Abstract
Maternal high-fat (HF) diet has long-term consequences on the metabolic phenotype of the offspring. Here, we determined the effects of postweaning exercise in offspring of rat dams fed HF diet during gestation and lactation. Pregnant Sprague-Dawley rats were maintained on chow or HF diet throughout gestation and lactation. All pups were weaned onto chow diet on postnatal day (PND) 21. At 4 wk of age, male pups were given free access to running wheels (RW) or remained sedentary (SED) for 3 wk, after which all rats remained sedentary, resulting in four groups: CHOW-SED, CHOW-RW, HF-SED, and HF-RW. Male HF offspring gained more body weight by PND7 compared with CHOW pups and maintained this weight difference through the entire experiment. Three weeks of postweaning exercise did not affect body weight gain in either CHOW or HF offspring, but reduced adiposity in HF offspring. Plasma leptin was decreased at the end of the 3-wk running period in HF-RW rats but was not different from HF-SED 9 wk after the exercise period ended. At 14 wk of age, intracerebroventricular injection of leptin suppressed food intake in CHOW-SED, CHOW-RW, and HF-RW, while it did not affect food intake in HF-SED group. At death, HF-RW rats also had higher leptin-induced phospho-STAT3 level in the arcuate nucleus than HF-SED rats. Both maternal HF diet and postweaning exercise had effects on hypothalamic neuropeptide and receptor mRNA expression in adult offspring. Our data suggest that postweaning exercise improves central leptin sensitivity and signaling in this model.
Keywords: maternal diet, obesity, development, exercise, leptin
the incidence of obesity in the developed and developing world has increased over the last decade (17, 27). The prevalence of childhood obesity is also increasing, suggesting that the obesity epidemic will continue to worsen (24, 33). Increasing evidence suggests that the early life environment can influence the development of obesity (15, 29). Maternal high-fat diet throughout gestation and suckling has been shown to have long-term consequences on offspring's metabolic phenotype (11, 13, 40).
It is well known that the hypothalamus plays an important role in controlling feeding and regulating energy balance. Neurons in the arcuate nucleus of the hypothalamus (ARC), which express neuropeptide Y (NPY), agouti-related peptide (AgRP), and proopiomelanocortin (POMC) play an important role in this regulation (8). In rodents, hypothalamic neurogenesis occurs during midgestation, while the neural projections between different nuclei develop during early postnatal life (7). Environmental changes during these critical periods may affect hypothalamic neurogenesis or neural projections and have long-term consequences on the offspring's metabolism (8).
Exercise has been known to affect energy balance (38, 46). Chronic exercise lowers the defended body weight gain and adiposity in diet-induced obese rats (22). Since obesity has become a worldwide health problem, weight loss therapy is particularly important, especially in children. It has been demonstrated that 3 wk of early-onset exercise in diet-induced obese (DIO) rats prolongs obesity resistance and increases central leptin sensitivity and signaling (28, 30).
We previously reported that maternal high-fat (HF) diet throughout gestation and suckling resulted in rat offspring with increased body weight, adiposity, leptin concentrations, and impaired glucose tolerance at weaning and greater susceptibility to diet-induced obesity in adulthood (40). Our studies also show that maternal HF diet during gestation or suckling impairs offspring leptin sensitivity prior to weaning (39). In this study, we sought to determine whether early postweaning exercise improves central leptin sensitivity in rat offspring exposed to maternal HF diet. We measured body weight and food intake, tested glucose tolerance, and assessed central leptin sensitivity and signaling in the offspring.
MATERIALS AND METHODS
Animals and Diet
Pregnant female Sprague-Dawley rats (Charles River, Kingston, NY) were received on gestation day 2. Animals were individually housed in conventional tub cages with access to food and water ad libitum. The room was maintained on a 12:12-h light-dark cycle with light onset at 0600. All animal procedures were approved by the Institutional Animal Care and Use Committee of the Johns Hopkins University School of Medicine.
Pregnant rats were divided into two groups and provided with one of two diets: standard chow diet (CHOW; LabDiet, 5001, 13.5% kcal from fat; n = 12) or high-fat diet (HF; Research Diets, D12492, 60% kcal from fat; n = 12). They remained on their respective diets from gestation day 2 throughout gestation and the suckling period. Dams' body weight and food intake were measured daily throughout gestation. The day the dams gave birth was designated postnatal day (PND) 0. On PND 1, litters were culled to 10 pups each (5 males and 5 females). Pups and dams were weighed once a week on PND 1, 7, 14, and 21. Dams' food intake was measured daily throughout the suckling period. On PND 21, all pups were weaned to standard chow diet. In all experiments, food spillage was recorded and accounted for in all food intake data presented.
Wheel Running
At weaning, two male pups per litter were individually housed in running-wheel cages (Mini Mitter, Bend, OR) with locked running wheels. One week later, at 4 wk of age, the running wheel for one male pup per litter was unlocked, while the second one remained locked. The pups were given free access to running wheels (RW) or remained sedentary (SED) for 3 wk, after which the running wheels were removed and rats remained sedentary. There were four groups, according to dams' diet and pups' exercise: CHOW-SED (n = 12), CHOW-RW (n = 12), HF-SED (n = 12), and HF-RW (n = 12). Body weight and food intake were measured daily during the running period. The running activity was monitored by VitalView Data Acquisition System (Mini Mitter, Bend, OR). At 11 wk of age, all of the rats were divided into two cohorts: the first cohort (n = 6 per group) was used for lateral ventricle cannulation and testing central leptin sensitivity; the second cohort (n = 6 per group) was euthanized at 14 wk of age for gene expression and body composition studies. Tail blood from the first cohort was collected (0900) for plasma leptin measurement at 6 wk (after 17 days of running) and 16 wk (sedentary for 9 wk after running) of age. For body composition, fat pads (dorsosubcutaneous, inguinal, and retroperitoneal) were unilaterally dissected and weighed.
Experiment 1
Glucose tolerance test and endocrine assays.
At 7 wk (right after 3 wk running) or 10 wk (3 wk running + 3 wk sedentary) of age, rats were food-deprived overnight for 16 h with only water available. A baseline blood sample (∼200 μl blood) was taken via a small tail nick for determination of plasma insulin. Baseline fasted blood glucose was determined at the same time by a hand-held glucose meter (Freestyle; TheraSense, Alameda, CA). Glucose (2.0 g/kg body wt, 20% glucose in sterile water solution) was then administered by oral gavage. Blood samples were collected at 15, 30, 45, 60, and 120 min after glucose gavage to determine plasma insulin levels. Blood glucose was determined at each time point using the glucometer. Plasma hormone concentrations were determined by commercially available radioimmunoassay kits for leptin and insulin (both for rats; Millipore, Billerca, MA).
Cannulation surgery.
At 11 wk of age, rats were implanted with cannulas into right lateral ventricle. The coordinates for right lateral ventricular injection were anterior-posterior (from bregma): −1.0 mm; dorsal-ventral (from skull surface): −4.3 mm; and medial-lateral: −2.0 mm. Rats recovered for 1 wk after surgery, during which time they were handled daily. Cannula placement was validated by intracerebroventricular injection of ANG II (5 nmol in 5 μl) and those rats that consumed ≥5 ml water within 30 min were used in the experiment. Because 3 of the 24 rats drank less than 5 ml water, they were not used in the food intake experiment.
Leptin effects on food intake.
Four days after the ANG II test (at 14 wk of age), food was removed 2 h prior to dark onset (1200). At dark onset (1400), all rats received intracerebroventricular injection of either 2 μl saline or leptin (A. F. Parlow, National Hormone and Peptide Program, Torrance, CA; 10 μg in 2 μl) in a counter-balanced manner. Food was returned, and food intake was measured at 4 and 22 h after injection. There was 7-day rest between the two injections.
Leptin responsiveness.
Seven days after the last injection (at 17 wk of age), rats were fasted overnight. At dark onset, rats received intracerebroventricular injection of either 2 μl saline (n = 3 per group) or leptin (10 μg in 2 μl; n = 3 per group). Three hours later, rats were killed by decapitation. Brains were removed and immediately frozen on powdered dry ice and stored at −80°C. The ARC was isolated from 600-μm-thick frozen coronal sections using a blunted 16-gauge stainless-steel needle (inner diameter = 1.65 mm) based on the coordinates described in Ref. 31.
Western blotting.
ARC samples were homogenized in lysis buffer (Sigma, St. Louis, MO) with protease inhibitor cocktail (Roche) and phosphatase inhibitor cocktail (Roche). After lysis on ice for 2 h, samples were centrifuged at 12,000 rpm at 4°C for 15 min. Protein concentration was determined using a protein assay kit (Thermo Scientific, Waltham, MA). Proteins (30 μg) were run on a 3–8% Tris-acetate gel and transferred onto PVDF membranes. Blots were blocked with 5% nonfat dry milk for 2 h. Phospho-STAT3 and STAT3 were determined using corresponding antibodies from Cell Signaling (Beverly, MA). Targeted proteins were revealed using SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific) and exposed to film (GE Healthcare, New York, NY). The intensity of bands was quantified using Scion Image Software (Scion, Frederick, MD). The ratio of the intensity of pSTAT3 to STAT3 was calculated to represent the level of phosphorylation. β-actin (Sigma, St. Louis, MO) was used as the loading control.
Experiment 2
Hypothalamic neuropeptide and receptor mRNA assays by real-time PCR.
At 14 wk of age (second cohort), rats were fasted 4 h (0900–1300) and were killed by decapitation at 1300. Brains were removed and immediately frozen on powdered dry ice and stored at −80°C. The paraventricular nucleus (PVN), ARC, ventromedial nucleus (VMN), dorsomedial hypothalamic nucleus (DMN), and lateral hypothalamus (LH) were punched from four 600-μm-thick frozen coronal sections using a blunted 16-gauge stainless-steel needle (inner diameter = 1.65 mm) based on the coordinates described in Refs. 14 and 31. Punches of PVN, ARC, VMN, DMN, and LH were put into QIAzol lysis reagent (Qiagen, Valencia, CA) and homogenized immediately with a sterile pipet tip. RNA was extracted with the RNeasy Mini Kit (Qiagen). For each individual sample, 500 ng of total RNA was used in reverse transcription using the QuantiTect reverse transcription kit (Qiagen). Expression of target genes was determined by real-time PCR using gene-specific TaqMan probes (Applied Biosystems, Foster City, CA) with TaqMan Gene Expression Master Mix (Applied Biosystems) on the ABI 7900HT Fast real-time PCR system set for 40 PCR cycles. Probes used for RT-PCR are listed in Table 1. To determine relative expression values, the −ΔΔCt method (Applied Biosystems) was used, where triplicate Ct values for each sample were averaged and subtracted from those derived from the housekeeping gene Actb.
Table 1.
TaqMan probes used for gene expression assays
| Gene Symbol | Gene Name | Refseq mRNA | TaqMan Assay ID |
|---|---|---|---|
| Bdnf | brain-derived neurotrophic factor | NM_012513.3 | Rn02531967_s1 |
| Mc4r | melanocortin 4 receptor | NM_013099.2 | Rn01491866_s1 |
| LepRb | long-form of the leptin receptor | NM_012596.1 | Rn01433205_m1 |
| Npy | neuropeptide Y | NM_012614.1 | Rn01410145_m1 |
| Pomc | proopiomelanocortin | NM_139326.2 | Rn00595020_m1 |
| Hcrt* | hypocretin | NM_013179.1 | Rn00565995_m1 |
| Pmch | pro-melanin-concentrating hormone | NM_012625.1 | Rn00561766_g1 |
| Actb | actin, beta | NM_031144.2 | Rn00667869_m1 |
Hrc denotes orexin.
Statistical analysis.
Data were analyzed by ANOVA, repeated-measures ANOVA, or Student's t-tests for independent samples as appropriate, using SPSS (SPSS 13.0; SPSS, Chicago, IL). Subsequent comparisons between groups used Newman-Keuls procedures. Data are presented as the means ± SE.
RESULTS
Dams' body weight and food intake during gestation and male pups' body weight before weaning.
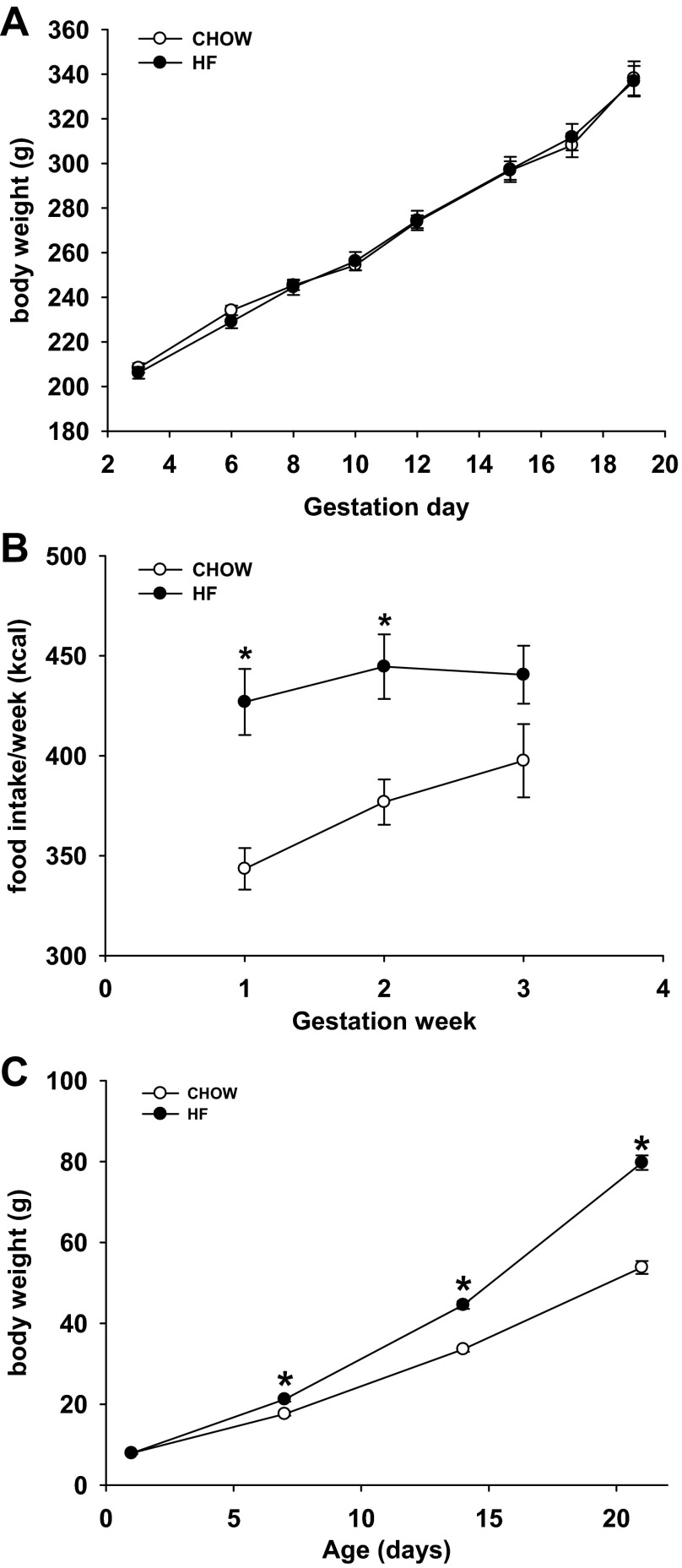
There were no significant differences in maternal body weight between the two dietary groups during the gestation period (Fig. 1A). HF dams had higher caloric intake during the first and second week of gestation than CHOW dams (P < 0.05) (Fig. 1B). By PND 7, male HF offspring were significantly heavier than those with maternal chow diet, and this difference persisted through weaning on PND 21 (P < 0.05) (Fig. 1C).
Fig. 1.
Body weight and food intake of dams during gestation and male pups' body weight before weaning. A: dams' body weight during gestation. B: dams' food intake per week during gestation. C: male pups' body weight before weaning. CHOW, n = 12; HF, n = 12. *P < 0.05 vs. CHOW group.
Effect of maternal HF diet and postweaning exercise on body weight, food intake, body composition, leptin concentration, and glucose tolerance.
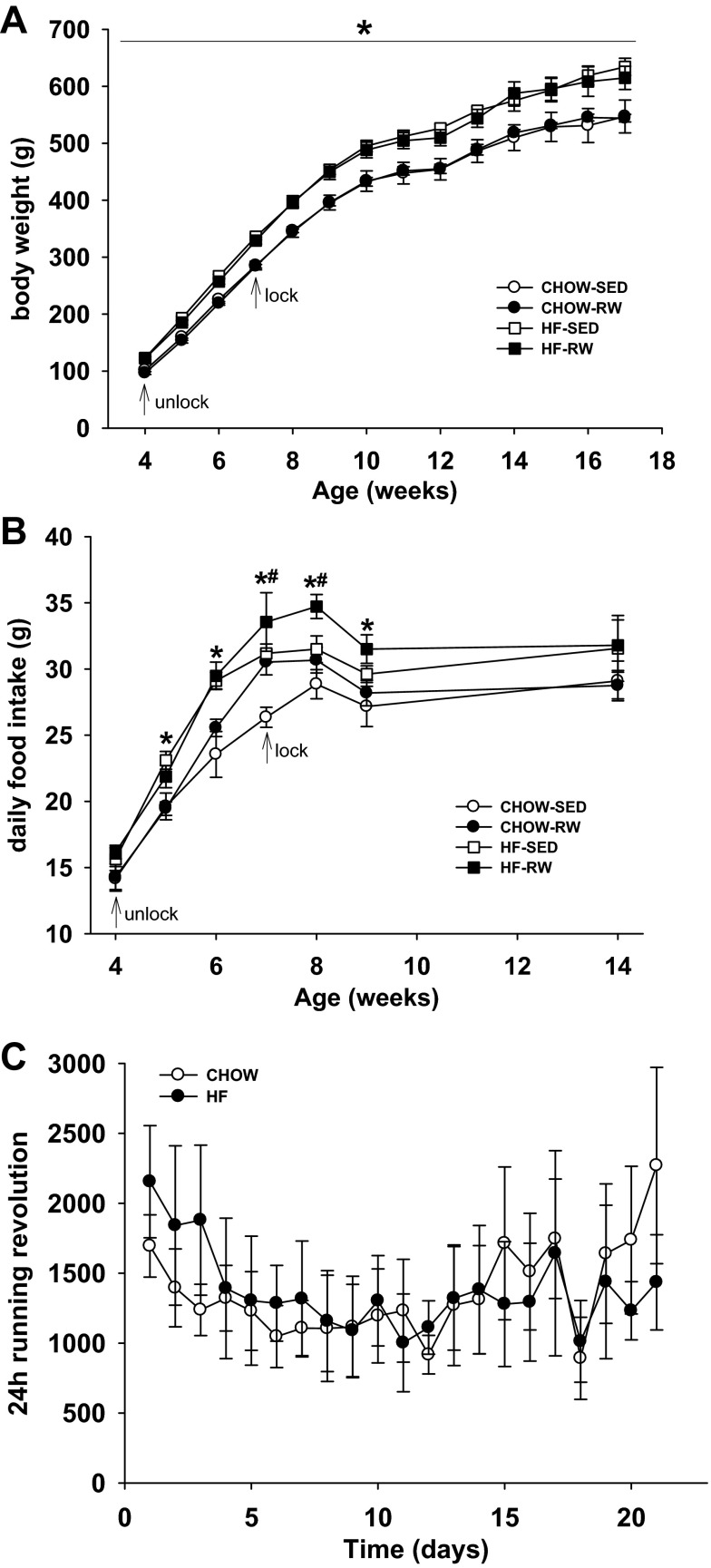
At 4 wk of age, HF pups were ∼24% heavier than CHOW pups (Fig. 2A). There was no difference in daily running revolution between the two dietary groups during 3 wk of running (Fig. 2C). There was no effect of exercise on body weight in either CHOW or HF rats (Fig. 2A). During the 21-day running period, HF rats consumed more food than CHOW rats (P < 0.05) (Fig. 2B). In the last week of running, exercised rats had greater food intake than sedentary rats in both CHOW and HF groups (P < 0.05), and this persisted until 1 wk after running (Fig. 2B).
Fig. 2.
At 4 wk of age, male offspring from CHOW or HF dams were left sedentary or given free access to exercise on a running wheel. RW groups were allowed to exercise for 3 wk, after which time their wheels were removed and all rats remained sedentary. A: body weight during and after running. B: daily food intake during and after running. C: daily running revolution during 3 wk of running. *Main effect of maternal HF diet, P < 0.05 vs. maternal chow diet. #Main effect of running, P < 0.05 vs. sedentary group.
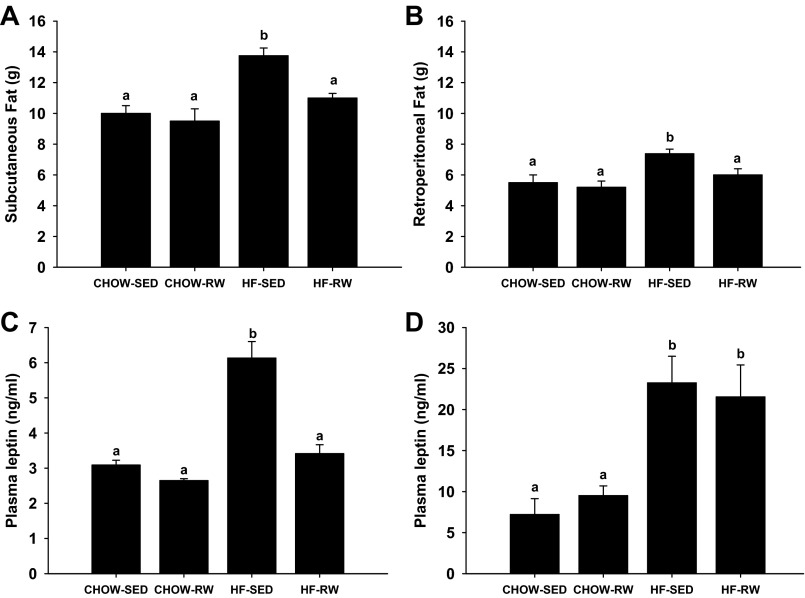
At 14 wk of age, HF rats had more subcutaneous and retroperitoneal fat than CHOW rats (Fig. 3, A and B). Three weeks of postweaning exercise had no effect on adipose depots in CHOW rats, while it decreased both subcutaneous and retroperitoneal fat in HF rats (Fig. 3, A and B). Running reduced plasma leptin levels in HF rats (P < 0.05), but not in CHOW rats (Fig. 3C). At 16 wk of age (sedentary for 9 wk after running), HF rats had higher leptin than CHOW rats, but there was no effect of exercise on plasma leptin concentration in either CHOW or HF rats (Fig. 3D).
Fig. 3.
Adipose depots (A and B) at 14 wk of age and plasma leptin concentration at 6 wk (after 17 days running) (C) and 16 wk (sedentary for 9 wk after running) (D) of age. a,bGroups with different superscript letters differ from each other at P < 0.05 level.
At 7 wk (right after 3 wk of running) and 10 wk (sedentary for 3 wk after running) of age, rats were challenged with a glucose tolerance test. At 7 wk, there were no differences among the groups in glucose clearance (Fig. 4A). However, HF rats had higher insulin level at 0, 30, 45, 60, and 120 min (P < 0.05). There was a main effect of exercise on insulin level at 0 and 120 min (P < 0.05). Rats in HF-SED group had higher insulin level than the other three groups at 0 and 120 min (P < 0.05) (Fig. 4C). At 10 wk, there were no differences among the groups in glucose clearance and insulin level (Fig. 4, B and D).
Fig. 4.
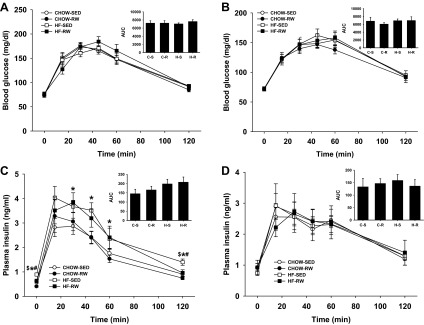
Glucose tolerance test (2.0 g/kg, oral gavage) at 7 wk (right after 3 wk running) (A and C) and 10 wk (sedentary for 3 wk after running) (B and D) of age. Blood glucose (A and B) and plasma insulin (C and D) were determined for 2 h after administration of glucose. The integrated area under the curve (AUC) was determined for glucose and insulin using the trapezoidal method. *Main effect of maternal HF diet, P < 0.05 vs. maternal CHOW diet. #Main effect of running, P < 0.05 vs. sedentary group. $P < 0.05, HF-SED vs. other three groups. C-S, CHOW-SED; C-R, CHOW-RW; H-S, HF-SED; H-R, HF-RW.
Effect of maternal HF diet and postweaning exercise on central leptin sensitivity and signaling.
To assess whether 3 wk of postweaning exercise alters central leptin sensitivity, rats received intracerebroventricular injection of saline or 10 μg leptin at 14 wk of age, and food intake was measured at 4 h and 22 h. Leptin suppressed cumulative food intake in CHOW-SED, CHOW-RW, and HF-RW groups at both 4 h and 22 h (P < 0.05) but did not significantly affect food intake in HF-SED group (Fig. 5). These results demonstrate that maternal HF diet decreased offspring's leptin sensitivity in adulthood, and postweaning exercise can rescue it.
Fig. 5.
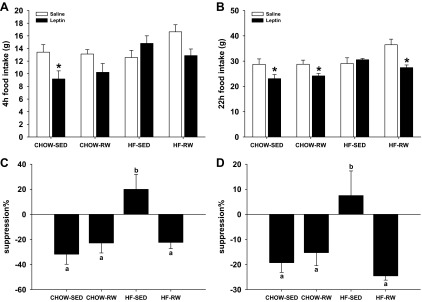
At 14 wk of age, rats received intracerebroventricular injection of saline or 10 μg leptin, and food intake was monitored at 4 h (A and C) and 22 h (B and D). Cumulative food intake (A and B) and suppression of food intake in response to leptin (C and D) were analyzed. *P < 0.05 vs. saline group; a,bGroups with different superscript letters differ from each other at P < 0.05 level.
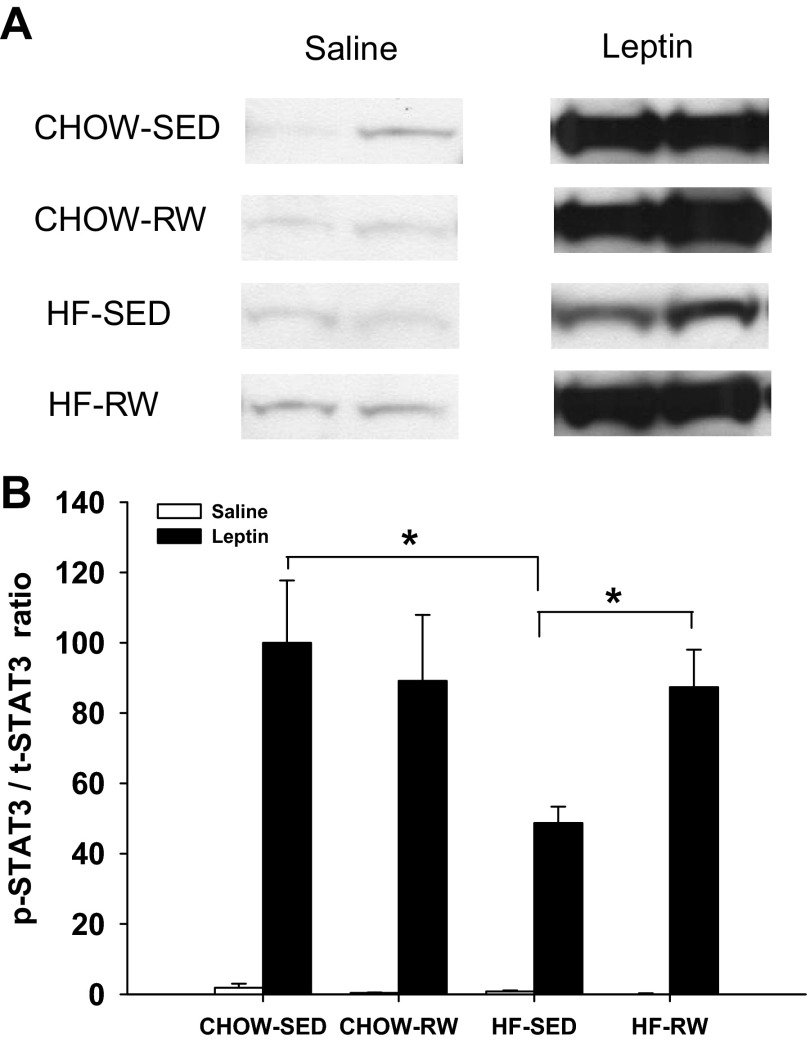
Since postweaning exercise led to an increase in the anorectic effect of leptin in HF rats, we thought that this would be related with an increase in leptin signaling in the hypothalamus. At 17 wk of age, after an overnight fast, rats received intracerebroventricular injection of saline or 10 μg leptin at dark onset and were decapitated 3 h later. We measured p-STAT3 protein in ARC as a marker of leptin signaling activation. There were no differences in baseline levels of p-STAT3/t-STAT3 ratios among the groups. Leptin injection significantly increased p-STAT3 level in ARC in all four groups (P < 0.05) (Fig. 6B). However, leptin induced significantly less p-STAT3 in the ARC of HF-SED rats compared with that induced in CHOW-SED rats (P < 0.05), indicating that maternal HF diet reduces leptin-induced activation of STAT3 in adult offspring. There was no difference in leptin-induced p-STAT3 between CHOW-SED and CHOW-RW groups, while rats in HF-RW group had higher p-STAT3 level after leptin challenge compared with HF-SED group (P < 0.05), demonstrating that postweaning exercise improves leptin-induced activation of STAT3 in HF rats.
Fig. 6.
At 17 wk of age, after overnight fast, rats received intracerebroventricular injection of saline (n = 3) or 10 μg leptin (n = 3) at dark onset and were decapitated 3 h later. p-STAT3 and t-STAT3 were measured in ARC by Western blot. A: band for p-STAT3. B: p-STAT3/t-STAT3 ratio (compared with leptin-injected rats in CHOW-SED group). *P < 0.05.
Effects of maternal HF diet and postweaning exercise on hypothalamic neuropeptide and receptor mRNA expression.
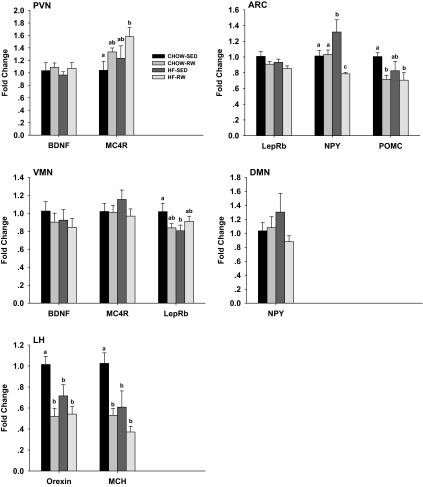
We measured neuropeptide and receptor mRNA expression in the brain of offspring (Fig. 7). At 14 wk of age, rats in HF-RW group had 50% higher mRNA expression of PVN MC4R (P = 0.034) compared with CHOW-SED group. Male HF offspring that were sedentary (HF-SED) had a 30% increase (P = 0.001) in ARC NPY mRNA expression compared with CHOW-SED rats, while postweaning exercise (HF-RW) resulted in a 40% decrease in ARC NPY mRNA expression compared with HF-SED rats. Rats in CHOW-RW and HF-RW groups both had 30% lower (P = 0.043) mRNA expression of ARC POMC compared with CHOW-SED rats. HF-SED had a 20% decrease (P = 0.044) in VMN leptin receptor-b mRNA expression compared with CHOW-SED rats. Rats in CHOW-RW, HF-SED, and HF-RW groups all had lower mRNA expression of LH orexin (P = 0.001) and melanin-concentrating hormone (MCH; P = 0.001) compared with CHOW-SED group. There were no other significant differences in other genes assessed among these groups.
Fig. 7.
Gene expression in the hypothalamus. At 14 wk of age, after a 4-h fast, rats were killed by decapitation (n = 6 per group). Tissue from the paraventricular nucleus (PVN), arcuate nucleus (ARC), ventromedial nucleus (VMN), dorsomedial hypothalamic nucleus (DMN), and lateral hypothalamus (LH) were punched and assayed by quantitative RT-PCR. BDNF, brain-derived neurotropic factor; MC4R, melanocortin-4 receptor; LepRb, leptin receptor-b; NPY, neuropeptide Y; POMC, proopiomelanocortin; MCH, melanin concentrating hormone. Expression level of each gene examined was normalized to β-actin mRNA levels. a,b,cParameters with different superscript letters differ from each other at P < 0.05 level.
DISCUSSION
We previously found that maternal HF diet can increase body weight and adiposity, impair glucose tolerance, and alter leptin sensitivity in neonatal rats (40). Exercise is often considered to be an effective treatment for obesity. Previous studies have shown that 3 wk of early-onset exercise in DIO rats prolongs obesity resistance and increases central leptin sensitivity and signaling (28, 30). In this study, we used a different obese model-obese offspring from dams fed HF diet during gestation and lactation to determine whether early-onset exercise can reduce body weight or improve central leptin sensitivity in these rats. Our data suggest that postweaning exercise improves central leptin sensitivity and signaling without affecting body weight in male HF offspring.
Consistent with previous studies using HF diet only during gestation, those dams fed the HF diet consumed more calories during gestation and lactation, but body weight did not differ significantly between the dietary groups throughout the experiment, perhaps suggesting that the HF-fed dams may have increased their energy expenditure during this time (1). These data are also consistent with our previous studies (39, 40), which also showed similar results.
The offspring from HF dams in this study were heavier than that of chow-fed dams at weaning, and the difference persisted through adulthood after being weaned on a chow diet. This is in contrast with our previous study (40), in which body weight of HF offspring was higher at weaning, but was not different from CHOW offspring in adulthood when weaned on chow diet. Only those pups that were weaned on HF diet were heavier than control pups, suggesting that HF offspring were more susceptible to diet-induced obesity. The chow diet used in this study (LabDiet 5001) contains sugars (4.22%), while the diet used previously did not. HF offspring were hyperphagic on the LabDiet 5001 in this study, while those on Harlan Teklad 2018 in our previous study were not. Thus, the difference in body weight outcomes in this study compared with our previous study may be due to greater palatability of the chow diet used. Others have demonstrated deficits in the dopamine and opioid reward systems of HF offspring (41), and this is a possibility in our study but remains to be tested directly.
In this experiment, we found no significant difference in body weight between the SED group and RW group, suggesting that the exercise provided no protection against gains in body mass. Patterson et al.'s 2007 study found that 3 wk of early-onset exercise can decrease body weight gain in DIO rats (30); Bi et al. (6) also have shown that voluntary running activity beginning at 8 wk of age normalized body weight in Otsuka Long-Evans Tokushima fatty (OLETF) rats. However, several other studies have also reported access to a running wheel had little impact on body mass (9, 16, 18, 21). Studies with varying experimental factors, including strain, duration, and type of exercise, dietary fat content, and palatability, reported that exercise is associated with increased (26), decreased (19), or no change (22) in food intake. In our experiment, exercise did not affect food intake in the first 2 wk, and increased food intake in the last week of running. One possible explanation for this phenomenon is that the offspring were only 4 wk of age when exercise started, and they were in the period of rapid growth. In this case, exercise did not reduce food intake in these animals.
Although 3 wk postweaning exercise provided no protection against gains in body weight in our experiment, we found that exercise reduced both subcutaneous and retroperitoneal fat in adult HF rats. Schroeder et al. (35) showed that postweaning voluntary exercise reduced inguinal and retroperitoneal fat in male OLETF rats. Patterson et al. (30) also found that postweaning exercise reduced fat depot in DIO rats. A similar study by Rajia et al. (32) showed that voluntary postweaning exercise reduced fat mass in female offspring of HF dams. Thus, although postweaning exercise did not alter food intake or body weight of HF offspring, our data and those of others suggest that the exercise has a long-term effect in decreasing adiposity. These distinct differences in the response an individual has to exercise may stem from the underlying cause of the obesity to start with (i.e., genetic, dietary, and environmental). Use of multiple models may help facilitate elucidation of the specific mechanisms that mediate the beneficial effects of exercise against obesity and associated metabolic diseases.
Our previous study shows that maternal HF diet throughout gestation and suckling resulted in rat offspring with increased adiposity and plasma leptin concentrations at weaning (40). In this study, male HF offspring continue to have higher plasma leptin concentration in adulthood, even after weaned onto chow diet, consistent with their greater body weight. We show that 3 wk of running reduces plasma leptin in HF offspring during the running period, which is consistent with other previous studies (6, 28). A study by Patterson et al. (28) showed that 3 wk of postweaning exercise in DIO rats did not modify the development of terminal density of ARC AgRP or α-MSH fibers innervating the PVN. However, they report increased leptin receptor (Lepr-b) binding and greater leptin-induced signaling downstream of the receptor without changes in Lepr-b mRNA expression (28).
Many previous studies showed that wheel running increased leptin signaling in several brain regions (28, 34, 37) and changed hypothalamic neuropeptide expression involved in the regulation of energy balance (6, 25, 30). Although exercise provided no protection against gains in body mass in our experiment, we found that 3 wk of postweaning exercise improved central leptin sensitivity and signaling in HF offspring. Our previous study showed that maternal HF diet during gestation or suckling altered offspring leptin sensitivity prior to weaning (39). In this study, maternal HF diet throughout gestation and suckling continues to reduce central leptin sensitivity in adulthood, even after weaned onto a chow diet, which is consistent with the study by Ferezou-Viala et al. (13). Patterson et al. (28) showed that 3 wk of postweaning exercise in DIO rats increased the anorectic effect of leptin at 4 wk after exercise cessation, while neither the previously exercised, nor the continuously sedentary rats responded to leptin by reducing their food intake by 10 wk after exercise cessation. Our results show that 3 wk of postweaning exercise increases the anorectic effect of leptin at 7 wk after exercise cessation (14 wk of age) in HF offspring. There are several differences between these two studies: First, the DIO rats that Patterson used were weaned onto the HF diet, which may cause leptin resistance after prolonged exposure, while our HF offspring were weaned onto a chow diet; Second, the leptin challenge used was an intraperitoneal injection in Patterson's study, in which leptin resistance may be due to reduced leptin transport across the blood-brain barrier (BBB) associated with the development of obesity on an HF diet in DIO rats (23), while the leptin challenge used was an intracerebroventricular injection in our study. The increased anorectic effect of leptin is consistent with increased central leptin signaling in ARC in our experiment.
One possible explanation for changed central leptin sensitivity and signaling is alterations in the central pathways involved in the regulation of energy balance. In this experiment, maternal HF diet increased ARC NPY expression, while postweaning exercise lowered ARC NPY expression in these rats. Kozak et al. (20) similarly found that HF offspring tended to have higher ARC NPY concentration in adulthood. Patterson et al. (30) showed that postweaning exercise had no effect on ARC NPY expression in DIO rats. The NPY expression in DMN had a similar trend with ARC NPY, but there was no significant difference. In contrast with other studies (30), we found RW rats had a decreased ARC POMC expression. If it were associated with a decreased release of the anorectic peptide α-MSH, it could increase food intake in RW rats, which was consistent with our food intake result—RW rats had more food intake than SED rats in the last week of running, and this persisted 1 wk after running. Further research will be done to see whether there is a change of ARC POMC expression right after running. There was no difference in the long form leptin receptor (LepRb) expression in ARC, while HF offspring had lower VMN LepRb expression, which may be a reason for reduced central leptin sensitivity. Many studies have shown that brain-derived neurotrophic factor (BDNF) in PVN and VMN reduces energy intake and increases energy expenditure (42–45), which may work through interacting with MC4R (48). We did not see any change in PVN BDNF, VMN BDNF, or VMN MC4R expression, while RW rats tended to have higher PVN MC4R, which may increase energy expenditure in RW rats even after running. LH orexin and MCH have been shown to be involved in both homeostatic and reward-based food intake (10, 12), and what we found in this experiment was that both maternal HF diet and postweaning exercise decreased LH orexin and MCH expression, although the reason for this decrease is unclear. However, changes in mRNA expression are not necessarily representative of parallel changes in peptide release or receptor function. Further research will be done at the protein level.
Finally, it is interesting to see that in HF offspring, postweaning exercise improved central leptin sensitivity and signaling, and it changed mRNA expression of hypothalamic neuropeptide and receptor, which are involved in energy balance, while it provided no protection against gains in body mass. One possible explanation is that HF offspring have impaired BBB transport (2–5), and postweaning exercise did not improve it. Therefore, although HF-RW rats have normal central leptin sensitivity and signaling, peripheral leptin cannot pass through the BBB to activate the leptin receptor. Further studies with peripheral leptin injection may be helpful in understanding this. Another consideration is that all offspring were weaned on a standard chow diet. Weaning on a HF diet may reveal the protective effects of exercise in HF offspring against weight gain compared with those that remain sedentary. Although postweaning exercise provided no protection against gains in body weight, it reduced adipose depots in HF offspring, which may have a relationship with improved central leptin sensitivity and changed hypothalamic neuropeptide expression (36, 47).
Perspectives and Significance
Early life environment can influence the development of obesity, and a growing body of evidence suggests that maternal HF diet during gestation and suckling has long-term consequences on offspring's metabolic phenotype. We demonstrate here that 3 wk of early postweaning exercise improved central leptin sensitivity and signaling, changed hypothalamic mRNA expression, and reduced fat depots in HF offspring, while it had no effect on body weight. Further studies are needed to elucidate these mechanisms, including BBB transport of leptin, HF diet challenge after weaning, measurement of energy expenditure, and so on. These data provide a better understanding for how maternal HF diet affects adult offspring's metabolic phenotype and how early life exercise can partially moderate it.
GRANTS
This study was supported by National Institutes of Health grant HD-055030 and DK-077623. Bo Sun was supported by an Exchanging Scholarship from the China Scholarship Council of the Ministry of Education of China.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.S., J.Y., and K.L.K.T. conception and design of research; B.S., N.-C.L., E.R.E., R.H.P., G.J.B., and K.L.K.T. performed experiments; B.S. analyzed data; B.S., N.-C.L., and K.L.K.T. interpreted results of experiments; B.S. prepared figures; B.S. and K.L.K.T. drafted manuscript; B.S., N.-C.L., E.R.E., R.H.P., G.J.B., J.Y., and K.L.K.T. approved final version of manuscript; N.-C.L., G.J.B., J.Y., and K.L.K.T. edited and revised manuscript.
ACKNOWLEDGMENTS
We thank Dr. Su Gao (Scripps Institute, Jupiter, FL) for technical assistance.
REFERENCES
- 1.Ainge H, Thompson C, Ozanne SE, Rooney KB. A systematic review on animal models of maternal high fat feeding and offspring glycaemic control. Int J Obes (Lond) 35: 325–335, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Banks WA. Is obesity a disease of the blood-brain barrier? Physiological, pathological, and evolutionary considerations. Curr Pharm Des 9: 801–809, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Banks WA, Coon AB, Robinson SM, Moinuddin A, Shultz JM, Nakaoke R, Morley JE. Triglycerides induce leptin resistance at the blood-brain barrier. Diabetes 53: 1253–1260, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Banks WA, DiPalma CR, Farrell CL. Impaired transport of leptin across the blood-brain barrier in obesity. Peptides 20: 1341–1345, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Banks WA, Farrell CL. Impaired transport of leptin across the blood-brain barrier in obesity is acquired and reversible. Am J Physiol Endocrinol Metab 285: E10–E15, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Bi S, Scott KA, Hyun J, Ladenheim EE, Moran TH. Running wheel activity prevents hyperphagia and obesity in Otsuka Long-Evans Tokushima Fatty rats: role of hypothalamic signaling. Endocrinology 146: 1676–1685, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Bouret SG. Development of hypothalamic neural networks controlling appetite. Forum Nutr 63: 84–93, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Bouret SG. Early life origins of obesity: role of hypothalamic programming. J Pediatr Gastroenterol Nutr 48 Suppl 1: S31–S38, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Brownlow BS, Petro A, Feinglos MN, Surwit RS. The role of motor activity in diet-induced obesity in C57BL/6J mice. Physiol Behav 60: 37–41, 1996 [DOI] [PubMed] [Google Scholar]
- 10.Cason AM, Smith RJ, Tahsili-Fahadan P, Moorman DE, Sartor GC, Aston-Jones G. Role of orexin/hypocretin in reward-seeking and addiction: implications for obesity. Physiol Behav 100: 419–428, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen H, Simar D, Morris MJ. Hypothalamic neuroendocrine circuitry is programmed by maternal obesity: interaction with postnatal nutritional environment. PLos One 4: e6259, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DiLeone RJ, Georgescu D, Nestler EJ. Lateral hypothalamic neuropeptides in reward and drug addiction. Life Sci 73: 759–768, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Ferezou-Viala J, Roy AF, Serougne C, Gripois D, Parquet M, Bailleux V, Gertler A, Delplanque B, Djiane J, Riottot M, Taouis M. Long-term consequences of maternal high-fat feeding on hypothalamic leptin sensitivity and diet-induced obesity in the offspring. Am J Physiol Regul Integr Comp Physiol 293: R1056–R1062, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Gao S, Kinzig KP, Aja S, Scott KA, Keung W, Kelly S, Strynadka K, Chohnan S, Smith WW, Tamashiro KL, Ladenheim EE, Ronnett GV, Tu Y, Birnbaum MJ, Lopaschuk GD, Moran TH. Leptin activates hypothalamic acetyl-CoA carboxylase to inhibit food intake. Proc Natl Acad Sci USA 104: 17358–17363, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glavas MM, Kirigiti MA, Xiao XQ, Enriori PJ, Fisher SK, Evans AE, Grayson BE, Cowley MA, Smith MS, Grove KL. Early overnutrition results in early-onset arcuate leptin resistance and increased sensitivity to high-fat diet. Endocrinology 151: 1598–1610, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harri M, Lindblom J, Malinen H, Hyttinen M, Lapvetelainen T, Eskola S, Helminen HJ. Effect of access to a running wheel on behavior of C57BL/6J mice. Lab Anim Sci 49: 401–405, 1999 [PubMed] [Google Scholar]
- 17.Haslam DW, James WP. Obesity. Lancet 366: 1197–1209, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Jung AP, Luthin DR. Wheel access does not attenuate weight gain in mice fed high-fat or high-CHO diets. Med Sci Sports Exer 42: 355–360, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kibenge MT, Chan CB. The effects of high-fat diet on exercise-induced changes in metabolic parameters in Zucker fa/fa rats. Metabolism 51: 708–715, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Kozak R, Burlet A, Burlet C, Beck B. Dietary composition during fetal and neonatal life affects neuropeptide Y functioning in adult offspring. Brain Res 125: 75–82, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Laker RC, Gallo LA, Wlodek ME, Siebel AL, Wadley GD, McConell GK. Short-term exercise training early in life restores deficits in pancreatic β-cell mass associated with growth restriction in adult male rats. Am J Physiol Endocrinol Metab 301: E931–E940, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Levin BE, Dunn-Meynell AA. Chronic exercise lowers the defended body weight gain and adiposity in diet-induced obese rats. Am J Physiol Regul Integr Comp Physiol 286: R771–R778, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Levin BE, Dunn-Meynell AA, Banks WA. Obesity-prone rats have normal blood-brain barrier transport but defective central leptin signaling before obesity onset. Am J Physiol Regul Integr Comp Physiol 286: R143–R150, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Molinari-Buchi B, Barth J, Janner M, Frey P. [Overweight and obesity in children: known facts and new trends]. Revue Med Suisse 6: 1022–1025, 2010 [PubMed] [Google Scholar]
- 25.Molteni R, Wu A, Vaynman S, Ying Z, Barnard RJ, Gomez-Pinilla F. Exercise reverses the harmful effects of consumption of a high-fat diet on synaptic and behavioral plasticity associated to the action of brain-derived neurotrophic factor. Neuroscience 123: 429–440, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Monda M, Amaro S, De Luca B. The influence of exercise on energy balance changes induced by ventromedial hypothalamic lesion in the rat. Physiol Behav 54: 1057–1061, 1993 [DOI] [PubMed] [Google Scholar]
- 27.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 295: 1549–1555, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Patterson CM, Bouret SG, Dunn-Meynell AA, Levin BE. Three weeks of postweaning exercise in DIO rats produces prolonged increases in central leptin sensitivity and signaling. Am J Physiol Regul Integr Comp Physiol 296: R537–R548, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patterson CM, Bouret SG, Park S, Irani BG, Dunn-Meynell AA, Levin BE. Large litter rearing enhances leptin sensitivity and protects selectively bred diet-induced obese rats from becoming obese. Endocrinology 151: 4270–4279, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patterson CM, Dunn-Meynell AA, Levin BE. Three weeks of early-onset exercise prolongs obesity resistance in DIO rats after exercise cessation. Am J Physiol Regul Integr Comp Physiol 294: R290–R301, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Rajia S, Chen H, Morris MJ. Voluntary post weaning exercise restores metabolic homeostasis in offspring of obese rats. Nutr Metab Cardiovasc Dis 23: 574–581, 2013 [DOI] [PubMed] [Google Scholar]
- 33.Rocchini AP. Childhood obesity and a diabetes epidemic. N Engl J Med 346: 854–855, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Scarpace PJ, Matheny M, Zhang Y. Wheel running eliminates high-fat preference and enhances leptin signaling in the ventral tegmental area. Physiol Behav 100: 173–179, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schroeder M, Shbiro L, Gelber V, Weller A. Post-weaning voluntary exercise exerts long-term moderation of adiposity in males but not in females in an animal model of early-onset obesity. Horm Behav 57: 496–505, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature 404: 661–671, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Shapiro A, Cheng KY, Gao Y, Seo DO, Anton S, Carter CS, Zhang Y, Tumer N, Scarpace PJ. The act of voluntary wheel running reverses dietary hyperphagia and increases leptin signaling in ventral tegmental area of aged obese rats. Gerontology 57: 335–342, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stubbs RJ, Sepp A, Hughes DA, Johnstone AM, King N, Horgan G, Blundell JE. The effect of graded levels of exercise on energy intake and balance in free-living women. Int J Obes Relat Metab Disord 26: 866–869, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Sun B, Purcell RH, Terrillion CE, Yan J, Moran TH, Tamashiro KL. Maternal high-fat diet during gestation or suckling differentially affects offspring leptin sensitivity and obesity. Diabetes 61: 2833–2841, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tamashiro KL, Terrillion CE, Hyun J, Koenig JI, Moran TH. Prenatal stress or high-fat diet increases susceptibility to diet-induced obesity in rat offspring. Diabetes 58: 1116–1125, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vucetic Z, Kimmel J, Totoki K, Hollenbeck E, Reyes TM. Maternal high-fat diet alters methylation and gene expression of dopamine and opioid-related genes. Endocrinology 151: 4756–4764, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang C, Bomberg E, Billington C, Levine A, Kotz CM. Brain-derived neurotrophic factor in the hypothalamic paraventricular nucleus increases energy expenditure by elevating metabolic rate. Am J Physiol Regul Integr Comp Physiol 293: R992–R1002, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Wang C, Bomberg E, Billington C, Levine A, Kotz CM. Brain-derived neurotrophic factor in the hypothalamic paraventricular nucleus reduces energy intake. Am J Physiol Regul Integr Comp Physiol 293: R1003–R1012, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Wang C, Bomberg E, Levine A, Billington C, Kotz CM. Brain-derived neurotrophic factor in the ventromedial nucleus of the hypothalamus reduces energy intake. Am J Physiol Regul Integr Comp Physiol 293: R1037–R1045, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Wang C, Godar RJ, Billington CJ, Kotz CM. Chronic administration of brain-derived neurotrophic factor in the hypothalamic paraventricular nucleus reverses obesity induced by high-fat diet. Am J Physiol Regul Integr Comp Physiol 298: R1320–R1332, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wing RR. Physical activity in the treatment of the adulthood overweight and obesity: current evidence and research issues. Med Sci Sports Exer 31: S547–S552, 1999 [DOI] [PubMed] [Google Scholar]
- 47.Woods SC, Seeley RJ, Porte D, Jr, Schwartz MW. Signals that regulate food intake and energy homeostasis. Science 280: 1378–1383, 1998 [DOI] [PubMed] [Google Scholar]
- 48.Xu B, Goulding EH, Zang K, Cepoi D, Cone RD, Jones KR, Tecott LH, Reichardt LF. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat Neurosci 6: 736–742, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]