Abstract
Introduction
Over the past three years numerous patents and patent applications have been published relating to scientific advances in the use of the green tea polyphenol epigallocatechin gallate (EGCG) (the most abundant, and bioactive compound in green tea) and its analogs as anticancer agents. EGCG affects multiple molecular targets involved in cancer cell proliferation and survival; however, polyphenolic catechins, such as EGCG, generally exhibit poor oral bioavailability. Since the anticancer activity of polyphenols largely depends on their susceptibility to biotransformation reactions, numerous EGCG derivatives, analogs and prodrugs have been designed to improve the stability, bioavailability and anticancer potency of the native compound.
Areas covered
This review focuses on the applications of EGCG and its analogs, derivatives and prodrugs in the prevention and treatment of human cancers. A comprehensive description of patents related to EGCG and its derivatives, analogs and prodrugs and their uses as anticancer agents is included.
Expert opinion
EGCG targets multiple essential survival proteins and pathways in human cancer cells. Because it is unstable physiologically, numerous alterations to the EGCG molecule have been patented, either to improve the integrity of the native compound or to generate a more stable yet similarly efficacious molecule. EGCG and its derivatives, analogs and prodrugs could be developed into future drugs for chemoprevention, chemosensitization, radiosensitization and/or cancer interception.
Keywords: apoptosis, biotransformation, combination therapies, EGCG, EGCG analogs, EGCG prodrugs, proteasome inhibitor, synthetic EGCG
1. Introduction
Cancer is a global health concern without geographical, racial or ethnic borders. In the United States, in 2012, it is predicted that 1.6 million people will receive a new cancer diagnosis and 577,000 deaths will occur due to cancer [1]. While chemotherapy is often the most effective cancer treatment, patient toxicity and drug-resistant tumor cells are common obstacles in achieving and maintaining a cancer-free status [2], especially in advanced disease [3,4]. For this reason, anticancer agents have evolved from chemotherapy that kills proliferating cells indiscriminately to molecular-targeting agents that inhibit or alter individual molecules to selectively and effectively destroy cancer cells.
Asian and islander populations demonstrate significantly lower cancer incidence and mortality compared to other populations. This seems to correlate with extensive dietary intake of green tea [5,6]. Since tea consumption is generally nontoxic, the cancer preventive and therapeutic benefits of tea compounds and components have been assessed in a number of in vitro and in vivo model systems.
The major components of green tea include the polyphenolic catechins (−)-epigallocatechin-3-gallate [(−)-EGCG], (−)-epigallocatechin [(−)-EGC], (−)-epicatechin-3-gallate [(−)-ECG] and (−)-epicatechin [(−)-EC] [7]. (−)-EGCG (Figure 1A) is the most abundant and biologically active anti-cancer polyphenol in green tea [8,9] due to its ability to affect and alter several cancer-related survival targets and pathways. (−)-EGCG inhibits the MAPK pathway and activator protein-1 (AP-1) activity in human colon cancer cells [10], PI3K in the mouse TRAMP model [11], angiogenesis through suppressing VEGF phosphorylation [12,13], and potentially, urokinase-plasminogen activator (uPA) activity in cancer cells [14]. Additionally, (−)-EGCG inhibits telomerase activity in mice bearing colon tumors [15], DNA methyltransferase in colon and prostate cancer cells [16] and dihydrofolate reductase (DHFR) in cancer cells [17]. Finally, ester bond-containing tea polyphenols (e.g., (−)-EGCG) potently and specifically inhibit the proteasomal chymotrypsin-like (β5) and PGPH-like (β1), but not trypsin-like (β2), activities of the proteasome [18]. These anticancer activities of (−)-EGCG reduce proliferation of tumor cells and the size of tumors.
Figure 1. GTPs and the chemotherapeutics used in combination with them.
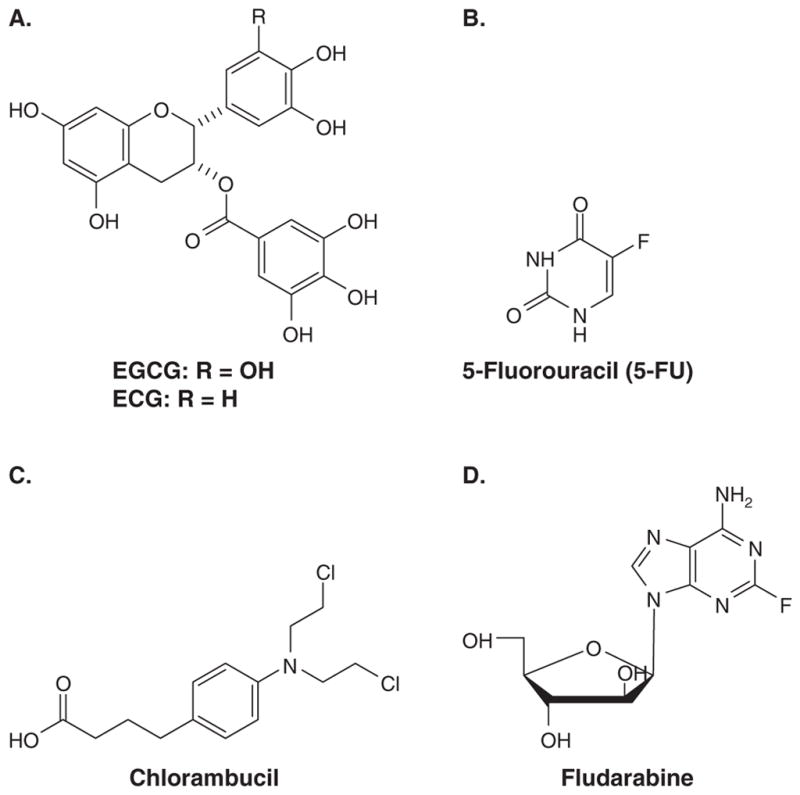
(A) EGCG and ECG, (B) 5-Fluorouracil, (C) Chlorambucil and (D) Fludarabine.
The numerous studies mentioned above describe the anti-cancer effects of nutritional compounds, such as (−)-EGCG. However, polyphenolic catechins generally exhibit poor oral bioavailability in vivo, possibly due to their inability to pass through the gut intact and into the circulation. Biochemically, the efficacy of ingested tea polyphenols may be limited due to conjugation of the free hydroxyl groups surrounding the molecules (Figure 1A) [19,20]. In fact, the green tea polyphenol (GTP) (−)-EGCG is relatively unstable under neutral or alkaline conditions and could be rapidly degraded, via deprotonation of hydroxyl (-OH) groups on the phenol rings [21]. Furthermore, these -OH groups can be modified through major biotransformation reactions, including methylation, glucuronidation and sulfonation, resulting in reduced biological activity [19]. Since the anticancer activity of polyphenols largely depends on their susceptibility to biotransformation reactions, numerous (−)-EGCG derivatives, analogs and prodrugs have been designed in order to improve the stability or enhance the activity of the native compound. The purpose of this review is to summarize the patent literature involving EGCG and its derivatives, analogs and prodrugs as potential anticancer agents. We searched the WIPO, EPO and USPTO databases with keywords such as ‘EGCG’, ‘gallate’ and ‘polyphenol’ in titles, abstracts and claims, with publication dates after 2009. US patent numbers are included in the citations in preference to WO patent numbers when possible. Equivalent patents and patent applications may exist in other jurisdictions. We were unable to review a small number of patents and patent applications that have been published in Japanese or Chinese. The patents and patent applications cited in this review are classified by: i) green tea extract containing EGCG, ii) EGCG alone, iii) EGCG in combination with another chemotherapeutic agent(s), iv) synthetically modified EGCG and v) ester derivatives and prodrugs of EGCG and other catechins.
2. Patents involving EGCG compounds filed during 2009 – 2012
2.1 Patents involving green tea extracts containing EGCG
A mixture of compounds that included at least 80% total catechins with at least 50% of the total catechins being EGCG was used to prevent the onset of prostate cancer in cultured cells, an animal model and clinical trial in persons diagnosed with a preneoplastic lesion (PIN) [22]. The patent summarizes data that was published separately [23,24]. The inventors treated three cell lines with EGCG: normal human prostate epithelia cells, immortalized cells stably transfected with SV40 (PNT1a) and human prostate cancer cells isolated from bone metastases (PC-3). PNT1a cells were more sensitive to treatment with EGCG than the other cell lines with regard to cell proliferation and apoptosis [23]. The data suggests that EGCG and catechins are particularly effective in inhibiting the initial phases of neoplastic transformation. In order to verify this hypothesis, the inventors treated transgenic TRAMP mice (the patent erroneously states rats) with a formulation of catechins extracted from green tea in drinking water [23]. The animal study showed an 80% reduction in the onset of neoplasia in the treated mice [23]. Furthermore, in a clinical trial, subjects with high-grade PIN were treated with a formulation containing 600 mg multicatechins or placebo, daily, for 1 year. The results showed that in treated subjects, no cases of prostate cancer were detected after 6 months from the beginning of treatment, whereas in subjects treated with placebo, the onset of prostate cancer was detected in 20% of patients after 6 months [24] and 30% after 1 year [22].
MediGene AG claimed a method of treating patients with precancerous lesions of the skin (actinic keratosis) using topical administration of a mixture of catechins from green tea extract [25]. The mixture contained as much as 20% (w/w) of polyphenol. Patients with actinic keratoses were treated using various schedules and treatment periods, but in the two cases described in this patent, the keratoses were no longer evident after 12 or 16 weeks of treatment. Of note, Polyphenon E™® (an ointment containing green tea extract) has been approved by the United States Food and Drug Administration (FDA) for topical application in the treatment of external genital warts and is marketed as Veregen™ by MediGene AG.
2.2 Patents on EGCG alone
The anticancer activities of EGCG are widely supported by empirical data from cell culture and animal models. EGCG and/or ECG (Figure 1A) were found to inhibit nucleoside diphosphate kinase-B (NDPK-B) [26] and to repress signaling of the Sonic Hedgehog pathway [27].
The NDPK-B enzyme functions to transfer ATP from various cellular compartments. The ATP that is generated from endothelial cells contributes to the regulation of angiogenesis – an event that is required for the growth of cancer cells and cancer metastasis [28,29]. NDPK-B that is generated by aggressive tumor cells may also enhance the metastatic properties of those cells [26]. Inhibition of NDPK-B activity by both EGCG and ECG (IC50 values of 150 μM and 170 μM, respectively) could therefore reduce angiogenesis to nascent tumors.
EGCG has been shown to impart antiproliferation effects on cancer cells through inhibition of the Hedgehog signaling pathway. There is ample evidence to suggest that proliferation and differentiation of cancer cells are controlled by Hedgehog signaling [30,] and one of the key effectors of Hedgehog signaling, the GLI1 gene, has been found to be upregulated in many tumors [31]. EGCG (25 μM) was able to suppress nearly 50% of the GLI1 gene expression in TRAMP-C2 mouse prostate cancer cells [27].
2.3 Patents involving EGCG in combination with another chemotherapeutic agent(s)
There exist a large number of patent applications claiming combinations of compounds including EGCG in methods of use to treat cancer. However, we have only found one issued United States Patent over the past three years involving such a combination. This involves the use of EGCG in treating CD33 positive neoplastic cells together with a cell-killing agent [32]. No human clinical trials have yet been conducted with pure EGCG alone or in combination with other chemotherapeutic agents. The following comments relate to a few patent applications claiming methods using EGCG in combination with other approaches to treat cancer.
2.3.1 EGCG in combination with 5-fluorouracil
The S-phase DNA damaging agent 5-fluorouracil (5-FU) (Figure 1B) is a well-established treatment for various cancers [33–35] yet numerous toxicities limit its ubiquitous use [36]. A treatment regimen consisting of 5-FU and of the tea polyphenol EGCG (Figure 1A) has been claimed for decreasing cell viability, altering the cell cycle and increasing the expression of the tumor suppressor protein p53 in various human cancer cell lines [37]. The ratio of the DNA damaging agent to the tea polyphenols is between 2 and 10%, preferably 4%. Treating the human liver cancer cell line Sk-Hep-1 with a combination of 5-FU (10 μM) and EGCG (50 – 500 μM) was more effective in reducing cell viability and inducing cell cycle arrest at the G1 phase than 5-FU (10 μM) or EGCG (50 – 500 μM) treatment alone. These claims are further supported by numerous other studies [38–40].
2.3.2 EGCG in combination with a purine nucleoside analog and an alkylating agent
Alkylating agents and purine nucleoside analogs both essentially damage DNA to limit replication of cells. However, toxicities are common with both agents used singly and in combination [41]. A method of treating B-cell chronic lymphocytic leukemia (B-CLL) using EGCG in combination with a purine nucleoside analog (e.g., fludarabine (Figure 1D)) and/or an alkylating agent (e.g., chlorambucil (Figure 1C)) has been described [42]. B-CLL cells were harvested from patients and treated with EGCG, chlorambucil and fludarabine either singly, or with a combination of two or all three agents. The combination index [43] showed that the combination of chlorambucil and EGCG exhibited an additive effect on the decreased viability of B-CLL cells tested, whereas the combination of fludarabine and EGCG produced a mixed effect (additive and synergistic). The combination of fludarabine/chlorambucil/EGCG had a synergistic effect on the decreased viability of B-CLL cells. The ranges of concentrations tested were 0 – 1.0 μM for fludarabine, 0 – 20 μM for chlorambucil and 0 – 200 μM for EGCG. The in vitro data suggested that a dose of 1:20 fludarabine/chlorambucil (μM) with 4 μM EGCG was effective to reduce B-CLL cell viability to below 10%.
2.3.3 EGCG in combination with a DNA vaccine
Immunotherapy has been shown to be promising for the treatment of a number of tumors and hyperproliferative diseases. In particular, DNA vaccine systems for human papillomavirus (HPV)-associated cervical neoplasia and HPV-associated head and neck cancers have been developed. However, the utility of the immunotherapy approach is limited in situations where the tumor is relatively large or rapidly growing [44]. Through a multimodality treatment regimen that combines immunotherapy, such as DNA vaccination, with an apoptosis-inducing agent, such as EGCG, this limitation can be overcome. EGCG may be administered orally before the first dose of the DNA vaccine. In mice bearing TC-1-derived lung tumors, a great decrease in tumor burden was shown when treated with 0.5 mg/ml EGCG in combination with a pcDNA3-Sig/E7/LAMP-1 DNA vaccine [44,45]. Furthermore, the number of E7-specific IFN-γ-secreting CD8+ T cells was also increased [44], indicating an antitumor response [46].
2.4 Patents on synthetically modified EGCG
2.4.1 Synthetic oligomers of EGCG
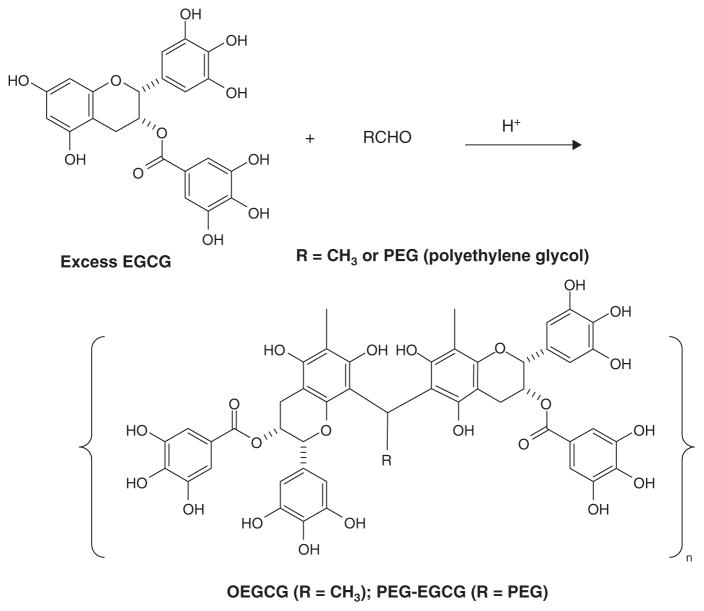
In order to better deliver high doses of EGCG (or other flavonoids) to tumor cells within a tissue, conversion to oligomers by conjugation with aldehydes (Scheme 1) can be achieved. For example, coupling of excess EGCG with acetaldehyde in acetic acid/water/DMSO or ethanol, gives oligomeric epigallocatechin gallate (OEGCG) that has an estimated molecular weight of 4000 after acetylation and Mw/Mn = 1.2 [47]. Similarly, aldehyde-terminated polyethylene glycol (PEG-CHO, Mw = 5000, 0.35 g) can be coupled with EGCG (0.65 g) to give PEG-EGCG (Mw = 7900, Mw/Mn = 1.2). PEG-OEGCG (Mw = 10100, Mw/Mn = 1.1) can be obtained from a similar conjugation of PEG-CHO with OEGCG. Finally, the aldehyde can be aldehyde-derivatized hyaluronic acid.
Scheme 1.
Condensation of EGCG with aldehydes to give OEGCG or PEG-EGCG.
A two-step conjugation similar to PEG-OEGCG leads to a micellar nanocomplex with the flavonoid-rich OEGCG in the central core and the PEG on the external core. These micellar nanocomplexes may be used to deliver high doses of the flavonoids, such as EGCG, and may also be used to deliver an additional bioactive agent [47].
In a subsequent patent application, it is claimed that a flavonoid such as EGCG can be used as a delivery agent for anticancer compounds [48]. In fact, OEGCG may be coupled to an anticancer agent that is protein, nucleic acid, small molecule or drug, to generate a micellar nanocomplex with PEG-EGCG as an external core. These anticancer agents conjugated to OEGCG are not effective when the anticancer agent is assembled in the micellar nanocomplex, but their activity is unmasked when the anticancer agent is released from the micellar nanocomplex. An example that was demonstrated in the patent application was the use of Herceptin® (trastuzumab)-loaded micellar nanocomplex. Herceptin® is a humanized monoclonal antibody against the HER2/neu receptor that induces regression of HER2-overexpressing metastatic breast cancer tumors. It was demonstrated that OEGCG combined with Herceptin® in the PEG-EGCG micellar nanocomplex reduced cell proliferation of SK-BR-3 mammary adenocarcinoma cells compared to OECG alone or Herceptin® alone [48].
2.4.2 Synthetic EGCG derivatives
The proteasome is a multicatalytic, multisubunit protease complex responsible for the vast majority of protein degradation, biological homeostasis and the regulation of numerous cellular processes in both normal and cancer cells. It has been found that naturally occurring (−)-EGCG inhibits the chymotrypsin-like activity of the proteasome in vitro (IC50 = 86 – 194 nM) and in vivo (IC50 = 1 – 10 μM) [18]. Furthermore, an ester bond within EGCG is critical for its ability to inhibit proteasome activity effectively and to act as an anticancer agent [18]. Additional options for enhanced proteasome inhibition warranted synthesis of GTP enantiomers.
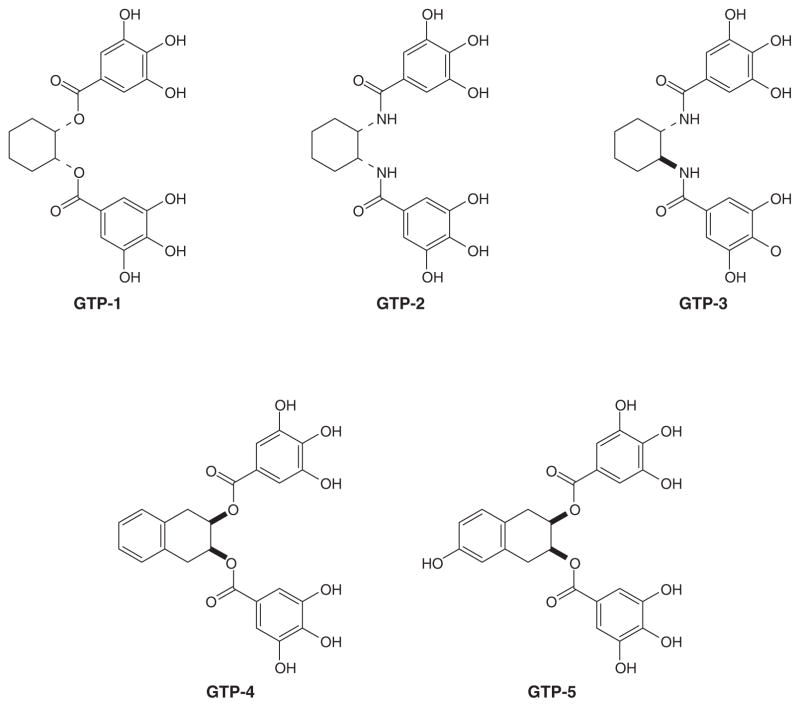
The naturally occurring polyphenols extracted from green tea are (−)-EGCG, (−)-ECG (Figure 1A) and their trans-epimers are (−)-GCG and (−)-CG (Figure 2A). The unnatural enantiomers of these GTPs, (+)-EGCG and (+)-GCG have been synthesized (Figure 2) [49]. Surprisingly, the unnatural enantiomers are more or equally potent as the natural GTPs in their ability to inhibit the proteasome (Table 1). Similarly, the (−)-EGCG-amide and the (+)-EGCG-amide (Figure 2B) display nearly the same IC50 against proteasome activity (Table 1) [49]. The IC50 values are in the submicromolar range and are within the plasma concentrations of GTPs after green tea consumption [50]. Furthermore, because the natural and the unnatural enantiomers show nearly the same IC50s, it suggest that the active site of the chymotrypsin-like, β5 subunit of the proteasome may interact with GTPs in a pseudosymmetric fashion.
Figure 2. Synthetic EGCG derivatives.
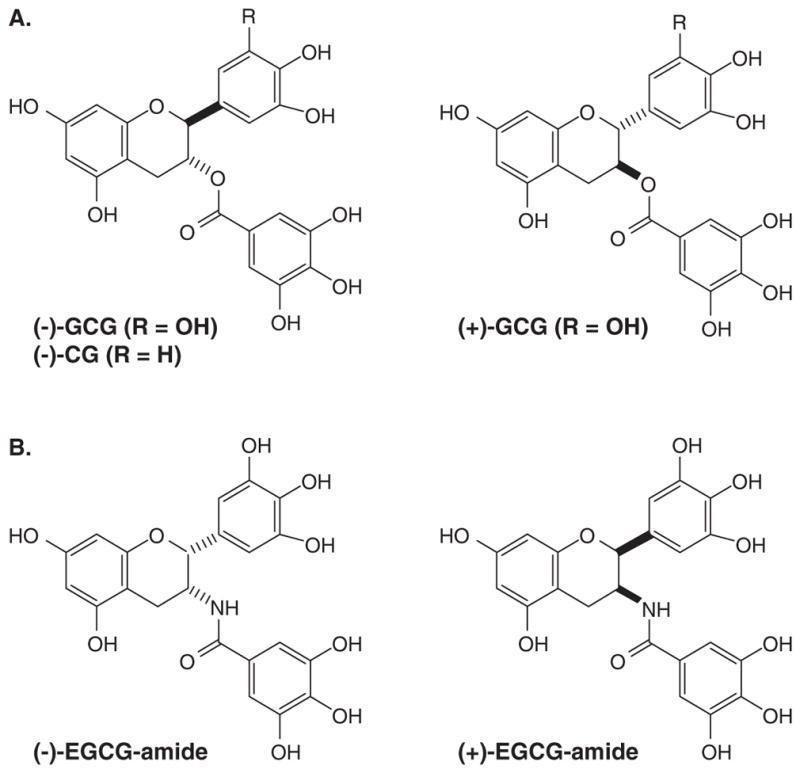
(A) The trans-epimers of naturally occurring tea polyphenols [(−)-GCG and (−)-CG], (B) EGCG-amides.
Table 1.
IC50 of the 20S eukaryotic proteasome by selected compounds.
| Compound | IC50 | Compound | IC50 |
|---|---|---|---|
| (−)-EGCG | 0.205 μM | GTP-1 | 1.9 μM |
| (+)-EGCG | 0.170 μM | GTP-2 | 2.5 μM |
| (−)-GCG | 0.610 μM | GTP-3 | 1.5 μM |
| (+)-GCG | 0.270 μM | GTP-4 | 0.34 μM |
| (−)-EGCG-amide | 0.320 μM | GTP-5 | 0.73 μM |
| (+)-EGCG-amide | 0.405 μM |
2.4.3 Synthetic EGCG analogs
Based on the assumption that the active site is pseudosymmetric, a series of synthetic EGCG analogs have been synthesized [49,51,52] (GTP-1 to -5, Figure 3). Their IC50 values in proteasome inhibition were determined (Table 1) and found to be comparable to natural GTPs. In particular, GTP-4 showed a similar proteasome inhibitory potency as (−)-EGCG. These synthetic polyphenol compounds potently inhibited proteasomal chymotrypsin-like activity in different human cancer cells as measured by Western-blot and activity assays. These synthetic compounds also inhibited colony formation and induced cell cycle arrest at the G1 phase in human cancer cells.
Figure 3. Synthetic EGCG analogs.
GTP-1 to -5.
Under physiological conditions, biotransformation reactions, such as methylation, can modify GTPs and therefore limit their in vivo anticancer and cancer-preventive activities. We hypothesized that methylated GTPs decreased proteasome-inhibitory abilities [50]. To test this hypothesis, methylated (−)-EGCG and (−)-ECG analogs that can be found in vivo were synthesized and studied for their structure–activity relationships (SARs) using a purified 20S proteasome. The addition of a single methyl group on (−)-EGCG or (−)-ECG led to decreased proteasome inhibition and, as the number of methyl groups increased, the inhibitory potencies further decreased. These SARs were supported by our findings from in silico docking analysis. Peracetate-protected forms of methylated GTPs were also tested in intact Jurkat T cells to observe the intracellular effects of methylation. Peracetate-protected, monomethylated (−)-EGCG induced greater cellular proteasome inhibition and apoptosis than did peracetate-protected, trimethylated (−)-EGCG, consistent with the potencies of the parent methylated analogs against a purified 20S proteasome. Therefore, methylation on GTPs, under physiological conditions, could decrease their proteasome-inhibitory activity, contributing to decreased cancer-preventive effects of tea consumption [50].
The 1,2-benzenediol structure of natural EGCG allows it to be a substrate of the human enzyme catechol-O-methyl-transferase (COMT), and the methylated metabolites of EGCG were found to be much less active in proteasome inhibition [53,54]. Therefore, EGCG analogs have been synthesized which do not have the 1,2-benzenediol structure, but a 1,3-benzenediol or other structure (Figure 3).
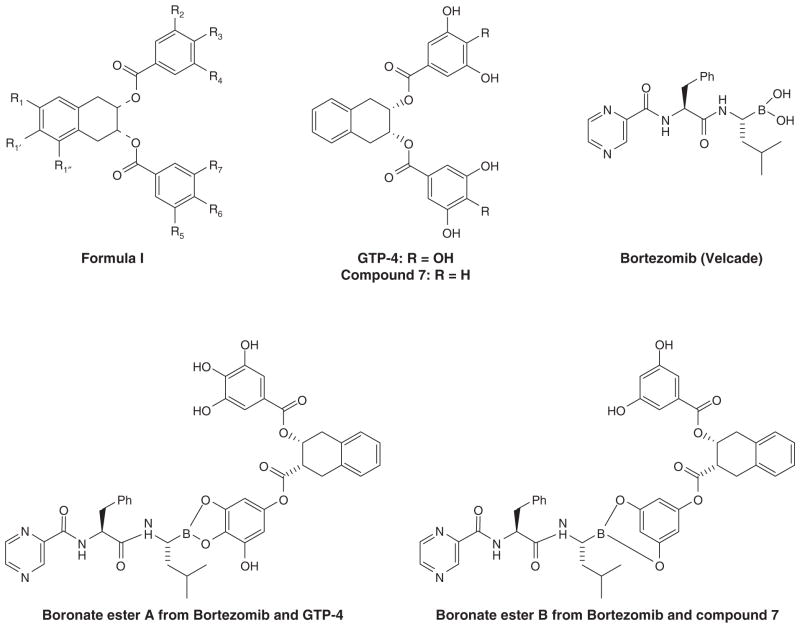
EGCG alone possesses anticancer effects and can also enhance therapeutic effects of other anticancer drugs such as 5-FU, chlorambucil and fludarabine. Unexpectedly, it was found that EGCG antagonized the antitumor effect of bortezomib (Velcade®) [55], which is the first proteasome inhibitor approved by the FDA for the treatment of cancer. EGCG could effectively prevent tumor cell death induced by bortezomib in preclinical in vitro and in vivo model systems. EGCG inhibited the antiproliferative effects of bortezomib in cell lines, prevented bortezomib from inhibiting the proteasome and inducing caspase-7 cleavage and protected xenografts from proapoptotic effects [55]. A possible mechanism for this phenomenon involves a direct chemical interaction between EGCG and bortezomib. The likely chemical interaction is the formation of a cyclic boronate ester from the 1,2-benzenediol structure of EGCG and the boronic acid structure of bortezomib (Figure 4).
Figure 4. Interactions of synthetic EGCG analog GTP-4, but not compound 7 with bortezomib.
GTP-4 has the 3,4,5-trihydroxy substitution in the benzoate moiety whereas compound 7 has the 3,5-dihydroxy substitution thus lacking an ortho-catechol structural feature. GTP-4 could therefore easily interact with bortezomib, forming a boronate ester A, resulting in inhibition of bortezomib’s activity. However, compound 7 is difficult to form a boronate ester B with bortezomib because of the geometrical constraint, resulting in a synergetic effect when both are used together.
Another series of EGCG analogs have been synthesized based on the general formula I, where R1, R1′ and R1″ are each independently selected from the group of H, alkyl and aryl and R2 to R7 are each independently H, alkyl, halogen, OH, acyloxy, amino, etc. (Figure 4) [56]. Of particular interest is the comparison of GTP-4 with compound 7. These compounds differ only in the 3,4,5-trihydroxy substitution in the benzoate moiety (GTP-4) versus the 3,5-dihydroxy substitution (compound 7) thus lacking an ortho-catechol structural feature. When human multiple myeloma ARP cells or OPM1 cells were treated with GTP-4 or compound 7 alone or in combination with bortezomib, the effect of GTP-4 appeared to block the effect of bortezomib on the proliferation of the cells. On the other hand, the combination of compound 7 with bortezomib appeared to synergistically inhibit cell proliferation of the cancer cells. The difference in the combined effect of GTP-4 or compound 7 with bortezomib may be attributed to the ease of formation of GTP-4 with bortezomib to form a boronate ester A. In contrast, because of the geometrical constraint, compound 7 is believed to not form the boronate ester B with bortezomib (Figure 4) [57]. The results from these EGCG analogs clearly showed that the analog without the 1,2-benzenediol structure (compound 7) was able to enhance the cytotoxicity of bortezomib against human multiple myeloma cells. This was attributed to the 1,3-benzenediol structure and its inability to form a cyclic boronate ester with bortezomib. Meanwhile, compound 7 was insensitive to modification by COMT, and its structure contributed to its synergistic effect with bortezomib on human multiple myeloma cells.
Synthetic EGCG analogs likely have multiple molecular targets in cancer cells and AMP-activated protein kinase (AMPK) would be one of them. AMPK is a critical monitor of cellular energy status and also controls processes related to tumor development, including cell cycle progression, protein synthesis, cell growth and survival. Therefore AMPK as an anticancer target has received great attention recently. It has been reported that metformin, an antidiabetic drug, could inhibit tumor cell growth in vitro and in vivo [58–60], and could also selectively kill cancer stem cells [61]. Our group reported that synthetic EGCG analogs (Figure 4, Formula I, R1=R1′=R1″=R2=R4=R5=R7=H, R3=R6=NH2 [56]) were more potent AMPK activators compared with metformin [62]. Activation of AMPK by these EGCG analogs resulted in inhibition of cell proliferation, upregulation of the p53 – p21 axis, downregulation of mTOR pathway, and suppression of the stem cell population in human breast cancer cells [62].
2.5 Patents involving ester derivatives and prodrugs of EGCG and other catechins
Patents have been issued relating to EGCG derivatives and prodrugs for inhibiting the proteasome, a proven molecular target for cancer chemotherapy [63,64]. The ubiquitin-proteasome system is an important molecular target for the bioactive flavonoid, EGCG, which is an irreversible, mechanism-based, inhibitor of the chymotrypsin-like activity of the proteasome [18] and a docking model to describe this interaction has been established [65]. However, it is well recognized that EGCG displays poor bioavailability in vivo [66]. For example, following intragastric administration of decaffeinated green tea to mice, the absolute plasma bioavailability of EGCG, EGC and EC was 0.1, 14 and 31%, respectively. The pharmacokinetics of tea polyphenols in humans has also been reported [50,67,68]. Oral administration of green tea at a dose of 20 mg/kg body weight resulted in a plasma Cmax of 78 ng/ml for EGCG, a concentration far below the micromolar concentration usually required for the in vitro activity reported for GTPs.
Drug design efforts to increase the bioavailability of EGCG have resulted in the design of an EGCG prodrug, as well as prodrugs of EGCG analogs [63,64]. These issued patents claim the octaacetate of (−)-EGCG (Pro-EGCG, Figure 5A). Pro-EGCG was found to be much more stable than EGCG in a physiological solution of pH = 8 and was converted intracellularly into EGCG, presumably by cellular esterases. In a cellular environment, these compounds were metabolized to release the native EGCG molecule, inhibit proteasome chymotrypsin-like activity and result in potent antiproliferative and proapoptotic effects. In fact, when cultured human breast cancer MDA-MB-231 cells were treated with Pro-EGCG, accumulation of both Pro-EGCG and EGCG were found inside the cells [69]. Furthermore, intragastric administration of Pro-EGCG to CF-1 mice led to higher bioavailability in plasma, small intestine and colon compared with administration of equimolar doses of EGCG [70]. Even though Pro-EGCG is not an inhibitor of the proteasome in cell-free system, Pro-EGCG is more potent than EGCG at inhibiting the proteasomal chymotrypsin-like activity in MDA-MB-231 cells [69], presumably because Pro-EGCG converts to EGCG intracellularly. In mouse xenograft models, Pro-EGCG was found to be more effective than EGCG in inhibiting tumor growth of MDA-MB-231 breast tumors [69] and CWR22R androgen-independent prostate cancer [71]. Pro-EGCG has also been demonstrated to be more potent than EGCG in preventing colon tumorigenesis in a mouse azoxymethane and dextran sulfate sodium-induced model [72].
Figure 5. Ester derivatives and prodrugs of EGCG.
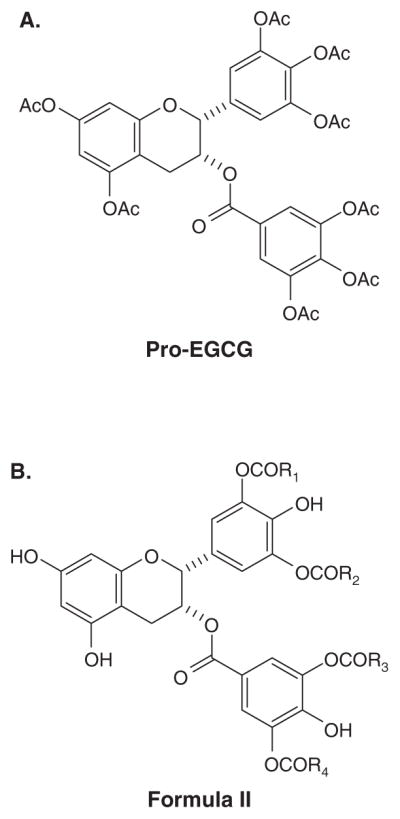
(A) Pro-EGCG, (B) Formula II of EGCG esterified with long chain fatty acids.
The biological activity of Pro-EGCG has been studied most extensively. We have also demonstrated that Pro-EGCG can synergize with currently used cancer chemotherapeutic agents to reduce cell proliferation and increase apoptosis in human cancer cell lines (data unpublished). Pro-EGCG appears to demonstrate advantages over other proteasome inhibitors, thus providing the foundation for future clinical strategies.
In another effort to increase the bioavailability of EGCG, the esterification of EGCG with fatty acids has been developed. In this patent application, compounds of formula II (Figure 5B) were claimed where R1, R2, R3 and R4 are saturated or unsaturated long chain fatty acids [73]. These esterified EGCG compounds were soluble in oil and emulsion systems and were claimed to improve cellular absorption.
3. Conclusions
The health-beneficial effects of green tea and its main constituent EGCG are widely supported by results from epidemiological, cell culture, animal and clinical studies. This review summarizes the patent literature (2009 – present) relating to EGCG and its analogs, derivatives and prodrugs as potential anticancer agents, and discusses recent advances on the biological effects and potential cancer-related molecular targets of these compounds. It is clear that EGCG and some of its analogs effect multiple essential survival proteins and pathways in human cancer cells (Table 2). However, biotransformation reactions (such as methylation) under physiological conditions could affect the biological activity and cancer-preventive effects of EGCG and GTPs.
Table 2.
Summary of different cancer cell lines inhibited by (−)-EGCG and its analogs.
| Compounds | Cell lines | Cancer type | Comments | Ref. |
|---|---|---|---|---|
| (−)-EGCG | Caco2 | Colon | Inhibition of MAPK pathway and activator protein-1 | [10] |
| HCT116 | Colon | |||
| HT29 | Colon | |||
| SW480 | Colon | |||
| SW837 | Colon | |||
| U937 | Leukemia | Telomerase inhibition | [15] | |
| HT29 | Colon | Telomerase inhibition | ||
| KYSE 510 | Esophageal | DNA methyltransferase | [16] | |
| HT29 | Colon | DNA methyltransferase | ||
| PC-3 | Prostate | DNA methyltransferase | ||
| LNCaP, PC-3 | Prostate | Proteasome inhibition | [18] | |
| MDA-MB-435 | Breast | Inhibition of NDPK-B | [26] | |
| GTP-4 analog | LNCaP | Prostate | Proteasome inhibition | [51] |
| Compound-7 analog | ARP, OPM1 | Multiple myeloma | Inhibiting cell proliferation | [56,57] |
| EGCG analog 4 | MDA-MB-231 | Breast | Activating AMPK pathway | [56,62] |
| EGCG analog 6 | MDA-MB-231 | Breast | Activating AMPK pathway | [56,62] |
| Pro-EGCG | MDA-MB-231 | Breast | Proteasome inhibition | [63,64] |
| Pro-EGCG | CWR22R | Prostate | Proteasome inhibition | [71] |
4. Expert opinion
An EGCG prodrug has been developed to improve the stability of EGCG under physiological conditions [63,64]. The peracetate-protected or prodrug form of EGCG increases the bioavailability, stability and proteasome-inhibitory and anticancer potency of EGCG in numerous cancer cell culture models and several mouse models, suggesting its potential use for cancer prevention and treatment. In comparison, another notable patent describes the protection of EGCG (linked to other chemotherapeutic agents) in a micellar nanocomplex [47]. These two approaches are of particular importance because they eloquently address the most serious problem with EGCG as a therapeutic agent, its bioavailability and instability in vivo. Lastly, the esterification of EGCG with fatty acids [73] to increase its solubility in oil and permeability into cells is notable. However, prior work relating to this approach needs to be considered [74,75].
One relevant concern of many of the studies described herein pertains to the very high concentrations of EGCG and polyphenols used in observing a significant biological effect. In fact, the IC50 for the inhibition of NDPK-B activity was reported to be 150 and 170 μM for EGCG and ECG, respectively [26]. Such an IC50 concentration is far higher than the normal plasma concentration of EGCG of less than 1 μM after green tea consumption [68]. The anticancer effects observed after treatment with EGCG (50 – 500 μM) in combination with 5-FU were very high and nonphysiological [37]. Furthermore, a surprising and unexplained finding was that an additive effect was observed with lower concentration of EGCG, while the highest EGCG concentrations appeared to effectively reduce cell viability with very little enhancement from 5 FU [37]. Lastly, EGCG used in combination with a DNA vaccine, was given orally at a very high dose of ~109 μM [44]. Accordingly, patents or patent applications based on in vitro data resulting from the use of very high, nonphysiological concentrations of EGCG are unlikely to result in clinical or commercial applications.
Another interesting patent application described the use of EGCG in combination with chemotherapeutic agents such as a purine nucleoside analog (e.g., fludarabine) and/or an alkylating agent (e.g., chlorambucil) [42]. While the concentration of EGCG tested was as high as 200 μM, it was found to be effective at much lower doses as well (4 μM). Although this is still above a physiologically relevant concentration, it is not nearly as high as several of the other patents or patent applications involving the use of EGCG. It is not yet known, whether EGCG or its analogs, derivatives or prodrugs will find clinical use as a monotherapy or in combination with other agents to treat cancer. Combination treatments may not always result in synergies (e.g., with bortezomib). However, based on the scientific literature, further investigations are warranted.
Initial clinical studies involving green tea extracts in treating patients with high-grade prostatic intraepithelial neoplasia [76] or early stage chronic lymphocytic leukemia [77] have demonstrated encouraging biological responses without significant toxicities. However, the poor oral bioavailability of EGCG, patient compliance (the need to take many pills) and lack of differentiation between many green tea extracts available on the market, will likely limit the further development of oral green tea extracts to treat cancer. In general, strong patent positions are required in order to justify the significant expenditures associated with clinical development. EGCG itself is not protected by patent composition of matter claims, and although it has been studied in numerous animal models, pure EGCG has not yet been studied in cancer patients. EGCG derivatives, analogs or prodrugs with patent protection and improved bioavailability and potency have started to emerge over the past 8 years and represent a more commercially viable approach to exploiting the considerable health benefits demonstrated in the large body of scientific literature surrounding the use of green tea, green tea extracts and EGCG. In the case of cancer, we expect future studies involving EGCG derivatives, analogs and prodrugs to expand their foundation for clinical application as chemopreventive compounds, monotherapy in cancer interception strategies or in combination with other agents or modalities in chemosensitization or radiosensitization strategies.
Article highlights.
EGCG affects multiple molecular targets involved in cancer cell proliferation and survival.
EGCG exhibits poor oral bioavailability due to poor absorption and biotransformation reactions.
Numerous alterations to the EGCG molecule have been patented either to improve the integrity of the native compound or to generate a more stable yet similarly efficacious molecule.
EGCG and its derivatives, analogs and prodrugs could be developed into future drugs for chemoprevention, chemosensitization, radiosensitization and/or cancer interception.
This box summarizes key points contained in the article.
Acknowledgments
Declaration of interest
K Landis-Piwowar is the co-inventor of one of the patents mentioned in the review and has received support from the American Society of Clinical Laboratory Sciences. R Foldes is President of Cognovie Inc., D Chen, T-H Chan & QP Dou are the inventors of several patents cited in the review, patents that have been optioned by Cognovie, Inc., Canada. T-H Chan has received support from The Hong Kong Research Grants Council; The Natural Science and Engineering Research Council of Canada. QP Dou has received support from the National Cancer Institute at the National Institutes of Health (5R01CA127258-05, 1R01CA120009 and 3R01CA120009-04S1).
Contributor Information
Kristin Landis-Piwowar, Email: landispi@oakland.edu.
Qing Ping Dou, Email: doup@karmanos.org.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Landis-Piwowar KR, Milacic V, Chen D, et al. The proteasome as a potential target for novel anticancer drugs and chemosensitizers. Drug Resist Updat. 2006;9:263–73. doi: 10.1016/j.drup.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Bendell J, Goldberg RM. Targeted agents in the treatment of pancreatic cancer: history and lessons learned. Curr Opin Oncol. 2007;19:390–5. doi: 10.1097/CCO.0b013e32816f76f0. [DOI] [PubMed] [Google Scholar]
- 4.Carver JR, Shapiro CL, Ng A, et al. American Society of Clinical Oncology clinical evidence review on the ongoing care of adult cancer survivors: cardiac and pulmonary late effects. J Clin Oncol. 2007;25:3991–4008. doi: 10.1200/JCO.2007.10.9777. [DOI] [PubMed] [Google Scholar]
- 5.Sasazuki S, Tamakoshi A, Matsuo K, et al. Green tea consumption and gastric cancer risk: an evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Jpn J Clin Oncol. 2012;42:335–46. doi: 10.1093/jjco/hys009. [DOI] [PubMed] [Google Scholar]
- 6.Yang G, Zheng W, Xiang YB, et al. Green tea consumption and colorectal cancer risk: a report from the Shanghai Men’s Health Study. Carcinogenesis. 2011;32:1684–8. doi: 10.1093/carcin/bgr186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta S, Hussain T, Mukhtar H. Molecular pathway for (−)-epigallocatechin-3-gallate-induced cell cycle arrest and apoptosis of human prostate carcinoma cells. Arch Biochem Biophys. 2003;410:177–85. doi: 10.1016/s0003-9861(02)00668-9. [DOI] [PubMed] [Google Scholar]
- 8.Kemberling JK, Hampton JA, Keck RW, et al. Inhibition of bladder tumor growth by the green tea derivative epigallocatechin-3-gallate. J Urol. 2003;170:773–6. doi: 10.1097/01.ju.0000081278.64511.96. [DOI] [PubMed] [Google Scholar]
- 9.Shimizu M, Deguchi A, Lim JT, et al. (−)-Epigallocatechin gallate and polyphenon E inhibit growth and activation of the epidermal growth factor receptor and human epidermal growth factor receptor-2 signaling pathways in human colon cancer cells. Clin Cancer Res. 2005;11:2735–46. doi: 10.1158/1078-0432.CCR-04-2014. [DOI] [PubMed] [Google Scholar]
- 10.Shimizu M, Deguchi A, Lim JT, et al. (−)-Epigallocatechin gallate and polyphenon E inhibit growth and activation of the epidermal growth factor receptor and human epidermal growth factor receptor-2 signaling pathways in human colon cancer cells. Clin Cancer Res. 2005;11:2735–46. doi: 10.1158/1078-0432.CCR-04-2014. [DOI] [PubMed] [Google Scholar]
- 11.Adhami VM, Siddiqui IA, Ahmad N, et al. Oral consumption of green tea polyphenols inhibits insulin-like growth factor-I-induced signaling in an autochthonous mouse model of prostate cancer. Cancer Res. 2004;64:8715–22. doi: 10.1158/0008-5472.CAN-04-2840. [DOI] [PubMed] [Google Scholar]
- 12.Lee HJ, Wang CJ, Kuo HC, et al. Induction apoptosis of luteolin in human hepatoma HepG2 cells involving mitochondria translocation of Bax/Bak and activation of JNK. Toxicol Appl Pharmacol. 2005;203:124–31. doi: 10.1016/j.taap.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 13.Neuhaus T, Pabst S, Stier S, et al. Inhibition of the vascular-endothelial growth factor-induced intracellular signaling and mitogenesis of human endothelial cells by epigallocatechin-3 gallate. Eur J Pharmacol. 2004;483:223–7. doi: 10.1016/j.ejphar.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 14.Jankun J, Selman SH, Swiercz R, Skrzypczak-Jankun E. Why drinking green tea could prevent cancer. Nature. 1997;387:561. doi: 10.1038/42381. [DOI] [PubMed] [Google Scholar]
- 15.Naasani I, Seimiya H, Tsuruo T. Telomerase inhibition, telomere shortening, and senescence of cancer cells by tea catechins. Biochem Biophys Res Commun. 1998;249:391–6. doi: 10.1006/bbrc.1998.9075. [DOI] [PubMed] [Google Scholar]
- 16.Fang MZ, Wang Y, Ai N, et al. Tea polyphenol (−)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Res. 2003;63:7563–70. [PubMed] [Google Scholar]
- 17.Navarro-Peran E, Cabezas-Herrera J, Garcia-Canovas F, et al. The antifolate activity of tea catechins. Cancer Res. 2005;65:2059–64. doi: 10.1158/0008-5472.CAN-04-3469. [DOI] [PubMed] [Google Scholar]
- 18.Nam S, Smith DM, Dou QP. Ester bond-containing tea polyphenols potently inhibit proteasome activity in vitro and in vivo. J Biol Chem. 2001;276:13322–30. doi: 10.1074/jbc.M004209200. [DOI] [PubMed] [Google Scholar]
- 19.Lu H, Meng X, Yang CS. Enzymology of methylation of tea catechins and inhibition of catechol-O-methyltransferase by (−)-epigallocatechin gallate. Drug Metab Dispos. 2003;31:572–9. doi: 10.1124/dmd.31.5.572. [DOI] [PubMed] [Google Scholar]
- 20.Manach C, Donovan JL. Pharmacokinetics and metabolism of dietary flavonoids in humans. Free Radic Res. 2004;38:771–85. doi: 10.1080/10715760410001727858. [DOI] [PubMed] [Google Scholar]
- 21.Chen Z, Zhu QY, Tsang D, Huang Y. Degradation of green tea catechins in tea drinks. J Agric Food Chem. 2001;49:477–82. doi: 10.1021/jf000877h. [DOI] [PubMed] [Google Scholar]
- 22.Bettuzzi S, Corti A, Corvetta S. US8044095. Mixture of catechins or rather polyphenols extracted from chinese green tea or other vegetables for the prevention of prostate cancer and for the treatment of prostate hypertrophy (BPH) 2011
- 23.Caporali A, Davalli P, Astancolle S, et al. The chemopreventive action of catechins in the TRAMP mouse model of prostate carcinogenesis is accompanied by clusterin over-expression. Carcinogenesis. 2004;25:2217–24. doi: 10.1093/carcin/bgh235. [DOI] [PubMed] [Google Scholar]
- 24.Bettuzzi S, Brausi M, Rizzi F, et al. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study. Cancer Res. 2006;66:1234–40. doi: 10.1158/0008-5472.CAN-05-1145. [DOI] [PubMed] [Google Scholar]
- 25.Stockfleth E. US7910138. Use of a Polyphenol for the Treatment of a Cancerous or Precancerous Lesion of the Skin. 2011
- 26.Buxton I. US0175834. Polyphenol inhibition of nucleoside diphosphate kinase-B activity and cancer metastasis. 2010
- 27.Lubahn DB, Slusarz A, Shenouda N, Sakla MS. US0054517. Phytoestrogens an regulators of hedgehog signaling and methods of their use in cancer treatment. 2009
- 28.Yang S, Cheek DJ, Westfall DP, Buxton IL. Purinergic axis in cardiac blood vessels. Agonist-mediated release of ATP from cardiac endothelial cells. Circ Res. 1994;74:401–7. doi: 10.1161/01.res.74.3.401. [DOI] [PubMed] [Google Scholar]
- 29.Buxton IL, Kaiser RA, Oxhorn BC, Cheek DJ. Evidence supporting the nucleotide axis hypothesis: ATP release and metabolism by coronary endothelium. American journal of physiology. Heart Circ Physiol. 2001;281:H1657–66. doi: 10.1152/ajpheart.2001.281.4.H1657. [DOI] [PubMed] [Google Scholar]
- 30.Evangelista M, Tian H, de Sauvage FJ. The hedgehog signaling pathway in cancer. Clin Cancer Res. 2006;12:5924–8. doi: 10.1158/1078-0432.CCR-06-1736. [DOI] [PubMed] [Google Scholar]
- 31.Kinzler KW, Bigner SH, Bigner DD, et al. Identification of an amplified, highly expressed gene in a human glioma. Science. 1987;236:70–3. doi: 10.1126/science.3563490. [DOI] [PubMed] [Google Scholar]
- 32.Ball ED, Balaian L. US7977320. Method of increasing efficacy of tumor cell killing using combinations of anti-neoplastic agents. 2011
- 33.Shah MA, Schwartz GK. Cell cycle-mediated drug resistance: an emerging concept in cancer therapy. Clin Cancer Res. 2001;7:2168–81. [PubMed] [Google Scholar]
- 34.Ishida K, Nishizuka SS, Chiba T, et al. Molecular marker identification for relapse prediction in 5-FU-based adjuvant chemotherapy in gastric and colorectal cancers. PLoS one. 2012;7:e43236. doi: 10.1371/journal.pone.0043236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Satoh T, Sakata Y. S-1 for the treatment of gastrointestinal cancer. Expert Opin Pharmacother. 2012;13:1943–59. doi: 10.1517/14656566.2012.709234. [DOI] [PubMed] [Google Scholar]
- 36.Ciccolini J, Gross E, Dahan L, et al. Routine dihydropyrimidine dehydrogenase testing for anticipating 5-fluorouracil-related severe toxicities: hype or hope? Clin Colorectal Cancer. 2010;9:224–8. doi: 10.3816/CCC.2010.n.033. [DOI] [PubMed] [Google Scholar]
- 37.Wei J. US0068295. Composition for treating cancer and method of using the same. 2009
- 38.Yang XW, Wang XL, Cao LQ, et al. Green tea polyphenol epigallocatechin-3-gallate enhances 5-fluorouracil-induced cell growth inhibition of hepatocellular carcinoma cells. Hepatol Res. 2012;42:494–501. doi: 10.1111/j.1872-034X.2011.00947.x. [DOI] [PubMed] [Google Scholar]
- 39.Norwood AA, Tucci M, Benghuzzi H. A comparison of 5-fluorouracil and natural chemotherapeutic agents, EGCG and thymoquinone, delivered by sustained drug delivery on colon cancer cells. Biomed Sci Instrum. 2007;43:272–7. [PubMed] [Google Scholar]
- 40.Masuda M, Suzui M, Weinstein IB. Effects of epigallocatechin-3-gallate on growth, epidermal growth factor receptor signaling pathways, gene expression, and chemosensitivity in human head and neck squamous cell carcinoma cell lines. Clin Cancer Res. 2001;7:4220–9. [PubMed] [Google Scholar]
- 41.Nabhan C, Gartenhaus RB, Tallman MS. Purine nucleoside analogues and combination therapies in B-cell chronic lymphocytic leukemia: dawn of a new era. Leuk Res. 2004;28:429–42. doi: 10.1016/j.leukres.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 42••.Kay NE, Shanafelt TD. US0190638. Methods of treating hematologic cancers. 2012 This patent describes a highly potent combination of low concentration of EGCG with chemotherapeutic compounds.
- 43.Chang TT, Gulati SC, Chou TC, et al. Synergistic effect of 4-hydroperoxycyclophosphamide and etoposide on a human promyelocytic leukemia cell line (HL-60) demonstrated by computer analysis. Cancer Res. 1985;45:2434–9. [PubMed] [Google Scholar]
- 44.Wu T, Hung C. US20100330105. Anticancer Combination therapies. 2010
- 45.Kim TW, Hung CF, Ling M, et al. Enhancing DNA vaccine potency by coadministration of DNA encoding antiapoptotic proteins. J Clin Invest. 2003;112:109–17. doi: 10.1172/JCI17293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheng WF, Hung CF, Pai SI, et al. Repeated DNA vaccinations elicited qualitatively different cytotoxic T lymphocytes and improved protective antitumor effects. J Biomed Sci. 2002;9:675–87. doi: 10.1159/000067285. [DOI] [PubMed] [Google Scholar]
- 47••.Chung JE, Kurisawa M, Yang YY, Zhuo L. US7858080. Aldehyde conjugated flavonoid preparations. 2010 This patent describes a unique and innovative technique to house EGCG in a micellar complex.
- 48.Ying JY, Chung JE, Kurisawa M, Ng SP. US0044992. Method of delivering an anti-cancer agent to a cell. 2011
- 49.Dou QP, Chan T, Smith DM. US8058310. Polyphenol proteasome inhibitors, synthesis, and methods of use. 2012
- 50.Chow HH, Cai Y, Alberts DS, et al. Phase I pharmacokinetic study of tea polyphenols following single-dose administration of epigallocatechin gallate and polyphenon E. Cancer Epidemiol Biomarkers Prev. 2001;10:53–8. [PubMed] [Google Scholar]
- 51.Dou QP, Chan T, Smith DM. US7767711. Polyphenol proteasome inhibitors, synthesis, and methods of use. 2010
- 52.Dou QP, Chan T, Smith DM. US0029067. Polyphenol proteasome inhibitors, synthesis, and methods of use. 2012
- 53.Wu AH, Tseng CC, Van Den Berg D, Yu MC. Tea intake, COMT genotype, and breast cancer in Asian-American women. Cancer Res. 2003;63:7526–9. [PubMed] [Google Scholar]
- 54.Landis-Piwowar KR, Wan SB, Wiegand RA, et al. Methylation suppresses the proteasome-inhibitory function of green tea polyphenols. J Cell Physiol. 2007;213:252–60. doi: 10.1002/jcp.21124. [DOI] [PubMed] [Google Scholar]
- 55.Golden EB, Lam PY, Kardosh A, et al. Green tea polyphenols block the anticancer effects of bortezomib and other boronic acid-based proteasome inhibitors. Blood. 2009;113:5927–37. doi: 10.1182/blood-2008-07-171389. [DOI] [PubMed] [Google Scholar]
- 56.Chan T, Dou QP. US0152210. Polyphenol compounds for inhibiting proteasome and uses thereof. 2011
- 57.Pamu S, Chen D, Morin F, et al. Inhibitory effect of bortezomib on human multiple myeloma cells when combined with epigallocatechin-gallate (EGCG) analogs. Med Chem Comm. 2012;3:229–32. [Google Scholar]
- 58.Liu B, Fan Z, Edgerton SM, et al. Metformin induces unique biological and molecular responses in triple negative breast cancer cells. Cell Cycle. 2009;8:2031–40. doi: 10.4161/cc.8.13.8814. [DOI] [PubMed] [Google Scholar]
- 59.Alimova IN, Liu B, Fan Z, et al. Metformin inhibits breast cancer cell growth, colony formation and induces cell cycle arrest in vitro. Cell Cycle. 2009;8:909–15. doi: 10.4161/cc.8.6.7933. [DOI] [PubMed] [Google Scholar]
- 60.Zakikhani M, Dowling R, Fantus IG, et al. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res. 2006;66:10269–73. doi: 10.1158/0008-5472.CAN-06-1500. [DOI] [PubMed] [Google Scholar]
- 61.Hirsch HA, Iliopoulos D, Tsichlis PN, Struhl K. Metformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remission. Cancer Res. 2009;69:7507–11. doi: 10.1158/0008-5472.CAN-09-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen D, Pamu S, Cui Q, et al. Novel epigallocatechin gallate (EGCG) analogs activate AMP-activated protein kinase pathway and target cancer stem cells. Bioorg Med Chem. 2012;20:3031–7. doi: 10.1016/j.bmc.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63••.Chan T, Lam W, Chow LM, et al. US20080176931. Epigallocatechin gallate derivatives for inhibiting proteasome. 2008 This patent describes the novel use of a prodrug form of EGCG.
- 64••.Chan T, Lam W, Chow LM, et al. US8193377. Epigallocatechin gallate derivatives for inhibiting proteasome. 2012 This patent describes the novel use of a prodrug form of EGCG.
- 65.Kazi A, Daniel KG, Smith DM, et al. Inhibition of the proteasome activity, a novel mechanism associated with the tumor cell apoptosis-inducing ability of genistein. Biochem Pharmacol. 2003;66:965–76. doi: 10.1016/s0006-2952(03)00414-3. [DOI] [PubMed] [Google Scholar]
- 66.Lambert JD, Lee MJ, Lu H, et al. Epigallocatechin-3-gallate is absorbed but extensively glucuronidated following oral administration to mice. J Nutr. 2003;133:4172–7. doi: 10.1093/jn/133.12.4172. [DOI] [PubMed] [Google Scholar]
- 67.Chow HH, Cai Y, Hakim IA, et al. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and polyphenon E in healthy individuals. Clin Cancer Res. 2003;9:3312–19. [PubMed] [Google Scholar]
- 68.Yang CS, Chen L, Lee MJ, et al. Blood and urine levels of tea catechins after ingestion of different amounts of green tea by human volunteers. Cancer Epidemiol Biomarkers Prev. 1998;7:351–4. [PubMed] [Google Scholar]
- 69.Landis-Piwowar KR, Huo C, Chen D, et al. A novel prodrug of the green tea polyphenol (−)-epigallocatechin-3-gallate as a potential anticancer agent. Cancer Res. 2007;67:4303–10. doi: 10.1158/0008-5472.CAN-06-4699. [DOI] [PubMed] [Google Scholar]
- 70.Lambert JD, Sang S, Hong J, et al. Peracetylation as a means of enhancing in vitro bioactivity and bioavailability of epigallocatechin-3-gallate. Drug Metab Dispos. 2006;34:2111–16. doi: 10.1124/dmd.106.011460. [DOI] [PubMed] [Google Scholar]
- 71.Lee SC, Chan WK, Lee TW, et al. Effect of a prodrug of the green tea polyphenol (−)-epigallocatechin-3-gallate on the growth of androgen-independent prostate cancer in vivo. Nutr Cancer. 2008;60:483–91. doi: 10.1080/01635580801947674. [DOI] [PubMed] [Google Scholar]
- 72.Chiou YS, Ma NJ, Sang S, et al. Peracetylated (−)-epigallocatechin-3-gallate (AcEGCG) potently suppresses dextran sulfate sodium-induced colitis and colon tumorigenesis in mice. J Agric Food Chem. 2012;60:3441–51. doi: 10.1021/jf300441p. [DOI] [PubMed] [Google Scholar]
- 73•.Fereidoon S, Zhong Y. WO123942. Fatty acid derivatives of catechins and methods of their use. 2011 This is an interesting method of increasing the ability of EGCG to cross cell membranes.
- 74.Mori S, Miyake S, Kobe T, et al. Enhanced anti-influenza A virus activity of (−)-epigallocatechin-3-O-gallate fatty acid monoester derivatives: effect of alkyl chain length. Bioorg Med Chem Lett. 2008;18:4249–52. doi: 10.1016/j.bmcl.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 75.Matsumura K, Kaihatsu K, Mori S, et al. Enhanced antitumor activities of (−)-epigallocatechin-3-O-gallate fatty acid monoester derivatives in vitro and in vivo. Biochem Biophys Res Commun. 2008;377:1118–22. doi: 10.1016/j.bbrc.2008.10.128. [DOI] [PubMed] [Google Scholar]
- 76.Bettuzzi S, Brausi M, Rizzi F, et al. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study. Cancer Res. 2006;66:1234–40. doi: 10.1158/0008-5472.CAN-05-1145. [DOI] [PubMed] [Google Scholar]
- 77.Shanafelt TD, Call TG, Zent CS, et al. Phase 2 trial of daily, oral polyphenon E in patients with asymptomatic, Rai stage 0 to II chronic lymphocytic leukemia. Cancer. 2012 doi: 10.1002/cncr.27719. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]