In a large prospective study in Kampala, Uganda, we found that case investigation can be effective (10% yield) in early detection of active tuberculosis in children <15 years old, with 71% culture confirmation.
Keywords: pediatric, child, tuberculosis, risk factors, contact tracing
Abstract
Background. Tuberculosis is a large source of morbidity and mortality among children. However, limited studies characterize childhood tuberculosis disease, and contact investigation is rarely implemented in high-burden settings. In one of the largest pediatric tuberculosis contact investigation studies in a resource-limited setting, we assessed the yield of contact tracing on childhood tuberculosis and indicators for disease progression in Uganda.
Methods. Child contacts aged <15 years in Kampala, Uganda, were enrolled from July 2002 to June 2009 and evaluated for tuberculosis disease via clinical, radiographic, and laboratory methods for up to 24 months.
Results. Seven hundred sixty-one child contacts were included in the analysis. Prevalence of tuberculosis in our child population was 10%, of which 71% were culture-confirmed positive. There were no cases of disseminated tuberculosis, and 483 of 490 children (99%) started on isoniazid preventative therapy did not develop disease. Multivariable testing suggested risk factors including human immunodeficiency virus (HIV) status (odds ratio [OR], 7.90; P < .001), and baseline positive tuberculin skin test (OR, 2.21; P = .03); BCG vaccination was particularly protective, especially among children aged ≤5 years (OR, 0.23; P < .001). Adult index characteristics such as sex, HIV status, and extent or severity of disease were not associated with childhood disease.
Conclusions. Contact tracing for children in high-burden settings is able to identify a large percentage of culture-confirmed positive tuberculosis cases before dissemination of disease, while suggesting factors for disease progression to identify who may benefit from targeted screening.
(See the Editorial Commentary by Graham and Triasih on pages 1693–4.)
Childhood tuberculosis remains a neglected area of research despite the severe risk of morbidity and mortality in this population [1]. In 2011, there were an estimated 490 000 episodes of childhood tuberculosis worldwide, or 6% of the total incident cases, with 64 000 deaths [1]. Children have a higher risk of progression to active tuberculosis than adults and an increased risk of extrapulmonary complications including tuberculosis meningitis [2, 3]. Diagnosis is more complicated in children, who can present with nonspecific symptoms, paucibacillary sputum, and limited cavitation on chest radiography, placing them at further risk of misdiagnosis and delayed care [2]. Consequently, the World Health Organization (WHO) has called for greater epidemiological studies to characterize the burden and risk factors for progression of childhood tuberculosis disease [4, 5].
Children are often infected through a household contact or close relative [6]. Because of the high risk of disease, fast progression, and diagnostic challenge, contact tracing for children is recommended. However, this is rarely implemented in high-burden settings, and past contact tracing studies are limited, with short follow-up time, small sample size, or greater emphasis on risk factors for infection rather than disease [7]. We present one of the largest contact investigations to determine the yield of active tuberculosis in child contacts aged <15 years in Kampala, Uganda. In addition, we identified risk factors for progression to disease among children exposed to tuberculosis, to guide clinicians and public health practitioners on subpopulations that may benefit the most from contact tracing.
METHODS
Study Setting and Participants
The Kawempe Community Health Study is an ongoing prospective study started in July 2002 that investigates determinants of tuberculosis infection and disease in adult tuberculosis index cases and their household contacts. Adult (aged ≥18 years) index cases with an initial episode of pulmonary tuberculosis living for at least 3 months in the Kawempe and surrounding divisions of Kampala, Uganda, were recruited from the National Tuberculosis and Leprosy Programme at Old Mulago Hospital and local community clinics. A household contact was defined as an individual living in the same household (same building or a portion of the building) as an index case for at least 1 week in the last 3 months. Consenting adult and child contacts were enrolled within 3 weeks of a confirmed index case and evaluated for tuberculosis infection and disease for 24 months [8].
Child Contact Evaluation
Children were defined as individuals aged <15 years. Younger children were defined as those aged ≤5 years, and older children were those aged >5 to <15 years. Study visit evaluation for latent and active tuberculosis occurred at enrollment and months 3, 6, 12, 18, and 24, and included a history and physical examination, risk assessment survey, human immunodeficiency virus (HIV) test, sputum microscopy and culture, complete blood count, chest radiography, measurement of liver enzymes, and drug susceptibility testing for any recovered isolate.
BCG status was evaluated by the presence of a deltoid scar. Material for acid-fast bacilli smear and culture was obtained if the child was symptomatic or had suggestive findings on chest radiography. If a sputum sample could not be adequately collected, other potential sites included gastric aspirates, pleural fluid, cerebrospinal fluid, and lymph node aspirates. Culture was performed (LJ slants/BACTEC) in triplicate at baseline evaluation and sick visits, as well as at initiation of therapy and then months 1, 2, and 5 and the end of treatment.
Anthropometric measurements consisted of height (centimeters) and weight (kilograms), and height-for-age, weight-for-age, body mass index (BMI)–for-age and weight-for-height z scores were calculated according to WHO and Centers for Disease Control and Prevention growth standards [9–11]. Stunting, underweight, low BMI, and wasting were defined as a z score less than −2 for height-for-age, weight-for-age, BMI-for-age, and weight-for-height, respectively. Wasting was only indicated for children aged ≤5 years, for which weight-for-height z scores are only available. Bioelectric impedance analysis was used as previously described to measure body composition [12]. To calculate lean tissue mass, the following equation was used: [0.59 (H2/R) + 0.065W + 0.04]/[0.769 – 0.0025A – 0.19S], where H is height (centimeters); W, weight (kilograms); R, resistance (ohms); S, sex (males = 1, females = 0); A, age (years) [13]. This has been shown to be an accurate measure of lean tissue mass in both HIV-positive and -negative children [14].
HIV testing was conducted via enzyme immunoassay. Tuberculin skin testing (TST) was performed by Mantoux method (5 tuberculin units, Tubersol) and size of induration was read 48–72 hours later with digital calipers, recorded in millimeters. A positive TST was defined as an induration of ≥10 mm if the individual was aged ≥5 years and HIV negative, or ≥5 mm if the person was HIV positive or was <5 years of age due to contact with an active tuberculosis case.
Clinical diagnosis was based on positive Mycobacterium tuberculosis culture from at least 1 site, or at least 2 of the following in the context of a positive response to tuberculosis therapy: (1) symptoms of tuberculosis including fever, cough for >2 weeks, and weight loss; (2) a positive TST; (3) chest radiography consistent with active tuberculosis; or (4) failure to respond to empiric antibiotics in 2 weeks. Cases of suspected tuberculosis were reviewed by a study outcome committee consisting of at least 2 clinicians, and classified according to American Thoracic Society definitions of definite, probable, possible, and unlikely tuberculosis [15]. Definite cases were those with either (1) a positive culture with radiographic evidence; (2) positive molecular diagnosis of tuberculosis with signs/symptoms, suggestive chest radiography, or positive smear; or (3) ≥2 separate positive cultures. Probable cases were those with a positive culture but no radiographic evidence, with ≥2 of the following: clinical symptoms, positive smear, or response to active therapy.
Isoniazid (10–20 mg/kg or a maximum dose of 300 mg/day) prophylaxis for 9 months was offered to all child contacts aged <5 years, all HIV-infected contacts, and to children who were TST positive at enrollment or converted during the study period. Adherence was evaluated at follow-up visits via self-report (if an older child) or by the caretaker, and defined as completion of at least 180 daily doses in the 9 months. Child contacts clinically diagnosed with tuberculosis disease received daily isoniazid (10–20 mg/kg, maximum 300 mg), rifampin (10–20 mg/kg, maximum 600 mg), and pyrazinamide (15–30 mg/kg, maximum 2 g) for 2 months followed by daily isoniazid and rifampin for 4 months.
Analytical Strategy
Household contacts aged <15 years were included for analysis. Exclusion criteria were children who did not complete baseline evaluation or did not have at least 1 follow-up visit. The primary outcome of our analysis was a child contact classified with definite or probable active tuberculosis.
Baseline variables were given as median and interquartile range (IQR) if continuous, and categorical variables were described in frequency and percentage. Simple and multiple logistic regression was used to evaluate for risk factors for active tuberculosis. Variables that were significant in univariate testing were included in the multivariable model. Individuals with missing values were excluded from the analysis. Significance was defined as P < .05. All analyses were conducted with Stata software, version 12 (StataCorp, College Station, Texas).
Ethical Considerations
Informed consent was obtained for all index cases, parents or guardians provided written consent for the child contacts, and children aged ≥8 years provided additional assent. This study was approved by the institutional review boards of Makerere University and Case Western Reserve University, and the Uganda National Council for Science and Technology.
RESULTS
Baseline Characteristics
Seven hundred sixty-six child contacts were included from July 2002 to June 2009. Five of these children did not have baseline TST measurements and were excluded from the analysis, for a final sample of 761 children from 351 households. Baseline characteristics are outlined in Table 1, stratified by age group. Ninety-four percent of child contacts spent ≥4 days with the index case. A BCG scar suggesting vaccination was present in 82%, TST was positive in 65% (median positive TST, 15.5 mm [IQR, 13.3–17.5 mm] overall; 14.8 mm [IQR, 12.4–17.2 mm] for children aged ≤5 years; and 16 mm [IQR, 14.5–18 mm] for older children), and the HIV-positive rate was 3%. Nutritional assessment found that 6% of children were underweight, 16% were stunted, and 4% had low BMI. Wasting was found in 3% of younger children.
Table 1.
Baseline Characteristics
| Characteristic | Age ≤5 y | Age >5 y | All Children |
|---|---|---|---|
| No. | 389 (51) | 372 (49) | 761 |
| Age, y, median (IQR) | 2.80 (3.00) | 9.00 (5.00) | 6 (6) |
| Male sex | 204 (54) | 198 (51) | 402 (53) |
| HIV positive | 15 (4) | 8 (2) | 23 (3) |
| BCG scar present | 274 (83) | 273 (80) | 547 (82) |
| Baseline positive TST | 248 (67) | 245 (63) | 493 (65) |
| BMI, kg/m2, median (IQR) | 16.37 (2.34) | 15.75 (2.53) | 16.06 (2.44) |
| Low BMI | 8 (2) | 19 (5) | 27 (4) |
| Underweight | 18 (5) | 30 (8) | 48 (6) |
| Stunting | 78 (21) | 46 (12) | 124 (16) |
| Wasting | 10 (3) | … | … |
| Lean tissue mass, kg, median (IQR) | 10.65 (5.42) | 25.85 (12.64) | 16.29 (15.53) |
| Sleeping in the same bed as the index case | 127 (34) | 21 (5) | 148 (20) |
| In contact with index case ≥4 d per wk | 359 (97) | 360 (93) | 719 (94) |
Continuous variables are shown as median (IQR), categorical variables as frequency (%).
Abbreviations: BCG, Bacille Calmette Guérin; BMI, body mass index; HIV, human immunodeficiency virus; IQR, interquartile range; TST, tuberculin skin test.
Active Tuberculosis Prevalence
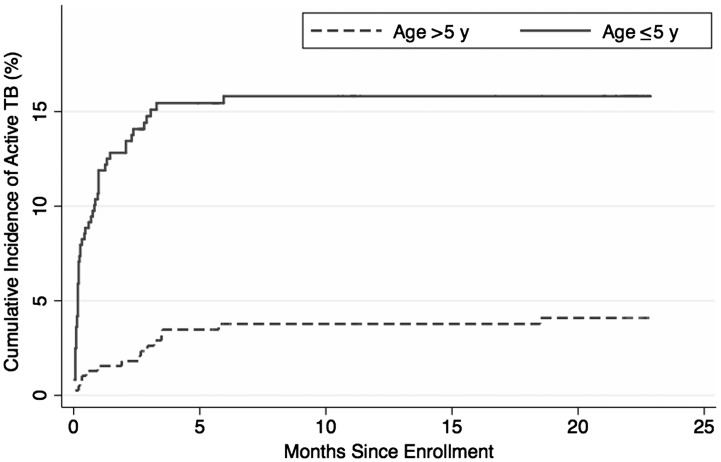
Contact investigation yielded 79 cases of active tuberculosis for a prevalence of 10%. Cases were significantly higher among younger children compared to older children (61 cases [16.4%] vs 18 cases [4.6%]; P < .001). The median time to diagnosis from enrollment was 10 days (IQR, 3–70 days), with significantly shorter time for younger than for older children (6 days [IQR, 2–30 days] vs 85 days [IQR, 15–175 days]; P < .001; Figure 1). Fifty-six (71%) of the cases had at least 1 positive culture from initial suspicion to start of treatment, and 18 (23%) had a positive smear. The number and frequency of diagnostic findings for the active cases are summarized in Table 2. The most common symptoms were cough (51 [65%]) and fever (30 [38%]), with radiographic findings suggesting extent 2 or greater in 21 (27%) of cases and bronchial adenopathy in 21 (27%) of the children with active tuberculosis. The least common symptoms were dyspnea (1%) and purulent sputum (1%). There were no cases of disseminated or extrapulmonary tuberculosis. Of the 682 children who did not develop active tuberculosis, 483 (71%) were initiated on isoniazid preventive therapy (IPT). Adherence over the first 6 months was 74%. Four children were started on IPT, but then began definitive therapy shortly after confirmation of active tuberculosis. Two tuberculosis disease cases occurred while on IPT (median days after initiation of IPT, 64.5 [IQR, 32–97 days]), and 1 case occurred after completion of 9 months of IPT (882 days later). Thus, 483 of 490 children (99%) started on IPT did not develop active tuberculosis.
Figure 1.
Cumulative incidence of childhood tuberculosis (TB) in the 24 months after enrollment.
Table 2.
Frequency of Diagnostic Findings in Active Tuberculosis Cases
| No. (%) |
|||
|---|---|---|---|
| Finding | Age ≤5 y | Age >5 y | All Children |
| Signs and symptoms | |||
| Loss of appetite | 14 (23) | 0 | 14 (18) |
| Cough | 42 (70) | 9 (50) | 51 (65) |
| Dyspnea | 1 (2) | 0 | 1 (1) |
| Fever | 26 (43) | 4 (22) | 30 (38) |
| Weight loss | 19 (32) | 0 | 19 (24) |
| Productive sputum | 14 (23) | 7 (39) | 21 (27) |
| Purulent sputum | 1 (2) | 0 | 1 (1) |
| Sweating | 6 (10) | 3 (17) | 9 (11) |
| Lymphadenopathy | 20 (33) | 2 (11) | 22 (28) |
| Abnormal respiratory exam | 26 (43) | 4 (22) | 30 (38) |
| Chest radiographya,b | |||
| Extent 2 or greater | 16 (27) | 5 (29) | 21 (27) |
| Upper lung involvement | 18 (30) | 4 (26) | 22 (29) |
| Lower lung involvement | 21 (35) | 5 (29) | 26 (34) |
| Fibrosis present | 0 | 0 | 0 |
| Cavitation | 1 (2) | 2 (12) | 3 (4) |
| Miliary | 1 (2) | 0 | 1 (1) |
| Bronchial adenopathy | 14 (23) | 7 (41) | 21 (27) |
| Effusion | 1 (2) | 1 (6) | 2 (3) |
| Lung thickening | 0 | 0 | 0 |
| Microscopyc | |||
| Culture positive | 45 (74) | 11 (61) | 56 (71) |
| Smear positive | 11 (18) | 7 (39) | 18 (23) |
a Two cases had an inadequate or missing chest radiograph at time of diagnosis.
b Based on chest radiographic findings at suspect date. If not available, findings based on radiography prior to start of treatment.
c Defined as a positive culture or smear from any site during the period from active tuberculosis suspect until initiation of tuberculosis therapy.
Risk Factors for Active Tuberculosis in Child Contacts
Univariate logistic regression for active tuberculosis among child contacts suggested that younger age, HIV infection, baseline positive TST, low lean tissue mass, and sleeping in the same bed as the index case were associated with active tuberculosis (Table 3). Multivariable logistic regression found that younger age, HIV infection (odds ratio [OR], 7.9; 95% confidence interval [CI], 2.48–25.13; P < .001), and baseline positive TST (2.21; 95% CI, 1.09–4.46; P = .03) were associated with active tuberculosis independent of sex, BCG scar, and nutritional status (stunting and lean tissue mass) (Table 4). BCG scar continued to indicate a level of protection (OR, 0.37; 95% CI, .19–.70; P = .002). When stratified by age group, HIV and baseline positive TST remained significant risk factors in the younger age group, with BCG scar as a protective factor (OR, 0.23; 95% CI, .11–.52; P < .001). For older children, the only significant risk factor was sleeping in the same bed as the index case (OR, 14.87; 95% CI, 3.54–62.45; P < .001).
Table 3.
Simple Logistic Regression of Risk Factors for Active Tuberculosis Disease (N = 761)
| Risk Factor | No Tuberculosisa,b | Active Tuberculosisa | Age ≤5 y |
Age >5 y |
All Children |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (n = 682 [90%]) | (n = 79 [10%]) | OR | 95% CI | P Value | OR | 95% CI | P Value | OR | 95% CI | P Value | |
| Age, y, median (IQR) | 6 (7) | 2 (4) | 0.72 | .59–.88 | .001 | 0.79 | .64–.98 | .03 | 0.79 | .73–.85 | <.001 |
| Age group ≤5 y | 311 (46) | 61 (77) | 4.04 | 2.34–6.99 | <.001 | ||||||
| Male sex | 364 (53) | 38 (48) | 0.7 | .41–1.22 | .21 | 0.96 | .37–2.48 | .94 | 0.81 | .51–1.29 | .38 |
| Positive HIV status | 15 (2) | 8 (11) | 4.74 | 1.64–13.63 | .004 | 3 | .35–25.78 | .32 | 4.95 | 2.03–12.14 | <.001 |
| BCG scar | 499 (83) | 48 (69) | 0.31 | .16–.59 | <.001 | 0.82 | .22–3.08 | .77 | 0.44 | .26–.76 | .003 |
| Baseline positive TST | 433 (63) | 60 (76) | 2.59 | 1.30–5.18 | .01 | 0.72 | .28–1.88 | .51 | 1.82 | 1.06–3.11 | .03 |
| Underweightc | 40 (6) | 8 (10) | 3.54 | 1.31–9.52 | .01 | 0.69 | .09–5.38 | .73 | 1.81 | .81–4.01 | .15 |
| Stuntingc | 105 (15) | 19 (24) | 1.58 | .85–2.96 | .15 | 0.93 | .21–4.18 | .92 | 1.74 | .98–3.04 | .05 |
| Low BMIc | 24 (4) | 3 (4) | 3.17 | .74–13.61 | .12 | … | … | … | 1.08 | .32–3.68 | .9 |
| Wastingc | 5 (2) | 5 (8) | 5.46 | 1.53–19.49 | .01 | ||||||
| Lean tissue mass, kg, median (IQR) | 17.36 (16.04) | 11.37 (7.88) | 0.95 | .89–1.02 | .16 | 0.96 | .90–1.02 | .2 | 0.92 | .89–.95 | <.001 |
| Sleep in same bed as index case | 119 (18) | 29 (38) | 1.35 | .76–2.38 | .31 | 9.11 | 2.86–29.0 | <.001 | 2.83 | 1.72–4.68 | <.001 |
| ≥4 d per wk contact with index case in last 1 mod | 643 (94) | 76 (96) | 1.08 | .23–5.01 | .92 | 1.39 | .18–10.82 | .75 | 1.54 | .46–5.09 | .48 |
| Index case, male sex | 235 (40) | 30 (44) | 1.6 | .88–2.92 | .12 | 0.44 | .14–1.37 | .16 | 1.17 | .70–1.93 | .55 |
| Index smear grade 3 or 4 | 312 (46) | 30 (39) | 0.78 | .44–1.38 | .4 | 0.64 | .23–1.77 | .39 | 0.76 | .47–1.23 | .26 |
| Index CXR extent 2 or 3 | 492 (85) | 59 (87) | 1.57 | .59–4.19 | .37 | 0.57 | .18–1.82 | .34 | 1.13 | .54–1.37 | .74 |
| Index baseline cavitation | 351 (61) | 45 (66) | 1.41 | .74–2.67 | .29 | 0.92 | .34–2.48 | .87 | 1.26 | .74–2.14 | .39 |
Abbreviations: BCG, Bacille Calmette Guérin; BMI, body mass index; CI, confidence interval; CXR, chest radiograph; HIV, human immunodeficiency virus; IQR, interquartile range; OR, odds ratio; TST, tuberculin skin test.
a Values given as frequency (%) unless noted.
b No tuberculosis refers to those with latent or no tuberculosis infection.
c Nutritional categories defined as a z score less than −2.
d Index refers to adult household member with active tuberculosis.
Table 4.
Multivariable Logistic Regression
| Variable | Age ≤5 y |
Age >5 y |
All Children |
||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | OR | 95% CI | P Value | |
| Age ≤5 y | … | … | … | … | … | … | 5.89 | 2.24–15.52 | <.001 |
| Male vs female | 0.72 | .36–1.41 | .34 | 0.6 | .14–2.64 | .5 | 0.73 | .40–1.31 | .29 |
| HIV positive | 14.72 | 3.26–66.55 | <.001 | 4.3 | .36–52.0 | .25 | 7.9 | 2.48–25.13 | <.001 |
| BCG scar | 0.23 | .11–.52 | <.001 | 0.42 | .09–1.90 | .26 | 0.3 | .15–.59 | <.001 |
| Baseline positive TST | 4.21 | 1.71–10.40 | .002 | 0.52 | .13–1.99 | .34 | 2.21 | 1.09–4.46 | .03 |
| Stunting | 1.61 | .74–3.52 | .23 | 0.92 | .08–10.22 | .94 | 1.51 | .75–3.03 | .25 |
| Lean tissue mass | 0.98 | .92–1.05 | .61 | 1.01 | .95–1.08 | .73 | 1 | .95–1.04 | .84 |
| Sleep in same bed as index case | 1.28 | .64–2.56 | .48 | 14.87 | 3.54–62.45 | <.001 | 1.86 | .99–3.51 | .05 |
Abbreviations: BCG, Bacille Calmette Guérin; CI, confidence interval; HIV, human immunodeficiency virus; OR, odds ratio; TST, tuberculin skin test.
DISCUSSION
Uganda is one of the 22 high-burden countries that encompass 25%–40% of all new childhood tuberculosis cases [2]. Despite the high risk of infection among children, contact tracing is rarely implemented in similar settings. Reasons include the already large need to address active disease, staff limitations, and challenges of screening in rural locations [7, 16, 17]. However, active case investigation may increase detection of tuberculosis cases in children to begin full therapy, and find more children who would qualify for IPT [18]. Therefore, we conducted one of the few large, longitudinal contact studies with children in an urban, resource-limited setting.
We found a 10% prevalence of active tuberculosis in children aged <15 years. A pooled analysis of contact studies in low and middle-income countries demonstrated a yield of 7% [19]. This suggests a high burden of disease in our population, and promotes the use of contact tracing to prevent morbidity and mortality from childhood tuberculosis. In addition to the high yield, we were able to obtain at least 1 positive culture in 71% of cases. This is greater than other studies, which can range from 30%–40% [20]. Reasons include our investigation at multiple sites and time points, and our stricter definition of definite and probable disease. We also did not have any cases of extrapulmonary or disseminated disease. Taken together, our findings suggest that active case detection for pediatric tuberculosis in Uganda is able to identify and confirm early pediatric tuberculosis cases and begin treatment, which may be able to prevent further disease complications. At the same time, only 2 children on IPT developed disease, further promoting the use of contact tracing for disease prevention.
Studies have shown that identifying key characteristics may allow for more targeted screening of children most at risk of disease progression [21, 22]. We therefore determined risk factors for active disease in our population, which may serve as an aid in tuberculosis risk stratification. Past contact studies in children have focused primarily on risk factors for tuberculosis infection but not disease. These are generally related to exposure variables, including crowding, poor ventilation, and index characteristics such as severity of disease and positive sputum smear [23, 24]. In contrast, our study found that exposure variables were not significant risk factors for disease progression. The only exposure factor associated with disease was sleeping in the same bed with the index case among older children, which may be a surrogate for socioeconomic factors or living conditions that may increase their risk of disease. Further studies are required to investigate how exposure modulates disease progression in children.
Young children aged ≤5 years were at particular at risk of developing disease, and these findings support those of past studies in developed countries that pathogenesis is different in younger than older children. [6, 25]. As opposed to older children who are at risk of reactivation of latent infection, younger children are usually at risk of primary disease after infection from the index case [2]. This is further supported from our finding that baseline TST positivity predicted tuberculosis disease in younger but not older children. Baseline positive TST in younger children is likely reflective of primary infection from the index case, and consequently their conversion may be a better marker of progression than for older children who may have been infected at an earlier age or an outside source. The rapid progression of disease in young children is presumed to be a consequence of an incompletely developed immune system [6]. A positive TST in young children may thus be an important factor to raise the index of suspicion and prompt greater investigation for active disease.
At the same time, BCG showed a 77% decrease in risk of tuberculosis disease in younger children, suggesting a significant level of early protection with vaccination. BCG has traditionally been thought to protect against severe disease during infancy [26]. However, BCG's effect on the immune response is poorly understood; recent studies suggest that BCG vaccination is able to induce strong CD4 Th1-type and CD8 responses in the first year of life [27, 28]. Tuberculosis vaccine investigations have suggested recombinant BCG as a prime vaccine in heterologous prime and boost strategies for infant vaccination [26].
HIV-positive children had almost 8 times the risk of disease and were the greatest at risk for progression. The HIV and tuberculosis syndemic is well established, transforming a 10% lifetime tuberculosis disease risk into a 10% annual risk [29]. HIV-positive young children are particularly affected, and have been shown to be at considerable risk of mortality from tuberculosis disease [30]. Whereas older children had an increased but nonsignificant risk from HIV status, only 8 older children were HIV positive, and so we may have not had the sample size to see a significant effect.
The WHO states that nutritional status is a risk factor for tuberculosis disease, although there are limited studies to support this in children [31]. Although univariate testing suggested a relationship between stunting and lean tissue mass on progression, multivariable analysis did not find that nutritional status was significantly associated with progression to disease in our population. Our results suggest several differences on the interaction of tuberculosis and nutritional status between adults and children. BMI has been shown to be a significant predictor of active tuberculosis in adults; however, our results demonstrate that BMI may not be as useful an indicator in children [32]. In addition, body composition analysis in Ugandan adults with tuberculosis found that men have a greater loss of lean tissue to fat mass compared to women, whereas we did not see differences by sex in children [12]. One of the challenges in understanding the role of nutrition and tuberculosis is establishing a direction of causality; malnutrition is thought to weaken immunity and thus increase risk of tuberculosis, but tuberculosis in itself creates an inflammatory state that worsens nutritional status [31]. Further prospective studies are required to investigate how malnutrition contributes to the risk of active tuberculosis.
Compared to others, our study had the strengths of being one of the largest prospective contact studies with 2-year follow-up in a high-risk, low-resource setting. In addition, we conducted a comprehensive investigation for pediatric tuberculosis that included clinical, laboratory, and radiographic evaluation. However, we could have benefited from a greater number of cases in identifying predictors, although our higher burden allowed us greater comparison than past studies. In addition, many of the children were suspected of tuberculosis in the initial evaluation, and thus there are limitations in determining an incidence rate and overall direction of causality. However, this further supports that progression occurs early and that case investigation can identify a large number of cases.
CONCLUSIONS
In this prospective contact study among children aged <15 years, we evaluated the yield of contract tracing in a high-burden setting and indicators for progression of disease. We found a high percentage of cases in our cohort, and were able to confirm disease with positive cultures in >70% of cases. This study represents one of the few prospective investigations of children at risk of tuberculosis in a resource-limited setting. Because of the greater severity and high rate of disease progression in children, a strong understanding of the risk profile and interventions that can effectively identify cases early are critical to their care.
Notes
Acknowledgments. The authors thank Dr Robert Salata for his support and guidance. We acknowledge the invaluable contributions made by the study medical officers, health visitors, and laboratory and data personnel: Dr Lorna Nshuti, Dr Roy Mugerwa, Dr Christina Hirsch, Allan Chiunda, Bonnie Thiel, Mark Breda, Dennis Dobbs, Mary Rutaro, Albert Muganda, Richard Bamuhimbisa, Yusuf Mulumba, Deborah Nsamba, Barbara Kyeyune, Faith Kintu, Gladys Mpalanyi, Janet Mukose, Grace Tumusiime, Pierre Peters, Keith Chervenak, Karen Morgan, Alfred Etwom, Micheal Angel Mugerwa, and Lisa Kucharski. We acknowledge and thank Dr Francis Adatu Engwau, Head of the Uganda National Tuberculosis and Leprosy Program, for his support of this project. We acknowledge the medical officers, nurses, and counselors at the National Tuberculosis Treatment Centre, Mulago Hospital, the Ugandan National Tuberculosis and Leprosy Program, and the Uganda Tuberculosis Investigation Bacteriological Unit, Wandegeya, for their contributions to this study. This study would not have been possible without the generous participation of the Ugandan patients and families. We also greatly thank the Tuberculosis Research Unit at Case Western Reserve University and the Fogarty International Clinical Research Scholars and Fellows Support Center at Vanderbilt University.
Financial support. This work was supported by the National Institutes of Health (NIH) Office of the Director, Fogarty International Center, Office of AIDS Research, National Cancer Center, National Eye Institute, National Heart, Blood, and Lung Institute, National Institute of Dental and Craniofacial Research, National Institute on Drug Abuse, National Institute of Mental Health, National Institute of Allergy and Infectious Diseases (NIAID), and NIH Office of Women's Health and Research through the Fogarty International Clinical Research Scholars and Fellows Program at Vanderbilt University (R24 TW007988), and the American Relief and Recovery Act. The Kawempe Community Health Study is funded by the Tuberculosis Research Unit, established with federal funds from the NIAID and the NIH (contract numbers N01-AI95383, HHSN266200700022C/N01-AI70022, and AI32414).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.World Health Organization. Geneva, Switzerland: WHO; 2012. Global tuberculosis report 2012. [Google Scholar]
- 2.Newton SM, Brent AJ, Anderson S, Whittaker E, Kampmann B. Pediatric tuberculosis. Lancet Infect Dis. 2008;8:498–510. doi: 10.1016/S1473-3099(08)70182-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harries AD, Hargreaves NJ, Graham SM, et al. Childhood tuberculosis in Malawi: nationwide case-finding and treatment outcomes. Int J Tuberc Lung Dis. 2002;6:424–31. [PubMed] [Google Scholar]
- 4.Peter D, Maher D, Qazi SA. A research agenda for childhood tuberculosis: improving the management of childhood tuberculosis within national tuberculosis programmes: research priorities based on a literature review. Geneva, Switzerland: World Health Organization; 2007. [Google Scholar]
- 5.Stop TB Partnership Childhood TB Subgroup, World Health Organization. Guidance for national tuberculosis programmes on the management of tuberculosis in children. Chapter 1: introduction and diagnosis of tuberculosis in children. Int J Tuberc Lung Dis. 2006;10:1091–7. [PubMed] [Google Scholar]
- 6.Marais BJ, Gie RP, Schaaf HS, et al. The natural history of childhood intra-thoracic tuberculosis: a critical review of literature from the pre-chemotherapy era. Int J Tuberc Lung Dis. 2004;8:392–402. [PubMed] [Google Scholar]
- 7.Singh V, Patra S. A relook at preventive therapy for tuberculosis in children. Indian J Pediatr. 2011;78:205–10. doi: 10.1007/s12098-010-0257-0. [DOI] [PubMed] [Google Scholar]
- 8.Guwatudde D, Nakakeeto M, Jones-Lopez EC, et al. Tuberculosis in household contacts of infectious cases in Kampala, Uganda. Am J Epidemiol. 2003;158:887–98. doi: 10.1093/aje/kwg227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization Multicentre Growth Reference Study Group. Geneva, Switzerland: WHO; 2006. WHO Child growth standards: length/ height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: methods and development. [Google Scholar]
- 10.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC growth charts for the United States: methods and development. National Center for Health Statistics. Vital Health Stat 11. 2002;246:1. [PubMed] [Google Scholar]
- 11.de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007;85:660–7. doi: 10.2471/BLT.07.043497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mupere E, Zalwango S, Chiunda A, Okwera A, Mugerwa R, Whalen C. Body composition among HIV-seropositive and HIV-seronegative adult patients with pulmonary tuberculosis in Uganda. Ann Epidemiol. 2010;20:210–6. doi: 10.1016/j.annepidem.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goran MI, Kaskoun MC, Carpenter WH, Poehlman ET, Ravussin E, Fontvieille A-M. Estimating body composition of young children by using bioelectrical resistance. J Appl Physiol. 1993;75:1776–80. doi: 10.1152/jappl.1993.75.4.1776. [DOI] [PubMed] [Google Scholar]
- 14.Horlick M, Arpadi SM, Bethel J, et al. Bioelectrical impedance analysis models for prediction of total body water and fat-free mass in healthy and HIV-infected children and adolescents. Am J Clin Nutr. 2002;76:991–9. doi: 10.1093/ajcn/76.5.991. [DOI] [PubMed] [Google Scholar]
- 15.American Thoracic Society and the Centers for Disease Control and Prevention. Diagnostic standards and classification of tuberculosis in adults and children. Am J Respir Crit Care Med. 2000;161:1376–95. doi: 10.1164/ajrccm.161.4.16141. [DOI] [PubMed] [Google Scholar]
- 16.Pothukuchi M, Nagaraja SB, Kelamane S, et al. Tuberculosis contact screening and isoniazid preventive therapy in a south Indian district: operational issues for programmatic consideration. PLoS One. 2011;6:e22500. doi: 10.1371/journal.pone.0022500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Wyk SS, Reid AJ, Mandalakas AM, et al. Operational challenges in managing isoniazid preventive therapy in child contacts: a high-burden setting perspective. BMC Public Health. 2011;11:544. doi: 10.1186/1471-2458-11-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zachariah R, Spielmann MP, Harries AD, et al. Passive versus active tuberculosis case finding and isoniazid preventive therapy among household contacts in a rural district of Malawi. Int J Tuberc Lung Dis. 2003;7:1033–9. [PubMed] [Google Scholar]
- 19.Morrison J, Pai M, Hopewell PC. Tuberculosis and latent tuberculosis infection in close contacts of people with pulmonary tuberculosis in low-income and middle-income countries: a systematic review and meta-analysis. Lancet Infect Dis. 2008;8:359–68. doi: 10.1016/S1473-3099(08)70071-9. [DOI] [PubMed] [Google Scholar]
- 20.Swaminathan S, Rekha B. Pediatric tuberculosis: global overview and challenges. Clin Infect Dis. 2010;50:S184–94. doi: 10.1086/651490. [DOI] [PubMed] [Google Scholar]
- 21.Kruk A, Gie RP, Schaaf HS, Marais BJ. Symptom-based screening of child tuberculosis contacts: improved feasibility in resource-limited settings. Pediatrics. 2008;121:e1646–52. doi: 10.1542/peds.2007-3138. [DOI] [PubMed] [Google Scholar]
- 22.Marais BJ, Gie RP, Hesseling AC, Schaaf HS, Enarson DA, Beyers N. Radiographic signs and symptoms in children treated for tuberculosis. Pediatr Infect Dis J. 2006;25:237–40. doi: 10.1097/01.inf.0000202140.76368.74. [DOI] [PubMed] [Google Scholar]
- 23.Lienhardt C, Sillah J, Fielding K, et al. Risk factors for tuberculosis infection in children in contact with infectious tuberculosis cases in The Gambia, West Africa. Pediatrics. 2003;111:e608–14. doi: 10.1542/peds.111.5.e608. [DOI] [PubMed] [Google Scholar]
- 24.Singh M, Mynak ML, Kumar L, Mathew JL, Jindal SK. Prevalence and risk factors for transmission of infection among children in household contact with adults having pulmonary tuberculosis. Arch Dis Child. 2005;90:624–8. doi: 10.1136/adc.2003.044255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morán-Mendoza O, Marion SA, Elwood K, Patrick D, FitzGerald JM. Risk factors for developing tuberculosis: a 12-year follow-up of contacts of tuberculosis cases. Int J Tuberc Lung Dis. 2010;14:1112–9. [PubMed] [Google Scholar]
- 26.Hatherill M. Prospects for elimination of childhood tuberculosis: the role of new vaccines. Arch Dis Child. 2011;96:851–6. doi: 10.1136/adc.2011.214494. [DOI] [PubMed] [Google Scholar]
- 27.Soares AP, Kwong Chung CK, et al. Longitudinal changes in CD4+ T-cell memory responses induced by BCG vaccination of newborns. J Infect Dis. 2013;207:1084–94. doi: 10.1093/infdis/jis941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murray RA, Mansoor N, Harbacheuski R, et al. Bacillus Calmette Guerin vaccination of human newborns induces a specific, functional CD8+ T cell response. J Immunol. 2006;177:5647–51. doi: 10.4049/jimmunol.177.8.5647. [DOI] [PubMed] [Google Scholar]
- 29.Verhagen LM, Warris A, van Soolingen D, de Groot R, Hermans PW. Human immunodeficiency virus and tuberculosis coinfection in children: challenges in diagnosis and treatment. Pediatr Infect Dis J. 2010;29:e63–70. doi: 10.1097/INF.0b013e3181ee23ae. [DOI] [PubMed] [Google Scholar]
- 30.Cavanaugh J, Genga K, Marigu I, Laserson K, Ackers M, Cain K. Tuberculosis among children in Kenya: epidemiology and impact of HIV in two provinces. J Trop Pediatr. 2012;58:292–6. doi: 10.1093/tropej/fmr098. [DOI] [PubMed] [Google Scholar]
- 31.Jaganath D, Mupere E. Childhood tuberculosis and malnutrition. J Infect Dis. 2012;206:1809–15. doi: 10.1093/infdis/jis608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lönnroth K, Williams BG, Cegielski P, Dye C. A consistent log-linear relationship between tuberculosis incidence and body mass index. Int J Epidemiol. 2010;39:149–55. doi: 10.1093/ije/dyp308. [DOI] [PubMed] [Google Scholar]