Abstract
Mild decrease of core temperature (32–34°C), also known as therapeutic hypothermia, is a highly effective strategy of neuroprotection from ischemia and holds significant promise in the treatment of stroke. However, induction of hypothermia in conscious stroke patients is complicated by cold-defensive responses, such as shivering and tachycardia. Although multiple thermoregulatory responses may be altered by modulators of thermosensitive ion channels, TRPM8 (transient receptor potential melastatin 8) and TRPV1 (TRP vanilloid 1), it is unknown whether these agents affect cold-induced shivering and tachycardia. The current study aimed to determine the effects of TRPM8 inhibition and TRPV1 activation on the shivering and tachycardic responses to external cooling. Conscious mice were treated with TRPM8 inhibitor compound 5 or TRPV1 agonist dihydrocapsaicin (DHC) and exposed to cooling at 10°C. Shivering was measured by electromyography using implanted electrodes in back muscles, tachycardic response by electrocardiography, and core temperature by wireless transmitters in the abdominal cavity. The role of TRPM8 was further determined using TRPM8 KO mice. TRPM8 ablation had no effect on total electromyographic muscle activity (vehicle: 24.0 ± 1.8; compound 5: 23.8 ± 2.0; TRPM8 KO: 19.7 ± 1.9 V·s/min), tachycardia (ΔHR = 124 ± 31; 121 ± 13; 121 ± 31 beats/min) and drop in core temperature (−3.6 ± 0.1; −3.4 ± 0.4; −3.6 ± 0.5°C) during cold exposure. TRPV1 activation substantially suppressed muscle activity (vehicle: 25.6 ± 3.0 vs. DHC: 5.1 ± 2.0 V·s/min), tachycardia (ΔHR = 204 ± 25 vs. 3 ± 35 beats/min) and produced a profound drop in core temperature (−2.2 ± 0.6 vs. −8.9 ± 0.6°C). In conclusion, external cooling-induced shivering and tachycardia are suppressed by TRPV1 activation, but not by TRPM8 inhibition. This suggests that TRPV1 agonists may be combined with external physical cooling to achieve more rapid and effective hypothermia.
Keywords: therapeutic hypothermia, pharmacological hypothermia, shivering, transient receptor potential, physical cooling, electromyography, dihydrocapsaicin
mild decrease of core temperature to 32–34°C has been shown to effectively protect the brain from ischemia and has become a basis for the clinical method of therapeutic hypothermia (TH) (5). Following two successful clinical trials, TH has become a standard of care in patients resuscitated after cardiac arrest (1a). Ample evidence from animal studies strongly suggests that TH is also beneficial in acute ischemic stroke (37). However, so far, TH has been successfully applied only to unconscious mechanically ventilated patients. Because stroke patients are typically conscious, the use of TH in the treatment of stroke requires developing methods of lowering core temperature in conscious subjects (5).
Within current protocols, hypothermia is induced through application of physical cooling (e.g., by cold water-perfused blankets or intravascular heat exchangers), with the resulting increase in heat loss (19). In conscious subjects, however, the decrease in both ambient and core temperatures activates compensatory responses to generate and retain heat, such as shivering and peripheral vasoconstriction, which make physical cooling ineffective in achieving hypothermia (32). Although compensatory cold-defensive responses can be overcome by muscle-paralyzing and sedative drugs, these agents are not amenable for use in conscious subjects, including the majority of stroke victims (5).
A promising novel approach to induce hypothermia is the use of pharmacological agents acting on specific molecular targets within the thermoregulatory system. In particular, inhibitors of the transient receptor potential (TRP) melastatin 8 (TRPM8) ion channel and activators of the TRP vanilloid 1 (TRPV1) ion channel were shown to decrease core temperature in conscious organisms of a number of species (1, 10, 11, 14, 17). TRPM8 is a molecular cold sensor of the primary cold-sensitive neurons, while TRPV1 is a heat and pain receptor expressed within peripheral and central sensory nervous system (23, 30). Modulators of the TRPM8 and TRPV1 channels decrease heat generation and increase heat dissipation, even at typical room ambient temperatures in the absence of physical cooling (1, 14, 30). Because the rate of heat loss is directly proportional to the gradient between the core and ambient temperatures, the drop in core temperature may be achieved faster if subjects are also exposed to external cooling by physical methods. However, as mentioned earlier, physical cooling in conscious subjects activates compensatory heat generation, to a large extent through shivering thermogenesis (32). It is unknown whether TRPM8 inhibitors or TRPV1 agonists suppress the shivering response to external cooling. Furthermore, physical cooling leads to an increase in heart rate, which may exacerbate the cardiac rhythm abnormalities often seen in stroke patients. The involvement of TRPV1 and TRPM8 in cold-induced tachycardia is also unknown.
Answering these questions is important because it may provide conceptual support to the idea of combining TRP modulators with forced physical cooling. In case these agents do suppress the shivering response, such combined treatment is predicted to increase the rate of heat loss and lead to a faster and larger drop in core temperature. Alternatively, if TRP modulators have no effect on the external cooling-induced shivering, combining them with external cooling is predicted to have no net effect on the rate of heat loss, but instead to increase metabolic rate, which is detrimental for the neuroprotection offered by hypothermia. Therefore, the current study was designed to answer the basic biological question of whether TRPM8 inhibition or agonist-induced TRPV1 activation suppress the shivering and tachycardic responses to external cooling in a conscious subject using mice as a model of the regulation of the thermoregulatory responses by molecular thermoreceptors.
MATERIALS AND METHODS
Animals.
All animal experiments were performed in accordance with the National Institutes of Health's Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at Baylor College of Medicine. Male mice of the C57BL/6 strain were used, 12–18 wk of age, weighing 25–42 g. TRPM8 knockout (KO) male and female mice (weighing 21–39 g) on C57BL/6J background were obtained from Jackson Laboratories (Bar Harbor, ME). Mice were raised and housed at ∼22°C under 12:12-h light-dark cycle (lights on at 6 AM) and had free access to standard chow and water. Experiments were started at 12 PM. Mice were housed singly or in groups of 2–5 mice before the instrumentation, and singly after instrumentation and throughout the experiments. There were no statistical differences in weights of mice across experimental groups.
Drugs.
Compound 5, 3-[7-trifluoromethyl-5-(2-trifluoromethyl-phenyl)-1H-benzimidazol-2-yl]-1-oxa-2-azaspiro[4.5]dec-2-ene hydrochloride, a selective TRPM8 antagonist kindly provided by Janssen Research & Development (28), was prepared in 5% 1-methyl-2-pyrrolidinone (Sigma, St. Louis, MO) and 10% Solutol HS-15 (Sigma) and was injected intraperitoneally at 20 mg/kg in a volume of 10 ml/kg body wt.
DHC (Cayman Chemical, Ann Arbor, MI), a TRPV1 agonist, was dissolved in 10% dimethyl sulfoxide per 90% sterile saline and was injected intraperitoneally at 4 mg/kg in a volume of 10 ml/kg body wt.
For the in vivo TRPM8 behavioral assay, icilin (Cayman Chemical), a TRPM8 agonist, was suspended in 20% dimethyl sulfoxide per 80% sterile saline and injected intraperitoneally at 20 mg/kg in a volume of 10 ml/kg body wt.
Icilin-induced behavioral assay for TRPM8 activity.
Intraperitoneal injections of icilin in mice produce a number of characteristic TRPM8-dependent behaviors, of which “wet-dog shakes” are the most prominent example (6, 24). To demonstrate the inhibition of TRPM8 by a recently developed antagonist, compound 5 (28), the number of icilin-induced behavioral events was calculated in mice after pretreatment with compound 5. TRPM8 KO mice were used as a positive control, as they are expected to demonstrate a complete absence of icilin-induced TRPM8-dependent behavioral events in this assay. Wild-type mice were pretreated with compound 5 (20 mg/kg ip) or vehicle 60 min prior to icilin injections. An additional group of TRPM8 KO mice was pretreated with vehicle only. After 60 min, mice were injected with icilin (20 mg/kg ip), placed individually into plastic observation chambers, and recorded on video for the next 10 min for offline analysis of the behavioral events. The number of icilin-induced behavioral events (“wet-dog shakes”, shivering bursts, jumping, scratching and grooming) was counted during the 10th min after icilin injection.
Surgical implantation of the electromyography electrodes.
To measure the shivering response to external cooling, the total muscle activity from electromyographic (EMG) recordings was calculated. To capture EMG recordings, mice were implanted with electrodes in the muscles of the back. For surgical electrode implantation, mice were anesthetized with ketamine/xylazine/acepromazine anesthetic cocktail (35/6/1 mg/kg body wt ip). A small area of the skin on the back was shaved, and skin incisions were performed for attaching and exteriorizing EMG electrodes. Spinotrapezius muscles were exposed and bluntly dissected to provide a space for inserting electrode tips. Insulated EMG wires with ∼1 mm exposed stainless-steel tips were inserted ambilaterally in the spinotrapezius muscles ∼3 mm cranially and medially from the scapulae spines and sutured to the muscles using 6–0 silk sutures. An additional reference electrode was placed under the skin ∼2 cm caudally from the recording electrodes along the midline. All electrodes terminated with a microconnector (Omnetics Connector, Minneapolis, MN), which protruded ∼2 cm above the skin. The microconnector allowed for a mouse to be unrestrained during postsurgery recovery and was used during the experiment to connect the EMG electrodes to the recording equipment. The skin incision was closed by 5–0 polypropylene sutures. Mice were also implanted with miniature wireless implantable programmable temperature transponders (IPTT-300; BioMedic Data Systems, Seaford, DE). The biocompatible transponders have cylindrical shape and are 14 mm in length × 2 mm in diameter. Transponders have an accuracy of ±0.5°C, have a resolution of 0.1°C, and were factory-calibrated. A skin and muscle incision (∼3 mm in length) was made in the abdomen, and transponders were inserted into the abdominal cavity. Muscle and skin incisions were closed with 6–0 silk and 5–0 polypropylene sutures, respectively. Mice received buprenorphine (0.1 mg/kg sc) before and 6 h after surgery for analgesia. Mice were kept on the heating pad during and after surgery until ambulatory and then transferred to individual cages until the start of experiments. All experiments started at least 24 h after surgery. The average recovery time from surgery to the start of experiments was 48 h. To exclude the possibility that the instrumentation and incomplete recovery of the thermoregulatory response of mice affected the results, an additional experiment with visual observation of shivering was performed in naïve noninstrumented, nonanesthetized mice. There was no observable qualitative difference in both the shivering response and in the effects of the experimental treatment in noninstrumented mice compared with the instrumented mice, which recovered for 24 h.
Temperature-controlled chamber.
A custom-built temperature-controlled chamber was used to expose mice to external cooling by forced cooled air. The chamber consisted of a Plexiglas enclosure (20 × 20 × 12 cm), built around a Peltier element (area = 20 × 12 cm), equipped with heat exchangers and fans (TE Technology, Traverse City, MI). The air inside the chamber was recirculated by a computer fan (∼8 cm in diameter) directed over the heat exchange element and away from the mice, ensuring a uniform distribution of air temperature. The Peltier element was regulated by a temperature controller interfaced with a computer, allowing air temperature control to within 0.1°C.
Experimental protocol.
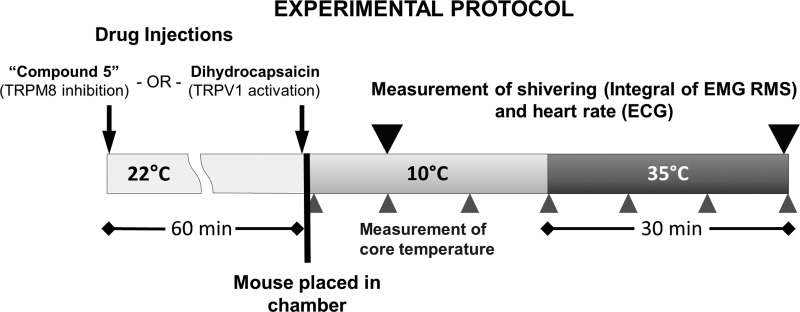
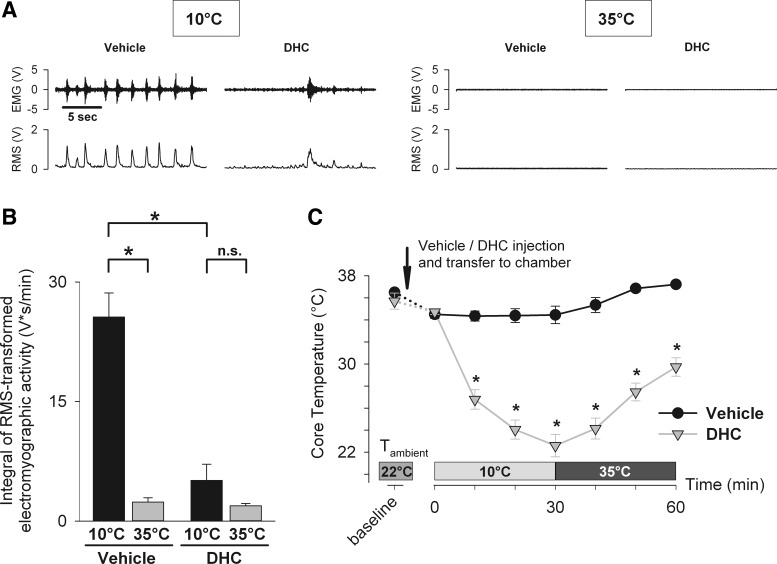
The experimental protocol is schematically demonstrated in Fig. 1. To determine the effects of TRPM8 ablation on cold-defensive responses, wild-type and TRPM8 KO mice were instrumented with EMG electrodes and abdominal wireless temperature transmitters. On the day of the experiment, mice were injected with compound 5 (20 mg/kg ip) or vehicle 60 min before cold exposure. This timing of injection with compound 5 was dictated by the experiment with icilin, in which it was determined that TRPM8 activity was blocked by compound 5 at ∼70 min postinjection. After 1 h, mice were lightly anesthetized with isoflurane, quickly transferred to the temperature-controlled chamber, with an ambient temperature set to 10°C, and connected to the biopotential amplifier (DAM-50, World Precision Instruments, Sarasota, FL) and the data acquisition system (Powerlab 4/SP, AD Instruments, Colorado Springs, CO). Brief (less than 30 s) isoflurane anesthesia was required to avoid stress and trauma associated with handling of the implanted wire during its connection to the recording equipment. Mice were held at 10°C for 30 min to measure cold-induced responses, with all measurements taken during the 10th min of the cold exposure. Next, the temperature in the chamber was set to 35°C, which was reached within ∼10 min. Mice were then rewarmed for the next 30 min to obtain reference values for the measured parameters, which also served as the positive control, indicative of complete absence of the cold-defensive responses. Although the temperature of 35°C might present a mild heat stress for mice, short durations of this slightly above neutral temperature were employed to ensure complete absence of cold-defensive responses. EMG recordings were taken from the implanted electrodes continuously while mice were in the temperature-controlled chamber using LabChart 7 Pro software (ADInstruments, Colorado Springs, CO). The EMG signal was amplified by 10,000×, bandpass filtered (1 Hz–3 kHz), and acquired at a sampling rate of 4 kHz. In addition, mice were recorded on video while in the chamber to detect their gross movements, which might create nonshivering artifacts on the EMG recordings. Subsequently, only those periods in which mice did not demonstrate locomotion or other gross voluntary movements (such as grooming and scratching) were included for analysis. Core temperature was measured wirelessly by a reader device from the implanted transponders every 10 min. Biopotential recordings were analyzed offline in LabChart 7 Pro. Representative biopotential recordings are demonstrated in parts A of Figs. 3–6.
Fig. 1.
The schematic illustration of the experimental protocol. See detailed description in the materials and methods.
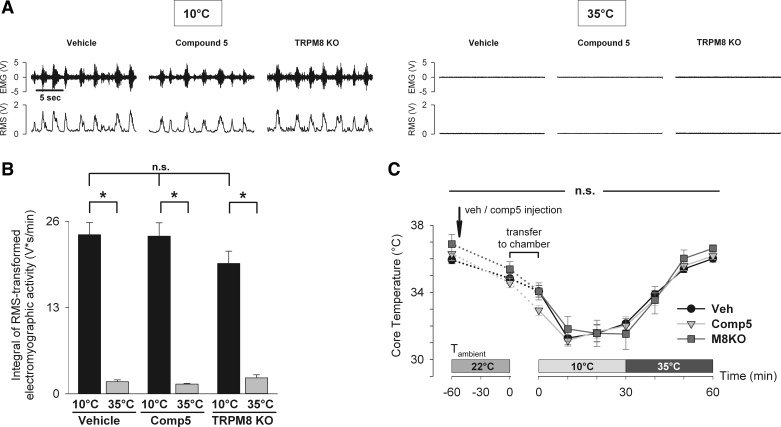
Fig. 3.
Pharmacological inhibition by compound 5 and genetic deletion of TRPM8 do not suppress the shivering response to external cooling. Total electromyographic muscle activity as a measure of the shivering response was determined in wild-type mice pretreated with vehicle (n = 6) or TRPM8 antagonist compound 5 (n = 6), as well as in TRPM8 KO mice (n = 5). Mice were exposed to cold (10°C for 30 min) and warm temperatures (35°C for 30 min). Muscle activity was calculated as a 1-min integral of the root-mean-square (RMS) transformed EMG signal during the 10th min of 10°C exposure and during the 30th min of 35°C exposure. A: representative electromyograms demonstrate raw EMG signal (top) and RMS-transformed signal (bottom). B: there was no difference in muscle activity among all groups at 10°C, as well as at 35°C [two-way repeated-measures ANOVA, effect of the treatment group: F(2,14) = 0.956, P = 0.408, n.s.]. Within each group, muscle activity at 35°C was significantly lower than at 10°C (Holm-Sidak test, *P < 0.001). C: core temperature was measured by wireless transponders in abdominal cavity in the same groups of mice throughout the experimental protocol. There was no difference in core temperature across all groups at all timepoints [two-way repeated-measures ANOVA, effect of the treatment group: F(2,14) = 0.34, P = 0.717, n.s.].
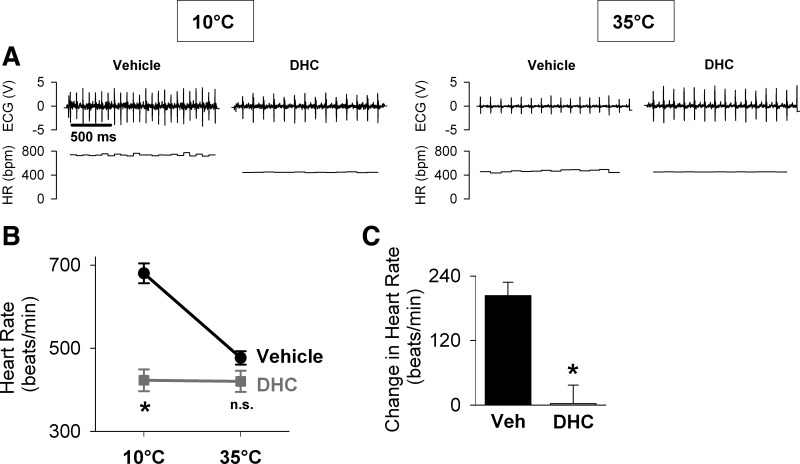
Fig. 6.
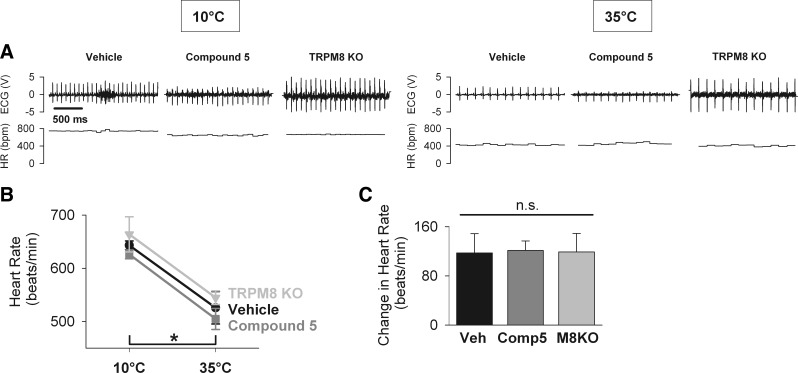
TRPV1 activation by DHC blunts the tachycardic response to external cooling. Heart rate was calculated from electrocardiographic recordings in mice pretreated with vehicle (n = 6) or DHC (n = 6), as described in Fig. 5. A: representative recordings demonstrate ECG signal (top) and calculated beat-to-beat heart rate (HR; bottom). B: heart rate in DHC-treated mice was significantly lower at 10°C compared with vehicle-treated mice [two-way repeated-measures ANOVA, effect of the treatment group: F(1,5) = 151.168, P < 0.001; Holm-Sidak test for the effect of the treatment group within 10°C, *P < 0.001], but not different at 35°C (Holm-Sidak test, P = 0.096, n.s.). C: tachycardic response, calculated as the difference in heart rate of mice exposed to cold (10°C) and warm (35°C) temperatures, was significantly decreased in DHC-treated mice compared with vehicle-treated mice (Wilcoxon signed rank test, *P = 0.031).
To determine the effects of TRPV1 activation by an agonist, immediately before being placed into the temperature-controlled chamber, instrumented mice were injected with DHC (4 mg/kg ip) or vehicle while under brief isoflurane anesthesia and subjected to the same protocol described above.
In these experiments, a paired design was used, in which each subject received both experimental and control treatments on two different days. Each subject underwent only one experiment per day. The order of treatments was balanced (i.e., half of the subjects received experimental treatment on the first day and vehicle control treatment on the next day, and the other half vice versa). For the experiment evaluating TRPM8 modulation of responses, an additional group of TRPM8 KO mice injected with vehicle for compound 5 was studied under the same protocol.
Analysis of the EMG recordings to determine muscle activity associated with shivering.
To calculate the total muscle activity as the measure of shivering, EMG recordings were analyzed offline in LabChart 7 Pro. The EMG signal was additionally software-filtered (bandpass, 500–1,000 Hz) and root-mean-square (RMS) transformed with smoothing over 0.1 s. The integral of the RMS-transformed signal over the 10th min of the cold exposure was used as a measure of muscle activity associated with the shivering response. The minimum RMS value of the respective epoch was subtracted from the RMS signal prior to integral calculation to eliminate baseline electrical noise. The 10-min time point was selected because the data from the icilin-induced behavioral assay indicated that at this time point, compound 5 effectively blocks TRPM8 activity in vivo (considering that compound 5 was injected 60 min prior to the start of the recording, this 10-min time point corresponds to 70 min after compound 5 injection). As a reference value for the muscle activity at warm temperature, the RMS integral during the 30th min after switching the temperature to 35°C was also determined. After mice were exposed to warm temperature (i.e., 35°C), they displayed no visual indication of shivering. Therefore, the muscle activity at 35°C was considered to be representative of the complete absence of shivering.
Measurement of the heart rate by ECG.
To determine the effects of TRPM8 inhibition and TRPV1 activation on the tachycardic response to external cooling, the heart rate from ECG recordings was calculated. The ECG signal was extracted from the EMG signal obtained in the experiments with the measurement of shivering. The original biopotential signal was filtered (low pass, 300 Hz), which allowed the “R” waves of the ECG to be resolved. Because the position of the electrodes was not suitable for high-quality ECG recordings (electrodes in back muscle instead of typical ECG lead configurations), which required significant amplification and filtering of the signal, the shape of the electrocardiogram was extremely variable across subjects and not amenable to meaningful and more detailed analysis. Nevertheless, the resolution of the R waves allowed quantifying the R-R interval. A cyclic measurement algorithm was applied to the ECG signal (LabChart 7 Pro) to determine heart rate. Heart rate was measured as a 1-min average over the 10th min of the cold exposure (10°C) and during the 30th min of the rewarming phase (35°C). The tachycardic response was defined as the difference between heart rates at 10°C (during cold exposure) and 35°C (during rewarming) in each mouse. Although the more correct definition of the tachycardic response would involve the difference between the heart rate at 10°C and 35°C prior to cooling, in the current experimental protocol, it was not practical to obtain resting heart rate of mice before cooling. Accordingly, the steady-state heart rate at 35°C after rewarming was assumed to be equal to and used instead of the heart rate at 35°C prior to cooling.
Measurement of the core temperature by wireless transponders.
Core temperature was measured wirelessly from the temperature transponders in the abdominal cavity using a handheld reader device every 10 min. To determine the relative drop in temperature due to cold exposure, a within-subject difference in core temperature was calculated between the prechamber values and after 10 min at 10°C.
In the experiment investigating the effects of compound 5 on core temperature during mild cooling (Fig. 2B), the temperature-controlled chamber was separated into two parts by a plastic mesh divider, and two mice at a time were placed into the separate parts of the chamber and studied simultaneously. This paired design reduced variability in uncontrolled factors associated with the cooling. Mice were implanted with wireless temperature transmitters, as described above, on average ∼7 days before the experiment. The chamber was set to 18°C to provide mild cooling. After a baseline period of 30 min in the chamber at 18°C, mice were briefly removed from the chamber, injected with compound 5 (20 mg/kg ip) or vehicle, and placed back into the chamber. Injections were performed in conscious mice and completed within 2 min. Next, mice were held at 18°C for an additional 2 h. Core temperature was measured, while mice were in their home cage (baseline), every 5 min during the first 60 min of the experiment, and every 10 min for the rest of the experiment.
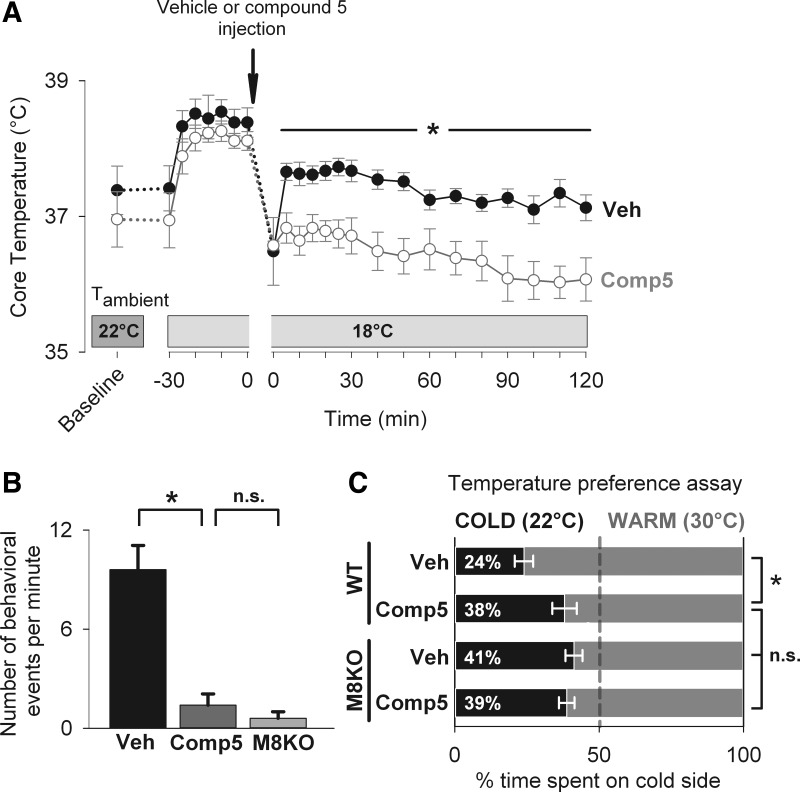
Fig. 2.
Compound 5 effectively inhibits TRPM8 activity in vivo. A: effects of compound 5 treatment on core temperature of conscious wild-type mice exposed to mild cooling (18°C). Mice were held at 18°C for 30 min, then briefly removed from the chamber, injected with compound 5 (n = 7) or vehicle (n = 7) and exposed to mild cooling at 18°C for an additional 2 h. Compound 5 treatment led to a statistically significant decrease in core temperature during 5–120 min of the cold exposure [two-way repeated-measures ANOVA, effect of the treatment group: F(1,12) = 14.646, P = 0.002; Holm-Sidak test for the effect of the treatment group within 5–120-min timepoints, *P < 0.05]. B: wild-type mice pretreated with vehicle (Veh, n = 5) or compound 5 (Comp5, n = 5) 60 min earlier, as well as TRPM8 KO mice (TRPM8 KO, n = 5), were injected with TRPM8 agonist icilin (20 mg/kg ip). The number of icilin-induced behaviors, counted during the 10th min posti-icilin (equal to 70th min after administration of compound 5), in mice pretreated with compound 5 was significantly lower compared with vehicle-treated mice and not different compared with TRPM8 KO mice. One-way ANOVA across three groups, F(2,12) = 26.78, P < 0.001. Student-Newman-Keuls post hoc test: *P < 0.001 for Veh vs. Comp5 and Veh vs. TRPM8 KO; P = 0.568 (not significant, n.s.) for Comp5 vs. TRPM8 KO. C: effects of compound 5 treatment on cold sensitivity of mice in the temperature preference assay. Wild-type mice (WT, n = 8), as well as TRPM8 KO mice (M8KO, n = 9) were pretreated with vehicle (Veh) or compound 5 (Comp5) 60 min earlier and then allowed to explore the contiguous surface, two sides of which had cold (22°C) or warm (30°C) temperature, for 6 min. The percentage of time spent on each side was determined. The percentage of time spent on the cold side by compound 5-treated WT mice was significantly higher compared with vehicle-treated WT mice and not different from vehicle-treated M8KO mice. There was no increase in time on the cold side in compound 5-treated M8KO above that in vehicle-treated M8KO mice. One-way ANOVA across four groups, F(3,30) = 5.52, P = 0.004. Student-Newman-Keuls post hoc test: *P < 0.01 for WT Veh vs. WT Comp5, M8KO Veh, and M8KO Comp5 each; P > 0.05 (n.s.) for WT Comp5, M8KO Veh, and M8KO Comp5 pairwise.
Temperature preference assay.
A temperature preference assay was used to determine whether compound 5 decreases cold sensitivity of mice, indicative of an effective block of the TRPM8 activity (2). The assay was performed using a custom-built setup, which consisted of two adjacent water-jacketed Plexiglas platforms. The temperature of each platform base was independently controlled using two circulating water baths. Cardboard walls were constructed around these two platforms, creating a contiguous surface of two different temperatures. For these studies, one side was maintained at 30°C (“warm”) and the other at 22°C (“cold”). Mice were introduced and maintained on the warm side for the first 30 s. After 30 s, the separator keeping the mice on the warm side was removed and mice were allowed to freely move between warm and cold sides. Mice were then recorded on video for 6 min, during which time spent on each temperature platform was measured. In pilot studies, it was found that some mice never ventured onto the cold surface. Therefore, to ensure that mice experienced both sides, mice were gently nudged to the opposite side of the chamber if they failed to change sides within a 60-s period. A lower percentage of time spent on the cold side represents the sensitivity and aversion to cold in this paradigm. Accordingly, the loss of cold sensitivity leads to a higher percentage of time spent on the cold side. A paired design was used, with each mouse receiving vehicle injection on one experimental day and compound 5 (20 mg/kg ip) on another experimental day. A group of wild-type male mice and another group of both male and female TRPM8 KO mice were used in this experiment. Mice were placed into the temperature preference setup 60 min after the injection, as in the shivering experiment. Mice recovered for 2 wk between the two experimental days. The order of the injections was balanced.
Statistics.
All results are reported as means ± SE. Error bars in graphs denote means ± SE. To test the effects of vehicle, compound 5, and TRPM8 gene KO on icilin-induced behaviors (Fig. 2B), as well as to test the difference in the percentage of time spent on the cold side in the temperature preference assay (Fig. 2C), One-way ANOVA followed by Student-Newman-Keuls post-hoc test for pairwise comparisons was used. To test the effects of vehicle or experimental treatment (compound 5 and TRPM8 gene KO or DHC) on the shivering response at 10°C and 35°C (Figs. 3B and 4B), on heart rate at 10°C and 35°C (Figs. 5B and 6B), and on core temperature at different timepoints (Figs. 2A, 3C, and 4C), two-way repeated-measures ANOVA was used, with treatment group as a fixed factor and temperature (10°C and 35°C) or timepoint as a repeated-measures factor, followed by Holm-Sidak test for multiple pairwise comparisons within each treatment group and temperature/time level. To test the difference in the tachycardic response between vehicle, compound 5 and TRPM8 KO groups (Fig. 5C) one-way ANOVA was used. To test the difference in the tachycardic response between DHC and vehicle group (Fig. 6C), the nonparametric Wilcoxon signed rank test was used, due to non-normally distributed data. The differences were considered statistically significant at P < 0.05. Statistical analysis was performed in SigmaPlot version 12 (Systat Software, San Jose, CA).
Fig. 4.
TRPV1 activation by dihydrocapsaicin (DHC) potently suppresses the shivering response to external cooling. Total electromyographic muscle activity as a measure of the shivering response was determined in mice pretreated with vehicle (n = 6) or TRPV1 agonist DHC (n = 6) as described in Fig. 3. A: representative electromyograms demonstrate raw EMG signal (top) and RMS-transformed signal (bottom). B: muscle activity at 10°C in the DHC group was significantly lower compared with the vehicle control group [two-way repeated-measures ANOVA, effect of the treatment group: F(1,5) =19.03, P = 0.007; Holm-Sidak test for the effect of the treatment group within 10°C, *P < 0.001]. In the vehicle group, muscle activity at 35°C was significantly lower than at 10°C (Holm-Sidak test, *P < 0.001). In the DHC-treated group, there was no difference in muscle activity at 35°C compared with 10° (Holm-Sidak test, P = 0.214, n.s.). C: core temperature was measured by wireless transponders in abdominal cavity in the same groups of mice throughout the experimental protocol. DHC-treated mice had a significantly lower core temperature compared with vehicle-treated mice [two-way repeated-measures ANOVA, effect of the treatment group: F(1,5) = 93.37, P < 0.001; Holm-Sidak test for the effect of the treatment group within 5–60-min timepoints, *P < 0.001].
Fig. 5.
Pharmacological inhibition by compound 5 and genetic deletion of TRPM8 do not blunt the tachycardic response to external cooling. Heart rate was calculated from electrocardiographic recordings in wild-type mice pretreated with vehicle (n = 6) or TRPM8 antagonist compound 5 (n = 6), as well as in TRPM8 KO mice (n = 5). Mice were exposed to cold (10°C for 30 min) and warm temperatures (35°C for 30 min), and heart rate was averaged over the 10th min of the 10°C exposure and over the 30th min of the 35°C exposure. A: representative recordings demonstrate ECG signal (top) and calculated beat-to-beat heart rate (HR; bottom). B: there was no difference in the heart rate among all groups at 10°C, as well as at 35°C [two-way repeated-measures ANOVA, effect of the treatment group: F(2,14) = 1.394, P = 0.28, n.s.]. In each group, heart rate was higher at 10°C compared with 35°C (Holm-Sidak test, *P < 0.001). C: tachycardic response, calculated as the difference in heart rate of mice exposed to cold (10°C) and warm (35°C) temperatures, was not different among all groups [one-way ANOVA, F(2,14) = 0.01, P = 0.994, n.s.].
RESULTS
Compound 5 effectively inhibits TRPM8 activity in vivo.
To confirm the inhibition of TRPM8 activity in vivo by a recently developed TRPM8 antagonist compound 5 (28), we determined the core temperature of mice treated with vehicle and compound 5 (at a dose of 20 mg/kg ip) exposed to mild cooling at 18°C for 2 h. Mice treated with compound 5 had ∼1°C lower core temperature throughout the cooling session (Fig. 2A). The drop in core temperature after 70 min at 18°C relative to the preinjection value was −1.1 ± 0.1 vs. −1.7 ± 0.2 in vehicle- and compound 5-treated mice, respectively (P = 0.02). The hypothermic effect of compound 5 observed here is in agreement with the effects of other reported TRPM8 inhibitors (1, 11, 17), which is consistent with the inhibition of TRPM8 activity by compound 5.
To demonstrate the TRPM8 inhibition by compound 5 more specifically, TRPM8-dependent icilin-induced behaviors were measured in mice pretreated with compound 5. In vehicle-treated mice with intact TRPM8 activity, used as a negative control in this assay, 9.6 ± 1.5 events/min was observed. In TRPM8 KO mice, used as a positive control for full suppression of the TRPM8 activity, 0.6 ± 0.4 events/min was observed. The number of behavioral events in mice pretreated with compound 5 (1.4 ± 0.7 events/min) was significantly lower compared with vehicle-treated mice and not different compared with TRPM8 KO mice (Fig. 2B). These data demonstrate that compound 5 effectively inhibits TRPM8 activity in vivo.
The inhibition of TRPM8 by compound 5 was additionally determined in the temperature preference assay. The loss of cold sensitivity in this assay is a well-established consequence of the genetic deletion of TRPM8 (2, 6, 8). Wild-type mice were injected with vehicle or compound 5 (20 mg/kg ip) 60 min prior to the experiment and placed in the temperature preference setup, where they were allowed to explore and select between two sides of a surface set at 22°C (cold side) and the other set at 30°C (warm side). The percentage of time spent on the cold side was measured as an inverse measure of cold sensitivity. Cold sensitivity was also determined in TRPM8 KO mice used as a positive control for full ablation of TRPM8 activity. Compound 5-treated wild-type mice spent significantly more time on the cold side compared with vehicle-treated mice (38 ± 4% vs. 24 ± 3%, respectively, Fig. 2C). Cold sensitivity was not different in compound 5-treated wild-type mice and vehicle-treated TRPM8 KO mice (41 ± 3%). To determine whether the effect of compound 5 is specific to TRPM8, cold sensitivity was determined in compound 5-injected TRPM8 KO mice. Compound 5 injection did not increase the time on the cold side above that of vehicle-treated TRPM8 KO mice (39 ± 3%). These data demonstrate that compound 5 decreases cold sensitivity of mice by effectively and specifically inhibiting TRPM8 activity.
Pharmacologic antagonism or genetic deletion of TRPM8 does not lead to a decrease in the shivering response.
To assess the potential involvement of TRPM8 in the shivering response to external cooling, the total EMG muscle activity was measured in conscious mice pretreated with compound 5 and exposed to external cooling in a temperature-controlled chamber (Fig. 3, A and B). External cooling at 10°C resulted in a robust EMG response in vehicle-pretreated mice (24.0 ± 1.8 V·s/min). Pretreatment with compound 5 at a dose of 20 mg/kg ip did not significantly reduce the EMG activity produced by external cooling compared with vehicle control (23.8 ± 2.0 V·s/min). Similarly, TRPM8 KO mice also showed no statistically significant decrease in EMG activity in response to external cooling (19.7 ± 1.9 V·s/min) compared with wild-type, vehicle-treated mice. As a positive control for full shivering suppression, mice were exposed to 35°C, at which no shivering should occur. Mice in all three groups had a significantly decreased EMG activity at 35°C compared with 10°C, consistent with the absence of shivering (vehicle control: 1.8 ± 0.3 V·s/min; compound 5: 1.5 ± 0.1 V·s/min; TRPM8 KO: 2.4 ± 0.5 V·s/min). These results demonstrate that neither pharmacological antagonism nor genetic ablation of TRPM8 results in suppression of the shivering response to external cooling.
In addition, the effect of TRPM8 ablation on core temperature during cooling at 10°C was measured, as an indication of the total cold-induced thermogenesis. Pharmacologic antagonism and genetic deletion of TRPM8 had no effect on absolute core temperature during both cooling and rewarming phase (Fig. 3C). The relative drop in core temperature after 10 min at 10°C was −3.6 ± 0.1°C, −3.4 ± 0.4°C, and −3.6 ± 0.5°C in the vehicle control, compound 5 and TRPM8 KO groups, respectively (P = 0.96, data not shown).
TRPV1 activation suppresses the shivering response to external cooling.
To determine the effects of TRPV1 activation by DHC on the shivering response, the total EMG muscle activity in conscious mice exposed to cold was measured, as described previously (Fig. 4, A and B). EMG activity at 10°C in mice pretreated with DHC (4 mg/kg ip) was significantly decreased compared with vehicle-pretreated control mice (5.1 ± 2.0 vs. 25.6 ± 3.0 V·s/min, respectively). The suppression of the shivering response was accompanied by the substantially lower core temperature in DHC-treated mice (Fig. 4C). The relative drop in core temperature after 10 min at 10°C was −8.9 ± 0.6°C vs. −2.2 ± 0.6°C, in DHC- and vehicle-treated mice, respectively (P < 0.001, data not shown). These results demonstrate that agonist-induced activation of TRPV1 during external cooling at 10°C significantly decreases the shivering response and produces a profound drop in core temperature.
TRPM8 ablation does not blunt the tachycardic response to external cooling.
To determine the role of TRPM8 in the tachycardic response to cold, heart rate was measured by ECG in conscious mice pretreated with compound 5, as well as in TRPM8 KO mice (Fig. 5). There was no significant difference in heart rate measured at 10°C among all groups (vehicle control: 643 ± 8 beats/min; compound 5: 626 ± 8 beats/min; TRPM8 KO: 664 ± 13 beats/min) or after rewarming to 35°C (vehicle control: 527 ± 30 beats/min; compound 5: 512 ± 18 beats/min; TRPM8 KO: 547 ± 60 beats/min; Fig. 5B). At 10°C, heart rate was significantly higher in all three groups compared with 35°C. The tachycardic response to external cooling, defined as the difference in heart rate measured at 10°C and 35°C, was not significantly different either in compound 5-treated or TRPM8 KO mice, compared with vehicle-treated, wild-type mice (control: ΔHR = 124 ± 31 beats/min; compound 5: ΔHR=121 ± 13 beats/min; TRPM8 KO: ΔHR=121 ± 31 beats/min; Fig. 5C). These results demonstrate that neither pharmacological antagonism nor genetic ablation of TRPM8 affects the tachycardic response to external cooling.
TRPV1 activation completely blunts the tachycardic response to external cooling.
To determine the effects of TRPV1 activation by DHC on the tachycardic response to cold, heart rate was measured by ECG in DHC- and vehicle-treated conscious mice during external cooling (Fig. 6). After 10 min at 10°C, heart rate was substantially lower in the DHC-treated mice compared with vehicle-treated mice (423 ± 26 vs. 680 ± 24 beats/min, respectively; Fig. 6B). Following 30 min of rewarming at 35°C, there was no significant difference in heart rate between DHC- and vehicle-treated mice (421 ± 26 vs. 477 ± 16 beats/min, respectively). The tachycardic response (i.e., the difference in heart rate measured at 10°C and 35°C) was significantly lower in DHC-treated mice compared with vehicle-treated mice (control: ΔHR = 204 ± 25 beats/min; DHC: ΔHR = 3 ± 35 beats/min; Fig. 6C). In fact, the tachycardic response in DHC-treated mice was effectively absent. These results demonstrate that TRPV1 activation by DHC completely blunts the tachycardic response to external cooling.
DISCUSSION
TH is emerging as one of the most effective strategies of neuroprotection from ischemia and, therefore, holds significant promise as a novel treatment for acute ischemic stroke (5). The current protocols for inducing hypothermia rely on increasing heat loss by applying physical cooling, which is provided by surface or intravascular cooling devices (19). However, in conscious nonsedated subjects, cooling triggers compensatory cold-defensive responses, such as shivering, which increases metabolic rate and oxygen demand, thereby counteracting the cooling effect and preventing the drop in core temperature (19). Therefore, the successful use of TH for the treatment of stroke requires novel methods of lowering core temperature, which can be used in awake patients outside of intensive care units.
One of the promising novel approaches to the induction of hypothermia is pharmacological targeting of specific molecular components of the sensory nervous system that affect thermoregulation. In particular, such targets include two temperature-sensitive ion channels from the TRP superfamily, namely TRPM8 and TRPV1, which are expressed within the afferent pathways for temperature sensation (23, 30). Pharmacologic inhibition of TRPM8 and activation of TRPV1 alter the afferent signals from thermoreceptors to the neural centers of thermoregulation, thereby modifying thermoregulatory responses and decreasing core temperature in conscious organisms of a number of species (1, 10, 14, 17). Accordingly, it has been proposed to use TRPV1 agonists and TRPM8 antagonists to induce or modulate TH in human patients (1, 10, 26).
The driving force behind any method of hypothermia is the loss of heat to the environment. The rate of heat loss, as well as the rate of decrease in core temperature, is directly proportional to the gradient between ambient and core temperatures. Therefore, pharmacologically induced hypothermia may be achieved faster if combined with the decrease in ambient temperature, or, in other words, with external cooling by physical methods. However, as stated earlier, external cooling in conscious subjects results in compensatory cold-defensive responses, which, if left intact, negate the added benefit of external cooling (32). Therefore, to predict whether external cooling enhances the hypothermic effect of a pharmacological agent, it is important to know whether this agent is able to suppress the cold-defensive responses.
Mammals utilize two major autonomic cold-defensive responses: shivering and brown adipose tissue (BAT) thermogenesis. Previously, it was assumed that modern adult humans rely mostly, if not exclusively, on shivering for compensatory heat generation in response to external cooling, but recently, this idea was challenged by the exciting discovery of brown adipose tissue in adult humans (20). Thus, it was established that the contribution of BAT to the cold-induced thermogenesis is significant, depends on factors such as cold acclimatization and the degree of acute cold exposure and can reach up to 30% (20). However, even considering the latest evidence, shivering must still be considered a major contributor to compensatory heat generation and an important impediment to the use of physical cooling in therapeutic hypothermia. Therefore, the current study investigated the ability of a TRPM8 inhibitor and a TRPV1 agonist to suppress the shivering response to external cooling.
From the clinical perspective, it would be important to determine whether combining TRP modulators with external physical cooling enhances the drop in core temperature in human patients. However, the absolute changes in core temperature cannot always be directly and meaningfully translated from rodents to humans, because core temperature is a complex resultant measure of multiple interacting species-specific processes. The current study, therefore, employed a more reductionist and mechanistic approach and specifically focused on an individual thermoeffector response, i.e., shivering, which substantially contributes to the cold-induced heat generation in humans and can still be reliably measured in mice.
TRPM8 is not required for shivering.
TRPM8 is a nonspecific cation channel from the TRP superfamily, which is directly activated by cold temperature in the range of 10–27°C (24, 29). TRPM8 has been shown to be responsible for the cold sensitivity of a subset of cold-responsive primary afferent neurons that innervate the skin (2, 6, 8). Accordingly, a series of recent in vivo studies using pharmacological antagonism and/or genetic deletion have established that TRPM8 plays a significant role in most individual thermoregulatory responses to cold: behavioral thermoregulation, BAT activation, peripheral vasoconstriction, and total metabolic response to cold (1, 2, 6, 8, 17, 33). TRPM8 was also implicated in shivering thermogenesis in two studies using menthol as a TRPM8 agonist, which, however, were not conclusive due to possible TRPM8-independent actions of menthol (15, 18, 21, 34). Despite this caveat, these and other studies seemed to have formed a prevailing implicit view in the literature that TRPM8 is a “universal” cold sensor, insofar as it controls every physiological response to cold. However, the role of TRPM8 specifically in shivering, a critical missing piece in this puzzle, was never directly confirmed.
Interestingly, it was found in the current study that pharmacological inhibition and genetic ablation of TRPM8 ion channel did not suppress the increase in electromyographic activity in response to external cooling, which suggests that TRPM8 is not required for external cooling-induced shivering. This result may be reconciled with existing data by considering that in TRPM8 KO mice a subset of primary sensory neurons still responds to cold (2, 6, 8). Therefore, it is conceivable that a distinct population of TRPM8-independent primary sensory neurons forms the afferent portion of the pathway for the shivering response to external cooling. According to the emerging concept of “thermoeffector loops”, different thermoeffectors are functionally independent and use anatomically distinct pathways (22, 31). The existence of such a shivering-specific primary neuron population would serve as additional anatomical evidence in support of this concept, particularly in relation to the afferent pathways.
Is TRPM8 required for maintaining core temperature during cold exposure?
The current study also explored the effects of TRPM8 inhibition on core temperature during external cooling. Because mice have higher rates of heat loss and are more labile thermoregulators, the findings about the absolute changes in core temperature of mice should not be directly translated to humans (12). Nevertheless, these findings should still provide useful mechanistic insights into the involvement of TRPM8 in shivering. Laboratory rodents, exclusively used in relevant previous studies, rely on two autonomic thermogenic mechanisms for maintaining core temperature during cold exposure: BAT activation and shivering. As mentioned earlier, TRPM8 was definitively shown to be involved in BAT activation (1, 33). In the event that TRPM8 was also substantially involved in shivering, TRPM8 ablation would be predicted to severely compromise both thermogenic mechanisms and make an organism unable to maintain its temperature in a cold environment. However, the existing data do not support this prediction. Several studies, which explored the effects of genetic deletion and pharmacological inhibition of TRPM8 on core temperature during mild and moderate cooling (ambient temperatures ranging from 10 to 22°C), consistently report ∼1°C lower core temperatures after TRPM8 ablation compared with controls (1, 11, 17, 33). In agreement, in the current study a ∼1°C difference was observed in mice treated with TRPM8 inhibitor compound 5 and exposed to mild cooling at 18°C for 2 h, compared with vehicle-treated mice. Taken together, these data indeed leave little doubt that TRPM8 ablation decreases the ability to maintain core temperature during cold exposure and mildly lowers it. However, the magnitude of this effect is less than would be predicted if TRPM8 had a major role in triggering shivering.
It should be noted that in another experiment of this study designed to measure the shivering response, TRPM8 ablation had no effect on core temperature of mice exposed to more intense cooling at 10°C. However, this result might have been affected by the necessary adjustments in experimental conditions, such as instrumentation with EMG leads and brief anesthesia with isoflurane. Nevertheless, if TRPM8 ablation had the potential to produce a substantial drop in core temperature, it would still have been detected in this setup, as was the case in the experiment with TRPV1 agonist stimulation, which was performed in the exact same conditions and revealed ∼7°C larger drop in core temperature in DHC over the vehicle control group.
On the basis of the effects of TRPM8 ablation on thermoregulatory responses and core temperature during cold exposure, observed in the present study and reported by others, we propose the model, wherein TRPM8 is required for behavioral responses to cold, nonshivering thermogenesis, and peripheral vasoconstriction but not for the shivering thermogenesis. Within this model, the slight drop in core temperature observed with TRPM8 inhibition at mildly subneutral ambient temperatures is explained by suppression of the first three TRPM8-dependent processes, which are responsible for basal heat generation and retention. At the same time, the proposed model explains why TRPM8 ablation produces only a minor decrease in core temperature even during intense cooling, namely, because the heat needed to maintain core temperature in these conditions is instead being supplied primarily by TRPM8-independent shivering thermogenesis.
TRPV1 activation suppresses shivering.
TRPV1 is a temperature-sensitive TRP channel that is activated above 43°C in vitro and is expressed in neurons on multiple levels within the peripheral and central sensory nervous system (30). It is well established that agonist-stimulated TRPV1 activation leads to a profound drop in core temperature in various mammalian species through activation of heat loss mechanisms, such as peripheral vasodilation, sweating, panting, saliva spreading, and warm-seeking behaviors, as well as suppression of heat gain mechanisms (14). However, the suppression of the shivering thermogenesis specifically in response to external cooling following TRPV1 activation was never directly demonstrated. The present study, using an objective shivering measurement method based on EMG recordings, provides the first substantiated report demonstrating that TRPV1 activation potently suppresses the shivering response to external cooling.
In agreement with potent suppression of shivering, here, it was also found that TRPV1 agonist administration is accompanied by a profound drop in core temperature during cold exposure. These findings are consistent with the current understanding of the actions of TRPV1 agonists on the thermoregulatory system (14, 30), which include a coordinated activation of heat loss responses with reciprocal inhibition of heat-generating responses.
Hypothermic and shivering-suppressive actions of TRPV1 agonists make them promising agents for inducing or modulating TH. Indeed, a recent study by Fosgerau et al. (10) demonstrated the feasibility of using the TRPV1 agonist DHC for long-term sustained hypothermia in several species, including rats, monkeys, and cattle (10). Furthermore, evidence suggests that hypothermia induced by the TRPV1 agonists resiniferatoxin and DHC provides neuroprotection in mouse models of acute ischemic stroke (3, 26). In these studies, TRPV1 agonists were administered at room temperature, which removes the external signal for triggering cold-defensive responses, such as shivering, and precludes possible complications associated with external physical cooling. However, the findings in the current study suggest that TRPV1 activation by itself prevents these complications. Therefore, we propose that combining TRPV1 agonists with physical cooling methods does not result in substantial triggering of the cold-defensive responses and might bring an additional benefit of reducing the time needed to achieve the target temperature, especially in larger mammals, or reducing the dose of the agonist and associated side effects.
TRPV1 activation, but not TRPM8 inhibition, blunts the tachycardic response to cooling.
Cold exposure in conscious mammals also leads to an increase in heart rate, accompanied by an increased heat generation by the working heart (25). Although the contribution of the cardiac thermogenesis to the regulation of core temperature is unclear, tachycardia, as one of the physiological responses to cooling, has important implications for the clinical method of TH in the treatment of stroke. Stroke patients are at high risk of cardiac arrhythmias, and pronounced tachycardia may increase this risk even further (7). Therefore, we examined whether TRPM8 or TRPV1 modulators with potential application in TH have an additional benefit of blunting the tachycardic response to cold. It was found that TRPM8 is not involved in cold-induced tachycardia. Therefore, tachycardia seems to be another response to cold, along with shivering, which is controlled by the TRPM8-independent neuronal population.
It was also found that TRPV1 activation by the agonist DHC completely eliminated the tachycardic response to cold. This finding is consistent with the view that TRPV1 activation broadly suppresses all cold-defensive responses. It is, thus, tempting to speculate that using pharmacological TRPV1 agonists to produce or enhance therapeutic hypothermia may bring an additional benefit of blunting tachycardia, limiting the load on the heart and preventing cardiac tachyarrhythmias. However, TRPV1 agonists seem to have complex and variable effects on the cardiovascular system at baseline conditions in the absence of cooling (4, 9, 13, 35, 36). Since there is no clear and unified picture of how TRPV1 activation affects heart function, this area deserves further study.
Limitations.
Our conclusion relies on the assumption that both pharmacological antagonism using compound 5 and TRPM8 gene deletion would result in the substantial decrease in TRPM8 activity. Although compound 5 was shown to be a specific and effective TRPM8 inhibitor in a thorough panel of in vitro tests (28), as well as in several in vivo assays in rats (28) and mice (the current study), there is still some possibility that the antagonist, at the concentration and delivery route used here, does not effectively block the activity of some population of the TRPM8-expressing neurons within the shivering pathway. Likewise, a whole body TRPM8 KO mouse may have developed compensatory mechanisms for shivering control and, therefore, may no longer be a physiologically relevant model, even though a pronounced phenotype of the TRPM8 KO mouse argues against compensatory changes in this genetic model. (2, 6, 8, 33). Similar results obtained with pharmacological and genetic approaches increase the reliability of conclusions in the current study. Nevertheless, it would be important to confirm these findings by measuring the shivering response in mice treated with other developed TRPM8 inhibitors (1, 11, 17), as well as in conditional KO mice with acute genetic deletion of TRPM8 gene. Finally, because not all possible scenarios can be tested, we cannot completely rule out the possibility that TRPM8 still plays at least a minor role in shivering in some specific set of conditions (including specific species, ambient temperature, method of cooling, etc.) However, the results presented here strongly argue against a major and practically relevant involvement of TRPM8 in the shivering response to external cooling.
Perspectives and Significance
The broad implication of our findings is that the molecular organization of autonomic temperature sensation is more complex than previously thought and that, although all thermoeffectors share a common goal to protect thermal homeostasis, they may rely on distinct afferent neuronal populations with distinct molecular cold-sensing mechanisms. Because the cold receptor TRPM8 was not found to be required for cold-induced shivering and tachycardia, it remains to be determined whether and what dedicated cold-sensing mechanism regulates these important responses. Therefore, it might be useful to explore the role of other known cold-sensitive channels, such as TRPA1 (16), TRPC5 (38), and TREK-1/TRAAK (27), in shivering and tachycardia. On the other hand, a heat and capsaicin receptor TRPV1 seems to play a less thermoeffector-specific role within the thermoregulatory system. From the clinical perspective, pharmacological TRPV1 activation might, therefore, be used in conscious subjects to broadly suppress both the basal heat-generating mechanisms and the responses to physical cooling, such as shivering and tachycardia. Although this broader action makes TRPV1 agonists effective hypothermic agents, it may also lead to unwanted off-target effects on autonomic cardiovascular and somatic nociceptive systems. Therefore, considerably more study is needed before the clinical use of TRPV1 agonists for hypothermia induction could be considered.
Conclusion.
The present study for the first time demonstrates that the molecular cold receptor TRPM8, with well-established functions in behavioral, nonshivering thermogenic, and vasoconstriction responses to cold, is not substantially involved in external cooling-induced shivering and tachycardia. This suggests that complementing TRPM8 inhibitors with external physical cooling may not prevent significant compensatory shivering response and, therefore, may not be beneficial over each treatment used individually for inducing TH in human patients. Furthermore, it is demonstrated that systemic pharmacological TRPV1 activation suppresses the shivering and tachycardic responses to cold. Therefore, we propose that TRPV1 activation may be used in combination with traditional physical cooling methods for more rapid and efficient achievement of the low target core temperature.
GRANTS
These studies were supported by the grants from the National Institute of Neurological Disorders and Stroke (R21 NS-077413) and the National Heart, Lung, and Blood Institute (R01 HL-088435) to S.P.M.
DISCLOSURES
Dr. Christopher M. Flores and Dr. Mark R. Player are employed by Janssen Research & Development, LLC, a pharmaceutical company.
AUTHOR CONTRIBUTIONS
Author contributions: V.V.F. and S.P.M. conception and design of research; V.V.F. and A.B. performed experiments; V.V.F., A.B., and S.P.M. analyzed data; V.V.F. and S.P.M. interpreted results of experiments; V.V.F. prepared figures; V.V.F. drafted manuscript; V.V.F., C.M.F., M.R.P., and S.P.M. edited and revised manuscript; V.V.F., C.M.F., M.R.P., and S.P.M. approved final version of manuscript.
REFERENCES
- 1.Almeida MC, Hew-Butler T, Soriano RN, Rao S, Wang W, Wang J, Tamayo N, Oliveira DL, Nucci TB, Aryal P, Garami A, Bautista D, Gavva NR, Romanovsky AA. Pharmacological blockade of the cold receptor TRPM8 attenuates autonomic and behavioral cold defenses and decreases deep body temperature. J Neurosci 32: 2086–2099, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1a.American Heart Association American Heart Association Guidelines for Cardiopulmonary Resuscitation, and Emergency Cardiovascular Care. Circulation 112: IV1–IV203, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, Julius D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 448: 204–208, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Cao Z, Balasubramanian A, Marrelli SP. Hijacking the thermoregulatory system to promote therapeutic hypothermia in the treatment of acute cerebral ischemic stroke. In: First Annual Cardiovascular Research Symposium. Houston, Texas, USA: Baylor College of Medicine, 2013, p. 67 [Google Scholar]
- 4.Chanda S, Mould A, Esmail A, Bley K. Toxicity studies with pure trans-capsaicin delivered to dogs via intravenous administration. Regul Toxicol Pharmacol 43: 66–75, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Choi HA, Badjatia N, Mayer SA. Hypothermia for acute brain injury—mechanisms and practical aspects. Nat Rev Neurol 8: 214–222, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Colburn RW, Lubin Lou M, Stone DJ, Wang Y, Lawrence D, D'Andrea MR, Brandt MR, Liu Y, Flores CM, Qin N. Attenuated cold sensitivity in TRPM8-null mice. Neuron 54: 379–386, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Daniele O, Caravaglios G, Fierro B, Natalè E. Stroke and cardiac arrhythmias. J Stroke Cerebrovasc Dis 11: 28–33, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Dhaka A, Murray AN, Mathur J, Earley TJ, Petrus MJ, Patapoutian A. TRPM8 is required for cold sensation in mice. Neuron 54: 371–378, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Fosgerau K, Ristagno G, Jayatissa M, Axelsen M, Gotfredsen JW, Weber UJ, Køber L, Torp-Pedersen C, Videbaek C. Increased susceptibility to cardiovascular effects of dihydrocapcaicin in resuscitated rats. Cardiovascular effects of dihydrocapsaicin BMC. Cardiovasc Disord 10: 39, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fosgerau K, Weber UJ, Gotfredsen JW, Jayatissa M, Buus C, Kristensen NB, Vestergaard M, Teschendorf P, Schneider A, Hansen P, Raunsø J, Køber L, Torp-Pedersen C, Videbaek C. Drug-induced mild therapeutic hypothermia obtained by administration of a transient receptor potential vanilloid type 1 agonist. BMC Cardiovasc Disord 10: 51, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gavva NR, Davis C, Lehto SG, Rao S, Wang W, Zhu DXD. Transient receptor potential melastatin 8 (TRPM8) channels are involved in body temperature regulation. Mol Pain 8: 36, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gordon CJ. Thermal physiology of laboratory mice. Defining thermoneutrality. J Thermal Biol 37: 654–685, 2012 [Google Scholar]
- 13.Hancock JC, Hoover DB. Capsaicin-evoked bradycardia in anesthetized guinea pigs is mediated by endogenous tachykinins. Regul Pept 147: 19–24, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Hori T. Capsaicin and central control of thermoregulation. Pharmacol Ther 26: 389–416, 1984 [DOI] [PubMed] [Google Scholar]
- 15.Karashima Y, Damann N, Prenen J, Talavera K, Segal A, Voets T, Nilius B. Bimodal action of menthol on the transient receptor potential channel TRPA1. J Neurosci 27: 9874–9884, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karashima Y, Talavera K, Everaerts W, Janssens A, Kwan KY, Vennekens R, Nilius B, Voets T. TRPA1 acts as a cold sensor in vitro and in vivo. Proc Natl Acad Sci USA 106: 1273–1278, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knowlton WM, Daniels RL, Palkar R, McCoy DD, McKemy DD. Pharmacological blockade of TRPM8 ion channels alters cold and cold pain responses in mice. PLos One 6: e25894, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kozyreva TV, Kozaruk VP, Tkachenko EY, Khramova GM. Agonist of TRPM8 channel, menthol, facilitates the initiation of thermoregulatory responses to external cooling. J Therm Biol 35: 428–434, 2010 [Google Scholar]
- 19.Lay C, Badjatia N. Therapeutic hypothermia after cardiac arrest. Curr Atheroscler Rep 12: 336–342, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Lee P, Swarbrick MM, Ho KKY. Brown adipose tissue in adult humans: a metabolic renaissance. Endocr Rev 34: 413–438, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Mahieu F, Owsianik G, Verbert L, Janssens A, De Smedt H, Nilius B, Voets T. TRPM8-independent menthol-induced Ca2+ release from endoplasmic reticulum and Golgi. J Biol Chem 282: 3325–36, 2007 [DOI] [PubMed] [Google Scholar]
- 22.McAllen RM, Tanaka M, Ootsuka Y, McKinley MJ. Multiple thermoregulatory effectors with independent central controls. Eur J Appl Physiol 109: 27–33, 2010 [DOI] [PubMed] [Google Scholar]
- 23.McCoy DD, Knowlton WM, McKemy DD. Scraping through the ice: uncovering the role of TRPM8 in cold transduction. Am J Physiol Regul Integr Comp Physiol 300: R1278–R1287, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 416: 52–58, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Morrison SF, Nakamura K. Central neural pathways for thermoregulation. Front Biosci 16: 74–104, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muzzi M, Felici R, Cavone L, Gerace E, Minassi A, Appendino G, Moroni F, Chiarugi A. Ischemic neuroprotection by TRPV1 receptor-induced hypothermia. J Cereb Blood Flow Metab 32: 978–982, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noël J, Zimmermann K, Busserolles J, Deval E, Alloui A, Diochot S, Guy N, Borsotto M, Reeh P, Eschalier A, Lazdunski M. The mechano-activated K+ channels TRAAK and TREK-1 control both warm and cold perception. EMBO J 28: 1308–1318, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parks DJ, Parsons WH, Colburn RW, Meegalla SK, Ballentine SK, Illig CR, Qin N, Liu Y, Hutchinson TL, Lubin Lou M, Stone DJ, Baker JF, Schneider CR, Ma J, Damiano BP, Flores CM, Player MR. Design and optimization of benzimidazole-containing transient receptor potential melastatin 8 (TRPM8) antagonists. J Med Chem 8: 233–247, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson a D, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A. A TRP channel that senses cold stimuli and menthol. Cell 108: 705–715, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Romanovsky AA, Almeida MC, Garami A, Steiner AA, Norman MH, Morrison SF, Nakamura K, Burmeister JJ, Nucci TB. The transient receptor potential vanilloid-1 channel in thermoregulation: a thermosensor it is not. Pharmacol Rev 61: 228–261, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romanovsky AA. Thermoregulation: some concepts have changed. Functional architecture of the thermoregulatory system. Am J Physiol Regul Integr Comp Physiol 292: R37–R46, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Sessler DI. Thermoregulatory defense mechanisms. Crit Care Med 37: S203–S210, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Tajino K, Hosokawa H, Maegawa S, Matsumura K, Dhaka A, Kobayashi S. Cooling-sensitive TRPM8 is thermostat of skin temperature against cooling. PLos One 6: e17504, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tajino K, Matsumura K, Kosada K, Shibakusa T, Inoue K, Fushiki T, Hosokawa H, Kobayashi S. Application of menthol to the skin of whole trunk in mice induces autonomic and behavioral heat-gain responses. Am J Physiol Regul Integr Comp Physiol 293: R2128–R2135, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Toda N, Usui H, Nishino N, Fujiwara M. Cardiovascular effects of capsaicin in dogs and rabbits. J Pharmacol Exp Ther 181: 512–521, 1972 [PubMed] [Google Scholar]
- 36.Varga K, Lake KD, Huangfu D, Guyenet PG, Kunos G. Mechanism of the hypotensive action of anandamide in anesthetized rats. Hypertension 28: 682–686, 1996 [DOI] [PubMed] [Google Scholar]
- 37.Van der Worp HB, Sena ES, Donnan a G, Howells DW, Macleod MR. Hypothermia in animal models of acute ischaemic stroke: a systematic review and meta-analysis. Brain 130: 3063–3074, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Zimmermann K, Lennerz JK, Hein A, Link AS, Kaczmarek JS, Delling M, Uysal S, Pfeifer JD, Riccio A, Clapham DE. Transient receptor potential cation channel, subfamily C, member 5 (TRPC5) is a cold-transducer in the peripheral nervous system. Proc Natl Acad Sci USA 108: 18114–18119, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]