Abstract
Age-related decline in mammalian circadian rhythm has been recognized for decades, but the underlying molecular mechanisms have remained elusive. In this issue of Cell, Chang and Guarente use brain-specific SIRT1 knockout mice and transgenic mice overexpressing SIRT1 to develop an enticing model for how SIRT1 helps maintain the robustness of the aging circadian clock.
Nearly half a century ago, Pittendrigh and Daan (1974) noted that the period of the circadian activity cycle shortens as mice, hamsters, and deer mice age. Although this study may have sprung from Pittendrigh’s personal experience as an aging academic or from the difficulties associated with getting his Stanford University undergraduates to attend 8:00 a.m. lectures, it most likely arose out of his earlier work on fruit flies. For Drosophila, being reared in noncircadian day lengths has an adverse affect on longevity (Pittendrigh and Minis, 1972). Since these early studies, other reports have documented an interconnection between the circadian clock and longevity along with age-related changes in normal rhythms in physiology, endocrinology, and metabolism. Despite all these studies, the mechanistic and molecular factors that establish or regulate this connection have remained elusive. In this issue of Cell, Chang and Guarente (2013) suggest that SIRT1 bridges clock function with aging in the mammalian brain.
In fungi and animals, the core of the circadian oscillator is a transcription-translation negative feedback loop in which a heterodimeric transcriptional activator drives the expression of genes encoding proteins that turn down the activity of the heterodimer. Although some components of the loop have come and gone over the course of evolution, this regulatory architecture appears to be conserved, and, in mammals, BMAL1 and CLOCK form the heterodimer that drives expression of the Period and Cryptochrome clock gene paralogs whose products form the negative feedback elements. Although not essential for oscillations, other nested feedback loops assembled from clock-associated gene products, such as REV-ERBa and RORa, influence BMAL1 expression and contribute to robustness and the free-running period of the loop (e.g., Preitner et al., 2002; Sato et al., 2004). The work of Chang and Guarente (2013) involves SIRT1, a regulator of the loop containing RORa.
SIRT1, like its eponymous antecedent Sir2p from yeast, is first and foremost an NAD+-dependent deacetylase, but it has garnered a great deal of attention as a factor implicated in longevity (Bishop and Guarente, 2007). A SIRT1-circadian link was initiallymade in 2008 with the discovery that SIRT1 can modulate the amplitude of circadian clock-controlled genes and deacetylate both PER2 (Asher et al., 2008) and BMAL1 (Nakahata et al., 2008). Thus, the previous connection of SIRT1 to aging was joined to the clock, providing the first hint that SIRT1 is a potential modulator of clock-dependent aging effects. Also, clock-regulated metabolic rhythms in NAD+ levels derive from the CLOCK-dependent transcription of Nampt (Nakahata et al., 2009; Ramsey et al., 2009), which encodes nicotinamide phosphoribosyltransferase, the rate-limiting enzyme in NAD+ biosynthesis. In turn, these rhythms contribute to the reported SIRT1 enzymatic rhythms (Nakahata et al., 2008).
Fast forward to the present where Chang and Guarente (2013) characterize circadian activity rhythms in mice overexpressing or lacking SIRT1 in the brain, a tiny part of which contains the suprachiasmatic nuclei (SCN), which comprises the cells that form the dominant pace-maker for circadian activity. SIRT1 has a modest and reciprocal effect on period length (e.g., less SIRT1 equals a longer period) but a sizable effect on the duration of “jet lag,” which doubles upon the loss of SIRT1 in young mice. Similar effects— longer period and worse jet lag—have been seen in old mice (Valentinuzzi et al., 1997) although, intriguingly, not in old hamsters or even always in old mice (Pittendrigh and Daan, 1974). The effects now seen in aged mice are exacerbated by the loss of SIRT1 and partly amelio-rated by the overexpression of SIRT1. This correlation provided the entry point in this story, made with all the attendant caveats of whole-brain knockouts, and the rest of the paper tests this connection.
SIRT1 in the SCN declines with age, and, in general, the expression level of clock genes (CLOCK, Bmal1, Per2, and Cry1) and clock-associated genes (Rev-Erbα and RORα) track SIRT1 changes with age, knockout, or overexpression. A clue to the underlying mechanism arose when the authors noted similarities between the Sirt1 brain-specific knockout mice and Pgc-1α null mice in the expression of Bmal1 and recalled that RORa activation of Bmal1 transcription requires the SIRT1 substrate PGC-1a (Liu et al., 2007; Sato et al., 2004). Thus, the hypothesis was laid out, and it was consistent with the knockdown and overexpression data from cell lines that showed that SIRT1 functions to deacetylate and, thereby, activate PGC-1a at the Bmal1 promoter and that PGC-1a assists RORa expression of Bmal1. Then, Chang and Guarente (2013) confirmed, via chromatin immunoprecipitation, the previously reported binding of PGC-1a to the Bmal1 promoter (Liu et al., 2007) and showed that SIRT1 acts cooperatively in this binding.
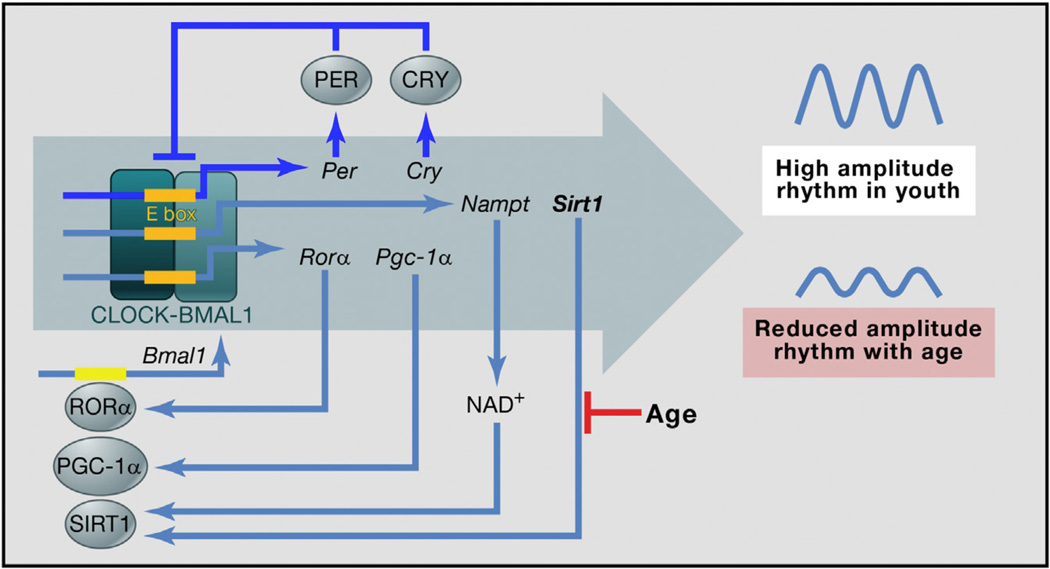
Altogether, the data are consistent with a model in which clock-driven Nampt expression creates a rhythm in NAD+ that synergizes with a rhythm in SIRT1, which fosters the rhythmic deacetylation and activation of PGC-1a and the rhythmic coactivation with RORa to drive robust rhythms in Bmal1 expression (see Figure 1). This positive feedback loop could provide resiliency to the clock oscillatory network. As the model would predict, under light-dark (LD) cycles, there are cycles in Sirt1, Pgc-1a, and Nampt messenger RNA in the part of the brain containing the SCN. The model predicts that age-related reduction in SIRT1 would attenuate this amplifying loop and result in a lower-amplitude core circadian transcription-translation feedback loop. Thus, circadian rhythms would decline with age partly because of the loss of SIRT1.
Figure 1. SIRT1 in an Aging Circadian Clock.
CLOCK-BMAL1 binds to E boxes in many promoters. Then, the products of some of their target genes inhibit these transcription factors (shown in blue for the multiple Per and Cry gene paralogs), resulting in rhythmic CLOCK-BMAL1 activity. This feedback loop is essential for circadian rhythms. Rhythmic CLOCK-BMAL1 activity drives (perhaps indirectly) the rhythmic expression of genes encoding clock-associated factors, including RORα and PGC-1α, which coactivate (gray arrows) the expression of Bmal1, Sirt1 (whose product uses NAD+ to deacetylate and activate PGC-1α), and Nampt (whose product helps make NAD+). These and other modulatory feedback loops (in gray) are helpful, but are not essential, for circadian rhythms. In the model, the effects of age-related reduction in SIRT1 (shown in black) propagate through these interrelated feedback loops, resulting in a reduction in the amplitude of all the CLOCKBMAL1-driven rhythms in aged animals, thereby influencing overt circadian parameters, including period length and jet lag.
It is reasonable to assert that, if the amplifying loop works and responds to age in this way in mice, then it may also do so in people, and, if so, then the results, as suggested in the title of this piece, are of real interest. That is, any dietary or pharmacological interventions that could increase SIRT1 activity—perhaps resveratrol in red wine—should help to reverse the effects of aging on the circadian system (if you can consume enough without causing other alcohol-related damage). And, although many unknowns remain (such as to what extent the observed molecular rhythms are dependent on the LD cycles that were used, why SIRT1 declines with age, and the extent of differences among species as with Pittendrigh’s hamsters and deer mice), the point is that the circadian system does change with age, and reciprocal feedback with metabolism influences this change. In the realm of physiological crosstalk among aging, metabolism, and environmental factors, the circadian clock appears to be a keystone.
REFERENCES
- Asher G, Gatfield D, Stratmann M, Reinke H, Dibner C, Kreppel F, Mostoslavsky R, Alt FW, Schibler U. Cell. 2008;134:317–328. doi: 10.1016/j.cell.2008.06.050. [DOI] [PubMed] [Google Scholar]
- Bishop NA, Guarente L. Nat. Rev. Genet. 2007;8:835–844. doi: 10.1038/nrg2188. [DOI] [PubMed] [Google Scholar]
- Chang H-C, Guarente L. Cell. 2013;153(this issue):1448–1460. doi: 10.1016/j.cell.2013.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Li S, Liu T, Borjigin J, Lin JD. Nature. 2007;447:477–481. doi: 10.1038/nature05767. [DOI] [PubMed] [Google Scholar]
- Nakahata Y, Kaluzova,M.,Grimaldi B, Sahar S, Hirayama J, Chen D, Guarente LP, Sassone-Corsi P. Cell. 2008;134:329–340. doi: 10.1016/j.cell.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahata Y, Sahar S, Astarita G, Kaluzova M, Sassone-Corsi P. Science. 2009;324:654–657. doi: 10.1126/science.1170803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittendrigh CS, Daan S. Science. 1974;186:548–550. doi: 10.1126/science.186.4163.548. [DOI] [PubMed] [Google Scholar]
- Pittendrigh CS, Minis DH. Proc. Natl. Acad. Sci. USA. 1972;69:1537–1539. doi: 10.1073/pnas.69.6.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preitner N, Damiola F, Lopez-Molina L, Zakany J, Duboule D, Albrecht U, Schibler U. Cell. 2002;110:251–260. doi: 10.1016/s0092-8674(02)00825-5. [DOI] [PubMed] [Google Scholar]
- Ramsey KM, Yoshino J, Brace CS, Abrassart D, Kobayashi Y, Marcheva B, Hong HK, Chong JL, Buhr ED, Lee C, et al. Science. 2009;324:651–654. doi: 10.1126/science.1171641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato TK, Panda S, Miraglia LJ, Reyes TM, Rudic RD, McNamara P, Naik KA, FitzGerald GA, Kay SA, Hogenesch JB. Neuron. 2004;43:527–537. doi: 10.1016/j.neuron.2004.07.018. [DOI] [PubMed] [Google Scholar]
- Valentinuzzi VS, Scarbrough K, Takahashi JS, Turek FW. Am. J. Physiol. 1997;273:R1957–R1964. doi: 10.1152/ajpregu.1997.273.6.R1957. [DOI] [PubMed] [Google Scholar]