Abstract
Noradrenergic A2 neurons in nucleus tractus solitarius (NTS) respond to stressors such as hypoxia. We hypothesize that tyrosine hydroxylase (TH) knockdown in NTS reduces cardiovascular responses to chronic intermittent hypoxia (CIH), a model of the arterial hypoxemia observed during sleep apnea in humans. Adult male Sprague-Dawley rats were implanted with radiotelemetry transmitters and adeno-associated viral constructs with green fluorescent protein (GFP) reporter having either short hairpin RNA (shRNA) for TH or scrambled virus (scRNA) were injected into caudal NTS. Virus-injected rats were exposed to 7 days of CIH (alternating periods of 10% O2 and of 21% O2 from 8 AM to 4 PM; from 4 PM to 8 AM rats were exposed to 21% O2). CIH increased mean arterial pressure (MAP) and heart rate (HR) during the day in both the scRNA (n = 14, P < 0.001 MAP and HR) and shRNA (n = 13, P < 0.001 MAP and HR) groups. During the night, MAP and HR remained elevated in the scRNA rats (P < 0.001 MAP and HR) but not in the shRNA group. TH immunoreactivity and protein were reduced in the shRNA group. FosB/ΔFosB immunoreactivity was decreased in paraventricular nucleus (PVN) of shRNA group (P < 0.001). However, the shRNA group did not show any change in the FosB/ΔFosB immunoreactivity in the rostral ventrolateral medulla. Exposure to CIH increased MAP which persisted beyond the period of exposure to CIH. Knockdown of TH in the NTS reduced this CIH-induced persistent increase in MAP and reduced the transcriptional activation of PVN. This indicates that NTS A2 neurons play a role in the cardiovascular responses to CIH.
Keywords: A2 neurons, nucleus of the solitary tract, tyrosine hydroxylase and chronic intermittent hypoxia
sleep apnea is a condition where periodic, repetitive cessation of ventilation results in intermittent nocturnal episodes of arterial hypoxemia. This activates arterial chemoreceptors (CR) leading to increased sympathetic nerve activity (SNA), which contributes to increased arterial pressure (AP) (55). This elevated SNA and AP persist during the daytime when the patients are not experiencing apneic episodes (5). An estimated 15 million Americans suffer from various forms of sleep apnea (67).
The nucleus of the solitary tract (NTS) is the first integrative site of CR input termination within the central nervous system (11, 27), and neurons responding to CR activation are found throughout the NTS (35). However, chemoreceptor afferent integration appears to be more prevalent in the caudal aspects of the NTS, more precisely in the commissural region caudal to the calamus scriptorius (35, 36, 66), an area rich in catecholaminergic neurons, also known as A2 noradrenergic neurons. A2 neurons are activated by chemoreceptor afferents during systemic hypoxia and carotid sinus nerve stimulation (14). A2 neurons mediate responses to a variety of stressors (49) and project to sympathoregulatory sites throughout the neuraxis (43, 49, 65). Among these regions is the paraventricular nucleus (PVN), which receives a major projection from A2 neurons (64), and studies have found that the cardiovascular response to activation of peripheral chemoreceptors involves activation of PVN neurons (42, 48). PVN projections to the rostral ventrolateral medulla (RVLM) have been shown to contribute to the cardiovascular changes occurring during hypoxia exposure in rats (28).
To simulate the hypoxemia that occurs during sleep apnea, a number of labs have chronically exposed rats to intermittent hypoxia (CIH), which results in a persistently elevated AP (18, 29), as seen in humans with sleep apnea. The mechanisms involved in the genesis of hypertension induced by sleep apnea and CIH appear to involve a sequence of events starting with increased arterial chemoreceptor function (16, 30), stimulation, and activation of neurons of central nervous system (CNS) (4, 14, 29, 61) leading to augmented SNA (2, 17). Studies have used accumulation of FosB or its more stable splice variant ΔFosB, a member of activator protein-1 (AP-1) transcription factor family, as a marker for chronic or intermittent activation of CNS neurons (23, 41) following CIH (29). The goal of these studies was to test the hypothesis that depletion of tyrosine hydroxylase (TH) in A2 neurons would attenuate the elevated AP observed during 7 day exposure to CIH by reducing the transcriptional activation of neurons in sympathoregulatory sites like PVN and RVLM.
MATERIALS AND METHODS
Animals.
Experiments were conducted on adult male Sprague-Dawley rats (250–350 g, Charles River Laboratories, Wilmington, MA). Rats were housed in a thermostatically regulated room (23°C) with 12-h light:12 h dark cycle (12 L:12 D, on at 7 AM, off at 7 PM). A 1-wk acclimatization period was provided for the rats before any procedures were performed. Rats were provided with food and water ad libitum. All experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of North Texas Health Science Center.
Telemetry implantation.
Mean arterial pressure (MAP), heart rate (HR), and respiratory frequency (RF) were monitored in conscious rats using a radiotelemetry system (DSI, St. Paul, MN). Under 2% isoflurane inhalation anesthesia and aseptic conditions, all rats were implanted with an abdominal aortic catheter attached to a CA11PA-C40 radiotelemetry transmitter. The transmitter was secured to the abdominal muscle.
NTS microinjections.
Rats were randomly divided into two groups: a group injected with an adeno-associated virus (AAV) with a green fluorescent protein (GFP) reporter and short hairpin RNA for TH (shRNA; n = 15), and a group injected with an AAV with a GFP reporter and scrambled RNA, which is a control for the shRNA virus (scRNA; n = 14). The viral constructs were commercially available (Genedetect) and synthesized at titer 1.0 × 1012 genomic particles/ml using recently published sequences (63). After 1 wk of recovery from telemetry implantation surgery, under 2% isoflurane inhalation anesthesia, rats were placed in a stereotaxic frame and a limited occipital craniotomy conducted to expose the caudal medulla region. Three 100-nl injections of either shRNA or scRNA were performed with glass micropipette (tip diameter, 50 μm) using a pneumatic picopump (PV 800, WPI, Sarasota, FL) over a 5-min period, at the calamus scriptorius and bilaterally at 0.5 mm rostral and 0.5 mm lateral to calamus to cover the caudal NTS. Because of technical difficulties, telemetry data were recorded from 13 rats in shRNA group.
Chronic intermittent hypoxia exposure.
One week after the AAV injections, rats were transferred to a commercially available hypoxia chamber system where O2 concentration was varied using 100% N2, 100% O2 and a computerized system (Oxycycler, Biospherix, NY). Seven days of baseline MAP, HR, and RF were recorded during which the chambers were maintained at 21% O2. This was followed by a 7-day exposure to CIH, as described previously (70). Setting the cycles at 9% O2 for 6 min and 21% O2 for 4 min resulted in an O2 concentration of 10% for 3 of the 6 min. The rats were exposed to the CIH during the light phase, which coincides with their sleeping period, from 8 AM to 4 PM (8 h). From 4 PM to 8 AM the rats were exposed to 21% O2. After the last day of CIH exposure the rats were euthanized and the brain of each rat was collected for either immunohistochemistry or Western blotting.
Immunohistochemistry.
Rats were anesthetized with thiobutabarbital (100 mg/kg ip, Inactin; Sigma, St. Louis, MO) on morning of the day following the last CIH exposure and perfused transcardially with phosphate-buffered saline followed by 4% paraformaldehyde. Brains were then postfixed for 1–2 h before cryoprotecting in 30% sucrose at 4°C. Three sets of coronal 40-μm sections were collected and stored in cryoprotectant at −20°C until processed for immunohistochemistry. Separate sets of serial sections from the brain stem were processed either for TH-dopamine β-hydroxylase (DBH) double labeling or FosB-TH double labeling. Forebrain sections containing the PVN were processed only for FosB. For TH-DBH double labeling, the sections were processed with a primary antibody cocktail of rabbit anti-TH (1:1,000, AB152; Millipore, Billerica, MA) and mouse anti-DBH (1:1,000, MAB308; Millipore) followed by a secondary antibody cocktail with CY3-labeled anti-rabbit (1:250, 711-165-152; Jackson Immunoresearch) and AMCA-labeled anti-mouse (1:100, 715-156-150; Jackson Immunoresearch). For FosB-TH double labeling, a separate set of brain stem sections were first processed for FosB using a goat anti-FosB primary antibody (1:5,000, sc-48-G, Santa Cruz Biotechnology, Santa Cruz, CA) and a biotinylated horse anti-goat IgG (1:200, BA-9500; Vector labs Burlingame, CA). Next the sections were reacted with an avidin-peroxidase conjugate (Vectastain ABC kit, PK-4000; Vector labs) and PBS containing 0.04% 3,3′-diaminobenzidine hydrochloride and 0.04% nickel ammonium sulfate for 10–11 min. After rinsing was completed, these FosB-stained brain stem sections were processed with mouse anti-TH primary antibody(1:1,000, MAB318; Millipore) and a CY3-labeled donkey anti-mouse secondary antibody (1:250, 715–165-150; Jackson ImmunoResearch). Forebrain serial sections were only stained for FosB as described above. The FosB primary antibody used in this study does not discriminate ΔFosB from FosB; therefore the immunoreactivity will be referred to as FosB/ΔFosB.
Imaging and cell counts.
Imaging of TH-DBH immunoreactive neurons and GFP expression in the NTS was performed using an Olympus IX-2 DSU confocal microscope (Olympus, Tokyo, Japan) equipped for epifluorescence. Sections double labeled for TH-DBH were used to count the number of TH and DBH immunoreactive neurons and to construct the images in Figs. 2 and 3. FosB immunolabeled forebrain sections were used to count the number of FosB/ΔFosB immunoreactive neurons in PVN and for images in Fig. 5. Sections double labeled for TH-FosB were used to count FosB/ΔFosB immunoreactivity in RVLM and also to acquire images for Fig. 6 using Olympus microscope (BX41) equipped for epifluorescence, and an Olympus DP70 digital camera with DP manager software (version 2.2.1). ImageJ software (version 1.44, NIH, Bethesda, MD) was used to count the number of TH, DBH (shRNA, n = 9 and scRNA, n = 8), FosB/ΔFosB in PVN, and RVLM (shRNA, n = 7 and scRNA, n = 7) immunoreactive neurons in 9 to 10 sections bilaterally for NTS and RVLM; 5 to 6 sections for PVN [2 to 3 sections per rat/subnuclei dorsal parvocellular (dp), medial parvocellular (mp), lateral parvocellular (lp), and posterior magnocellular (pm)] from each rat and the average number of immunoreactive neurons per section calculated for each rat. The regions of interest were identified using the rat brain stereotaxic atlas (45) and within the regions, the counts were conducted in areas as previously described (29).
Fig. 2.
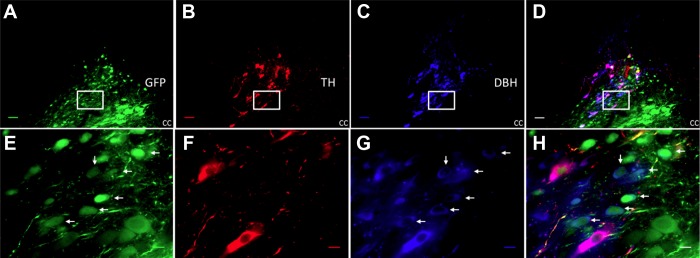
shRNA selective knockdown of tyrosine hydroxylase (TH) in A2 neurons (A) green fluorescent protein (GFP) staining showing the adeno-associated virus (AAV)-TH-shRNA-infected cells in the nucleus tractus solitarius (NTS). B: red-colored Cy3-labeled A2 neurons expressing TH. C: AMCA-labeled blue-colored dopamine β-hydroxylase (DBH)-positive cells. D: superimposed image of A, B and C. E, F, G, and H are the high-magnification images of the area in box of A, B, C, and D, respectively. Arrows point at the cells where in shRNA successfully knocked down TH without affecting DBH's expression. cc, central canal. Scale bar, 50 μm in low-magnification images and 10 μm in high-magnification images. Sections from subpostremal NTS.
Fig. 3.
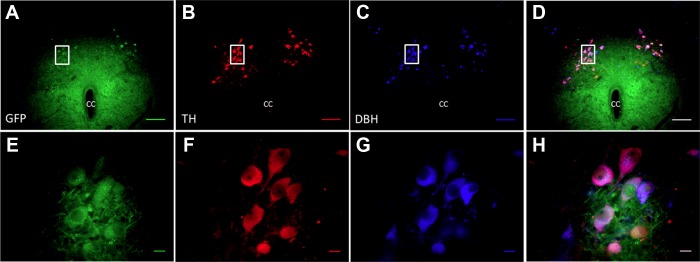
Scrambled virus does not affect the expression of TH in A2 neurons. A: GFP staining showing the AAV-Sc virus-infected cells in the NTS. B: red-colored Cy3-labeled A2 neurons expressing TH. C: AMCA-labeled blue-colored DBH positive cells. D: composite image of A, B and C. E, F, G, and H are the high-magnification images of the area in box of A, B, C, and D, respectively. Superimposed image of E, F, and G in H showing mostly purple-colored cells, where in both TH and DBH are intact in scrambled virus-infected cells. cc, central canal. Scale bar, 100 μm in low-magnification images and 10 μm in high-magnification images. Sections from caudal NTS.
Fig. 5.
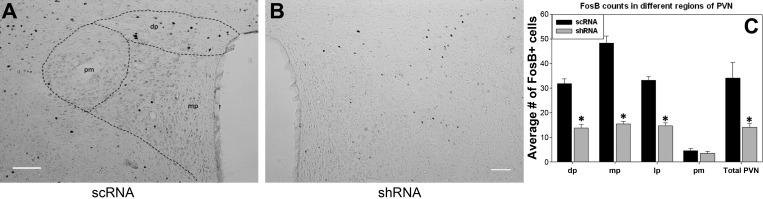
Representative digital images of FosB/ΔFosB staining on one side in PVN regions of scRNA (A) and shRNA (B) rats. Parvocellular and magnocellular subdivisions are diagrammed in the scRNA (A). Image C indicates the mean no. of FosB/ΔFosB-positive cells counted in the different regions with in paraventricular nucleus (PVN). *P value <0.05 vs. scRNA; n = 8/group. dp, Dorsal parvocellular; mp, medial parvocellular; pm, posterior magnocellular; lp, lateral parvocellular (not shown in image A, as it is found at different level of PVN), Scale bar = 100 μm.
Fig. 6.
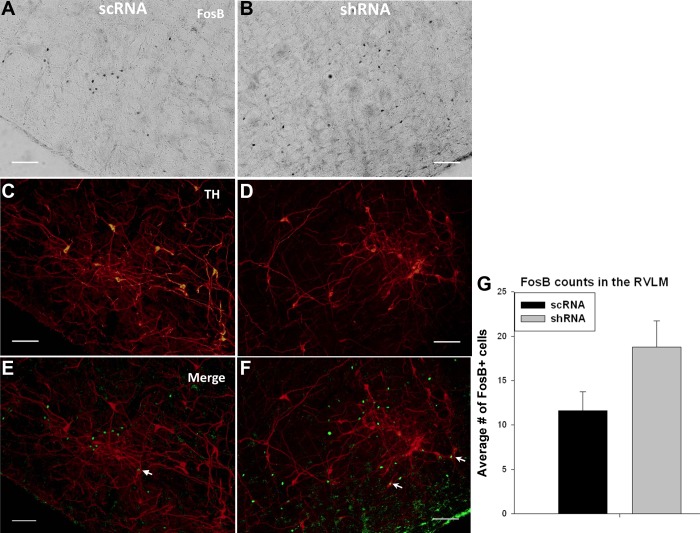
Representative digital images of FosB/ΔFosB staining on one side in RVLM region of scRNA (A) and shRNA (B). C and D are the TH images of scRNA and shRNA, respectively, E and F are the colocalized images of FosB/ΔFosB and TH images of scRNA and shRNA, respectively. G: mean no. of FosB/ΔFosB-positive cells counted in the rostral ventrolateral medulla (RVLM). Arrows point at the TH-FosB/ΔFosB colocalization. n = 7/group. Scale bar = 100 μm.
Western blot.
Six rats each from shRNA and scRNA groups were anesthetized with thiobutabarbital (100 mg/kg ip Inactin; Sigma) and decapitated quickly. The brain stem was removed and snap frozen in isopentane on dry ice and placed in a brain matrix (Stoelting, Wood Dale, IL) to cut 1-mm thick slices. Caudal and subpostremal NTS regions were dissected from the frozen sections and subjected to sonication in modified radioimmunoprecipitation buffer supplemented with protease and phosphatase inhibitors followed by centrifugation at 10,000 g at 4°C to obtain a clear supernatant of protein. Bradford assay was conducted to determine the total protein concentration. Fifty micrograms of tissue lysate per sample were loaded onto sodium dodecyl sulfate (SDS)-10% acrylamide gel and electrophorosed before transferring to polyvinylidene fluoride (PVDF) membrane. The membrane was then blocked with 5% (wt/vol) nonfat milk in Tris-buffered saline 0.05% (vol/vol) Tween 20 (TBS-Tween; 50 mM Tris base, 200 mM NaCl, 0.05% Tween 20) followed by overnight incubation with primary antibodies against TH (1:1,000 mouse anti-TH, MAB318; Millipore) and/or (glyceraldehyde-3-phosphate dehydrogenase) GAPDH (1:1,000, mouse anti-GAPDH, MAB374; Millipore). Peroxidase-Conjugated AffiniPure sheep anti-mouse IgG (1:5,000, A5906; Sigma Aldrich) was used as a secondary antibody. Immunoreactive bands were detected by enhanced chemiluminescence (ECL reagents, Amersham, Piscataway, NJ) by acquiring digital gel images using Syngene G-box (Frederick, MD). ImageJ software was used to analyze the densitometry of immunoreactive bands.
Telemetry data analysis.
MAP (sampled at 250 Hz), HR, and RF were recorded for 10 s every 10 min as previously described (29, 70). Pulse interval and fluctuations obtained from the AP waveform were used to calculate HR and RF respectively (Dataquest, DSI, MN). MAP, HR, and RF were averaged for every hour in the 24-h period and the 1-h averages averaged during the light phase (period of exposure) during CIH (8 AM to 4 PM) and during the dark period (7 PM to 7 AM).
Statistical analysis.
All data are presented as means ± SE. Effects of CIH on MAP, HR, and RF during different periods of the day (light with CIH and dark with normoxia) in shRNA and scRNA were determined by two-way ANOVA with repeated measures (SigmaPlot, Systat Software, San Jose, CA). Fisher LSD post hoc test was used to identify significant difference among mean values. One-way ANOVA was used to determine any differences between the control day baseline averages of the three parameters between two groups, differences between the scRNA and shRNA groups TH-immunoreacive (ir) cell counts, DBH cell counts, FosB/ΔFosB immunoreactivity in PVN and RVLM, and Western blot means values. P value <0.05 was considered statistically significant.
RESULTS
Responses to CIH in conscious rats.
Figure 1 shows the average changes from baseline MAP, HR, and RF in the light period (A, C, and E) and the dark period (B, D, and F) in conscious rats during CIH. Seven days of control baseline values were recorded in both groups of rats. The average baseline values during the control period of MAP (mmHg), HR (beats/min), and RF (breaths/min) during light phase and dark phase are presented in Table 1. There was no significant difference between the average control days MAP and RF of shRNA and scRNA groups during light or dark phase. However, HR was greater in shRNA compared with scRNA (P < 0.001) during both the light and dark phases.
Fig. 1.
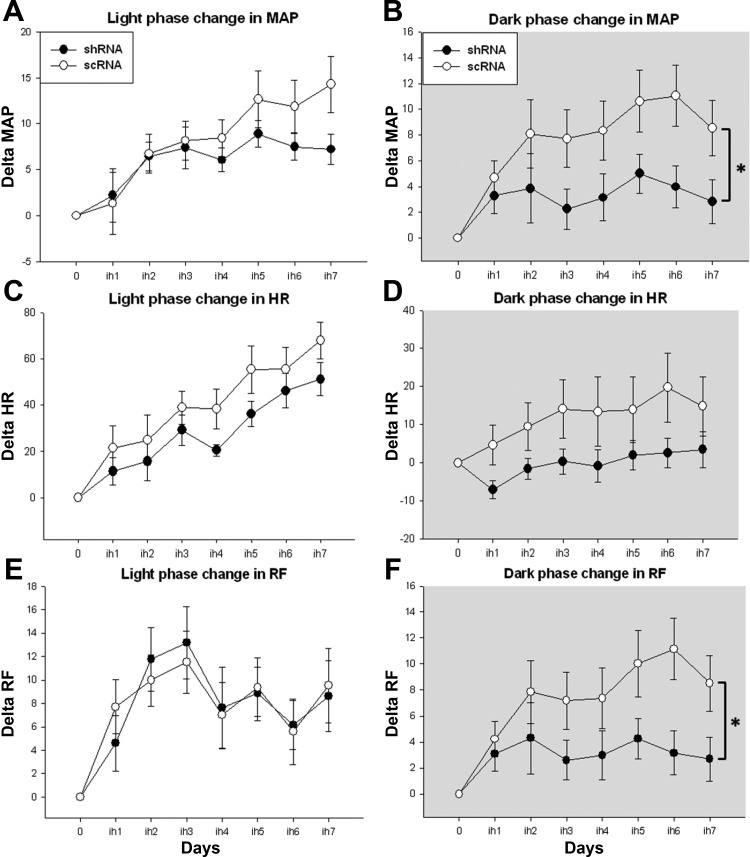
Graphs are presented as differences from baseline with the baseline absolute values represented as zero. 7 days of chronic intermittent hypoxia (CIH) are presented on x-axis as ih1 to ih7. A: CIH caused a significant elevation in mean arterial pressure (MAP) starting day 2 in both short hairpin RNA (shRNA) and scrambled (scRNA) virus-injected groups, compared with their baseline, during light phase. B: sustained hypertension during normoxic dark phase has been significantly reduced in shRNA virus-injected rats. C: CIH significantly increased the heart rate (HR) in both the virus-injected groups. D: during the dark phase, shRNA moderately decreased the HR elevation noticed in scRNA group. E: light phase rrespirationn frequency (RF) significantly increased in shRNA and scRNA viruses-injected groups. F: This elevation in RF was significantly decreased by shRNA. (shRNA; n = 13 and scRNA; n = 14); *shRNA group significantly different from scRNA group. P value <0.05 was considered significant.
Table 1.
Average baseline values of physiological parameters in shRNA and scRNA groups
| Light Phase |
Dark Phase |
|||
|---|---|---|---|---|
| scRNA | shRNA | scRNA | shRNA | |
| MAP, mmHg | 100.9 ± 3.4 | 100.9 ± 2.3 | 104.1 ± 4.2 | 104.9 ± 2.7 |
| HR, beats/min | 311.0 ± 6.1* | 320.5 ± 5.4 | 363.3 ± 7.6* | 378.4 ± 6.3 |
| RF, breaths/min | 95.4 ± 1.3 | 95.0 ± 1.5 | 94.4 ± 4.5 | 96.4 ± 2.9 |
Values are means ± SE.
cRNA, scrambled RNA; shRNA, short hairpinRNA; MAP, mean arterial pressure; HR, heart rate; RF, respiration frequency.
Significantly different from respective phase HR of shRNA.
The average of MAP absolute values of each CIH day during light phase, compared with the baseline values in the Table 1, were significantly higher on all days of CIH except day 1 in both shRNA and scRNA groups (P < 0.05). In the dark phase of CIH, MAP of all the 7 days were significantly higher in scRNA group but were only significantly elevated on days 2 and 5 in shRNA group compared with their respective baseline values. When the changes in MAP were compared, the shRNA-treated rats showed a significantly reduced CIH-induced increase in MAP compared with scRNA-treated rats during the dark phase (Fig. 1B; P < 0.05).
HR on all the days of CIH was significantly higher compared with the baseline in both scRNA and shRNA groups, during the light phase (P < 0.05). This elevation in HR persisted into the normoxic dark phase on all CIH days except day 1 in the scRNA group but not in the shRNA group. The differences in HR between the groups were not significantly different (P = 0.127) (Fig. 1D).
All the days in the scRNA group and the shRNA group, except day 1, showed a significant increase in RF during light phase on CIH days compared with the baseline. During the dark phase, all the days in scRNA-treated rats showed an increased RF compared with the baseline, whereas only days 2 and 5 showed an increase in the shRNA group. Changes in RF during dark phase of CIH days in the shRNA group were significantly lower compared with the scRNA group (Fig. 1F; P < 0.05).
AAV-TH-shRNA reduces the number of TH-ir NTS neurons.
Figure 2 illustrates that the shRNA construct reduced the number of TH-ir neurons in NTS without altering the DBH immunoreactivity. The arrows in Fig. 2, E–H, point out to the neurons where virus-infected cells showed no TH-ir, but the DBH was intact. The number of TH-ir neurons was reduced by 20% in sections with GFP fluorescence in shRNA group; shRNA 28.6 ± 1.5 cells/section compared with scRNA 35.5 ± 1.4 cells/section (P value: 0.005). The number of DBH-immunoreactive neurons was not different between groups (shRNA: 43.8 ± 2.4 cells/section; scRNA: 45.4 ± 2.4 cells/section). The scrambled RNA treatments did not alter the TH-ir in the virus-infected (GFP-expressing) neurons (Fig. 3). There was no apparent difference in the reduction of TH-ir by the shRNA comparing subpostremal and caudal regions of NTS.
Caudal and subpostremal regions showed a reduced TH protein levels in shRNA group.
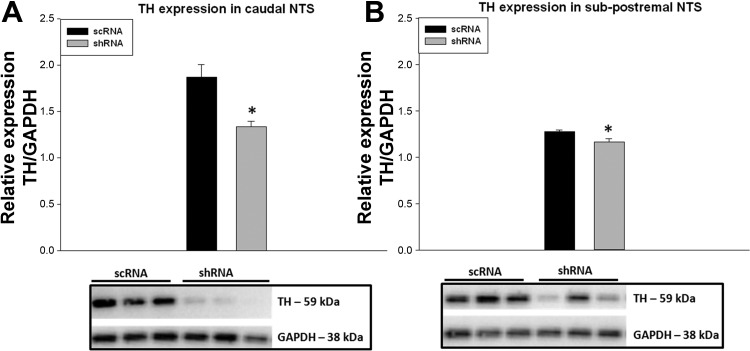
Counting TH-ir cells is a binary measure, a cell either possesses TH-ir or it does not, and cannot discern partial reductions in the TH content of individual neurons. To confirm TH knockdown as indicated by counting cells with TH-ir, we performed Western blots. In the shRNA group, TH levels in caudal (P value: 0.005; n = 6 in both groups) and subpostremal (P value: 0.02; n = 5, shRNA, n = 6, scRNA) NTS were reduced by 30% and 10%, respectively, compared with scRNA group (Fig. 4).
Fig. 4.
Western blot analysis shows a significant reduction of TH protein levels in caudal NTS (A) and subpostremal NTS (B) of shRNA virus-injected rats compared with scRNA. TH expression was normalized using GAPDH. *P value <0.05 vs. scRNA. Caudal NTS: n = 6/group; subpostremal: n = 5 in shRNA and n = 6 in scRNA.
shRNA group showed a reduced FosB/ΔFosB staining in different regions of PVN.
There was a significant reduction in the number of FosB/ΔFosB-positive cells in the PVN of the shRNA group compared with the scRNA-injected group (P value: 0.006; shRNA: 14 ± 1; scRNA: 34 ± 6, n = 8 in both groups). Further analysis of different regions of PVN displayed a reduction in FosB/ΔFosB-positive cells in dorsal parvocellular (dp; P value: <0.001; shRNA: 14 ± 1; scRNA: 35 ± 2), medial parvocellular (mp; P value: <0.001; shRNA: 15 ± 1; scRNA: 48 ± 3), and lateral parvocellular (lp; P value: <0.001; shRNA: 15 ± 1; scRNA: 33 ± 2) subnuclei. There was little to no FosB/ΔFosB staining in the posterior magnocellular region in either group (pm; shRNA: 3 ± 1; scRNA: 5 ± 1) with no difference between them (Fig. 5).
No change in FosB/ΔFosB staining between the groups in RVLM.
A moderate increase in the number of FosB/ΔFosB-positive cells in the RVLM of the shRNA group compared with the scRNA group (P value: 0.07; shRNA: 19 ± 3; scRNA: 12 ± 2) was not significant. While the FosB/ΔFosB staining in RVLM was intermingled with TH-positive neurons, the increase in FosB/ΔFosB staining was not associated with significant colocalization of FosB/ΔFosB with TH (shRNA: 1.8 ± 0.3; scRNA: 1.6 ± 0.2) (Fig. 6).
DISCUSSION
Sleep apnea is an increasingly recognized contributor to cardiovascular disease and mortality. Sleep apnea patients have elevated blood pressures during both the day and the night, indicating that the effects of sleep apnea persist beyond the actual exposure to nighttime apneas. The model of CIH used in the present study replicates this aspect of the sleep apnea phenotype. It is interesting to note that CIH induces a persistent increase in MAP in the absence of hypercapnia, which accompanies the hypoxia in sleep apnea patients. In fact, due to the increases in ventilation during intermittent hypoxia (IH) in CIH models, the hypoxia is accompanied by hypocapnia. Previous studies in humans (8, 54, 68) and rodents (15, 62) indicate that IH-induced increases in MAP and/or SNA are independent of end-tidal CO2, being the same whether the subject is hypocapnic, isocapnic, or hypercapnic. The present study provides new insights that suggest catecholaminergic A2 neurons in the NTS are major contributors to the CIH-induced persistent increase in MAP.
A2 neurons are activated by a variety of stressors, including systemic hypoxia (14). A2 projections to sympathoregulatory sites within the CNS (49) suggesting that A2 neurons play a role in the cardiovascular and sympathetic responses to systemic hypoxia. A 7-day exposure to IH produced no change in TH enzymatic activity or protein level in the brain stem; however, this study did not examine specific areas within the brain stem (20). Chronic hypoxia increased TH expression within the A2 cell group (13, 57), and this increase was abolished after carotid sinus nerve section (56). Therefore, the variations in TH expression observed in A2 group are not solely the direct effect of tissue hypoxia but are dependent on afferent chemoreceptor inputs to NTS. A2 neurons also play a role in the hypothalamic-pituitary-adrenal (HPA) axis reactivity as they are found to express glucocorticoid receptors (19, 22) and project to regions important in regulation of HPA axis function (49). Our group has shown that CIH sensitizes HPA axis reactivity (31), and since glucocorticoids have shown to increase arterial pressure in response to acute stress (51, 52), a hyperreactive stress response may also contribute to the CIH induced increase in MAP. Exposure to CIH has been reported to increase the plasma corticosterone levels (71); however, in that study plasma was collected for measurement of corticosterone at euthanasia so the elevated corticosterone level may reflect enhanced stress reactivity.
In this study, we reduced TH levels in A2 neurons using AAV vector delivery of shRNAs directed toward TH and observed MAP, HR, and RF during exposure to CIH. This approach has been used to reduce TH levels in specific regions of the brain in mice (26) and rats (63). Genetic models are widely used to uncover the molecular mechanisms of a disease, but the use of transgenic and knockout models is limited by developmental effects, genetic compensation, and lack of regional specificity. The use of shRNA to knockdown genes of interest in the whole brain (44) and in specific regions (26, 50, 69) has provided a useful adjunct approach to the use of genetic models. Unlike saporin toxin studies (10, 60) the use of shRNA reduces TH levels and does not kill the A2 neurons. To address concerns about the toxicity of these viral constructs and possible knock down of other genes, a previously used construct (63) at a safe titer (>1 × 1012 genomic particles/ml) was used. In addition, immunohistochemistry was performed for DBH, which like TH, is also specific for catecholaminergic neurons. Figures 2 and 3 illustrate that the viral constructs are not toxic and do not suppress DBH levels. Uniform sized bands for the housekeeping gene GAPDH in the Western blots in both shRNA and scrambled groups also suggest no nonspecific changes in protein levels.
Our finding of a CIH-induced persistent increase in respiratory frequency was surprising as central catecholaminergic neurons are considered to exert a weak, tonic inhibition of ventilation (34). However, the cited study produced a global reduction in neuronal catecholaminergic content, and more selective and site-specific reductions in neuronal catecholamine content might produce different results. It has been suggested that CIH-induced hypertension might be due to increased respiratory drive to sympathoexcitatory neurons in the brain stem (37).
Previous studies from our group have shown that within the first week of exposure CIH increased MAP during the light phase, when the rats are exposed to the intermittent hypoxia, and the increase in MAP persisted into the normoxic dark phase (7, 24, 29). The results of this study demonstrate that TH knockdown in A2 neurons decreased the persistent increase in MAP and RF during the normoxic dark phase. These results demonstrate that A2 neurons contribute to the cardiovascular and respiratory responses to intermittent hypoxia without altering baseline blood pressure.
Consistent with this finding of a reduced response to CIH, a physiological stress, are the results of a recent study that found that injections of DBH-saporin into caudal NTS reduced the number of catecholaminergic neurons and reduced chronic stress-induced elevations in dark phase blood pressure (10). This study and others have shown increases or no change in baseline blood pressure following lesion of A2 neurons with either DBH-saporin (10, 60) or after silencing of A2 neurons by overexpressing potassium channels (12). Our results indicate no change in baseline blood pressure in shRNA-injected rats. This may be result of a more modest reduction in TH in our study; however, such modest reductions could represent a degree of knock down that might happen physiologically. It could also reflect the fact that the shRNA does not kill the A2 neurons. The contribution of A2 neurons to baseline blood pressure is important to understand and requires further study.
To define potential sites in the CNS that might be involved in the reduction in MAP during normoxic dark phase of CIH following decreased TH levels in NTS, we examined FosB/ΔFosB in sympathoregulatory sites like PVN and RVLM. Increased expression of FosB during CIH is an important finding because FosB/ΔFosB has been linked to neuronal adaptations and plasticity under conditions such as drug addiction, epilepsy, and long-term potentiation (6, 32, 33, 38–41). The present results confirm our previous studies (29) demonstrating increased levels of FosB/ΔFosB in CNS neurons following CIH which may mediate changes in gene expression that alter neuronal function. PVN neurons have been shown to increase levels of FosB/ΔFosB immunoreactivity following exposure to CIH (29). PVN parvocellular neurons project to RVLM (46, 47, 53, 58) and intermediolateral cell column of spinal cord (47, 53, 58, 59), the location of sympathetic preganglionic neurons. The reduction in the number of FosB/ΔFosB immunoreactive neurons in the dorsal parvocellular (dp), medial parvocellular (mp) and lateral parvocellular (lp) (Fig. 5) in the shRNA rats, compared with scRNA rats, indicates that TH knockdown in the NTS reduced the transcriptional activation of PVN, most likely by reducing A2 neuron excitation of PVN neurons (49). This indicates that the sympathetic drive from the PVN (9, 21) might have been decreased, causing the reduction in the CIH-induced persistent increase in MAP during the normoxic dark phase.
The observation that shRNA knockdown of TH in the NTS was not associated with a change in the FosB/ΔFosB immunoreactivity in RVLM (Fig. 6) was not expected as decreased MAP was expected to be associated with decreased transcriptional activation of RVLM. However, A2 neurons do not project directly to the RVLM (3, 25). Optogenetic stimulation of catecholaminergic neurons in RVLM in the rat increases SNA and blood pressure (1). Noncatecholaminergic neurons in RVLM serve a variety of functions (e.g., thermoregulation, respiration, cardiovascular). The reduced activity of PVN parvocellular neurons and their projections to the intermediolateral cell column of spinal cord might be the driving force behind the reduction in the dark phase MAP elevation observed after reductions in TH in the caudal NTS.
Perspectives and Significance
This study shows that it is plausible to knockdown TH using viral vectors without affecting the cell vitality and other protein expression. These results suggest that activation of NTS A2 neurons during CIH mediates the increased sympathetic outflow that has been shown to underlie CIH-induced persistent hypertension. The observations that FosB was not different between treatment groups in the RVLM and that the increase in blood pressure during the light phase was also not different between groups raises the possibility that PVN sympathoexcitatory neurons might have a greater contribution to the dark phase elevation in baseline blood pressure, while the RVLM might be more involved during the light phase exposures to hypoxia.
GRANTS
This study was funded by the National Heart, Lung, and Blood Institute Grant HL-088052.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.S.B. and S.W.M. conception and design of research; C.S.B., A.R., and M.F. performed experiments; C.S.B., A.R., K.Y., and J.T.C. analyzed data; C.S.B. and S.W.M. interpreted results of experiments; C.S.B. and A.R. prepared figures; C.S.B. drafted manuscript; C.S.B., J.T.C., and S.W.M. edited and revised manuscript; C.S.B., A.R., M.F., K.Y., J.T.C., and S.W.M. approved final version of manuscript.
REFERENCES
- 1.Abbott SB, Stornetta RL, Socolovsky CS, West GH, Guyenet PG. Photostimulation of channelrhodopsin-2 expressing ventrolateral medullary neurons increases sympathetic nerve activity and blood pressure in rats. J Physiol 587: 5613–5631, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bao G, Metreveli N, Li R, Taylor A, Fletcher EC. Blood pressure response to chronic episodic hypoxia: role of the sympathetic nervous system. J Appl Physiol 83: 95–101, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Blessing WW, Hedger SC, Joh TH, Willoughby JO. Neurons in the area postrema are the only catecholamine-synthesizing cells in the medulla or pons with projections to the rostral ventrolateral medulla (C1-area) in the rabbit. Brain Res 419: 336–340, 1987 [DOI] [PubMed] [Google Scholar]
- 4.Buller KM, Smith DW, Day TA. NTS catecholamine cell recruitment by hemorrhage and hypoxia. Neuroreport 10: 3853–3856, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Carlson JT, Hedner J, Elam M, Ejnell H, Sellgren J, Wallin BG. Augmented resting sympathetic activity in awake patients with obstructive sleep apnea. Chest 103: 1763–1768, 1993 [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Zhang Y, Kelz MB, Steffen C, Ang ES, Zeng L, Nestler EJ. Induction of cyclin-dependent kinase 5 in the hippocampus by chronic electroconvulsive seizures: role of [Delta]FosB. J Neurosci 20: 8965–8971, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cunningham JT, Knight WD, Mifflin SW, Nestler EJ. An Essential role for DeltaFosB in the median preoptic nucleus in the sustained hypertensive effects of chronic intermittent hypoxia. Hypertension 60: 179–187, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cutler MJ, Swift NM, Keller DM, Wasmund WL, Burk JR, Smith ML. Periods of intermittent hypoxic apnea can alter chemoreflex control of sympathetic nerve activity in humans. Am J Physiol Heart Circ Physiol 287: H2054–H2060, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Dampney RA, Horiuchi J. Functional organisation of central cardiovascular pathways: studies using c-fos gene expression. Prog Neurobiol 71: 359–384, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Daubert DL, McCowan M, Erdos B, Scheuer DA. Nucleus of the solitary tract catecholaminergic neurons modulate the cardiovascular response to psychological stress in rats. J Physiol 590: 4881–4895, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donoghue S, Felder RB, Jordan D, Spyer KM. The central projections of carotid baroreceptors and chemoreceptors in the cat: a neurophysiological study. J Physiol 347: 397–409, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duale H, Waki H, Howorth P, Kasparov S, Teschemacher AG, Paton JF. Restraining influence of A2 neurons in chronic control of arterial pressure in spontaneously hypertensive rats. Cardiovasc Res 76: 184–193, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Dumas S, Pequignot JM, Ghilini G, Mallet J, Denavit-Saubie M. Plasticity of tyrosine hydroxylase gene expression in the rat nucleus tractus solitarius after ventilatory acclimatization to hypoxia. Brain Res Mol Brain Res 40: 188–194, 1996 [DOI] [PubMed] [Google Scholar]
- 14.Erickson JT, Millhorn DE. Hypoxia and electrical stimulation of the carotid sinus nerve induce Fos-like immunoreactivity within catecholaminergic and serotoninergic neurons of the rat brainstem. J Comp Neurol 348: 161–182, 1994 [DOI] [PubMed] [Google Scholar]
- 15.Fletcher EC, Bao G. Effect of episodic eucapnic and hypocapnic hypoxia on systemic blood pressure in hypertension-prone rats. J Appl Physiol 81: 2088–2094, 1996 [DOI] [PubMed] [Google Scholar]
- 16.Fletcher EC, Lesske J, Behm R, Miller CC, 3rd, Stauss H, Unger T. Carotid chemoreceptors, systemic blood pressure, and chronic episodic hypoxia mimicking sleep apnea. J Appl Physiol 72: 1978–1984, 1992 [DOI] [PubMed] [Google Scholar]
- 17.Fletcher EC, Lesske J, Culman J, Miller CC, Unger T. Sympathetic denervation blocks blood pressure elevation in episodic hypoxia. Hypertension 20: 612–619, 1992 [DOI] [PubMed] [Google Scholar]
- 18.Fletcher EC, Lesske J, Qian W, Miller CC, 3rd, Unger T. Repetitive, episodic hypoxia causes diurnal elevation of blood pressure in rats. Hypertension 19: 555–561, 1992 [DOI] [PubMed] [Google Scholar]
- 19.Fuxe K, Cintra A, Agnati LF, Harfstrand A, Wikstrom AC, Okret S, Zoli M, Miller LS, Greene JL, Gustafsson JA. Studies on the cellular localization and distribution of glucocorticoid receptor and estrogen receptor immunoreactivity in the central nervous system of the rat and their relationship to the monoaminergic and peptidergic neurons of the brain. J Steroid Biochem 27: 159–170, 1987 [DOI] [PubMed] [Google Scholar]
- 20.Gozal E, Shah ZA, Pequignot JM, Pequignot J, Sachleben LR, Czyzyk-Krzeska MF, Li RC, Guo SZ, Gozal D. Tyrosine hydroxylase expression and activity in the rat brain: differential regulation after long-term intermittent or sustained hypoxia. J Appl Physiol 99: 642–649, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci 7: 335–346, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Harfstrand A, Fuxe K, Cintra A, Agnati LF, Zini I, Wikstrom AC, Okret S, Yu ZY, Goldstein M, Steinbusch H. Glucocorticoid receptor immunoreactivity in monoaminergic neurons of rat brain. Proc Natl Acad Sci USA 83: 9779–9783, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herdegen T, Leah JD. Inducible and constitutive transcription factors in the mammalian nervous system: control of gene expression by Jun, Fos and Krox, and CREB/ATF proteins. Brain Res Brain Res Rev 28: 370–490, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Hinojosa-Laborde C, Mifflin SW. Sex differences in blood pressure response to intermittent hypoxia in rats. Hypertension 46: 1016–1021, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Hirooka Y, Polson JW, Potts PD, Dampney RA. Hypoxia-induced Fos expression in neurons projecting to the pressor region in the rostral ventrolateral medulla. Neuroscience 80: 1209–1224, 1997 [DOI] [PubMed] [Google Scholar]
- 26.Hommel JD, Sears RM, Georgescu D, Simmons DL, DiLeone RJ. Local gene knockdown in the brain using viral-mediated RNA interference. Nat Med 9: 1539–1544, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Housley GD, Martin-Body RL, Dawson NJ, Sinclair JD. Brain stem projections of the glossopharyngeal nerve and its carotid sinus branch in the rat. Neuroscience 22: 237–250, 1987 [DOI] [PubMed] [Google Scholar]
- 28.Kc P, Balan KV, Tjoe SS, Martin RJ, Lamanna JC, Haxhiu MA, Dick TE. Increased vasopressin transmission from the paraventricular nucleus to the rostral medulla augments cardiorespiratory outflow in chronic intermittent hypoxia-conditioned rats. J Physiol 588: 725–740, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knight WD, Little JT, Carreno FR, Toney GM, Mifflin SW, Cunningham JT. Chronic intermittent hypoxia increases blood pressure and expression of FosB/DeltaFosB in central autonomic regions. Am J Physiol Regul Integr Comp Physiol 301: R131–R139, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lesske J, Fletcher EC, Bao G, Unger T. Hypertension caused by chronic intermittent hypoxia–influence of chemoreceptors and sympathetic nervous system. J Hypertens 15: 1593–1603, 1997 [DOI] [PubMed] [Google Scholar]
- 31.Ma S, Mifflin SW, Cunningham JT, Morilak DA. Chronic intermittent hypoxia sensitizes acute hypothalamic-pituitary-adrenal stress reactivity and Fos induction in the rat locus coeruleus in response to subsequent immobilization stress. Neuroscience 154: 1639–1647, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Madsen TM, Bolwig TG, Mikkelsen JD. Differential regulation of c-Fos and FosB in the rat brain after amygdala kindling. Cell Mol Neurobiol 26: 87–100, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McClung CA, Ulery PG, Perrotti LI, Zachariou V, Berton O, Nestler EJ. DeltaFosB: a molecular switch for long-term adaptation in the brain. Brain Res Mol Brain Res 132: 146–154, 2004 [DOI] [PubMed] [Google Scholar]
- 34.McCrimmon DR, Dempsey JA, Olson EB., Jr Effect of catecholamine depletion on ventilatory control in unanesthetized normoxic and hypoxic rats. J Appl Physiol 55: 522–528, 1983 [DOI] [PubMed] [Google Scholar]
- 35.Mifflin SW. Arterial chemoreceptor input to nucleus tractus solitarius. Am J Physiol Regul Integr Comp Physiol 263: R368–R375, 1992 [DOI] [PubMed] [Google Scholar]
- 36.Mifflin SW. Inhibition of chemoreceptor inputs to nucleus of tractus solitarius neurons during baroreceptor stimulation. Am J Physiol Regul Integr Comp Physiol 265: R14–R20, 1993 [DOI] [PubMed] [Google Scholar]
- 37.Moraes DJ, Zoccal DB, Machado BH. Medullary respiratory network drives sympathetic overactivity and hypertension in rats submitted to chronic intermittent hypoxia. Hypertension 60: 1374–1380, 2012 [DOI] [PubMed] [Google Scholar]
- 38.Nestler EJ. Is there a common molecular pathway for addiction? Nat Neurosci 8: 1445–1449, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci 2: 119–128, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Nestler EJ. Molecular neurobiology of addiction. Am J Addict 10: 201–217, 2001 [DOI] [PubMed] [Google Scholar]
- 41.Nestler EJ, Barrot M, Self DW. DeltaFosB: a sustained molecular switch for addiction. Proc Natl Acad Sci USA 98: 11042–11046, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olivan MV, Bonagamba LG, Machado BH. Involvement of the paraventricular nucleus of the hypothalamus in the pressor response to chemoreflex activation in awake rats. Brain Res 895: 167–172, 2001 [DOI] [PubMed] [Google Scholar]
- 43.Otake K, Reis DJ, Ruggiero DA. Afferents to the midline thalamus issue collaterals to the nucleus tractus solitarii: an anatomical basis for thalamic and visceral reflex integration. J Neurosci 14: 5694–5707, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pardridge WM. shRNA and siRNA delivery to the brain. Adv Drug Deliv Rev 59: 141–152, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paxinos G, Watson CR, Emson PC. AChE-stained horizontal sections of the rat brain in stereotaxic coordinates. J Neurosci Methods 3: 129–149, 1980 [DOI] [PubMed] [Google Scholar]
- 46.Pyner S, Coote JH. Identification of an efferent projection from the paraventricular nucleus of the hypothalamus terminating close to spinally projecting rostral ventrolateral medullary neurons. Neuroscience 88: 949–957, 1999 [DOI] [PubMed] [Google Scholar]
- 47.Pyner S, Coote JH. Identification of branching paraventricular neurons of the hypothalamus that project to the rostroventrolateral medulla and spinal cord. Neuroscience 100: 549–556, 2000 [DOI] [PubMed] [Google Scholar]
- 48.Reddy MK, Patel KP, Schultz HD. Differential role of the paraventricular nucleus of the hypothalamus in modulating the sympathoexcitatory component of peripheral and central chemoreflexes. Am J Physiol Regul Integr Comp Physiol 289: R789–R797, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Rinaman L. Hindbrain noradrenergic A2 neurons: diverse roles in autonomic, endocrine, cognitive, and behavioral functions. Am J Physiol Regul Integr Comp Physiol 300: R222–R235, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Salahpour A, Medvedev IO, Beaulieu JM, Gainetdinov RR, Caron MG. Local knockdown of genes in the brain using small interfering RNA: a phenotypic comparison with knockout animals. Biol Psychiatry 61: 65–69, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Scheuer DA, Bechtold AG, Shank SS, Akana SF. Glucocorticoids act in the dorsal hindbrain to increase arterial pressure. Am J Physiol Heart Circ Physiol 286: H458–H467, 2004 [DOI] [PubMed] [Google Scholar]
- 52.Scheuer DA, Bechtold AG, Vernon KA. Chronic activation of dorsal hindbrain corticosteroid receptors augments the arterial pressure response to acute stress. Hypertension 49: 127–133, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shafton AD, Ryan A, Badoer E. Neurons in the hypothalamic paraventricular nucleus send collaterals to the spinal cord and to the rostral ventrolateral medulla in the rat. Brain Res 801: 239–243, 1998 [DOI] [PubMed] [Google Scholar]
- 54.Smith ML, Pacchia CF. Sleep apnoea and hypertension: role of chemoreflexes in humans. Exp Physiol 92: 45–50, 2007 [DOI] [PubMed] [Google Scholar]
- 55.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 96: 1897–1904, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Soulier V, Cottet-Emard JM, Pequignot J, Hanchin F, Peyrin L, Pequignot JM. Differential effects of long-term hypoxia on norepinephrine turnover in brain stem cell groups. J Appl Physiol 73: 1810–1814, 1992 [DOI] [PubMed] [Google Scholar]
- 57.Soulier V, Dalmaz Y, Cottet-Emard JM, Kitahama K, Pequignot JM. Delayed increase of tyrosine hydroxylation in the rat A2 medullary neurons upon long-term hypoxia. Brain Res 674: 188–195, 1995 [DOI] [PubMed] [Google Scholar]
- 58.Stocker SD, Cunningham JT, Toney GM. Water deprivation increases Fos immunoreactivity in PVN autonomic neurons with projections to the spinal cord and rostral ventrolateral medulla. Am J Physiol Regul Integr Comp Physiol 287: R1172–R1183, 2004 [DOI] [PubMed] [Google Scholar]
- 59.Swanson LW, Kuypers HG. The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol 194: 555–570, 1980 [DOI] [PubMed] [Google Scholar]
- 60.Talman WT, Dragon DN, Jones SY, Moore SA, Lin LH. Sudden death and myocardial lesions after damage to catecholamine neurons of the nucleus tractus solitarii in rat. Cell Mol Neurobiol 32: 1119–1126, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Teppema LJ, Veening JG, Kranenburg A, Dahan A, Berkenbosch A, Olievier C. Expression of c-fos in the rat brainstem after exposure to hypoxia and to normoxic and hyperoxic hypercapnia. J Comp Neurol 388: 169–190, 1997 [DOI] [PubMed] [Google Scholar]
- 62.Toney GMCJ, Mifflin SW. Early neural adaptations to intermittent hypoxia: Triggers for the pathophysiology of sleep apnea. In: Recent Advances in Cardiovascular Research: From Sleep to Exercise, edited by Ally A, Maher TJ, Wyss JM, Kerala, India: Transworld Research Network, 2010, p. 75–96 [Google Scholar]
- 63.Ulusoy A, Sahin G, Bjorklund T, Aebischer P, Kirik D. Dose optimization for long-term rAAV-mediated RNA interference in the nigrostriatal projection neurons. Mol Ther 17: 1574–1584, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Valentine JD, Matta SG, Sharp BM. Nicotine-induced cFos expression in the hypothalamic paraventricular nucleus is dependent on brainstem effects: correlations with cFos in catecholaminergic and noncatecholaminergic neurons in the nucleus tractus solitarius. Endocrinology 137: 622–630, 1996 [DOI] [PubMed] [Google Scholar]
- 65.van der Kooy D, Koda LY, McGinty JF, Gerfen CR, Bloom FE. The organization of projections from the cortex, amygdala, and hypothalamus to the nucleus of the solitary tract in rat. J Comp Neurol 224: 1–24, 1984 [DOI] [PubMed] [Google Scholar]
- 66.Vardhan A, Kachroo A, Sapru HN. Excitatory amino acid receptors in commissural nucleus of the NTS mediate carotid chemoreceptor responses. Am J Physiol Regul Integr Comp Physiol 264: R41–R50, 1993 [DOI] [PubMed] [Google Scholar]
- 67.Wolk R, Shamsuzzaman AS, Somers VK. Obesity, sleep apnea, hypertension. Hypertension 42: 1067–1074, 2003 [DOI] [PubMed] [Google Scholar]
- 68.Xie A, Skatrud JB, Crabtree DC, Puleo DS, Goodman BM, Morgan BJ. Neurocirculatory consequences of intermittent asphyxia in humans. J Appl Physiol 89: 1333–1339, 2000 [DOI] [PubMed] [Google Scholar]
- 69.Xue B, Beltz TG, Yu Y, Guo F, Gomez-Sanchez CE, Hay M, Johnson AK. Central interactions of aldosterone and angiotensin II in aldosterone- and angiotensin II-induced hypertension. Am J Physiol Heart Circ Physiol 300: H555–H564, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yamamoto K, Eubank W, Franzke M, Mifflin S. Resetting of the sympathetic baroreflex is associated with the onset of hypertension during chronic intermittent hypoxia. Auton Neurosci 173: 22–27, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zoccal DB, Bonagamba LG, Antunes-Rodrigues J, Machado BH. Plasma corticosterone levels is elevated in rats submitted to chronic intermittent hypoxia. Auton Neurosci 134: 115–117, 2007 [DOI] [PubMed] [Google Scholar]