Abstract
OBJECTIVES:
Estrogen has been shown to play an important protective role in non-reproductive systems, such as the cardiovascular system. Our aim was to observe gender differences in vivo with regard to the increase in macromolecular permeability and leukocyte-endothelium interaction induced by ischemia/reperfusion as well as in microvascular reactivity to vasoactive substances using the hamster cheek pouch preparation.
METHODS:
Thirty-six male and 36 female hamsters, 21 weeks old, were selected for this study, and their cheek pouches were prepared for intravital microscopy. An increase in the macromolecular permeability of post-capillary venules was quantified as a leakage of intravenously injected fluorescein-labeled dextran, and the leukocyte-endothelium interaction was measured as the number of fluorescent rolling leukocytes or leukocytes adherent to the venular wall, labeled with rhodamin G, during reperfusion after 30 min of local ischemia. For microvascular reactivity, the mean internal diameter of arterioles was evaluated after the topical application of different concentrations of two vasoconstrictors, phenylephrine (α1-agonist) and endothelin-1, and two vasodilators, acetylcholine (endothelial-dependent) and sodium nitroprusside (endothelial-independent).
RESULTS:
The increase in macromolecular permeability induced by ischemia/reperfusion was significantly lower in females compared with males [19 (17–22) leaks/cm2 vs. 124 (123–128) leaks/cm2, respectively, p<0.001), but the number of rolling or adherent leukocytes was not different between the groups. Phenylephrine-induced arteriolar constriction was significantly lower in females compared with males [77 (73–102)% vs. 64 (55–69)%, p<0.04], but there were no detectable differences in endothelin-1-dependent vasoreactivity. Additionally, arteriolar vasodilatation elicited by acetylcholine or sodium nitroprusside did not differ between the groups.
CONCLUSION:
The female gender could have a direct protective role in microvascular reactivity and the increase in macromolecular permeability induced by ischemia/reperfusion.
Keywords: Gender Differences, Microcirculation, Hamster Cheek Pouch, Microvascular Reactivity, Macromolecular Permeability
INTRODUCTION
The incidence of cardiovascular disease differs significantly between men and women during their fertile period. Epidemiological studies have revealed that atherosclerosis, hypertension, and peripheral vascular and coronary artery diseases occur with greater prevalence in men and postmenopausal women compared with women at a fertile age (1,2). The reasons for these differences are subject to debate, but it is conceivable that the absolute levels of estradiol and the different degrees of estrogen receptor regulation are the two major determinant factors. However, genetic differences may also exert effects independently of gonadal function (3).
Estrogen has been shown to play an important protective role in non-reproductive systems such as the cardiovascular system. Estrogen causes vasodilation through non-genomic actions, resulting in rapid increases in nitric oxide (NO) production as well as through genomic actions by inducing transcription of NO synthase. These effects on NO bioavailability provide estrogen with the ability to improve arterial wall responsiveness following vascular injury and to inhibit the development of atherosclerosis by promoting re-endothelization, inhibition of smooth muscle cell proliferation, and matrix deposition (4). Estrogen may also decrease systemic vascular resistance, improve coronary and peripheral endothelial function, and prevent coronary artery spasm in women with and without coronary atherosclerosis (5). The endothelium effects on the modulation of vasorelaxation are thought to also occur through an endothelium-derived hyperpolarizing factor by inducing vasodilator prostanoids and by inhibiting endothelin-1 production (6,7). Additionally, endothelium action could modulate myogenic vascular responses, resulting in the reduction of microvessel basal tone (8). The majority of these effects have been attributed to estrogen acting on two ligand-activated transcription factors, ERα and ERβ, and one G-protein coupled receptor (GPR30 or GPER) expressed on vascular endothelial and smooth muscle cells (4).
The purpose of this study was to further explore the previous findings detailing the cardiovascular protective effects of endogenous estrogens and to focus on gender specificities in the in vivo microcirculation.
METHODS
Experiments were performed on 36 male and 36 female hamsters (Mesocricetus auratus), which were divided into six animals/group. Group I was used to evaluate macromolecular permeability, group II was used to evaluate leukocyte-endothelium interactions, and the subsequent groups were used to assess microvascular reactivity as follows: group III, phenylephrine (α1-agonist with vasoconstrictor properties); group IV, endothelin-1 (potent endothelial-derived vasoconstrictor); group V, acetylcholine (endothelial-dependent vasodilator); and group VI, sodium nitroprusside (endothelial-independent vasodilator). All animals were at a fertile age and were matched for age, without any attempt to classify females according to their menstrual cycle. Experiments were performed using animals at 21 weeks of age, according to the Guide for Care and Use of Laboratory Animals published by the US National Institute of Health (NIH publication No. 85-23, revised in 1996), and the protocol was approved by the Ethical Committee of the State University of Rio de Janeiro. All animals received the same appropriate isocaloric laboratory diet (Nuvital from Nuvilab, Curitiba, PR, Brazil).
On the day of the experiment, anesthesia was induced with 0.1-0.2 ml of sodium pentobarbital (Pentobarbital sodique, Sanofi, Paris, France, 60 mg/ml), which was administered intraperitoneally and maintained with α-chloralose [1,2-O-(2,2,2-trichlorethyliden) α-D-glucofuranose, Merck, Darmstadt, Germany] at a dose of 150 mg/kg body weight/h, administered through a femoral vein catheter. The femoral artery was also cannulated to enable pressure measurements. Throughout and following surgery, the temperature of the animals was kept at 37.5 °C with a heating pad controlled by a rectal thermistor (LTB 750 Thermostat System, Uppsala process data AB, Sweden). A tracheal tube was inserted to facilitate spontaneous breathing.
Conceptually, the hamster cheek pouch has both skeletal muscle and cutaneous microcirculatory beds, and in this study, experiments were performed with the cutaneous tissue to facilitate the comparison with data available in the literature (9,10). This part of the pouch is highly vascularized, and all classes of microcirculatory vessels can usually be visualized in the microscopic field with good clarity, thereby allowing for the comparison of effects in different microvascular segments.
Cheek pouch preparation
Cheek pouch preparations were dissected according to the protocol of Duling (11), as modified by Bouskela and Grampp (12), and mounted in an experimental chamber, where they were continuously superfused at a rate of 4 ml/min by a HEPES-supported HCO−3 buffered saline solution (composition in mM: NaCl 110.0, KCl 4.7, CaCl2 2.0, MgSO4 1.2, NaHCO3 18.0, HEPES 15.39, and HEPES Na+-salt 14.61) bubbled with 5% CO2/95% N2 to keep the pH of the superfusate at 7.40. Throughout the experiments, the same gas mixture was also gently blown over the experimental chamber to keep the pO2 level at 12–15 mmHg, monitored by an O2 electrode in the superfusion solution, as previously described (12). The temperature of the solution was kept at 36.0±0.5 °C with a circulating bath (model 8005, Polyscience, Niles, Illinois, USA) and (Leitz optical magnification 400×, Wetzlar, Hessen, Germany) coupled to a closed-circuit TV system and allowed to rest for 30 min. The cheek pouch preparation is stable with respect to microvessel diameter, spontaneous arteriolar vasomotion, arteriolar blood flow, and functional capillary density for at least 4 h of microscopic observation (12).
Ischemia/reperfusion of the cheek pouch
Local ischemia of the cheek pouch was obtained by a cuff made of a thin latex tubing that was mounted around the neck of the everted pouch where it exits the mouth of the hamster; this procedure was previously validated in our laboratory (9,10). The placement of the cuff can be made without any visible interference with local blood flow. The intratubular pressure can be rapidly increased by air compression using a syringe and also rapidly decreased at evacuation. An intratubular pressure of 200-220 mmHg in the cuff resulted in a complete arrest of microvascular blood flow, which eventually returned to a level similar to that observed before occlusion. In these experiments, the duration of the ischemia was 30 min.
Increase in macromolecular permeability after ischemia/reperfusion
To quantify the increase in macromolecular permeability, 30 min after completion of the preparative procedure, fluorescein isothiocyanate (FITC)-dextran, with a substitution of two FITC molecules per 1,000 glucose molecules in the polysaccharide chain, was administered at a dose of 25 mg/100 g as an intravenous injection of a 5% solution in 0.9% saline. The sites of leakage were defined as fluorescent spots with a diameter of approximately 40 μm found near post-capillary venules. Extravasated material is continuously washed away by the superfusion solution, and consequently, the fluorescent spots gradually decrease (13). Before the ischemic period, hamsters in which the prepared area showed spontaneous nonfading leaks or more than 10 fading leaks were discarded. The number of leaks was counted 10 min after the ischemic period because it was shown that the maximum number of leaks is observed at this time (9,14).
Leukocytes rolling or sticking after ischemia/reperfusion
Three venular segments with a diameter of approximately 40 μm each were selected for observation. Leukocytes were labeled in vivo with rhodamine G (10 mg/100 g body mass) immediately before observation, and images were recorded on VHS tapes for later analysis. Rolling was defined as the occurrence of contact between the leukocyte and the venular wall, i.e., when the speed of the leukocyte in the bloodstream was lower than that observed for red blood cells. A leukocyte was considered as adherent when it did not show any visible movement for at least 30 s (15).
Measurements of arteriolar reactivity
For the evaluation of microvascular reactivity, a preparation was considered suitable for experimentation if, under control conditions, there was an indication of good vascular tone (which, in 15 separate control tests, implied that the arteriolar diameter could be increased by 56±3% through topical application of papaverine, 10 μg/ml), a brisk blood flow in all parts of the vascular bed including the larger veins (where individual erythrocytes should not be discernible in the blood stream images), and no tendency for leukocytes to adhere to the venular wall. Phenylephrine, endothelin-1, acetylcholine, and sodium nitroprusside (Sigma Chemicals, St. Louis, MO, USA) were freshly prepared for each experiment and applied topically, added to the superfusion solution, to the cheek pouch with a syringe infusion pump 22 (model 25-2222, Harvard Apparatus, MA, USA) to avoid systemic effects, such as changes in blood pressure, which could result in biased results. For consecutive measurements, three arterioles were selected in each preparation, taking into account the potential for returning them to exactly the same site (based on, e.g., the presence of fat cells and bifurcations). Experiments were performed by taking 3-min videotape recordings of selected microvessels under initial control conditions (before the addition of any drug) and 10–15 min after each experimental intervention. The cumulative concentrations of phenylephrine and endothelin-1 used were 10−9, 10−7, and 10−5 M, in order to create a dose-response curve, while the concentrations of acetylcholine and sodium nitroprusside used were 10−8, 10−6, and 10−4 M. Diameter measurements were performed using an Image Shearing Device (model 907, Bela Vista, CA, USA).
Statistical analysis
StatSoft Statistica 8.0 software was used for statistical analysis, and variables were tested regarding the possible problems associated with data distribution (i.e., normality, kurtosis, skewness, and homoscedasticity).
Results are shown as medians and quartile ranges, with the exception of weight and blood pressure, which are shown as the means and standard deviations. Increases in macromolecular permeability, number of adherent or rolling leukocytes, and arteriolar diameters were analyzed by gender using the pairwise non-parametric Mann-Whitney U test, and non-parametric analysis of variance using the Kruskal-Wallis and Dunn's tests for post hoc comparisons was performed for comparisons of different concentrations and times. The significance level (α) used in all tests was 0.05.
Arteriolar diameter values were transformed to percentages relative to the initial diameter measurements taken as baseline values, i.e., before the addition of any drug to each arteriole associated with each preparation.
RESULTS
Seventy-two animals (21 weeks old), 36 males and 36 females, with a mean body mass and blood pressure, respectively, of 124.7±24.2 vs. 141.1±39.5 g (NS) and 95.2±1.2 vs. 95.0±0.8 mmHg (NS). All six study groups were compared according to gender, and the only group that presented any difference in baseline parameters was group V (acetylcholine), in which females had a higher body mass than males (187.7±10.4 vs. 145.2±7.2 g; p<0.01).
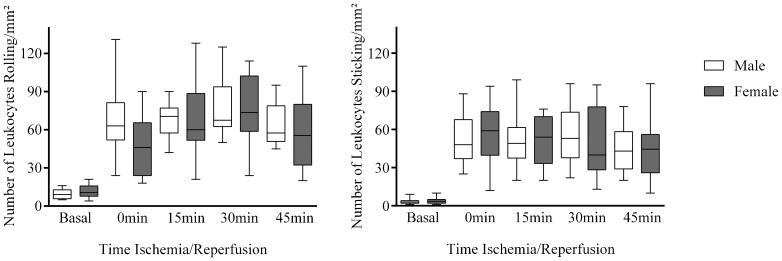
The increase in macromolecular permeability induced by ischemia/reperfusion was significantly lower in females than in males [19 (17–22) vs. 124 (123–128) leaks/cm2; p<0.001; Figure 1], while no gender difference was noted in the number of rolling (p = 0.386) or adherent (p = 0.732) leukocytes (Figure 2).
Figure 1.
An increase in macromolecular permeability induced by ischemia/reperfusion.
Figure 2.
The number of rolling/adherent leukocytes induced by ischemia/reperfusion.
Before the addition of any drug, the baseline arteriolar diameters were similar between the groups. Males and females showed, respectively, the following initial arteriolar diameters: phenylephrine group, 34.6 (27.0–55.1) vs. 30.2 (25.8–36.7) μm (NS); endothelin-1 group, 79.9 (52.7–104.7) vs. 79.80 (59.0–103.5) μm (NS); acetylcholine group, 14.4 (8.7–19.0) vs. 13.5 (11.8–20.1) μm (NS); and sodium nitroprusside group, 14.5 (11.4–22.3) vs. 13.8 (9.7–21.6) μm (NS).
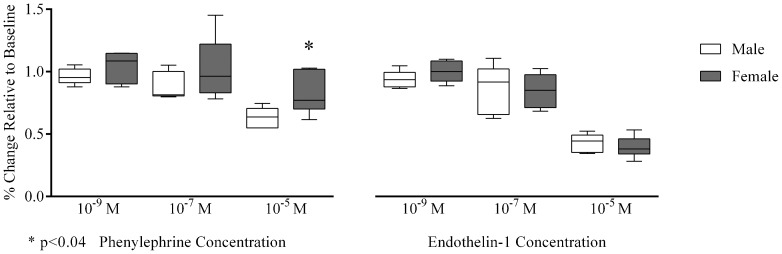
Of note, we observed that arteriolar phenylephrine-induced vasoconstriction was significantly lower in females than in males at 10-5 M [77 (73–102) vs. 64 (55–69)%, p≤0.04], while there were no significant differences at other concentrations [10−9 M (p = 0.39), 10−7M (p = 0.31); Figure 3]. In contrast, arteriolar vasoconstriction due to endothelin-1 revealed no gender differences at all concentrations tested [10−9 M (p = 0.24), 10−7 M (p = 0.94), 10−5 M (p = 0.70); Figure 3].
Figure 3.
Arteriolar vasoconstriction induced by phenylephrine and endothelin-1 according to dose.
Arteriolar vasodilation elicited by acetylcholine was not significantly different between males and females at all concentrations tested [10−8 M (p = 0.82), 10−6 M (p = 0.82), 10−4 M (p = 0.70); Figure 4], similar to the results associated with sodium nitroprusside [10−8 M (p = 0.82), 10−6 M (p = 0.70), 10−4 M (p = 0.82); Figure 4].
Figure 4.
Arteriolar vasodilation induced by acetylcholine and sodium nitroprusside according to dose.
DISCUSSION
Ischemia of the cheek pouch for 30 min followed by reperfusion is able to induce leukocyte adhesion and increase plasma extravasations in post-capillary venules (16). In this study, we observed a significant decrease in microvascular permeability to macromolecules in females compared with males without a concomitant reduction in the number of rolling or adherent leukocytes.
Endothelial cells (ECs) form a barrier between blood and tissue environments and control the movement of blood and immune cells, plasma fluid, small molecules, and proteins between the vascular compartment and the extracellular space. In continuous, non-fenestrated endothelium, the routes of passage are (a) transcellular through the EC membranes and cytoplasm, (b) transcellular via vesicles that traverse the endothelium, and (c) paracellular at junctions between ECs (17). The paracellular pathway is the most understood route, where junctional complexes are composed of tight (TJ), adherens (AJ), and gap junctions that create a physiological intercellular barrier. It has become increasingly clear that the changes/shifts in the function and regulation of these respective TJ and AJ proteins contribute to paracellular regulation and are important for vascular integrity and function (18,19). Additionally, the role of estrogens in these structures is now under investigation.
Mitochondrial oxidative stress caused by reactive oxygen species (ROS) generation after ischemia/reperfusion injury has been shown to regulate the mitochondrial release of cytochrome C into the cytosol, leading to the activation of caspase-3, which cleaves the endothelial cell AJ protein β-catenin (20). The cleavage of β-catenin is thought to be a key regulator of cell–cell adhesion and therefore the loss of this important AJ protein results in barrier dysfunction and hyperpermeability. 17β-estradiol has been shown to inhibit important components of the intrinsic apoptotic signaling pathway, causing increased mitochondrial (ROS) formation, decreased mitochondrial transmembrane potential, mitochondrial release of cytochrome C, and activation of caspase-3 (21). In humans, another mechanism contributing to the antioxidant effect of estrogens was observed in young women recruited for in vitro fertilization, where increased estrogen levels led to enhanced manganese superoxide dismutase (SOD) and extracellular (EC)-SOD expression in circulating monocytes (22). It should also be noted that a previous study demonstrated that the administration of SOD isoforms, specifically EC-SOD and copper-zinc SOD, was able to reduce the post-ischemic permeability increase in the hamster cheek pouch, demonstrating that reperfusion injury could be related to the formation of ROS (23).
Although we have not been able to prove the mechanisms involved in estrogen protection, our results suggest that the decreased microvascular permeability observed in females could be dependent on an up-regulation of transmembrane proteins, leading to reinforcement of the structural integrity of TJs and AJs, as well as inhibition of all important components of the intrinsic apoptotic signaling pathway. However, this hypothesis remains to be validated.
The control of reactivity and tonus strongly depends on endothelial and smooth muscle cells and their interactions. These cells express adrenergic receptors (α, e, β, and their subtypes) in variable amounts, depending on the vascular bed. Sympathetic neurons release catecholamines, and their effects depend on the type of receptor activated; for example, stimulation of α1 receptors (e.g., by phenylephrine) promotes vasoconstriction (24). Animal and human models have shown that there are gender differences in adrenergic vasomotor responses, although the results are conflicting. Some studies did not show significant differences in vasoreactivity after adrenergic stimuli between genders, while other studies demonstrated that the female vasculature is less responsive to norepinephrine- and phenylephrine-induced vasoconstriction (25-27). It is possible that these conflicting results could be explained by different concentrations of circulating sex hormones.
Sex hormone receptors are present in many vascular beds and act through genomic and non-genomic mechanisms. Among these receptors, it appears that the estrogen receptor has the most prominent action due to its potent endothelial-dependent vasodilatation effect and vasoconstriction resistance (28). A comparison of castrated and non-castrated male rats demonstrated that there was no difference in vascular contraction following castration; however, when comparing intact and ovarectomized female rats, the latter demonstrated increased vasoconstriction in response to stimuli (8).
The present study showed that, in both genders, there was significant, dose-dependent vasoconstriction induced by phenylephrine but that this effect was less pronounced in females, thereby supporting previous findings (25,26). Contrary to previous studies (29-31), we did not detect any gender differences with regard to secondary vascular responses to acetylcholine, sodium nitroprusside, and endothelin-1. These findings could be due to the heterogeneity observed in the microcirculation in vivo, where several physiological mechanisms are involved simultaneously and may reveal different results. Additionally, there are controversial studies concerning the action of endothelin-1, with some researchers observing reduced vasoconstriction in males (30) and other researchers observing it in females (32). In our study, the vasoconstriction elicited by endothelin-1 was not significantly different between males and females. With respect to microvascular reactivity after acetylcholine administration, the literature has more consistent positive findings in females (29), which we were unable to reproduce in our study. It is possible that the higher body mass of females compared with males may have biased our results in acetylcholine group. Finally, it is well-known that the action of an endothelium-independent stimulus is less evident when an atherosclerotic process is already present. It is possible that if the animals had been older or had a pro-atherosclerotic disease, such as diabetes mellitus, we may have found gender differences at microcirculation after sodium nitroprusside administration. Other limitations of the study should also be highlighted. The unawareness of the menstrual cycle period of females may have influenced the microvascular reactivity (33), although some researchers still consider this a controversial issue (34). Moreover, the reduced number of animals in each group may have limited our ability to observe differences.
Gender differences in the microvascular bed were observed in this study, and according to our findings, we conclude that the female gender seemed to exhibit microvascular protection, exhibited as lower microvascular permeability to macromolecules induced by ischemia/reperfusion and reduced microvascular constriction elicited by a phenylephrine stimulus.
ACKNOWLEDGMENTS
This study was supported by grants from the National Research Council of Brazil (CNPq) and the Agency for Research Support of Rio de Janeiro State (FAPERJ). The authors do not have any conflicts of interest to declare.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Lerner DJ, Kannel WB. Patterns of coronary heart disease morbidity and mortality in the sexes: a 26-year follow-up of the Framingham population. Am Heart J. 1986;111(2):383–90. doi: 10.1016/0002-8703(86)90155-9. [DOI] [PubMed] [Google Scholar]
- 2.Kuvin JT, Patel AR, Sliney KA, Pandian NG, Rand WM, Udelson JE, et al. Peripheral vascular endothelial function testing as a noninvasive indicator of coronary artery disease. J Am Coll Cardiol. 2001;38(7):1843–9. doi: 10.1016/s0735-1097(01)01657-6. [DOI] [PubMed] [Google Scholar]
- 3.Wang J, Bingaman S, Huxley VH. Intrinsic sex-specific differences in microvascular endothelial cell phosphodiesterases. Am J Physiol Heart Circ Physiol. 2010;298(4):H1146–54. doi: 10.1152/ajpheart.00252.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mendelsohn ME, Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science. 2005;308(5728):1583–7. doi: 10.1126/science.1112062. [DOI] [PubMed] [Google Scholar]
- 5.Leonardo F, Medeirus C, Rosano GM, Pereira WI, Sheiban I, Gebara O, et al. Am J Cardiol. 1997;80(6):791–3. doi: 10.1016/s0002-9149(97)00520-1. [DOI] [PubMed] [Google Scholar]
- 6.Villar IC, Hobbs AJ, Ahluwalia A. Sex differences in vascular function: implication of endothelium-derived hyperpolarizing factor. J Endocrinol. 2008;197(3):447–62. doi: 10.1677/JOE-08-0070. [DOI] [PubMed] [Google Scholar]
- 7.Pearson LJ, Yandle TG, Nicholls MG, Evans JJ. Regulation of endothelin-1 release from human endothelial cells by sex steroids and angiotensin-II. Peptides. 2008;29(6):1057–61. doi: 10.1016/j.peptides.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Orshal JM, Khalil RA. Gender, sex hormones, and vascular tone. Am J Physiol Regul Integr Comp Physiol. 2004;286(2):R233–49. doi: 10.1152/ajpregu.00338.2003. [DOI] [PubMed] [Google Scholar]
- 9.Simoes C, Svensjo E, Bouskela E. Effects of L-NA and sodium nitroprusside on ischemia/reperfusion-induced leukocyte adhesion and macromolecular leakage in hamster cheek pouch venules. Microvasc Res. 2001;62(2):128–35. doi: 10.1006/mvre.2001.2324. [DOI] [PubMed] [Google Scholar]
- 10.Bouskela E, Cyrino FZ, Lerond L. Leukocyte adhesion after oxidant challenge in the hamster cheek pouch microcirculation. J Vasc Res. 1999;36Suppl 1:11–4. doi: 10.1159/000054069. [DOI] [PubMed] [Google Scholar]
- 11.Duling BR. The preparation and use of the hamster cheek pouch for studies of the microcirculation. Microvasc Res. 1973;5(3):423–9. doi: 10.1016/0026-2862(73)90059-9. [DOI] [PubMed] [Google Scholar]
- 12.Bouskela E, Grampp W. Spontaneous vasomotion in hamster cheek pouch arterioles in varying experimental conditions. Am J Physiol. 1992;262(2 Pt 2):H478–85. doi: 10.1152/ajpheart.1992.262.2.H478. [DOI] [PubMed] [Google Scholar]
- 13.Svensjo E. Bradykinin and prostaglandin E1, E2 and F2alpha-induced macromolecular leakage in the hamster cheek pouch. Prostaglandins Med. 1978;1(5):397–410. doi: 10.1016/0161-4630(78)90126-x. [DOI] [PubMed] [Google Scholar]
- 14.Bouskela E, Cyrino FZ, Conde CM, Garcia AA. Microvascular permeability with sulfonylureas in normal and diabetic hamsters. Metabolism. 1997;46(12 Suppl 1):26–30. doi: 10.1016/s0026-0495(97)90313-9. [DOI] [PubMed] [Google Scholar]
- 15.Zeintl H, Sack FU, Intaglietta M, Messmer K. Computer assisted leukocyte adhesion measurement in intravital microscopy. Int J Microcirc Clin Exp. 1989;8(3):293–302. [PubMed] [Google Scholar]
- 16.Persson NH, Erlansson M, Svensjo E, Takolander R, Bergqvist D. The hamster cheek pouch--an experimental model to study postischemic macromolecular permeability. Int J Microcirc Clin Exp. 1985;4(3):257–63. [PubMed] [Google Scholar]
- 17.Bazzoni G, Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev. 2004;84(3):869–901. doi: 10.1152/physrev.00035.2003. [DOI] [PubMed] [Google Scholar]
- 18.Harhaj NS, Antonetti DA. Regulation of tight junctions and loss of barrier function in pathophysiology. Int J Biochem Cell Biol. 2004;36(7):1206–37. doi: 10.1016/j.biocel.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 19.Beyer EC, Gemel J, Seul KH, Larson DM, Banach K, Brink PR. Modulation of intercellular communication by differential regulation and heteromeric mixing of co-expressed connexins. Braz J Med Biol Res. 2000;33(4):391–7. doi: 10.1590/s0100-879x2000000400004. [DOI] [PubMed] [Google Scholar]
- 20.Sugawara T, Noshita N, Lewen A, Gasche Y, Ferrand-Drake M, Fujimura M, et al. Overexpression of copper/zinc superoxide dismutase in transgenic rats protects vulnerable neurons against ischemic damage by blocking the mitochondrial pathway of caspase activation. J Neurosci. 2002;22(1):209–17. doi: 10.1523/JNEUROSCI.22-01-00209.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu A, Frink M, Choudhry MA, Schwacha MG, Hubbard WJ, Rue LW, III, et al. Mitochondria play an important role in 17beta-estradiol attenuation of H(2)O(2)-induced rat endothelial cell apoptosis. Am J Physiol Endocrinol Metab. 2007;292(2):E585–93. doi: 10.1152/ajpendo.00413.2006. [DOI] [PubMed] [Google Scholar]
- 22.Strehlow K, Rotter S, Wassmann S, Adam O, Grohe C, Laufs K, et al. Modulation of antioxidant enzyme expression and function by estrogen. Circ Res. 2003;93(2):170–7. doi: 10.1161/01.RES.0000082334.17947.11. [DOI] [PubMed] [Google Scholar]
- 23.Erlansson M, Bergqvist D, Marklund SL, Persson NH, Svensjo E. Superoxide dismutase as an inhibitor of postischemic microvascular permeability increase in the hamster. Free Radic Biol Med. 1990;9(1):59–65. doi: 10.1016/0891-5849(90)90050-s. [DOI] [PubMed] [Google Scholar]
- 24.Guimaraes S, Moura D. Vascular adrenoceptors: an update. Pharmacol Rev. 2001;53(2):319–56. [PubMed] [Google Scholar]
- 25.Altura BM. Sex as a factor influencing the responsiveness of arterioles to catecholamines. Eur J Pharmacol. 1972;20(3):261–5. doi: 10.1016/0014-2999(72)90183-5. [DOI] [PubMed] [Google Scholar]
- 26.Kalsner S. Steroid potentiation of responses to sympathomimetic amines in aortic strips. Br J Pharmacol. 1969;36(3):582–93. doi: 10.1111/j.1476-5381.1969.tb08013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Z, Krause DN, Doolen S, Duckles SP. Ovariectomy eliminates sex differences in rat tail artery response to adrenergic nerve stimulation. Am J Physiol. 1997;272(4 Pt 2):H1819–25. doi: 10.1152/ajpheart.1997.272.4.H1819. [DOI] [PubMed] [Google Scholar]
- 28.Binko J, Murphy TV, Majewski H. 17Beta-oestradiol enhances nitric oxide synthase activity in endothelium-denuded rat aorta. Clin Exp Pharmacol Physiol. 1998;25(2):120–7. doi: 10.1111/j.1440-1681.1998.tb02188.x. [DOI] [PubMed] [Google Scholar]
- 29.Sader MA, Celermajer DS. Endothelial function, vascular reactivity and gender differences in the cardiovascular system. Cardiovasc Res. 2002;53(3):597–604. doi: 10.1016/s0008-6363(01)00473-4. [DOI] [PubMed] [Google Scholar]
- 30.Barber DA, Sieck GC, Fitzpatrick LA, Miller VM. Endothelin receptors are modulated in association with endogenous fluctuations in estrogen. Am J Physiol. 1996;271(5 Pt 2):H1999–2006. doi: 10.1152/ajpheart.1996.271.5.H1999. [DOI] [PubMed] [Google Scholar]
- 31.Hayashi T, Fukuto JM, Ignarro LJ, Chaudhuri G. Basal release of nitric oxide from aortic rings is greater in female rabbits than in male rabbits: implications for atherosclerosis. Proc Natl Acad Sci U S A. 1992;89(23):11259–63. doi: 10.1073/pnas.89.23.11259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MacIntyre JN, Slusar JE, Zhu J, Dong AX, Howlett SE, Kelly ME. Age-associated alterations in retinal arteriole reactivity to endothelin-1 differ between the sexes. Mech Ageing Dev. 2012;133(9-10):611–9. doi: 10.1016/j.mad.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 33.Bungum L, Kvernebo K, Oian P, Maltau JM. Laser doppler-recorded reactive hyperaemia in the forearm skin during the menstrual cycle. Br J Obstet Gynaecol. 1996;103(1):70–5. doi: 10.1111/j.1471-0528.1996.tb09517.x. [DOI] [PubMed] [Google Scholar]
- 34.Ketel IJ, Stehouwer CD, Serne EH, Poel DM, Groot L, Kager C, et al. Microvascular function has no menstrual-cycle-dependent variation in healthy ovulatory women. Microcirculation. 2009;16(8):714–24. doi: 10.3109/10739680903199186. [DOI] [PubMed] [Google Scholar]