Abstract
OBJECTIVES:
To identify predictors of in-hospital mortality in patients with acute myocardial infarction undergoing pharmacoinvasive treatment.
METHODS:
This was an observational, prospective study that included 398 patients admitted to a tertiary center for percutaneous coronary intervention within 3 to 24 hours after thrombolysis with tenecteplase. ClinicalTrials.gov: NCT01791764
RESULTS:
The overall in-hospital mortality rate was 5.8%. Compared with patients who survived, patients who died were more likely to be older, have higher rates of diabetes and chronic renal failure, have a lower left ventricular ejection fraction, and demonstrate more evidence of heart failure (Killip class III or IV). Patients who died had significantly lower rates of successful thrombolysis (39% vs. 68%; p = 0.005) and final myocardial blush grade 3 (13.0% vs. 61.9%; p<0.0001). Based on the multivariate analysis, the Global Registry of Acute Coronary Events score (odds ratio 1.05, 95% confidence interval (CI) 1.02-1.09; p = 0.001), left ventricular ejection fraction (odds ratio 0.9, 95% CI 0.89-0.97; p = 0.001), and final myocardial blush grade of 0-2 (odds ratio 8.85, 95% CI 1.34-58.57; p = 0.02) were independent predictors of mortality.
CONCLUSIONS:
In this prospective study that evaluated patients with ST-segment elevation myocardial infarction treated by a pharmacoinvasive strategy, the in-hospital mortality rate was 5.8%. The Global Registry of Acute Coronary Events score, left ventricular ejection fraction, and myocardial blush were independent predictors of mortality in this high-risk group of acute coronary syndrome patients.
Keywords: Myocardial Infarction, Mortality, Pharmacoinvasive Therapy
INTRODUCTION
Primary percutaneous coronary intervention (pPCI) is the standard of care reperfusion therapy for patients with evolving ST-segment elevation myocardial infarction (STEMI) (1-3). Although individual centers have performed pPCI for many years, a significant number of patients with STEMI either do not receive this optimal treatment or do not receive it in a timely manner due to the small number of tertiary care centers with an around-the-clock ('24/7') service. Large urban centers and rural areas, where tertiary care hospitals are restricted to specific regions, are less likely to have the capacity to deliver this treatment to the population as a whole (4).
Thus, a pharmacoinvasive treatment strategy has emerged as an alternative for STEMI patients admitted to primary care centers. A pharmacoinvasive strategy consists of the use of intravenous thrombolytic therapy in a primary care center followed by immediate transfer to a tertiary hospital, where early, systematic coronary angiography and percutaneous coronary angioplasty should be performed within 3 to 24 hours, even in cases of successful reperfusion (5).
In Brazil, despite recent social and medical therapeutic advances, the number of acute myocardial infarctions in the population still reaches 300,000 to 400,000 per year, with STEMI representing 30% to 40% of all cases. Furthermore, mortality rates are still high outside major referral centers, with an estimated 1 death for every 5 to 7 cases (6). Therefore, we recently developed a public STEMI network based on a pharmacoinvasive treatment strategy in the metropolitan area of São Paulo as a possible alternative to pPCI. In the present study, we sought to evaluate the rate and predictors of in-hospital mortality in a large population of STEMI patients who underwent pharmacoinvasive treatment.
METHODS
Patients and study protocol
Between November 2010 and October 2012, 567 patients with STEMI were transferred to a tertiary hospital (Hospital São Paulo, Escola Paulista de Medicina at Federal University of São Paulo) as part of a municipal primary care program for STEMI. The structured health care network for patients with STEMI has been previously described in detail elsewhere (7). In brief, patients are first admitted to a primary emergency room facility or rescued by the Emergency Mobile Healthcare Service (SAMU) at home and then referred to our tertiary PCI center. Patients were either treated with thrombolysis or underwent pPCI as appropriate and according to current guidelines (1-3). Those patients receiving thrombolysis were referred for an early invasive coronary angiography procedure regardless of successful reperfusion 3 to 24 hours after thrombolysis (i.e., elective or rescue PCI) according to clinical and electrocardiographic criteria. Coronary angiographies were performed by femoral access, and only bare metal stents were used. After excluding patients who did not receive thrombolysis, those who underwent pPCI, and those with no acute myocardial infarction, 398 patients remained for the present analysis.
Study objective and definitions
The primary objective of this study was to evaluate the rate and predictors of in-hospital mortality in a population of STEMI patients who underwent pharmacoinvasive treatment. Patients were consecutively included and divided into two groups according to mortality (patients who died during the index hospitalization from all causes and patients who survived) and compared according to their clinical, angiographic, and procedural variables as appropriate. Demographic and clinical data were entered into a database prospectively, and all quantitative coronary angiography data were analyzed by one experienced interventional cardiologist. Thrombolysis in myocardial infarction (TIMI) flow and blush grade were assessed as previously reported (8,9). Coronary artery disease (CAD) was defined as single- or multi-vessel disease according to the number of epicardial arteries with at least 1 lesion measuring ≥70% of the diameter of stenosis.
Chronic renal failure was defined as creatinine clearance <60 mL/min, estimated by the Cockcroft-Gault formula (10). Anemia was defined as plasma hemoglobin measuring less than 13 mg/dL for males or 12 mg/dL for females (11). The Global Registry of Acute Coronary Events (GRACE) score (12) was calculated at admission, and the left ventricular ejection fraction (LVEF) was determined by echocardiography during hospitalization. Successful thrombolysis was defined as a culprit artery with TIMI flow grade 2-3 at the time of coronary angiography. Time intervals between chest pain and the administration of Tenecteplase (TNK; pain-to-needle time) and between the administration of TNK and coronary angiography (needle-to-balloon time) are expressed in hours. This study was conducted in compliance with the ethical principles of the Helsinki Declaration (2008), as well as local applicable laws and regulations. It was registered in ClinicalTrials.gov with the identifier number NCT01791764.
Statistical analysis
Continuous variables are expressed as the mean and standard deviation, and categorical variables are expressed as absolute numbers and percentages. Fisher's exact test, Pearson's chi-square test, or Student's t-test were used when appropriate.
A multivariate logistic regression model was used to identify potential independent predictors of mortality. Odds ratios (ORs) and their respective confidence intervals (95% CIs) were used to quantify the effects. The variables included in the model were age, gender, hemoglobin level, diabetes mellitus, hypertension, current smoking status, chronic renal failure, GRACE score, Killip class, baseline and final TIMI flow, and final myocardial blush grade. SPSS® software version 20 (IBM, Armonk, NY, USA) was used for all analyses, and a final p value less than 0.05 was considered significant.
RESULTS
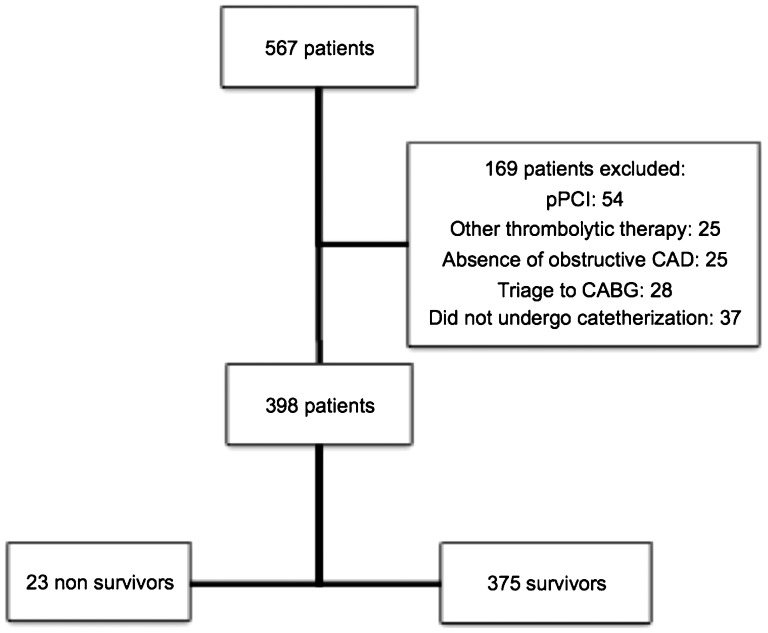
The study flowchart is shown in Figure 1. After excluding pPCI patients, patients without CAD, patients triaged to CABG, and patients treated with thrombolytics other than TNK, a total of 398 patients were selected for the current analysis. The mean pain-to-needle and needle-to-balloon times were 4.5±3.8 and 16.4±20.6 hours, respectively. Overall, the in-hospital mortality rate was 5.8% (compared with 6.5% of the entire population of 567 patients), and the mean GRACE score was 148.2±36.9. A comparison of demographic and clinical characteristics in non-survivors and survivors is provided in Table 1. Compared with patients who survived, non-survivors were more likely to be older and to have higher rates of diabetes and chronic renal failure, higher GRACE scores, lower LVEFs, and more evidence of heart failure (Killip class III or IV). Additionally, patients who died had a significantly lower incidence of baseline TIMI grade 2 or 3 flow (39% vs. 68%; p = 0.005) and a lower final myocardial blush grade (Table 1).
Figure 1.
Flowchart of patients included in the study.
Table 1.
Baseline, clinical, and procedural characteristics of non-survivors vs. survivors.
| Variables | Non-survivors (23) | Survivors (375) | p-value |
| Age (years) | 63.78±11.2 | 57.63±11.5 | 0.011 |
| Male | 13 (56.5%) | 260 (69.3%) | 0.199 |
| CrCl (<60 ml/min) | 10 (43.5%) | 63 (16.8%) | 0.001 |
| Diabetes mellitus | 14 (60.9%) | 105 (28%) | 0.001 |
| Hypertension | 16 (69.5%) | 234 (58.7%) | 0.330 |
| Dyslipidemia | 14 (60.9%) | 220 (58.6%) | 0.846 |
| Currently smoking | 14 (60.9%) | 248 (66.1%) | 0.797 |
| Anemia | 6 (26.1%) | 61 (16.3%) | 0.222 |
| Anterior AMI | 6 (26.1%) | 97 (25.9%) | 0.981 |
| Inferior AMI + RV | 5 (21.7%) | 36 (9.6%) | 0.075 |
| Killip class III or IV | 10 (43.5%) | 29 (7.7%) | <0.0001 |
| GRACE risk score | 206.2±39.2 | 144.6±33.7 | <0.0001 |
| LVEF (%) | 33.9±20.2 | 49.9±12.5 | <0.0001 |
| Pain-to-needle time (h) | 4.8±3.1 | 4.4±3.9 | 0.69 |
| Needle-to-balloon time (h) | 8.0±6.3 | 17.0±21.1 | 0.053 |
| Successful thrombolysis | 9 (39.1%) | 255 (68%) | 0.005 |
| Multi-vessel CAD | 20 (87%) | 240 (64%) | 0.024 |
| Final TIMI 2, 3 | 20 (87%) | 343 (91.4%) | 0.442 |
| Final Blush 0-2 | 20 (87%) | 143 (38.1%) | <0.0001 |
CrCl: creatinine clearance; AMI: acute myocardial infarction; RV: right ventricular involvement.
Two patients (0.5%) had a hemorrhagic stroke, 16 (4%) required blood transfusion, and vascular complications related to the access site were observed in 26 (6.5%) patients. A more detailed analysis of the vascular access and bleeding complications in patients undergoing pharmacoinvasive strategy in our institution has been previously reported (13).
Predictors of in-hospital mortality
Based on the multivariate regression analysis, only GRACE score, LVEF, and final blush grade 0-2 were independent predictors of mortality (Table 2).
Table 2.
Independent predictors of mortality according to multivariate logistic regression analysis.
| Variables | OR | p-value | 95% CI |
| GRACE score | 1.057 | 0.001 | 1.023-1.091 |
| LVEF | 0.930 | 0.001 | 0.890-0.972 |
| Final Blush 0-2 | 8.857 | 0.02 | 1.339-58.570 |
DISCUSSION
Providing optimal care for STEMI patients is challenging in any country, regardless of its wealth and developmental level. This is especially true in large urban centers or rural areas, where a lack of access to tertiary healthcare may make the performance of timely pPCI difficult. The so-called pharmacoinvasive treatment strategy, consisting of thrombolysis with TNK and contemporary antithrombotic therapy administered before transport to a PCI-capable hospital, has emerged as an alternative to pPCI. The recommendation of prompt transfer to a referral center with a catheterization laboratory is deemed fundamental to the strategy's success, allowing for (i) immediate reperfusion in cases when thrombolysis fails; (ii) treatment for early reocclusion, which can affect as many as 30% of cases (14); and, ultimately, (iii) early PCI (<24 hours) for patients with successful thrombolytic reperfusion but with severe residual stenosis. Compared with pPCI, this strategy has shown similar clinical outcomes (15-17). Accordingly, this strategy is strongly recommended by the European Society of Cardiology and ACC/AHA guidelines (class I and IIa, respectively) (1,3).
The present study represents one of the largest real-world studies relevant to this subject thus far and shows an in-hospital all-cause mortality rate of 5.8%. Other national, multicenter studies have reported mortality rates of 3.3% in patients who underwent elective PCI after thrombolysis, 7.6% in patients who underwent rescue PCI, and 5.6% in pPCI patients (18,19). In previous randomized multicenter studies comparing a pharmacoinvasive strategy with ischemia-driven PCI, in-hospital mortality rates ranged from 3.0% to 4.5%. These studies, however, also demonstrated shorter pain-to-needle and needle-to-balloon times (2 and 4 hours, respectively), which may explain, at least in part, their lower mortality rates (14,20). A remarkably low mortality rate was also observed in a recently published STREAM trial (21), which compared pPCI with a pharmacoinvasive approach. This study randomized STEMI patients who had a clinical presentation for less than 3 hours and who were at least 60 minutes away from pPCI centers and found an overall mortality rate of 4.7% for both groups. Similar to previous results, such as those obtained in the TRANSFER AMI study (14), and our own results, one-third of the STREAM patients required rescue catheterization.
In the present analysis, LVEF and the GRACE score emerged as significant predictors of mortality. Successful thrombolysis was achieved in over 65% of patients, similar to the rate reported in the STREAM trial, with a mortality rate of only 3.4% in this subgroup (significantly lower than the rate of 10.4% in patients with a baseline TIMI flow of 0-1). Therefore, this study showing a low mortality rate in patients who underwent successful thrombolysis is encouraging and stresses the potential importance of a pharmacoinvasive treatment strategy, especially in public hospitals and developing countries.
Regardless of achieving a similar optimal final TIMI flow after PCI (as a surrogate endpoint for PCI angiographic success) in both groups, grade 3 myocardial blush was observed significantly less often in non-survivors; thus, myocardial blush grade was also an important independent predictorof mortality. Restoring coronary microcirculation through the use of several available strategies and in light of ongoing studies (e.g., intracoronary abciximab and thrombectomy) has been a challenge for interventional cardiologists over the last several decades. Studies evaluating patients who underwent thrombolytic and mechanical reperfusion during STEMI showed a direct relationship between the degree of myocardial perfusion, as detected by the blush angiographic method, and mortality in short and long clinical follow-up periods (22,23).
Restoring microcirculation extends beyond the restoration of epicardial flow and depends on the optimization of adjunctive therapy, especially antiplatelet and antithrombotic therapy. Compared with the STREAM trial population, we achieved similar rates of final TIMI flow 2-3 even in a setting of a longer pain-to-needle time. Therefore, achieving a baseline TIMI flow of 3 along with a myocardial blush grade of 3 should be considered to indicate optimal reperfusion. This finding is in accordance with previous studies demonstrating a normal myocardial blush grade as a predictor of survival and left ventricular recovery in patients who undergo pPCI and rescue angioplasty (24-28).
Hemorrhagic complications are still a concern associated with this strategy. However, no significant difference was observed in randomized trials between this treatment strategy and other treatments (14,20,21). In the STREAM trial, after the amendment of halving the TNK dosage in patients older than 75 years, there was no significant difference in hemorrhagic events between the groups. Hemorrhagic stroke and blood transfusion occurred in 0.5% and 2.9% of patients, respectively; in our study, these rates were 0.5% and 4.0%, respectively.
Although far from ideal because pPCI is considered the standard of care for STEMI patients, a pharmacoinvasive treatment strategy could be an alternative for achieving short pain-to-balloon times and, coupled with a structured healthcare network, could represent a highly important improvement in public health management of STEMI in Brazil. The present study is a step forward in adjusting the healthcare system in accordance with global standards to optimally assist the largest possible number of patients.
This study has several limitations. It was an observational and single-center study. A femoral artery approach was used in all cases, and bleeding criteria may underestimate the actual impact of this important complication on mortality. In addition, the use of radial access in pPCI has been associated with lower mortality rates (29,30). The multivariate analysis performed in this study cannot exclude residual confounding due to unmeasured or unknown variables. Finally, the generalizability of this study is limited because it can only be applied to STEMI patients who undergo a pharmacoinvasive treatment strategy according to the methods described in our protocol.
In this real-world, prospective study that included STEMI patients who underwent a pharmacoinvasive treatment strategy, in-hospital mortality rates were low at 5.8%. The GRACE score, LVEF, and myocardial blush grade were powerful predictors of mortality after administration of this pharmacoinvasive treatment strategy in the contemporary reperfusion era.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Steg PG, James SK, Atar D, Badano LP, Blomstrom-Lundqvist C, Borger MA, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33(20):2569–619. doi: 10.1093/eurheartj/ehs215. [DOI] [PubMed] [Google Scholar]
- 2.Piegas LS, Feitosa G, Mattos LA, Nicolau JC, Rossi Neto JM, Timerman A, et al. Sociedade Brasileira de Cardiologia. Diretriz da Sociedade Brasileira de Cardiologia sobre Tratamento do Infarto agudo do Miocárdio com Supradesnível do Segmento ST. Arq Bras Cardiol. 2009;93(6 supl.2):e179–e264. [PubMed] [Google Scholar]
- 3.O'Gara PT, Kushner FG, Ascheim DD, Casey DE, Jr, Chung MK, de Lemos JA, et al. ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction: Executive Summary: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61(4):485–510. doi: 10.1016/j.jacc.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 4.Boden WE, Eagle K, Granger CB. Reperfusion Strategies in Acute ST-Segment Elevation Myocardial Infarction. A Comprehensive Review of Contemporary Management Options. J Am Coll Cardiol. 2007;50(10):917–29. doi: 10.1016/j.jacc.2007.04.084. [DOI] [PubMed] [Google Scholar]
- 5.Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011:e574–e651. doi: 10.1161/CIR.0b013e31823ba622. [DOI] [PubMed] [Google Scholar]
- 6.Linha do Cuidado do Infarto Agudo do Miocárdio na Rede de Atenção às Urgências [Path of Care of Acute Myocardial Infarction in Emergency Care Networks, guidelines developed by The Brazilian Health Secretary]. Available at: http://portal.saude.gov.br/portal/arquivos/pdf/protocolo_sindrome_coronaria.pdf. (Accessed on December 20, 2012). [Google Scholar]
- 7.Caluza ACV, Barbosa AH, Gonçalves I, Oliveira CAL, Matos LN, Zeefried C, et al. Rede de Infarto com Supradesnivelamento de ST: Sistematização em 205 Casos Diminui Eventos Clínicos na Rede Pública [ST-Elevation Myocardial Infarction Network: Systematization in 205 Cases Reduces Clinical Events in the Public Health Care System] Arq Bras Cardiol. 2012;99(5):1040–8. doi: 10.1590/s0066-782x2012005000100. [DOI] [PubMed] [Google Scholar]
- 8.TIMI Study Group. The Thrombolysis in Myocardial Infarction (TIMI) trial. Phase I findings. N Engl J Med. 1985;312(14):932–6. doi: 10.1056/NEJM198504043121437. [DOI] [PubMed] [Google Scholar]
- 9.van 't Hof AW, Liem A, Suryapranata H, Hoorntje JC, de Boer MJ, Zijlstra F. Angiographic assessment of myocardial reperfusion in patients treated with primary angioplasty for acute myocardial infarction: myocardial blush grade. Zwolle Myocardial Infarction Study Group. Circulation. 1998;97(23):2302–6. doi: 10.1161/01.cir.97.23.2302. [DOI] [PubMed] [Google Scholar]
- 10.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 11.WHO. Vitamin and Mineral Nutrition Information System. Geneva: World Health Organization; 2011. Hemoglobin concentrations for the diagnosis of anemia and assessment of severity. (WHO/NMH/NHD/MNM/11.1). Available at: http://www.who.int/vmnis/indicators/haemoglobin.pdf. (Accessed on December 20, 2012). [Google Scholar]
- 12.Granger CB, Goldberg RJ, Dabbous O, Pieper KS, Eagle KA, Cannon CP, et al. Predictors of hospital mortality in the global registry of acute coronary events. Arch Intern Med. 2003;163(19):2345–53. doi: 10.1001/archinte.163.19.2345. [DOI] [PubMed] [Google Scholar]
- 13.Gomes Júnior MPM, Falcão FJA, Alves CMR, Sousa JMA, Herrmann JL, Moreno ACC, et al. Complicações Vasculares em Pacientes Submetidos a Intervenção Coronária Percutânea Precoce por Via Femoral após Fibrinólise com Tenecteplase: Registro de 199 Pacientes. Rev Bras Cardiol Invasiva. 2012;20(3):274–81. [Google Scholar]
- 14.Cantor WJ, Fitchett D, Borgundvaag B, Ducas J, Heffernan M, Cohen EA, et al. Routine early angioplasty after fibrinolysis for acute myocardial infarction. N Engl J Med. 2009;360(26):2705–18. doi: 10.1056/NEJMoa0808276. [DOI] [PubMed] [Google Scholar]
- 15.Bodí V, Rumiz E, Merlos P, Nunez J, López-Lereu MP, Monmeneu JV, et al. One-week and 6-month cardiovascular magnetic resonance outcome of the pharmacoinvasive strategy and primary angioplasty for the reperfusion of ST-segment elevation myocardial infarction. Revista Española de Cardiología. 2011;4(2):111–20. doi: 10.1016/j.recesp.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Danchin N, Coste P, Ferrieres J, Steg PG, Cottin Y, Blanchard D, et al. Comparison of thrombolysis followed by broad use of percutaneous coronary intervention with primary percutaneous coronary intervention for ST-segment-elevation acute myocardial infarction: data from the french registry on acute ST-elevation myocardial infarction (FAST-MI) Circulation. 2008;118(3):268–76. doi: 10.1161/CIRCULATIONAHA.107.762765. [DOI] [PubMed] [Google Scholar]
- 17.Armstrong P. WEST Steering Committee. A comparison of pharmacologic therapy with/without timely coronary intervention vs primary percutaneous intervention early after ST-elevation myocardial infarction: the WEST (Which Early ST-elevation myocardial infarction Therapy) Study. Eur Heart J. 2006;27(13):1530–8. doi: 10.1093/eurheartj/ehl088. [DOI] [PubMed] [Google Scholar]
- 18.Lima EC, Nascimento GA, Pena MI, Vasconcelos VSA, Crepaldi RJQ, Rabelo W, et al. Intervenção Coronária Percutânea Eletiva após Fibrinólise: Dados do REMAT (Registro Madre Teresa) [Elective Percutaneous Coronary Intervention after Fibrinolysis: REMAT Data (Madre Teresa Registry)] Rev Bras Cardiol Invasiva. 2011;19(4):373–8. [Google Scholar]
- 19.Mattos LA, Sousa AGMR, Pinto IMF, Silva ER, Carneiro JK, Sousa JE, et al. Uma comparação entre a intervenção coronariana percutânea de resgate e primária realizadas no infarto agudo do miocárdio: relato multicêntrico de 9.371 pacientes. Arq Bras Cardiol. 2004;82(5):434–9. [Google Scholar]
- 20.Di Mario C, Dudek D, Piscione F, Mielecki W, Savonitto S, Murena E, et al. Immediate angioplasty versus standard therapy with rescue angioplasty after thrombolysis in the Combined Abciximab REteplase Stent Study in Acute Myocardial Infarction (CARESS-in-AMI): an open, prospective, randomized, multicentre trial. Lancet. 2008;371(9612):559–68. doi: 10.1016/S0140-6736(08)60268-8. [DOI] [PubMed] [Google Scholar]
- 21.Armstrong PW, Gershlick AH, Goldstein P, Wilcox R, Danays T, Lambert Y, et al. Fibrinolysis or primary PCI in ST-Segment elevation myocardial infarction. N Engl J Med. 2013;368(15):1379–87. doi: 10.1056/NEJMoa1301092. [DOI] [PubMed] [Google Scholar]
- 22.Ito H, Maruyama A, Iwakura K, Takiuchi S, Masuyama T, Hori M, et al. Clinical Implications of the No ‘Reflow' Phenomenon: A Predictor of Complications and Left Ventricular Remodeling in Reperfused Anterior Wall Myocardial Infarction. Circulation. 1996;93(2):223–8. doi: 10.1161/01.cir.93.2.223. [DOI] [PubMed] [Google Scholar]
- 23.Henriques JP, Zijlstra L, van ?t Hof AW, de Boer MJ, Dambrink JH, Gosselink M, et al. Angiographic assessment of reperfusion in acute myocardial infarction by myocardial blush grade. Circulation. 2003;107(16):2115–9. doi: 10.1161/01.CIR.0000065221.06430.ED. [DOI] [PubMed] [Google Scholar]
- 24.Gibson CM, Murphy SA, Morrow DA, Aroesty JM, Gibbons RJ, Gourlay SG, et al. Angiographic perfusion score: an angiographic variable that integrates both epicardial and tissue level perfusion before and after facilitated percutaneous coronary intervention in acute myocardial infarction. Am Heart J. 2004;148(2):336–40. doi: 10.1016/j.ahj.2003.12.044. [DOI] [PubMed] [Google Scholar]
- 25.Henriques JP, Zijlstra F, van 't Hof AW, de Boer MJ, Dambrink JH, Gosselink M, et al. Angiographic assessment of reperfusion in acute myocardial infarction by myocardial blush grade. Circulation. 2003;107(16):2115–9. doi: 10.1161/01.CIR.0000065221.06430.ED. [DOI] [PubMed] [Google Scholar]
- 26.Costantini CO, Stone GW, Mehran R, Aymong E, Grines CL, Cox DA, et al. Frequency, correlates, and clinical implications of myocardial perfusion after primary angioplasty and stenting, with and without glycoprotein IIb/IIIa inhibition, in acute myocardial infarction. J Am Coll Cardiol. 2004;44(2):305–12. doi: 10.1016/j.jacc.2004.03.058. [DOI] [PubMed] [Google Scholar]
- 27.Di Nucci TP, Pimentel Filho WA, Correia MB, Abdalla Filho R, Bocchi EA, Custodio WB, et al. Correlação entre o Grau de Perfusão Miocárdica e a Evolução Clínica Tardia de Pacientes Submetidos a Trombólise e Implante de Stent. Rev Bras Cardiol Invas. 2006;14(1):56–62. [Google Scholar]
- 28.Bellandi F, Leoncini M, Maioli M, Toso A, Gallopin M, Piero Dabizzi R. Markers of myocardial reperfusion as predictors of left ventricular function recovery in acute myocardial infarction treated with primary angioplasty. Clinical Cardiology. 2004;27(12):683–8. doi: 10.1002/clc.4960271205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jolly SS, Yusuf S, Cairns J, Niemela K, Xavier D, Widimsky P, et al. Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL): a randomized, parallel group, multicentre trial. Lancet. 2011;377(9775):1409–20. doi: 10.1016/S0140-6736(11)60404-2. [DOI] [PubMed] [Google Scholar]
- 30.Romagnoli E, Biondi-Zoccai G, Sciahbasi A, Politi L, Rigattieri S, Pendenza G, et al. Radial Versus Femoral Randomized Investigation in ST-Segment Elevation Acute Coronary Syndrome: The RIFLE-STEACS (Radial Versus Femoral Randomized Investigation in ST-Elevation Acute Coronary Syndrome) Study. J Am Coll Cardiol. 2012;60(24):2481–97. doi: 10.1016/j.jacc.2012.06.017. [DOI] [PubMed] [Google Scholar]