Abstract
Background
Parenteral nutrition (PN), with the lack of enteral feeding, compromises mucosal immune function and increases the risk of infections. We developed an ex vivo intestinal segment culture (EVISC) model to study the ex vivo effects of PN on susceptibility of the ileum to invasion by extraintestinal pathogenic Escherichia coli (ExPEC) and on ileal secretion of antimicrobial secretory phospholipase A2 (sPLA2) in response to the pathogen.
Materials and Methods
Study1: Using mouse (n=7) ileal tissue, we examined the effects of ileal region (proximal vs. distal) and varying ExPEC inoculum concentrations on ex vivo susceptibility to ExPEC invasion and sPLA2 secretion. Study2: Ten mice were randomized to oral chow or intravenous PN-feeding for 5 days (n=5/group). Using the EVISC model, we compared the susceptibility of ileal tissue to invasion by ExPEC and sPLA2 secretion in response to the pathogen.
Results
Study1: The proximal ileum was more susceptible to invasion (P<0.0001) and secreted lower amounts of sPLA2 (P=0.0002) than the distal ileum. Study2: Ileal tissue from PN-fed animals was more susceptible (approximately 4-fold, P=0.018) to invasion than those from chow-fed animals. Ileal tissue from PN-fed animals secreted less sPLA2 (P<0.02) than those from chow-fed animals.
Conclusions
The data illustrate EVISC as a reproducible model for studying host-pathogen interactions and the effects of diet on susceptibility to infections. Specifically, the findings support our hypothesis that PN with the lack of enteral feeding decreases mucosal responsiveness to pathogen exposure and provides a plausible mechanism by which PN is associated with increased risk of infectious complication.
Keywords: Parenteral Nutrition, Enteroinvasion, Extraintestinal Pathogens, Escherichia coli, secretory Phospholipase A2, Paneth cells, Ex Vivo, polarized In Vitro Organ Culture
INTRODUCTION
The use of parenteral nutrition (PN), without enteral feeding, is associated with an increased incidence of respiratory and intra-abdominal infections in critically injured patients (1, 2). Evidence suggests that impairment of mucosal and innate immunity following PN, without enteral nutrition compromises the mucosal barrier, resulting in abnormal bacterial-host interactions (3, 4). This impairment of the mucosa is multi-factorial; it includes altered tight junction proteins, reduced release of antimicrobial peptides by Paneth cells, lowered secretion of mucus by goblet cells, lower levels of luminal secretory IgA (sIgA) and increased virulence of luminal bacteria (5-8). Slowed intestinal motility (ileus) with PN also allows bacteria to migrate into the proximal intestine (9), a region that is typically sparsely colonized (<104 CFU/mL intestinal content). The treatment of these infections is often further complicated by the involvement of pathogens resistant to antibiotics (10, 11).
While clinical advances in diagnosis and therapy can identify and treat infections with certain degree of success, an understanding of host-associated vulnerabilities at the mucosal level is necessary for the development of preventive strategies. Studies utilizing cell monolayers provide mechanistic insights into how bacteria invade the tissues, but no insight into the complex in vivo host-pathogen interactions that occur at the mucosal interface composed of multiple cell types (12, 13). Understanding these processes in a closely modeled system would allow for insights into the susceptibility of patients on PN feeding alone to infections and for the development of more effective prophylactic treatments.
More complex models such as conventional and polarized in vitro organ cultures (IVOC or pIVOC) (14) using human tissue from biopsies have investigated the interactions between intestinal mucosal and enteric pathogens. Specifically, pIVOC of human pediatric specimens have been utilized to qualitatively study attaching-effacing bacteria that damage mucosal surfaces; however, the methodology did not provide quantitative assessment of the pathogen-host interactions. pIVOC in Ussing chambers, on the other hand, have been use to quantitatively investigate bacterial translocation across the mucosa (3, 15). Based on the underpinnings of the pIVOC model, we developed an ex vivo intestinal segment culture (EVISC) model that uniquely allows reproducible and high-throughput quantitative studies of bacterial invasion of the mucosal epithelium, a key step that facilitates the survival and persistence of many intestinal and extraintestinal pathogens, and their subsequent systemic translocation (16). Additionally, the EVISC model allows for the measurement of innate intestinal response to bacteria in context of the secretion of antimicrobial protein such as secretory phospholipase A2 (sPLA2).
In this work, we assessed the effect of PN without enteral feeding upon the susceptibility of murine intestinal tissues to enteroinvasion by extraintestinal pathogenic Escherichia coli (ExPEC) and the secretion of mucosal sPLA2 in response to ExPEC exposure. Since PN without enteral feeding is associated with a greater risk of infection and impaired gut-derived immune responses, we hypothesized that PN would result in greater enteroinvasion by ExPEC and reduced sPLA2 secretion by the small intestinal tissue.
MATERIALS AND METHODS
Animals
All protocols were approved by the Animal Care and Use Committee of the University of Wisconsin-Madison, and the William S. Middleton Memorial Veterans Hospital. Male Institute of Cancer Research (ICR) mice were purchased from Harlan (Indianapolis, IN) and housed 5 per microisolater-top cage in a temperature and humidity controlled environment with a 12h/12h light/dark cycle at an Association for Assessment and Accreditation of Laboratory Animal Care-accredited conventional facility. Animals were fed a standard pelleted mouse chow (Rodent Diet 5001; LabDiet, PMI Nutrition International, St. Louis, MO) and given ad libitum Chow and water for 1 week prior to initiation of the study protocol.
Study 1: Effect of ileal region and ExPEC inoculum concentration on susceptibility of ileal tissue to invasion by ExPEC and sPLA2 secretion by the tissue in response to pathogen exposure
Seven male ICR mice (7–8 weeks old) were anesthetized with intraperitoneal administration of ketamine (100 mg/kg) and acepromazine (10 mg/kg), and then euthanized by exsanguinated via left axillary artery transection. The small intestine from each mouse was removed and cleaned of mesenteric fat and vascular tissue. The lumen was rinsed with 60 mL HBSS and then with 60 mL RPMI media. Ileum segments (1.5 cm) devoid of Peyer’s patches were isolated, placed in RPMI solution until use in the experiment, within 30 minutes. For regionalization studies, segments from the distal ileum (adjacent to the ileal-cecal junction) and proximal ileum (15 cm proximal to “distal ileum”) were exposed to varying ExPEC inoculum concentrations; enteroinvasion by ExPEC and secretion of sPLA2 in response to ExPEC exposure were measured as described in detail in later sections.
Study 2: Effects of PN-feeding on susceptibility of ileal tissue to invasion by ExPEC and sPLA2 secretion by the tissue in response to pathogen exposure
Ten male ICR mice (7–8 weeks old) were anesthetized as in Study 1, weighed and catheterized by the placement of a silicon rubber catheter (0.012-inch I.D./0.025-inch O.D.; Helix Medical, Inc., Carpinteria, CA) in the vena cava via the right external jugular vein. The catheter was tunneled subcutaneously from the neck site, over the back, finally exiting mid-tail. The mice were partially restrained by the tail for the remainder of the study to protect the catheter during infusions; this partial restraint technique does not induce significant stress in the mice (17). The mice were also housed individually in metal wire-bottomed cages to prevent coprophagia and ingestion of bedding.
The catheterized mice were connected to infusion pumps and allowed to recover for 48 h while receiving 4 mL/d of saline (0.9%) via the catheter. The mice also received ad libitum chow and water. This period allows for surgical recovery as previously determined by normalization of serum cytokines and food intake. Following the recovery period, the mice were randomized (n = 5 / group) to receive oral chow or intravenous PN. The chow-fed mice were given ad libitum chow and water, and continued to receive 0.9% saline at 4 mL/d via the intravenous catheter. The PN mice received PN solution at 4 mL/d (day 1), 7 mL/d (day 2) and 10 mL/d (days 3-5) as well as ad libitum water throughout the study. We previously demonstrated that a graded infusion period was necessary for the mice to adapt to the glucose and fluid loads. The PN solution [Table 1] includes 6.0% amino acids, 35.6% dextrose, electrolytes, and multivitamins, with a non-protein calorie to nitrogen ratio of 128:1 (1440 kcal/L). These values meet the calculated nutrient requirements of mice weighing 25 to 30 g (18). After 5 days of chow or PN feeding, the mice were anesthetized, euthanized and the intestinal segments were collected as described in Study 1. The segments of the distal ileum from chow and PN animals were exposed to an optimal ExPEC inoculum concentration (determined in Study 1); enteroinvasion by ExPEC and secretion of sPLA2 in response to ExPEC exposure were measured as described in detail in later sections.
Table 1.
Formulation of Parenteral Nutrition (PN) Solution
| Component | Amount (per 1 L) |
|---|---|
| Dextrose | 356.0 g |
| Amino acids (Clinisol) | 60.0 g |
| Sodium chloride | 32.0 mEq |
| Sodium phosphate | 36 mmol |
| Potassium chloride | 16 mEq |
| Calcium gluconate | 37.5 mEq |
| Potassium acetate | 44.0 mEq |
| Magnesium sulfate | 8.0 mEq |
| Manganese | 0.8 mg |
| Copper | 0.5 μg |
| Zinc | 2.0 mg |
| Vitamin C | 200 mg |
| Vitamin A | 3300 IU |
| Vitamin D3 | 200 IU |
| Thiamine | 6 mg |
| Riboflavan | 3.6 mg |
| Pyridoxine HCl | 6 mg |
| Niacinamide | 40 mg |
| Folic Acid | 600 mcg |
| Biotin | 60 mcg |
| Cyanocobalamin | 5 mcg |
| Vitamin E | 10 IU |
| (dl-α-tocopheryl Acetate) | |
| Vitamin K1 | 150 mcg |
| Dexpanthenol | 15 mg |
IU, international units.
ExPEC Preparation
The ExPEC strain (ExPEC Strain-5011) used in our studies was isolated from a clinical fecal sample and characterized to be genotypically representative of ExPECs. The strain expresses both P- and type-1 fimbriae. To aid in the selection of the strain (over other strains that may be present in the mouse tissue), the microbe was transformed to constitutively express luciferase (Lux) using highly stable, custom low-copy plasmid, pGEN-Lux with resistance to ampicillin. The ampicillin resistant ExPEC were cultured under static conditions in 40 mL tryptose broth (100 μg/mL ampicillin) for 48 hours at 37°C. An aliquot (1 mL) was taken from the surface of the culture and used to inoculate a new culture grown under static conditions for 24 hours at 37°C. This culturing procedure optimizes the expression of virulence factors. On the day of the EVISC experiment, the culture was centrifuged at 1780 × g for 11 minutes to obtain a bacterial pellet. The pellet was washed twice in 40 mL DPBS, and then re-suspended in 1 mL DPBS to obtain the bacterial stock solution used in the experiments. The colony forming units (CFU) of the stock solution was determined by obtaining the optical density on a spectrophotometer (DU640B, Beckman, Brea, CA) at 450 nm wavelength and plotting it on previously established growth curves.
Ex Vivo Intestinal Segment Culture (EVISC)
The intestinal segments were placed on a sterile surface, carefully opened apical side up, and kept moist with RPMI during all procedures. Tissue glue (Dermabond, Ethicon, Cornelia, GA) was applied lightly on one side of a polystyrene spacer (9 mm outer diameter and a 6 mm internal aperture) that was fabricated in the laboratory. The spacer was then placed onto the apical side of the intestinal segment with gentle pressure. Once the tissue glue set (approximately 10 seconds), the bonded tissue-spacer was turned over. A second spacer was lightly coated with tissue glue and placed on the serosal side of the intestinal segment. Then a light layer of tissue glue was applied to the bottom of the serosal spacer and the intestinal segment with the attached spacers was lowered into a cell culture insert (Cat 353292, 3.0 μM pore, 12-well format, BD Bioscience, NJ). Gentle pressure was applied to ensure adherence of the bottom spacer to the cell culture insert. A schematic of spacer, intestinal segment, and cell culture insert is shown in Figure 1. The entire insert containing the sandwiched tissue segment was placed into a well of a 12-well plate prefilled with 1 mL RPMI containing ampicillin (100 μg/mL).
Figure 1. A graphical representation of the EVISC model setup.
Figure shows the assembly of the spacer and the intestinal segment into each cell culture insert that is then placed into a well of a 12-well cell culture plate.
The ExPEC inoculum were delivered in 400 μL of RPMI containing ampicillin (100 μg/mL) to obtain final concentrations of 0 (Control), 0.4, 2, 4, 20, and 40 ×107 CFU/well in Study 1 and 4×107 CFU/well in Study 2. Once the inoculums were added, the plate was incubated for 1 hour at 37°C. At the end of the incubation period, the media in each well was collected and centrifuged at 14,000 × g for 2 minutes to obtain supernatant devoid of bacteria; the supernatant was stored at −80°C for analysis of mucosal secretions (sPLA2). Then, each well was washed three times with 500 μL of DPBS. Then 700 μL of RPMI containing gentamicin (100 μg/mL) was added to each well and incubated for 1 hour at 37°C to kill bacteria remaining in the well or adhered to the mucosal surface; at the concentration and incubation time utilized, the gentamicin cannot enter the cells of the tissue. The RPMI + gentamicin was removed and the tissue was washed three times with 500 μL of DPBS. The cells of the tissue segments in the wells were lysed to release the invaded ExPEC by adding 400 μL/well of 0.1% Triton-X in DPBS and agitating the plate on an orbital shaker (175 rpm; New Brunswick Scientific Classic Series C1 Shaker) for 30 minutes at room temperature; at this concentration, Triton-X has no effect on the viability of the ExPEC. Serial dilutions (101-107) of the cell lysate were made in DPBS and plated on Luria broth (LB) agar plates containing ampicillin (100 μg/mL), and then incubated for 18 hours at 37°C. Enteroinvasion was assessed by enumerating CFUs of ExPEC grown on the plates. Bacterial invasivity was calculated by dividing the total invaded CFUs by the total inoculum CFUs.
Scanning electron microcopy
Segments of distal ileal tissue were sandwiched between spacers, attached to cell culture inserts and placed into wells of a culture plate prefilled with 1 mL RPMI containing ampicillin (100 μg/mL) as described in previous sections. The wells were inoculated with ExPEC (4×107 CFU/well) for 1 hour at 37°C. Then, each well was washed three times with 500 μL of DPBS. The tissues in the wells were fixed overnight at 4°C using 2% gluteraldehyde in 0.1M phosphate buffer. Cells were then washed twice for 10 minutes in 0.1M phosphate buffer. The tissues sandwiched between spacers were removed from the apparatus; the spacers were left intact to ensure that the tissues maintained their flat orientation. Tissues were incubated in step-wise increasing concentrations of ethanol (30-100%) for specific durations of time and then placed in the critical point dryer chamber with molecular sieve-dried ethanol. The dryer was cooled and maintained at 10°C using CO2. The tissues were purged of ethanol for 2 minutes and incubated with CO2 for 10 minutes; the process was repeated twice. After another 2-minute purge, the dryer chamber was heated to 35°C, increasing the pressure on the samples to approximately 1250 psi. The pressure was slowly decreased (~100psi/min) until 0 psi was reached. The tissues were removed from the dryer and placed in a desiccator. After desiccation, the tissues specimens were platinum coated with IBS/TM200S ion-beam sputter coater (VCR Group, San Francisco, CA) to a thickness of 2.5 nm. The tissue samples were viewed and imaged on a Field-Emission Scanning Electron Microscope Hitach S-900 (Hitachi Instruments, Santa Clara, CA) at 8keV.
Determination of sPLA2 activity
sPLA2 activity was performed as described previously by Tsao et al (19), with some modification to the substrate preparation (20). This assay uses a quenched fluorophore, bis-BODIPY®-FL C11-PC (1,2-bis-(4,4-difluoro-5,7-dimethyl-4-bora-3a,4a-diaza-s-indacene-3-undecanoyl)-sn-glycero-3-phosphocholine (Molecular Probes, Eugene, OR), which becomes unquenched (fluoresces) when the sn-2 ester bond of is cleaved by sPLA2. This method was developed as a high-throughput assay to rapidly analyze sPLA2 activity. Briefly, the substrate was prepared by mixing 10 μL of bis-BODIPY-FL C11-PC with 1 mL aliquot of phosphatidylglycerol (2 mg/mL; Sigma, St. Louis, MO) dissolved in chloroform. The choloroform was evaporated under nitrogen, and the remaining mixture of bis-BODIPY-FL C11-PC and phosphatidylglycerol were re-dissolved in 100% ethanol, and used as substrate. The assay reaction mixture was prepared in a glass tube on ice by mixing 10 μL of substrate solution (20 μg of phospholipids) and 10 μL of sample, and then bringing up the reaction volume to 1 mL with 0.01 M Tris-HCl (pH 7.4) buffer containing 10 mM Ca2. An aliquot (0.3 mL) of the reaction mixture was promptly transferred in triplicate to the wells of a white polystyrene microplate (Porvair PS White, PerkinElmer Instruments, Norwalk, CT). The microplate was placed in a temperature-controlled (30°C) microplate reader attached to a PerkinElmer Luminescence Spectrometer LS50B. The fluorescence intensity (FI) in each well was recorded every 10 sec for 70 cycles at 488 nm excitation (excitation slit: 2.5 nm) and 530 nm emission (emission slit: 5.0 nm). After the enzymatic reactions reached equilibrium temperature, the reaction curve was fit to a second-order polynomial equation and the first-degree coefficient was taken as the initial rate of reaction (expressed as change in FI/min/μL sample). This initial rate is taken to be the activity of sPLA2 in the sample. Blank wells containing only substrate and buffer were used to determined non-enzymatic background activity.
Statistical analysis
A fixed effects ANOVA model was fit for each measured parameter (total invasion, invasivity and sPLA2 secretion) using the PROC MIXED function (SAS Software (Version 8), SAS Institute Inc, Cary, NC) to test for significant effects of bacterial inoculum concentration, ileal regionality, and/or diet. Second-order interactions between these factors were also included in the model whenever appropriate. The correlations between observations taken between factors were modeled using a diagonal covariance structure. For each measured parameter, the model was fit using the untransformed data, and the residuals were evaluated to ensure that standard ANOVA assumptions of constant variance and normality were reasonably met. Transformations of the data were performed if required to improve adherence to these assumptions. Type III tests were then performed to evaluate the significance of the effects of interest for each measured parameter, and least-square means were calculated. The data are reported as least-square mean ± standard error of mean (SEM). Statistical significance was accepted at P < 0.05.
RESULTS
Study 1: Effects of ileal region (proximal vs. distal ileal segments) and ExPEC inoculum concentration on susceptibility of ileal tissue to invasion by ExPEC
Overall Analysis
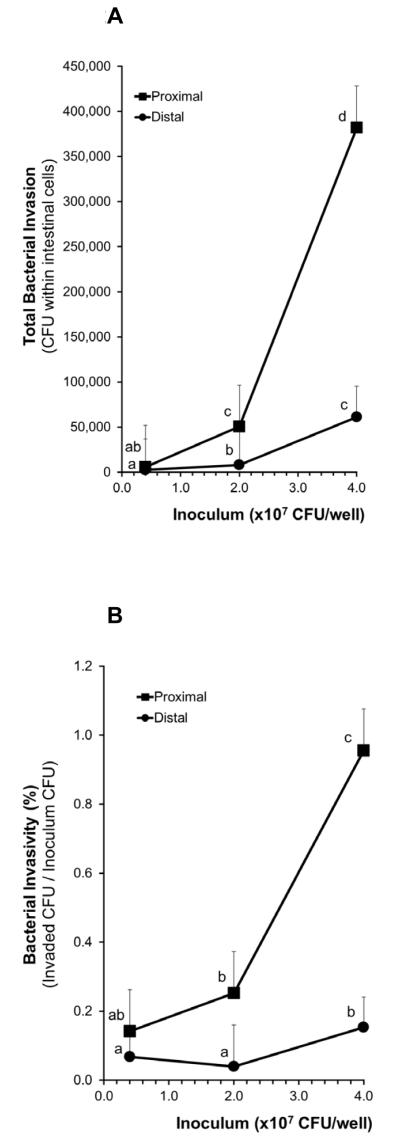
There were significant differences (P<0.0001) between the susceptibilities of the two ileal regions to enteroinvasion by ExPEC (as determined by total invasion and invasivity); The proximal ileum was more susceptible to invasion than the distal ileum (Figures 2A and 2B). The concentration of the ExPEC inoculums also had a significant effect on enteroinvasion (P<0.0001 and P=0.0001 for total invasion and invasivity, respectively; Figures 2A and 2B). However, there were no significant interactions between the two factors, ileal regionality and inoculum concentration. That is, the relationship between increasing inoculum concentration and total invasion (or invasivity) in the proximal region was not significantly different from the relationship between increasing inoculum concentrations and total invasion (or invasivity) in the distal region.
Figure 2. Effects of ileal region (proximal vs. distal ileal segments) and ExPEC inoculum concentration on susceptibility of ileal tissue to invasion by ExPEC.
Figures show (A) total invasion and (B) invasivity (Invaded CFU/Inoculum CFU). Means without a common letter differ, P < 0.05.
Total invasion
The two highest concentrations of ExPEC inoculums, 20 and 40 ×107 CFU/well induced degradation of tissue and thus were excluded from further studies (data not shown in figure due to tissue degradation). In the distal ileal segments: The total invasion produced by the ExPEC inoculum concentration of 2×107 CFU/well (7,848 ± 46,031 CFU of ExPEC within cells) was significantly greater than that produced by 0.4×107 CFU/well inoculum (2,708 ± 33,996 CFU; P < 0.05; Figure 2A). The total invasion produced by 4×107 CFU/well inoculum (61,125 ± 33,996 CFU within cells) was significantly greater than those produced by either 0.4×107 (2,708 ± 33,996 CFU; P < 0.0001) or 2×107 (7,848 ± 46,031 CFU, P < 0.01) CFU/well inoculums. In the proximal ileal segments: The total invasion produced by the ExPEC inoculum concentration of 2×107 CFU/well (50,422 ± 46,031 CFU of ExPEC within cells) was significantly greater than that produced by the 0.4×107 CFU/well inoculum (5,675 ± 46,031 CFU; P < 0.005). The total invasion produced by 4×107 CFU/well inoculum (381,933 ± 46,031 CFU) was significantly greater than those produced by either 0.4×107 (5,675 ± 46,031 CFU; P < 0.0001) or 2×107 (50,422 ± 46,031 CFU; P < 0.01) CFU/well inoculums. When comparing the two ileal regions: total invasion of the proximal region was significantly greater than that of the distal region with 2×107 (P < 0.01) and 4×107 (P < 0.005) CFU/well inoculums.
Invasivity
In the distal ileal segments: The invasivity of the ExPEC was significantly greater at an inoculum concentration of 4×107 CFU/well (0.15 ± 0.09 %) than at 0.4×107 (0.07 ± 0.09 %; P < 0.05) or 2×107 (0.04 ± 0.12 %; P < 0.05; Figure 2B) CFU/well. In the proximal ileal segments: The invasivity of the ExPEC at an inoculum concentration of 4×107 CFU/well (0.95 ± 0.12 %) was significantly greater than that at 0.4×107 (0.14 ± 0.12 %; P < 0.01) or 2×107 (0.25 ± 0.12 %; P = 0.09) CFU/well. When comparing the two ileal regions: The invasivity of the ExPEC was significantly greater in the proximal region than in the distal region with 2×107 (P < 0.01) and 4×107 (P < 0.0005) CFU/well inoculums.
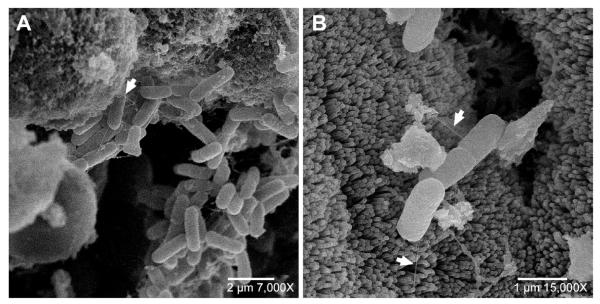
Scanning electron microscopy
The examination of ExPEC interaction with the ileal tissue by scanning electron microscopy revealed ExPECs attaching to the apical surface of the ileal tissue via fimbriae and then initiating entry into the epithelial cells (Figure 3).
Figure 3. Representative scanning electron photomicrographs of the invasion of ileal epithelial cells by ExPEC.
(A) Shows the adhesion of ExPEC to apical surface of cells via surface virulence factor (fimbriae; example indicated by white arrow), (B) Shows ExPEC invasion of the epithelium cells by initiating uptake, a mechanism commonly utilized by microbial pathogens to gain entry into non-phagocytic host cells (37, 38). The observed photomicrographs are identical to what we have previously observed in our studies exploring the invasion of enterocytes (Caco-2 cells) in culture by ExPEC (data not shown).
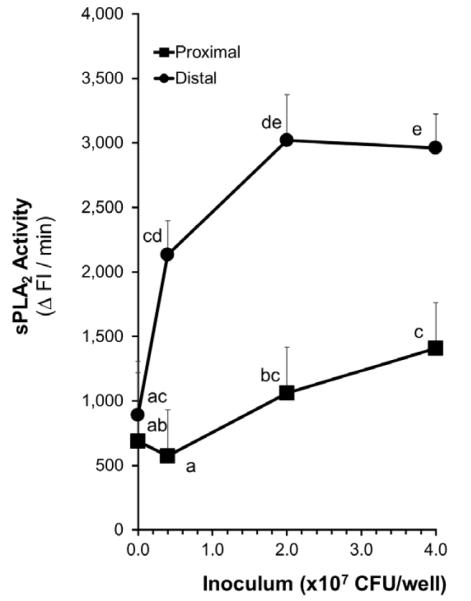
Study 1: Effects of ileal region (proximal vs. distal ileal segments) and ExPEC inoculum concentration on sPLA2 secretion by mucosal tissue in response to pathogen exposure
Overall Analysis
There were significant differences (P=0.0002) between the secretion of sPLA2 by the two ileal regions in response to ExPEC exposure; The proximal ileum produced significantly lower sPLA2 than the distal ileum in response to the pathogen (Figure 4). The concentration of the ExPEC inoculums also had a significant effect on the secretion of sPLA2 (P<0.0001). However, there were no significant interactions between the two factors, ileal regionality and inoculum concentration. That is, the relationship between increasing inoculum concentration and secretion of sPLA2 in the proximal region was not significantly different from the relationship between increasing inoculum concentrations and secretion of sPLA2 in the distal region.
Figure 4. Effects of ileal region (proximal vs. distal ileal segments) and ExPEC inoculum concentration on sPLA2 secretion by mucosal tissue in response to pathogen exposure.
sPLA2 activity in the culture media is taken to be a measure of sPLA2 secretion. Means without a common letter differ, P < 0.05.
A baseline level of sPLA2 secretion by ileal tissue was detected regardless of the presence or absence of bacteria or bacterial inoculum (Figure 4). In the distal ileal segments: The sPLA2 secretion induced by the ExPEC inoculum concentrations of 2×107 CFU/well (3019 ± 355 ΔFI/min; P<0.05) and 4×107 CFU/well (2,961 ± 262 ΔFI/min; P<0.05) were significantly greater than the baseline level (892 ± 329 ΔFI/min) secreted by the tissue. In the proximal ileal segments: Only the sPLA2 secretion induced by the inoculum concentrations of 4×107 CFU/well (1,407 ± 355 ΔFI/min; P<0.05) was significantly greater than the baseline level (689 ± 615 ΔFI/min) secreted by the tissue. When comparing the two ileal regions: The baseline sPLA2 secretion by the two ileal segments did not significantly differ. However, the sPLA2 secretion induced by the distal ileal segments were significantly higher than those secreted by the proximal ileal segments in response to 0.4, 2, and 4 ×107 CFU/well (P<0.0005, P=0.001, and P<0.005, respectively).
Study 2: Effects of PN-feeding on susceptibility of ileal tissue to invasion by ExPEC and sPLA2 secretion by the tissue in response to pathogen exposure
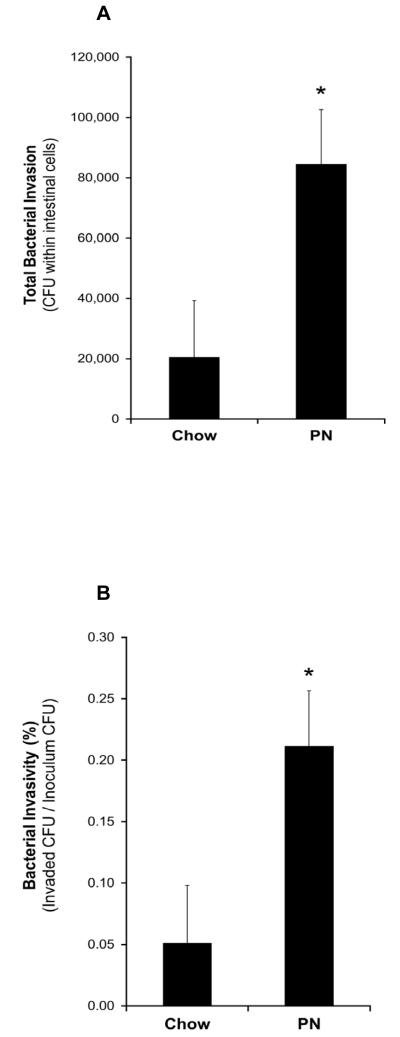
Overall Analysis
Since the distal bowel was more resistant to bacterial enteroinvasion, we decided to focus on the distal ileal segments when examining the effects of chow and PN feeding. This was done to test the hypothesis that PN feeding (or the lack of enteral feeding) would impair the ileal region that is more resistant to bacterial challenge under chow feeding. We observed that the ileal tissue from PN-fed animals was significantly more susceptible (approximately 4-fold) to enteroinvasion than those from chow-fed animals (total invasion: 84,584 ± 18,047 vs. 20,455 ± 18,784 CFU of ExPEC within cells, P=0018; invasivity: 0.21 ± 0.05 % vs. 0.05 ± 0.05 %, P=0018; Figures 5A and 5B). Ileal tissue from PN-fed animals secreted significantly lower levels of sPLA2 than those from chow-fed animals (1,959 ± 552 vs. 4,161 ± 552 ΔFI/min, P < 0.02; Figure 6).
Figure 5. Effect of chow- and PN- feeding on susceptibility of ileal tissue to invasion by ExPEC.
Figures show (A) total invasion and (B) invasivity (Invaded CFU/Inoculum CFU). * Denotes statistical difference (P=0.0018) between mucosal tissues from PN- and chow-fed mice.
Figure 6. Effect of chow- and PN- feeding on sPLA2 secretion by mucosal tissue in response to pathogen exposure.
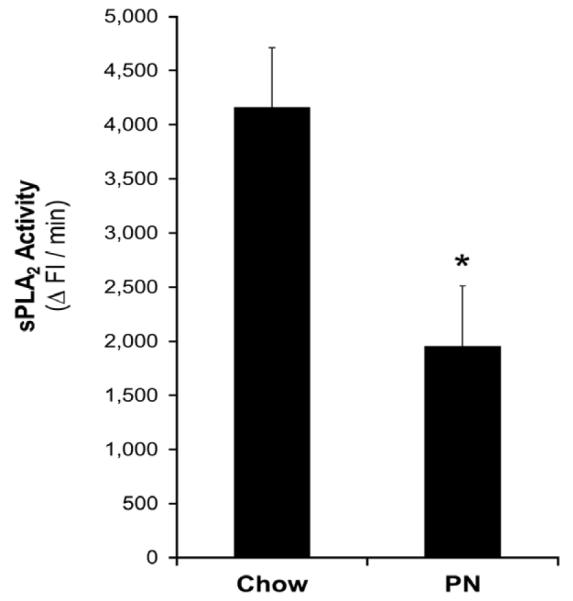
sPLA2 activity in the culture media is taken to be a measure of sPLA2 secretion. * Denotes statistical difference (P=0.01) between secretions by mucosal tissues from PN- and chow-fed mice.
DISCUSSION
PN is a necessity for the prevention of malnutrition in patients who are unable to be fed enterally. However, its use is associated with increased risk of infection when compared to enteral feeding. PN without enteral stimulation alters host immune defenses in part by changes to the gutassociated lymphoid tissue and the mucosal barrier (3, 4, 8, 21, 22). It also affects bacterial virulence within the gut lumen due to decreased nutrient availability (6, 7), decreased pH (5), and reduced bacterial diversity (23). Current evidence suggests that under such conditions, the bacterial invasion of the mucosal epithelium may to be an initial step in inducing a systemic inflammatory response that originates from the gastrointestinal system (16, 24, 25).
In patients hospitalized for 5 days or longer, the causative organisms of hospital acquired infections include facultative anaerobes, such as Escherichia coli (E. coli), Klebsiella, Streptococcus, and Enterobacter species, as well as anaerobes, such as Bacteroides, Peptostreptococcus, and Clostridium species (26). The preservation of mucosal barrier integrity is paramount in host defense against these microbial pathogens (3), especially in the face of increasing antibiotic resistance (10).
PN without enteral nutrients not only compromises the integrity of the physical mucosal barrier, but also alters the innate molecular defense capabilities of the barrier by reducing the secretion of mucin, antimicrobial peptides and sIgA. We recently reported that PN feeding alone reduces levels of the Paneth cell protein, sPLA2, in the small intestinal lumen (20). sPLA2 plays an important role in antimicrobial defense, in addition to the wide array of other antimicrobial peptides and proteins, such as lysozymes, defensins and RegIIIγ, produced by the Paneth cells (27, 28). We and others have demonstrated the antimicrobial action of sPLA2 (29-34). Due to the cationic charge on sPLA2, negatively charged bacterial cell membranes attract the protein which cleaves fatty acids from the sn-2 position of phospholipid glycerol backbones on the bacterial cell wall (35, 36). This process induces membrane permeability and subsequent lysis of the microbe.
Existing cell culture models are of limited use for investigating the complex bacterial-host interactions that occur at gut mucosa, especially for elucidating the mechanisms that affect host vulnerability to infections. While these models allow the investigator to focus on specific virulent mechanisms employed by bacteria to invade the host, they exclude the complex immune responses generated by an intact host mucosal barrier in response to pathogenic bacteria (12, 13). Perhaps, more importantly, these models do not allow for the study of the effects of types and route of diet, such as PN alone, on mucosal immunity as it relates to susceptibility to infections.
In this work, based on the pIVOC model (14) used by Schuller et al., we developed the EVISC model for studying the effects of PN without enteral feeding on bacterial invasion of the epithelium and the secretory response of the mucosa to pathogen exposure. Since E. coli are the most commonly cultured genera of bacteria in intra-abdominal abscess and since extraintestinal pathogenic E. coli (ExPEC), common to gastrointestinal tract, are often a significant threat of infection when displaced from the tract, we utilized a strain of ExPEC isolated from clinical fecal samples. The ExPEC strain was transformed to express luciferase and ampicillin resistance, allowing us to recover and enumerate the specific strain in our studies with intestinal segments that may contain other bacterial strains.
Our studies, in general, indicated that increasing concentrations of ExPEC inoculum, up to 4×107 CFU/well, produced increasing enteroinvasion and stimulation of sPLA2 secretion by the tissue. The two highest inoculum concentrations, 2×108 and 4×108 CFU/well, induced observable sloughing of tissue segments and thus the data were excluded from figures. The scanning electron microscopy of ExPEC interaction with the ileal tissue revealed ExPEC attaching to the apical surface of the ileal tissue via fimbriae and then initiating entry into the epithelial cells. This mechanism of invasion is commonly utilized by microbial pathogens to gain entry into non-phagocytic host cells (37, 38). The observations were also identical to what we have previously observed in our studies exploring the invasion of enterocytes (Caco-2 cells) in culture by ExPEC (data not shown). The studies of the effects of ileal regionality on bacterial invasion showed that the proximal ileum was significantly more susceptible to enteroinvasion than the distal ileum. Additionally, the proximal region secreted less sPLA2 in response to pathogen exposure compared to the distal region, suggesting the differences in susceptibility of the regions to enteroinvasion may be due to a decreased immune response, such as the decreased secretion of sPLA2 and possibly other antimicrobial proteins. This finding supports the view that the distal ileum is more resistant to pathogens and is consistent with the higher in vivo bacterial concentrations commonly reported in the distal intestine (39). While the current study suggests an inverse relationship between the secretion of sPLA2 and the susceptibility to enteroinvasion, additional studies are needed to elucidate the mechanistic link between the two parameters. The EVISC model, which revealed the regional differences in mucosal immunity that exist in vivo and the potential role of sPLA2 secretion in this context, is also a suitable tool for further studies to elucidate mechanisms responsible for the observations.
Our studies with ileal tissue from PN- and chow-fed animals showed that PN without enteral nutrition increases the susceptibility of the distal ileum to enteroinvasion by ExPEC; bacterial invasion of the tissue from PN-fed animals was approximately four times higher than in that from chow-fed animals. The distal ileum, as opposed to the proximal region, was chosen to study the effects of PN without enteral feeding since the distal bowel was more resistant to bacterial enteroinvasion in our previous experiments, and we were particularly interested in what effects PN would have on this resistance. Increased susceptibility to enteroinvasion in the distal region following PN with the lack of enteral feeding suggests impaired mucosal defense in what is normally the more protected region of intestine. The increased susceptibility may, in part, be due to the decreased secretion of sPLA2 in response to the pathogen that was observed in tissue from PN-fed animals. These observations not only suggest why PN without enteral feeding is associated with increased risk of infection in patients, but also illustrate the value of the EVISC model for understanding the impact of diet on susceptibility to infections and for elucidating the mechanisms by which diets alter mucosal immunity in this context.
In summary, our studies illustrate the usefulness of EVISC as a reproducible model of the heterogeneity of mucosal tissue and some of the complexity of host-pathogen interactions for studying the effect of type and route of diet in context of infections. More specifically, the data supports our hypothesis that reduced enteral stimulation decreases the effective responsiveness of mucosal immunity to a pathogen exposure, and provide a potential mechanism by which PN without enteral feeding is associated with an increased risk of infectious complications in patients. We have further studies in this model underway to examine how stimulation of individual components of the host defense, such as Paneth cell production of sPLA2, lysozymes, and defensins, lamina propria production of IgA, and goblet cell production and release of mucins, influence response to a pathogen.
ACKNOWLEDGEMENTS
We would like to thank Kristin M. Kohlmann for her technical assistance with bacterial culturing and preparations, and Joseph A. Heintz for his technical assistance with electron microscopy. We would also like to thank Dr. Walter J. Hopkins (Department of Urology, University of Wisconsin School of Medicine and Public Health) for providing ExPEC Strain-5011 that was used in our studies.
Abbreviations used
- PN
parenteral nutrition
- CFUs
colony forming units
- EVISC
ex vivo intestinal segment culture
- sPLA2
secretory phospholipase A2
- pIVOC
polarized in vitro organ culture
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Moore FA, Feliciano DV, Andrassy RJ, McArdle AH, Booth FV, Morgenstein-Wagner TB, Kellum JM, Welling RE, Moore EE. Early enteral feeding, compared with parenteral, reduces postoperative septic complications. The results of a meta-analysis. Ann Surg. 1992;216:172–183. doi: 10.1097/00000658-199208000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kudsk KA, Croce MA, Fabian TC, Minard G, Tolley EA, Poret HA, Kuhl MR, Brown RO. Enteral versus parenteral feeding. Effects on septic morbidity after blunt and penetrating abdominal trauma. Ann Surg. 1992;215:503–511. doi: 10.1097/00000658-199205000-00013. discussion 511-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deitch EA, Xu D, Naruhn MB, Deitch DC, Lu Q, Marino AA. Elemental diet and IV-TPN-induced bacterial translocation is associated with loss of intestinal mucosal barrier function against bacteria. Ann Surg. 1995;221:299–307. doi: 10.1097/00000658-199503000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gatt M, MacFie J. Randomized clinical trial of gut-specific nutrients in critically ill surgical patients. Br J Surg. 2010;97:1629–1636. doi: 10.1002/bjs.7155. [DOI] [PubMed] [Google Scholar]
- 5.Romanowski K, Zaborin A, Fernandez H, Poroyko V, Valuckaite V, Gerdes S, Liu DC, Zaborina OY, Alverdy JC. Prevention of siderophore- mediated gut-derived sepsis due to P. aeruginosa can be achieved without iron provision by maintaining local phosphate abundance: role of pH. BMC Microbiol. 2011;11:212. doi: 10.1186/1471-2180-11-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romanowski K, Zaborin A, Valuckaite V, Rolfes RJ, Babrowski T, Bethel C, Olivas A, Zaborina O, Alverdy JC. Candida albicans isolates from the gut of critically ill patients respond to phosphate limitation by expressing filaments and a lethal phenotype. PLoS One. 2012;7:e30119. doi: 10.1371/journal.pone.0030119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Long J, Zaborina O, Holbrook C, Zaborin A, Alverdy J. Depletion of intestinal phosphate after operative injury activates the virulence of P aeruginosa causing lethal gut-derived sepsis. Surgery. 2008;144:189–197. doi: 10.1016/j.surg.2008.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spaeth G, Gottwald T, Specian RD, Mainous MR, Berg RD, Deitch EA. Secretory immunoglobulin A, intestinal mucin, and mucosal permeability in nutritionally induced bacterial translocation in rats. Ann Surg. 1994;220:798–808. doi: 10.1097/00000658-199412000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belyansky LS, Sayenko VF, Furmanov JA, Churilova TJ. Bacterial translocation as a cause for septic complications in obstructive colonic ileus. Acta Chir Belg. 2002;102:75–77. doi: 10.1080/00015458.2002.11679270. [DOI] [PubMed] [Google Scholar]
- 10.Barie PS. Multidrug-resistant organisms and antibiotic management. Surg Clin North Am. 2012;92:345–391. ix–x. doi: 10.1016/j.suc.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 11.Flanagan JL, Brodie EL, Weng L, Lynch SV, Garcia O, Brown R, Hugenholtz P, DeSantis TZ, Andersen GL, Wiener-Kronish JP, Bristow J. Loss of bacterial diversity during antibiotic treatment of intubated patients colonized with Pseudomonas aeruginosa. J Clin Microbiol. 2007;45:1954–1962. doi: 10.1128/JCM.02187-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bai L, Schuller S, Whale A, Mousnier A, Marches O, Wang L, Ooka T, Heuschkel R, Torrente F, Kaper JB, Gomes TA, Xu J, Phillips AD, Frankel G. Enteropathogenic Escherichia coli O125:H6 triggers attaching and effacing lesions on human intestinal biopsy specimens independently of Nck and TccP/TccP2. Infect Immun. 2008;76:361–368. doi: 10.1128/IAI.01199-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schuller S, Chong Y, Lewin J, Kenny B, Frankel G, Phillips AD. Tir phosphorylation and Nck/N-WASP recruitment by enteropathogenic and enterohaemorrhagic Escherichia coli during ex vivo colonization of human intestinal mucosa is different to cell culture models. Cell Microbiol. 2007;9:1352–1364. doi: 10.1111/j.1462-5822.2006.00879.x. [DOI] [PubMed] [Google Scholar]
- 14.Schuller S, Lucas M, Kaper JB, Giron JA, Phillips AD. The ex vivo response of human intestinal mucosa to enteropathogenic Escherichia coli infection. Cell Microbiol. 2009;11:521–530. doi: 10.1111/j.1462-5822.2008.01275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mosenthal AC, Xu D, Deitch EA. Elemental and intravenous total parenteral nutrition diet-induced gut barrier failure is intestinal site specific and can be prevented by feeding nonfermentable fiber. Crit Care Med. 2002;30:396–402. doi: 10.1097/00003246-200202000-00022. [DOI] [PubMed] [Google Scholar]
- 16.Reddy BS, MacFie J, Gatt M, Macfarlane-Smith L, Bitzopoulou K, Snelling AM. Commensal bacteria do translocate across the intestinal barrier in surgical patients. Clin Nutr. 2007;26:208–215. doi: 10.1016/j.clnu.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Sitren HS, Heller PA, Bailey LB, Cerda JJ. Total parenteral nutrition in the mouse: development of a technique. JPEN J Parenter Enteral Nutr. 1983;7:582–586. doi: 10.1177/0148607183007006582. [DOI] [PubMed] [Google Scholar]
- 18.National Academy of Science: Nutrient Requirements of Laboratory Animals. National Academy of Science; Washington, DC: 1978. National Research Publication No. 10. [Google Scholar]
- 19.Tsao FH, Shanmuganayagam D, Zachman DK, Khosravi M, Folts JD, Meyer KC. A continuous fluorescence assay for the determination of calcium-dependent secretory phospholipase A2 activity in serum. Clin Chim Acta. 2007;379:119–126. doi: 10.1016/j.cca.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 20.Pierre JF, Heneghan AF, Tsao FH, Sano Y, Jonker MA, Omata J, Lan J, Kudsk KA. Route and type of nutrition and surgical stress influence secretory phospholipase A2 secretion of the murine small intestine. JPEN J Parenter Enteral Nutr. 2011;35:748–756. doi: 10.1177/0148607111414025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.King BK, Li J, Kudsk KA. A temporal study of TPN-induced changes in gut-associated lymphoid tissue and mucosal immunity. Arch Surg. 1997;132:1303–1309. doi: 10.1001/archsurg.1997.01430360049009. [DOI] [PubMed] [Google Scholar]
- 22.Li J, Kudsk KA, Gocinski B, Dent D, Glezer J, Langkamp-Henken B. Effects of parenteral and enteral nutrition on gut-associated lymphoid tissue. J Trauma. 1995;39:44–51. doi: 10.1097/00005373-199507000-00006. discussion 51-42. [DOI] [PubMed] [Google Scholar]
- 23.Schneider SM, Le Gall P, Girard-Pipau F, Piche T, Pompei A, Nano JL, Hebuterne X, Rampal P. Total artificial nutrition is associated with major changes in the fecal flora. Eur J Nutr. 2000;39:248–255. doi: 10.1007/s003940070003. [DOI] [PubMed] [Google Scholar]
- 24.Mazuski JE, Solomkin JS. Intra-abdominal infections. Surg Clin North Am. 2009;89:421–437. ix. doi: 10.1016/j.suc.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 25.Kristof K, Madach K, Sandor N, Ivanyi Z, Kiraly A, Erdei A, Tulassay E, Gal J, Bajtay Z. Impact of molecular mimicry on the clinical course and outcome of sepsis syndrome. Mol Immunol. 2011;49:512–517. doi: 10.1016/j.molimm.2011.09.023. [DOI] [PubMed] [Google Scholar]
- 26.Brook I. Microbiology and management of abdominal infections. Dig Dis Sci. 2008;53:2585–2591. doi: 10.1007/s10620-007-0194-6. [DOI] [PubMed] [Google Scholar]
- 27.Ouellette AJ, Selsted ME. Paneth cell defensins: endogenous peptide components of intestinal host defense. FASEB J. 1996;10:1280–1289. doi: 10.1096/fasebj.10.11.8836041. [DOI] [PubMed] [Google Scholar]
- 28.Porter EM, Bevins CL, Ghosh D, Ganz T. The multifaceted Paneth cell. Cell Mol Life Sci. 2002;59:156–170. doi: 10.1007/s00018-002-8412-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Omata J, Pierre JF, Heneghan AF, Tsao FH, Sano Y, Jonker MA, Kudsk KA. Parenteral nutrition suppresses the bactericidal response of the small intestine. Surgery. 2012 doi: 10.1016/j.surg.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harwig SS, Tan L, Qu XD, Cho Y, Eisenhauer PB, Lehrer RI. Bactericidal properties of murine intestinal phospholipase A2. J Clin Invest. 1995;95:603–610. doi: 10.1172/JCI117704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beers SA, Buckland AG, Koduri RS, Cho W, Gelb MH, Wilton DC. The antibacterial properties of secreted phospholipases A2: a major physiological role for the group IIA enzyme that depends on the very high pI of the enzyme to allow penetration of the bacterial cell wall. J Biol Chem. 2002;277:1788–1793. doi: 10.1074/jbc.M109777200. [DOI] [PubMed] [Google Scholar]
- 32.Weinrauch Y, Elsbach P, Madsen LM, Foreman A, Weiss J. The potent anti-Staphylococcus aureus activity of a sterile rabbit inflammatory fluid is due to a 14-kD phospholipase A2. J Clin Invest. 1996;97:250–257. doi: 10.1172/JCI118399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weinrauch Y, Abad C, Liang NS, Lowry SF, Weiss J. Mobilization of potent plasma bactericidal activity during systemic bacterial challenge. Role of group IIA phospholipase A2. J Clin Invest. 1998;102:633–638. doi: 10.1172/JCI3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ayabe T, Satchell DP, Wilson CL, Parks WC, Selsted ME, Ouellette AJ. Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria. Nat Immunol. 2000;1:113–118. doi: 10.1038/77783. [DOI] [PubMed] [Google Scholar]
- 35.Selsted ME, Ouellette AJ. Mammalian defensins in the antimicrobial immune response. Nat Immunol. 2005;6:551–557. doi: 10.1038/ni1206. [DOI] [PubMed] [Google Scholar]
- 36.Mukherjee S, Vaishnava S, Hooper LV. Multi-layered regulation of intestinal antimicrobial defense. Cell Mol Life Sci. 2008;65:3019–3027. doi: 10.1007/s00018-008-8182-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bower JM, Eto DS, Mulvey MA. Covert operations of uropathogenic Escherichia coli within the urinary tract. Traffic. 2005;6:18–31. doi: 10.1111/j.1600-0854.2004.00251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cossart P, Sansonetti PJ. Bacterial invasion: the paradigms of enteroinvasive pathogens. Science. 2004;304:242–248. doi: 10.1126/science.1090124. [DOI] [PubMed] [Google Scholar]
- 39.Hao WL, Lee YK. Microflora of the gastrointestinal tract: a review. Methods Mol Biol. 2004;268:491–502. doi: 10.1385/1-59259-766-1:491. [DOI] [PubMed] [Google Scholar]