Abstract
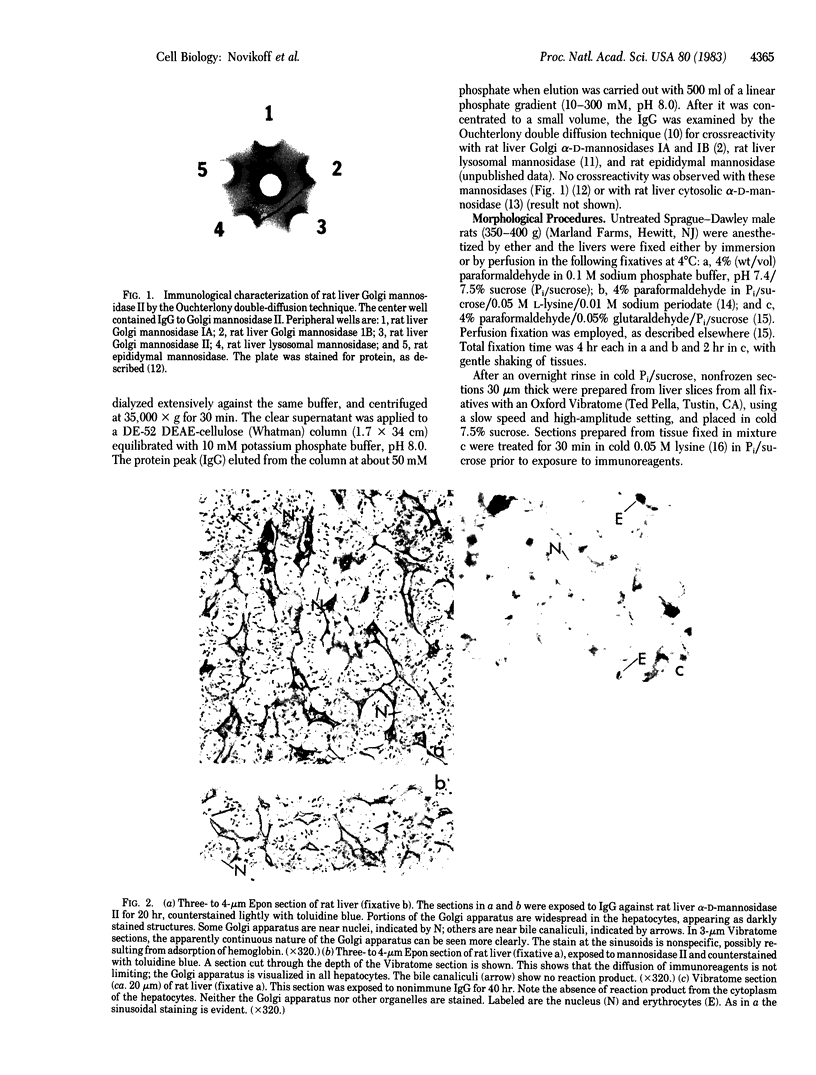
Mannosidase II is involved in the trimming of alpha-1,6-mannosyl residues during the biosynthesis of glycoproteins containing N-linked oligosaccharides of the complex type. A highly specific polyclonal antibody (IgG) was isolated from rabbits immunized with a homogeneous preparation of mannosidase II prepared from rat liver. With this antibody, light and electron microscopic immunocytochemical studies on rat liver reveal that essentially all mannosidase II in hepatocytes is localized in the Golgi apparatus, the only other site with reaction product being the endoplasmic reticulum. The indirect immunocytochemical method used in this study involved three major steps: exposure of aldehyde-fixed tissue to immune and nonimmune IgG, treatment with staphylococcal protein A labeled with horseradish peroxidase, and incubation in diaminobenzidine to reveal sites of peroxidase activity. The procedures described overcome major problems in immunocytochemistry, allowing preservation of antigenic sites and maintaining adequate ultrastructural integrity. The in situ localization of other carbohydrate-processing enzymes, involved in either trimming or attachment of sugar residues, should be possible with this procedure. Because biosynthetic precursors of the processing enzymes may be revealed by an immunocytochemical approach, it is potentially significant that mannosidase II reaction product is present in areas of the endoplasmic reticulum as well as in the Golgi apparatus.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cheetham R. D., Morré D. J., Pannek C., Friend D. S. Isolation of a Golgi apparatus-rich fraction from rat liver. IV. Thiamine pyrophosphatase. J Cell Biol. 1971 Jun;49(3):899–905. doi: 10.1083/jcb.49.3.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois-Dalcq M., McFarland H., McFarlin D. Protein A-peroxidase: a valluable tool for the localization of antigens. J Histochem Cytochem. 1977 Nov;25(11):1201–1206. doi: 10.1177/25.11.199666. [DOI] [PubMed] [Google Scholar]
- Dunphy W. G., Fries E., Urbani L. J., Rothman J. E. Early and late functions associated with the Golgi apparatus reside in distinct compartments. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7453–7457. doi: 10.1073/pnas.78.12.7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenreich J. H., Bergeron J. J., Siekevitz P., Palade G. E. Golgi fractions prepared from rat liver homogenates. I. Isolation procedure and morphological characterization. J Cell Biol. 1973 Oct;59(1):45–72. doi: 10.1083/jcb.59.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar M. G., Palade G. E. Cell junctions in amphibian skin. J Cell Biol. 1965 Jul;26(1):263–291. doi: 10.1083/jcb.26.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godelaine D., Spiro M. J., Spiro R. G. Processing of the carbohydrate units of thyroglobulin. J Biol Chem. 1981 Oct 10;256(19):10161–10168. [PubMed] [Google Scholar]
- Goldberg D. E., Kornfeld S. Evidence for extensive subcellular organization of asparagine-linked oligosaccharide processing and lysosomal enzyme phosphorylation. J Biol Chem. 1983 Mar 10;258(5):3159–3165. [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Harpaz N., Schachter H. Control of glycoprotein synthesis. Processing of asparagine-linked oligosaccharides by one or more rat liver Golgi alpha-D-mannosidases dependent on the prior action of UDP-N-acetylglucosamine: alpha-D-mannoside beta 2-N-acetylglucosaminyltransferase I. J Biol Chem. 1980 May 25;255(10):4894–4902. [PubMed] [Google Scholar]
- Hino Y., Asano A., Sato R. Biochemical studies on rat liver Golgi apparatus. II. Further characterization of isolated Golgi fraction. J Biochem. 1978 Apr;83(4):925–934. doi: 10.1093/oxfordjournals.jbchem.a132019. [DOI] [PubMed] [Google Scholar]
- Hubbard S. C., Robbins P. W. Synthesis and processing of protein-linked oligosaccharides in vivo. J Biol Chem. 1979 Jun 10;254(11):4568–4576. [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Molin S. O., Nygren H., Hansson H. A. Binding of glutaraldehyde reacted peroxidase to cell surfaces--a source of error in immunocytochemistry. J Histochem Cytochem. 1978 Apr;26(4):325–326. doi: 10.1177/26.4.96176. [DOI] [PubMed] [Google Scholar]
- NOVIKOFF A. B., ESSNER E. Pathological changes in cytoplasmic organelles. Fed Proc. 1962 Nov-Dec;21:1130–1142. [PubMed] [Google Scholar]
- NOVIKOFF A. B., GOLDFISCHER S. Nucleosidediphosphatase activity in the Golgi apparatus and its usefulness for cytological studies. Proc Natl Acad Sci U S A. 1961 Jun 15;47:802–810. doi: 10.1073/pnas.47.6.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novikoff A. B. DAB cytochemistry: artifact problems in its current uses. J Histochem Cytochem. 1980 Sep;28(9):1036–1038. doi: 10.1177/28.9.7410815. [DOI] [PubMed] [Google Scholar]
- Novikoff A. B., Novikoff P. M., Stockert R. J., Becker F. F., Yam A., Poruchynsky M. S., Levin W., Thomas P. E. Immunocytochemical localization of epoxide hydrase in hyperplastic nodules induced in rat liver by 2-acetylaminofluorene. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5207–5211. doi: 10.1073/pnas.76.10.5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novikoff P. M., Yam A. Sites of lipoprotein particles in normal rat hepatocytes. J Cell Biol. 1978 Jan;76(1):1–11. doi: 10.1083/jcb.76.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novikoff P. M., Yam A. The cytochemical demonstration of GERL in rat hepatocytes during lipoprotein mobilization. J Histochem Cytochem. 1978 Jan;26(1):1–13. doi: 10.1177/26.1.563889. [DOI] [PubMed] [Google Scholar]
- Opheim D. J., Touster O. Lysosomal alpha-D-mannosidase of rat liver. Purification and comparison with the golgi and cytosolic alpha-D-mannosidases. J Biol Chem. 1978 Feb 25;253(4):1017–1023. [PubMed] [Google Scholar]
- Pohlmann R., Waheed A., Hasilik A., von Figura K. Synthesis of phosphorylated recognition marker in lysosomal enzymes is located in the cis part of Golgi apparatus. J Biol Chem. 1982 May 25;257(10):5323–5325. [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini D. D., Kreibich G., Morimoto T., Adesnik M. Mechanisms for the incorporation of proteins in membranes and organelles. J Cell Biol. 1982 Jan;92(1):1–22. doi: 10.1083/jcb.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoup V. A., Touster O. Purification and characterization of the alpha-D-mannosidase of rat liver cytosol. J Biol Chem. 1976 Jul 10;251(13):3845–3852. [PubMed] [Google Scholar]
- Tabas I., Kornfeld S. Purification and characterization of a rat liver Golgi alpha-mannosidase capable of processing asparagine-linked oligosaccharides. J Biol Chem. 1979 Nov 25;254(22):11655–11663. [PubMed] [Google Scholar]
- Tabas I., Kornfeld S. The synthesis of complex-type oligosaccharides. III. Identification of an alpha-D-mannosidase activity involved in a late stage of processing of complex-type oligosaccharides. J Biol Chem. 1978 Nov 10;253(21):7779–7786. [PubMed] [Google Scholar]
- Tulsiani D. R., Hubbard S. C., Robbins P. W., Touster O. alpha-D-Mannosidases of rat liver Golgi membranes. Mannosidase II is the GlcNAcMAN5-cleaving enzyme in glycoprotein biosynthesis and mannosidases Ia and IB are the enzymes converting Man9 precursors to Man5 intermediates. J Biol Chem. 1982 Apr 10;257(7):3660–3668. [PubMed] [Google Scholar]
- Tulsiani D. R., Opheim D. J., Touster O. Purification and characterization of alpha-D-mannosidase from rat liver golgi membranes. J Biol Chem. 1977 May 25;252(10):3227–3233. [PubMed] [Google Scholar]
- Tulsiani D. R., Touster Resolution and partial characterization of two aldehyde reductases of mammalian liver. J Biol Chem. 1977 Apr 25;252(8):2545–2550. [PubMed] [Google Scholar]