Abstract
Background: Anaphylaxis is a potentially fatal condition requiring immediate resuscitation. Data regarding the epidemiology of anaphylaxis are limited and inconsistent. A reason for the variability was unavailability of a universally acceptable case definition till 2005. We reviewed cases using this new definition
Aim: To review the incidence, clinical presentation, cause and outcome of anaphylaxis at a tertiary-care centre in a low-income country.
Design: Retrospective, case series
Methods: Chart review of all patients discharged from Aga Khan University Hospital between January 1988 and December 2012 (24 years) with anaphylaxis definition as per second National Institute of Allergy and Infection disease/Food Allergy and Anaphylaxis Network Symposium
Results: Total of 129 cases were found with mean age of 41.6 years (SD 18.8). Majority of patients had cutaneous features (76.7%), followed by respiratory (68.9%), cardiac (64.3%) and gastrointestinal (20.9%) symptoms, respectively. About 22.4% of patients had positive history for allergens out of which 31% (n = 9) were exposed to the same allergens. The common causes identified for anaphylaxis were drugs (60.5%), food (16.3%) and intravenous contrast (10.9%), respectively. Only 22.5% of cases received epinephrine as a part of their initial management. In four patients (3.1%) the cause of death was attributed to anaphylaxis.
Conclusion: Anaphylaxis is a rare but life-threatening condition. Though cutaneous features are most common, their absence does not exclude the diagnosis. Drugs were the most common cause and epinephrine was not commonly used as first-line agent for its management.
Introduction
Anaphylaxis is a life-threatening medical emergency requiring prompt recognition and resuscitation. Data on the epidemiology, incidence and prevalence of anaphylaxis is limited globally. Studies have reported variable incidence ranging from 10.5 to 21 cases per 100 000 person-years.1,2 Studies from Emergency departments showed the incidence of anaphylaxis as high as 0.4% in an Italian study3 to as low as 0.04% in an English study.4 The overall risk of death with anaphylaxis has been estimated to be ∼1%.5 One of the limitations of these studies is non-standardized case definition of anaphylaxis leading to variability in case selections and eventually in their outcome.
Second National Institute of Allergy and Infection disease/Food Allergy and Anaphylaxis Network Symposium (NIAID and FAAN) in 2005 defined the criteria for diagnosis of anaphylaxis. Their complete definition aims to capture >95% of all the clinical cases with three diagnostic criteria (Table 1). Criterion 1 will identify at least 80% of the anaphylaxis cases, even if the allergic status of the patient and potential cause of the reaction is unknown, as majority of anaphylactic reactions include skin symptoms. Criterion 2 is anaphylaxis which requires a known allergic history and a possible exposure that could occur in the absence of cutaneous features such as a child with food allergy. Gastrointestinal symptoms are also included. Criterion 3 captures the rare patient with an acute hypotensive episode after exposure to a known allergen.6
Table 1.
Definition of anaphylaxis—clinical criteria for Diagnosis
Anaphylaxis is highly likely when any one of the following three criteria are fulfilled:
|
PEF, Peak expiratory flow; BP, blood pressure. *Low systolic blood pressure for children is defined as <70 mmHg from 1 month to 1 year; <(70 mmHg + [2 × age]) from 1 to 10 years; and < 90 mmHg from 11 to 17 years.28
Our search for studies on anaphylaxis in low- and middle-income countries (LMIC) revealed only few case reports,7–15 as there was a scarcity of comprehensive work on the overall diagnosis and management of this life-threatening condition. In this study we aim to find out the incidence, causes, clinical features and outcome of anaphylaxis in a tertiary-care hospital of a low-income country using the new case definition described by NIAID and FAAN.
Methods
This was retrospective case series of all anaphylaxis cases discharged from the Aga Khan University Hospital during the 24 years period from January 1988 to December 2012. Aga Khan University Hospital is a 570 bedded private, tertiary-care university-affiliated teaching hospital in Karachi, Pakistan.
The cases were selected through the hospital information management system using a computerized search of all patients with discharge diagnostic codes, as per International Classification of Diseases–9th revision–Clinical Modification (ICD-9-CM). Aga Khan University Hospital is using ICD-9-CM since 1988 till date. The codes included anaphylaxis, anaphylactic reaction, anaphylactic shock specified (995.0) and allergic reaction not otherwise (995.3). Those cases that fulfilled the NIAID and FAAN criteria for anaphylaxis were selected for final review.
The data were collected on a standard questionnaire by a research assistant who was a medical graduate and included information on patient’s demographics, clinical findings, previous history of allergy, causative agent (if known), treatment and length of stay in the hospital. These variables were then reviewed for inconsistencies by the primary investigator. Data were stored in password protected computers after removal of all personal information.
Descriptive statistics were calculated and results were expressed in either means ± SD, or percentages. IBM SPSS 19 was used to perform the analysis.
The study was reviewed by the Ethical Review Committee of Aga Khan University Hospital and was given an exemption for an ethical approval.
Results
During the study period there were 1 04 6321 patients admitted and discharged from all causes across age groups. A total of 146 cases with discharge diagnostic codes were found, as per International Classification of Diseases–9th revision–Clinical Modification (ICD-9-CM), of anaphylaxis, anaphylactic reaction, anaphylactic shock and allergic reaction not otherwise specified. Out of which 17 charts had insufficient documentation for anaphylaxis to be clearly defined as per NIAID and FAAN criteria (described above) and hence were excluded. This left a total of 129 cases with an incidence of 0.01%.
Out of these 129 cases of anaphylaxis, 3 patients came twice with anaphylaxis. However, each episode of anaphylaxis was considered as a separate entity. The mean age of study population was 41.62 years (SD, 18.78; range, 1–83 years). Only 16 cases (12.4%) belonged to paediatric age group (<18 years). Females outnumbered males by a ratio of 1.35.
Out of a total of 129 cases, 90 (69.8%) cases presented to the emergency room with anaphylaxis, 19 (14.7%) cases occurred in medicine department followed by 9 (7%) in radiology department. Altogether 47 (36.4%) patients presented with the sole diagnosis of anaphylaxis with no other systemic involvement.
Clinical features
The most common were cutaneous findings observed in 99 patients (76.7%) out of which 83 patients (64.3%) presented with urticaria while 47 patients (36.4%) had erythema. Respiratory abnormality was present in 89 patients (68.9%). In paediatric population, respiratory symptoms were more common comprising of 87.5% of the total. Cardiac features were present in 83 patients (64.3%), most common of which was hypotension seen in 52 patients (40.3%). One female patient aged 32 years old had ventricular fibrillation after intravenous (IV) contrast and later died. Vomiting was the most common gastrointestinal symptom found in 17 patients (13.2%). The clinical features of patients presenting with anaphylaxis are shown in Table 2.
Table 2.
Clinical features of patients presenting with anaphylaxis
| No. of patients (%) | |
|---|---|
| Cutaneous features | |
| Urticaria | 83 (64.3) |
| Angioedema | 47 (36.4) |
| Erythema | 38 (29.5) |
| Local oedema | 9 (6.9) |
| Conjunctivitis | 2 (1.5) |
| Respiratory features | |
| Dyspnoea | 79 (61.2) |
| Wheeze/ Bronchospasm | 21 (16.2) |
| Hoarseness | 2 (1.5) |
| Stridor | 1 (0.8) |
| Gasping | 1 (0.8) |
| Cardiovascular features | |
| Hypotension | 52 (40.3) |
| Tachycardia | 26 (20.1) |
| Syncope/ LOC | 24 (18.6) |
| Crepitation | 4 (3.1) |
| Cyanosis | 4 (3.1) |
| Bradycardia | 3 (2.3) |
| Ventricular fibrillation | 1 (0.8) |
| Gastrointestinal features | |
| Vomiting | 17 (13.2) |
| Abdominal pain | 11 (8.5) |
| Diarrhoea | 6 (4.6) |
| Tongue swelling | 1 (0.8) |
Causative agents
The causative agent was identified as per history in 115 patients (89.1%). More than half of the patients were allergic to drugs n= 74 (57.4%); followed by food n= 21 (16.3%) and IV contrast n= 14 (10.9%). The details of causative agents are shown in Table 3. Most common drugs involved were antibiotics (mainly include penicillin and cephalosporin) and non-steroidal anti-inflammatory agents. Interestingly, one of the patients was allergic to Pheniramine (HI antagonist) which is used in the management of anaphylaxis.
Table 3.
Causative agent in patients with anaphylaxis
| Causative agent | No. of patients (%) |
|---|---|
| Drugs | 78 (60.5) |
| Antibiotics | 22 (17) |
| Penicillin | 10 (7.8) |
| Cephalosporin | 6 (4.7) |
| Other Antibiotics | 6 (4.7) |
| NSAIDS | 21 (16.3) |
| Diclofenac | 7 (5.4) |
| Dispirin | 5 (3.9) |
| Other NSAIDS | 9 (7) |
| Paracetamol | 1 (0.8) |
| HI antagonist | 1 (0.8) |
| Other drugs | 33 (25.6) |
| Food | 21 (16.3) |
| Dry fruits (peanut, etc.) | 2 (1.6) |
| Sea food (prawn, shrimps, lobsters, etc.) | 9 (7) |
| Egg | 1 (0.8) |
| Beef/ chicken | 4 (3.1) |
| Others (burger, haleem, cake, etc.) | 5 (3.9) |
| Intravenous contrast | 14 (10.9) |
| Blood and blood products | 3 (2.3) |
| Sting/bites | 2 (1.6) |
| Others | 3 (2.3) |
| Anti-lice shampoo | 1 (0.8) |
| Hydatid cyst | 2 (1.6) |
Out of 21 patients who developed anaphylaxis due to food allergy, 3 were known food allergic; for e.g. a patient who was allergic to peanuts consumed a peanut-containing ice-cream by mistake and developed anaphylaxis.
There was 1 patient who died out of 14 patients who developed anaphylaxis secondary to IV contrast. Interestingly two patients with hydatid cyst developed anaphylaxis, one after aspiration and other preoperatively while operating the cyst.
Altogether 29 (22.4%) patients had a known history of different allergens, out of which 26 (20.1%) had drug allergies and 8 (6.2%) had food allergies. Common drug allergies were related to NSAIDS n= 10 (7.7%) and antibiotics n= 8 (6.2%). Common food allergens were eggs n= 3 (2.3%) and sea food n= 2 (1.5%).
Treatment and outcome
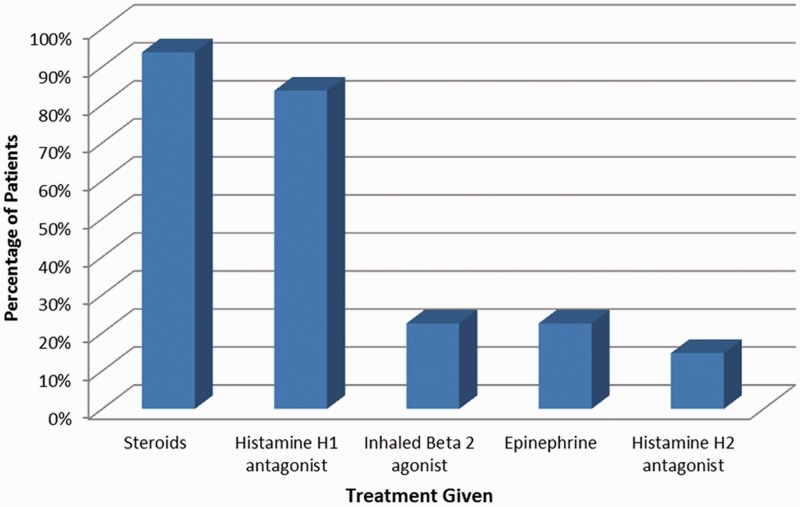
Majority of the patients n= 58 (45%) received a combination of steroid and Histamine HI antagonist as the mainstay of their treatment. Epinephrine was used in only 29 patients (22.5%). Figure 1 shows bar representation of the treatment data for patients with anaphylaxis
Figure 1.
Bar representation of treatment received by patients with anaphylaxis.
Median hospital stay of patient was 2 days (range 1–32 days). Around half of the patients (n= 61; 47.3%) were discharged from the hospital within 24 h, 8 (6.2%) patients died during the hospital course; out of which four cases were attributed to anaphylaxis as mentioned in Table 4.
Table 4.
Details of patients expired due to anaphylaxis
| Age (years) | Sex | Symptoms | Presumed cause | Risk factors | Associated diagnosis | Treatment |
|---|---|---|---|---|---|---|
| 32 | Female | Tachypnoea, hypotension, ventricular fibrillation | Intravenous contrast for CT Scan | Rapid intravenous contrast | Renal calculi | Epinephrine |
| 37 | Female | Stridor, hypotension, tachy cardia, urticaria, angioedema | Hakimi medications | Nil | Interstitial nephritis secondary to Hakimi drugs | Steroid |
| Composition? | ||||||
| 60 | Male | Wheeze, Hypertension, tachycardia, haemoptysis | Multivitamin | Cardiac patient on β-blocker | Disseminated intravascular coagulation | Epinephrine, steroid, hista mine H1 antagonist, histamine H2 antagonist, inhaled β 2 agonist |
| 83 | Male | Dyspnoea, hypertension, tachycardia, urticaria, diarrhoea, seizures, loss of consciousness | Ceftriaxone | Nil | Cortical venous thrombosis | Epinephrine, steroid, histamine H1 antagonist |
The median time after anaphylaxis when the patient sought medical advice or was given treatment was 3 h. The prevalence of asthma in our study population was found to be 10.1% (n= 13). However; we did not specifically record other atopic conditions.
Discussion
According to the best of our knowledge, this is the first reported case series of anaphylaxis using the current criteria from a tertiary-care centre in Karachi in the last 24 years
Our study highlighted the mortality rate of 3.1%, which is much higher than findings reported in other studies which range from 0.002%16 to 0.65%.2 Delay in both diagnosis and subsequent use of epinephrine or reluctance of physicians to its use might be the factors involved. A history of known asthma was recorded in 10.1% of our patients with anaphylaxis which is much lower than atopy rates reported in other studies; 22%,17 23%,18 33%,2 respectively. Generally, patients with asthma and cardiac disease and those on beta blockers and who receive rapid IV allergen have a worse course. Since none of the patients died had asthma, therefore this was not the contributory factor in our patients. One patient died after rapid IV contrast, as rapid IV allergen could cause severe reaction and contribute to mortality. One patient had pre-existing cardiac disease and was on beta blocker, this could have been the factor leading to mortality.
Moreover, we only found 129 patients in 24 years which could be an underestimation. Since we included only inpatient data, patients presented in Emergency department with anaphylaxis who were successfully treated and discharged were not included. Similarly, patients presented as dead on arrival in Emergency department or in cardiac arrest who could not be revived were also missed. Majority of our patients were females; a finding similar to other studies.
Overall, 76.7% of our patients with anaphylaxis had cutaneous features; in contrast, some authors have reported that all of their patients had cutaneous manifestations.2,17 Patients with acute anaphylaxis might indeed present without cutaneous features because of treatment before hospital presentation, spontaneous resolution, or complete absence of such signs, particularly in those presenting with the rapid onset of laryngeal oedema or circulatory shock2; similar to our findings. We found increased respiratory features in paediatric population; a similar finding to a study by Braganza et al.19
Majority of the causative agents in our setting were drugs (60.5%); a finding similar to Sheikh et al.20 though other authors have recognized an increasing role of food-induced anaphylaxis.17,21,22 One of the reasons could be that the patients are already aware of their food allergies and thus avoid them. Antibiotics and NSAIDS were causal for most drug-induced anaphylaxis which is in agreement with recent studies.2,22,23 Food was the second most common cause that included fish, prawn, milk, eggs and peanuts comparable to other studies.14,23,24 We also encountered two cases of anaphylaxis attributed to hydatid cyst as evident in literature.13 Around one tenth of cases (10.8%) in our study had unidentified cause of anaphylaxis; which is greater than 5.3% reported by Helbling et al. in Switzerland23 but much lower than the numbers reported by Yocum et al. (32%)2 and Kemp et al. (37%) in United States.22
A pre-existing allergy to the causative agent was known in 7% of the patients, which is much lower than the rate of 23.2%,18 and 24%4 reported in other ED series in Australia and England, respectively. However, major concern is the fact that six of our patients were already known to be allergic to a drug and still were prescribed the same drug by their family practitioner. We were unable to determine whether this was due to failure of patient record documentation, an inadequate or absent physician inquiry, or simply insufficient importance given to the history of allergens. All of these iatrogenic cases were avoidable as they serve to emphasize the need for taking a comprehensive allergy history in every ED patient.
Out of 21 patients who developed anaphylaxis due to food, 3 were already known to be allergic to the same food. We think it could be unintentional as generally our food labels are incomplete or missing altogether. It also emphasizes the need of teaching patients to avoid allergen-containing food.
The use of H1 anti-histamines and steroids was far greater than epinephrine which was used in only 22.5% of the patients similar to other studies.25,26 One of the reasons could be that in our setting epinephrine is available in bedside crash cart and can be given as a verbal order in case of emergency and hence documentation might be missing in the medical record of the patient. Epinephrine is considered to be the first-line therapy of anaphylaxis and must be used appropriately in acute anaphylaxis.4,27–29 Arguments about recommended doses, route, dilution and timing should not obscure epinephrine’s vital role.30–32 This study found that most of our patients who received epinephrine had severe anaphylactic reaction. Some of our patients with syncope, dizziness and altered conscious levels were recovered spontaneously before hospital presentation and others with wheeze responded to inhaled β-2 agonists both of which are documented in literature.4 However, the subjective nature of the symptoms and the likelihood of panic or hyperventilation in some patients might have distorted the data. The additional use of H2 with H1 antihistamines reflected local ED policy, supported in a randomized controlled trial33 but still controversial.27,28
Limitations of our study include a relatively small number of patients, anaphylactic reactions from a single institution and the retrospective design which is prone to reporting bias. One more limitation to this study could be selection bias since AKUH is a private, fee for service hospital catering to only those who can afford it. One of the other limitations of our study was that we were only able to retrieve the data of hospitalized patients and unable to identify patients managed and discharged from ED thus underestimating the true incidence.
Lastly, we suggest that an educational program is necessary to increase awareness in high risk groups like in patients with severe reaction, unknown causative agent and recurrent attacks.
Conclusion
Anaphylaxis is a rare but life-threatening condition with high-mortality rates. Though cutaneous features are most common, their absence does not exclude the diagnosis which is based on clinical criteria. A detailed history of known allergens is important for physician to avoid iatrogenic causes and for giving instructions to patients for future avoidance. Epinephrine is not commonly used as first line agent for its management in everyday medical practice and hence efforts are needed to educate physicians about its use.
Acknowledgement
Authors NUK, URK and JAR were partially supported through the “Johns Hopkins-Pakistan International Collaborative Trauma and Injury Research Training program”, Grant Number 2D43-TW007-292 from the Fogarty International Center of the United States National Institutes of Health. The content is solely the responsibility of the authors and do not represent the views of Fogarty or NIH.
Conflict of interest: None declared.
References
- 1.Bohlke K, Davis RL, DeStefano F, Marcy SM, Braun MM, Thompson RS. Epidemiology of anaphylaxis among children and adolescents enrolled in a health maintenance organization. J Allergy Clin Immunol. 2004;113:536–42. doi: 10.1016/j.jaci.2003.11.033. [DOI] [PubMed] [Google Scholar]
- 2.Yocum MW, Butterfield JH, Klein JS, Volcheck GW, Schroeder DR, Silverstein MD. Epidemiology of anaphylaxis in Olmsted County: a population-based study. J Allergy Clin Immunol. 1999;104:452–6. doi: 10.1016/s0091-6749(99)70392-1. [DOI] [PubMed] [Google Scholar]
- 3.Pastorello EA, Rivolta F, Bianchi M, Mauro M, Pravettoni V. Incidence of anaphylaxis in the emergency department of a general hospital in Milan. J Chromatogr B Biomed Sci Appl. 2001;756:11–7. doi: 10.1016/s0378-4347(01)00067-6. [DOI] [PubMed] [Google Scholar]
- 4.Stewart AG, Ewan PW. The incidence, aetiology and management of anaphylaxis presenting to an accident and emergency department. QJM. 1996;89:859–64. doi: 10.1093/qjmed/89.11.859. [DOI] [PubMed] [Google Scholar]
- 5.Neugut AI, Ghatak AT, Miller RL. Anaphylaxis in the United States: an investigation into its epidemiology. Arch Int Med. 2001;161:15. doi: 10.1001/archinte.161.1.15. [DOI] [PubMed] [Google Scholar]
- 6.Sampson HA, Muñoz-Furlong A, Campbell RL, Adkinson NF, Jr, Bock SA, Branum A, et al. Second symposium on the definition and management of anaphylaxis: summary report–Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. Ann Emerg Med. 2006;47:373–80. doi: 10.1016/j.annemergmed.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 7.Ahmed A, Kumar A. Severe anaphylactic reaction at induction of anaesthesia. JPMA The Journal of the Pakistan Medical Association. 2007;57:463. [PubMed] [Google Scholar]
- 8.Budagoda B, Kodikara KAS, Kularatne WKS, Mudiyanse RM, Edussuriya DH, Edirisinghe JP, et al. Giant Asian honeybee or Bambara stings causing myocardial infarction, bowel gangrene and fatal anaphylaxis in Sri Lanka: a case series. Asian Pac J Trop Med. 2010;3:586–8. [Google Scholar]
- 9.Gul A, Masood N, Imaam M. Acute Anaphylaxis with Lignocaine. Pak Armed Forces Med J. 2007;57:324–6. [Google Scholar]
- 10.Havaldar PV, Patil SS, Phandnu C. Anaphylaxis due to Red Fire ant bite. Indian Pediatr. 2012;49:237–8. [PubMed] [Google Scholar]
- 11.Hegde VL, Venkatesh YP. Anaphylaxis following ingestion of mango fruit. J Investig Allergol Clin Immunol. 2007;17:341. [PubMed] [Google Scholar]
- 12.Kazim SF, Shahid M. Losartan associated anaphylaxis and angioneurotic oedema. J Pak Med Ass. 2010;60:685–6. [PubMed] [Google Scholar]
- 13.Maqbool B, Anwar MS. Hepatic hydatid cyst presenting as anaphylaxis. JCPSP. 2007;17:224. [PubMed] [Google Scholar]
- 14.Shamim SM. Peanut allergy: case report. J Pak Ass Dermatol. 2006;16:183–6. [Google Scholar]
- 15.Siddiqi R, Khan MA, Dar NR. Anaphylaxis during anesthesia-role of skin prick testing in diagnosing multiple sensitivities. JCPSP. 2007;17:219. [PubMed] [Google Scholar]
- 16.Idsoe O, Guthe T, Willcox RR, de Weck AL. Nature and extent of penicillin side-reactions, with particular reference to fatalities from anaphylactic shock. Bull World Health Organ. 1968;38:159–88. [PMC free article] [PubMed] [Google Scholar]
- 17.Piromrat K, Chinratanapisit S, Trathong S. Anaphylaxis in an emergency department: a 2-year study in a tertiary-care hospital. Asian Pac J Allergy Immunol. 2008;26:121–8. [PubMed] [Google Scholar]
- 18.Brown AFT, McKinnon D, Chu K. Emergency department anaphylaxis: a review of 142 patients in a single year. J allergy Clin Immunol. 2001;108:861–6. doi: 10.1067/mai.2001.119028. [DOI] [PubMed] [Google Scholar]
- 19.Braganza SC, Acworth JP, McKinnon DRL, Peake JE, Brown AFT. Paediatric emergency department anaphylaxis: different patterns from adults. Arch Dis Child. 2006;91:159–63. doi: 10.1136/adc.2004.069914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sheikh A, Alves B. Hospital admissions for acute anaphylaxis: time trend study. BMJ. 2000;320:1441. doi: 10.1136/bmj.320.7247.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ewan PW. Clinical study of peanut and nut allergy in 62 consecutive patients: new features and associations. BMJ. 1996;312:1074–8. doi: 10.1136/bmj.312.7038.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kemp SF, Lockey RF, Wolf BL, Lieberman P. Anaphylaxis. A review of 266 cases. Arch Intern Med. 1995;155:1749–54. doi: 10.1001/archinte.155.16.1749. [DOI] [PubMed] [Google Scholar]
- 23.Helbling A, Hurni T, Mueller UR, Pichler WJ. Incidence of anaphylaxis with circulatory symptoms: a study over a 3-year period comprising 940,000 inhabitants of the Swiss Canton Bern. Clin Exp Allergy. 2004;34:285–90. doi: 10.1111/j.1365-2222.2004.01882.x. [DOI] [PubMed] [Google Scholar]
- 24.Wimalasiri YSG, Ratnayake RMU, Karunaratne TDN, Ranaweera K. Food allergy and anaphylaxis – 2063. Identification of foods causing hypersensitivity/allergy among school children in two sub-urban schools in Colombo District, Sri Lanka. World Allergy Org J. 2013;6:P146. [Google Scholar]
- 25.Cianferoni A, Novembre E, Mugnaini L, Lombardi E, Bernardini R, Pucci N, et al. Clinical features of acute anaphylaxis in patients admitted to a university hospital: an 11-year retrospective review (1985-1996) Ann Allergy Asthma Immunol. 2001;87:27–32. doi: 10.1016/S1081-1206(10)62318-6. [DOI] [PubMed] [Google Scholar]
- 26.Rudders SA, Banerji A, Corel B, Clark S, Camargo CA. Multicenter study of repeat epinephrine treatments for food-related anaphylaxis. Pediatrics. 2010;125:e711–8. doi: 10.1542/peds.2009-2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown AF. Current management of anaphylaxis. Emergencias. 2009;21:213–23. [Google Scholar]
- 28.Sampson HA, Munoz-Furlong A, Campbell RL, Adkinson NF, Jr, Bock SA, Branum A, et al. Second symposium on the definition and management of anaphylaxis: summary report–Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006;117:391–7. doi: 10.1016/j.jaci.2005.12.1303. [DOI] [PubMed] [Google Scholar]
- 29.O’Brien J, Howell JM. Allergic emergencies and anaphylaxis: how to avoid getting stung. Emerg Med Pract. 2000;2:1–20. [Google Scholar]
- 30.Brown AF. Intramuscular or intravenous adrenaline in acute, severe anaphylaxis? J Accid Emerg Med. 2000;17:152. doi: 10.1136/emj.17.2.152. author reply -3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jowett NI. Speed of treatment affects outcome in anaphylaxis. BMJ. 2000;321:571. [PMC free article] [PubMed] [Google Scholar]
- 32.Sadana A, O'Donnell C, Hunt MT, Gavalas M. Managing acute anaphylaxis. Intravenous adrenaline should be considered because of the urgency of the condition. BMJ. 2000;320:937–8. [PMC free article] [PubMed] [Google Scholar]
- 33.Lin RY, Curry A, Pesola GR, Knight RJ, Lee HS, Bakalchuk L, et al. Improved outcomes in patients with acute allergic syndromes who are treated with combined H1 and H2 antagonists. Ann Emerg Med. 2000;36:462–8. doi: 10.1067/mem.2000.109445. [DOI] [PubMed] [Google Scholar]