Abstract
Significance
Tissue elasticity is severely compromised in aging skin, lungs, and blood vessels. In the vascular and pulmonary systems, respectively, loss of mechanical function is linked to hypertension, which in turn is a risk factor for heart and renal failure, stroke, and aortic aneurysms, and to an increased risk of mortality as a result of acute lung infections.
Recent Advances
Although cellular mechanisms were thought to play an important role in mediating tissue aging, the reason for the apparent sensitivity of elastic fibers to age-related degradation remained unclear. We have recently demonstrated that compared with type I collagen, a key component of the elastic fiber system, the cysteine-rich fibrillin microfibril is highly susceptible to direct UV exposure in a cell-free environment. We hypothesized therefore that, as a consequence of both their remarkable longevity and cysteine-rich composition, many elastic fiber-associated components will be susceptible to the accumulation of damage by both direct UV radiation and reactive oxygen species-mediated oxidation.
Critical Issues
Although elastic fiber remodeling is a common feature of aging dynamic tissues, the inaccessibility of most human tissues has hampered attempts to define the molecular causes.
Clinical Care Relevance
Although, currently, the localized repair of damaged elastic fibers may be effected by the topical application of retinoids and some cosmetic products, future studies may extend the application of systemic transforming growth factor β antagonists, which can prevent cardiovascular remodeling in murine Marfan syndrome, to aging humans. Acellular mechanisms may be key mediators of elastic fiber remodeling and hence age-related tissue stiffening.

Michael J. Sherratt, BSc, MSc, PhD
Scope
The components of skin, blood vessels, and lungs are required to withstand diverse mechanical environments. Skin, for example, is subjected to continuous stresses, which act in multiple directions,1 but major arteries must resist and dampen cyclic variations in blood pressure.2 Despite these dissimilar physiological requirements, the mechanical properties of most soft biological tissues are dominated by an extracellular matrix (ECM) in which fibrillar collagens and hydrated proteoglycans resist tensile and compressive forces and elastic fibers confer both compliance and passive recoil. Although elastic fibers are complex multicomponent assemblies, their structure is dominated by two major constituents: highly crosslinked elastin, a molecular spring that stores and then releases mechanical energy, and fibrillin microfibrils, which form the interface between the elastic fiber and the surrounding tissue.3 These microfibrils perform multiple physiological functions by acting as (i) an important tissue repository for cell signaling molecules, (ii) the adhesive interface between the elastic fiber and adjacent cells and ECM molecules, and potentially (iii) a mechanical reinforcing element.3–5 In both humans and other mammals, however, dermal, cardiovascular, and pulmonary tissues become increasingly stiff and less able to recoil with age, leading to the hypothesis that age-related failure of elastic fibers may underpin the apparent 100–120-year limit on human life expectancy.6
In contrast to intracellular proteins that are continuously synthesized and recycled, ECM proteins, in general, and elastic fiber proteins, in particular, are remarkably long-lived. In both small mammals and humans, individual elastic fiber proteins remain within the tissue and hence are required to perform their mechanical role, for the lifetime of the organism.7 Specifically, during the course of 70 years, human aortic elastic fibers must undergo over 3 billion (3 × 109) extension and recoil cycles without repair or replacement. As a consequence, these assemblies are vulnerable to the accumulation of damage, and although the key cause(s) of elastic fiber degradation remain to be determined, potential degradative mechanisms include glucose-mediated cross-linking, calcium and lipid accumulation, the time-dependent modification of aspartic acid residues, reactive oxygen species (ROS)-mediated oxidation, and enzymatic proteolysis by the large family of matrix metalloproteinases (MMPs). These proteases are expressed both constitutively and as a consequence of age-related inflammatory conditions such as emphysema, atherosclerosis, and skin photoaging.8 However, it is not clear how these enzymes, each of which is capable of degrading multiple ECM components, could mediate the specific remodeling of the elastic fiber system in chronically UV-exposed skin. We recently suggested therefore that acellular mechanisms (either UVR [ultraviolet radiation] alone or UVR-induced ROS) may be key players in elastic fiber degradation and hence loss of tissue elasticity.
Target Articles.
1. Sherratt MJ: Tissue elasticity and the ageing elastic fibre. Age 2009; 31: 305.
2. Sherratt MJ, Bayley CP, Reilly SM, Gibbs NK, Griffiths CEM, and Watson REB: Low-dose ultraviolet radiation selectively degrades chromophore-rich extracellular matrix components. J Pathol 2010; 222: 32.
3. Naylor EC, Watson REB, and Sherratt MJ. Molecular aspects of skin ageing. Maturitas 2011; 69: 249.
Translational Relevance
The elastic fiber system in skin is highly ordered. In the reticular dermis, large-diameter elastic fibers lie parallel to the skin surface. These fibers are connected to smaller-diameter elaunin fibers in the papillary dermis and eventually to oxytalan fibers, which connect with the dermal–epidermal junction (DEJ). The aging process has a profound effect on the structure of the elastic fiber system and in areas exposed to UVR; acute or “extrinsic” aging processes are superimposed on underlying chronic or “intrinsic” aging mechanisms.9,10 These two processes have distinct cosmetic and structural consequences. The smooth appearance and fine wrinkles of intrinsically aged skin are associated with a gradual fragmentation of the elastic fiber network, whereas severely photoaged skin appears roughened and deeply wrinkled and is characterized histologically by the deposition of disorganized elastic fiber material. Early in the photoaging process, however, fibrillin microfibrils and the elastic fiber-associated protein fibulin-5 are lost from the papillary dermis.11,12 Although the exposure of skin to low-dose UVR is sufficient to induce the expression and/or activation of MMP-1, -2, -3, -7, -9, -12, and -13, collectively these enzymes are capable of degrading more than 20 ECM proteins, including the abundant skin components collagen types I, III, IV, and VII, fibronectin, and elastin in addition to fibrillin.13 In our 2010 study,14 we suggested therefore that enzyme-mediated pathways alone are unlikely to be responsible for the differential degradation elastic fiber components in early photoaging and that direct exposure to UVR may play a key role.
Although previous studies have demonstrated that isolated ECM components such as type I collagen and elastin may be degraded by UV irradiation, the required doses far exceed physiologically attainable exposures.15 However, as only a subset of the 20 amino acid residues found in human proteins absorb UV-B radiation (i.e., act as UV chromophores),14,16 we hypothesized that UV chromophore-rich ECM components will be susceptible to much lower radiation doses. We tested this hypothesis by exposing type I collagen, fibronectin, and isolated fibrillin microfibrils (UV chromophore content 2.2%, 12.9%, and 21.0%, respectively) to physiologically relevant doses of UV-B radiation.
Clinical Relevance
Age-related changes in the biomechanical properties of the vascular and pulmonary systems profoundly influence human morbidity and mortality. Specifically, reduced arterial compliance (arteriosclerosis) is linked to hypertension, which in turn is a risk factor for heart and renal failure, stroke, and aortic aneurysms, whilst loss of pulmonary elasticity within the aging population increases the risk of mortality as a result of acute infectious diseases in the elderly population.2,8,17 Unfortunately, progress in understanding these diseases, and hence in developing strategies to limit or reverse age-related stiffening, is severely hampered by the inaccessibility of human cardiovascular and pulmonary tissues. However, in common with lungs and blood vessels, the mechanical properties of skin are not only determined by fibrillar collagens and elastic fibers but also undergo profound changes with age.8,9 In turn, this age-related loss of cutaneous elasticity is associated with remodeling of the dermal ECM and an increased incidence of both skin tears and pressure ulcers.18,19 As a consequence, insights into the mechanisms that degrade ECM proteins isolated from accessible dermal tissues and cultured cells may be directly relevant to the systemic processes that mediate aberrant tissue remodeling in skin, blood vessels, and lungs.
Experimental Model or Material: Advantages and Limitations
Attempts to characterize the causative mechanisms of age-related tissue stiffening have been severely hampered by the inaccessibility and complexity of many human tissues. In contrast, skin is a uniquely accessible tissue that is composed of the same structural components (fibrillar and nonfibrillar collagens, elastic fiber proteins and proteoglycans) that comprise the ECM of blood vessels and lungs. In turn, many of these components may be isolated as intact macromolecular assemblies or obtained from commercial suppliers and hence studied in a simplified cell-free environment. In our 2010 study, we characterized the relative susceptibility of three key isolated ECM components to UVR. As UVR-induced protein degradation may act directly (via the absorption of energy) and/or indirectly (via the generation and subsequent action of ROS), these observations have implications for the relative susceptibility of proteins within the cardiovascular and pulmonary systems to systemic oxidation.14 Such in vitro systems, however, are not without potential drawbacks: (i) the structure of recombinant or isolated proteins may not truly reflect their in vivo structure, (ii) acellular degradative mechanisms are unlikely to operate in isolation, and (iii) the degree and nature of protein degradation will vary between tissues.
Discussion of Findings and Relevant Literature
Fibrillin microfibrils, whether isolated from cultured cells or human dermis, adopt a characteristic morphology, appearing, when visualized by atomic force microscopy (AFM), as uniform, semirigid, beaded filaments with a periodicity of 56 nm.3 However, exposure to UV-B doses as low as 20 mJ/cm2 (less than half the dose required to cause minimal skin reddening) induced microfibril fragmentation and significantly altered both periodicity and average mass.14,20 In many cases, the microfibrils appeared to be highly flexible and prone to self-association/aggregation. These physiologically attainable doses of UV-B radiation were between 200 and 5,000 less than the reported doses, which induce measurable structural changes in fibrillar collagens.15 To understand this disparity in the experimental susceptibility of major ECM proteins to UV-B radiation, we quantified the relative amino acid composition of 49 common dermal ECM proteins including collagens, elastic fiber-associated proteins, and adhesive glycoproteins. Of the 20 amino acid residues that comprise human proteins, only the aromatic ring-containing amino acid residues His, Phe, Trp, and Tyr and sulphur-containing Cys absorb UV-B radiation.16 Our survey of published protein amino acid sequences demonstrated that these UV-B chromophores are unevenly distributed in dermal ECM proteins. In general, fibrillar collagens and elastin are chromophore poor, whereas the other elastic fiber-associated proteins (the fibrillins, fibulins, and latent transforming growth factor-β-binding proteins [LTBPs]) are UV-B chromophore rich. This commonality of amino acid composition within elastic fiber-associated proteins is primarily due to a shared cysteine-rich structural motif, the calcium-binding epidermal growth factor-like domain, which dominates the structures of the fibrillins, fibulins, and LTBPs.3 Having demonstrated that UV-chromophore-rich fibrillin microfibrils are UV labile, and based on our survey of UV chromophore content, we hypothesized that the adhesive glycoprotein fibronectin (12.9% UV-B chromophores), but not type I collagen (2.2% UV-B chromophores), would be also UV labile. Following exposure to UV-B doses of up to 10 times the minimal erythemal dose, we observed no change in the electrophoretic mobility (and hence molecular structure) of type I collagen in either reducing or native gel conditions. In contrast, fibronectin underwent dose-dependent aggregation, which was evident not only by gel electrophoresis but also by disruption of the characteristic morphology when imaged by AFM.
Innovation
In this collection of target articles, our research group highlights the longevity of elastic fiber proteins and the ubiquitous nature of elastic fiber remodeling in aging tissues. We demonstrate that a key elastic fiber protein is susceptible to UVR-mediated degradation and present a hypothesis based on relative amino acid composition, which predicts that the cysteine-rich elastic fiber-associated proteins will be particularly UV labile. Although the suggestion that acellular mechanisms such as UVR may be capable of degrading ECM proteins is not new, our hypothesis explains the observations made by previous investigators that to induce significant structural changes in fibrillar collagens and elastin (which are both UV chromophore poor), exposure to physiologically unattainable radiation doses is required.
Take-Home Message.
Basic science advances
• Tissue elasticity (both compliance and recoil), which is normally conferred by long-lived elastic fibers, is severely compromised in aging tissues including skin, lungs, and blood vessels.
• Whilst the elastic fiber system undergoes profound remodeling in each of these aging tissues, there is mounting evidence to suggest that it is the organization, rather than relative composition of the ECM that is mediating mechanical properties.
• Multiple mechanisms, including glucose-mediated cross-linking, calcium and lipid accumulation, aspartic acid racemization, ROS-mediated oxidation, and enzymatic proteolysis, have all been proposed as drivers of elastic fiber remodeling.
• We have demonstrated that ECM proteins, with distinct amino acids compositions, are differentially susceptible to physiologically attainable doses of UVR. We therefore hypothesize that because of their cysteine-rich composition, elastic fiber-associated proteins will be particularly susceptible to degradation by both UVR (in skin) and ROS (in skin and internal tissues).
Clinical science advances
• The topical administration of both clinically prescribed vitamin A derivatives (retinoids) and over-the-counter cosmetic products can induce the deposition of fibrillin microfibrils in the papillary dermis, thus potentially reestablishing a physical link between superficial skin layers and mature elastic fibers in the deep dermis.
• The systemic administration of a transforming growth factor β (TGF-β) antagonist in an animal model of Marfan syndrome (which is caused by heritable mutations in fibrillin-1) prevents the extensive elastic fiber remodeling and life-threatening cardiovascular manifestations, which characterize the disorder in animals and humans.
Relevance to clinical care
• Methods to prevent either the oxidation of elastic fiber proteins or the subsequent aberrant tissue remodeling (as a consequence of MMP upregulation or TGF-β-mediated collagen fibrosis) may find future clinical applications in treating age-related tissue stiffening.
• In the immediate future, topical treatments will be available, which can significantly alter the clinical appearance and histological organization of aged skin.
Summary Illustration
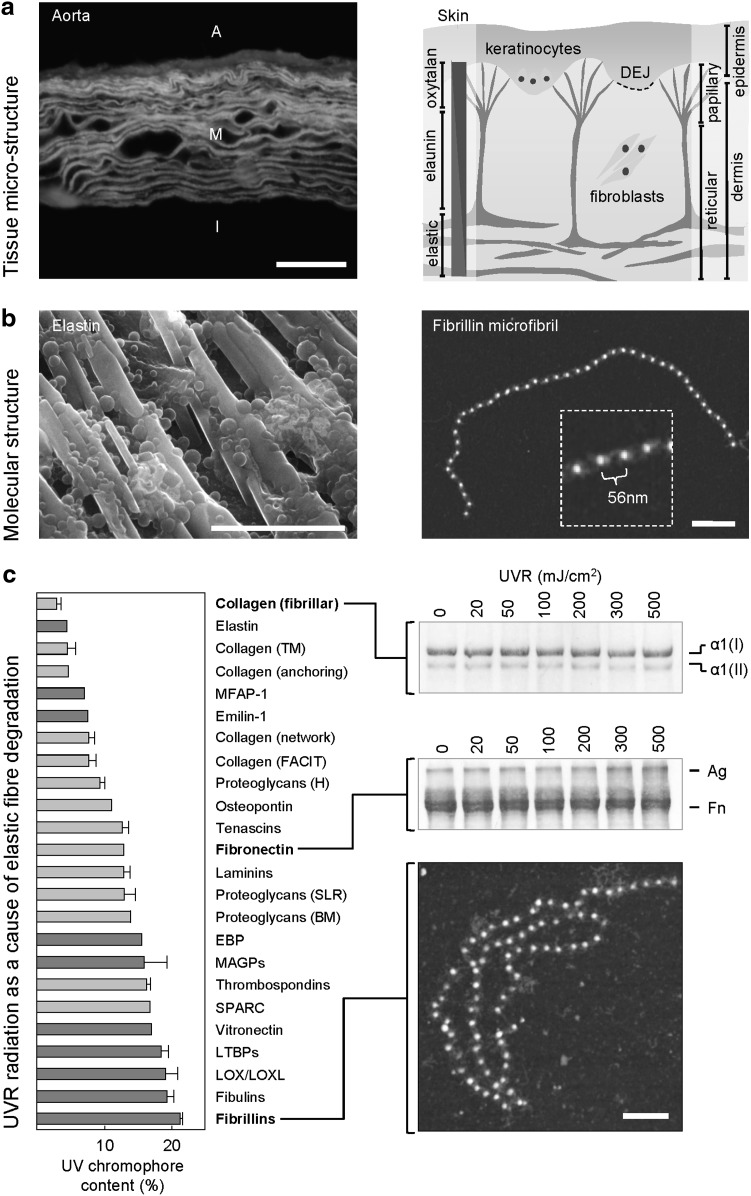
The figure below (adapted from Refs.8,14) illustrates (i) the distinct architectures adopted by elastic fibers in large arteries and skin, (ii) the macromolecular structures of the key elastic fiber components elastin and fibrillin, and (iii) the relationship between amino acid composition and susceptibility to UVR of three key ECM components. At micrometer length scales (a), elastic fibers in a fluorescence microscope image of ferret aorta are arranged into concentric lamellae in central medial layer (M). The outer advential and inner intimal layers are indicated by A and I, respectively. However, in skin, large elastic fibers, which are composed of both elastin and fibrillin, run parallel to the tissue surface in the deep reticular dermis, whereas oxytalan fibers, which are composed of fibrillin microfibrils only, intercalate with the DEJ. Regardless of tissue source, the major elastic fiber components, elastin and fibrillin, adopt similar molecular architectures (b). Recombinant tropoelastin forms linear arrays and globules when imaged by environmental scanning electron microscopy and fibrillin microfibrils appear as characteristic “beads-on-a-string” when imaged by AFM. With the exception of elastin itself (c), elastic fiber-associated proteins (dark gray) are rich in the UV-B chromophores cysteine, histidine, phenylalanine, tryptophan, and tyrosine. As a consequence, fibrillin microfibrils undergo extensive structural reorganization following exposure to 100 mJ/cm2 of UV-B radiation. Similarly, fibronectin (UV chromophore content 12.9%) undergoes UV-B dose-dependent aggregation (Ag). In contrast, the electrophoretic mobility of UV-B chromophore-poor type I collagen remains unaffected by doses of up to 500 mJ/cm2. Scale bars = 50 μm (a), 20 μm (b, left), 200 nm (b, right; c).
Caution, Critical Remarks, and Recomendations
Although our data suggest that ECM proteins are differentially susceptible to UVR exposure in vitro, it remains to be determined whether the accumulation of damage by repeated exposure to low doses of radiation is an important factor in mediating aberrant tissue remodeling in vivo. Even if direct exposure to UVR is capable of selectively degrading elastic fiber proteins within tissues, such events may be just an initial trigger, which leads to further degradation as microfibril fragments induce the expression of MMPs and/or tissue fibrosis and hence stiffening as a result of the uncontrolled release of previously microfibril-bound TGF-β. These enzymatic mechanisms (and their functional consequences) in combination with alternative degradative pathways including aberrant protein glycation, oxidation, and calcification may play key roles in mediating elastic fiber degradation in both UVR-exposed and UVR-protected tissues.8,10 With regard to potential interventions to reverse age-related ECM remodeling, although both topical (retinoids and some cosmetic preparations) and systemic (TGF-β antagonists) treatments are capable of influencing tissue structure, matrix components such as elastic fibers and fibrillin microfibrils are highly complex macromolecular assemblies and hence existing and newly synthesized assemblies may not be functionally equivalent.21,22
Future Development of Interest
We are currently carrying out investigations to determine whether the UVR-mediated degradation of elastic fiber components occurs directly (via interaction of the incident radiation with amino acid residues) or indirectly (via the UV-induced production of ROS, which in turn cause oxidative damage to nearby proteins). Regardless of the precise causative mechanism(s), however, to understand and quantify both the pathological effects of tissue stiffening and the efficacy of treatments, these tissues must be characterized mechanically. As we discuss in our 2009 review, the mechanical properties of tissues differ from macroscopic to microscopic length scales.8 Therefore, we are currently developing novel nano-indentation and scanning acoustic microscopy approaches to localize the cause of tissue stiffening to individual micrometer-scale ECM components in young, old, healthy, diseased, and treated tissues.23
Abbreviations and Acronyms
- AFM
atomic force microscope (microscopy)
- DEJ
dermal-epidermal junction
- ECM
extracellular matrix
- LTBP
latent transforming growth factor β binding protein
- MMP
matrix metalloproteinase
- ROS
reactive oxygen species
- TGF-β
transforming growth factor β
- UVR
ultraviolet radiation
Acknowledgments and Funding Sources
The author is grateful to Research into Ageing (Age UK) for support via a senior research fellowship and to Alliance Boots, Nottingham, United Kingdom, for funding ongoing work in the author's laboratory.
Author Disclosure and Ghostwriting
Alliance Boots exerted no editorial control over the contents of this article. This article was written solely by the author.
References
- 1.Escoffier C. de Rigal J. Rochefort A. Vasselet R. Leveque JL. Agache PG. Age-related mechanical properties of human skin: an in vivo study. J Invest Dermatol. 1989;93:353. [PubMed] [Google Scholar]
- 2.O'Rourke MF. Hashimoto J. Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol. 2007;50:1. doi: 10.1016/j.jacc.2006.12.050. [DOI] [PubMed] [Google Scholar]
- 3.Kielty CM. Sherratt MJ. Shuttleworth CA. Elastic fibres. J Cell Sci. 2002;115:2817. doi: 10.1242/jcs.115.14.2817. [DOI] [PubMed] [Google Scholar]
- 4.Habashi JP. Judge DP. Holm TM. Cohn RD. Loeys BL. Cooper TK, et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science. 2006;312:117. doi: 10.1126/science.1124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sherratt MJ. Baldock C. Haston JL. Holmes DF. Jones CJP. Shuttleworth CA, et al. Fibrillin microfibrils are stiff reinforcing fibres in compliant tissues. J Mol Biol. 2003;332:183. doi: 10.1016/s0022-2836(03)00829-5. [DOI] [PubMed] [Google Scholar]
- 6.Robert L. Robert AM. Fulop T. Rapid increase in human life expectancy: will it soon be limited by the aging of elastin? Biogerontology. 2008;9:119. doi: 10.1007/s10522-007-9122-6. [DOI] [PubMed] [Google Scholar]
- 7.Shapiro SD. Endicott SK. Province MA. Pierce JA. Campbell EJ. Marked longevity of human lung parenchymal elastic fibers deduced from prevalence of D-aspartate and nuclear weapons-related radiocarbon. J Clin Invest. 1991;87:1828. doi: 10.1172/JCI115204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sherratt MJ. Tissue elasticity and the ageing elastic fibre. Age. 2009;31:305. doi: 10.1007/s11357-009-9103-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El-Domyati M. Attia S. Saleh F. Brown D. Birk DE. Gasparro F, et al. Intrinsic aging vs. photoaging: a comparative histopathological, immunohistochemical, and ultrastructural study of skin. Exp Dermatol. 2002;11:398. doi: 10.1034/j.1600-0625.2002.110502.x. [DOI] [PubMed] [Google Scholar]
- 10.Naylor EC. Watson REB. Sherratt MJ. Molecular aspects of skin ageing. Maturitas. 2011;69:249. doi: 10.1016/j.maturitas.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 11.Watson REB. Griffiths CEM. Craven NM. Shuttleworth CA. Kielty CM. Fibrillin-rich microfibrils are reduced in photoaged skin. Distribution at the dermal-epidermal junction. J Invest Dermatol. 1999;112:782. doi: 10.1046/j.1523-1747.1999.00562.x. [DOI] [PubMed] [Google Scholar]
- 12.Kadoya K. Sasaki T. Kostka G. Timpl R. Matsuzaki K. Kumagai N, et al. Fibulin-5 deposition in human skin: decrease with ageing and ultraviolet B exposure and increase in solar elastosis. Br J Dermatol. 2005;153:607. doi: 10.1111/j.1365-2133.2005.06716.x. [DOI] [PubMed] [Google Scholar]
- 13.Chakraborti S. Mandal M. Das S. Mandal A. Chakraborti T. Regulation of matrix metalloproteinases: an overview. Mol Cell Bioche. 2003;253:269. doi: 10.1023/a:1026028303196. [DOI] [PubMed] [Google Scholar]
- 14.Sherratt MJ. Bayley CP. Reilly SM. Gibbs NK. Griffiths CEM. Watson REB. Low-dose ultraviolet radiation selectively degrades chromophore-rich extracellular matrix components. J Pathol. 2010;222:32. doi: 10.1002/path.2730. [DOI] [PubMed] [Google Scholar]
- 15.Menter JM. Cornelison LM. Cannick L. Patta AM. Dowdy JC. Sayre RM, et al. Effect of UV on the susceptibility of acid-soluble Skh-1 hairless mouse collagen to collagenase. Photodermatol Photoimmunol Photomed. 2003;19:28. doi: 10.1034/j.1600-0781.2003.00004.x. [DOI] [PubMed] [Google Scholar]
- 16.Du H. Fuh RCA. Li JZ. Corkan LA. Lindsey JS. PhotochemCAD: a computer-aided design and research tool in photochemistry. Photoche Photobiol. 1998;68:141. [Google Scholar]
- 17.Janssens JP. Pache JC. Nicod LP. Physiological changes in respiratory function associated with ageing. Eur Respir J. 1999;13:197. doi: 10.1034/j.1399-3003.1999.13a36.x. [DOI] [PubMed] [Google Scholar]
- 18.Baranoski S. Skin tears: the enemy of frail skin. Adv Skin Wound Care. 2000;13(3 Pt 1):123. [PubMed] [Google Scholar]
- 19.Farage MA. Miller KW. Berardesca E. Maibach HI. Clinical implications of aging skin: cutaneous disorders in the elderly. Am J Clin Dermatol. 2009;10:73. doi: 10.2165/00128071-200910020-00001. [DOI] [PubMed] [Google Scholar]
- 20.Fisher GJ. Datta SC. Talwar HS. Wang ZQ. Varani J. Kang S, et al. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature. 1996;379:335. doi: 10.1038/379335a0. [DOI] [PubMed] [Google Scholar]
- 21.Weiss JS. Ellis CN. Headington JT. Tincoff T. Hamilton TA. Voorhees JJ. Topical tretinoin improves photoaged skin—a double-blind vehicle-controlled study. JAMA. 1988;259:527. [PubMed] [Google Scholar]
- 22.Watson REB. Ogden S. Cotterell LF. Bowden JJ. Bastrilles JY. Long SP, et al. A cosmetic ‘anti-ageing’ product improves photoaged skin: a double-blind, randomized controlled trial. Br J Dermatol. 2009;161:419. doi: 10.1111/j.1365-2133.2009.09216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graham HK. Akhtar R. Kridiotis C. Derby B. Kundu T. Trafford AW. Sherratt MJ. Localised micro-mechanical stiffening in the ageing aorta. Mech Ageing Dev. 2011;132:459. doi: 10.1016/j.mad.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]