Abstract
Little is known about the expression of Pax2 in mature retina or optic nerve. Here we probed for the expression of Pax2 in late stages of embryonic development and in mature chick retina. We find two distinct Pax2 isoforms expressed by cells within the retina and optic nerve. Surprisingly, Müller glia in central regions of the retina express Pax2, and levels of expression are decreased with increasing distance from the nerve head. In Müller glia, the expression levels of Pax2 are increased by acute retinal damage or treatment with growth factors. At the optic nerve, Pax2 is expressed by peripapillary glia, at the junction of the neural retina and optic nerve head and by glia within the optic nerve. In addition, we assayed for Pax2 expression in glial cells in mammalian retinas. In mammalian retinas, unlike the case in chick retina, the Müller glia do not express Pax2. Pax2-expressing cells are found in the optic nerve and astrocytes within the mouse retina. By comparison, Pax2-positive cells are not found within the guinea pig retina; Pax2-expressing glia are confined to the optic nerve. In dog and monkey (Macaca fascicularis), Pax2 is expressed by astrocytes that are scattered across inner retinal layers and by numerous glia within the optic nerve. Interestingly, Pax2-positive glial cells are found at the peripheral edge of the dog retina, but only in older animals. We conclude that the expression of Pax2 in the vertebrate eye is restricted to retinal astrocytes, peripapillary glia, and glia within the optic nerve.
INDEXING TERMS: retina, Pax2, Müller glia, astrocytes, optic nerve
Pax2 is a paired box transcription factor known to play important roles in the development of neural tube, inner ear, eyes, and kidneys (Dressler et al., 1990; Soukkarieh et al., 2007). Pax2 is a member of the paired-type, class 3 transcription factor family based on distinguishing motifs, including a paired domain, an octapeptide sequence, and an incomplete homeodomain (Walther et al., 1991). The paired domain contains three α-helices, the third of which can be deleted in certain Pax proteins without affecting recognition of E box DNA sequences (Chalepakis et al., 1991; Treisman et al., 1991), whereas the third α-helix is required by other Pax proteins for DNA binding (Czerny et al., 1993). The transcriptional activation domain of Pax2 is in the C-terminal region (Lechner and Dressler, 1996; Treisman et al., 1991), whereas transcriptional repression is mediated by the octapeptide (Lechner and Dressler, 1996). The different functions of Pax2 may, in part, be manifested through alternative splicing. To date, many splice variants of Pax2 have been identified, although their functional significance has yet to be determined. In Xenopus, Pax2 has at least nine splice variants (Heller and Brandli, 1997), and five variants have been identified in human (Busse et al., 2009; Dressler and Douglass, 1992). Mutations in Pax2 can result in retinal coloboma syndrome manifest by the failure of optic fissure histogenesis as well as renal abnormalities (Nishimoto et al., 2001; Sanyanusin et al., 1995; Tellier et al., 2000).
The mechanisms that regulate the patterns and levels of Pax2 expression are slowly being revealed. The expression of Pax2 in ventral optic structures is stimulated by sonic hedgehog (Shh), a secreted growth factor that is produced by the notochord during early eye-field patterning (Dakubo et al., 2003). Recently, the expression of Pax2 in the optic stalk has been shown to be regulated by interactions between the hedgehog and bone morphogenetic protein (BMP) cell signaling pathways (Sehgal et al., 2009). Sehgal and colleagues (2009) demonstrated that second messenger effectors of BMP and Shh pathways are expressed in the developing optic stalk and together inhibit the Pax2-suppressing influence of TLX. Perturbations in ventral midline Shh signaling results in loss of Pax2 expression and expansion of Pax6 expression in neural retina into the presumptive optic stalk region (Dakubo et al., 2003; Macdonald et al., 1995; Wang et al., 2002). The reciprocal inhibition of Pax2 and Pax6 in the ventral regions of the developing eye segregates precursor cells into gliogenic (Pax2) and neurogenic (Pax6) populations (for review see Pichaud and Desplan, 2002). Taken together, these findings suggest that Pax2 suppresses the neurogenic potential of progenitors in the optic stalk.
Although a great deal is known about the expression patterns and roles of Pax2 in the developing eye, nothing is known about Pax2 in the mature eye. Accordingly, the purpose of this study was to examine Pax2 expression in late-stage embryonic and posthatch chick eyes. Furthermore, we assay whether Pax2 expression is affected by acute retinal damage or treatments known to influence the activity of retinal glia. We examine, based on findings in the chick retina, the expression of Pax2 in the mature retina of different mammalian species, including mice, guinea pigs, dogs, and nonhuman primates.
METHODS AND MATERIALS
Animals
The use of animals in these experiments was in accordance with the guidelines established by the National Institutes of Health and The Ohio State University. Newly hatched leghorn chickens (Gallus gallus domesticus) were obtained from the Department of Animal Sciences at The Ohio State University and kept on a cycle of 12 hours light, 12 hours dark (lights on at 7:00 am). Chicks were housed in a stainless-steel brooder at 25°C and received water and Purina chick starter ad libitum. Eyes were obtained post-mortem from mice (Mus musculus), guinea pigs (Cavia porcellus), dogs (Canis familiarus), and monkeys (Macaca fascicularis).
Embryonic staging
The developmental stage of chick embryos was determined according to the guidelines established by Hamburger and Hamilton (1951, 1992).
Intraocular injections
Intraocular injections were performed as described elsewhere (Fischer et al., 1998). In brief, animals were anesthetized with 2.5% isofluorane in oxygen at a flow rate of 1.5 liters/minute. Injections of 20 μl were made with a Hamilton syringe with a 26-gauge needle through the dorsal eyelid into the vitreous chamber. Treated eyes were injected with insulin-like growth factor (IGF; 200 ng), fibroblast growth factor-2 (FGF2; 200 ng), or N-methyl-D-aspartate (NMDA; 200 nmol or 2 μmol) dissolved in vehicle (0.9% NaCl in dH2O). Control eyes received injections of vehicle.
Fixation, sectioning, and immunofluorescence
Eyes were enucleated and fixed in 4% paraformaldehyde in 0.1 M PB, pH 7.4, with 3% sucrose for 30 minutes. After washing in PBS (0.05 M phosphate buffer + 0.154 mM NaCl), eyecups were cryoprotected by soaking in 30% sucrose in PBS with NaN3 overnight. Transverse retinal sections were cut at 10 μm and thaw mounted onto Superfrost-Plus slides (Fisher Scientific, Fair Lawn, NJ). The slides were air dried and stored at −20°C until use. Sections prepared for in situ hybridization (ISH) protocols were prepared as described above with the following modifications: fixation was carried out overnight at 4°C, sections were cut at 16 μm, and slides were stored at −80°C for less than 2 weeks until use.
Sections were warmed to room temperature and ringed with rubber cement. After being washed with PBS, slides were incubated overnight under 200 μl primary antibody (antisera diluted in PBS with 0.2% Triton-X with 0.01% NaN3 and 5% blocking serum). For bromodeoxyuridine (BrdU) immunolabeling, slides were washed for 7 minutes in 4 N HCl and washed in PBS prior to incubation overnight under primary antibody solution.
The antisera used in this study included the following. 1) Rabbit anti-Pax2 was used at 1:250 (PRB-276; Covance, Berkeley, CA). The antibody was raised to amino acids (188–385) of human Pax2 and is known to recognize both Pax2a and Pax2b isoforms (manufacturer’s information). The specificity of the Pax2 antibody was assessed by Western blot analysis and by comparison of patterns of immunofluorescence with those seen with in situ hybridization (current study). The Pax2 antibody produced a cellular pattern and distribution of labeling that was consistent with previous studies regarding optic nerve glia in the developing chick retina (Sehgal et al., 2008). 2) Mouse anti-glial fibrillairy acidic protein (GFAP) was used at 1:1,000 (G-3893; Sigma-Aldrich, St. Louis, MO). The antibody was raised to purified GFAP from porcine spinal cord and recognizes a single 52-kDa band in Western blot analysis (manufacturer’s information). The GFAP monoclonal antibody produced a cellular pattern and distribution of labeling that was consistent with previous studies of astrocytes in the mammalian CNS (Franke et al., 1991; Tran et al., 2007). 3) Goat anti-Sox2 was used at 1:1,000 (Y-17; Santa Cruz Biotechnology, Santa Cruz, CA). The antibody was raised to the recombinant C-terminus of human Sox2 and recognizes a single 34-kDa band in Western blot analysis of lysate from mouse embryonic stem cells (manufacturer’s information). The Sox2 antibody is known to recognize amino acids 277–293 of human Sox2, as determined by preabsorption controls and mass spectrometry analysis of blocking peptide (Poche et al., 2008). The Sox2 antibody produced a pattern of labeling in the nuclei of progenitors, Müller glia, and cholinergic amacrine cells in the retina consistent with previous reports (Fischer et al., 2009a–c; Poche et al., 2008). 4) Mouse anti-Pax6 was used at 1:50 [PAX6; Developmental Studies Hybridoma Bank (DSHB)]. The antibody was raised to chick Pax6 (amino acids 1–233). Western blot analysis of retinal extracts of embryonic chickens indicated that the Pax6 monoclonal labels two major (~55 kDa) and two minor (~40 kDa) bands at the expected molecular masses of known Pax6 splice variants (Kawakami et al., 1997). The Pax6 antibody produced a cellular pattern and distribution of labeling that was consistent with previous studies in the chicken retina (Fischer and Reh, 2000, 2001; Fischer et al., 2007). 5) Mouse anti-vimentin was used at 1:50 (40E-C; DSHB). This antibody was raised to homogenized adult canary brain, and the specificity has been confirmed, with the detection of a single band at ~50 kDa, by using Western blot analysis (Alvarez-Buylla et al., 1987). This monoclonal produced a cellular pattern and distribution of labeling that were consistent with those of vimentin expression in astrocytes in the mammalian CNS (Alvarez-Buylla et al., 1987, 1988; Alvarez-Buylla and Nottebohm, 1988). 6) Mouse anti-BrdU was used at 1:100 (G3G4; DSHB) and 7) rat anti-BrdU was used at 1:200 (OBT0030S; Accurate Chemicals/Serrotec). The antibodies to BrdU failed to label cells in tissues that were not exposed to BrdU. Nuclei were visualized with ToPro3 (Invitrogen, Carlsbad, CA) diluted in 1 μM in PBS and incubated for 15 minutes, DRAQ5 (Biostatus Limited) diluted 1:1,000 and added to the secondary antibody solution and incubated for 1 hour, or DAPI (Sigma-Aldrich) diluted to 1 μg/ml in PBS and incubated for 7 minutes.
Microscopy, cell counts, and quantitiative immunofluoresence
High-resolution (5.4 MP) micrographs were taken with a Leica DC500 camera and a DM5000B microscope. Confocal images were obtained by using a Zeiss LSM 510 at the Hunt-Curtis Imaging Facility. Cell counts were made from at least five different animals and means and standard deviations calculated on data sets. To avoid the possibility of region-specific differences within the retina, cell counts were consistently made from the same region of retina for each data set. Images were optimized for color, brightness, and contrast; multiple-channel images were overlaid; and figures were constructed in Adobe Photoshop 6.
ImagePro 6.2 was used to measure immunofluorescence as described previously (Fischer et al., 2009a,b; Ghai et al., 2009). Images obtained from control and treated samples were obtained by using identical illumination, microscope, and camera settings. Areas (1,600 × 500 pixels or 464 × 145 μm) were sampled from 5.4-MP digital images. These areas were randomly sampled over the inner nuclear layer (INL) where the nuclei of the Müller glia were observed. Measurements were made from areas containing pixel intensities greater than 80 (0 = black, 255 = saturation). Total area, density sum, and average pixel intensities were calculated for pixel intensities within the threshold range of 80–225. These calculations were determined for INL regions sampled from six different retinas for each experimental condition.
RT-PCR
Standard methods and procedures were used, as described previously (Fischer et al., 2004a,b). In brief, total RNA was extracted using the Trizol method from pooled samples of three optic nerve heads (ONH), central retina (CR), and peripheral retina (PR) and reverse transcribed using RT-SSIII (Invitrogen) with poly-dT primers. To detect Pax2 transcripts, primers (listed in Table 1) were designed using the Pax2 cDNA in Genebank (ASSN: AB026496) and Primer3 software from the Whitehead Institute for Biomedical Research (http://frodo.wi.mi-t.edu/). cDNA was amplified using an Eppendorf thermocycler for 40 cycles with an annealing temperature of 56°C. Products were verified on a 1% agarose gel with added ethidium bromide.
TABLE 1.
Pax2 Primer Pairs
| Primer name | Sequence | Product size (bp) |
|---|---|---|
| isPax-F | 5′-gctggaagctttagatcgag-3′ | 975 |
| isPax2-R | 5′-ccttctcttgtttgccagat-3′ | |
| Pax2-FM | 5′-gatccagtgggctcttactc-3′ | 693 |
| Pax2-MF1 | 5′-atcgtctgaaagggtcatgt-3′ | |
| Pax2-MR1 | 5′-acatgaccctttcagacgat-3′ | 1,102 |
| Pax2-RM | 5′-cttctcttgtttgccagatg-3′ |
Primers anneal to the same sequence on the forward and reverse strands.
ISH
PCR products were cloned into Topo TA vector (pCR-II; Invitrogen). RNA polymerases (T7 and SP6) were used to generate digoxigenin-labeled riboprobes by using a kit provided by Roche and stored at −80°C until use. Ocular tissues were dissected in RNase-free Hanks balanced salt solution (HBSS; Invitrogen), fixed overnight at 4°C in 4% PFA in 0.1 M sodium phosphate, and embedded in OCT compound (Tissue-Tek). Cryosections were processed for ISH with riboprobes as described previously (Fischer et al., 2002a, 2004a). Hybridization was detected by using Fab fragments to digoxigenin that were conjugated to alkaline phosphatase (anti-DIG-AP; Roche) diluted in MABT (0.05 M maleic acid buffer, 0.1% Tween-20) plus 10% normal goat serum, 10 mM levamisole, and 10 mM glycine. Nitro-blue tetrazolium (NBT) and 5-bromo-4-chloro-3′-indolyphosphate p-toluidine (BCIP) in 0.1 M NaCl, 0.1 M Tris-HCl, pH 9.5, 0.05 M MgCl2 and 0.01% Tween-20 were used to precipitate chromophore from the anti-digoxigenin-AP. With sufficient levels of chromophore developed, the sections were washed in PBS and fixed in 4% paraformaldehyde in PBS for 15 minutes.
Sequence analysis
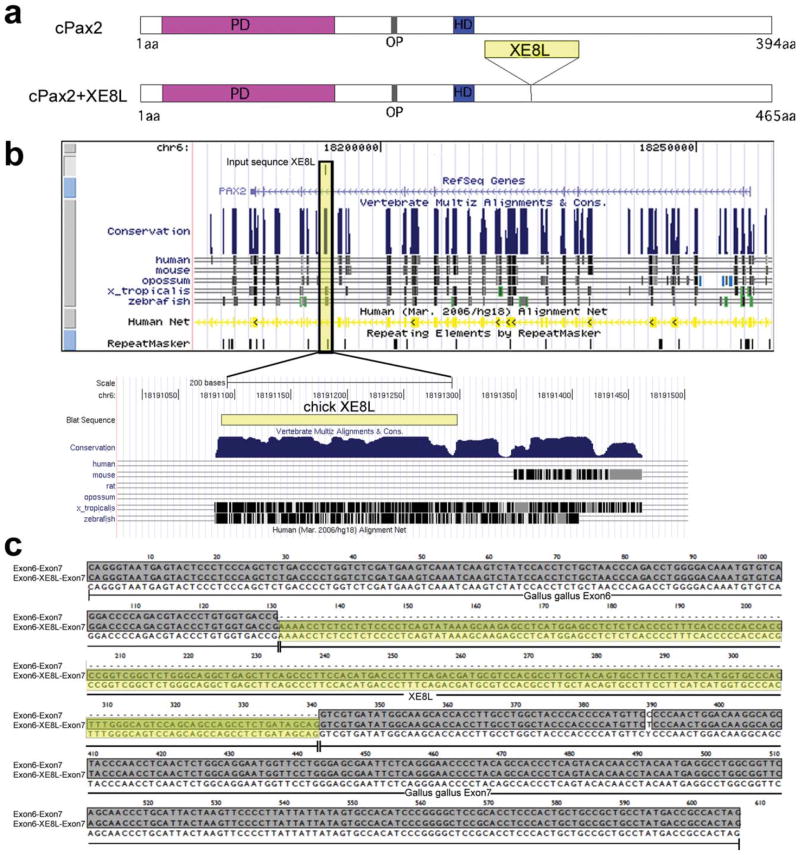
Two regions of Pax2 cDNA were amplified and sequenced; these cDNAs were centered on a novel exon, found between exons 6 and 7, that we later define as Xenopus exon 8-like (XE8L). The regions of Pax2 that were amplified included cDNA upstream of XE8L to exon 4 and a region ~1,100 kb downstream of XEL8 (see Fig. 4). The PCR primers that we used are presented in Table 1. The XE8L-specific primers (Pax2-FM and Pax2-MR) were designed against complementary sequence in order to facilitate PCR amplification and sequencing of upstream and downstream regions, respectively.
Figure 4.
Sequence of the cPax2-XL8E splice variant and cross-species alignments of Pax2. a: Graphic representation of the protein domains of Pax2. The paired domain is indicated by magenta (PD), the octapeptide domain is gray (OD), the partial hoomeodomain is blue (HD), and the XE8L is yellow. b: Cross-species alignment of Pax2 and the XE8L, in yellow. c: Sequence of the XE8L variant with flanking exons. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Alignments of sequences were performed using BLAST (Basic Local Alignment Search Tool; NCBI). We verified the sequence of transcripts that included the XE8L insertion from the TA clones by performing ClustalW analysis in MacVector (add NCBI accessions of the known Gallus gallus Pax2 gene). We obtained a complete sequence across Exon4-NE-3′DSR by performing contig analysis using sequences obtained from multiple TA clones of PCR products made with Pax2FM:Pax2-MF and Pax2MR: Pax2RM and the sequence of the XE8L-insertion in Seq-Man software (Lasergene 8 software suite).
Protein extraction and Western blot
Retinas were dissected in cold HBSS. Central and peripheral retina and the optic nerve head were isolated and proteins were extracted using a standard extraction buffer added with Complete protease inhibitor Cocktail (Roche). Tissues were disrupted by sonication, extracts were centrifuged at 1,000g, and the supernatant was aspirated. Protein concentration was determined by using bicinchoninic acid (BCA) Protein Assay Reagent (Pierce, Pittsburgh, PA) and stored at −20°C diluted 1:1 in Lamellis’ buffer. Proteins (20 μg) were separated on a gradient (4–20%) polyacrylamide gel and transfered to nitrocellulose membranes. Membranes were incubated with primary antisera overnight in 5% milk powder in Tris-buffered saline, pH 7.4, + .01% Tween-20. Membranes were washed and incubated for 1 hour under horseradish peroxidase (HRP)-conjugated antibodies. ECL chemiluminescent substrates (Pierce) were added to membranes, as per the manufacturer’s instructions, and fluorescence was detected on Kodak X-ray film with minimal exposure.
RESULTS
Pax2 is expressed in retinas of late-stage chick embryos
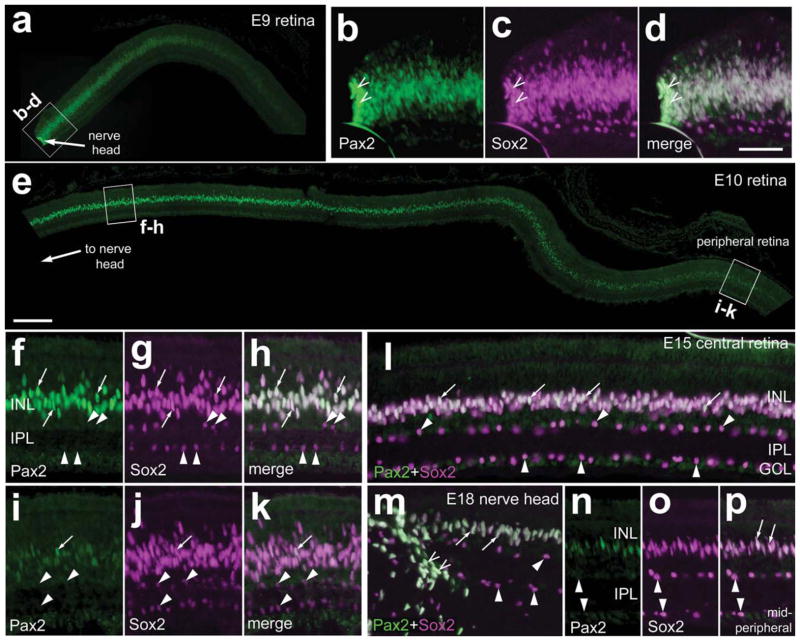
Expression of Pax2 in the chick retina during early stages of embryonic development has been described previously (Hidalgo-Sanchez et al., 1999; Sehgal et al., 2008; Soukkarieh et al., 2007; Zhang and Yang, 2001). However, the expression of Pax2 has not been described beyond embryonic day 8 (E8). Accordingly, we assayed for Pax2 expression in the ocular tissues of embryos at developmental stages after E8. For eyes from E9 embryos, we detected intense Pax2 immunoreactivity in cells adjacent to the optic nerve head (ONH; Fig. 1a). The Pax2 immunoreactivity was confined to nuclei and overlapped with labeling for Sox2 (Fig. 1b–d), a transcription factor that is expressed by retinal progenitors, mature Müller glia, and cholinergic amacrine cells (Fischer et al., 2009a,b; Hayes et al., 2007; Karl et al., 2008; Lin et al., 2009). In E9 retinas, immunoreactivity for Pax2 was diminished with increasing distance from the ONH, extending less than 500 μm into the retina (Fig. 1a). At E10, Pax2 immunoreactivity appeared elevated in retinal areas surrounding the nerve head, and expression levels decreased toward more peripheral regions (Fig. 1e). All of the fusiform Pax2+ nuclei in the inner nuclear layer (INL) were colabeled for Sox2, whereas Pax2 was not observed in Sox2+ cells found in the sclerad INL or ganglion cell layer (GCL; Fig. 1f–k). Sox2 is expressed by retinal progenitors and mature Müller glia, so it was not possible to make the distinction whether Pax2 was expressed by late-stage progenitors or postmitotic, differentiating Müller glia. However, in E10 retinas, there is little or no proliferation of progenitors within central regions of the retina (Ghai et al., 2008; Prada et al., 1991). Therefore, we propose that the Sox2+/Pax2+ nuclei in the INL of central regions of E10 retinas were those of differentiating Müller glia. Low levels of Pax2 expression were seen in far peripheral regions of the retina at E10 (Fig. 1i–k). Similar to the case at earlier stages of embryonic development, diminished levels of Pax2 immunoreactivity were observed in Sox2+ Müller glia in peripheral regions of the retina at E15 and E18 (Fig. 1l,n–p). In E18 eyes, 3 days before hatching, Pax2 immunoreactivity remained prevalent in the nuclei of Sox2+ cells within the ONH and in Müller glia in central regions of the retina (Fig. 1m).
Figure 1.
Pax2 is expressed in the chick retina during late stages of embryonic development. Vertical sections of retinas from E9 (a–d), E10 (e–k), E15 (l), and E18 (m–p) chick embryos were labeled with antibodies to Pax2 and Sox2. b–d, f–h, and i–k are threefold enlargements of the boxed areas in a and e. m: Micrograph of the optic nerve head and retina from an E18 eye. n–p are micrographs of the midperipheral retina about 2 mm peripheral to the optic nerve in m. Arrowheads indicate cholinergic amacrine cells labeled for Sox2 alone; arrows indicate cells labeled for Sox2 and Pax2; carets indicate peripapillary cells. INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer. Scale bars = 50 μm in e (applies to a,e); 50 μm in d (applies b–d,f–p). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
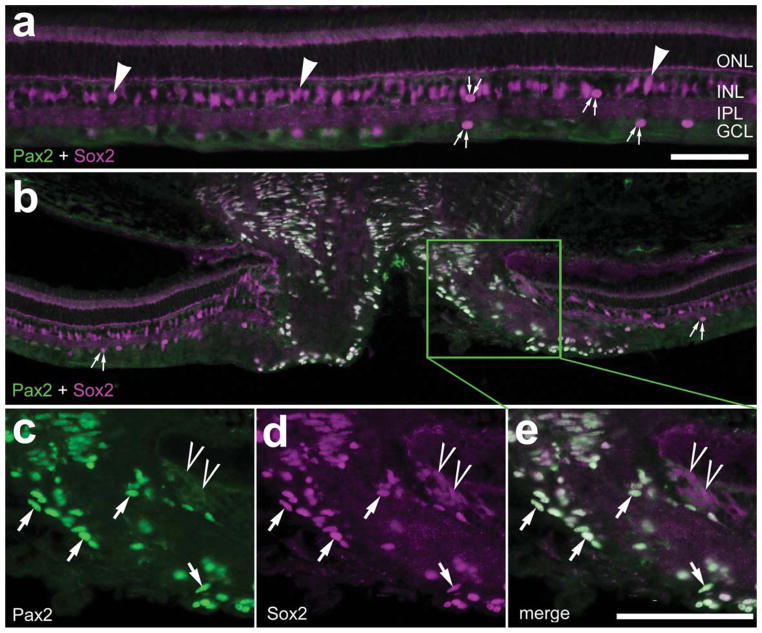
In the posthatch chick retina, several weeks after the completion of retinal histogenesis, intense Pax2 immunoreactivity was found in the nuclei of cells that reside in the ONH (Fig. 2a). In addition, we observed many Pax2+/Sox2+ cells within the optic nerve and ONH; some of these cells extended up to 50 μm into the retina and were located in the nerve fiber layer (NFL) or GCL (Fig. 2b). In addition, we found Pax2 expression within the neural retina of posthatch chick (Fig. 2b). Nuclei weakly immunoreactive for Pax2 were present in a single lamina within the INL; these oblong nuclei were those of Müller glia (Fig. 2c). The Pax2 immunoreactivity in Müller glia gradually decreased with increasing distances from the ONH (Fig. 2c–f). Very few Pax2-positive nuclei were seen in midperipheral regions of the retina, and levels of Pax2 were further reduced in far peripheral regions of the retina (Fig. 2e,f). All of the Pax2+ cells in the optic nerve, ONH, and retina were colabeled for Sox2 (Fig. 2g–l). However, not all Sox2+ cells in the optic nerve and retina were labeled for Pax2 (Fig. 2g–l); these cells likely included cholinergic amacrine cells and nonastrocytic inner retinal glia-like (NIRG) cells that we have recently described (Fischer et al., 2009c).
Figure 2.
Pax2 is expressed in the neural retina and optic nerve of the posthatch chick. Levels of Pax2 expression in Müller glia decrease with increasing distances from the ONH. Confocal microscopy (a–f) and widefield microscopy (g–l) were used to obtain micrographs. a–f: Representative micrographs of vertical sections of the optic nerve head (a,b) and retina (c–f) labeled with antibodies to Pax2. Images were taken in retinal regions adjacent to the optic nerve head (b) and in central (c), midperipheral (d), midfar peripheral (e), and far peripheral (f) retina. g–l: Vertical sections of the central retina and optic nerve labeled with antibodies to Pax2 (green) and Sox2 (magenta). Arrowheads indicate cholinergic amacrine cells labeled for Sox2 alone; small double arrows indicate putative NIRG cells labeled for Sox2 alone; single arrows indicate cells labeled for Sox2 and Pax2; carets indicate peripapillary cells; asterisks in a indicate blood vessels. ONL, outer nuclear layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer; ON, optic nerve; ONH, optic nerve head. Scale bars = 50 μm in a; 50 μm in f (applies to b–f); 50 μm in i (applies to g–i); 50 μm in l (applies to j–l). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Alternative splicing of Pax2
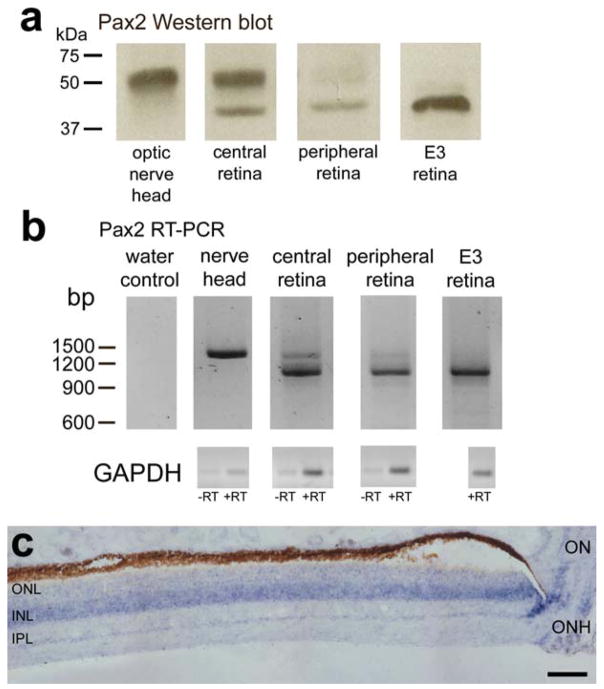
We next sought to characterize better the specificity of the antibody to Pax2. In Western blot preparations, the Pax2 antibody detected two distinct bands, one at 44 kDa and another at ~51 kDa (Fig. 3a). The band at 44 kDa matches the predicted mass of Pax2, whereas the band at ~51 kDa was significantly larger. The Pax2 antibody used in this study was raised to a recombinant protein spanning amino acids 188–385 of human Pax2 (Dressler and Douglass, 1992) and is expected to recognize both Pax2a, which includes exon 6, and Pax2b, which does not include exon 6 (Dressler and Douglass, 1992; Tavassoli et al., 1997).
Figure 3.
Western blot and RT-PCR indicate that cells in the retina and optic nerve express two distinct variants of Pax2. a: Western blot of proteins extracted from the optic nerve, central retina, and peripheral retina of posthatch chick eyes and embryonic day 3 (E3) ocular tissues as a positive control. b: Ethidium bromide-stained gel for RT-PCR products for mRNA obtained from the optic nerve head, central retina, and peripheral retina of posthatch chick eyes and E3 ocular tissues as a positive control. c: Vertical section through the retina and optic nerve head that was hybridized with riboprobes to Pax2. INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer; ONH, optic nerve head. Scale bar = 50 μm. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
To validate the patterns of Pax2 immunolabeling in the eye, we used RT-PCR and ISH. RT-PCR revealed two distinct products in cDNA pools from mature retina and ONH (Fig. 3b). The shorter PCR product was detected in retinal cDNA from E3 embryos (Fig. 3b). The PCR products were TOPO cloned, sequenced, and riboprobes generated for ISH. ISH revealed a pattern of Pax2 expression that was consistent with that seen for Pax2 immunofluorescence in the posthatch chick eye. The ISH signal was highest near the nerve head and decreased with increasing distances from the ONH (Fig. 3c). The Pax2 ISH signal was highest in the INL, consistent with the location of Pax2 immunofluorescence in the nuclei of Müller glia.
By using RT-PCR, we found that the Pax2 splice variant detected in the ONH contained 210 extra nucleotides (Fig. 4a). Primers were designed to span different introns, one set with the forward primer in exon 4 and the reverse primer in the 210-bp insertion and a second set with the forward primer in the 210-bp insertion and the reverse primer in the 3′ UTR of the transcript, several exons downstream (Table 2). PCR products were used to generate a contig spanning the novel exon. BLAT (BLAST-Like Alignment Tool) alignment revealed that the 210-bp insertion occurs between exon 6 and exon 7 of the chicken Pax2 gene. Cross-species sequence alignment demonstrated that the 210-bp insertion was very similar to sequences described for Xenopus tropicalis and Danio rerio, but similar sequences were not found in mouse or human (Fig. 4b). The Gallus protein database was searched using the contig consensus sequence using tBLASTx. As expected, identity was confirmed for Gallus Pax2; however, high homology was also seen with Pax5 and Pax3, because of the paired domain shared in this family. Further alignments to human and Xenopus Pax2 sequences revealed that the reported chicken Pax2 (NM_204793) most likely codes for Pax2b (cPax2b) based on the absence of the sequence that codes exon 6 in Xenopus, mouse, and human (Heller and Brandli, 1997). The insertion of the 210 bp reveals an exon and a splice variant with sequence homology to exon 8 of Xenopus Pax2 (Fig. 4b). Accordingly, we termed this variant cPax2+Xenopus-exon 8-like (cPax2+XE8L). The translation of cPax2b+XE8L aligns most closely with Xenopus isoform 8. The translation of the contig including the XE8L revealed that the protein remains in frame.
TABLE 2.
Pax2 Expression in the Retina of Various Species1
| Species | Nerve head | Peripapillary glia | Central retina (INL/MG) | Peripheral retina (INL/MG) | Astrocytes (NFL) |
|---|---|---|---|---|---|
| Chick | + | + | + | − | − |
| Guinea pig | + | + | − | − | − |
| Mouse | + | + | − | − | + |
| Dog | + | − | − | + | + |
| Monkey | nd | nd | − | − | + |
INL, inner nuclear layer; MG, Müller glia; NFL, nerve fiber layer; nd, not done.
Pax2 is expressed and up-regulated in Müller glia that reenter the cell cycle
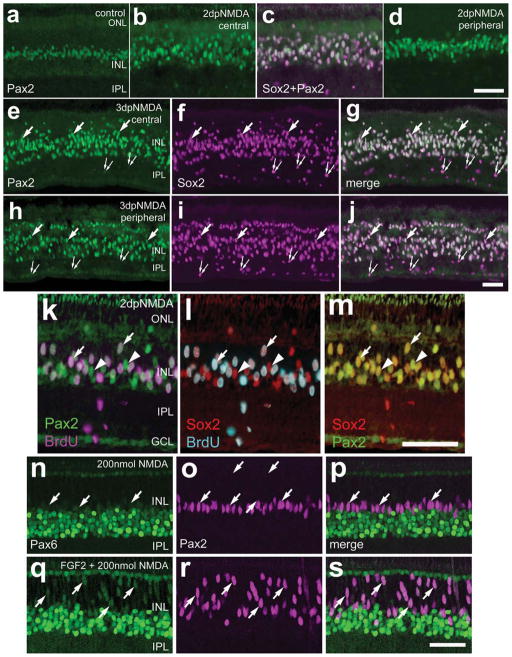
Müller glia have been shown to dedifferentiate and proliferate after an acute retinal insult of sufficient magnitude (Fischer and Reh, 2001, 2003). To examine the expression of Pax2 in Müller glia in injured retinas, NMDA was used to destroy amacrine and bipolar cells, as described in prior reports (Fischer et al., 2002a,b; Fischer and Reh, 2001, 2002). To assay for the expression of Pax2 in Müller glia, we applied antibodies to Pax2 and Sox2. In undamaged retinas, Pax2+ glial nuclei were observed in the middle of the INL (Fig. 5a). Two days after NMDA treatment, the Pax2+/Sox2+ nuclei in the INL had delaminated, and levels of Pax2 immunofluorescence appeared elevated, particularly in peripheral regions of the retina (Fig. 5b–d). To determine whether proliferating Müller glia express Pax2, we injected BrdU 6 hours prior to sacrifice at 2 days after NMDA treatment. More than half (58.3% ± 7.5%) of the Sox2+ Müller glia nuclei incorporated BrdU (Fig. 5k–m). The vast majority (~83%) of the BrdU+ cells were Sox2+/Pax2+ cells, whereas few (~17%) of the BrdU+ cells were labeled for Sox2 alone. The Sox2+/Pax2− cells were scattered across the IPL and GCL. The Sox2+ nuclei of the IPL and GCL are those of NIRG cells, which are known to proliferate in response to injury (Fischer et al., 2009c); the Sox2+ cholinergic amacrine cells are destroyed by the NMDA treatment.
Figure 5.
a–s: Pax2 is expressed by Müller glia that reenter the cell cycle in acutely damaged retinas. Vertical sections of chick retina were labeled with antibodies to Sox2 (magenta in a–I; red in l and m), Pax2 (green in a–m; magenta in n–s), BrdU (magenta in k; turquoise in l), and Pax6 (green in n–s). Retinas were obtained from eyes that were injected with saline (a), 2,000 nmol NMDA (b–m), or 200 ng FGF2 at P5 and P6 and 200 nmol NMDA at P7 (n–s). Retinas were harvested 6 hours after injection of BrdU at 2 (b–d and k–m) and 3 (e–j) days after NMDA treatment or 24 hours after injection of 200 nmol NMDA with FGF2 pretreatment (n–s). Widefield (a–m) and confocal (n–s) microscopy were used to obtain images. Arrows indicate nuclei of Müller glia that are labeled for Pax2 and BrdU, Sox2, or Pax6; small double arrows indicate the nuclei of presumptive NIRG cells that are labeled for Sox2 alone (e–j); arrowheads indicate nuclei of Müller glia that are labeled for Pax2 and Sox2 but not BrdU (k–m). INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer. Scale bars = 50 μm in d (applies to a–d); 50 μm in j (applies to e–j); 50 μm in m (applies to k–m); 50 μm in s (applies to o–s). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Although significant numbers of amacrine and bipolar cells are destroyed by lower doses (<1,000 nmol) of NMDA, these lower doses fail to stimulate the proliferation of Müller glia (Fischer et al., 2004b; Fischer and Reh, 2001). However, activation of the MAPK pathway by FGF2 in conjunction with low levels of retinal damage or with coinjection of insulin, or IGF1, results in a dramatic increase in glial proliferation and transdifferentiation (Fischer et al., 2002b, 2009b,c). Thus, we sought to determine whether the expression of Pax2 is influenced by treatment with low doses of NMDA (200 nmol) and FGF2. Low doses of NMDA increased the expression of Pax2, but not Pax6, in the Müller glia (Fig. 5n–p), without inducing proliferation (data not shown). By comparison, treatment with FGF2 before a low dose of NMDA stimulated glial proliferation (Fischer et al., 2009b) and appeared to increase expression levels of Pax2 and Pax6 in the nuclei of Müller glia that delaminated from the center of the INL (Fig. 5q–s).
Pax2 is expressed by Müller glia that are treated with IGF1 and FGF2
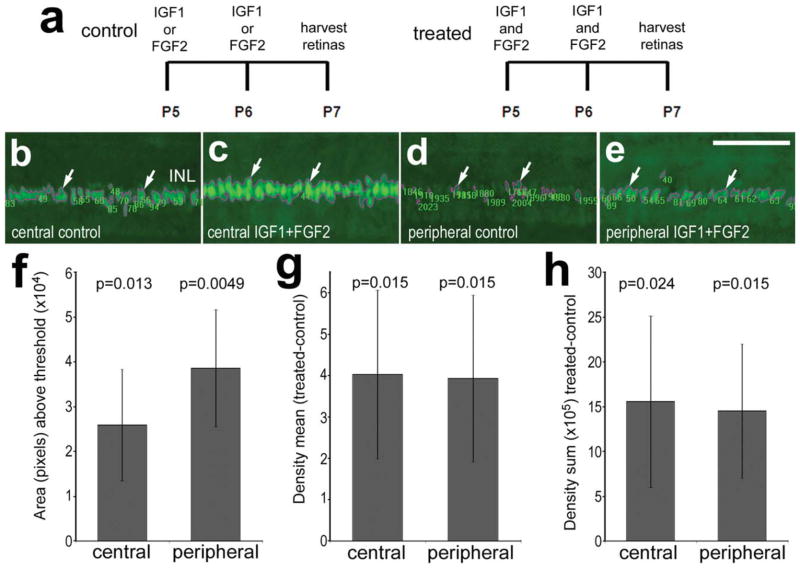
Combined and sustained exposure to insulin and FGF2 has been shown to stimulate the proliferation and trans-differentiation of Müller glia without damaging retinal neurons (Fischer et al., 2002b). In this paradigm, insulin has been assumed to act at IGF1 receptors. Thus, we next sought to determine whether the combination of FGF2 and IGF1 influences the expression of Pax2 in the Müller glia. After two consecutive daily injections of FGF2 and IGF1, there were greater levels of Pax2 immunoreactivity in the nuclei of Müller glia in both the central and the peripheral regions of the retina compared with Pax2 levels in vehicle-treated retinas (Fig. 6). Treatment with either factor alone did not significantly influence the expression of Pax2 in the Müller glia (data not shown).
Figure 6.
a–h: Intraocular injections of FGF2 and IGF1 stimulate the expression of Pax2 in the Müller glia in undamaged retinas. Retinas were obtained from eyes that had received consecutive daily injections of IGF1 or FGF2 alone (a,b,d) or the combination of IGF1 and FGF2 (a,c,e). Vertical sections of central (b,c) and peripheral (d,e) regions of the retina were labeled with antibodies to Pax2 (b–e). The nuclei outlined in magenta with associated numbers indicate the areas with pixel values above threshold as designated by Image Pro 6.2 (as described in Materials and Methods). The histograms indicate the mean (±SD) area (f), density (g), and density sum (h) for Pax2 immunofluorescence above threshold in the INL. P values were determined by using a paired Student’s t-test. INL, inner nuclear layer. Scale bar = 50 μm. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Pax2 is expressed in the retinas of adult mammals
Because Pax2 was expressed by glial cells in the chick retina, we sought to examine the expression of Pax2 in the retinas of different species, beginning with the guinea pig. Unlike the Müller glia in the chick retina, the Müller glia in the guinea pig retina did not express Pax2 (Fig. 7a). Immunoreactivity for Pax2 was not detected in any cells within the neural retina (Fig. 7a). Similar to the patterns of expression seen in the chick retina, Sox2 immunoreactivity was detected in the nuclei of Müller glia and presumptive cholinergic amacrine cells (Fig. 7a,b). Pax2 expression was detected in the nuclei of cells in the ONH and optic nerve (Fig. 7b–e). Cells within the ONH were intensely immunoreactive for both Pax2 and Sox2 (Fig. 7b–e), suggesting that these cells were glial. Some of the Pax2-expressing cells were found a short distance into the retina in the NFL (Fig. 7b–e). Cells closer to the vit-read surface of the ONH appeared to express higher levels of Pax2 compared with those farther away from the retina (Fig. 7b).
Figure 7.
In the guinea pig eye, Pax2 is expressed by cells in the optic nerve head but not by cells within the retina. Transverse sections of eye were labeled with antibodies to Pax2 and Sox2. Images were obtained for central regions of retina (a) and at the optic nerve head (b). Arrowheads indicate Sox2-positive nuclei in Müller glia; small double arrows indicate Sox2-positive nuclei in cholinergic amacrine cells. c–e are twofold enlargements of the boxed area in b. Arrows indicate optic nerve glia labeled for Pax2 and Sox2 in the peripapillary region of the optic nerve; carets in c–e indicate a population of cells in the peripapillary region that is Pax2 negative and weakly Sox2 positive. ONL, outer nuclear layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer. Scale bars = 50 μm in a; 50 μm in e (applies to c–e). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Similar to the expression patterns seen in the eyes of chicks and guinea pigs, Pax2 expression in the mouse was seen within glial cells that are scattered throughout the optic nerve and nerve head (Fig. 8a–d). These glial cells expressed both Pax2 and Sox2 (Fig. 8a–g). Unlike the expression patterns seen in the retinas of chicks and guinea pigs, Pax2 was expressed by astrocytes within the vitread layers of the mouse retina. All of the astrocytes found within the optic nerve, GCL, and NFL were immunoreactive for Pax2 and Sox2 (Fig. 8h–j) or Pax2 and GFAP (Fig. 8k). Identical to the patterns of expression observed in chick and guinea pig, Sox2 was present in the nuclei of Müller glia, stratified in the middle of the INL and the nuclei of cholinergic amacrine cells in the sclerad INL and displaced to the GCL in the mouse retina (Fig. 8e–j). The Sox2+ cholinergic cells were identified based on the position and distribution within the retina and colabeling for the transcription factor Islet1 (not shown). Pax2 was found in all GFAP+ astrocytes that were found within the GCL or NFL across central and peripheral regions of the retina (Fig. 8k).
Figure 8.
a–k: In the mouse eye, Pax2 is expressed by cells in the optic nerve head and retinal astrocytes. Transverse sections of the eye were labeled with DAPI (blue in a,j) and antibodies to Pax2 (green), Sox2 (magenta in a–j), and GFAP (magenta in k). Images were obtained using widefield (a–j) and confocal (k) microscopy. b–g: Twofold enlargements of the boxed areas in a. Arrowheads indicate Sox2+ nuclei of Müller glia; carets indicate Sox2+/Pax2+ peripapillary glia; small double arrows indicate the Sox2+ nuclei of cholinergic amacrine cells; arrows indicate astrocytes in the GCL or NFL in the retina; k includes orthogonal projections. ONL, outer nuclear layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer; NFL, nerve fiber layer. Scale bar = 50 μm for a. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
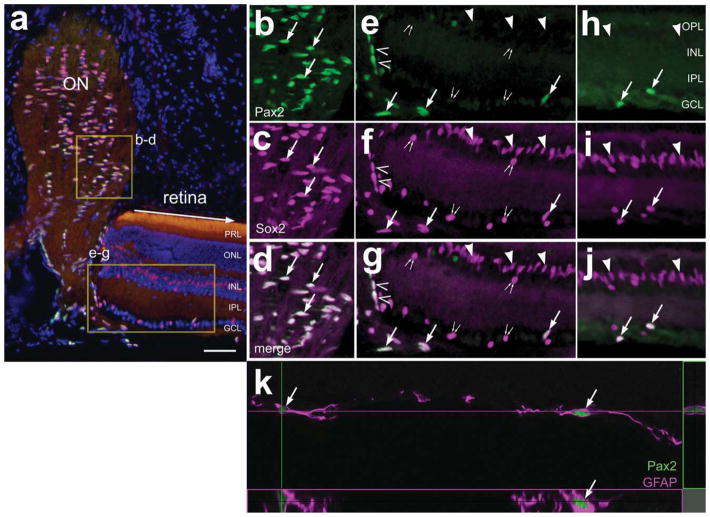
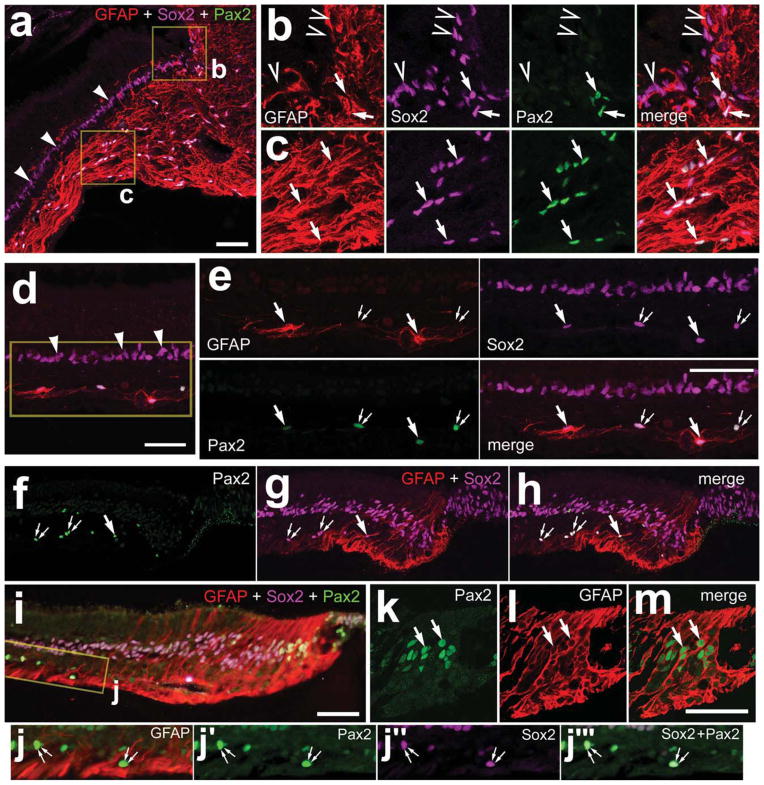
The expression of Pax2 within the dog retina was similar to that observed in the mouse retina. As in the rodent, numerous Pax2+ cells were observed in the optic nerve and nerve head (Fig. 9a–c). However, Pax2 was not observed in the peripapillary glia at the transition between the neural retina and the optic nerve, whereas these cells were immunoreactive for Sox2 and GFAP (Fig. 9a–c). All of the Pax2+ cells in the optic nerve and nerve head were immunoreactive for Sox2 (Fig. 9a–c). Within the retina, many Pax2+ cells were scattered across inner layers of the retina (Fig. 9d–f). In addition to Sox2 labeling in the nuclei of presumptive Müller glia in the INL, Sox2 colocalized to the nuclei of all Pax2+ cells in the GCL and NFL (Fig. 9d,e,j). Approximately half (46.1% ± 6.2%) of the Pax2+ cells in the GCL and NFL were immunoreactive for GFAP, whereas many of the Pax2+ cells did not contain detectable levels of GFAP (Fig. 9d,e,j). The identity of the Pax2+/GFAP− cells remains uncertain. We observed, in the eyes of younger (<2 years of age) and older (>5 years of age) dogs, a zone of vertically oriented GFAP+/ Sox2+ cells at the peripheral edge of the retina (Fig. 9f–h). In younger animals, the zone of GFAP+ cells was relatively narrow, and these cells were negative for Pax2 (Fig. 9f–h). By comparison, this zone of GFAP+ cells was wider in older animals, and many of these cells expressed Pax2 and Sox2 (Fig. 9i–m).
Figure 9.
In the dog eye, Pax2 is expressed by cells at the peripheral margin and by retinal astrocytes. Transverse sections of the dog retina and optic nerve head were labeled with antibodies to GFAP (red), Pax2 (green), and Sox2 (magenta). Images were obtained at the optic nerve head (a–c), midperipheral retina (d,e), and far peripheral retina (f–m). Tissues were obtained from younger (<2 years of age; a–h) and older (>5 years of age; i–m) animals. b,c: Threefold enlargements of the boxed areas in a; e: 1.5-fold enlargements of the boxed area in d; j: twofold enlargements of the boxed area in i. Arrows indicate astrocytes labeled for Pax2, Sox2, and GFAP; arrowheads indicate Sox2+ nuclei of Müller glia; small double arrows indicate cells in the GCL and/or NFL labeled for Sox2 and Pax2 (not GFAP); carets indicate peripapillary glia. NFL, nerve fiber layer; GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer. Scale bars = 50 μm in a; 50 μm in d; 50 μm in e; 50 μm in i (applies to f–i); 50 μm in m (applies to k–m). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
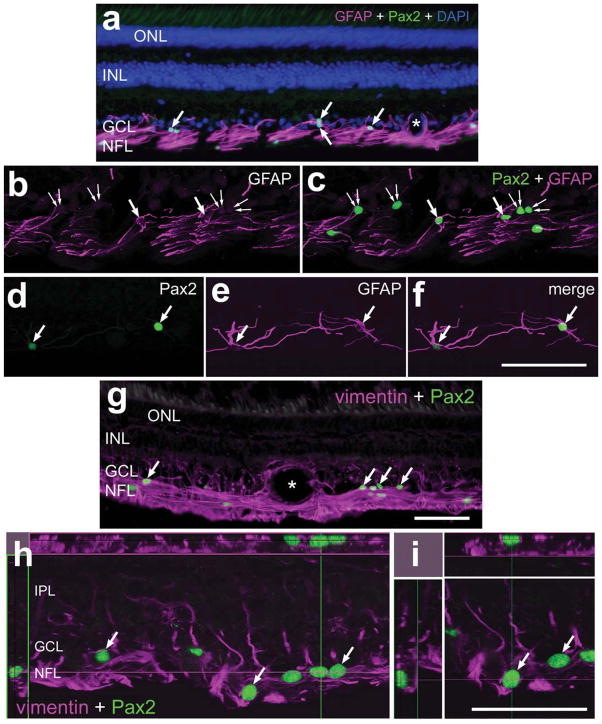
We next probed for Pax2 expression in the adult monkey retina. Similar to the patterns of labeling in the retinas of other species, the monkey retina contained Pax2+ cells scattered across the GCL and NFL (Fig. 10a,g). In addition, Sox2 immunoreactivity was seen in all Müller glia and all of the Pax2+ cells in the inner retinal layers (not shown). Many (42.6% ± 7.1%) of the Pax2+ cells in the GCL and NFL were immunoreactive for GFAP, indicating that these cells were astrocytes (Fig. 10b–f). The identity of Pax2+/GFAP− cells remains uncertain (Fig. 10b,c). The Pax2+ cells were found in central (Fig. 10a–c) and peripheral (Fig. 10d–f) regions of the retina. The Pax2+ cells did not express detectable levels of vimentin but were surrounded by vimentin+ end-feet of Müller glia (Fig. 10g–i). Unlike the case in the dog, we failed to find a cluster of Pax2-positive cells at the peripheral edge of the monkey retina (data not shown).
Figure 10.
In the monkey eye, Pax2 is expressed by retinal astrocytes. Vertical sections of the retina were labeled with antibodies to Pax2 (green) and GFAP (magenta in a–f) or vimentin (magenta in g–i). Nuclei were labeled with DAPI (blue in a). Widefield (a,g) and confocal (b–f,h,i) microscopy were used to obtain images. Orthogonal projections were reconstructed from Z-series stacks of confocal images (h,i). The orthogonal reconstructions are centered on the nuclei of Pax2+/vimentin− astrocytes. Arrows indicate Pax2+ astrocytes colabeled for GFAP; small double arrows indicate cells labeled for Pax2 alone. NFL, nerve fiber layer; GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer. Scale bars = 50 μm in f (applies to b–f); 50 μm in g (applies to a,g); 50 μm in i (applies to h,i). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
DISCUSSION
This paper describes the patterns of expression of Pax2 in the developing and mature chick retina and in the retinas of different species of adult mammals. In all species surveyed, Pax2 is expressed by presumptive astrocytes within the optic nerve and nerve head. All of the different types of Pax2+ glial cells in the optic nerve and retina also expressed Sox2, a transcription factor known to be expressed by neural stem cells and glia (Fischer et al., 2009a; Lin et al., 2009; Van Raay et al., 2005). Unique to chick retina, Pax2 is expressed by Müller glia in a central-to-peripheral gradient that becomes more pronounced as development proceeds. After acute injury or sustained exposure to IGF1 and FGF2, Pax2 is up-regulated in the Müller glia. Unlike the retinas of chicks and guinea pigs, Pax2 is expressed by the astrocytes within the NFL and GCL in monkey, dog, and mouse. Unique among the mammalian retinas that we studied, Pax2+/ Sox2+ astrocytes were restricted to the optic nerve and nerve head of the guinea pig eye. No Pax2 expression was seen in the Müller glia of guinea pig, mouse, dog, and monkey. However, we found a small patch of reactive glia-like cells at the peripheral edge of the retina of older dogs. A summary of Pax2 expression in the mature retina and optic nerve is provided in Table 2.
Variants of Pax2
We identified two isoforms of Pax2 in the chick retina and optic nerve, but we cannot exclude the possibility of additional isoforms. The Pax2 antibody that we used was raised to amino acids 188–385 of a recombinant human Pax2 and is known to recognize two distinct isoforms, Pax2a and Pax2b (Dressler and Douglass, 1992). The Pax2 antibody recognized two distinct isoforms in our Western blot studies. It is likely that the polyclonal antibody recognized both cPax2b and cPax2b+XE8L given that the primary amino acid sequence to which the antibody was raised includes residues up- and downstream of the splice insertion. However, we cannot exclude the possibility that there are Pax2 variants within ocular tissues that the antibody fails to recognize, because the variations change the antigenicity. Furthermore, it is possible that chick retina contains Pax2 splice variants in addition to those that we detected using RT-PCR. The splice variants amplified by the primers that we designed included only Pax2b variants. Reports have indicated the potential for many different Pax2 splice variants. The first description of Pax2 indicated a splice variant that contains an additional 69 nucleotide sequence, now known as Pax2a (Dressler et al., 1990). Additional studies have shown that regions of the Pax2 paired domain that mediate DNA binding can be interrupted through alternative splicing. The C-terminus of Pax2 contains the transactivation domain (Lechner and Dressler, 1996), and it is possible that alternative splicing creates a frame shift that may interrupt this domain and render the transactivation domain less potent. In Xenopus, there are as many as 13 exons and nine different splice variants of the Pax2 transcript, all of which maintain the reading frame of Pax2 (Heller and Brandli, 1997). Alignments of cPax2b-XE8L with Xenopus sequences demonstrate that homology with the XE8L exon lies between exons 8 and 9 of the Xenopus gene. Given that we did not detect sequence for exon 6 in Pax2 transcripts in the chicken retina, and that human and Xenopus Pax2 genes have at least 10 exons, it seems likely that additional exons reside downstream of the primer sets that we used.
Significance of Pax2 in Müller glia
Müller glia that are “primed” to proliferate express increased levels of Pax2. Recently, it has been shown that FGF2 predisposes the Müller glia to reenter the cell cycle (Fischer et al., 2009b). After 2 days of consecutive doses of FGF2 and IGF1, a dose regimen that is insufficient to drive the glia back into the cell cycle, increased expression of Pax2 was seen in the Müller glia. Under the “priming” conditions of FGF2 treatment, Müller glia are known to reenter the cell cycle in far peripheral regions of the retina after a modest excitotoxic insult that otherwise would not stimulate glial proliferation (Fischer et al., 2009b). It is worth noting that, in response to injury and exogenous insulin and FGF2, Müller glia in the peripheral regions of the chick retina have a greater capacity to proliferate and produce new neurons (Fischer, 2005; Fischer et al., 2002b, 2009b). Coincidently, levels of Pax2 in the Müller glia are low or undetectable in peripheral regions of the retina. This correlation suggests that Pax2 may act to suppress the transdifferentiation of Müller glia or to promote the glial phenotype in central regions of the retina.
Pax2 in retinal astrocytes and optic nerve glia
Regardless of the species, Pax2 is expressed by cells within the optic nerve and nerve head. All of the Pax2+ glial cells coexpress Sox2 and are most likely to be glial cells. Glial cells in the optic nerve are known to express transcription factors in addition to Pax2 and Sox2. For example, Vax1 and Vax2 are expressed by differentiating glial cells in the optic nerve (Mui et al., 2005). However, it remains uncertain whether the expression of Vax transcription factors is maintained in mature glial cells in the optic nerve. In addition, there are many reports that nuclear factor-κB is normally expressed by astrocytes in the optic nerve (Agapova et al., 2006). Furthermore, we have observed that presumptive astrocytes in the optic nerves of different classes of vertebrates express Sox9 and Nkx2.2 (Fischer, Zelinka, and Scott, unpublished observations). It seems likely that the transcription factors expressed by optic nerve glia function to maintain the phenotype of these cells under normal and disease conditions.
The astrocytes that migrate into the retina from the optic nerve share similar phenotypes. All of these glia express Pax2 and Sox2, whereas the expression of GFAP was variable. Although all of the Pax2+/Sox2+ astrocytes in the mouse retina are intensely immunoreactive for GFAP, about half of the Pax2+/Sox2+ cells in the retinas of dogs and monkeys are negative for GFAP. We propose that the Pax2+/GFAP− cells within the retinas of dogs and monkeys are some type of nonastrocytic inner retinal glia-like cells. For example, we have recently identified a novel type of glial cell, termed nonastrocytic inner retinal glia-like (NIRG) cells, in the chick retina (Fischer et al., 2009c). The NIRG cells are distinctly different from astrocytes and normally express Sox2, Sox9, Nkx2.2, vimentin, and transitin (nestin) but not other glial markers such as GFAP, TopAP, and glutamine synthetase (Fischer et al., 2009c). However, our data indicate that Pax2 is expressed only by Müller glia, and not by other cells types, including NIRG cells, in the chick retina.
Many studies have demonstrated that Pax2 promotes a glial phenotype in the developing optic nerve (Schulte et al., 1999; Sehgal et al., 2008; Washbourne and McAllister, 2002). Consistent with this notion, a recent report has demonstrated the expression of Pax2 in a subset of astrocytes in the optic nerve of adult goldfish where ongoing remodeling occurs with the addition new ganglion cell axons throughout the life of the animal (Parrilla et al., 2009). The mechanisms by which Pax2 promotes the differentiation of glial cells in the optic stalk are slowly being revealed. Pax2 may inhibit neuronal phenotypes by suppressing the expression of proneural genes and by inhibiting the expression of Pax6, which promotes the multipotency of retinal progenitors (for review see Marquardt, 2003). Our findings suggest that Pax2 may function to maintain glial phenotypes in mature eyes. Little or no Pax2 was seen in Müller glia in peripheral regions of the retina, where these glia are known to be more plastic, proliferative, and neurogenic compared with glia in central regions of the retina (for review see Fischer, 2005). However, the majority of cells produced by Müller glia-derived progenitors do not differentiate into neurons but remain as progenitor-like cells or form new glia (Fischer et al., 2002b; Fischer and Reh, 2001). Collectively, these observations suggest that Pax2 may maintain glial phenotype, and suppress neurogenic potential, in Müller cells in the chick retina. We propose that increased expression levels of Pax2 in Müller glia treated with IGF1 and FGF2 may act to suppress neurogenesis and maintain glial phenotype.
Coexpression of Pax6 and Pax2
The mutual inhibition between Pax6 and Pax2 is likely to be context specific. For example, the reciprocal suppression between Pax6 and Pax2 in ventral optic structures is known to be important for proper patterning of the optic nerve and nerve head (Schwarz et al., 2000). However, we find that Pax6 and Pax2 are coexpressed in Müller glia that have reentered the cell cycle in response to FGF2 and acute retinal damage. This finding suggests that the mechanisms that mediate reciprocal inhibition between Pax2 and Pax6 are not active within stimulated Müller glia. The notion that the reciprocal inhibition of Pax2 and Pax6 is context specific is supported by several reports. For example, coexpression of Pax2 and Pax6 is seen in early optic vesicle, with segregation of these transcription factors occurring at about E12.5 as the optic nerve matures (Nornes et al., 1990). Similarly, Pax2 and Pax6 are coexpressed by prospective RPE cells, and these factors function redundantly and additively to promote early stages of RPE differentiation (Baumer et al., 2003). It seems likely that different combinations of binding partners, phosphorylation states, DNA/promoter methylation, and possibly splice variants determine whether reciprocal inhibition of Pax6 and Pax2 occurs. It is also possible that the Pax2 isoform expressed by Müller glia lacks the capacity to inhibit Pax6 expression. Further studies are required to understand better the roles of Pax2 and Pax6 in the Müller glia of the chick retina.
CONCLUSIONS
We conclude that patterns of Pax2 expression in mammalian eyes are similar across species. These patterns include Pax2 expression by glial cells in the optic nerve, peripapillary glia surrounding the ONH, and astrocytes within the retina. The notable exception for Pax2 expression in retinal astrocytes is the guinea pig, in which this glial cell type does not migrate into the neural retina. Another exception to the uniform patterns of expression seen in mammalian eyes is the presence of Pax2 in Müller glia in central regions of the mature chick retina. We conclude that two distinct isoforms of Pax2 are expressed in the chick retina and optic nerve and that one of these isoforms includes a splice variant with an insertion between exons 6 and 7 that resembles the sequence for Xenopus exon 8.
Acknowledgments
Grant sponsor: National Institutes of Health, National Eye Institute; Grant number: EY016043 (to A.J.F.); Grant number: EY015480 (to H.M.E.); Grant sponsor: OSU Graduate School Presidential Fellowship (2008, to J.S.).
We thank Dr. John Buford (The Ohio State University) for providing for providing primate tissues, Dr. Simon Petersen-Jones (Michigan State University) for providing canine tissues, Dr. Karl Obrietan (The Ohio State University) for providing murine tissues, and Dr. Jackie Wood (The Ohio State University) for providing guinea pig tissues. The Pax6, BrdU, and vimentin antibodies developed by Drs. A. Kawakami, S.J. Kaufman, and A. Alvarez-Buylla, respectively, were obtained from the Developmental Studies Hybridoma Bank developed under auspices of the NICHD and maintained by the Department of Biological Sciences, University of Iowa (Iowa City, IA 52242). Confocal microscopy was performed at the Hunt-Curtis Imaging Facility at the Department of Neuroscience of The Ohio State University. Additionally, the authors thank Megan Cermak, Sarada Eleswarpu, and Christopher Zelinka for providing technical support.
LITERATURE CITED
- Agapova OA, Kaufman PL, Hernandez MR. Androgen receptor and NFκB expression in human normal and glaucomatous optic nerve head astrocytes in vitro and in experimental glaucoma. Exp Eye Res. 2006;82:1053–1059. doi: 10.1016/j.exer.2005.10.021. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Nottebohm F. Migration of young neurons in adult avian brain. Nature. 1988;335:353–354. doi: 10.1038/335353a0. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Buskirk DR, Nottebohm F. Monoclonal antibody reveals radial glia in adult avian brain. J Comp Neurol. 1987;264:159–170. doi: 10.1002/cne.902640203. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Theelen M, Nottebohm F. Mapping of radial glia and of a new cell type in adult canary brain. J Neurosci. 1988;8:2707–2712. doi: 10.1523/JNEUROSCI.08-08-02707.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumer N, Marquardt T, Stoykova A, Spieler D, Treichel D, Ashery-Padan R, Gruss P. Retinal pigmented epithelium determination requires the redundant activities of Pax2 and Pax6. Development. 2003;130:2903–2915. doi: 10.1242/dev.00450. [DOI] [PubMed] [Google Scholar]
- Busse A, Rietz A, Schwartz S, Thiel E, Keilholz U. An intron 9 containing splice variant of PAX2. J Transl Med. 2009;7:36. doi: 10.1186/1479-5876-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalepakis G, Fritsch R, Fickenscher H, Deutsch U, Goulding M, Gruss P. The molecular basis of the undulated/ Pax-1 mutation. Cell. 1991;66:873–884. doi: 10.1016/0092-8674(91)90434-z. [DOI] [PubMed] [Google Scholar]
- Czerny T, Schaffner G, Busslinger M. DNA sequence recognition by Pax proteins: bipartite structure of the paired domain and its binding site. Genes Dev. 1993;7:2048–2061. doi: 10.1101/gad.7.10.2048. [DOI] [PubMed] [Google Scholar]
- Dakubo GD, Wang YP, Mazerolle C, Campsall K, McMahon AP, Wallace VA. Retinal ganglion cell-derived sonic hedgehog signaling is required for optic disc and stalk neuroepithelial cell development. Development. 2003;130:2967–2980. doi: 10.1242/dev.00515. [DOI] [PubMed] [Google Scholar]
- Dressler GR, Douglass EC. Pax-2 is a DNA-binding protein expressed in embryonic kidney and Wilms tumor. Proc Natl Acad Sci USA. 1992;89:1179–1183. doi: 10.1073/pnas.89.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressler GR, Deutsch U, Chowdhury K, Nornes HO, Gruss P. Pax2, a new murine paired-box-containing gene and its expression in the developing excretory system. Development. 1990;109:787–795. doi: 10.1242/dev.109.4.787. [DOI] [PubMed] [Google Scholar]
- Fischer AJ. Neural regeneration in the chick retina. Prog Ret Eye Res. 2005;24:161–182. doi: 10.1016/j.preteyeres.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Reh TA. Identification of a proliferating marginal zone of retinal progenitors in postnatal chickens. Dev Biol. 2000;220:197–210. doi: 10.1006/dbio.2000.9640. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Reh TA. Muller glia are a potential source of neural regeneration in the postnatal chicken retina. Nat Neurosci. 2001;4:247–252. doi: 10.1038/85090. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Reh TA. Exogenous growth factors stimulate the regeneration of ganglion cells in the chicken retina. Dev Biol. 2002;251:367–379. doi: 10.1006/dbio.2002.0813. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Reh TA. Potential of Muller glia to become neurogenic retinal progenitor cells. Glia. 2003;43:70–76. doi: 10.1002/glia.10218. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Seltner RL, Poon J, Stell WK. Immunocytochemical characterization of quisqualic acid- and N-methyl-D-aspartate-induced excitotoxicity in the retina of chicks. J Comp Neurol. 1998;393:1–15. [PubMed] [Google Scholar]
- Fischer AJ, Dierks BD, Reh TA. Exogenous growth factors induce the production of ganglion cells at the retinal margin. Development. 2002a;129:2283–2291. doi: 10.1242/dev.129.9.2283. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, McGuire CR, Dierks BD, Reh TA. Insulin and fibroblast growth factor 2 activate a neurogenic program in Muller glia of the chicken retina. J Neurosci. 2002b;22:9387–9398. doi: 10.1523/JNEUROSCI.22-21-09387.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ, Omar G, Eubanks J, McGuire CR, Dierks BD, Reh TA. Different aspects of gliosis in retinal Muller glia can be induced by CNTF, insulin, and FGF2 in the absence of damage. Mol Vis. 2004a;10:973–986. [PubMed] [Google Scholar]
- Fischer AJ, Schmidt M, Omar G, Reh TA. BMP4 and CNTF are neuroprotective and suppress damage-induced proliferation of Muller glia in the retina. Mol Cell Neurosci. 2004b;27:531–542. doi: 10.1016/j.mcn.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Stanke JJ, Aloisio G, Hoy H, Stell WK. Heterogeniety of horizontal cells in the chicken retina. J Comp Neurol. 2007;500:1154–1171. doi: 10.1002/cne.21236. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Scott MA, Ritchey ER, Sherwood P. Mitogen-activated protein kinase-signaling regulates the ability of Muller glia to proliferate and protect retinal neurons against excitotoxicity. Glia. 2009a;57:1538–1552. doi: 10.1002/glia.20868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ, Scott MA, Tuten W. Mitogen-activated protein kinase-signaling stimulates Muller glia to proliferate in acutely damaged chicken retina. Glia. 2009b;57:166–181. doi: 10.1002/glia.20743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ, Scott MA, Zelinka C, Sherwood P. A novel type of glial cell in the retina is stimulated by insulin-like growth factor 1 to proliferate and exacerbate damage to neurons and Müller glia. Glia. 2009c doi: 10.1002/glia.20950. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke FE, Schachenmayr W, Osborn M, Altmannsberger M. Unexpected immunoreactivities of intermediate filament antibodies in human brain and brain tumors. Am J Pathol. 1991;139:67–79. [PMC free article] [PubMed] [Google Scholar]
- Ghai K, Stanke JJ, Fischer AJ. Patterning of the circumferential marginal zone of progenitors in the chicken retina. Brain Res. 2008;1192:76–89. doi: 10.1016/j.brainres.2007.01.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghai K, Zelinka C, Fischer AJ. Serotonin released from amacrine neurons is scavenged and degraded in bipolar neurons in the retina. J Neurochem. 2009 doi: 10.1111/j.1471-4159.2009.06270.x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. Dev Dyn. 1992;195:231–272. doi: 10.1002/aja.1001950404. [DOI] [PubMed] [Google Scholar]
- Hayes S, Nelson BR, Buckingham B, Reh TA. Notch signaling regulates regeneration in the avian retina. Dev Biol. 2007;312:300–311. doi: 10.1016/j.ydbio.2007.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller N, Brandli AW. Xenopus Pax-2 displays multiple splice forms during embryogenesis and pronephric kidney development. Mech Dev. 1997;69:83–104. doi: 10.1016/s0925-4773(97)00158-5. [DOI] [PubMed] [Google Scholar]
- Hidalgo-Sanchez M, Millet S, Simeone A, Alvarado-Mallart RM. Comparative analysis of Otx2, Gbx2, Pax2, Fgf8 and Wnt1 gene expressions during the formation of the chick midbrain/hindbrain domain. Mech Dev. 1999;81:175–178. doi: 10.1016/s0925-4773(98)00224-x. [DOI] [PubMed] [Google Scholar]
- Karl MO, Hayes S, Nelson BR, Tan K, Buckingham B, Reh TA. Stimulation of neural regeneration in the mouse retina. Proc Natl Acad Sci USA. 2008;105:19508–19513. doi: 10.1073/pnas.0807453105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami A, Kimura-Kawakami M, Nomura T, Fuhisawa H. Distribution of PAX6 and PAX7 proteins suggest their involvement in both early and late phases of chick brain development. Mech Dev. 1997;66:119–130. doi: 10.1016/s0925-4773(97)00097-x. [DOI] [PubMed] [Google Scholar]
- Lechner MS, Dressler GR. Mapping of Pax-2 transcription activation domains. J Biol Chem. 1996;271:21088–21093. doi: 10.1074/jbc.271.35.21088. [DOI] [PubMed] [Google Scholar]
- Lin YP, Ouchi Y, Satoh S, Watanabe S. Sox2 plays a role in the induction of amacrine and Muller glial cells in mouse retinal progenitor cells. Invest Ophthalmol Vis Sci. 2009;50:68–74. doi: 10.1167/iovs.07-1619. [DOI] [PubMed] [Google Scholar]
- Macdonald R, Barth KA, Xu Q, Holder N, Mikkola I, Wilson SW. Midline signalling is required for Pax gene regulation and patterning of the eyes. Development. 1995;121:3267–3278. doi: 10.1242/dev.121.10.3267. [DOI] [PubMed] [Google Scholar]
- Marquardt T. Transcriptional control of neuronal diversification in the retina. Prog Ret Eye Res. 2003;22:567–577. doi: 10.1016/s1350-9462(03)00036-3. [DOI] [PubMed] [Google Scholar]
- Mui SH, Kim JW, Lemke G, Bertuzzi S. Vax genes ventralize the embryonic eye. Genes Dev. 2005;19:1249–1259. doi: 10.1101/gad.1276605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto K, Iijima K, Shirakawa T, Kitagawa K, Satomura K, Nakamura H, Yoshikawa N. PAX2 gene mutation in a family with isolated renal hypoplasia. J Am Soc Nephrol. 2001;12:1769–1772. doi: 10.1681/ASN.V1281769. [DOI] [PubMed] [Google Scholar]
- Nornes HO, Dressler GR, Knapik EW, Deutsch U, Gruss P. Spatially and temporally restricted expression of Pax2 during murine neurogenesis. Development. 1990;109:797–809. doi: 10.1242/dev.109.4.797. [DOI] [PubMed] [Google Scholar]
- Parrilla M, Lillo C, Herrero-Turrion MJ, Arevalo R, Lara JM, Aijon J, Velasco A. Pax2 in the optic nerve of the goldfish, a model of continuous growth. Brain Res. 2009;1255:75–88. doi: 10.1016/j.brainres.2008.12.010. [DOI] [PubMed] [Google Scholar]
- Pichaud F, Desplan C. Pax genes and eye organogenesis. Curr Opin Genet Dev. 2002;12:430–434. doi: 10.1016/s0959-437x(02)00321-0. [DOI] [PubMed] [Google Scholar]
- Poche RA, Furuta Y, Chaboissier MC, Schedl A, Behringer RR. Sox9 is expressed in mouse multipotent retinal progenitor cells and functions in Muller glial cell development. J Comp Neurol. 2008;510:237–250. doi: 10.1002/cne.21746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prada C, Puga J, Perez-Mendez L, Lopez R, Ramirez G. Spatial and temporal patterns of neurogenesis in the chick retina. Eur J Neurosci. 1991;3:559–569. doi: 10.1111/j.1460-9568.1991.tb00843.x. [DOI] [PubMed] [Google Scholar]
- Sanyanusin P, Schimmenti LA, McNoe LA, Ward TA, Pierpont ME, Sullivan MJ, Dobyns WB, Eccles MR. Mutation of the PAX2 gene in a family with optic nerve colobomas, renal anomalies and vesicoureteral reflux. Nat Genet. 1995;9:358–364. doi: 10.1038/ng0495-358. [DOI] [PubMed] [Google Scholar]
- Schulte D, Furukawa T, Peters MA, Kozak CA, Cepko CL. Misexpression of the Emx-related homeobox genes cVax and mVax2 ventralizes the retina and perturbs the retinotectal map. Neuron. 1999;24:541–553. doi: 10.1016/s0896-6273(00)81111-3. [DOI] [PubMed] [Google Scholar]
- Schwarz M, Cecconi F, Bernier G, Andrejewski N, Kammandel B, Wagner M, Gruss P. Spatial specification of mammalian eye territories by reciprocal transcriptional repression of Pax2 and Pax6. Development. 2000;127:4325–4334. doi: 10.1242/dev.127.20.4325. [DOI] [PubMed] [Google Scholar]
- Sehgal R, Karcavich R, Carlson S, Belecky-Adams TL. Ectopic Pax2 expression in chick ventral optic cup phenocopies loss of Pax2 expression. Dev Biol. 2008;319:23–33. doi: 10.1016/j.ydbio.2008.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal R, Sheibani N, Rhodes SJ, Belecky Adams TL. BMP7 and SHH regulate Pax2 in mouse retinal astrocytes by relieving TLX repression. Dev Biol. 2009;332:429–443. doi: 10.1016/j.ydbio.2009.05.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soukkarieh C, Agius E, Soula C, Cochard P. Pax2 regulates neuronal-glial cell fate choice in the embryonic optic nerve. Dev Biol. 2007;303:800–813. doi: 10.1016/j.ydbio.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Tavassoli K, Ruger W, Horst J. Alternative splicing in PAX2 generates a new reading frame and an extended conserved coding region at the carboxy terminus. Hum Genet. 1997;101:371–375. doi: 10.1007/s004390050644. [DOI] [PubMed] [Google Scholar]
- Tellier AL, Amiel J, Delezoide AL, Audollent S, Auge J, Esnault D, Encha-Razavi F, Munnich A, Lyonnet S, Vekemans M, Attie-Bitach T. Expression of the PAX2 gene in human embryos and exclusion in the CHARGE syndrome. Am J Med Genet. 2000;93:85–88. doi: 10.1002/1096-8628(20000717)93:2<85::aid-ajmg1>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Tran PB, Banisadr G, Ren D, Chenn A, Miller RJ. Chemokine receptor expression by neural progenitor cells in neurogenic regions of mouse brain. J Comp Neurol. 2007;500:1007–1033. doi: 10.1002/cne.21229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman J, Harris E, Desplan C. The paired box encodes a second DNA-binding domain in the paired homeo domain protein. Genes Dev. 1991;5:594–604. doi: 10.1101/gad.5.4.594. [DOI] [PubMed] [Google Scholar]
- Van Raay TJ, Moore KB, Iordanova I, Steele M, Jamrich M, Harris WA, Vetter ML. Frizzled 5 signaling governs the neural potential of progenitors in the developing Xenopus retina. Neuron. 2005;46:23–36. doi: 10.1016/j.neuron.2005.02.023. [DOI] [PubMed] [Google Scholar]
- Walther C, Guenet JL, Simon D, Deutsch U, Jostes B, Goulding MD, Plachov D, Balling R, Gruss P. Pax: a murine multigene family of paired box-containing genes. Genomics. 1991;11:424–434. doi: 10.1016/0888-7543(91)90151-4. [DOI] [PubMed] [Google Scholar]
- Wang YP, Dakubo G, Howley P, Campsall KD, Mazarolle CJ, Shiga SA, Lewis PM, McMahon AP, Wallace VA. Development of normal retinal organization depends on Sonic hedgehog signaling from ganglion cells. Nat Neurosci. 2002;5:831–832. doi: 10.1038/nn911. [DOI] [PubMed] [Google Scholar]
- Washbourne P, McAllister AK. Techniques for gene transfer into neurons. Curr Opin Neurobiol. 2002;12:566–573. doi: 10.1016/s0959-4388(02)00365-3. [DOI] [PubMed] [Google Scholar]
- Zhang XM, Yang XJ. Temporal and spatial effects of Sonic hedgehog signaling in chick eye morphogenesis. Dev Biol. 2001;233:271–290. doi: 10.1006/dbio.2000.0195. [DOI] [PMC free article] [PubMed] [Google Scholar]