Abstract
Significance
Skin exhibits direction-dependent biomechanical behavior, influenced by the structural orientation of its collagen-rich fibrous network and its viscous ground-substance matrix. Injury can affect the skin's structure and composition, thereby greatly influencing the biomechanics and directionality of the resulting scar tissue.
Recent Advances
A combination of stress-relaxation and tensile failure testing identifies both the tissue's physiologically relevant viscoelastic behavior and resistance to rupture. When studied in mutually orthogonal directions in the plane of the tissue, these measures give insight into the directional properties of healthy tissue, and how they change with injury. By controlling the biomechanics of the wound environment, a new force-modulating dressing has demonstrated the ability to improve healing and reduce scar formation.
Critical Issues
Skin and scar biomechanics are typically characterized by using tensile failure, which identifies the tissue's resistance to rupture but offers limited insight into its normal daily function. Characterizing physiologically relevant biomechanics of skin, and how they change with injury, is critical to understand the tissue's ability to resist elongation, bear load, and dissipate energy via viscous means.
Future Directions
Compared with uninjured skin, scar tissue demonstrates similar high-load stiffness, greatly reduced resistance to failure, reduced low-load compliance, and altered material directionality. These findings, identified through combined stress relaxation and failure testing, suggest morphological changes with injury that are consistent with the viscoelastic and directional changes observed biomechanically. A more complete understanding of the directional, physiologically relevant skin biomechanics can guide the design and critical functional assessment of wound treatments, scaffolds, and tissue-engineered skin replacements.

David T. Corr, PhD
Scope
Skin is a composite material consisting of a collagen-rich fibrous network embedded in a ground substance matrix. The proteoglycan-rich matrix provides skin its viscous nature at low loads.1 The main fibrous constituents, collagen and elastin, provide structural stiffness and elasticity to the skin.2,3 Skin exhibits anisotropic, time-dependent, viscoelastic material behavior, in which the direction of minimal extensibility correlates with the fibrous network's principal direction.4 Skin is also a dynamic tissue, changing with age and influenced by gender.
Much of our current understanding of skin behavior has come from biomechanical tensile testing, in which tissue is stretched and the force that develops is measured. Although failure properties have typically dominated previous tensile analyses, skin's viscoelastic behavior at low loads has gained recent interest due to its relevance to normal physiological functioning. An appreciation of these biomechanical properties identifies the skin's ability to resist elongation, bear load, and dissipate energy via viscous means.5 When studied in mutually orthogonal directions in the plane of skin and scar, these measures give insight into the directionality of healthy tissue, and how the directional properties change with injury.6 Similar analyses can be employed to functionally assess potential wound healing treatments and engineered tissue replacements.
Target Articles.
1. Corr DT, Gallant-Behm CL, Shrive NG, and Hart DA: Biomechanical behavior of scar tissue and uninjured skin in a porcine model. Wound Repair Regen 2009; 17: 250.
2. Gurtner GC, Dauskardt RH, Wong VW, Bhatt KA, Wu K, Vial IN, et al.: Improving cutaneous scar by controlling the mechanical environment: large animal and phase I studies. Ann Surg 2011; 254: 217.
Translational Relevance
In his pioneering work, Karl Langer identified that skin's load-deformation behavior consisted of a highly extensible initial phase followed by a linear response.7,8 The initial strain-stiffening phase, or “toe-in” region, occurs at low loads and is attributed to progressive recruitment of collagen fibers: due to straightening of slack fibers and rotation of collagen fibers to align with the direction of elongation.1 When strained, skin dissipates elastic energy stored in the fibrous network via viscous means, as shown through both creep and stress relaxation. This viscous behavior dominates skin biomechanics at physiologically relevant loadings, and is believed to result from water contained in the tissue, and be influenced by the presence of proteoglycans. Thus, the biomechanics of skin is greatly influenced by its structure and composition. Wounding can greatly alter both structure and composition, thus resulting in scar tissue with significant biomechanical differences.
Skin's fibrotic network exhibits a directionality that correlates with its biomechanical function. Thus, to more completely assess skin biomechanics, it is critical to characterize the directional differences in both the physiologically relevant viscoelastic properties and failure properties. This can be achieved by evaluating specimens obtained in orthogonal directions within the plane of the tissue, using a combination of stress-relaxation and tensile failure testing. When carried out in both skin and scar tissue, the influence of injury on the directionality and biomechanical function of skin can be identified. Understanding how injury affects skin function can help identify the corresponding structural and compositional changes, and provide valuable information for the design and functional assessment of clinical treatments and/or tissue-engineered skin replacements.
Biomechanics also influence the healing wound, with more pronounced scarring observed in anatomical locations of high tissue tension. It appears that this effect can be mitigated by controlling the wound mechanical environment with a new force-modulating dressing.
Clinical Relevance
Skin exhibits a highly directional response to stretch. It is least extensible in the fibrous network's principal direction, which greatly influences the viscoelastic properties displayed at low loads and physiologic displacements. Acute wounding alters the skin's fibrotic structure, thereby producing scar tissue with significant functional impairments. Exploring skin biomechanics, in both healthy skin and scars, provides insight into the directionality of healthy tissue, and how it changes with injury. A more complete understanding of how wounding affects the skin's physiologic and failure properties can help guide the design and functional assessment of more effective treatments or engineered materials to repair compromised skin.
Experimental Model or Material: Advantages and Limitations
Pigs offer a preclinical model for full-thickness excisional wounds, with striking structural and functional similarities to human skin.9–11 Moreover, different breeds of pig exhibit responses to wounding that parallel those observed among humans of different ethnicities. As such, porcine models serve as excellent experimental models to study various aspects of human wound healing. Evaluating specimens during the late remodeling phase addresses the properties of a stable wound, in which scars have become more normocellular, an extracellular matrix is well established, and further remodeling occurs at a slow rate. However, this does not provide direct insight into other healing phases or the temporal aspects of wound healing. Biomechanical evaluation using a combination of stress-relaxation followed by tensile failure grants insight into both the viscoelastic properties relevant to physiologic use and tissue failure properties.5,12 These properties correlate to compositional and structural aspects of the tissue. Thus, evaluating skin and scar specimens, in orthogonal directions within the tissue's plane, identifies skin's material properties and directionality, and how these change with injury and subsequent healing.
A new, force-modulating, polymer dressing can control the wound's mechanical environment to improve incision healing and reduce scarring.13 However, this dressing has not yet been applied to excisional wounds.
Discussion of Findings and Relevant Literature
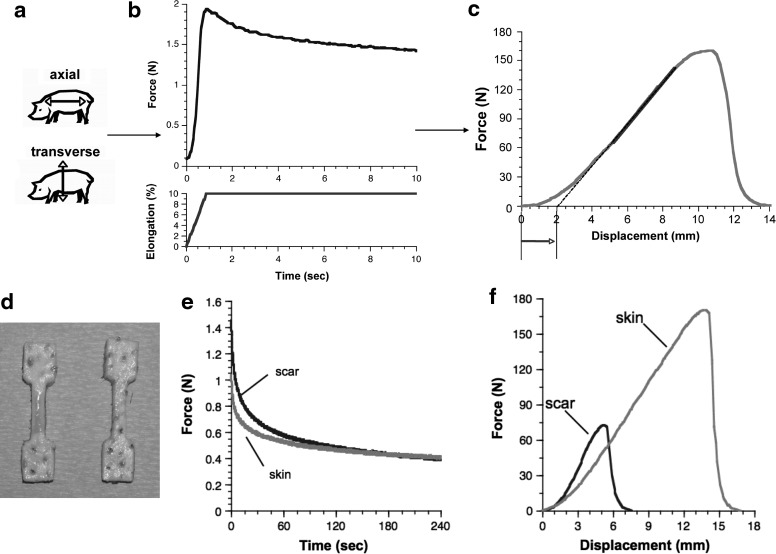
The influence of acute injury on dorsal skin biomechanics was explored by using porcine skin and scar tissue specimens, evaluated during the late remodeling phase of healing (70 days) after full-thickness excisional wounding.6 Test specimens were obtained in two orthogonal directions within the plane of skin: axial (cranial-caudal) and transverse (dorsal-ventral). Biomechanical characterization, using stress relaxation followed by tensile failure, provided insight into the tissue's viscoelastic response in physiologically relevant loadings, as well as its resistance to tensile rupture (Fig. 1).
Figure 1.
(a–c) Schematic of biomechanical protocol. (a) Mechanical test specimens are obtained by using a punch in both uninjured skin and scar, in two orthogonal directions: axial (cranial–caudal) and transverse (dorsal–ventral). (b) A stress-relaxation protocol (e.g., a 10% elongation, held for 4 min [first 10 s shown]) is used to characterize the low-load viscoelastic response, nondestructively. (c) Finally, the specimen is stretched to failure to obtain measures of the toe-in region (approximated by arrow), high-load stiffness (gray area of line), and failure properties. (d–f) Skin and scar data in a representative animal. (d) Mechanical test specimens obtained in scar (left) and uninjured skin (right). (e) Relaxation tests reveal that scar relaxes faster and is stiffer at lower loads. (f) Failure testing shows, despite similar high-load linear stiffness values, that scars exhibit stiffer low-load behavior, and greatly reduced resistance to rupture. This combined protocol identifies the physiologic and failure properties, as well as their planar directionality, and how these change with injury (adapted from Ref.6).
Biomechanical testing
For stress relaxation, specimens are stretched a small amount (e.g., 1.0 mm at 2.4 mm/s) and isometrically held for a period of time, during which tissue forces relax due to viscous energy dissipation. Data analysis can determine the peak force during relaxation (an indirect measure of low-load tissue stiffness), and rate of relaxation (indicative of tissue's viscous energy dissipation). For tensile failure, specimens are stretched at a constant rate (2.4 mm/s) until failure, while recording both displacement and force. Force-displacement data identify a number of important properties: load and displacement at failure, energy to failure (material “toughness”), linear stiffness (indicative of high-load tissue stiffness), and “toe-in” duration (extent of strain-stiffening behavior). At low loads, skin displays a nonlinear behavior in which it stiffens with increasing stretch. The extent of this strain-stiffening region, or toe-in, can be either directly calculated, or approximated by measuring the limit displacement. It is in this region that the skin, as well as most soft biologic tissues, typically operates.
Uninjured skin
Skin displayed comparable high-load behavior in both directions, exhibiting similar resistance to failure (loads, displacements, and energy at failure), as well as no directional difference in linear stiffness. However, at low loads, skin was more compliant in the axial direction than transverse: as evidenced by significantly lower peak relaxation forces and larger toe-in regions. Relaxation tests further identified that the skin relaxed much faster in the axial direction than in the transverse direction.
These behaviors are consistent with a tissue having principal orientation of collagen fibers biased toward the transverse direction. In such a structure, collagen fibers bear load at a lower tissue strain in the transverse specimens than the axial ones. As a result, transverse specimen “toe-in” would be smaller, low-load stiffness would be higher, and the collagen would experience a greater strain for a given displacement. Straightening of collagen fibers would cause fluids to exude from the tissue, thereby decreasing the viscous response.14 Thus, transverse specimens should display slower rates of relaxation than axial specimens, as was experimentally evidenced.
Effect of scarring
Scars showed significantly reduced failure properties (load, displacement, and energy), thus indicating their compromised bursting strength, extensibility, and toughness, with regard to uninjured skin.6,15 High-load linear stiffness was relatively unaffected by scarring,6 as previously observed in human hypertrophic scars,5 whereas the low-load behavior showed a more dramatic response. Scars relaxed faster, indicating greater viscous content, and exhibited reduced toe-in durations and stiffer responses at low loads. Scar tissue also showed strikingly similar viscoelastic behavior in both directions: with no directional differences in failure properties, low-load behavior, or relaxation rate.
Scar tissue's decreased failure resistance would suggest less total collagen in the tissue cross-section, due to either reduced size or number of collagen fibers. Since scars exhibit significantly stiffer responses at low loads, a greater strain would be expected in the collagen fibers, and, thus, a greater “wringing out” of the tissue. The increased viscous behavior observed in a tissue that should exude more water suggests that scars start with significantly higher water content, most likely due to increased proteoglycan content (quantitative), expression of different proteoglycans (qualitative), or a combination of both of these factors. Interestingly, similar elevated viscous behavior was observed in healing ligaments, although the specific cause(s) of this increase was not identified.16
The considerably compromised biomechanical function and decreased failure resistance of scars highlights the need for clinical treatments to restore normal viscoelastic behavior to the wound site. This would increase load transfer and strain compatibility between skin and scar, thereby leading to improved functionality and esthetics of the healed wound. Both skin and scar tissue structurally adapt to loading, exhibiting rapid parallel realignment of collagen in response to stretch.17 Additionally, clinical and preclinical data demonstrate pronounced scarring in anatomical areas with high tissue tension,18–20 thereby indicating that the wound environment's biomechanics can greatly influence healing outcomes. Significantly reducing tension in healing wounds could act to minimize scarring, as was recently demonstrated by using a force-modulating polymer wound dressing.13
Innovation
The first target article provides an innovative method to assess full-range mechanical properties of skin and scar tissue, as well as their directional differences.6 A protocol of stress relaxation followed by tensile failure characterizes the physiologically relevant, viscoelastic behavior, and failure properties. Evaluating orthogonal specimens determines the directionality of these properties. In skin, this approach identified significant changes with injury in failure and viscoelastic properties, as well as altered directionality in low-load response, which were consistent with observed structural changes (Fig. 2). This relatively simple approach can also characterize and assess functionality of various biomaterials, and engineered soft-tissue replacements.6
Figure 2.
Polarized light microscopy of skin (left) and scar (right), showing collagen network organization with regard to axial and transverse axes. Skin displays a collagen network with principal direction biased toward the transverse direction. Scars healed with considerable organization, in a fiber orientation with similar components in both directions, and exhibited much thinner fiber bundles than uninjured skin. These morphologic differences between skin and scar support the observed viscoelastic and failure biomechanics (modified from Ref.6).
Recent experiments, conducted in Yorkshire and red Duroc pigs, indicate that scar formation in ligaments,21 as well as in skin,22 leads to breed differences in both low- and high-load behavior.22–24 Thus, genetic influences can affect the biomechanics of healing skin and ligament.
The second target article13 developed a novel dressing that modulates the healing wound's biomechanical environment to reduce scarring. By exploiting its elastic recovery, the polymer sheet dressing can be adhered to skin with a prescribed amount of prestretch to deliver precise compressive loads to unload, or stress-shield, the wound. This provides an innovative potential treatment to control tissue loads during healing, which may help scars achieve structure, and directional properties similar to those of surrounding skin.
Take-Home Message.
Basic science advances
• Characterization using stress relaxation followed by tensile failure testing reveals both the physiologically relevant viscoelastic biomechanical behavior of the tissue and its resistance to rupture.
• Biomechanical evaluation of skin and scar tissue specimens, in orthogonal directions within the plane of skin, identifies the material properties and directionality of skin, and how these change with injury and subsequent healing.
• Precise measurement and analysis of low-load behavior uniquely identified changes in biomechanical behavior that were not appreciated through the traditional analysis of high-load linear stiffness and failure properties.
• Biomechanical differences between skin and scar tissue suggest morphological changes that may occur with injury which are consistent with the viscoelastic and directional changes observed.
• Genetic influences can affect the scarring response and biomechanics in healing ligament and skin.
• Controlling the mechanical forces on a healing wound can modify scar development and maturation.
Clinical science advances
• Characterizing both failure properties and low-load viscoelastic behavior provides insight into the skin's resistance to rupture, and the way it functions in normal, physiologic use.
• Identifying full-range tissue behaviors, and how they change with injury, provides a more complete understanding of the functional impairments of scar tissue.
• The porcine model exhibits similar structure, function, and healing response to normal human cutaneous healing, and as such, it is an attractive model to study human wound healing.
Relevance to clinical care
• Compared with uninjured skin, scar tissue demonstrates similar high-load stiffness, greatly reduced resistance to failure, reduced low-load compliance, and altered material directionality.
• It is critical to consider low-load viscoelastic behavior, in addition to failure properties, when assessing skin, scar tissue, or skin substitutes.
• The considerable reduction in biomechanical function coupled with decreased failure resistance of scars highlights the need for clinical treatments to restore the skin's viscoelastic behavior to the wound site.
• A more complete understanding of directional physiologically-relevant skin biomechanics can guide the design and critical functional assessment of potential wound treatments, scaffolds, grafting procedures, and tissue-engineered skin replacements.
• Biomechanical modulation of the wound environment by using a novel polymeric wound dressing has demonstrated the ability to reduce scar formation in skin.
Caution, Critical Remarks, and Recommendations
Skin and scar tissue biomechanics were explored in an established porcine model of wound healing,9–11 shown to exhibit a similar response to normal human cutaneous healing.9 Pigs (and humans) are tight-skinned animals that heal through a combination of re-epithelialization and wound contraction. Wound contractions associated with these domestic pigs (∼50%)9 are similar to those observed in normal human wound healing (25%–50%).10 In contrast, loose-skinned animals (e.g., rodents, lagomorphs) heal predominantly via contraction, with wound contractions approaching 90%.25 Porcine scars exhibited functional deficits similar to those previously observed in human skin injury; lower resistance to failure, and reduced toe-in region compared with uninjured skin.5,26 These similarities in structure, function, and healing response highlight the pig as an attractive model for studying human wound healing. Since these experiments study a single time point after acute full-thickness excision, they do not address the affect of age or time after injury as factors, and the results are limited to porcine skin healing in the late remodeling phase. Thus, the direct extrapolation of these findings to other models of wound healing (e.g., human healing, burns, incisions, body location, time postinjury, etc.) is cautioned. Similar caution is advised for extrapolating the reduced scarring observed in incisions treated with a force-modulating dressing, to the case of excisional wounds.
Future Developments of Interest
Further studies conducted at different developmental stages (e.g., juvenile, growing, sexually mature, and skeletally mature) would be necessary to identify and quantify any age-related differences in wound healing, and longitudinal studies could address temporal factors. Since skin cells,27 structure, and mechanical demands likely vary with location, future investigations using tissue from both dorsal and ventral wounds could determine whether location of injury plays a significant role in healing. Similarly, due to their contributions to low-load viscoelastic behavior, future studies exploring proteoglycans (qualitatively and quantitatively) may reveal their involvement in responses to injury, and give insights into potential therapies to restore normal viscous behavior to healing wounds. Furthermore, porcine incision scar formation has been significantly reduced by mechanical manipulation of the healing wound environment by using a dynamic stress-shielding dressing.13 Future efforts can utilize this force modulation to guide excisional scars to achieve structural alignment, and, thus, biomechanical directionality, similar to the surrounding uninjured skin.
Acknowledgments and Funding Sources
The authors acknowledge the financial support of The Alberta Ingenuity Fund (DTC), Ernst & Young Fellowship in Joint Injury and Arthritis Research (DTC), The Arthritis Society, CIHR, and NSERC. D.A.H. is the Calgary Foundation-Grace Glaum Professor.
Author Disclosure and Ghostwriting
The authors have no competing interests or disclosures relevant to this work, and no persons other than the authors contributed to the writing of this article.
References
- 1.Lanir Y. Skin mechanics. In: Skalak R, editor; Chien S., editor. Handbook of Bioengineering. Dallas, TX: McGraw-Hill; 1987. pp. 11.1–11.25. [Google Scholar]
- 2.Lanir Y. The fibrous structure of the skin, its relation to mechanical behaviour. In: Marks R, editor; Payne PA., editor. Bioengineering and the Skin. Hingham, MA: MTP Press; 1981. pp. 93–96. [Google Scholar]
- 3.Smith LT. Holbrook KA. Byers PH. Structure of the dermal matrix during development and in the adult. J Invest Dermatol. 1982;79(Suppl 1):93S. doi: 10.1111/1523-1747.ep12545877. [DOI] [PubMed] [Google Scholar]
- 4.Stark HL. Directional variations in the extensibility of human skin. Br J Plast Surg. 1977;30:105. doi: 10.1016/0007-1226(77)90001-7. [DOI] [PubMed] [Google Scholar]
- 5.Dunn MG. Silver FH. Swann DA. Mechanical analysis of hypertrophic scar tissue: structural basis for apparent increased rigidity. J Invest Dermatol. 1985;84:9. doi: 10.1111/1523-1747.ep12274528. [DOI] [PubMed] [Google Scholar]
- 6.Corr DT. Gallant-Behm CL. Shrive NG. Hart DA. Biomechanical behavior of scar tissue and uninjured skin in a porcine model. Wound Repair Regen. 2009;17:250. doi: 10.1111/j.1524-475X.2009.00463.x. [DOI] [PubMed] [Google Scholar]
- 7.Langer K. ur Anatomie und Physiologie der Haut. I. Über die Spaltbarkeit der Cutis. [The anatomy and physiology of the skin. I. About the cleavage of the cutis.] Sitzungsber Akad Wien. 1891;44:19. [Google Scholar]
- 8.Gibson T. Karl Langer (1819–1887) and his lines. Br J Plast Surg. 1978;31:1. doi: 10.1016/0007-1226(78)90002-4. [DOI] [PubMed] [Google Scholar]
- 9.Gallant-Behm CL. Reno C. Tsao H. Hart DA. Genetic involvement in skin wound healing and scarring in domestic pigs: assessment of molecular expression patterns in (Yorkshire x Red Duroc) x Yorkshire backcross animals. J Invest Dermatol. 2007;127:233. doi: 10.1038/sj.jid.5700482. [DOI] [PubMed] [Google Scholar]
- 10.Gallant-Behm CL. Hart DA. Genetic analysis of skin wound healing and scarring in a porcine model. Wound Repair Regen. 2006;14:46. doi: 10.1111/j.1743-6109.2005.00087.x. [DOI] [PubMed] [Google Scholar]
- 11.Wang JF. Olson ME. Reno CR. Wright JB. Hart DA. The pig as a model for excisional skin wound healing: characterization of the molecular and cellular biology, and bacteriology of the healing process. Comp Med. 2001;51:341. [PubMed] [Google Scholar]
- 12.Silver FH. Freeman JW. DeVore D. Viscoelastic properties of human skin and processed dermis. Skin Res Technol. 2001;7:18. doi: 10.1034/j.1600-0846.2001.007001018.x. [DOI] [PubMed] [Google Scholar]
- 13.Gurtner GC. Dauskardt RH. Wong VW. Bhatt KA. Wu K. Vial IN, et al. Improving cutaneous scar by controlling the mechanical environment: large animal and phase I studies. Ann Surg. 2011;254:217. doi: 10.1097/SLA.0b013e318220b159. [DOI] [PubMed] [Google Scholar]
- 14.Provenzano P. Lakes R. Keenan T. Vanderby R., Jr Nonlinear ligament viscoelasticity. Ann Biomed Eng. 2001;29:908. doi: 10.1114/1.1408926. [DOI] [PubMed] [Google Scholar]
- 15.White WL. Brody GS. Glaser AA. Marangoni RD. Beckwith TG. Must JS, et al. Tensiometric studies of unwounded and wounded skin: results using a standardized testing method. Ann Surg. 1971;173:19. doi: 10.1097/00000658-197101000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thornton GM. Leask GP. Shrive NG. Frank CB. Early medial collateral ligament scars have inferior creep behaviour. J Orthop Res. 2000;18:238. doi: 10.1002/jor.1100180211. [DOI] [PubMed] [Google Scholar]
- 17.Verhaegen PD. Schouten HJ. Tigchelaar-Gutter W. van Marle J. van Noorden CJ. Middelkoop E, et al. Adaptation of the dermal collagen structure of human skin and scar tissue in response to stretch: an experimental study. Wound Repair Regen. 2012;20:658. doi: 10.1111/j.1524-475X.2012.00827.x. [DOI] [PubMed] [Google Scholar]
- 18.Ogawa R. Keloid and hypertrophic scarring may result from a mechanoreceptor or mechanosensitive nociceptor disorder. Med Hypotheses. 2008;71:493. doi: 10.1016/j.mehy.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 19.Wray RC. Force required for wound closure and scar appearance. Plast Reconstr Surg. 1983;72:380. doi: 10.1097/00006534-198309000-00021. [DOI] [PubMed] [Google Scholar]
- 20.Ogawa R. Okai K. Tokumura F. Mori K. Ohmori Y. Huang C, et al. The relationship between skin stretching/contraction and pathologic scarring: the important role of mechanical forces in keloid generation. Wound Repair Regen. 2012;20:149. doi: 10.1111/j.1524-475X.2012.00766.x. [DOI] [PubMed] [Google Scholar]
- 21.Germscheid NM. Thornton GM. Hart DA. Hildebrand KA. Wound healing differences between Yorkshire and red Duroc porcine medial collateral ligaments identified by biomechanical assessment of scars. Clin Biomech. 2012;27:91. doi: 10.1016/j.clinbiomech.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Corr DT. Gallant-Behm CL. Shrive NG. Hart DA. The role of genetics in the biomechanical behavior of scar tissue and uninjured skin in a porcine model (Abstract) Wound Repair Regen. 2006;14:A58. doi: 10.1111/j.1524-475X.2009.00463.x. [DOI] [PubMed] [Google Scholar]
- 23.Corr DT. Gallant-Behm CL. Frank CB. Hart DA. Shrive NG. Proceedings of the 2005 BMES Annual Fall Meeting. Baltimore, MD: Biomedical Engineering Society; Sep 28, 2005. A comparison of the load behaviour in ligament, skin: tissue, scar (Abstract) –October 1. [Google Scholar]
- 24.Germscheid NM. Thornton GM. Hart DA. Hildebrand KA. A biomechanical assessment to evaluate breed differences in normal porcine medial collateral ligaments. J Biomech. 2011;44:725. doi: 10.1016/j.jbiomech.2010.10.036. [DOI] [PubMed] [Google Scholar]
- 25.Hayward PG. Robson MC. Animal models of wound contraction. Prog Clin Biol Res. 1991;365:301. [PubMed] [Google Scholar]
- 26.Clark JA. Cheng JC. Leung KS. Leung PC. Mechanical characterisation of human postburn hypertrophic skin during pressure therapy. J Biomech. 1987;20:397. doi: 10.1016/0021-9290(87)90047-9. [DOI] [PubMed] [Google Scholar]
- 27.de Hemptinne I. Gallant-Behm CL. Noack CL. Parreno J. Hart DA. Dermal fibroblasts from red Duroc and Yorkshire pigs exhibit intrinsic differences in the contraction of collagen gels. Wound Repair Regen. 2008;16:132. doi: 10.1111/j.1524-475X.2007.00340.x. [DOI] [PubMed] [Google Scholar]