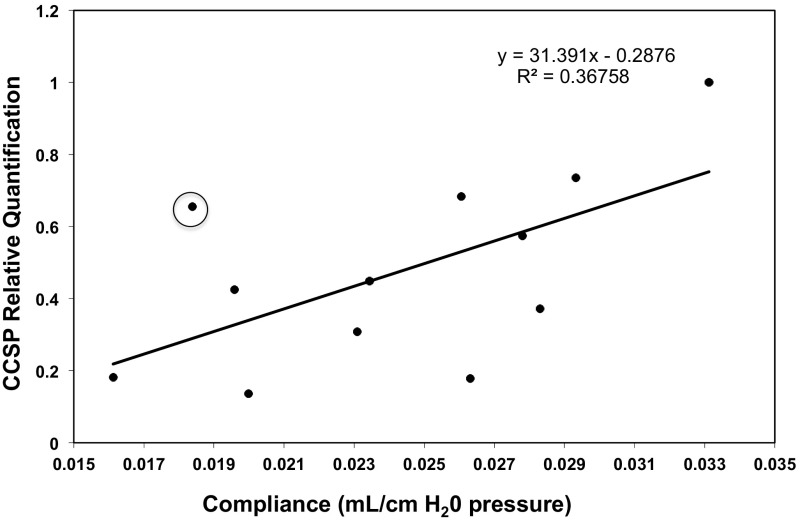
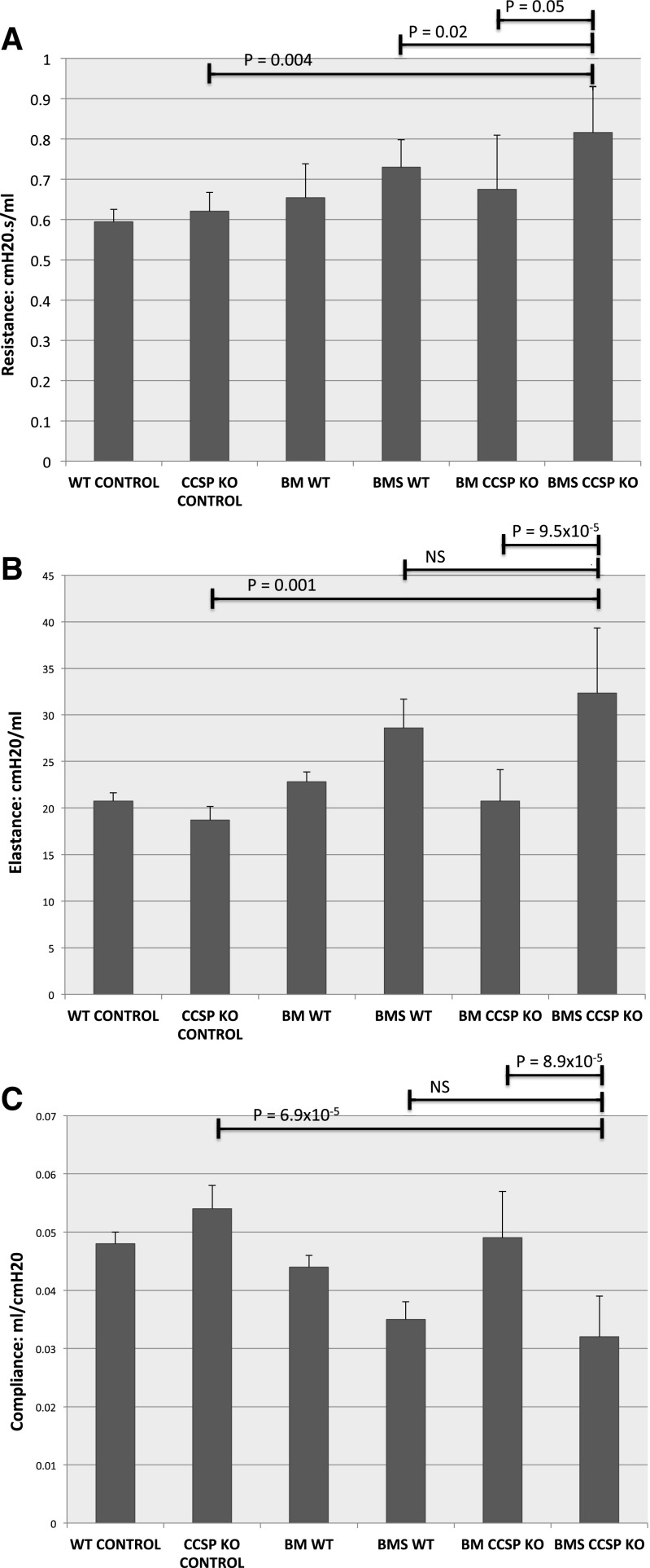
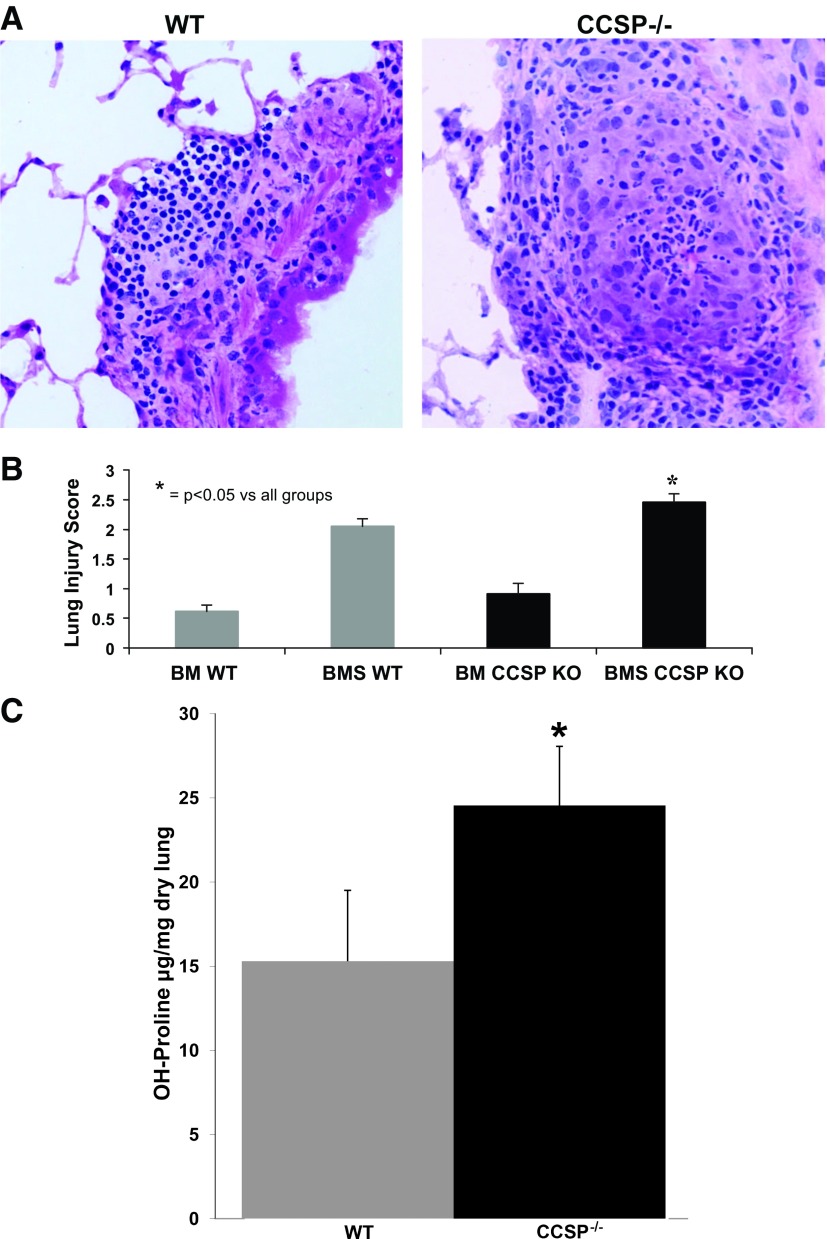
To further understand the role of CCSP in OB, we characterized lung function and histology in both WT and CCSP−/− mice after BMT. To determine the effect of CCSP deficiency on lung compliance, we performed pulmonary function tests on WT mice 60 days post-BMS. Following pulmonary function tests, the mice were euthanized, and CCSP gene expression was measured by qRT-PCR and correlated to lung compliance. We found a decrease in pulmonary compliance directly correlated with a decrease in the relative amount of CCSP gene expression (Fig. 2). We also found that CCSP−/− BMS mice demonstrated an increase in resistance compared with both CCSP−/− BM controls (P < 0.05) and WT BMS with OB (P < 0.02) (Fig. 3A). We did not find a significant difference in compliance or elastance in the OB models comparing CCSP−/− BMS mice with WT BMS. However, elastance is increased and compliance decreased in CCSP−/− BMS mice relative to their CCSP−/− BM controls (Fig. 3, B and C). The WT mice had a similar significant increase in resistance and elastance with concomitant decrease in compliance with the OB model (BMS). Histological scoring of the lungs at day 60 after BMS demonstrates a higher degree of injury in the CCSP−/− BMS mice relative to WT and CCSP−/− control BM transplant and WT BMS mice (Fig. 4, A and B). When collagen content of these lungs was measured, CCSP−/− mice had significantly higher levels of hydroxy proline (Fig. 4C). These results support a higher degree of injury and fibrosis in CCSP−/− recipients of BMS relative to WT recipients.
Fig. 2.
CCSP gene expression vs. compliance in WT mice on day 60 post-BMS. WT mice underwent a BMT with splenocytes (BMS), and at day 60 lung compliance was measured. The mice were killed after the compliance measurement, and CCSP gene expression was measured from whole lung homogenates by qRT-PCR and standardized to GADPH gene levels. CCSP gene expression is expressed relative to WT untreated controls. R2 = 0.8 with elimination of the circled data point.
Fig. 3.
Pulmonary function tests. A–C: pulmonary function tests were assessed by whole body plethysmography in anesthetized mice that were orally intubated. n = 12 for each WT BM and WT BMS, n = 8 for CCSP−/− BMS, and n = 5 for WT and CCSP−/− controls. All t-tests were with comparisons with CCSP−/− BMS. A: resistance. B: elastance. C: compliance.
Fig. 4.
CCSP−/− histology. Representative hematoxylin and eosin stains of lung demonstrating increased injury in WT vs. CCSP−/− mouse lung at day 60 after BMT with splenocytes (BMS), magnified ×200. B: histological scoring of mice that underwent a BM with splenocytes (BMS) and without splenocytes (BM) to induce OB in WT and CCSP KO mice at day 60 post-BM transplant. N = 12–19 per group in 3 separate pooled experiments. *P < 0.05 for BMS CCSP KO compared with all groups. C: hydroxy (OH)-proline levels measured from lung homogenates obtained at day 60 post-BMT plus splenocytes (BMS) in WT and CCSP−/− mice. Means ± SD are indicated for 6 mice per group pooled from 2 experiments. *P < 0.05.
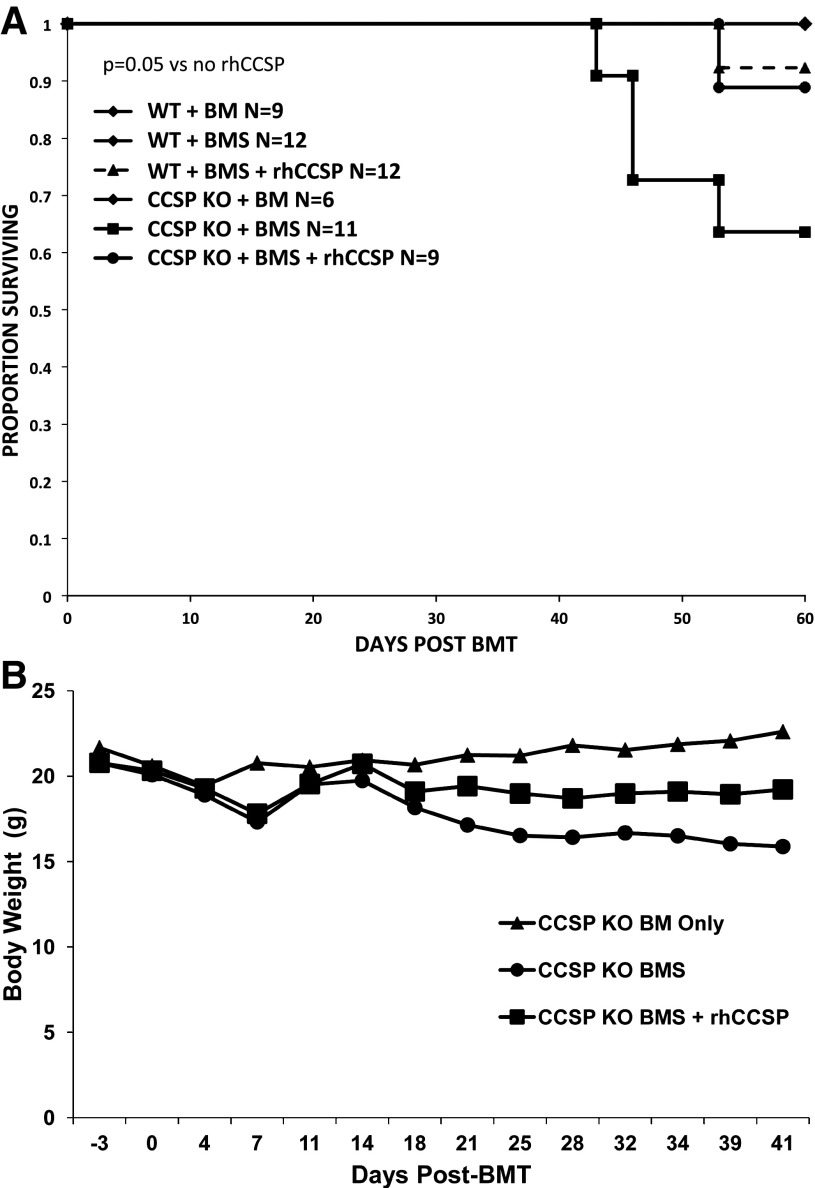
To determine whether replacement of CCSP attenuates the effects of BMS, CCSP−/− mice were administered intravenous hrCCSP weekly through day 60 following BMS. We found that the administration of hrCCSP improved the survival of CCSP−/− BMS mice with OB compared with CCSP−/− BMS mice that did not receive hrCCSP (P = 0.05, Fig. 5A). In addition, the rhCCSP-treated CCSP−/− BMS mice had a similar high 60-day survival compared with WT BMS mice with or without hrCCSP replacement. Daily weights of the mice demonstrated that the CCSP−/− BMS mice with OB had continued weight loss up to 40 days post-BMT compared with a stable weight in the CCSP−/− BM controls. The CCSP−/− BMS mice that were treated with hrCCSP initially lost weight (as all mice do in the immediate post-BMT period); however, weight stabilized slightly below that of controls (Fig. 5B).
Fig. 5.
Recombinant human (rhCCSP) replacement improves body weight and survival in CCSP−/− mice post-BMT. A: survival. CCSP−/− (KO) and WT mice underwent a BMT with splenocytes (BMS) to induce OB or without splenocytes (BM). Additional CCSP−/− (KO) and WT mice treated with splenocytes (BMS) also received weekly rhCCSP intravenous injections. P = 0.05 comparing CCSP−/− (KO) + BMS + rhCCSP with CCSP−/− (KO) + BMS without rhCCSP. B: Body weight. Body weight was measured in CCSP−/− (KO) mice that underwent a BMT without (BM) or with (BMS) splenocytes to induce OB. A subgroup of the BMS mice received weekly intravenous rhCCSP injections.
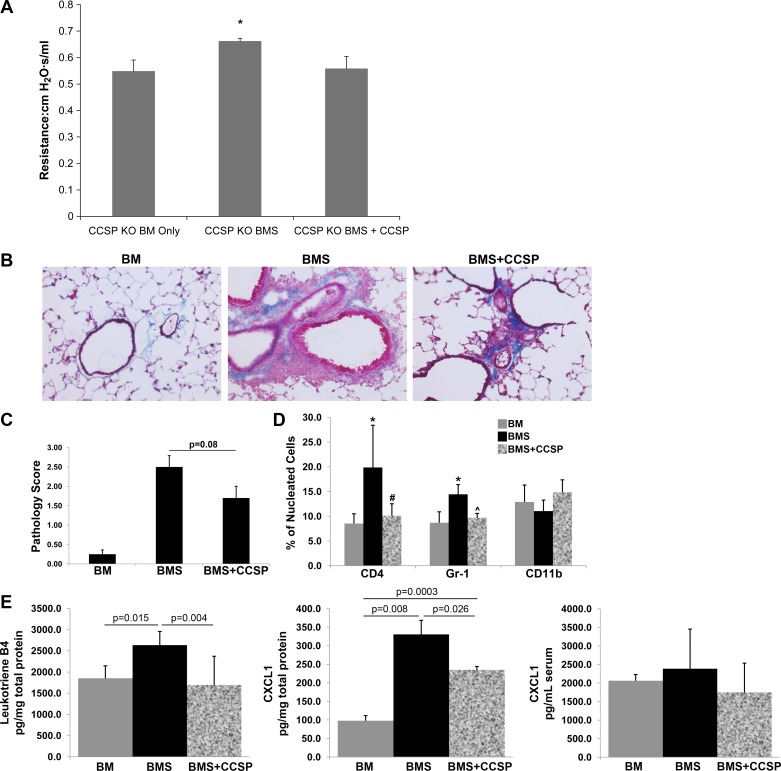
To determine whether the observed improvement in survival was associated with an improvement in lung function, we measured lung resistance in our CCSP−/− BMS mice with and without replacement of CCSP. We found that the CCSP−/− BMS mice receiving hrCCSP demonstrated an improvement in lung resistance (Fig. 6A). Trichrome staining of lung cryosections revealed perivascular inflammation and fibrosis that extended into the airways in CCSP−/− with BMS. This inflammation and fibrosis was attenuated following replacement rhCCSP treatment (Fig. 6B). Histological scoring of CCSP−/− mice with BMS also demonstrated a trend toward less injury in those that receive rhCCSP compared with those that did not receive replacement therapy (P = 0.08, Fig. 6C). Immunostaining of lung cryosections for helper T cells, mature granulocytes, and macrophages (CD4, Gr-1, and CD11b, respectively) revealed an increase in helper T cells and granulocytes with no significant change in macrophages in CCSP−/− with BMS. The inflammatory cells dissipated to that of the BM controls following CCSP weekly replacement treatments (Fig. 6C). In addition, CCSP−/− mice with BMS demonstrated a significant increase in the inflammatory markers, leukotriene B4 and neutrophil chemoattractant CXCL1, compared with BM alone (P = 0.015 and P = 0.008, Fig. 6E), whereas weekly treatments with hrCCSP in the CCSP−/− mice with BMS significantly decreased leukotriene B4 and CXCL1 compared with BMS without treatment (P = 0.004 and P = 0.026, Fig. 6E). These changes were limited to the lung, as we did not observe any significant changes in serum levels of CXCL1 with BMS or replacement treatment (Fig. 6E).
Fig. 6.
CCSP replacement improves lung function in CCSP−/− (KO) mice post-BMT. A: lung resistance was measured by whole body plethysmography in anesthetized CCSP−/− (KO) mice that were orally intubated. n = 6 for each CCSP−/− (KO) BM and CCSP−/− (KO) BMS, n = 5 for CCSP−/− (KO) BMS + rhCCSP. *P < 0.05 for CCSP KO BMS vs. all other groups. B: representative Trichrome stain of CCSP−/− mouse lung at day 60 post-BMT with and without splenocytes with or without hrCCSP replacement, magnified ×200. C: histological scoring for lung injury of CCSP−/− (KO) mice that underwent a BMT without (BM) and with (BMS) splenocytes to induce OB at day 60 post-BMT. A separate group of BMS mice underwent weekly treatment with rhCCSP (BMS + CCSP). n = 6 for each BM and BMS, n = 5 for BMS + rhCCSP. *P < 0.08 for BMS + CCSP compared with BMS. D: cell counts of helper T cells, mature granulocytes, and monocytes from lung cryosections stained for CD4, Gr-1, and CD11b, respectively. Cell counts represent positive stained cells as a percentage of total nucleated cells, n = 3. E: inflammatory markers. Leukotriene B4 (lung homogenates) and CXCL1 (lung homogenates and serum) levels from CCSP−/− (KO) mice that underwent a BMT without (BM) and with (BMS) splenocytes to induce OB at day 60 post-BMT. A separate group of BMS mice underwent weekly treatment with rhCCSP (BMS + CCSP), n = 5 for leukotriene B4 and n = 3 for CXCL1.
DISCUSSION
In this study, we demonstrate that CCSP is diminished in our murine BMT model of OB. In addition, deficiency of CCSP results in an increase in lung injury and fibrosis as measured by histological scoring and hydroxy proline content, respectively. This increased injury impairs lung mechanics and results in a decrease in survival. Replacement with intravenous rhCCSP improves survival, weight loss, and lung resistance of CCSP−/− BMS mice.
To identify biological processes present in our model, we performed protein profiling using iTRAQ with mass spectrometry. We identified 243 proteins by iTRAQ, 26 of which were significantly increased or decreased in our model relative to the BMT control. When these 26 proteins were entered into the annotation-clustering tool DAVID, we found them to be associated with wounding responses, cofactors in metabolic processes, and the homeostasis process. Among these proteins was a decrease in CCSP compared with BMT controls and healthy WT mice, a finding confirmed by CCSP ELISA. This decrease in CCSP is consistent with our and other previous reports in both human and animal models of OB (5, 16, 19, 34).
The complete role CCSP plays in the airways is unknown; however, in vitro studies link CCSP to lung homeostasis and wound healing, similar to our gene ontology analysis. Specifically, CCSP has potent immunosuppressor and anti-inflammatory roles while promoting wound healing through fibroblast migration (5, 21). In vitro, CCSP inhibits both monocyte and neutrophil chemotaxis in a dose-dependent manner. This is presumed to be secondary to CCSP inhibiting PLA2 activity. This PLA2 inhibition has been implicated in CCSP reducing integrin activation and subsequent abnormal fibronectin deposition and fibroblast migration (21, 35). This supports the findings in our model of increased injury associated with declining CCSP levels.
To elucidate the role of CCSP in vivo, Stripp, Reynolds, and colleagues (28, 29, 31) have generated CCSP-deficient mice that were used in our studies. Mice deficient for CCSP demonstrate a normal phenotype; however, they are prone to more severe lung inflammation following oxidant exposure with a propensity for fibrosis (10, 12). We used CCSP-deficient mice in our BMT model of OB to determine whether a primary deficiency of CCSP would accelerate the generation of OB. The CCSP−/− mice were treated with a lethal BMT-conditioning regimen and received rescue donor TCD bone marrow with (BMS model) or without (BM) donor splenocytes. It should be noted that the transplantation of WT donor bone marrow cells did not result in measurable CCSP serum levels in the BM recipients. We found that the CCSP−/− mice demonstrated a higher degree of histological injury and a higher collagen content, both suggesting an exaggerated inflammatory and fibroproliferative response. This higher degree of injury translated into poorer lung function and a higher mortality for the CCSP−/− mice compared with WT.
The association of low CCSP levels in lung disease states, along with a propensity toward more injury and fibrosis in its absence, has led to the investigation of disease modulation through administering therapeutic human recombinant CCSP. Miller and colleagues (13–15, 32) have demonstrated that both intravenous and inhaled hrCCSP can attenuate the inflammatory response and injury in animal models of acute lung injury. Human therapeutic applications of single doses of hrCCSP have been given safely in infants with respiratory distress with a demonstrated decrease in inflammation (11).
Our long-term model of OB following a BMT allows us to kinetically monitor the changes in lung function throughout the disease process and to assess for repair following the introduction of CCSP. With this in mind, we administered intravenous hrCCSP to mice weekly after receiving a BMT until they were evaluated at 60 days. We chose the intravenous over intratracheal route because previous studies have demonstrated a decrease in lung inflammation with the intravenous route, and it avoids confounders due to weekly sedation and intubation of the animals. We found that administration of hrCCSP significantly improved survival and body weight.
To determine whether the findings above were associated with lung-specific changes, we measured lung function and examined lung tissue for fibrosis and inflammation. We found that CCSP replacement improved lung resistance in CCSP−/− BMS mice. Histologically, CCSP−/− mice with BMS demonstrated perivascular inflammation and subsequent peribronchiolar areas of fibrosis that were diminished with hrCCSP replacement therapy. We also found less lung inflammation with a significant decrease in helper T cells and granulocytes in CCSP−/− mice with BMS that received replacement treatment. Coinciding with this, we demonstrated a decrease in the neutrophil chemoattractant CXCL1 with hrCCSP replacement. Interestingly, these effects on CXCL1 were limited to the lung, as serum levels were not significantly changed by BMS with or without replacement treatment.
CCSP has been reported to decrease PLA2 activity, a proinflammatory enzyme that hydrolyzes phospholipids to form arachidonic acid and lysophopholipids (33). These metabolites can be further metabolized to prostaglandins and leukotrienes. We found that replacement with CCSP in the CCSP−/− mice with BMS had a significant decrease in lung leukotriene B4 levels, consistent with a decrease in PLA2 activity. Although the individual effects of CCSP replacement were modest, the combined effects of systemic replacement of CCSP on lung mechanics, injury, and inflammation result in significant improvement in survival.
Currently, the therapeutic range and dosing frequency for rhCCSP delivery remains unknown, and this may require further optimization. Alternative dosing regimens or routes of administration, such as intratracheal, may be necessary to better target lung inflammation and thus preserve pulmonary function. Although the clinical use of intratracheal administration of rhCCSP has been reported (11), this was not chosen in our study due to the side effects and high mortality associated with repeated sedation and intubation of the mice.
Although the exact mechanism of action of CCSP has not been elucidated, these studies have highlighted the importance of this protein in lung function and repair in an OB model. There is no compelling evidence that the mice responded to the hrCCSP as a foreign substance because we would expect worsening of OB and not the observed improvements in lung injury, mechanics, and inflammation. Overall, our studies reveal the immunological relevance for CCSP in restoring pulmonary function.
GRANTS
This work was supported by NIH grant 1RO1 HL092171-01A1 (C. Wendt), NIH/NHLBI 2 T32 HL07741 (A. Stiehm), and HL-55209 (A. Panoskaltsis-Mortari), and the Children's Cancer Research Fund (A. Panoskaltsis-Mortari).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: C.H.W. and A.P.-M. conception and design of research; C.H.W., K.V.T., A.P.P., K.A.E., A.S., and A.P.-M. analyzed data; C.H.W., K.V.T., A.P.P., A.S., and A.P.-M. interpreted results of experiments; C.H.W., K.V.T., K.A.E., and A.P.-M. prepared figures; C.H.W., K.V.T., and A.P.-M. drafted manuscript; C.H.W. and A.P.-M. edited and revised manuscript; C.H.W. and A.P.-M. approved final version of manuscript; K.V.T., A.P.P., K.A.E., and A.S. performed experiments.
Supplementary Material
ACKNOWLEDGMENTS
The thank Dr. Sue Reynolds (National Jewish Hospital, Denver) for providing the CCSP−/− mouse, Trisha Becker for technical support, and Dr. April Pilon (Clarassance, Rockville, MD) for supplying the rhCCSP.
REFERENCES
- 1.Barth PJ, Koch S, Muller B, Unterstab F, von Wichert P, Moll R. Proliferation and number of Clara cell 10-kDa protein (CC10)-reactive epithelial cells and basal cells in normal, hyperplastic and metaplastic bronchial mucosa. Virchows Arch 437: 648–655, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Blazar BR, Taylor PA, Linsley PS, Vallera DA. In vivo blockade of CD28/CTLA4: B7/BB1 interaction with CTLA4-Ig reduces lethal murine graft-versus-host disease across the major histocompatibility complex barrier in mice. Blood 83: 3815–3825, 1994. [PubMed] [Google Scholar]
- 3.Blazar BR, Taylor PA, McElmurry R, Tian L, Panoskaltsis-Mortari A, Lam S, Lees C, Waldschmidt T, Vallera DA. Engraftment of severe combined immune deficient mice receiving allogeneic bone marrow via In utero or postnatal transfer. Blood 92: 3949–3959, 1998. [PubMed] [Google Scholar]
- 4.Bolton SJ, Pinnion K, Marshall CV, Wilson E, Barker JE, Oreffo V, Foster ML. Changes in Clara cell 10 kDa protein (CC10)-positive cell distribution in acute lung injury following repeated lipopolysaccharide challenge in the rat. Toxicol Pathol 36: 440–448, 2008. [DOI] [PubMed] [Google Scholar]
- 5.Broeckaert F, Clippe A, Knoops B, Hermans C, Bernard A. Clara cell secretory protein (CC16): features as a peripheral lung biomarker. Ann NY Acad Sci 923: 68–77, 2000. [DOI] [PubMed] [Google Scholar]
- 6.Davidson FF, Dennis EA. Biological relevance of lipocortins and related proteins as inhibitors of phospholipase A2. Biochem Pharmacol 38: 3645–3651, 1989. [DOI] [PubMed] [Google Scholar]
- 7.Holland HK, Wingard JR, Beschorner WE, Saral R, Santos GW. Bronchiolitis obliterans in bone marrow transplantation and its relationship to chronic graft-v-host disease and low serum IgG. Blood 72: 621–627, 1988. [PubMed] [Google Scholar]
- 8.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4: 44–57, 2009. [DOI] [PubMed] [Google Scholar]
- 9.Lakind JS, Holgate ST, Ownby DR, Mansur AH, Helms PJ, Pyatt D, Hays SM. A critical review of the use of Clara cell secretory protein (CC16) as a biomarker of acute or chronic pulmonary effects. Biomarkers 12: 445–467, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Lee YC, Zhang Z, Mukherjee AB. Mice lacking uteroglobin are highly susceptible to developing pulmonary fibrosis. FEBS Lett 580: 4515–4520, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Levine CR, Gewolb IH, Allen K, Welch RW, Melby JM, Pollack S, Shaffer T, Pilon AL, Davis JM. The safety, pharmacokinetics, and anti-inflammatory effects of intratracheal recombinant human Clara cell protein in premature infants with respiratory distress syndrome. Pediatr Res 58: 15–21, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Mango GW, Johnston CJ, Reynolds SD, Finkelstein JN, Plopper CG, Stripp BR. Clara cell secretory protein deficiency increases oxidant stress response in conducting airways. Am J Physiol Lung Cell Mol Physiol 275: L348–L356, 1998. [DOI] [PubMed] [Google Scholar]
- 13.Miller TL, Shashikant BN, Melby JM, Pilon AL, Shaffer TH, Wolfson MR. Recombinant human Clara cell secretory protein in acute lung injury of the rabbit: effect of route of administration. Pediatr Crit Care Med 6: 698–706, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Miller TL, Shashikant BN, Pilon AL, Pierce RA, Shaffer TH, Wolfson MR. Effects of an intratracheally delivered anti-inflammatory protein (rhCC10) on physiological and lung structural indices in a juvenile model of acute lung injury. Biol Neonate 89: 159–170, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Miller TL, Shashikant BN, Pilon AL, Pierce RA, Shaffer TH, Wolfson MR. Effects of recombinant Clara cell secretory protein (rhCC10) on inflammatory-related matrix metalloproteinase activity in a preterm lamb model of neonatal respiratory distress. Pediatr Crit Care Med 8: 40–46, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Nord M, Schubert K, Cassel TN, Andersson O, Riise GC. Decreased serum and bronchoalveolar lavage levels of Clara cell secretory protein (CC16) is associated with bronchiolitis obliterans syndrome and airway neutrophilia in lung transplant recipients. Transplantation 73: 1264–1269, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Panoskaltsis-Mortari A, Lacey DL, Vallera DA, Blazar BR. Keratinocyte growth factor administered before conditioning ameliorates graft-versus-host disease after allogeneic bone marrow transplantation in mice. Blood 92: 3960–3967, 1998. [PubMed] [Google Scholar]
- 18.Panoskaltsis-Mortari A, Taylor PA, Yaeger TM, Wangensteen OD, Bitterman PB, Ingbar DH, Vallera DA, Blazar BR. The critical early proinflammatory events associated with idiopathic pneumonia syndrome in irradiated murine allogeneic recipients are due to donor T cell infusion and potentiated by cyclophosphamide. J Clin Invest 100: 1015–1027, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Panoskaltsis-Mortari A, Tram KV, Price AP, Wendt CH, Blazar BR. A new murine model for bronchiolitis obliterans post-bone marrow transplant. Am J Respir Crit Care Med 176: 713–723, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paz HL, Crilley P, Patchefsky A, Schiffman RL, Brodsky I. Bronchiolitis obliterans after autologous bone marrow transplantation. Chest 101: 775–778, 1992. [DOI] [PubMed] [Google Scholar]
- 21.Reynolds SD, Mango GW, Gelein R, Boe IM, Lund J, Stripp BR. Normal function and lack of fibronectin accumulation in kidneys of Clara cell secretory protein/uteroglobin deficient mice. Am J Kidney Dis 33: 541–551, 1999. [DOI] [PubMed] [Google Scholar]
- 22.Reynolds SD, Reynolds PR, Snyder JC, Whyte F, Paavola KJ, Stripp BR. CCSP regulates cross talk between secretory cells and both ciliated cells and macrophages of the conducting airway. Am J Physiol Lung Cell Mol Physiol 293: L114–L123, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Singh G, Katyal SL. Clara cell proteins. Ann NY Acad Sci 923: 43–58, 2000. [DOI] [PubMed] [Google Scholar]
- 24.Snyder JC, Reynolds SD, Hollingsworth JW, Li Z, Kaminski N, Stripp BR. Clara cells attenuate the inflammatory response through regulation of macrophage behavior. Am J Respir Cell Mol Biol 42: 161–171, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soubani AO, Miller KB, Hassoun PM. Pulmonary complications of bone marrow transplantation. Chest 109: 1066–1077, 1996. [DOI] [PubMed] [Google Scholar]
- 26.Srinivasan M, Flynn R, Price A, Ranger A, Browning JL, Taylor PA, Ritz J, Antin JH, Murphy WJ, Luznik L, Shlomchik MJ, Panoskaltsis-Mortari A, Blazar BR. Donor B-cell alloantibody deposition and germinal center formation are required for the development of murine chronic GVHD and bronchiolitis obliterans. Blood 119: 1570–1580, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stripp BR, Lund J, Mango GW, Doyen KC, Johnston C, Hultenby K, Nord M, Whitsett JA. Clara cell secretory protein: a determinant of PCB bioaccumulation in mammals. Am J Physiol Lung Cell Mol Physiol 271: L656–L664, 1996. [DOI] [PubMed] [Google Scholar]
- 28.Stripp BR, Reynolds SD, Boe IM, Lund J, Power JH, Coppens JT, Wong V, Reynolds PR, Plopper CG. Clara cell secretory protein deficiency alters clara cell secretory apparatus and the protein composition of airway lining fluid. Am J Respir Cell Mol Biol 27: 170–178, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Stripp BR, Reynolds SD, Plopper CG, Boe IM, Lund J. Pulmonary phenotype of CCSP/UG deficient mice: a consequence of CCSP deficiency or altered Clara cell function? Ann NY Acad Sci 923: 202–209, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Wang SZ, Rosenberger CL, Bao YX, Stark JM, Harrod KS. Clara cell secretory protein modulates lung inflammatory and immune responses to respiratory syncytial virus infection. J Immunol 171: 1051–1060, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Watson TM, Reynolds SD, Mango GW, Boe IM, Lund J, Stripp BR. Altered lung gene expression in CCSP-null mice suggests immunoregulatory roles for Clara cells. Am J Physiol Lung Cell Mol Physiol 281: L1523–L1530, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Wolfson MR, Funanage VL, Kirwin SM, Pilon AL, Shashikant BN, Miller TL, Shaffer TH. Recombinant human Clara cell secretory protein treatment increases lung mRNA expression of surfactant proteins and vascular endothelial growth factor in a premature lamb model of respiratory distress syndrome. Am J Perinatol 25: 637–645, 2008. [DOI] [PubMed] [Google Scholar]
- 33.Yoshikawa S, Miyahara T, Reynolds SD, Stripp BR, Anghelescu M, Eyal FG, Parker JC. Clara cell secretory protein and phospholipase A2 activity modulate acute ventilator-induced lung injury in mice. J Appl Physiol 98: 1264–1271, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y, Wroblewski M, Hertz MI, Wendt CH, Cervenka TM, Nelsestuen GL. Analysis of chronic lung transplant rejection by MALDI-TOF profiles of bronchoalveolar lavage fluid. Proteomics 6: 1001–1010, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Z, Kundu GC, Yuan CJ, Ward JM, Lee EJ, DeMayo F, Westphal H, Mukherjee AB. Severe fibronectin-deposit renal glomerular disease in mice lacking uteroglobin. Science 276: 1408–1412, 1997. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.