Abstract
OBJECTIVES:
This study tests the hypothesis that local or remote ischemic preconditioning may protect the intestinal mucosa against ischemia and reperfusion injuries resulting from temporary supraceliac aortic clamping.
METHODS:
Twenty-eight Wistar rats were divided into four groups: the sham surgery group, the supraceliac aortic occlusion group, the local ischemic preconditioning prior to supraceliac aortic occlusion group, and the remote ischemic preconditioning prior to supraceliac aortic occlusion group. Tissue samples from the small bowel were used for quantitative morphometric analysis of mucosal injury, and blood samples were collected for laboratory analyses.
RESULTS:
Supraceliac aortic occlusion decreased intestinal mucosal length by reducing villous height and elevated serum lactic dehydrogenase and lactate levels. Both local and remote ischemic preconditioning mitigated these histopathological and laboratory changes.
CONCLUSIONS:
Both local and remote ischemic preconditioning protect intestinal mucosa against ischemia and reperfusion injury following supraceliac aortic clamping.
Keywords: Aorta, Reperfusion, Ischemic Preconditioning, Intestinal Mucosa, Rats
INTRODUCTION
Surgical correction of disease of the abdominal aorta involving its visceral branches produces intense and abrupt hemodynamic changes induced by aortic clamping and unclamping (1,2). However, the main consequences of temporary visceral aorta occlusion are related to the ischemia and reperfusion (I/R) injury of the splanchnic organs (3). Of the splanchnic organs, the intestine is the most sensitive to I/R injury and plays a pivotal role in the induction of systemic inflammation response syndrome (SIRS) and multiple organ dysfunction (MOD) (4,5), a major cause of morbidity in patients undergoing major aortic repair (6).
Strategies to reduce I/R injury are being extensively investigated, particularly ischemic preconditioning (IPC) (7). This strategy of submitting tissues to controlled periods of ischemia and reperfusion prior to the prolonged I/R injury is initially proven to be beneficial when applied to the same tissue (local) (8) and also when applied to a different tissue (remote) (9). Numerous investigators have described the protective effect of IPC on I/R injury in specific organs such as the heart (10) and liver (11). Currently, IPC is considered a ubiquitous phenomenon (12) that involves a complex mechanism of cell signaling (13), with clinical applicability that reaches beyond myocardial protection or organ transplantation (14).
Intestinal I/R injury and the use of IPC was initially evaluated by occluding the superior mesentery artery (15,16). The models used thus far typically employ this concept with different combinations of ischemic and reperfusion periods (17). Among studies, the preconditioning stimulus varies from 1 to 4 cycles of ischemia for 1 to 20 minutes and reperfusion for 5 to 10 minutes (18,19).
Supraceliac aortic occlusion has been evaluated with regard to the systemic inflammatory response resulting from I/R injury. The inhibition of tumor necrosis factor-α (TNF-α) and interleukin (IL) 1β attenuates pulmonary neutrophil infiltration (20). Additionally, IL-10 administration or its endogenous production seems to be protective (21,22).
Intestinal injury in the clinical context of total splanchnic and lower torso I/R has not been sufficiently described. Erling et al. identified that either local or remote IPC modulates the inflammatory response, reducing endothelial dysfunction in the mesenteric circulation after supraceliac aortic clamping (23). However, the possible protective effect of the IPC achieved in the intestine alone (24) has not been studied in the setting of supraceliac occlusion.
Using a supraceliac aortic clamping model, this study evaluated the morphologic alterations in the intestinal mucosa, variations in laboratory findings caused by multivisceral I/R injury, and the modulations in outcomes resulting from local or remote IPC.
MATERIAL AND METHODS
Animal model and surgical preparation
The experimental protocol was approved by the Ethical Committee of Federal University of Sao Paulo (CEP 1016/06) and was performed according to the National Institutes of Health guidelines on the use of experimental animals. Twenty-eight male Wistar rats weighting 190-250 g were kept in a non-stimulating environment for a week prior to the experiment. The subjects were fasted overnight prior to the procedure, with free access to water. Anesthesia was induced with intraperitoneal sodium pentobarbital (50 mg/kg). A tracheostomy was performed through a right anterior cervical incision to allow for airway control and spontaneous breathing. The common carotid artery and external jugular vein were dissected and cannulated with polyethylene catheters. Venous access was used to inject solutions, while arterial access was used to monitor mean arterial pressure (MAP) (MP 100, Biopac System Inc., Goleta, CA, USA). A midline abdominal incision was performed, and the aorta was dissected and controlled proximally at the supraceliac portion between the diaphragmatic crura and distally at the aortic bifurcation. The strings utilized for aortic control were used to create a 4 cm long Rumel tourniquet. The abdominal wall was exposured and controlled with the exteriorization of the tourniquets at the top and bottom of the incision to allow for aortic occlusion throughout the experiment. In this model, the supraceliac aortic occlusion produced ischemia in all splanchnic organs and the striate muscle of the lower torso, while the aortic bifurcation occlusion produced ischemia in the striate muscle of the lower torso and the caudal portion of the large bowel (25). Aortic occlusion and flow restoration, which were necessary in some experimental groups, were confirmed by an abrupt rise and fall in the MAP. Heparin (100 IU/kg) was administered intravenously, and the animals were kept warm during the experiment.
Experimental design and study groups
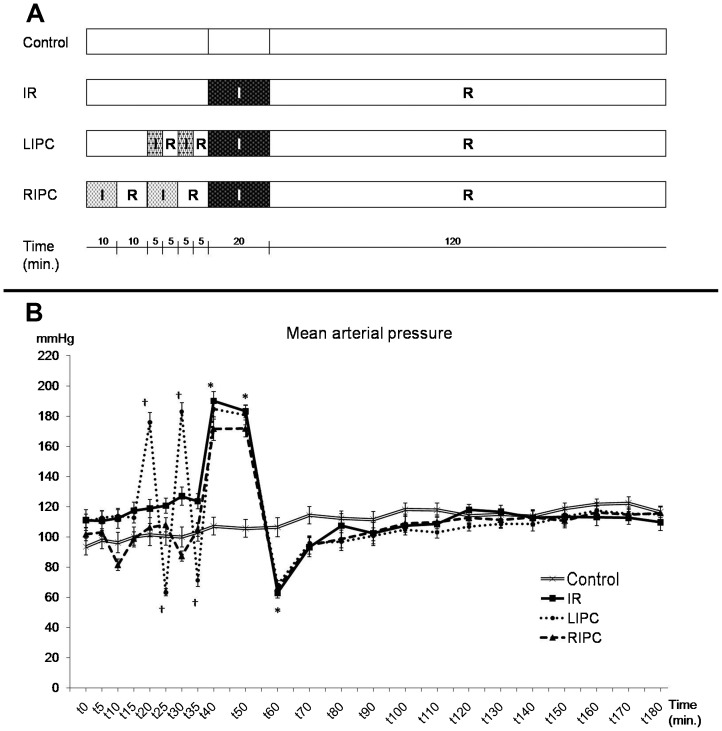
After identical initial surgical preparation and stabilization, the animals were allocated into four experimental groups according to the duration of ischemia and reperfusion. The control group was kept at rest during the entire experiment, without aortic occlusion. The IR (ischemia-reperfusion) group was submitted to 20 minutes of supraceliac aortic occlusion, followed by 120 minutes of reperfusion. The LIPC (local ischemic preconditioning) group was submitted to 2 cycles of supraceliac occlusion (5 minutes of ischemia and 5 minutes of reperfusion), followed by 20 minutes of supraceliac aortic occlusion and 120 minutes of reperfusion. The RIPC (remote ischemic preconditioning) group was submitted to 2 cycles of infrarenal occlusion (10 minutes of ischemia and 10 minutes of reperfusion), followed by 20 minutes of supraceliac aortic occlusion and 120 minutes of reperfusion (Figure 1A).
Figure 1.
Experimental protocol (A) and main arterial pressure variations during the experiment in the control, ischemia and reperfusion (IR), local ischemic preconditioning (LIPC), and remote ischemic preconditioning (RIPC) groups (B). Data are expressed as the mean ± SE for 7 animals per group. (*) p<0.001: IR, LIPC, and RIPC vs. control, (†) p<0.001: LIPC vs. control.
Hemodynamic stability at the time of supraceliac clamp opening (occluded for 20 minutes) was accomplished by an infusion of 1.5 mL of 0.9% saline. Subsequent doses of 0.5 mL of 0.9% saline were administered after 30, 60, 90, and 120 minutes of reperfusion. This volume expansion was provided at the same time point for all four groups. At the end of the experiment, the animals were exsanguinated by aortic puncture.
Blood and tissue sampling
Peripheral blood collected from the tail at the beginning and at the end of the experiment was used for hematocrit and leukogram determinations. Arterial blood samples for lactate and blood gas analysis were collected through the carotid catheter at surgical preparation and at the end of experiment. A blood sample for measuring lactic dehydrogenase (LDH) was collected directly by aortic puncture.
The bowel was stripped from its mesentery, and three samples of tissue were harvested from the proximal jejunum 3 cm distal to its origin, the middle portion of the small intestine, and the distal ileum 3 cm proximal to the cecum. Tissue was fixed in buffered 10% formalin for 24 hours and then embedded in paraffin wax. Sections of 5 μm were cut and stained with hematoxylin and eosin (HE). An independent pathologist blinded to the experimental group of the samples performed the histological analysis. Images were captured using a high-resolution Samsung camera coupled to a light Nikon E200 microscope and subsequently analyzed using AxioVision-Rel software (Zeiss). Total mucosal thickness, villous height, and crypt depth were evaluated. Each variable was measured three times for all three portions of the intestine, so the final value of a given variable for one specimen is the mean of these nine measurements.
Statistical analysis
All data are expressed as the mean ± standard error (SE). A paired t-test was used for repetitive measurements in the same group. Multiple comparisons between groups were performed using one-way ANOVA and post-hoc analysis with the Tukey test. The results were considered significant for p-values less than 0.05.
RESULTS
At baseline, there were no differences between groups regarding weight, MAP, or any other laboratory value (Table 1). The supraceliac aortic occlusion resulted in a significant MAP increase in all groups, while the aortic release significantly decreased MAP to values below the baseline or values at the corresponding time in the control group (Figure 1). Infrarenal aortic occlusion in the RIPC group did not increase MAP, and aortic release caused a transient but non-significant decrease in MAP (Figure 1).
Table 1.
Blood gas and hematologic profile, lactate, and lactate dehydrogenase levels.
| Control | IR | LIPC | RIPC | |||||
| t0 | t180 | t0 | t180 | t0 | t180 | t0 | t180 | |
| Hematocrit | 49.42%±1.84% | 49.28%±0.94% | 49.425±1.04% | 57.14%*†±1.20% | 49.71%±1.06% | 53.28%*±1.06% | 48.71%±0.96% | 52.28%*±1.04% |
| Total leucocyte (cells/mm3) | 12,685.7±963.5 | 16,664*±1,315.9 | 12,392±1,117.4 | 33,478*±4,296.6 | 13,435±786.7 | 29,278*±2,597.7 | 12,250±826.5 | 26,442*±3,500.4 |
| Polymorphonuclear leucocyte | 26.14%±2.28% | 68.57%*±6.65% | 20.42%±1.42% | 71.57%*±4.43% | 26.28%±3.15% | 75.42%*±2.77% | 22.42%±1.75% | 68.14%*±3.07% |
| Lymphocytes | 66.57%±2.25% | 28.28%*±6.28% | 71.71%±1.16% | 24.42%*±2.48% | 67.71%±3.41% | 20.85%*±2.48% | 69%±2.91% | 27.42%*±2.36% |
| Monocytes | 7%±0.72% | 2.71%*±0.47% | 7.42%±0.64% | 3.85%*±0.55% | 6%±0.69% | 3.42%*±0.71% | 7%±0.69% | 4.42%*±0.78% |
| pH | 7.387±0.015 | 7.375±0.017 | 7.425±0.019 | 7.280*†±0.035 | 7.406±0.014 | 7.330*±0.019 | 7.415±0.021 | 7.318*±0.024 |
| EB (mEq/L) | -2.21±0.77 | -6.25*±0.46 | -2.67±0.83 | -15.81*†±1.64 | -3.2±0.49 | -11.6*†±1.19 | -2.21±0.80 | -11.47*†±1.14 |
| pCO2 (mmHg) | 35.97±1.18 | 30.44*±1.70 | 31.45±0.94 | 19.98*†±1.27 | 32.57±1.84 | 21.61*†±0.89 | 33.62±1.57 | 22.62*†±1.61 |
| HCO3 (mEq/L) | 21.58±0.78 | 17.27*±0.44 | 20.25±0.55 | 9.3*†±0.95 | 20.1±0.71 | 11.77*†±0.59 | 21.07±0.59 | 11.8*†±0.80 |
| Lactate (mmol/L) | 1.18±0.08 | 1.65*±0.10 | 1.57±0.13 | 4.85*†±0.46 | 1.37±0.09 | 2.97*†§±0.25 | 1.3±0.13 | 3.24*†§±0.31 |
| LDH (U/L) | 1158±204 | 9460±977† | 6512±894†§ | 4812±1213†§ | ||||
The results of laboratory testing, summarized in Table 1, showed that there was an increase in hematocrit values at the end of the experiment in groups submitted to aortic occlusion, even in preconditioned animals (p≤0.036), compared with baseline values. Compared with the control group, this increase was higher in the IR (p = 0.015) group, but not in the LIPC (p = 0.208) or the RIPC (p = 0.443) groups. The total leukocyte counts at the end of the experiment were increased from baseline values in all groups (p≤0.004). Compared with the control group, leukocytosis was more pronounced in the IR group (p = 0.005) and less intense in the LIPC (p = 0.041) and RIPC (p = 0.149) groups. The percentage of polymorphonuclear (PMN) leukocytes increased at the end of the reperfusion compared with baseline values (p≤0.002) and increased similarly among all groups (p = 0.647). The results also showed decreases in the percentage of monocytes (p≤0.032) and lymphocytes (p≤0.002) in all groups at the end of the experiment.
The blood gas analysis showed that the pH was constant in the control group throughout the experiment but decreased in other groups (p<0.02). Arterial base excess (BE) decreased in all groups (p≤0.003). Compared with the control group, BE at the end of experiment was lower in the IR (p<0.001), LIPC (p = 0.020), and RIPC (p = 0.024) groups. The arterial partial pressure of CO2 and the HCO3 concentration also decreased during the experiment in all groups (p<0.04). Compared with the control group, both pCO2 and the HCO3 concentration at the end of experiment were lower in the IR, LIPC, and RIPC groups (p≤0.003).
Compared with the baseline, arterial lactate concentration increased in all groups (p<0.02) at the end of experiment. The increases in the IR, LIPC, and RIPC groups were higher than in the control group (p≤0.001), but the values were lower in the LIPC and RIPC groups than in the IR group (p<0.01). Compared with the control group, the LDH concentrations were higher in all three groups submitted to aortic occlusion. Compared with the IR group, the LDH concentrations were lower in the RIPC group (p = 0.007) but were not significantly different in the LIPC group (p = 0.129).
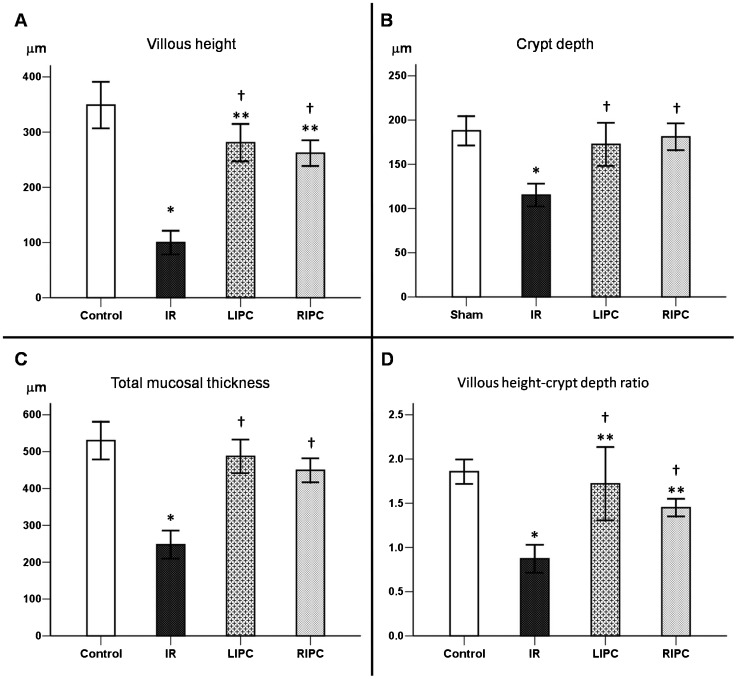
The histological analyses of the intestine showed a decrease in villous height in all groups submitted to aortic occlusion when compared with the control group (IR: p<0.001, LIPC: p = 0.013, and RIPC: p = 0.005) (Figure 2A). Crypt depth (Figure 2B) and total mucosal thickness (Figure 2C) were only reduced in the IR group (p<0.001). The villous height-crypt depth ratio was diminished in the IR, RIPC (p≤0.001), and LIPC (p<0.035) groups (Figure 2D). Compared with the IR group, the reductions in total mucosal thickness, villous height, crypt depth, and villous height-crypt depth ratio were lower in the LIPC and RIPC groups (p<0.001). Representative photomicrographs are presented in Figure 3.
Figure 2.
Histomorphometric analysis of the small bowel demonstrating villous height (A), crypt depth (B), total mucosal thickness (C), and villous height-crypt depth ratio (D) in the control, ischemia and reperfusion (IR), local ischemic preconditioning (LIPC), and remote ischemic preconditioning (RIPC) groups. Data are expressed as the mean ± SE for 7 animals per group. (*) p<0.001: IR vs. control, (**) p<0.05: LIPC and RIPC vs. control, (†) p<0.001: LIPC and RIPC vs. IR.
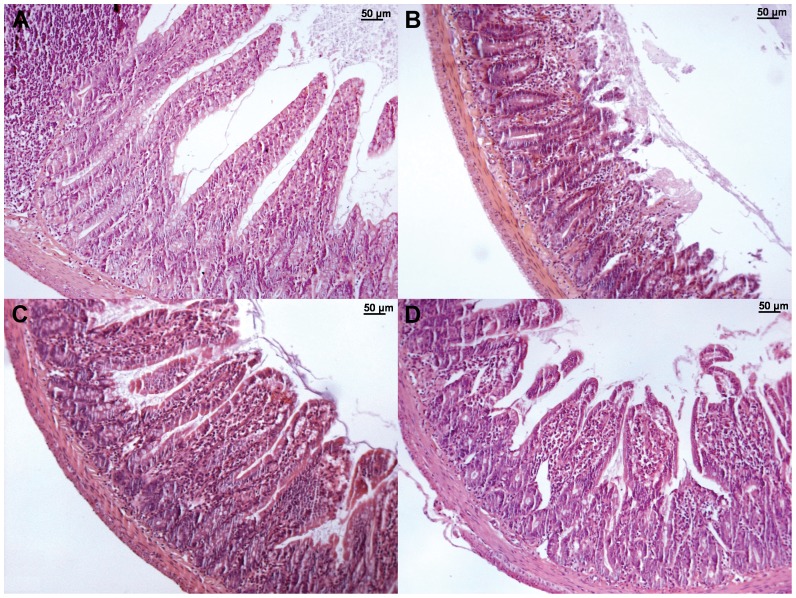
Figure 3.
Representative photomicrographs of the small bowel (HE, x100) from the control (A), IR (B), LIPC (C), and RIPC (D) groups. Normal small intestine architecture is shown in the control group (A). The IR group (B) demonstrates a marked loss of villus height, while the LIPC (C) and RIPC (D) groups have less intense findings.
DISCUSSION
This study indicates that local and remote IPC decreases the intestinal I/R injury resulting from supraceliac aortic clamping. The results of the LIPC group confirmed our hypothesis that the protection achieved in superior mesenteric artery (SMA) occlusion models of I/R and IPC could also apply to the total splanchnic I/R injury of aortic occlusion. The results of the RIPC group show that the method is also protective in this setting, extending the clinical applicability of IPC and strengthening the interest in this technique in aortic surgery.
This experiment mimicked the scenario of complex visceral aortic procedures. The redistribution of blood flow in the territories under ischemia (and also during reperfusion) in very different tissues with varying vascular bed resistance and tolerance to the I/R injury makes the local and systemic consequences of supraceliac aortic occlusion different from isolated SMA occlusion. This difference was demonstrated by the near 100% mortality rate in our pilot experiments after 1 h of reperfusion when supraceliac aortic clamping in excess of 20 minutes was tested, in contrast with the 90 minutes of SMA occlusion employed in some models (26,27). The duration and number of IPC cycles were chosen based on the most commonly published models (17,28), in accordance with the findings of our pilot experiments.
The intestine is very susceptible to I/R injury, and severe changes occur in the intestinal epithelium after this insult. We objectively measured and quantitatively assessed various components of the intestinal mucosa. This morphometric evaluation showed a reduction in mucosal thickness in the I/R groups. In accordance with other reports, this reduction was mainly attributable to the loss in villous height, with a relative sparing of the crypt depth (29,30). Our experiment also demonstrated that both forms of IPC consistently decrease the magnitude of the mucosal damage.
Laboratory parameters are useful clinical markers of advanced mesenteric ischemia. Lactate and LDH serum levels were also measured to evaluate the I/R injury and the effect of local IPC (31,32). We also observed these changes, with our results indicating markedly high levels of lactate and LDH in the IR group. The preconditioned groups had a reduced increase in the level of these variables, which is in agreement with the minor intestinal mucosal damage that occurred in these groups.
Arterial blood gas derangement and leukocytosis, which reflect an increased PMN count, demonstrated the systemic consequences of the I/R injury. Our results indicate that local and remote IPC can modulate the SIRS caused by supraceliac aortic occlusion. This phenomenon could be due to either the systemic anti-inflammatory effect of IPC or a reduced inflammatory response resulting from the halted visceral I/R injury after the IPC. Both mechanisms are likely causes and could take place simultaneously, although the definite protection mechanism of IPC is complex and still subject to debate (17,33), particularly with regard to a remote stimulus (34).
The first published clinical application of remote IPC in humans was in children undergoing cardiac surgery (35). Protection was achieved using four 5-minute cycles of lower limb ischemia using a blood pressure cuff. Using the same principle of cuff inflation, but with three 5-minute cycles of upper arm ischemia, remote IPC was tested in adult patients undergoing coronary artery bypass surgery. The primary outcome employed to assess myocardial injury was the “total area under the curve” troponin-T concentration during the 72-h postoperative period, and this value was significantly reduced by 43% in preconditioned subjects (36). The RIPC method also has a myocardial protective effect when used in percutaneous coronary intervention either for elective procedures (37) or for the management of acute ST-elevation myocardial infarctions (38).
In the field of aortic surgery, the first clinical investigation of RIPC was in open aneurysm repair. The authors applied two 10-minute cycles of ischemia by sequentially clamping the right and left common iliac arteries. Remote IPC reduces the incidence of myocardial injury, myocardial infarction, and renal impairment (14). Further studies showed that urinary biomarkers of renal injury are reduced with the RIPC method applied, which utilizes an inflatable tourniquet placed around the thigh, even during less invasive endovascular aneurysm repairs (39).
Our experimental protocol has some limitations. We used healthy rats, without the blood loss and fluid shifts that typically characterize aortic surgery in adults. Typically, cardiovascular, pulmonary, and renal dysfunction is present in these patients and contributes substantially to the high rate of observed complications. However, this well-standardized protocol allowed us to control the duration of ischemia and equally apply the method to all experimental groups. Another advantage of this animal model was the direct histopathological evaluation of mucosal injury, instead of using the substitutive outcomes that are usually used in human subjects.
In conclusion, this study demonstrates that IPC significantly reduces intestinal I/R injury in a clinically relevant model of supraceliac aortic clamping. The preconditioning stimulus is protective when applied locally or at remote sites. The low cost and simplicity of some forms of RIPC along with the favorable results of this practice highlight the need for further clinical and experimental studies to further elucidate the proper role of this promising strategy in aortic surgery.
ACKNOWLEDGMENTS
This work was supported by a grant from the Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP) 04/15964-6.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Poli de Figueiredo LF, Mathru M, Tao W, Solanki D, Uchida T, Kramer GC. Hemodynamic effects of isovolemic hemodilution during descending thoracic aortic cross clamping and lower torso reperfusion. Surgery. 1997;122(1):32–8. doi: 10.1016/s0039-6060(97)90261-0. [DOI] [PubMed] [Google Scholar]
- 2.Eide TO, Aasland J, Romundstad P, Stenseth R, Saether OD, Aadahl P, et al. Changes in hemodynamics and acid-base balance during cross-clamping of the descending thoracic aorta. A study in patients operated on for thoracic and thoracoabdominal aortic aneurysm. Eur Surg Res. 2005;37(6):330–4. doi: 10.1159/000090332. [DOI] [PubMed] [Google Scholar]
- 3.Cornet AD, Kingma SDK, Trof RJ, Wisselink W, Groeneveld ABJ. Hepatosplanchnic ischemia/reperfusion is a major determinant of lung vascular injury after aortic surgery. J Surg Res. 2009;157(1):48–54. doi: 10.1016/j.jss.2008.09.021. [DOI] [PubMed] [Google Scholar]
- 4.de Arruda MJC, Poggetti RS, Fontes B, Younes RN, Souza AL, Jr, Birolini D. Intestinal ischemia/reperfusion induces bronchial hyperreactivity and increases serum TNF-alpha in rats. Clinics. 2006;61(1):21–8. doi: 10.1590/s1807-59322006000100005. [DOI] [PubMed] [Google Scholar]
- 5.Zanoni FL, Benabou S, Greco KV, Moreno ACR, Cruz JWMC, Filgueira FP, et al. Mesenteric microcirculatory dysfunctions and translocation of indigenous bacteria in a rat model of strangulated small bowel obstruction. Clinics. 2009;64(9):911–9. doi: 10.1590/S1807-59322009000900013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Back MR, Bandyk M, Bradner M, Cuthbertson D, Johnson BL, Shames ML, et al. Critical analysis of outcome determinants affecting repair of intact aneurysms involving the visceral aorta. Ann Vasc Surg. 2005;19(5):648–56. doi: 10.1007/s10016-005-6843-3. [DOI] [PubMed] [Google Scholar]
- 7.Pasupathy S, Homer-Vanniasinkam S. Surgical implications of ischemic preconditioning. Arch Surg. 2005;140(4):405–9. doi: 10.1001/archsurg.140.4.405. discussion 410. [DOI] [PubMed] [Google Scholar]
- 8.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74(5):1124–36. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 9.Przyklenk K, Bauer B, Ovize M, Kloner RA, Whittaker P. Regional ischemic “preconditioning” protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation. 1993;87(3):893–9. doi: 10.1161/01.cir.87.3.893. [DOI] [PubMed] [Google Scholar]
- 10.Bolli R. Preconditioning: a paradigm shift in the biology of myocardial ischemia. Am J Physiol Heart Circ Physiol. 2007;292(1):H19–27. doi: 10.1152/ajpheart.00712.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaeschke H. Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am. J. Physiol. Gastrointest. Liver Physiol. 2003;284(1):G15–26. doi: 10.1152/ajpgi.00342.2002. [DOI] [PubMed] [Google Scholar]
- 12.Pasupathy S, Homer-Vanniasinkam S. Ischaemic preconditioning protects against ischaemia/reperfusion injury: emerging concepts. Eur J Vasc Endovasc Surg. 2005;29(2):106–15. doi: 10.1016/j.ejvs.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Eltzschig HK, Eckle T. Ischemia and reperfusion--from mechanism to translation. Nat Med. 2011;17(11):1391–401. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ali ZA, Callaghan CJ, Lim E, Ali AA, Nouraei SAR, Akthar AM, et al. Remote ischemic preconditioning reduces myocardial and renal injury after elective abdominal aortic aneurysm repair: a randomized controlled trial. Circulation. 2007;116(11 Suppl):I98–105. doi: 10.1161/circulationaha.106.679167. [DOI] [PubMed] [Google Scholar]
- 15.Hotter G, Closa D, Prados M, Fernández-Cruz L, Prats N, Gelpí E, et al. Intestinal preconditioning is mediated by a transient increase in nitric oxide. Biochem Biophys Res Commun. 1996;222(1):27–32. doi: 10.1006/bbrc.1996.0692. [DOI] [PubMed] [Google Scholar]
- 16.Neves J de S, Abrahão M de S, Salzedas Netto AA, Montero EF de S, Gonzalez AM. Effects of ischemic preconditioning associated to different preservation solutions in protecting the intestinal graft. Acta Cir Bras. 2011;26(5):396–403. doi: 10.1590/s0102-86502011000500013. [DOI] [PubMed] [Google Scholar]
- 17.Mallick IH, Yang W, Winslet MC, Seifalian AM. Ischemia-reperfusion injury of the intestine and protective strategies against injury. Dig Dis Sci. 2004;49(9):1359–77. doi: 10.1023/b:ddas.0000042232.98927.91. [DOI] [PubMed] [Google Scholar]
- 18.Tamion F, Richard V, Lacoume Y, Thuillez C. Intestinal preconditioning prevents systemic inflammatory response in hemorrhagic shock. Role of HO-1. Am J Physiol Gastrointest Liver Physiol. 2002;283(2):G408–414. doi: 10.1152/ajpgi.00348.2001. [DOI] [PubMed] [Google Scholar]
- 19.Wu B, Ootani A, Iwakiri R, Fujise T, Tsunada S, Toda S, et al. Ischemic preconditioning attenuates ischemia-reperfusion-induced mucosal apoptosis by inhibiting the mitochondria-dependent pathway in rat small intestine. Am J Physiol Gastrointest Liver Physiol. 2004;286(4):G580–7. doi: 10.1152/ajpgi.00335.2003. [DOI] [PubMed] [Google Scholar]
- 20.Welborn MB, 3rd, Douglas WG, Abouhamze Z, Auffenburg T, Abouhamze AS, Baumhofer J, et al. Visceral ischemia-reperfusion injury promotes tumor necrosis factor (TNF) and interleukin-1 (IL-1) dependent organ injury in the mouse. Shock. 1996;6(3):171–6. [PubMed] [Google Scholar]
- 21.Hess PJ, Seeger JM, Huber TS, Welborn MB, Martin TD, Harward TR, et al. Exogenously administered interleukin-10 decreases pulmonary neutrophil infiltration in a tumor necrosis factor-dependent murine model of acute visceral ischemia. J Vasc Surg. 1997;26(1):113–8. doi: 10.1016/s0741-5214(97)70154-x. [DOI] [PubMed] [Google Scholar]
- 22.Welborn MB, 3rd, Moldawer LL, Seeger JM, Minter RM, Huber TS. Role of endogenous interleukin-10 in local and distant organ injury after visceral ischemia-reperfusion. Shock. 2003;20(1):35–40. doi: 10.1097/01.SHK.0000071062.67193.b6. [DOI] [PubMed] [Google Scholar]
- 23.Erling N, Jr, Nakagawa NK, Costa Cruz JWM, Zanoni FL, Baptista-Silva JCC, Sannomiya P, et al. Microcirculatory effects of local and remote ischemic preconditioning in supraceliac aortic clamping. J Vasc Surg. 2010;52(5):1321–9. doi: 10.1016/j.jvs.2010.05.120. [DOI] [PubMed] [Google Scholar]
- 24.Moore-Olufemi SD, Kozar RA, Moore FA, Sato N, Hassoun HT, Cox CS, Jr, et al. Ischemic preconditioning protects against gut dysfunction and mucosal injury after ischemia/reperfusion injury. Shock. 2005;23(3):258–63. [PubMed] [Google Scholar]
- 25.DeSesso JM, Jacobson CF. Anatomical and physiological parameters affecting gastrointestinal absorption in humans and rats. Food Chem Toxicol. 2001;39(3):209–28. doi: 10.1016/s0278-6915(00)00136-8. [DOI] [PubMed] [Google Scholar]
- 26.Sola A, De Oca J, González R, Prats N, Roselló-Catafau J, Gelpí E, et al. Protective effect of ischemic preconditioning on cold preservation and reperfusion injury associated with rat intestinal transplantation. Ann Surg. 2001;234(1):98–106. doi: 10.1097/00000658-200107000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vlasov TD, Smirnov DA, Nutfullina GM. Preconditioning of the small intestine to ischemia in rats. Neurosci. Behav. Physiol. 2002;32(4):449–53. doi: 10.1023/a:1015896614819. [DOI] [PubMed] [Google Scholar]
- 28.Tapuria N, Kumar Y, Habib MM, Abu Amara M, Seifalian AM, Davidson BR. Remote ischemic preconditioning: a novel protective method from ischemia reperfusion injury--a review. J Surg Res. 2008;150(2):304–30. doi: 10.1016/j.jss.2007.12.747. [DOI] [PubMed] [Google Scholar]
- 29.Higa OH, Parra ER, Ab'Saber AM, Farhat C, Higa R, Capelozzi VL. Protective effects of ascorbic acid pretreatment in a rat model of intestinal ischemia-reperfusion injury: a histomorphometric study. Clinics. 2007;62(3):315–20. doi: 10.1590/s1807-59322007000300017. [DOI] [PubMed] [Google Scholar]
- 30.Harkin DW, D'Sa AA, Yassin MM, Hoper M, Halliday MI. Gut mucosal injury is attenuated by recombinant bactericidal/permeability-increasing protein in hind limb ischemia-reperfusion injury. Ann Vasc Surg. 2001;15(3):326–31. doi: 10.1007/s100160010087. [DOI] [PubMed] [Google Scholar]
- 31.Abrahão MS, Montero EFS, Junqueira VBC, Giavarotti L, Juliano Y, Fagundes DJ. Biochemical and morphological evaluation of ischemia-reperfusion injury in rat small bowel modulated by ischemic preconditioning. Transplant Proc. 2004;36(4):860–2. doi: 10.1016/j.transproceed.2004.03.046. [DOI] [PubMed] [Google Scholar]
- 32.Mallick IH, Yang W, Winslet MC, Seifalian AM. Ischaemic preconditioning improves microvascular perfusion and oxygenation following reperfusion injury of the intestine. Br J Surg. 2005;92(9):1169–76. doi: 10.1002/bjs.4988. [DOI] [PubMed] [Google Scholar]
- 33.Downey JM, Davis AM, Cohen MV. Signaling pathways in ischemic preconditioning. Heart Fail Rev. 2007;12(3-4):181–8. doi: 10.1007/s10741-007-9025-2. [DOI] [PubMed] [Google Scholar]
- 34.Kanoria S, Jalan R, Seifalian AM, Williams R, Davidson BR. Protocols and mechanisms for remote ischemic preconditioning: a novel method for reducing ischemia reperfusion injury. Transplantation. 2007;84(4):445–58. doi: 10.1097/01.tp.0000228235.55419.e8. [DOI] [PubMed] [Google Scholar]
- 35.Cheung MMH, Kharbanda RK, Konstantinov IE, Shimizu M, Frndova H, Li J, et al. Randomized controlled trial of the effects of remote ischemic preconditioning on children undergoing cardiac surgery: first clinical application in humans. J Am Coll Cardiol. 2006;47(11):2277–82. doi: 10.1016/j.jacc.2006.01.066. [DOI] [PubMed] [Google Scholar]
- 36.Hausenloy DJ, Mwamure PK, Venugopal V, Harris J, Barnard M, Grundy E, et al. Effect of remote ischaemic preconditioning on myocardial injury in patients undergoing coronary artery bypass graft surgery: a randomised controlled trial. Lancet. 2007;370(9587):575–9. doi: 10.1016/S0140-6736(07)61296-3. [DOI] [PubMed] [Google Scholar]
- 37.Hoole SP, Heck PM, Sharples L, Khan SN, Duehmke R, Densem CG, et al. Cardiac Remote Ischemic Preconditioning in Coronary Stenting (CRISP Stent) Study: a prospective, randomized control trial. Circulation. 2009;119(6):820–7. doi: 10.1161/CIRCULATIONAHA.108.809723. [DOI] [PubMed] [Google Scholar]
- 38.Bøtker HE, Kharbanda R, Schmidt MR, Bøttcher M, Kaltoft AK, Terkelsen CJ, et al. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet. 2010;375(9716):727–34. doi: 10.1016/S0140-6736(09)62001-8. [DOI] [PubMed] [Google Scholar]
- 39.Walsh SR, Boyle JR, Tang TY, Sadat U, Cooper DG, Lapsley M, et al. Remote ischemic preconditioning for renal and cardiac protection during endovascular aneurysm repair: a randomized controlled trial. J Endovasc Ther. 2009;16(6):680–9. doi: 10.1583/09-2817.1. [DOI] [PubMed] [Google Scholar]