Abstract
Previous research has demonstrated that sport-related concussions can have short-term effects on cognitive processes, but the long-term consequences are less understood and warrant more research. This study was the first to use event-related functional magnetic resonance imaging (fMRI) to examine long-term differences in neural activity during memory tasks in former athletes who have sustained multiple sport-related concussions. In an event-related fMRI study, former football players reporting multiple sport-related concussions (i.e., three or more) were compared with players who reported fewer than three concussions during a memory paradigm examining item memory (i.e., memory for the particular elements of an event) and relational memory (i.e., memory for the relationships between elements). Behaviorally, we observed that concussion history did not significantly affect behavioral performance, because persons in the low and high concussion groups had equivalent performance on both memory tasks, and in addition, that concussion history was not associated with any behavioral memory measures. Despite demonstrating equivalent behavioral performance, the two groups of former players demonstrated different neural recruitment patterns during relational memory retrieval, suggesting that multiple concussions may be associated with functional inefficiencies in the relational memory network. In addition, the number of previous concussions significantly correlated with functional activity in a number of brain regions, including the medial temporal lobe and inferior parietal lobe. Our results provide important insights in understanding the long-term functional consequences of sustaining multiple sports-related concussions.
Key words: cognitive function, head trauma, MRI
Introduction
Sport-related concussion has recently become a focus of public and academic concern, not to mention governmental intervention with congressional hearings on the topic in 2010 and 2011. The National Collegiate Athletic Association, National Football League (NFL), and National Hockey League have each responded by instituting new concussion guidelines and policies since 2010, and there has been a trickle-down effect to the youth levels of play. As of February 2013, 42 states have passed concussion laws for high school and youth athletes, with the first having just been passed in October 2009. Epidemiological studies comparing collegiate and high school sports have consistently shown that football has the highest concussion rates.1 In 2012, both the Ivy League and Pop Warner Football imposed restrictions on contact football practices in an attempt to minimize the number of head impacts to reduce the risk of concussion.
Few studies have investigated the concussion rates in professional sports or compared the rates between professional sports. Different data collection methods and reporting of concussion rates make such comparisons difficult. A review of professional football games, however, reported an average of 0.41 concussions per game.2 Given the estimate that nearly 50% of all concussions in football go unreported,3 it is highly probable that the concussion rate per NFL game approaches 1.00 per game. More broadly, the Centers for Disease Control and Prevention4 estimate that the incidence of sport-related concussion is 1.6 to 3.8 million cases per year, and the risk of recurrent concussion increases with each successive concussion to approach a four-fold risk of recurrent concussion once an athlete has sustained three or more concussions in a 5-year period.5 As such, it is essential to identify the cognitive and neural consequences of these injuries, as well as the subconcussive insults sustained in an athlete's career.
The current study focused on the long-term effects of sustaining multiple sport-related concussions. The literature is inconsistent regarding these effects6; although a 2005 meta-analysis suggested that athletes typically demonstrate a full neuropsychological recovery in 7–10 days after concussion,7 two recent studies demonstrated significant episodic memory impairments in middle-aged and older adults with a history of sport-related concussion.8,9 In a recent review, Broglio and colleagues6 suggested that such impairments are indicative of accelerated age-related cognitive decline from concussive and subconcussive head trauma. This hypothesis is consistent with a previous finding from our group showing a relationship between former NFL players' number of reported concussions and the diagnosis of mild cognitive impairment (MCI) later in life. Former players with multiple concussions were also more likely to report significant memory problems in their daily lives.10 Importantly, the association between number of concussions and MCI suggests that former athletes who have sustained multiple concussions may exhibit patterns of impairment that resemble those seen in MCI, but are less severe.
Although the long-term memory problems associated with multiple sport-related concussions have not been carefully evaluated, the memory changes that accompany MCI have been systematically investigated in recent years.11 Persons with MCI perform poorly on tests of episodic memory, defined as the encoding and conscious retrieval of contextually specific information.12 Performance on episodic memory tasks relies both on relational memory, the ability to integrate unrelated pieces of information, as well as item memory, which provides the basis for knowing that a stimulus has occurred.13 Over the last two decades, a number of cognitive studies in young adults have provided empirical evidence for the distinction between item and relational memory.14–17 Of relevance here, several recent reports suggest that persons with a diagnosis of MCI show a disproportionate decrease in relational memory,18,19 and that the relational memory deficits in this group can be predictive of future cognitive impairment.20,21
The current study was the first to examine whether dissociations between item and relational memory might exist in persons with multiple sport-related concussions. Because of the established link between multiple concussions and MCI diagnosis, it is possible that increased concussion history will be associated with disproportionate deficits in associative memory. Our analysis focused on the effects of sport-related concussion on neural recruitment during these tasks. Item and relational memory are thought to rely on distinct neuroanatomical regions within the medial temporal lobe, prefrontal cortex (PFC), and parietal lobe.22–26 The current study examined the effect of multiple sport-related concussions on recruitment of these regions.
Functional neuroimaging methodologies may be ideally suited to identify the underlying functional abnormalities resulting from repetitive head trauma.27,28 Electrophysiological measures (i.e., electroencephalography [EEG] and event-related potential [ERP]) have revealed functional effects of concussion as much as 3 years post-injury,29 even in the absence of behavioral effects.30 In addition, a number of recent studies have used functional magnetic resonance imaging (fMRI) to examine functional abnormalities in recently concussed athletes.28 Although these studies highlight short-term neural changes associated with sport-related head trauma, whether or not these changes persist in episodic memory (i.e., memory for day to day events) decades after a sport-related concussion is unclear. The current study addressed this issue.
The purpose of this study was to use event-related fMRI to examine long-term neural changes associated with multiple sport-related concussions. Participants engaged in item and relational memory tasks used in previous studies with healthy young and older adults31–33 and persons with a diagnosis of MCI.34 We hypothesized that increased concussion history would be associated with alterations in the memory retrieval network, including brain regions such as dorsolateral and ventrolateral prefrontal lobes, superior and inferior parietal lobes, and bilateral medial temporal lobes.
Methods
Participants
Participants in this study were 27 former professional NFL players who played a minimum of two seasons of professional football (M age=63.4 years; standard deviation [SD]=5.9; all male) and 14 healthy age and education-matched older adults with no history of concussion or memory impairment (M=62.2 years; SD=6.3; all male; Table 1). An additional five former football players (two from the low concussion group and three from the high concussion group) underwent functional MRI, but their data were excluded because of poor performance or difficulty following task instructions. Participants were all right-handed native English speakers without a history of psychiatric illness or neurological disorder. Before participating in the study, all persons gave written informed consent in accordance with the requirements of the Institutional Review Board at the University of North Carolina (UNC) at Chapel Hill.
Table 1.
Demographic Information and Neuropsychological Data for All Participants
| Controls (n=14) | Low concussion (n=15) | High concussion (n=12) | |
|---|---|---|---|
| Age | 62.2 (6.3) | 64.1 (6.8) | 62.6 (5.0) |
| Education | 16.4 (1.1) | 16.4 (1.4) | 16.2 (1.3) |
| Number of concussions | 1.07 (.96) | 6.5 (5.1) | |
| Survey composition score | 1.2 (.5) | 1.2 (.6) | |
| Telephone Interview for Cognitive Status (TICS) | 34.7 (4.8) | 35.2 (3.9) | |
| Mini-Mental State Exam (MMSE) | 27.1 (2.4) | 27.6 (1.2) | |
| Wechsler Adult Intelligence Scale (WAIS) | 114.0 (12.9) | 115.6 (19.5) | |
| Controlled Oral Word Association (COWA) | 43.9 (8.7) | 40.1 (10.4) | |
| Trail making Part B | 74 (15.9) | 87 (34.1) | |
| Boston Naming Test (BNT) | 51.7 (4.6) | 52.5 (6.1) | |
| Wechsler Test of Adult Reading (WTAR)-Standardized | 102.8 (10.3) | 94.8 (13.3) | |
| Geriatric Depression Scale (GDS) | 5.5 (4.6) | 8.8 (7.2) | |
Low concussion history: 0, 1, or 2 concussions; high concussion history: 3 or more concussions.
Values represent means for the group; standard deviations are in parentheses.
Former football players in the current study were divided into two groups based on self-reported concussion history. Previous research suggests that likelihood of future cognitive impairments increases for persons with three or more reported concussions.10 As such, the “low concussion” group included those participants with zero, one, or two reported concussions sustained during their professional playing years (n=15) and the “high concussion” group included those persons with three or more (n=12). The two groups of former football players were balanced in terms of primary playing position (linemen and skill positions). In addition, the two groups were matched with the third group, as well as with one another, in terms of age and education (Table 1). Former athletes with zero reported concussions were included in the low concussion group, because the tendency to underreport concussions makes it highly likely that these persons have sustained at least one concussion during their careers.35
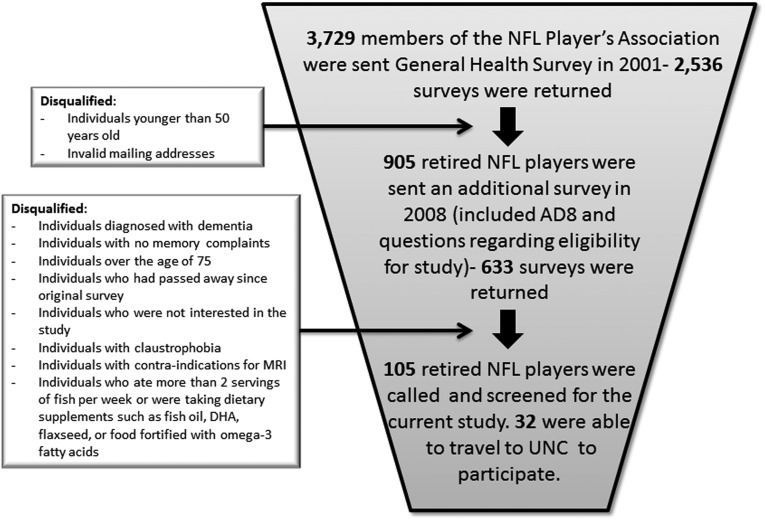
Recruitment of former professional football players (Fig. 1)
FIG. 1.
Visual depiction of the process of selecting former National Football League (NFL) players for the current study. UNC, University of North Carolina.
Potential members of the former football player sample were recruited from the Center for the Study of Retired Athletes at the UNC at Chapel Hill. In 2001, a General Health Survey was sent to all living members of the retired players' section of the NFL Players Association (n=3729), and 2536 (68%) of the surveys were completed and returned. Each person was asked a variety of questions about musculoskeletal, cardiovascular, and neurological conditions that the retired player may have experienced during or after his football career.
In 2008, an additional questionnaire focused on memory and issues related to MCI was sent to a subset of 905 retirees. The subset comprised all respondents from the General Health Survey who were 50 years and older in 2008 and had valid mailing addresses. The survey also included the AD-8 Dementia Screening Interview. In addition, duplicate forms were provided for a close family member to provide their opinions of the former player's symptoms.
A criterion score was used to identify subjects experiencing some level of memory impairment, especially those experiencing increasing memory problems over the past year. An algorithm was created that identified higher (worse) scores for selected questions on the Likert-style questionnaire for symptoms that were experienced “sometimes,” “quite a lot,” and “all the time.” Results from these memory impairment scores were combined with an indicated change in memory over the past year on the AD8. The composite survey scores ranged from 0–2, with 0 indicating no memory impairment and 2 indicating severe memory impairment.
The participants selected for this study had also agreed to participate in our clinical trial studying the effects of an omega-3 fatty acid supplementation as a potential treatment intervention. As such, of those who returned these surveys (n=633), we disqualified participants with no memory complaints (i.e., a score less than 0.13; n=224), or those who had a diagnosis of Alzheimer's disease or clinical dementia (n=36). In addition, persons who regularly ate more than two servings of fish per week or who were currently taking dietary supplements such as fish oil, docosahexaenoic acid, flaxseed, or food fortified with omega-3 fatty acids were disqualified from the study (n=211).
Potential participants were also disqualified because of disinterest in the study (n=18), being over 75 years of age (n=23), claustrophobia (n=4), contraindications for MRI research (e.g., metal implant or pacemaker; n=2), or being deceased (n=10). Of the 633 former professional football players who returned the survey, a total of 105 potential participants were called and screened. While multiple measures of neuropsychological function, behavior, and neuroimaging were taken over the course of a 9-month period, the focus of this article is on the baseline fMRI measures of those persons selected for participation in our clinical trial.
Recruitment of age-and-education–matched participants
Age-and-education–matched men were recruited from the UNC Cognitive Neuroscience of Memory Laboratory's volunteer database. Additional age-matched older adults were recruited via information e-mails in the UNC system. Participants were selected based on age, sex, and education, and were screened for psychiatric disorders, psychotropic medications, and contraindications for fMRI.
Neuropsychological assessment
Former football players were given neuropsychological measures to determine their level of cognitive status (see Table 1 for a summary).
Mini-Mental State Exam (MMSE)
This is a commonly used measure of orientation and gross cognitive functioning used by physicians and health care providers to screen for cognitive decline. The MMSE is a 30-question examination that is frequently used as a descriptor of gross cognitive function in studies of MCI and is useful in comparing samples of patients across studies. The presented score is out of 30 possible points, with high scores indicating higher cognitive performance.36
Wechsler Adult Intelligence Scale-3 (WAIS-3.)
This is the most widely used measure of general intellectual functioning. The Kaufman four-subtest short form is composed of Arithmetic, Similarities, Picture Completion, and Digit Symbol-Coding and is validated and recommended for dementia evaluations.37
Controlled Oral Word Association Test (COWA)
This is a well-known measure of phonemically controlled verbal fluency, sensitive to cognitive slowing and impairments of executive functioning, and is routinely used in dementia assessment and MCI studies. The participant is given three letters, one at a time, and asked to generate as many words that begin with that letter in 1 minute. The score is the total number of words they provide for all three letters.38
Trail making Test Part B (Trails B)
This is a complex measure of executive processing related to mental flexibility and working memory. The participant is presented with a page with numbers and letters. They are asked to begin at number one and draw a line from one to A, A to two, two to B and so on (alternating number, letter, number, letter). They are instructed to draw the lines connecting numbers and letters as quickly as possible. The presented score represents the time (in sec) needed for the participant to complete the task.39
Boston Naming Test (BNT)
This is a naming measure used to detect anomia or word-finding difficulties. These symptoms are common hallmarks of cognitive decline in elderly populations with mild cognitive impairment or early dementia. In this task, the participant is shown 60 pictures, ordered from easiest to most difficult, and given 20 seconds to name the picture. The participant receives a score (out of 60) representing the number of images correctly identified.40
Wechsler Test of Adult Reading (WTAR)
This is a reliable, standardized measure of word pronunciation that is also a strong predictor of pre-morbid general intellectual function. The examinee is asked to pronounce 50 words aloud. Their raw score is standardized by age (WTAR).41
Geriatric Depression Scale (GDS)
This is a brief, well-validated measure of depression in elderly populations. This measure is included to screen and help control for the possible effects of mild depression on other dependent variables. The GDS consists of 30 yes/no questions, with instructions for participants to respond “yes” or “no” to each question based on how they felt over the previous week. Lower scores represent lower levels of depression in the participant.42
Design and procedure
The memory paradigm involved encoding and retrieval of word pairs to examine item and relational memory.31–34 Stimuli included 288 one-to-three-syllable unrelated nouns (M Freq=56.3; SD=63.5). Participants engaged in extensive practice with the behavioral paradigm before placement in the scanner. During the practice session, participants were verbally provided with instructions for the entire task.
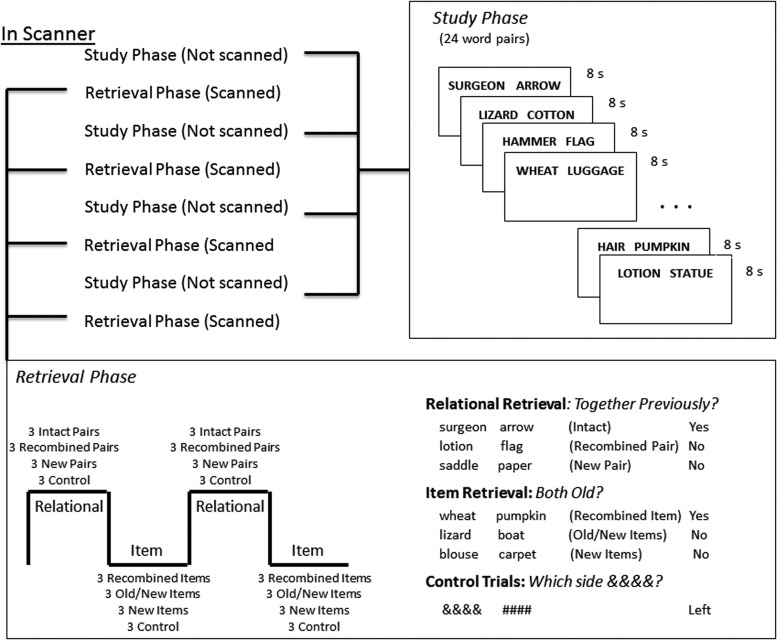
The experimental task consisted of four study lists and four test lists. Although only the test portion of the task was scanned, the entire task took place in the MRI scanner because of the alternating study-test-study-test pattern (Fig. 2). Each study list consisted of 24 unrelated word pairs (e.g., SURGEON ARROW) for a total of 96 studied word pairs across the entire experiment. Participants viewed word pairs for 8 sec each and were asked to covertly create a sentence that incorporated both words, ensuring that the word on the left side of the screen was used in the sentence before the word on the right side of the screen (e.g., “The surgeon removed the arrow”). Participants indicated via button press that they had created and encoded a sentence on each trial. This same encoding task was used for both memory tasks (i.e., participants encoded pairs tested in the item task and pairs tested in the relational task in the same manner), and the words used for the item and relational tasks were counterbalanced across subjects.
FIG. 2.
Each retrieval phase consisted of an event-related task design with alternating blocked task periods of relational retrieval (“together previously?”) and item retrieval (“both old?”). There were four study/retrieval phases. Imaging data were acquired during the retrieval phase only.
Immediately after each study task, participants engaged in a scanned retrieval task. Each retrieval session included an event-related design constructed of test-type blocks (4 blocks for each of the four sessions for 16 retrieval blocks total) that alternated between item and relational tasks (Fig. 2). During each of the 16 retrieval blocks, participants viewed nine word pairs and three control trials.
In each item retrieval block, participants saw three pairs of words previously seen, but not together (Recombined Items), three pairs consisting of one old word and one new word (Old/New Items), and three pairs consisting of two new words (New Items; for 6 pairs of each type in each retrieval session and 24 pairs of each type total). During the presentation of each pair, participants were asked to indicate whether both words were previously seen at any point during the previous study session (but not necessarily together; “Both Old?” 1=yes; 2=no). The pair appeared on the screen until the participant made a response. The pair was immediately followed by a fixation cross so that the total amount of time spent on a trial (i.e., viewing the pair plus viewing the fixation cross) equaled 6 sec.
In each relational retrieval block, participants were presented with three pairs of words previously seen together (Intact Pair), three pairs of words previously seen, but not together (Recombined Pair), and three pairs of new words (New Pair; for 6 pairs of each type in each retrieval session and 24 pairs of each type total). Each pair was presented for a total of 6 sec, during which participants were asked to indicate via button press whether the two words were previously seen together (“Together Previously?” 1=yes; 2=no).
Each retrieval block also included 3 control trials (for 48 control trials total). During the control task, participants viewed ampersands and number signs and were instructed to indicate via button press which side of the screen the ampersands appeared. Control trials were used to introduce jitter (3 to 12 sec) during each scanner run and were included to control for neural activity associated with visual activity, reading instructions, and executing a motor response. All trials were randomized within each task block.
fMRI data acquisition
MRIs were acquired using a Siemens Allegra 3-T scanner. Participants' heads were held in place using cushions and a headrest. An initial localizing scan was followed by a high resolution T1-weighted structural scan for anatomical visualization (160 1-mm slices, TR=1750 msec, TE=4.38 msec). Next, functional scans were collected during memory retrieval. Whole brain, gradient-echo, echo planar images (50 interleaved 3-mm slices, TR=3 sec, TE=30 msec, Flip angle=90 degrees, 3×3×3 mm) were acquired at an angle parallel to the long axis of the hippocampus, identified during the T1 scan.
fMRI data analysis
The purpose of this study was to use event-related fMRI to examine long-term neural changes associated with multiple sport-related concussions. To this end, whole-brain images were acquired every 3 sec during item and relational memory tasks, capturing the average hemodynamic response for each trial individually.43 The use of event-based fMRI enabled us to examine brain activity during item memory and relational memory trials under conditions of successful retrieval (i.e., correct responses only).
Images were preprocessed and analyzed using Statistical Parametic Mapping (SPM)8 (Wellcome Department of Cognitive Neurology, London, UK) software implemented as a suite of commands in MATLAB (MathWorks, Natick, MA). Images were co-registered with each person's anatomical scan, slice-time corrected, realigned, normalized, and smoothed using a Gaussian 8-mm kernel. Only trials in which participants responded correctly were modeled and analyzed. In the current task, a participant could respond correctly by indicating “old” to re-combinations of old items in the item task (recombined item hit), “new” to a combination of an old and new item in the item task (old/new item correct rejection), “new” to new items in the item task (new item correct rejection), “old” to intact pairs in the relational task (intact pair hit), “new” to re-combinations of old items in the relational task (recombined pair correct rejection), or “new” to new pairs in the relational task (new pair correct rejection).
Recombined item hits provide a measure of an person's ability to correctly identify two words that had been studied together, which may rely exclusively on item memory retrieval. New item correct rejections provide a measure of a person's ability to recognize that two words were never studied before, again relying on item memory retrieval. Importantly, in both cases, participants are required to make decisions using both words, equating memory load between this item memory task and the relational memory task. In the old/new item correct rejections, participants are presented one word that had previously been studied and one that had not been studied. Participants may correctly respond “new” if they remember one word as new, or if they mistakenly remember both words as new. Thus, it is not possible to pinpoint how participants make their responses in this condition, and as such, the condition was not analyzed and will not be discussed further.
Intact pair hits are a measure of a person's ability to remember that two words had been presented together previously, a decision that requires relational memory processes. Similarly, recombined pair correct rejections provide a measure of a person's ability to use relational memory processes to remember that words had not been presented together, even though both words had been presented at some point during the study phase. For new pair correct rejections, persons can use either item or relational memory processes to remember that the words had not been presented during the study. This condition is included during the task to better match the item and relational tasks, but was not included in the analysis because of this mixture of item and relational processes.
In the current analysis, we were interested in the behavioral and neural responses to relational and item memory conditions. Item memory is assessed as the difference in responses between recombined item and new items, whereas relational memory is the difference in responses between intact pairs and recombined pairs. Thus, in the current study, we examined responses to recombined item hits (i.e., correct responses to recombined items), new item correct rejections (i.e., correct responses to new items), intact pair hits (i.e., correct response to intact pairs), and recombined pair correct rejections (i.e., correct response to recombined pairs). As has been previously noted, old/new item correct rejections and new pair correct rejections were included in the study for design reasons, but are not meaningful conditions to examine at the neural level. As such, although all six response conditions were included in the model at the individual subject level, only the four conditions of interest were analyzed and will be discussed.
Each of the four conditions of interest was compared to the control condition using t tests (e.g., intact hits>control trials). The results of these four t tests were entered into group t tests at the group level: recombined item hits, new item correct rejections, intact pair hits, and recombined pair correct rejections. Activity during each of these four contrasts was examined for all three groups (i.e., the age-matched older adults, the low concussion group, and the high concussion group). In addition, two-group t tests were used to examine activity in a given condition that was greater for one group than another (e.g., low concussion intact hits>high concussion intact hits).
Conjunction analyses can be used to identify regions showing significant activation in multiple groups during a particular memory condition (i.e., low concussion intact hits and high concussion intact hits). In the current study, these analyses were performed using the masking function of SPM8 to identify neural overlap in mnemonic processing across concussion groups. To create a conjunction, once contrast of interest (e.g., low concussion intact hits) was used to create an inclusive mask at p<0.025 (i.e., a mask including all voxels reaching significance at p<0.025). This mask was applied to the second contrast of interest (e.g., high concussion intact hits) at p<0.025 with an extent of at least 10 contiguously activated voxels. Using the Fisher method of estimation,44,45 the probability that a voxel would be activated in both contrasts (i.e., the conjoint probability) is p<0.005.
Multiple regression analyses were conducted in SPM8 at the group level to identify regions in which the raw number of reported concussions in former football players significantly predicted neural activity in our four conditions of interest. All former players were included in these contrasts, with number of concussions included as a regressor of interest. In a final analysis, accuracy was included as a covariate of interest in the two-group t test comparing persons in the low and high concussion groups. This analysis identified regions preferentially engaged by persons in each group who performed well on the memory task. All activations were considered significant at p<0.005, k≥10, because this threshold has recently been shown to be an acceptable balance between Type I and Type II error in neuroimaging studies.46 For all analyses, the peak Montreal Neurological Institute coordinates of active regions (reported here) were converted to Talairach space and localized in reference to the Talairach and Tournoux atlas.47
Results
Behavioral results
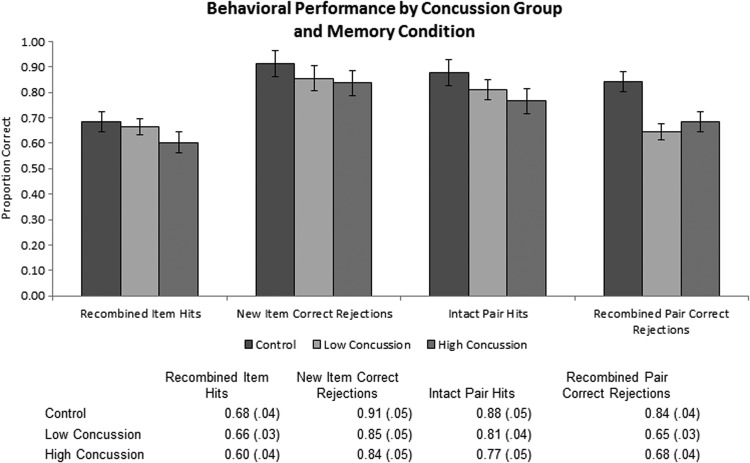
Recombined item hits, new item correct rejections, intact pair hits, and recombined pair correct rejections were compared across the three groups. On average, participants had 14.81 recombined item hits (out of a possible 24; SD=4.84), 18.89 new item correct rejections (SD=3.72), 18.63 intact pair hits (SD=3.70), and 14.26 recombined correct rejections (SD=5.07). The two concussion groups did not differ in any behavioral measures of item or relational memory (p>0.3 for all contrasts). In addition, the two concussion groups did not differ from the third group in either their proportions of recombined item hits or new item correct rejections (i.e., item memory measures; p>0.1 for all contrasts). Both groups, however, demonstrated relational memory impairments. The high concussion group was significantly worse at recognizing intact pairs as “old” (intact pair hits) relative to the age-matched group (t(24)=2.129, p<0.05), and both the low and high concussion groups had significantly fewer recombined pair correct rejections than the age-matched group (t(27)=2.871, p<0.05 and t(24)=2.916, p<0.05 for low and high concussion groups, respectively (Fig. 3). In addition, the item or relational memory measures were not correlated with the raw number of reported concussions in former football players (p>0.2 for all contrasts).
FIG. 3.
Behavioral performance presented by concussion group (i.e., control, low, and high) and memory condition.
Imaging results
The current analysis examined activity associated with recombined item hits, new item correct rejections, intact pair hits, and recombined pair correct rejections compared with control trials. Activity during each of these four contrasts was examined for all three groups (i.e., the age-matched group, the low concussion group, and the high concussion group). In addition, two-group t tests were used to examine activity in a given condition that was greater for one group than another (e.g., low concussion intact hits>high concussion intact hits). Finally, a conjunction analysis was performed to identify regions that were commonly activated by two groups during a particular memory condition (i.e., low concussion intact hits and high concussion intact hits).
Neural activity during recombined item hits
Neural activity associated with item hits, compared with activity associated with control trials, was examined in the age-matched group, low concussion group, and high concussion group. During item hits, the age-matched group recruited bilateral parietal and frontal regions. The low concussion group recruited bilateral parietal and frontal regions, as well as a more extensive network that included bilateral lateral and medial temporal lobes (left amygdala extending into the hippocampus and right parahippocampal gyrus). The high concussion group also recruited bilateral lateral and medial temporal lobes (specifically bilateral hippocampus), as well as parietal and frontal regions (Table 2).
Table 2.
Regions of Significant Activation during Recombined Item Hits
| |
|
|
MNI coordinates |
|
|
||
|---|---|---|---|---|---|---|---|
| Region of interest | Hemisphere | BA | x | y | z | t value | p value |
| Control group | |||||||
| Inferior parietal lobe | R | 40 | 56 | −54 | 44 | 4.26 | 0.000 |
| Middle frontal gyrus | R | 9 | 46 | 26 | 38 | 4.09 | 0.001 |
| L | 8 | −48 | 18 | 46 | 3.70 | 0.001 | |
| Supramarginal gyrus | L | 40 | −62 | −52 | 34 | 3.68 | 0.001 |
| Insula | L | 13 | −38 | 4 | 4 | 3.26 | 0.003 |
| Low concussion group | |||||||
| Inferior parietal lobe | L | 40 | −50 | −34 | 34 | 6.12 | 0.000 |
| −48 | −50 | 38 | 5.65 | 0.000 | |||
| R | 42 | −56 | 34 | 4.05 | 0.001 | ||
| Postcentral gyrus | L | 2 | −46 | −22 | 28 | 5.11 | 0.000 |
| 3 | −22 | −36 | 54 | 3.87 | 0.001 | ||
| R | 2 | 70 | −26 | 30 | 4.12 | 0.001 | |
| Amygdala/hippocampus | L | na | −32 | −8 | −14 | 6.08 | 0.000 |
| Inferior frontal gyrus | R | 47 | 58 | 32 | 0 | 5.99 | 0.000 |
| 52 | 36 | −12 | 4.65 | 0.000 | |||
| 45 | 60 | 20 | 18 | 3.29 | 0.003 | ||
| Precentral gyrus | R | 44 | 64 | 10 | 10 | 5.38 | 0.000 |
| 4 | 26 | −28 | 64 | 3.70 | 0.001 | ||
| L | 4 | −20 | −30 | 60 | 4.10 | 0.001 | |
| 9 | 46 | 18 | 44 | 3.72 | 0.001 | ||
| Superior temporal gyrus | L | 39 | −48 | −58 | 30 | 5.35 | 0.000 |
| 38 | −50 | 16 | −22 | 4.83 | 0.000 | ||
| 42 | −62 | −32 | 8 | 4.29 | 0.000 | ||
| R | 22 | 56 | −58 | 16 | 4.47 | 0.000 | |
| 38 | 36 | 12 | −40 | 3.75 | 0.001 | ||
| Medial frontal gyrus | R | 8 | 12 | 38 | 46 | 4.91 | 0.000 |
| 9 | 8 | 48 | 28 | 3.49 | 0.002 | ||
| L | 10 | −8 | 58 | 6 | 3.74 | 0.001 | |
| Superior frontal gyrus | R | 9 | 18 | 48 | 38 | 4.54 | 0.000 |
| R | 6 | 18 | 24 | 60 | 4.81 | 0.000 | |
| 10 | 22 | 48 | 18 | 4.20 | 0.001 | ||
| Middle frontal gyrus | R | 9 | 38 | 20 | 38 | 4.54 | 0.000 |
| Insula | R | 13 | 42 | −30 | 18 | 4.27 | 0.000 |
| 42 | −2 | −10 | 3.20 | 0.003 | |||
| L | −40 | −34 | 18 | 4.04 | 0.001 | ||
| −38 | −46 | 16 | 3.61 | 0.002 | |||
| Anterior cingulate | R | 32 | 22 | 40 | 10 | 4.08 | 0.001 |
| L | −8 | 38 | 24 | 3.64 | 0.001 | ||
| 24 | −4 | 36 | 4 | 3.29 | 0.003 | ||
| Middle temporal gyrus | L | 21 | −52 | 4 | −30 | 4.14 | 0.001 |
| −60 | −60 | 0 | 4.03 | 0.001 | |||
| −62 | −44 | −2 | 3.95 | 0.001 | |||
| Inferior temporal gyrus | L | 20 | −50 | −4 | −36 | 3.56 | 0.002 |
| −38 | 0 | −46 | 3.82 | 0.001 | |||
| Parahippocampal gyrus | R | 28 | 18 | −2 | −14 | 4.07 | 0.001 |
| 35 | 28 | −20 | −22 | 3.68 | 0.001 | ||
| Caudate | L | na | −22 | 14 | 22 | 3.82 | 0.001 |
| Precuneus | R | 7 | 26 | −62 | 28 | 3.79 | 0.001 |
| Fusiform gyrus | R | 20 | 50 | −14 | −30 | 3.53 | 0.002 |
| High concussion group | |||||||
| Amygdala/hippocampus | L | na | −36 | −6 | −20 | 7.71 | 0.000 |
| Inferior parietal lobe | R | 40 | 46 | −32 | 32 | 5.65 | 0.000 |
| Insula | L | 13 | −38 | −8 | 20 | 5.24 | 0.000 |
| Precentral gyrus | L | 6 | −40 | −18 | 36 | 5.03 | 0.000 |
| R | 54 | −6 | 22 | 4.16 | 0.001 | ||
| Hippocampus | R | na | 30 | −42 | −6 | 4.00 | 0.001 |
| Fusiform gyrus | R | 37 | 40 | −40 | −10 | 4.36 | 0.000 |
| Middle temporal gyrus | R | 21 | 48 | −38 | −8 | 4.28 | 0.000 |
| Inferior frontal gyrus | R | 45 | 56 | 28 | 4 | 4.01 | 0.001 |
| Superior temporal gyrus | L | 38 | −38 | 10 | −34 | 3.87 | 0.001 |
| Anterior cingulate | R | 32 | 6 | 40 | 24 | 3.76 | 0.001 |
| Postcentral gyrus | L | 40 | −56 | −28 | 20 | 3.58 | 0.002 |
| Low concussion group>high concussion group | |||||||
| Thalamus | R | na | 22 | −30 | 6 | 3.81 | 0.000 |
| Middle frontal gyrus | L | 10 | −44 | 54 | −2 | 3.42 | 0.001 |
| Effect of accuracy: low concussion group>high concussion group | |||||||
| Anterior cingulate | L | 32 | −22 | 34 | 10 | 4.04 | 0.000 |
| Insula | R | 13 | 28 | −44 | 20 | 3.70 | 0.001 |
| Middle frontal gyrus | L | 11 | −38 | 56 | −8 | 3.26 | 0.002 |
| High concussion group>low concussion group | |||||||
| Anterior cingulate | L | 32 | −12 | 18 | 42 | 3.65 | 0.001 |
| 24 | −16 | −2 | 42 | 3.47 | 0.001 | ||
| R | 24 | 20 | −16 | 36 | 3.24 | 0.002 | |
| Middle temporal gyrus | R | 21 | 46 | −36 | −8 | 3.58 | 0.001 |
| Precentral gyrus | R | 9 | 38 | 8 | 40 | 3.23 | 0.002 |
| Effect of accuracy: high concussion group>low concussion group | |||||||
| Thalamus | R | na | 10 | −34 | 4 | 5.36 | 0.000 |
| na | 20 | −16 | 12 | 4.14 | 0.000 | ||
| Caudate | L | na | −34 | −24 | −6 | 5.28 | 0.000 |
| Hippocampus | L | na | −34 | −12 | −14 | 3.52 | 0.001 |
| na | −24 | −38 | −4 | 4.44 | 0.000 | ||
| Parahippocampal gyrus | L | 36 | −42 | −20 | −16 | 3.42 | 0.001 |
| 30 | −6 | −40 | 8 | 3.56 | 0.001 | ||
| Anterior cingulate | L | 32 | −6 | 22 | 38 | 5.15 | 0.000 |
| Medial frontal gyrus | L | 32 | −14 | 10 | 50 | 5.00 | 0.000 |
| 6 | −16 | 30 | 40 | 3.39 | 0.001 | ||
| Superior temporal gyrus | L | 38 | −30 | 6 | −34 | 4.54 | 0.000 |
| R | 41 | 46 | −32 | 16 | 4.43 | 0.000 | |
| 22 | 48 | −8 | −8 | 3.93 | 0.000 | ||
| 38 | 46 | 6 | −12 | 3.11 | 0.002 | ||
| Precentral gyrus | R | 6 | 68 | −4 | 20 | 4.27 | 0.000 |
| Superior frontal gyrus | R | 10 | 10 | 62 | 32 | 4.26 | 0.000 |
| 6 | 22 | 10 | 60 | 3.44 | 0.001 | ||
| Precuneus | R | 39 | 40 | −66 | 38 | 4.23 | 0.000 |
| 31 | 16 | −48 | 44 | 3.57 | 0.001 | ||
| Middle temporal gyrus | R | 21 | 46 | 14 | −38 | 4.14 | 0.000 |
| 21 | 58 | 6 | −16 | 3.93 | 0.000 | ||
| Inferior frontal gyrus | L | 9 | −42 | 4 | 30 | 4.00 | 0.000 |
| L | 45 | −58 | 10 | 26 | 3.42 | 0.001 | |
| Inferior temporal gyrus | R | 21 | 60 | −10 | −16 | 3.17 | 0.002 |
| Supramarginal gyrus | L | 40 | −38 | −48 | 38 | 3.86 | 0.000 |
| Middle frontal gyrus | R | 6 | 24 | −6 | 44 | 3.51 | 0.001 |
| 6 | 28 | 12 | 54 | 3.36 | 0.001 | ||
| 6 | 30 | 20 | 60 | 3.26 | 0.002 | ||
| L | 9 | −42 | 20 | 34 | 3.31 | 0.002 | |
| Insula | L | 13 | −40 | −10 | 10 | 3.31 | 0.002 |
| Regions associated with a lesser number of reported concussions | |||||||
| Thalamus | R | na | 22 | −30 | 4 | 3.90 | 0.000 |
| Inferior frontal gyrus | R | 9 | 60 | 6 | 34 | 3.80 | 0.000 |
| Middle temporal gyrus | L | 22 | −58 | −40 | 2 | 3.80 | 0.000 |
| R | 22 | 58 | 34 | 2 | 3.40 | 0.001 | |
| Putamen | L | na | −12 | 10 | −6 | 3.10 | 0.002 |
| Regions associated with a greater number of reported concussions | |||||||
| Anterior cingulate | R | 24 | 12 | 8 | 30 | 3.10 | 0.002 |
| Low and high concussion groups | |||||||
| Inferior parietal lobe | L | 40 | −50 | −34 | 34 | 6.12 | 0.000 |
| Amygdala/hippocampus | L | na | −32 | −8 | −14 | 6.08 | 0.000 |
| Inferior frontal gyrus | R | 45 | 58 | 30 | 2 | 5.27 | 0.000 |
| Postcentral gyrus | L | 3 | −50 | −24 | 38 | 4.64 | 0.000 |
| 40 | −58 | −28 | 20 | 3.36 | 0.003 | ||
| Middle temporal gyrus | L | 21 | −52 | 4 | −30 | 4.14 | 0.001 |
| Middle frontal gyrus | R | 9 | 34 | 22 | 40 | 3.57 | 0.002 |
| Precentral gyrus | R | 9 | 44 | 24 | 40 | 3.24 | 0.003 |
| Medial frontal gyrus | R | 9 | 8 | 50 | 26 | 3.41 | 0.002 |
| Anterior cingulate | L | 32 | −4 | 38 | 24 | 3.36 | 0.003 |
| Middle temporal gyrus | L | 21 | −38 | 6 | −36 | 3.33 | 0.003 |
Regions significant at threshold of p<0.005 (uncorrected at the voxel level); k≥10.
BA, approximate Brodmann Area. Control, 0 concussions; low concussion, 0, 1, or 2 concussions; high concussion, 3 or more concussions.
MNI, Montreal Neurological Institute.
To test the hypothesis that concussion history was associated with alterations in memory networks, a comparison of activity was performed between groups. This analysis was conducted for the low relative to high concussion groups, eliminating the age-matched group. We did so for three reasons. First, the members of our age-matched group in the current study had highly heterogeneous activation patterns and did not recruit many episodic memory regions, even when the threshold was lowered to p<0.05. In addition, these age-and-education–matched participants did not activate any additional regions beyond those activated by the low and high concussion groups. Such results suggest that this group may be atypical in their neural recruitment. Second, the interpretation of neural differences is more straightforward when behavioral performance is equivalent, because this eliminates the possibility that observed differences are from the disparity in success rate. The behavioral memory measures and neuropsychological scores between the two concussion groups were equivalent, isolating concussion history as the only difference contributing to distinct neural recruitment. Although the age-matched older adults were significantly better than both concussion groups on some behavioral measures, the two concussion groups were equivalent in all respects. Finally, we were primarily interested in examining how sustaining multiple sport-related concussions might contribute to underlying differences in the neural architecture supporting episodic memory; the low versus high concussion group contrasts address that question directly.
The low versus high concussion group analysis examined regions commonly recruited by both groups, as well as regions preferentially recruited by each group compared with the other. The conjunction analysis revealed that the low and high concussion groups recruited many of the same regions in this analysis, including left lateral and medial temporal regions, and bilateral frontal and parietal lobes. With such a high degree of overlap, only a few regions were preferentially engaged by the low and high concussion groups. Regression analyses were also used to examine the effect of concussion history on activity. All regression analyses were also conducted with response time and accuracy as covariates, with highly similar results.
Relative to the high concussion group, the low concussion group showed greater activation in the right thalamus and the left middle frontal gyrus (Table 2 and Fig. 4). Similarly, the regression analysis identified the right thalamus and inferior frontal gyrus as regions in which activity was negatively associated with concussion history (i.e., increased concussion history was associated with decreased activity). In addition, these persons preferentially recruit bilateral lateral temporal lobes. Importantly, activity in the left middle frontal gyrus was associated with increased accuracy in this group, demonstrating that increased activity in this region in the low concussion group contributes to memory accuracy (Table 2 and Fig. 5).
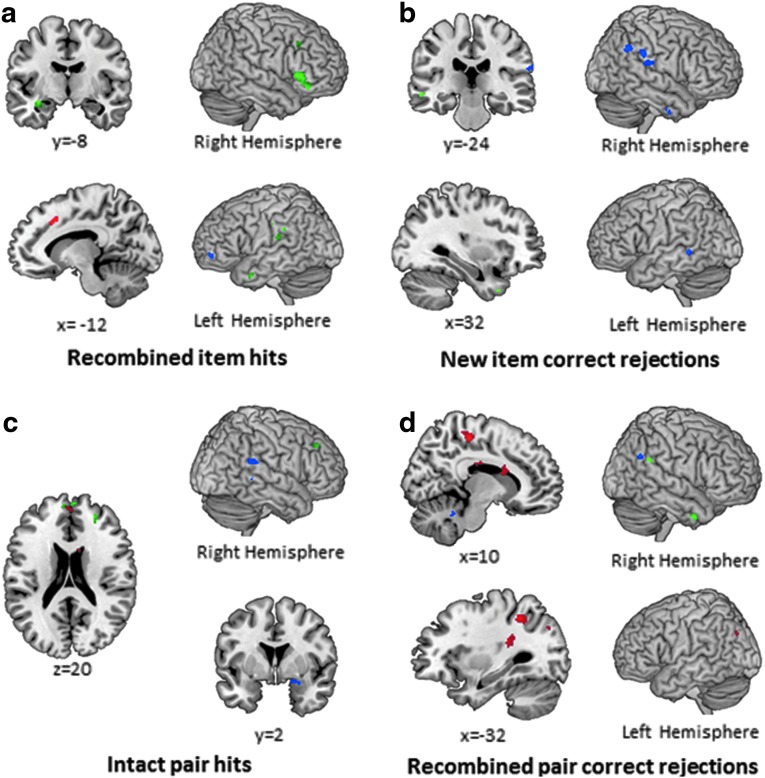
FIG. 4.
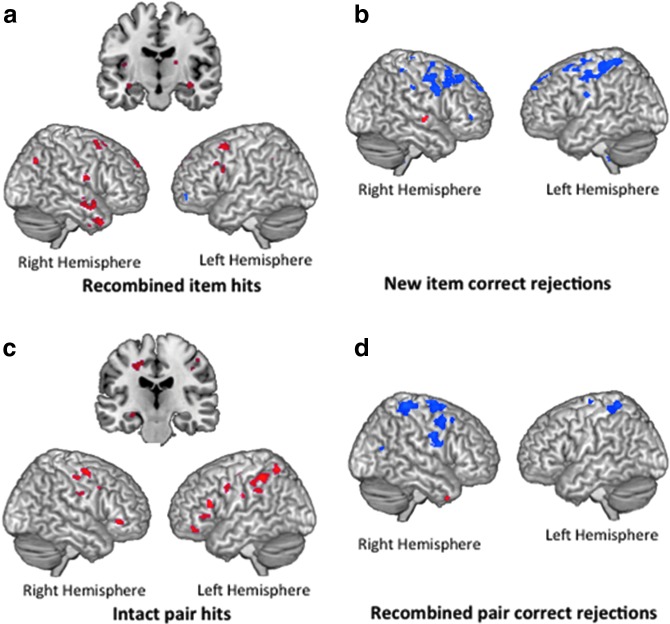
Regions of activation (at p<0.005, k≥10) preferentially recruited by the low concussion group relative to the high concussion group (blue), preferentially recruited by the high concussion group relative to the low concussion group (red), and commonly recruited by both low and high concussion groups (green). In recombined item hits (a), both groups engaged medial temporal and lateral frontal regions, but differentially engaged medial frontal regions. In new item correct rejections (b), the low concussion group preferentially recruited a number of parietal regions including BA40. In intact pair hits (c), the low concussion group preferentially engaged important relational memory regions, including right parahippocampal gyrus and inferior parietal gyrus, while the high concussion group recruited frontal regions. Finally, in recombined pair correct rejections (d), the high concussion group preferentially recruited a number of parietal, frontal, and temporal regions. Color image is available online at www.liebertpub.com/neu
FIG. 5.
Regions of activation (at p<0.005, k≥10) preferentially recruited by persons with increased memory accuracy in the low concussion group relative to the high concussion group (blue) and high concussion group relative to the low concussion group (red). In recombined item hits (a) and intact pair hits (c), accuracy was associated with increased activity in frontal regions in the high concussion group to a greater extent than the low concussion group. Conversely, in new item (b) and recombined pair (d) correct rejections, accuracy was associated with increased activity in frontal and parietal regions in the low concussion group to a greater extent than the high concussion group. Accuracy was associated with temporal regions in the high concussion group relative to the high concussion group in all four memory conditions. Color image is available online at www.liebertpub.com/neu
The high concussion group showed greater activation in the bilateral anterior cingulate and left middle temporal gyrus and precentral gyrus (Table 2 and Figure 4). As in the low concussion group, regions preferentially recruited by the high concussion group, such as left middle temporal gyrus and anterior cingulate, were associated with increased accuracy (Table 2 and Figure 5). These results suggest that the two concussion groups may rely on distinct neural regions to support memory performance.
Neural activity during new item correct rejections
Neural activity associated with item correct rejections, compared with activity associated with control trials, was examined in the age-matched group, low concussion group, and high concussion group. Only the low concussion group showed significant clusters at the threshold of p<0.005. The low concussion group engaged a large bilateral network of regions, including a number of frontal, parietal, and temporal lobe regions. Of interest, this network included bilateral medial temporal lobe activity (specifically, left hippocampus and right amygdala). The low and high concussion groups commonly engaged bilateral lateral temporal lobes in this condition (Table 3 and Figure 4).
Table 3.
Regions of Significant Activation during New Item Correct Rejections
| |
|
|
MNI coordinates |
|
|
||
|---|---|---|---|---|---|---|---|
| Region of interest | Hemisphere | BA | x | y | z | t value | p value |
| Control group | |||||||
| No significant clusters | |||||||
| Low concussion group | |||||||
| Middle frontal gyrus | R | 9 | 38 | 32 | 42 | 7.62 | 0.000 |
| 6 | 22 | 18 | 62 | 3.99 | 0.001 | ||
| 47 | 48 | 40 | 0 | 3.96 | 0.001 | ||
| 46 | 46 | 48 | 10 | 3.89 | 0.001 | ||
| L | 11 | −40 | 46 | −12 | 4.91 | 0.000 | |
| 10 | −28 | 54 | 24 | 3.82 | 0.001 | ||
| 9 | −40 | 26 | 42 | 3.52 | 0.002 | ||
| Superior frontal gyrus | R | 6 | 14 | 26 | 58 | 6.64 | 0.000 |
| 10 | 26 | 56 | 20 | 5.12 | 0.000 | ||
| 14 | 62 | 24 | 3.54 | 0.002 | |||
| L | 9 | −42 | 36 | 34 | 3.80 | 0.001 | |
| Middle temporal gyrus | R | 21 | 70 | −30 | 0 | 6.58 | 0.000 |
| 48 | 4 | −40 | 3.65 | 0.001 | |||
| 58 | 4 | −26 | 3.95 | 0.001 | |||
| L | −58 | −2 | −16 | 5.61 | 0.000 | ||
| −64 | −46 | 0 | 4.36 | 0.000 | |||
| 21 | −60 | −24 | −8 | 4.23 | 0.000 | ||
| Inferior parietal lobe | L | 40 | −52 | −56 | 40 | 5.78 | 0.000 |
| −52 | −30 | 30 | 4.71 | 0.000 | |||
| R | 66 | −30 | 32 | 5.37 | 0.000 | ||
| 32 | −38 | 44 | 4.62 | 0.000 | |||
| Supramarginal gyrus | R | 40 | 58 | −48 | 36 | 5.77 | 0.000 |
| L | −52 | −50 | 32 | 4.54 | 0.000 | ||
| Postcentral gyrus | R | 2 | 62 | −32 | 46 | 4.20 | 0.001 |
| L | 2 | −48 | −24 | 50 | 3.96 | 0.001 | |
| Inferior temporal gyrus | L | 20 | −40 | 2 | −48 | 4.96 | 0.000 |
| R | 42 | −2 | −42 | 4.40 | 0.000 | ||
| Superior temporal gyrus | L | 21 | −54 | −24 | −2 | 5.58 | 0.000 |
| 13 | −34 | −30 | 6 | 3.82 | 0.001 | ||
| R | 42 | 58 | −32 | 14 | 4.25 | 0.000 | |
| 22 | 62 | −56 | 6 | 4.21 | 0.001 | ||
| 39 | 50 | −62 | 26 | 3.85 | 0.001 | ||
| 13 | 52 | −50 | 16 | 3.55 | 0.002 | ||
| Posterior cingulate | R | 23 | 8 | −20 | 34 | 5.50 | 0.000 |
| L | 31 | −12 | −40 | 24 | 3.59 | 0.002 | |
| Anterior cingulate | L | 24 | −2 | −24 | 38 | 3.60 | 0.002 |
| 32 | −12 | 38 | 24 | 4.58 | 0.000 | ||
| R | 32 | 4 | 32 | 22 | 3.38 | 0.002 | |
| Insula | L | 13 | −32 | −20 | 22 | 5.44 | 0.000 |
| 13 | −36 | 6 | 14 | 4.72 | 0.000 | ||
| 13 | −42 | −12 | 26 | 3.33 | 0.003 | ||
| R | 13 | 32 | 16 | 16 | 4.52 | 0.000 | |
| 13 | 40 | −4 | 10 | 3.95 | 0.001 | ||
| 13 | 46 | −44 | 14 | 3.47 | 0.002 | ||
| Hippocampus | L | na | −28 | −24 | −18 | 5.40 | 0.000 |
| −28 | −10 | −28 | 4.38 | 0.000 | |||
| Medial frontal gyrus | R | 10 | 24 | 48 | 16 | 3.96 | 0.001 |
| 9 | 6 | 46 | 28 | 4.60 | 0.000 | ||
| L | 9 | −24 | 42 | 18 | 4.38 | 0.000 | |
| 10 | −6 | 58 | 8 | 3.80 | 0.001 | ||
| Precentral gyrus | R | 44 | 50 | 12 | 6 | 5.01 | 0.000 |
| 4 | 24 | −26 | 56 | 4.53 | 0.000 | ||
| 6 | 30 | −14 | 60 | 3.97 | 0.001 | ||
| Precentral gyrus | L | 6 | −40 | −14 | 50 | 3.63 | 0.002 |
| Inferior frontal gyrus | R | 47 | 40 | 24 | −18 | 4.93 | 0.000 |
| 50 | 46 | −12 | 3.97 | 0.001 | |||
| Amygdala | R | na | 26 | −4 | −18 | 4.59 | 0.000 |
| Precuneus | L | 31 | −10 | −48 | 28 | 4.20 | 0.000 |
| Thalamus | L | na | −2 | −28 | 18 | 3.71 | 0.001 |
| High concussion group | |||||||
| No significant clusters | |||||||
| Low concussion group>high concussion group | |||||||
| Middle temporal gyrus | R | 21 | 42 | −4 | −40 | 4.25 | 0.000 |
| L | 22 | −62 | −44 | 2 | 3.97 | 0.000 | |
| Supramarginal gyrus | R | 40 | 60 | −48 | 38 | 4.14 | 0.000 |
| Inferior parietal lobe | R | 40 | 66 | −30 | 32 | 3.94 | 0.000 |
| Postcentral gyrus | R | 40 | 66 | −24 | 20 | 3.32 | 0.001 |
| Effect of accuracy: low concussion group>high concussion group | |||||||
| Anterior cingulate | L | 24 | −16 | −18 | 42 | 5.56 | 0.000 |
| 24 | −10 | 12 | 28 | 4.76 | 0.000 | ||
| 32 | 0 | 28 | 36 | 3.11 | 0.002 | ||
| Middle frontal gyrus | R | 6 | 22 | −12 | 44 | 3.91 | 0.000 |
| 6 | 24 | 0 | 44 | 3.14 | 0.002 | ||
| L | 6 | −40 | 8 | 58 | 3.52 | 0.001 | |
| 6 | −20 | −4 | 66 | 3.32 | 0.001 | ||
| Precentral gyrus | L | 6 | −28 | −24 | 62 | 4.05 | 0.000 |
| 6 | −50 | −8 | 26 | 3.58 | 0.001 | ||
| Postcentral gyrus | L | 3 | −36 | −36 | 64 | 4.02 | 0.000 |
| 2 | −48 | −28 | 28 | 3.21 | 0.002 | ||
| R | 3 | 58 | −24 | 38 | 3.20 | 0.002 | |
| Inferior frontal gyrus | R | 9 | 58 | 6 | 32 | 3.99 | 0.000 |
| 13 | 40 | 26 | 10 | 3.26 | 0.002 | ||
| L | 13 | −38 | 24 | 10 | 3.87 | 0.000 | |
| Superior frontal gyrus | R | 8 | 22 | 34 | 50 | 3.89 | 0.000 |
| 6 | 4 | 20 | 62 | 3.60 | 0.001 | ||
| 9 | 4 | 60 | 36 | 3.29 | 0.002 | ||
| L | 6 | −8 | −2 | 68 | 3.43 | 0.001 | |
| 9 | −6 | 56 | 34 | 3.38 | 0.001 | ||
| 8 | −6 | 40 | 50 | 3.15 | 0.002 | ||
| Posterior cingulate | R | 31 | 4 | −44 | 26 | 3.89 | 0.000 |
| Insula | L | 13 | −40 | 16 | 14 | 3.29 | 0.002 |
| Paracentral lobe | L | 5 | −6 | −40 | 54 | 3.78 | 0.000 |
| Medial frontal gyrus | R | 10 | 12 | 54 | −8 | 3.61 | 0.001 |
| 9 | 6 | 52 | 44 | 3.22 | 0.002 | ||
| 8 | 4 | 42 | 50 | 3.24 | 0.002 | ||
| Thalamus | R | na | 18 | −22 | 12 | 3.57 | 0.001 |
| Insula | R | 13 | 40 | −4 | 4 | 3.22 | 0.002 |
| 13 | 36 | 6 | 20 | 3.20 | 0.002 | ||
| High concussion group>low concussion group | |||||||
| No significant clusters | |||||||
| Effect of accuracy: high concussion group>low concussion group | |||||||
| Parahippocampal gyrus | R | 19 | 22 | −44 | −6 | 3.60 | 0.001 |
| Superior temporal gyrus | R | 22 | 56 | −8 | 0 | 3.53 | 0.001 |
| Regions associated with a lesser number of reported concussions | |||||||
| Postcentral gyrus | R | 3 | 64 | −16 | 24 | 3.93 | 0.000 |
| 40 | 62 | −26 | 20 | 2.99 | 0.003 | ||
| Middle temporal gyrus | L | 21 | −62 | −44 | 0 | 3.90 | 0.000 |
| R | 21 | 62 | 0 | −14 | 3.64 | 0.001 | |
| Inferior parietal lobe | R | 40 | 66 | −28 | 36 | 3.69 | 0.001 |
| L | 40 | −62 | −32 | 40 | 3.33 | 0.001 | |
| Supramarginal gyrus | R | 40 | 54 | −42 | 32 | 3.68 | 0.001 |
| Posterior cingulate | L | 30 | −16 | −54 | 16 | 3.51 | 0.001 |
| Superior temporal gyrus | R | 38 | 54 | 6 | −8 | 3.45 | 0.001 |
| 39 | 44 | −56 | 20 | 3.10 | 0.002 | ||
| Regions associated with a greater number of reported concussions | |||||||
| No significant clusters | |||||||
| Low and high concussion groups | |||||||
| Middle temporal gyrus | L | 21 | −58 | −24 | −8 | 3.98 | 0.001 |
| Superior temporal gyrus | R | 38 | 32 | 16 | −40 | 3.72 | 0.001 |
Regions significant at threshold of p<0.005 (uncorrected at the voxel level); k≥10.
BA, approximate Brodmann Area. Control, 0 concussions; low concussion, 0, 1, or 2 concussions; high concussion, 3 or more concussions.
MNI, Montreal Neurological Institute.
Relative to the high concussion group, the low concussion group showed greater activation in bilateral middle temporal gyrus and inferior parietal lobe (Table 3 and Figure 4). Interestingly, these regions were not preferentially associated with accuracy in the low concussion group, suggesting that increased activation of these regions is not related to memory performance (Table 3 and Figure 5). The high concussion group did not show increased activation relative to the low concussion group in any regions (Table 3 and Figure 4).
Neural activity during intact pair hits
Neural activity during correct old responses to intact pairs (relational hits) was examined for all three groups (i.e., age-matched group, low concussion group, and high concussion group) separately. Of note, this contrast for age-matched older adults revealed no significant clusters. The same contrast in the low concussion group revealed extensive bilateral activation in frontal, temporal, and parietal regions. Activity in the high concussion group was primarily left lateralized along midline structures, in addition to activity in the left lateral temporal lobe. The conjunction analysis identified regions that were commonly activated by both concussion groups. Both groups rely heavily on medial frontal regions, including the anterior cingulate.
In the comparison between groups, the low concussion group recruited more posterior regions, including temporal and parietal regions, relative to the high concussion group. Importantly, the low concussion group engaged the right parahippocampal gyrus during retrieval of intact pairs to a greater extent than the high concussion group (Table 4 and Figure 4). The only region associated with accuracy in this group, however, was the posterior cingulate (Table 4 and Figure 5). The high concussion group recruited medial prefrontal regions to a greater extent than the low concussion group (Table 4 and Figure 4), and these regions were associated with increased memory performance. In addition, activity in the left parahippocampal gyrus was associated with increased accuracy in the high concussion group (Table 4 and Figure 5).
Table 4.
Regions of Significant Activation during Intact Pair Hits
| |
|
|
MNI coordinates |
|
|
||
|---|---|---|---|---|---|---|---|
| Region of interest | Hemisphere | BA | x | y | z | t value | p value |
| Control group | |||||||
| No significant clusters | |||||||
| Low concussion group | |||||||
| Insula | L | 13 | −40 | −8 | 22 | 6.63 | 0.000 |
| Precentral gyrus | L | 44 | −44 | 4 | 12 | 5.52 | 0.000 |
| R | 4 | 38 | −20 | 40 | 4.83 | 0.000 | |
| Middle frontal gyrus | L | 8 | −36 | 22 | 50 | 5.98 | 0.000 |
| 9 | −28 | 36 | 26 | 4.69 | 0.000 | ||
| 6 | −30 | −8 | 44 | 4.56 | 0.000 | ||
| 11 | −40 | 46 | −14 | 4.23 | 0.000 | ||
| 6 | −22 | 18 | 60 | 3.56 | 0.002 | ||
| R | 9 | 30 | 22 | 30 | 5.12 | 0.000 | |
| 8 | 48 | 20 | 44 | 4.69 | 0.000 | ||
| 9 | 40 | 34 | 40 | 3.54 | 0.002 | ||
| Supramarginal gyrus | R | 40 | 56 | −40 | 36 | 5.86 | 0.000 |
| L | −62 | −50 | 28 | 3.29 | 0.003 | ||
| Postcentral gyrus | R | 40 | 52 | −34 | 52 | 4.78 | 0.000 |
| 2 | 70 | −26 | 32 | 5.26 | 0.000 | ||
| L | 2 | −66 | −22 | 32 | 4.66 | 0.000 | |
| 40 | −50 | −34 | 52 | 3.45 | 0.002 | ||
| Inferior parietal lobe | R | 40 | 66 | −44 | 24 | 4.29 | 0.000 |
| L | −52 | −40 | 38 | 4.59 | 0.000 | ||
| −66 | −36 | 36 | 3.65 | 0.001 | |||
| Anterior cingulate | L | 24 | −14 | 38 | 8 | 5.42 | 0.000 |
| −6 | −24 | 42 | 4.01 | 0.001 | |||
| R | 4 | 24 | 26 | 4.31 | 0.000 | ||
| Medial frontal gyrus | R | 10 | 6 | 54 | 8 | 4.88 | 0.000 |
| 9 | 22 | 44 | 18 | 4.63 | 0.000 | ||
| 10 | 4 | 60 | 22 | 4.27 | 0.000 | ||
| Inferior frontal gyrus | R | 47 | 48 | 34 | −8 | 4.81 | 0.000 |
| 45 | 56 | 18 | 18 | 3.41 | 0.002 | ||
| Fusiform gyrus | L | 20 | −42 | −2 | −30 | 4.79 | 0.000 |
| Posterior cingulate | R | 23 | 12 | −22 | 34 | 4.52 | 0.000 |
| Superior frontal gyrus | R | 6 | 20 | 14 | 60 | 4.35 | 0.000 |
| Inferior temporal gyrus | R | 20 | 48 | 0 | −34 | 4.02 | 0.001 |
| Middle temporal gyrus | R | 22 | 56 | −42 | 2 | 4.00 | 0.001 |
| High concussion group | |||||||
| Fusiform gyrus | L | 20 | −46 | −2 | −30 | 5.63 | 0.000 |
| Posterior cingulate | L | 31 | −16 | −42 | 22 | 5.35 | 0.000 |
| Hippocampus | L | na | −32 | −10 | −18 | 4.39 | 0.000 |
| Anterior cingulate | L | 32 | −18 | 36 | 18 | 4.16 | 0.001 |
| Superior temporal gyrus | L | 22 | −46 | −22 | −10 | 3.13 | 0.004 |
| Superior frontal gyrus | R | 9 | 16 | 52 | 40 | 3.90 | 0.001 |
| Middle frontal gyrus | L | 10 | −30 | 46 | 0 | 3.87 | 0.001 |
| 9 | −4 | 56 | 20 | 3.54 | 0.002 | ||
| Low concussion group>high concussion group | |||||||
| Middle Temporal gyrus | R | 22 | 50 | −44 | 4 | 3.78 | 0.000 |
| Supramarginal gyrus | R | 40 | 62 | −48 | 24 | 3.47 | 0.001 |
| Posterior cingulate | L | 31 | −18 | −62 | 28 | 3.23 | 0.002 |
| Inferior parietal lobe | L | 40 | −50 | −32 | 30 | 3.14 | 0.002 |
| Parahippocampal gyrus | R | 34 | 24 | 2 | −16 | 3.10 | 0.002 |
| Effect of accuracy: low concussion group>high concussion group | |||||||
| Posterior cingulate | L | 31 | −10 | −66 | 14 | 3.30 | 0.002 |
| High concussion group>low concussion group | |||||||
| Anterior cingulate | R | 33 | 10 | 12 | 24 | 3.55 | 0.001 |
| Medial frontal gyrus | L | 9 | 0 | 54 | 20 | 3.07 | 0.003 |
| Effect of accuracy: high concussion group>low concussion group | |||||||
| Posterior cingulate | L | 31 | −26 | −26 | 44 | 5.06 | 0.000 |
| Anterior cingulate | L | 24 | −20 | −18 | 46 | 3.50 | 0.001 |
| 24 | −12 | 40 | 4 | 3.62 | 0.001 | ||
| 32 | −2 | 42 | 4 | 3.14 | 0.002 | ||
| 24 | −8 | −6 | 44 | 3.37 | 0.001 | ||
| 24 | −2 | −6 | 38 | 3.31 | 0.002 | ||
| 32 | 0 | 20 | 32 | 3.07 | 0.003 | ||
| R | 24 | 22 | 4 | 38 | 3.77 | 0.000 | |
| 24 | 18 | −4 | 42 | 3.08 | 0.003 | ||
| Inferior frontal gyrus | L | 45 | −56 | 24 | 18 | 4.37 | 0.000 |
| 47 | −54 | 32 | 2 | 3.29 | 0.002 | ||
| R | 47 | 28 | 10 | −18 | 3.61 | 0.001 | |
| 47 | 42 | 34 | −4 | 3.51 | 0.001 | ||
| Caudate | R | na | 8 | 4 | −2 | 4.09 | 0.000 |
| Inferior parietal lobule | L | 40 | −52 | −40 | 50 | 4.04 | 0.000 |
| 40 | −62 | −38 | 34 | 3.24 | 0.002 | ||
| Postcentral gyrus | L | 2 | −46 | −28 | 44 | 3.25 | 0.002 |
| 7 | −20 | −56 | 66 | 2.96 | 0.004 | ||
| R | 3 | 40 | −24 | 42 | 3.98 | 0.000 | |
| Insula | L | 13 | −36 | 16 | 18 | 3.85 | 0.000 |
| 13 | −36 | −8 | 26 | 3.39 | 0.001 | ||
| Superior parietal lobule | L | 7 | −32 | −60 | 60 | 3.72 | 0.001 |
| Middle frontal gyrus | R | 6 | 38 | −4 | 58 | 3.62 | 0.001 |
| 6 | 30 | −6 | 44 | 3.44 | 0.001 | ||
| 9 | 52 | 12 | 38 | 3.29 | 0.002 | ||
| L | 11 | −44 | 44 | −12 | 3.54 | 0.001 | |
| Precentral gyrus | R | 4 | 44 | −14 | 50 | 3.50 | 0.001 |
| 4 | 60 | −10 | 30 | 3.03 | 0.003 | ||
| L | 6 | −36 | −14 | 52 | 3.44 | 0.001 | |
| 6 | −50 | −16 | 28 | 3.43 | 0.001 | ||
| 6 | −52 | 2 | 36 | 3.21 | 0.002 | ||
| Medial frontal gyrus | R | 10 | 2 | 56 | −6 | 3.49 | 0.001 |
| Parahippocampal gyrus | L | 28 | −26 | −20 | −12 | 3.24 | 0.002 |
| Precuneus | R | 7 | 6 | −62 | 52 | 3.13 | 0.002 |
| Regions associated with a lesser number of reported concussions | |||||||
| Middle temporal gyrus | L | 22 | −54 | −40 | −2 | 4.66 | 0.000 |
| R | 21 | 40 | −2 | −38 | 3.66 | 0.001 | |
| 50 | −34 | 0 | 3.18 | 0.002 | |||
| Parahippocampal gyrus | R | 28 | 26 | −24 | −16 | 4.40 | 0.000 |
| 28 | 18 | −2 | −14 | 3.75 | 0.000 | ||
| 36 | 42 | −38 | −12 | 3.75 | 0.000 | ||
| Precentral gyrus | L | 6 | −36 | −12 | 44 | 3.82 | 0.000 |
| Anterior cingulate | L | 32 | −16 | 40 | 10 | 3.80 | 0.000 |
| Inferior parietal lobe | R | 40 | 62 | −46 | 24 | 3.65 | 0.001 |
| 68 | −30 | 32 | 3.34 | 0.001 | |||
| Insula | L | 13 | −40 | −10 | 22 | 3.65 | 0.001 |
| Lingual gyrus | R | 19 | 18 | −58 | −4 | 3.48 | 0.001 |
| Posterior cingulate | R | 31 | 26 | −66 | 16 | 3.42 | 0.001 |
| L | 30 | −30 | −72 | 16 | 3.38 | 0.001 | |
| Postcentral gyrus | L | 3 | −30 | −32 | 40 | 3.41 | 0.001 |
| Precuneus | L | 31 | −16 | −68 | 26 | 3.35 | 0.001 |
| R | 7 | 18 | −72 | 36 | 3.03 | 0.003 | |
| Regions associated with a greater number of reported concussions | |||||||
| No significant clusters | |||||||
| Low and high concussion groups | |||||||
| Anterior cingulate | L | 32 | −16 | 38 | 16 | 5.10 | 0.000 |
| Fusiform gyrus | L | 20 | −42 | −2 | −30 | 4.79 | 0.000 |
| Middle frontal gyrus | R | 9 | 30 | 26 | 30 | 4.32 | 0.000 |
| 40 | 34 | 40 | 3.54 | 0.002 | |||
| Medial frontal gyrus | R | 10 | 4 | 60 | 22 | 4.27 | 0.000 |
| 9 | 26 | 44 | 18 | 3.63 | 0.002 | ||
Regions significant at threshold of p<0.005 (uncorrected at the voxel level); k≥10.
BA, approximate Brodmann Area. Control, 0 concussions; low concussion, 0, 1, or 2 concussions; high concussion, 3 or more concussions.
MNI, Montreal Neurological Institute.
Neural activity during recombined pair correct rejections
During correct “new” responses to recombined pairs, the age-matched group recruited left caudate, thalamus, and middle frontal gyrus, as well as right precentral gyrus. The conjunction analysis revealed that the right inferior parietal lobe and middle temporal gyrus were commonly recruited by the low and high concussion groups in this task (Table 5 and Figure 4).
Table 5.
Regions of Significant Activation during Recombined Pair Correct Rejections
| |
|
|
MNI coordinates |
|
|
||
|---|---|---|---|---|---|---|---|
| Region of interest | Hemisphere | BA | x | y | z | t value | p value |
| Control group | |||||||
| Caudate | L | na | −14 | 4 | 22 | 5.73 | 0.000 |
| Middle frontal gyrus | L | 11 | −34 | 48 | −8 | 4.36 | 0.000 |
| 8 | −46 | 20 | 44 | 4.32 | 0.000 | ||
| 47 | 50 | 48 | −8 | 4.17 | 0.001 | ||
| Thalamus | L | na | −16 | −36 | 12 | 4.26 | 0.000 |
| 0 | −4 | 20 | 3.48 | 0.002 | |||
| Precentral gyrus | R | 6 | 58 | −4 | 22 | 3.77 | 0.001 |
| Low concussion group | |||||||
| Supramarginal gyrus | R | 40 | 66 | −46 | 30 | 4.88 | 0.000 |
| Inferior parietal lobe | R | 40 | 58 | −46 | 28 | 3.70 | 0.001 |
| 46 | −52 | 54 | 3.82 | 0.001 | |||
| High concussion group | |||||||
| Middle temporal gyrus | R | 21 | 44 | 8 | −38 | 5.30 | 0.000 |
| Caudate | L | na | −18 | −38 | 20 | 4.37 | 0.000 |
| R | 16 | −18 | 32 | 3.39 | 0.002 | ||
| Insula | L | 13 | −36 | −50 | 20 | 4.37 | 0.000 |
| 13 | −28 | −10 | 26 | 3.54 | 0.002 | ||
| Anterior cingulate | L | 24 | 0 | 30 | −4 | 4.05 | 0.001 |
| Superior temporal gyrus | L | 22 | −60 | −14 | 2 | 4.01 | 0.001 |
| R | 46 | −40 | 3.83 | 0.001 | |||
| Supramarginal gyrus | L | 40 | −54 | −52 | 30 | 3.68 | 0.001 |
| Inferior parietal lobe | R | 40 | 66 | −38 | 28 | 3.29 | 0.003 |
| Low concussion group>high concussion group | |||||||
| Supramarginal gyrus | R | 40 | 58 | −54 | 32 | 3.57 | 0.001 |
| Effect of accuracy: low concussion group>high concussion group | |||||||
| Precentral gyrus | R | 6 | 44 | −4 | 36 | 6.34 | 0.000 |
| 4 | 16 | −32 | 74 | 3.37 | 0.001 | ||
| 9 | 40 | 6 | 42 | 3.72 | 0.001 | ||
| L | 4 | −24 | −28 | 48 | 4.14 | 0.000 | |
| Inferior frontal gyrus | R | 44 | 52 | 2 | 22 | 4.29 | 0.000 |
| Postcentral gyrus | R | 3 | 28 | −38 | 68 | 5.24 | 0.000 |
| 3 | 12 | −42 | 70 | 3.69 | 0.001 | ||
| L | 3 | −28 | −38 | 62 | 3.53 | 0.001 | |
| Superior frontal gyrus | R | 6 | 10 | −10 | 74 | 4.78 | 0.000 |
| 9 | 20 | 42 | 38 | 2.95 | 0.004 | ||
| L | 6 | −16 | −14 | 70 | 3.16 | 0.002 | |
| Middle frontal gyrus | R | 6 | 26 | 0 | 66 | 4.25 | 0.000 |
| 6 | 42 | 8 | 52 | 4.06 | 0.000 | ||
| 8 | 30 | 16 | 50 | 3.36 | 0.001 | ||
| Superior parietal lobule | L | 5 | −18 | −46 | 68 | 4.54 | 0.000 |
| R | 41 | 42 | −38 | 16 | 4.27 | 0.000 | |
| Middle temporal gyrus | L | 22 | −48 | −42 | −4 | 4.06 | 0.000 |
| 39 | −34 | −68 | 20 | 3.49 | 0.001 | ||
| R | 39 | 44 | −66 | 18 | 3.33 | 0.001 | |
| Posterior cingulate | R | 31 | 18 | −38 | 46 | 3.94 | 0.000 |
| 31 | 4 | −24 | 46 | 3.84 | 0.000 | ||
| 31 | 10 | −30 | 38 | 3.18 | 0.002 | ||
| Medial frontal gyrus | R | 9 | 20 | 34 | 36 | 3.81 | 0.000 |
| 6 | 4 | −10 | 64 | 3.21 | 0.002 | ||
| 6 | 14 | −26 | 58 | 3.20 | 0.002 | ||
| Anterior cingulate | L | 32 | −22 | 16 | 34 | 3.62 | 0.001 |
| High concussion group>low concussion group | |||||||
| Paracentral lobe | R | 6 | 10 | −28 | 56 | 4.67 | 0.000 |
| Inferior parietal lobe | L | 40 | −32 | −46 | 46 | 4.27 | 0.000 |
| Caudate | L | na | −20 | −40 | 18 | 4.20 | 0.000 |
| R | 22 | −20 | 24 | 3.75 | 0.000 | ||
| 28 | −40 | 10 | 3.06 | 0.001 | |||
| 10 | 10 | 20 | 3.44 | 0.001 | |||
| Insula | R | 13 | 28 | −38 | 22 | 4.05 | 0.000 |
| L | 13 | −32 | −34 | 20 | 3.53 | 0.001 | |
| Thalamus | R | na | 16 | −32 | 16 | 3.06 | 0.003 |
| 14 | −22 | 22 | 3.31 | 0.001 | |||
| Anterior cingulate | R | 24 | 18 | −4 | 44 | 4.01 | 0.000 |
| Postcentral gyrus | R | 3 | 30 | −26 | 40 | 3.58 | 0.001 |
| Posterior cingulate | L | 23 | −6 | −14 | 30 | 3.50 | 0.001 |
| Precentral gyrus | R | 6 | 42 | −4 | 38 | 3.43 | 0.001 |
| Medial frontal gyrus | L | 6 | −2 | −18 | 66 | 3.41 | 0.001 |
| Superior temporal gyrus | L | 22 | −38 | −58 | 14 | 3.32 | 0.001 |
| Precuneus | L | 19 | −34 | −74 | 34 | 2.96 | 0.003 |
| Effect of accuracy: high concussion group>low concussion group | |||||||
| Middle temporal gyrus | R | 38 | 36 | 12 | −42 | 3.22 | 0.002 |
| Regions associated with a lesser number of reported concussions | |||||||
| No significant clusters | |||||||
| Regions associated with a greater number of reported concussions | |||||||
| No significant clusters | |||||||
| Low and high concussion groups | |||||||
| Inferior parietal lobe | R | 40 | 66 | −44 | 30 | 4.01 | 0.001 |
| Middle temporal gyrus | R | 21 | 46 | 6 | −38 | 3.15 | 0.004 |
Regions significant at threshold of p<0.005 (uncorrected at the voxel level); k≥10.
BA, approximate Brodmann Area. Control, 0 concussions; low concussion, 0, 1, or 2 concussions; high concussion, 3 or more concussions.
MNI, Montreal Neurological Institute.
The low concussion group recruited right parietal regions, with the supramarginal gyrus as the only region showing greater activation for the low concussion group relative to the high concussion group (Table 5 and Figure 4). A wide range of frontal and parietal regions were associated with increased accuracy in the low concussion group to the high concussion group (Table 5 and Figure 5). The high concussion group engaged a much more extensive bilateral network of regions, including frontal, temporal, and parietal regions. Relative to the low concussion group, persons with a high number of previous concussions showed greater activation in a number of midline regions, as well as a region in the inferior parietal lobe and lateral temporal lobe (Table 5 and Figure 4). Notably, only the right lateral temporal lobe was associated with increased accuracy in the high relative to the low concussion group (Table 5 and Figure 5).
Discussion
We found that increased concussion history in former NFL players was associated with changes in the recruitment of neural regions during memory tasks, despite not having a significant long-term behavioral effect, as measured by neuropsychological tests and item and relation memory tasks. These data suggest that sustaining multiple concussions may be associated with less efficient recruitment of neural regions during memory tasks, particularly during relational memory. Our results support the hypothesis that repeated exposure to concussions may lead to long-term alterations to the underlying functional architecture supporting memory.
The use of event-related fMRI provided the opportunity to limit the analysis to correct memory trials only, eliminating potential confounds because of differences in retrieval success. Previous research has examined functional reorganization during episodic memory retrieval in persons who have sustained severe traumatic brain injuries.48,49 These studies used a blocked analysis in which all trials (correct and incorrect) of a particular condition were analyzed together. Memory accuracy in the current study was equivalent between football players with a low and high concussion history, allowing us to identify potential differences in underlying retrieval processes that were not confounded by differences in behavioral performance.
In general, persons with low and high concussion histories did not recruit substantially different regions during correct retrieval of item information. During recombined item hits, both groups engaged a large network of regions commonly associated with memory retrieval, including left lateralized inferior parietal lobe, bilateral medial PFC, and left lateral and medial temporal lobe regions. Importantly, the high degree of overlap between the low and high concussion groups demonstrates that all former athletes in our study recruit typical memory regions during item retrieval, regardless of their concussion history.
Former football players with low and high concussion histories relied on more distinct neural networks during correct responses to the relational memory task than in the item memory task. The low concussion group showed greater activation in the right parahippocampal gyrus and bilateral inferior parietal cortex (BA 40), regions that have been implicated in successful retrieval of relational information, suggesting that the high concussion group might have some underlying changes associated with relational processing compared with the low concussion group. Of note, a recent study in our laboratory used this same paradigm to compare regions recruited during relational memory in healthy older adults and older adults with a diagnosis of MCI.34 Interestingly, healthy older adults recruited medial temporal and parietal regions to a greater extent relative to patients with MCI.34 In other words, the “healthy greater than MCI” contrast identified many of the same neural regions as our “low greater than high concussion group” comparison. This important parallel strengthens previous connections between repeated sport-related concussion and diagnosis of MCI.10
The high concussion group recruited medial PFC regions to a greater extent than the low concussion group. A recent study demonstrated that this region is functionally intact in healthy older adults during self-referential tasks.50 As such, it is possible that the high concussion group might be relying more heavily on self-referential processing to successfully retrieve intact relational pairs from impairments in regions that typically subserve relational memory processes.
A related line of research has proposed that the repetitive trauma associated with sport-related concussion and the accumulation of subconcussive impacts could lead to a progressive neurological disorder known as chronic traumatic encephalopathy (CTE).51,52 This disorder has been associated post-mortem tau protein deposits, most often in scattered frontal, temporal, and less so parietal locations, at the depths of sulci and perivascularly. Clinically, the persons present with cognitive changes, personality/behavioral changes, and movement abnormalities. Although the current study does not specifically investigate CTE, it is possible that the functional differences identified in our fMRI analysis may be indicative of structural changes identified in these recent reports.
We did not estimate exposure rates for subconcussive head impacts; however, our database of nearly 3000 former NFL players indicates that those in the high concussion group are more likely to be exposed to a higher frequency of subconcussive impacts during their career than those in the low concussion group. Likewise, those with longer playing careers are more likely to have a higher dose of both concussions and subconcussive impacts. Thus, we believe there is a relationship between propensity to sustain frequent subconcussive impacts and likelihood of sustaining multiple concussions over the course of a player's career. Our findings could provide a foundation for developing a prospective investigation of CTE, because this body of literature is currently limited by analyses of in-vitro brain tissue only. Establishing a database of the in-vivo clinical correlates predictive of CTE is necessary to advance our understanding of CTE.
Limitations
A secondary goal of the current study was to compare former NFL players with a group of healthy, age-matched adults. The paradigm used in the current study has been used successfully in a number of previous experiments with healthy older adults31,34 and young adults,33 revealing a number of regions recruited during memory tasks. As such, this task has been shown as a reliable method of activating both item and relational memory networks in both young and older adults. Specifically, older adults recruit right inferior parietal lobe and right medial temporal lobe during relational relative to item memory, and frontal regions in item relative to relational memory.34
We hypothesized that our healthy older adults would also activate these regions and to a greater extent than our former football players. The age-matched adults in the current study, however, did not activate these regions, suggesting that the age-matched older adults in our study were atypical in some way. As such, any direct comparisons between the age-matched group and either concussion group are uninformative. Results from such contrasts could be caused by functional changes associated with concussion history, or by an unexpected and unexplained difference between our age-matched group and the true healthy older adult population. Because our data cannot distinguish between these options, our analysis was limited to identifying differences between concussion groups (i.e., our first research question).
Investigating the long-term effects of sport-related concussion is necessarily accompanied by the loss of experimental control. For instance, our study relies on self-report of concussion history and subsequent symptoms. Although it is true that most athletes will underreport exposure to concussion, it is likely that this pattern is systematic across individuals. Our study also does not capture the subconcussive insults sustained by this group of former players, although it is very likely that those with a higher number of concussions are most prone to a higher exposure of impacts to the head, leading to a greater number of subconcussive insults. The current study divides participants into two distinct groups that are likely to be equivalent in their tendency to underreport concussions and subconcussive insults. This has been a consistent limitation of studies involving self-reported concussion history, but all of these studies have accepted the assumption that this cohort is clearly more at risk for having sustained repetitive subconcussive head traumas compared with their age matched counterparts in the general population.
Because of the highly specialized sample (i.e., former NFL players who are physically able and willing to participate in a 1 h MRI scan), our sample size was small in this study. Such small sample sizes can lead to low power in MR studies and, consequentially, an inability to identify activity in some conditions. Future studies are needed to replicate and extend our findings, but the results of the current study introduce an important first step in understanding the long-term function effects of multiple sport-related concussion.
Finally, the sample of former players in our study was biased by selection criteria because the study was interested in examining persons who expressed some degree of recent memory problems. Specifically, former players were recruited based on their self-reported mild memory impairments in their daily lives. As such, differences between athletes and healthy age-matched adults may not be surprising; however, the current study was primarily interested in differences between groups of former NFL players. Importantly, we found important neural differences between the low and high concussion groups who were equivalent on all measures, including selection criteria. Identifying differences in the underlying neural correlates of relational memory, even when behavioral performance was equivalent, suggests that significant functional reorganization may be occurring because of repeated sport-related concussion. In addition, we disqualified persons who reported taking supplements before the study began, potentially excluding a subset of persons who were already concerned about memory changes. Although we do not believe that including this subset would have altered our results, it is possible that our findings would not generalize to persons who have taken action to reduce memory impairment.
Conclusions
The current study serves as an important first step in understanding the long-term functional consequences of sport-related concussion. Behaviorally, we demonstrated that players who have low and high concussion histories have a specific relational memory deficit compared twith healthy age-matched adults. Importantly, concussion history does not appear to have an effect of behavioral performance in this task, with the two groups performing equivalently in both item and relational memory tasks. fMRI data suggest, however, that the high concussion group has different patterns of neural activity associated with relational, but not item, memory processing. Such differences suggest that repeated concussions and neural trauma may lead to inefficient recruitment of neural regions during the retrieval of novel relationships. Future research is needed to investigate these patterns in a randomly selected group of former players, across different playing positions, and inclusive of an estimate of both subconcussive and concussive insults to the head. Such a study will allow for a better understanding of the long-term effects of sport-related concussions and subconcussive impacts to the head on specific regions of the brain and may lead us to an understanding of possible interventions to mitigate the long-term effects.
Acknowledgments
The authors would like to thank Amy Matthews and Candice Goerger for their assistance in completing this work. This work was supported by National Institutes on Aging (AG028774 to KSG) and the Martek Biosciences Corporation (KMG).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Gessel L.M. Fields S.K. Collins C.L. Dick R.W. Comstock R.D. Concussions among United States high school and collegiate athletes. J. Athl Train. 2007;42:495–503. [PMC free article] [PubMed] [Google Scholar]
- 2.Pellman E. J. Powell J.W. Viano D.C. Casson I.R. Tucker A.M. Feuer H. Lovell M. Waeckerkle J.F. Robertson D.W. Concussion in professional football: epidemiological features of game injuries and review of the literature—Part 3. Neurosurgery. 2004;54:81–96. doi: 10.1227/01.neu.0000097267.54786.54. [DOI] [PubMed] [Google Scholar]
- 3.McCrea M. Hammeke T. Olsen G. Leo P. Guskiewicz K. Unreported concussion in high school football players: implications for prevention. Clin. J. Sport Med. 2004;14:13–17. doi: 10.1097/00042752-200401000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention, Atlanta. Nonfatal traumatic brain injuries from sports and recreational activities—United States 2001–2005. MMWR Morb. Mortal Wkly. Rep. 2007;56:733–737. [PubMed] [Google Scholar]
- 5.Guskiewicz K.M. McCrea M. Marshall S.W. Cantu R.C. Randolph C. Barr W. Onate J.A. Kelly J.P. Cumulative effects associated with recurrent concussion in collegiate football players: the NCAA Concussion Study. JAMA. 2003;290:2549–2555. doi: 10.1001/jama.290.19.2549. [DOI] [PubMed] [Google Scholar]
- 6.Broglio S.P. Eckner J.T. Paulson H.L. Kutcher J. Cognitive decline and aging: the role of concussive and subconcussive impacts. Exerc. Sport Sci. Rev. 2012;40:138–144. doi: 10.1097/JES.0b013e3182524273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belanger H.G. Vanderploeg R.D. The neuropsychological impact of sports-related concussion: a meta-analysis. J. Int. Neuropsychol. Soc. 2005;11:345–357. doi: 10.1017/s1355617705050411. [DOI] [PubMed] [Google Scholar]
- 8.De Beaumont L. Theoret H. Mongeon D. Messier J. Leclerc S. Tremblay S. Ellemberg D. Lassonde M. Brain function decline in healthy retired athletes who sustained their last sports concussion in early adulthood. Brain. 2009;132:695–708. doi: 10.1093/brain/awn347. [DOI] [PubMed] [Google Scholar]
- 9.Tremblay S. De Beaumont L. Henry L.C. Boulanger Y. Evans A.C. Bourgouin P. Poirier J. Theoret H. Lassonde M. Sports concussions and aging: A neuroimaging investigation. Cereb. Cortex. 2013;23:1159–1166. doi: 10.1093/cercor/bhs102. [DOI] [PubMed] [Google Scholar]
- 10.Guskiewicz K.M. Marshall S.W. Bailes J. McCrea M. Cantu R.C. Randolph C. Jordan B.D. Association between recurrent concussion and late-life cognitive impairment in retired professional football players. Neurosurgery. 2005;57:719–726. doi: 10.1093/neurosurgery/57.4.719. [DOI] [PubMed] [Google Scholar]
- 11.Petersen R.C. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 12.Tulving E. Elements of Episodic Memory. Clarendon Press; Oxford: 1983. [Google Scholar]
- 13.Yonelinas A.P. Consciousness, control, and confidence: The 3 Cs of recognition memory. J. Exp. Psychol. Gen. 2001;130:361–379. doi: 10.1037//0096-3445.130.3.361. [DOI] [PubMed] [Google Scholar]
- 14.Clark S.E. Word frequency effects in associative and item recognition. Mem. Cognit. 1992;20:231–243. doi: 10.3758/bf03199660. [DOI] [PubMed] [Google Scholar]
- 15.Hockley W.E. Item versus associative information: Further comparisons of forgetting rates. J. Exp. Psychol. 1992;18:1321. [Google Scholar]
- 16.Hockley W.E. Consoli A. Familiarity and recollection in item and associative recognition. Mem. Cognit. 1999;27:657–664. doi: 10.3758/bf03211559. [DOI] [PubMed] [Google Scholar]
- 17.Yonelinas A.P. Recognition memory ROCs for item and associative information: The contribution of recollection and familiarity. Mem. Cognit. 1997;25:747–763. doi: 10.3758/bf03211318. [DOI] [PubMed] [Google Scholar]
- 18.Anderson N.D. Ebert P.L. Jennings J.M. Grady C.L. Cabeza R. Graham S.J. Recollection- and familiarity-based memory in healthy aging and amnestic mild cognitive impairment. Neuropsychology. 2008;22:177–187. doi: 10.1037/0894-4105.22.2.177. [DOI] [PubMed] [Google Scholar]
- 19.Troyer A.K. Murphy K.J. Anderson N.D. Hayman-Abello B.A. Craik F.I. Moscovitch M. Item and associative memory in amnestic mild cognitive impairment: Performance on standardized memory tests. Neuropsychology. 2008;22:10–16. doi: 10.1037/0894-4105.22.1.10. [DOI] [PubMed] [Google Scholar]
- 20.Fowler K.S. Saling M.M. Conway E.L. Semple J.M. Louis W.J. Paired associate performance in the early detection of DAT. J. Int. Neuropsychol. Soc. 2002;8:58–71. [PubMed] [Google Scholar]
- 21.Ivanoiu A. Adam S. Van der Linden M. Salmon E. Juillerat A.C. Mulligan R. Seron X. Memory evaluation with a new cued recall test in patients with mild cognitive impairment and Alzheimer's disease. J. Neurol. 2005;252:47–55. doi: 10.1007/s00415-005-0597-2. [DOI] [PubMed] [Google Scholar]
- 22.Buckner R.L. Functional-anatomic correlates of control processes in memory. J Neurosci. 2003;23:3999–4004. doi: 10.1523/JNEUROSCI.23-10-03999.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eichenbaum H. Yonelinas A.P. Ranganath C. The medial temporal lobe and recognition memory. Ann. Rev. Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vilberg K.L. Rugg M.D. Left parietal cortex is modulated by amount of recollected verbal information. Neuroreport. 2009;20:1295–1299. doi: 10.1097/WNR.0b013e3283306798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wheeler M.E. Buckner R.L. Functional-anatomic correlates of remembering and knowing. Neuroimage. 2004;21:1337–1349. doi: 10.1016/j.neuroimage.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 26.Yonelinas A.P. Otten L.J. Shaw K.N. Rugg M.D. Separating the brain regions involved in recollection and familiarity in recognition memory. J. Neurosci. 2005;25:3002–3008. doi: 10.1523/JNEUROSCI.5295-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davis G.A. Iverson G.L. Guskiewicz K.M. Ptito A. Johnston K.M. Contributions of neuroimaging, balance testing, electrophysiology and blood markers to the assessment of sport-related concussion. Br. J. Sports Med. 2009;43(Suppl 1):i36–i45. doi: 10.1136/bjsm.2009.058123. [DOI] [PubMed] [Google Scholar]
- 28.Jantzen K.J. Functional magnetic resonance imaging of mild traumatic brain injury. J. Head Trauma Rehabil. 2010;25:256–266. doi: 10.1097/HTR.0b013e3181e5477c. [DOI] [PubMed] [Google Scholar]
- 29.Ellemberg D. Henry L.C. Macciocchi S.N. Guskiewicz K.M. Broglio S.P. Advances in sport concussion assessment: from behavioral to brain imaging measures. J. Neurotrauma. 2009;26:2365–2382. doi: 10.1089/neu.2009.0906. [DOI] [PubMed] [Google Scholar]
- 30.Broglio S.P. Pontifex M.B. O'Connor P. Hillman C.H. The persistent effects of concussion on neuroelectric indices of attention. J. Neurotrauma. 2009;26:1463–1470. doi: 10.1089/neu.2008.0766. [DOI] [PubMed] [Google Scholar]
- 31.Giovanello K.S. Schacter D.L. Reduced specificity of hippocampal and posterior ventrolateral prefrontal activity during relational retrieval in normal aging. J. Cogn. Neurosci. 2012;24:159–170. doi: 10.1162/jocn_a_00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giovanello K.S. Schnyer D. Verfaellie M. Distinct hippocampal regions make unique contributions to relational memory. Hippocampus. 2009;19:111–117. doi: 10.1002/hipo.20491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giovanello K. Schnyer D.M. Verfaellie M. A critical role for the anterior hippocampus in relational memory: Evidence from an fMRI study comparing associative and item recognition. Hippocampus. 2004;14:5–8. doi: 10.1002/hipo.10182. [DOI] [PubMed] [Google Scholar]
- 34.Giovannello K.S. De Brigard F. Ford J.H. Kaufer D.I. Burke J.R. Browndyke J.N. Welsh-Bohmer K.A. Event-related functional magnetic resonance imaging changes during relational retrieval in normal aging and amnestic mild cognitive impairment. J. Int. Neuropsychol. Soc. 2012;18:886–897. doi: 10.1017/S1355617712000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kerr Z.Y. Marshall S.W. Guskiewicz K.M. Reliability of concussion history in former professional football players. Med. Sci. Sports Exerc. 2012;44:377–382. doi: 10.1249/MSS.0b013e31823240f2. [DOI] [PubMed] [Google Scholar]
- 36.Folstein M.F. Folstein S.E. McHugh P.R. “Mini-Mental State”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 37.Donnell A. Rapidly-administered short forms of the Wechsler Adult Intelligence Scale-3rd edition. Arch. Clin. Neuropsychol. 2007;22:917–924. doi: 10.1016/j.acn.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 38.Benton A.L. Hamsher K. Multilingual Aphasia Examination. AJA Associates; Iowa City, Iowa: 1976. [Google Scholar]
- 39.Adjuntant General's Office. Army Individual Test Battery: Manual of Directions and Scoring. War Department; Washington, DC: 1944. [Google Scholar]
- 40.Kaplan E. Goodglass H. Weintrab S. The Boston Naming Test. Lea and Febiger; Philadelphia: 1983. [Google Scholar]
- 41.Wechsler D. Wechsler Test of Adult Reading. The Psychological Corporation; San Antonio, TX: 2001. [Google Scholar]
- 42.Sheikh J.I. Yesavage J.A. Clinical Gerontology : A Guide to Assessment and Intervention. The Haworth Press; New York: 1986. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version; pp. 165–173. [Google Scholar]
- 43.Burock M.A. Buckner R.L. Woldorff M.G. Rosen B.R. Dale A.M. Randomized event-related experimental designs allow for extremely rapid presentation rates using functional MRI. Neuroreport. 1998;9:3735–3739. doi: 10.1097/00001756-199811160-00030. [DOI] [PubMed] [Google Scholar]
- 44.Fisher S. 4th. Oliver and Boyd; London: 1932. Statistical Methods for Research Workers. [Google Scholar]
- 45.Lazar N.A. Luna B. Sweeney J.A. Eddy W.F. Combining brains: a survey of methods for statistical pooling of information. Neuroimage. 2002;16:538–550. doi: 10.1006/nimg.2002.1107. [DOI] [PubMed] [Google Scholar]
- 46.Lieberman M.D. Cunningham W.A. Type I and Type II error concerns in fMRI research: re-balancing the scale. Soc. Cogn. Affect. Neurosci. 2009;4:423–428. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Talairach J. Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. Thieme Medical Publishers; New York, NY: 1988. [Google Scholar]
- 48.Levine B. Cabeza R. McIntosh A. Black S.E. Grady C.L. Stuss D.T. Functional 37e organization of memory after traumatic brain injury: a study with H2150 positron emission tomography. J. Neurol. Neurosurg. Psychiatry. 2002;73:173–181. doi: 10.1136/jnnp.73.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ricker J.H. Müller R.A. Zafonte R.D. Black K.M. Millis S.R. Chugani H. Verbal recall and recognition following traumatic brain injury: a [0-15]-water positron emission tomography study. J. Clin. Exp. Neuropsychol. 2001;23:196–206. doi: 10.1076/jcen.23.2.196.1204. [DOI] [PubMed] [Google Scholar]
- 50.Gutchess A H. Kensinger E.A. Schacter D.L. Aging, self-referencing, and medial prefrontal cortex. Soc. Neurosci. 2007;2:117–133. doi: 10.1080/17470910701399029. [DOI] [PubMed] [Google Scholar]
- 51.McKee A C. Cantu R.C. Nowinski C.J. Hedley-Whyte E.T. Gavett B.E. Budson A.E. Santini V.E. Lee H.S. Kubilus C.A. Stern R.A. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J. Neuropathol Exp. Neurol. 2009;68:709–735. doi: 10.1097/NEN.0b013e3181a9d503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Omalu B. Bailes J. Hamilton R.L. Kamboh M.I. Hammers J. Case M. Fitzsimmons R. Emerging histomorphologic phenotypes of chronic traumatic encephalopathy in American athletes. Neurosurgery. 2011;69:173–183. doi: 10.1227/NEU.0b013e318212bc7b. [DOI] [PubMed] [Google Scholar]